Abstract
Background
Screening decreases colorectal cancer (CRC) morbidity and mortality, yet remains underutilized. Screening breakdowns arise from lack of uptake and failure to follow-up after a positive screening test.Objectives
Systems of support to increase colorectal cancer screening and follow-up (SOS) is a randomized trial designed to increase: (1) CRC screening and (2) follow-up of positive screening tests. The Chronic Care Model and the Preventive Health Model inform study design.Methods
The setting is a large nonprofit healthcare organization. In part-1 study, patients age 50-75 due for CRC screening are randomized to one of 4 study conditions. Arm 1 receives usual care. Arm 2 receives automated support (mailed information about screening choices and fecal occult blood tests (FOBT)). Arm 3 receives automated and assisted support (a medical assistant telephone call). Arm 4 receives automated, assisted, and care management support (a registered nurse provides behavioral activation and coordination of care). In part-2, study patients with a positive FOBT or adenomas on flexible sigmoidoscopy are randomized to receive either usual care or nurse care management. Primary outcomes are: 1) the proportion with CRC screening, 2) the proportion with a complete diagnostic evaluation after a positive screening test.Results
We sent recruitment letters to 15,414 patients and 4675 were randomized. Randomly assigned treatment groups were similar in age, sex, race, education, self-reported health, and CRC screening history.Conclusions
We will determine the effectiveness and cost effectiveness of stepped increases in systems of support to increase CRC screening and follow-up after a positive screening test over 2years.Free full text

Systems of Support to Increase Colorectal Cancer Screening and Follow-up Rates (SOS): design, challenges, and baseline characteristics of trial participants
Abstract
Background
Screening decreases colorectal cancer (CRC) morbidity and mortality, yet remains underutilized. Screening breakdowns arise from lack of uptake and failure to follow-up after a positive screening test.
Objectives
Systems of Support to Increase Colorectal Cancer Screening and Follow-up (SOS) is a randomized trial designed to increase: (1) CRC screening and (2) follow-up of positive screening tests. The Chronic Care Model and the Preventive Health Model inform study design.
Methods
The setting is a large nonprofit healthcare organization. In part-1 study, patients age 50-75 due for CRC screening are randomized to one of 4 study conditions. Arm 1 receives usual care. Arm 2 receives automated support (mailed information about screening choices and fecal occult blood tests (FOBT)). Arm 3 receives automated and assisted support (a medical assistant telephone call). Arm 4 receives automated, assisted, and care-management support (a registered nurse provides behavioral activation and coordination of care). In part-2, study patients with a positive FOBT or adenomas on flexible sigmoidoscopy are randomized to receive either usual care or nurse care-management. Primary outcomes are: 1) the proportion with CRC screening, 2) the proportion with a complete diagnostic evaluation after a positive screening test.
Results
We sent recruitment letters to 15,414 patients and 4,675 were randomized. Randomly assigned treatment groups were similar in age, sex, race, education, self-reported health, and CRC screening history.
Conclusions
We will determine the effectiveness and cost-effectiveness of stepped increases in systems of support to increase CRC screening and follow-up after a positive screening test over 2 years.
1. Introduction
Colorectal cancer (CRC) is the second leading cause of cancer mortality in the United States. Screening by fecal occult blood testing (FOBT), flexible sigmoidoscopy, and colonoscopy decreases colorectal cancer mortality and morbidity [1, 2]. Despite these benefits, only 50 - 60% of eligible adults are screened at recommended intervals, and many have never had any type of screening test [3, 4]. Screening failures occur not only from lack of screening but also from breakdowns in follow-up of positive tests. Strategies for improving CRC screening typically focus on either patients or health care providers, without addressing the infrastructure change, systems of support, and costs required to implement and sustain these changes.
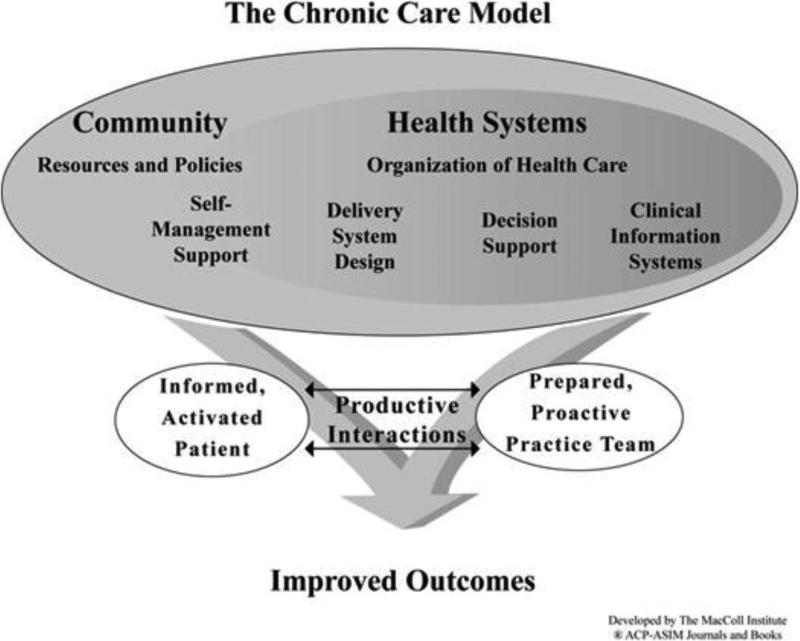
The Chronic Care Model was developed by Ed Wagner and others to address system-based deficiencies in the clinical care of chronic health conditions [5]. This model has also been applied to primary prevention interventions including improving breast cancer screening rates [6]. The model's 6 domains are evidence-based decision support, self-management support, delivery system redesign, clinical information systems, healthcare systems, and community resources. According to the model, paying attention to these domains and integrating them can produce system changes in which informed, activated patients interact collaboratively with prepared practice teams (figure 1). We describe here a two-part, randomized controlled trial based on the Chronic Care Model to improve CRC screening and follow-up rates. We follow the specifications of the revised Consolidated Standards of Reporting Trials (CONSORT) Statement for Randomized Trials of Nonpharmacologic Treatment [7] and the Standards for Quality Improvement Reporting Excellence (SQUIRE) [8] to report study methods.
2. Trial Design and Methods
2.1. Overview
Systems of Support to Improve Colorectal Cancer Screening Rates and Follow-up (SOS) is a two-part, randomized controlled trial designed to improve CRC screening and follow-up rates (figure 2). In part 1, patients age 50-75 due for CRC screening are randomized to one of four interventions with increasing stepwise support to complete CRC screening. Group 1 receives usual care (UC), which includes a birthday letter reminding patients to complete screening tests, immunizations, and chronic condition care as well as efforts by physicians and their teams at routine or preventive care visits to increase CRC screening. Group 2 receives usual care plus automated support (including mailed information on different options for CRC screening, mailed screening FOBT cards, mailed reminders, and a screening hotline to call if they have questions or screening preferences). Group 3 receives usual care, automated support, plus assisted support (including a telephone call from a medical assistant [MA] who documents choice and assists the patient with the resources already provided or sends requests to the patient's physician). Group 4 receives usual care, automated, assisted, and care management support from a registered nurse (including telephone-based counseling about screening test choices and an action plan and follow-up to assure completion of the chosen screening method). In part 2 of the trial, patients with a positive FOBT or flexible sigmoidoscopy screening test are randomized to either Group A usual care (UC) or Group B care management (usual care plus care management from a registered nurse). Patients enrolled in the trial receive interventions and follow-up for 2 years. We hypothesize that increasing systems of support (SOS) will lead to increased rates of CRC screening (including annual completion of FOBT) and increased rates of a complete diagnostic evaluation after a positive screening test.
2.2. Study setting
The study setting is Group Health, which is a large, nonprofit, integrated delivery system that provides both medical coverage and care to more than 600,000 members in Washington State and Idaho. This study is being conducted at 21 Group Health-owned primary care medical centers in the Puget Sound Region. Family practitioners (90%) and internists (10%), over 95% of whom are certified by their respective boards, provide primary care for adults. Each full-time physician cares for a defined panel of 1900 patients on average. Medical centers are divided into teams, which consist of three to four physicians, registered nurses (RNs), licensed practical nurses (LPNs), medical assistants (MAs), and a physician assistant or nurse practitioner. Almost all patients age 65 and older are Medicare patients, while most patients under age 65 are in commercial plans.
The demographic mix of Group Health enrollees is similar to the surrounding area, except that they are somewhat older (46% ≥ 45 years vs. 38% in the regional community), more likely to be employed, and more educated. African Americans 4%, Pacific Islander or American Indian 3%, and Hispanic 4%. Group Health is accredited by the National Committee for Quality Assurance (NCQA) and Healthcare Effectiveness Data and Information Set (HEDIS). CRCS rates in 2009 were 66.7% (FOBT in the measurement year, FS within 5 years, CS within 10 years, or barium enema within 5 years). However, HEDIS counts patients as screened if any code specific to FOBT submitted claim/encounter occurs, [9] regardless of the test being returned, so organizational estimates for CRCS are likely overestimated.
2.3 Participants
The Group Health Institutional Review Board (IRB) approved all study procedures. Participant recruitment began in July of 2008 and was completed in November of 2009. Subjects age 50 – 75 were eligible if continuously enrolled in Group Health for at least 2 years, and based on administrative data, also due for CRC screening (defined as no evidence of a FOBT in the last 8 months, no flexible sigmoidoscopy in the last 4 years, and no colonoscopy in the last 9 years). The recruitment sample pool was refreshed weekly to include or exclude potential participants based on their most recent CRC screening test. We also used administrative data to exclude patients with a history of the following: colorectal cancer, inflammatory bowel disease (Crohn's disease or ulcerative colitis), total colectomy, a myocardial infarction in the previous 3 months, and end stage or organ failure diseases (dementia, renal or liver failure, COPD on home oxygen, strokes, pacemakers or defibrillators), or active treatment for cancer with chemotherapy.
2.4 Telephone Recruitment
Figure 2 depicts recruitment and study flow. Those eligible based on automated data were sent recruitment letters to introduce the study. Research assistants then called potential participants to confirm eligibility, including their ability to converse in English over the telephone. Those no longer enrolled or planning to leave the health plan in the next year were excluded. Those reporting a flexible sigmoidoscopy in the prior 4 years or a colonoscopy in the prior 9 years were also excluded. Patients reporting a family history of colorectal cancer in a first degree relative age 60 or younger were also excluded and advised to discuss screening with their primary care physician. Patients who remained eligible and willing to participate (in both part 1 and 2 of the study) provide verbal consent (signed consent was not required). Then, they were sent a copy of the consent and a phone number to call if they decided not to participate after reading the form.
Approximately 25% of patients on the sample list were randomly pre-selected (prior to consent and randomization) to complete an additional survey with more detailed questions related to CRC screening knowledge, perceived risk for CRC, pros and cons of screening in general as well as for specific screening tests, and their self-efficacy and social support to complete screening. If their name is on this list and they consented to the longer questionnaire (20 minutes), they received $10 to thank them for their time. They did not need to complete the longer survey to participate in the trial. Participants completing the longer survey will be contacted again in 2 years to complete these questions again.
2.5 Study interventions
2.5.1. Study 1: Interventions to Increase CRC Screening Rates
Intervention components by study group are depicted in table 1. The components were selected based on the Chronic Care Model (figure 1) and its six domains: (1) evidence based guidelines (for CRC screening and follow-up and decision support tools); (2) information systems (electronic medical records, patient registries, automated mailings); (3) delivery design (the healthcare team and processes of care); (4) self-management support (patient information, informed decision making); (5) health care policy (insurance coverage, HEDIS measures [Health Employer Data Information Set]), and community resources (mass media, professional societies, worksite and community activities).
Table 1
Study 1 Intervention Components*
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| UC | UC + Auto | UC + Auto + Assist | UC + Auto + Assist + Care Management | |
| Evidence Based Guidelines and Decision Support | ||||
| ■ CRC screening guideline & Web based decision support for physicians | Yes | Yes | Yes | Yes |
| ■ Academic detailing of CRC screening guidelines | No | No | Yes | Yes |
| Information Systems | ||||
| ■ Registry of patients current CRC screening status | No | Yes | Yes | Yes |
| ■ Outreach screening and follow-up reminds tracking | No | Yes | Yes | Yes |
| Delivery System Design | ||||
| ■ Mailed FOBT screening kits with stamps and reminds | No | Yes | Yes | Yes |
| ■ Medical Assistant | No | No | Yes | Yes |
| - Proactive | ||||
| - Identifies Intent | ||||
| - Assists with Action Plan | ||||
| ■ Nurse Care | No | No | No | Yes |
| - Proactive | ||||
| - Decision Counseling/Preference Clarification | ||||
| - Arranges Action Plan (clinical assessments, orders test, coordination of care) | ||||
| Self-Management Support | ||||
| ■ Small media patient information (Web, paper) | Yes | Yes | Yes | Yes |
| ■ Access to cancer screening resource line | No | Yes | Yes | Yes |
| ■ Personalized screening schedule | No | Yes | Yes | Yes |
| ■ Mailed FOBT with simplified instructions | No | Yes | Yes | Yes |
| ■ Mailed reminds if screening not done | No | Yes | Yes | Yes |
| ■ Medical Assistant intent clarification and action planning | No | No | Yes | Yes |
| ■ Nursing care decision counseling/preference clarification, shared-decision making, assistance with preparation and completion of tests | No | No | No | Yes |
| Healthcare Policy and Community Resources | ||||
| ■ Workgroup of GH leaders, stakeholders, physicians, and researchers complete intervention protocols and processes of care | Yes | Yes | Yes | Yes |
| ■ HEDIS/Group Health CRCS at health plan level | Yes | Yes | Yes | Yes |
| ■ HEDIS/Group Health CRCS rates feedback at physician level | Yes | Yes | Yes | Yes |
| ■ Mass Media promoting CRCS | Yes | Yes | Yes | Yes |
The Chronic Care Model does not specify a specific behavioral construct for optimizing patient self-management but often uses an “action plan” to help patients define their health goals and to make a plan to attain these. We use the Preventive Health Model to guide patient-level behavioral interventions (figure 3) [10-12]. The Preventive Health Model is based on the Health Belief and Theory of Reasoned Action Models and describes both moderators and mediators (modifiable behaviors) related to completion of cancer screening. Potential moderators of screening behavior include patient demographic characteristics. Modifiable factors might include patient perceived susceptibility and worry or concerns about CRC, knowledge of screening and the different tests, pros and cons of the different tests, social influence, and screening self-efficacy. We hypothesize that both individual behavioral and systems of care factors might influence patient CRC screening “intent” and “action.”
Arm 1: Usual Care (UC)
Group Health recommends either yearly FOBT alone, FOBT yearly combined with flexible sigmoidoscopy every 5 years, or colonoscopy every 10 years. Usual care at Group Health already includes provider-level services to promote CRC screening including evidence based CRC screening guidelines, computer generated reports of patients who are not up to date for CRC screening and other quality measures. Usual care also includes patient-level paper information on CRC screening, online at a patient Web site, and in brochures in clinic. Additionally, all adult patients receive an annual letter from Group Health on their birthday listing dates of recommended immunization, screening tests, chronic condition care, and reminders if an item is due (including CRC screening).
Arm 2: Usual Care + Automated (Auto)
In addition to usual care, Arm 2 patients receive interventions delivered by a centralized automated registry system designed for the study. The registry is linked to Group Health clinical, laboratory, and claims databases and automatically tracks when screening is due and generates mailings accordingly. Approximately one week after randomization, Arm 2 patients receive a letter informing them they are due for CRC screening and a pamphlet with information on the different screening CRC tests and pros and cons of each. They are also informed FOBT cards will be mailed soon, but if they prefer another CRC screening test, they can call the SOS telephone line. Patients calling the SOS telephone line speak to a research assistant who informs them to make an appointment, call, or send a secure e-mail to their health care team to request an alternative to FOBT (entry of this information turns off subsequent year 1 mailings). Arm 2 patients not requesting alternative testing receive FOBT Hemoccult SENSA® cards (Beckman-Coulter Inc., Primary Care Diagnostics, Los Angeles, CA), simplified pictorial instructions for obtaining the specimens, and a postage-paid return envelope for returning them to the lab. FOBT results are entered into Group Health's laboratory databases with the SOS database capturing these results daily. If there is no evidence of FOBT completion after 3 weeks, a letter is sent to patients reminding them to complete the test or inquire about other types of CRC screening options. Automated systems are used to track interventions, record completion of CRC screening tests, and generate mailings. Quality processes are in place to assure proper labeling of the mailings and FOBT cards.
Arm 3: UC + Auto + Assisted (Assist)
In addition to automated support, Arm 3 patients receive telephone assistance from a medical assistant (MA) to complete the CRC test of their choice. The MA checks the registry database “to do list” to identify patients who have either called to request alternate screening or have not completed FOBT screening within 3 weeks of the mailings. She makes up to 3 attempts to contact the patient. Once she has contacted the patient, the MA determines the patient's CRC screening choice (such as planning to do the FOBT soon, prefers sigmoidoscopy or colonoscopy, or does not want to complete the test). If the patient needs assistance completing the cards or counseling related to making a screening choice, MAs can review the materials already sent to the patient (e.g. pictorial instructions for completing FOBTs and written information on risks and benefits of different screening tests). If the patient wants a flexible sigmoidoscopy or colonoscopy, the MA forwards an order to the patient's primary care physician. As per usual care, physicians may either sign and execute the order or refuse the order and suggest an alternate plan. If the patient still has clinical concerns or needs more information, the MA recommends making a telephone or in person appointment with their physician.
The MA documents all communications in the patient's electronic medical record (EMR) and updates the database. After the MA makes 3 attempts or contact is completed, a second code is used to remove the patient from the “to do” list. Quality of data entry and electronic medical records are monitored.
Arm 4: UC + Auto + Assist + Care Management
In addition to automated and assisted support, Arm 4 patients receive care management. Arm 4 patients who proactively call the SOS screening line with questions or requests for an alternative to FOBT are put directly in contact with an RN. Arm 4 patients not completing FOBT and not requesting an alternative to FOBT are initially contacted by the MA who makes up to 2 attempts to reach the patient and ascertain the patient's screening intent. The MA assists those who are still planning to do the FOBT (reminding, ordering more cards, or reviewing the pictorial instructions) and updates the database if the patient prefers not to do screening or an alternative test has been ordered by their physician. If the MA is unable to reach the patient after the second attempt, further attempts are made by the RN. The RN also contacts Arm 4 patients, who need assistance in making a screening choice, prefers an alternative to FOBT, or intend to do the FOBT, but after 3 weeks no FOBT laboratory results are in the database.
RN care management includes assessing patient risk for CRC and procedural risk; providing motivational counseling to assist with screening intent and completion of an action plan to complete this; assisting with referrals, appointments, and preparation for flexible sigmoidoscopy or colonoscopy; and tracking completion of the screening test. If the RN is unable to reach the patient, s/he sends the patient a secure e-mail (if they have secure access to the Group Health patient Web portal) or a letter (if they do not have or do not open their e-mails). The secure e-mail/letter reiterates the importance of completing CRC screening, gives brief information on the different options for screening, and provides the RN's phone number for assistance.
The RN uses the registry database “to do” list to track patients needing communication and to monitor completion of flexible sigmoidoscopy or colonoscopy. If the patient is waiting to have a colonoscopy, the RN can temporarily take the patient off the “to do” list until a time closer to testing. The RN also uses specific codes to document processes of care and an additional code to confirm that care has been completed. All communications and the assistance provided are documented in the patient's EMR.
Year 2 interventions (repeating annual FOBT screening)
Arms 2, 3, and 4 receive the same intervention in year 2 of the study as year 1. Exceptions to this include patients completing either a screening or diagnostic (after a positive screening test or for other reasons) colonoscopy in year 1.
2.6. Study 2: Interventions to Increase Follow-up After a Positive Test
Overview
All SOS study participants, regardless of their Study 1 screening intervention group, are potentially eligible for the second part of the study. Patients with a positive screening FOBT (captured via the study database) or a positive screening sigmoidoscopy (abstracted biopsy results with adenomatous or advanced adenomatous polyps) are randomized to Group A Usual Care (UC) or Group B UC + Nurse Care Management at the time of their positive FOBT or screening sigmoidoscopy.
Similar to Study 1, we use the Chronic Care and Preventive Health Models to plan interventions to increase follow-up after a positive CRC screening test (table 2). We hypothesize systems of support (a registry and RN care management) will lead to increased rates of follow-up after a positive CRC screening test.
Table 2
Part-2 Interventions to Increase Follow-up after a Positive Screening Test*
| Group A Follow-Up Positive Tests UC - Reminds | Group B Follow-Up Positive Tests UC Reminds + Nurse | |
|---|---|---|
| Decision Support | ||
| ■ GHC CRCS guideline & Web based decision support for physicians | Yes | Yes |
| ■ Academic detailing of CRC screening guidelines | No | Yes |
| Information Systems | ||
| ■ In reach – EMR reminds | Yes | Yes |
| ■ Lists of patients with unresolved positive FOBT sent to physicians | Yes | Yes |
| ■ Database to track patients with positive FOBT or FS with precancerous polyps | No | Yes |
| Delivery System Design | ||
| ■ Designated nursing staff to assist with scheduling and non-adherence | No | Yes |
| ■ Designated RN(s) to follow-up of positive FOBT and FS, and clinical issues related to CRC screening | No | Yes |
| Self-Management Support * | ||
| ■ Patient mailed and e-mail notification of lab results including +FOBT | Yes | Yes |
| ■ Nurse assistance with educational aspects of follow-up testing, test preparation, and issues related to non-adherence | No | Yes |
| Healthcare Policy and Community Resources | ||
| ■ Increasing recognition of the importance of follow-up after screening | Yes | Yes |
Group A: Usual Care (UC)
Patients with a positive FOBT or positive flexible sigmoidoscopy CRC screening test randomized to Group A receive usual care. Group Health keeps a registry of all patients with positive FOBT tests. Physicians receive reports quarterly (and, more recently, electronic staff messages) of their patients with a positive FOBT and no completion of a colonoscopy (preferred) or the combination of a flexible sigmoidoscopy and barium enema. Physicians can stop these reminders if they decide a colonoscopy is not indicated or there are other reasons for the patient not completing follow-up (such as patient refusal). Physicians or ancillary health care providers (physician assistants and nurse practitioners) who perform flexible sigmoidoscopy are responsible for follow-up after a positive biopsy. However, there is no centralized system of reminders to track completion of the follow-up.
Group B: Nurse Care Management
In addition to usual care, patients in Group B receive nurse case management. Study RNs receive notifications in the study database “to do” list when they are assigned a new Group B patient. They subsequently track and monitor the patient's progress and completion of diagnostic testing. They work with the patient's primary care physician and other health care providers to ensure the referral process goes smoothly and the patient is prepared and able to complete diagnostic testing. They have a number of resources that can be used if patients have barriers such as lack of transportation, language difficulties, or billing concerns. They also work with the referral gastroenterology office teams to assure referrals go smoothly. Using specific criteria, they identify patients at higher procedural risk who need an in-person evaluation prior to the procedure. All communications and the assistance provided are documented in the patient's EMR. For example, if the patient refuses a colonoscopy, the RN will work with the patient and his or her physician to make sure this is properly documented in the EMR. If the patient cannot be reached, a certified letter is sent requesting that they call the RN. The certified letter and its status after mailing (recipient signed or unable to find recipient and unsigned) is scanned into the EMR. The study RN also fills out a brief checklist documenting recommended processes of care.
Group B interventions last for 6 months. After 6 months, patients in Group A who have not yet had a colonoscopy receive the same care management as Group B patients to ensure optimal care processes for all SOS participants. Depending on the results of the colonoscopy (such as the presence of advanced adenomatous polyps), some patients may need follow-up colonoscopies or other tests. Group Health has a tracking system in place for this and often this is scheduled to occur after the end of the study. The SOS study does not track completion of repeat follow-up colonoscopies.
2.7. Hiring and Training of the MAs and RNs
Both MAs and RNs were required to already be Group Health employees and show competence in their use of the electronic medical records prior to work on the project. In our hiring process, we found that Group Health had many more licensed practical nurses (LPNs) than MAs. Both had similar job descriptions, except the LPNs have a few additional care privileges (for instance, the ability to give immunizations in clinic). Because of the similarities, we hired both MAs and LPNs for Arm 3 interventions. To simplify discussion, we label both LPNs and MAs as having the MA role. RNs have much more training than LPNs or MAs and have a larger, more complex set of clinical privileges.
The MAs and RNs were trained separately to avoid contamination. MAs received 2 half days of training. These sessions included reviewing study procedures, basic information on CRC screening guidelines as well as the materials sent to patients (CRC screening pamphlet and FOBT instructions) and practicing delivery of the telephone-based intervention. They were trained to use the database “to do list” including the use of a limited set of coded tasks documenting attempted and completed telephone contacts and documentation of the patient's screening “intent” (figure 4). MA patient contact continues to be periodically observed. Queries and audits of the database and EMR are performed quarterly to assure MAs are adhering to protocols and documenting care accurately.
The RNs received 3 half days of training. These sessions included reviewing study procedures as well as Group Health and national CRC screening guidelines, identifying patients at high risk for CRC or at risk for complications from endoscopic procedures, and reviewing the preparations required for endoscopy and rules related to clinical concerns. They were trained to use the database including how to use the “to do” list, how to document completed tasks (for instance, screening intent) and how to track the completion of action plans (figure 4). One half-day session was dedicated to role playing using different vignettes (for example, patients who are too busy or resistant because of perceived lack of risk). The RNs are trained to use motivational interviewing (MI) techniques including using open-ended questions and clarifying statements, knowing how to handle resistance in a non-judgmental manner, and facilitating patient-centered action plans for completing screening [13]. The RNs, project manager, and principal investigator meet by phone every other week to review concerns related to the study and patient care and to review and practice MI techniques. To ensure fidelity to the behavioral approach, patient communications are directly observed each quarter.
2.8 Physician Participation
Prior to recruitment, the principal investigator contacted each medical center's medical and administrative directors to review the study design and procedures including potential impacts on physician practices. Physicians were informed there will be requests for screening flexible sigmoidoscopies ,colonoscopies and FOBT results in their EMR inboxes. They were instructed not to assume that their patients will be cared for by the study and to continue best practices to ensure CRC screening and follow-up after a positive FOBT. Physicians received no other interventions during the trial.
2.9 Adverse Event Monitoring
Adverse events are monitored by the project manager and principal investigator. They review any death that occurs during the study period or any hospitalization within 30 days of a flexible sigmoidoscopy or colonoscopy. The protocol for reporting adverse and unanticipated events conforms to standards set by the IRB and the National Cancer Institute (NCI).
3. Objectives and Outcomes (Table 3)
Table 3
Outcomes measurements: tools, sources, and timing
| Aim | Outcome | Measurement | Data Sources | Collection Schedule |
|---|---|---|---|---|
| Primary Outcomes | ||||
| #1 | CRC screening rates | The proportion having CRC screening defined as: (1)Any CRC screening between T1-T3 (2)HEDIS annual screening rates T1-T2, T2-T3 (3)ACS screening rates T1-T3 | Automated data: claims and laboratory, OP and radiology procedures | T1-T2 T2-T3 T1-T3 |
| #2 | Follow-up rates after a positive FOBT or flexible sigmoidoscopy | Proportion of people with a positive FOBT or flexible sigmoidoscopy receiving a complete diagnostic evaluation at 6 months | “ | T4 |
| Secondary Outcomes | ||||
| #3 | Cognitive, Affective, and Social Factors related to CRC screening | Mean change from baseline (domains) Moderator effects on primary outcomes | Patient Long Survey* | T1, T3 |
| Satisfaction with medical services | Ware Scales | Patient Long Survey* | T1, T3 | |
| #4 | Utilization and costs | Utilization and costs | Automated data, cost of the interventions, costing data | T1 – T4 |
T1 = Baseline T2 = 12 months after randomization
T3 = 24 months after randomization
T4 = 6 months after a +FOBT or +flexible sigmoidoscopy
Aim #1
The primary objective of study 1 is to determine the proportion of patients screened for CRC. We use two definitions of completion of CRC screening: (1) any CRC screening between baseline and 24 months (T1 to T3); (2) The National Committee for Quality Assurance (NCQA) HEDIS definition [14] for annual completion of CRC screening (annual FOBT, flexible sigmoidoscopy within 5 years, or colonoscopy within 10 years) from T1 to T2 and T2 to T3. Receipt of CRC screening will be measured using the laboratory, encounter, and claims administrative databases at Group Health.
Aim #2
The primary objective for study 2 is to determine the proportion of participants with a complete diagnostic evaluation after a positive CRC screening test. Complete diagnostic evaluation (CDE) is defined as having either a colonoscopy (preferred) or a combination of flexible sigmoidoscopy and barium enema within 6 months of the date of the positive FOBT or flexible sigmoidoscopy. Automated data (procedure and claims codes) will be used to measure CDE receipt. There can be appropriate clinical reasons for patients not having a CDE including serious illness, frailty, death, or refusal. While we will describe these occurrences, these will be classified as non-receipt of a CDE.
Aim #3
Secondary aims include assessing patient behavioral components related to CRCS including: knowledge of CRC and CRCS, the pros and cons of CRCS testing, worries about and perceived risk of CRC, and self-efficacy for being able to complete testing. To measure psychosocial correlates related to screening, we use a questionnaire developed by Myers and Vernon based on the Preventive Health Model (which contains items related to pros, cons, social influence, and self-efficacy). Adaptations of this instrument have been used in diverse samples and have demonstrated good construct validity across different subgroups [12, 15-17].
Aim # 4
Secondary aims also include assessing the cost and incremental cost-effectiveness of each intervention condition. The centerpiece of this aim is a formal economic evaluation of the trial interventions; the trial design will facilitate assessment of the true incremental cost-effectiveness of the various study arms. Given the relatively short follow-up period for the trial, our utilization and cost measures will be directly linked to events that we expect to be observable within 30-months post randomization. For each study group, we will evaluate the quantity, type, and costs of utilization (primary care, specialty care, hospitalizations, laboratory, and pathology service). Group Health data are sufficiently detailed to allow us to isolate the reason for each visit (and telephone consultations), which allows us to identify visits for CRC testing as distinct from visits for all other purposes. Outcomes included in the economic evaluation will map directly to the trial results (for example, improvement in the proportion of patients screened for CRC).
4. Data Collection and Quality Control
We will account for the number of patients at every stage of our recruitment, randomization, and intervention processes so that we can report patient flow according to the CONSORT guidelines (figure 2). Ongoing queries, tests, and automated reports are run regularly to assure the accuracy of our data collection systems. Group Health electronic medical records and databases are very accurate as confirmed in other studies [18]. FOBTs are done by a centralized laboratory using a bar code system that identifies the patient, test, and status of the test. Flexible sigmoidoscopies are almost all performed within the group practice and available in EMR data. Colonoscopies are performed both Group Health and by contracted providers thus we rely on both EMR and claims data to collect these. To assess our accuracy in capturing colonoscopies with our automated database, we performed a chart audit of 150 records (100 randomly chosen from those without evidence of colonoscopy in claims or EMR data, and 50 randomly chosen from those with such evidence). Among these 150 records, we observed no classification errors for FOBT or flexible sigmoidoscopy receipt. We found one record with automated data evidence of colonoscopy but no information in the chart record that this was done. Thus if we consider the medical record to be correct, the SOS automated data capture for receipt of colonoscopy in this sample exhibited a positive predictive value (PPV) of 98% and a negative predictive value (NPV) of 100%
5. Sample Size and Power
The sample size selected (5000 total, 1250 in each of the 4 study arms), based on estimates needed to power study-2 (see below), provide good power to detect effects for Study 1 primary outcomes. With this sample size, assuming a 20% loss to follow-up, we will have 80% power to detect an 8% difference in screening during the first 2 years with 5% type-I error by adjusting for all possible (6) comparisons (alpha = 0.05/6) among the 4 randomized groups. The above power calculation is applicable to either FOBT or the combined screening of FOBT, flexible sigmoidoscopy, and colonoscopy. We expect groups 2, 3 and 4 to have effects greater than 10%. Two recently published studies including one that mailed FOBT cards to patients and the other that used automated telephone calls found an 8% absolute difference between those offered the active intervention and controls, and we will have sufficient power to find a similar difference.[19, 20]
Much larger sample sizes are needed to detect effects on Study 2 follow-up rates because only a small proportion in each group will have positive tests requiring diagnostic follow-up. Based on internal data and published reports, we assume that 6% of completed FOBT and 10% of flexible sigmoidoscopies are positive [21, 22]. Some patients will complete two FOBTs between T1 and T3 and have a 6-12% chance of receiving a positive test result. We conservatively estimate that 8% of patients who complete a FOBT or flexible sigmoidoscopy during the study will have a positive test. With 5000 patients in study 1, we predict we will have approximately 225 positive CRC screening tests. If we assume a 10% loss to follow-up, we will have 80% power to detect a 13% difference in the proportion receiving a complete diagnostic evaluation.
To assess differences between the groups for outcomes assessed using the “longer” patient interview, smaller sample sizes are adequate. Therefore, at baseline, we chose at random a sample of approximately 300 participants per group to receive the long interviews at baseline (all participants complete a shorter interview at baseline) and who we will attempt to interview again 24-months post-randomization. This sample size will give us 80% power to detect a 15% difference on a binary outcome (like the % rating their care as excellent), assuming a 40% rate in group 1 and a corresponding 55% rate in group 2, 3, or 4. The above calculation has taken into consideration multiple comparisons of Group 1 versus the other 3 groups. We assume that 80% of those who complete a baseline survey will complete the follow-up survey.
6. Randomization
6.1 Study 1 randomization
Patients were randomly assigned to one of three intervention arms or a usual care control arm automatically within the computer database. We waited 7 days after receiving verbal consent from each participant before assigning him or her to a group to allow patients time to withdraw their consent after viewing a mailed copy of the consent form. Randomization was stratified on medical center, age group (age 50 to <65 or age 65 to <75), and receipt of CRC screening as self-reported on the baseline telephone survey. Assignments in all strata were blocked in groups of 8; for each stratum we created a list of treatment assignments to issue consecutively, each comprised of random permutations of the numbers 1 through 4 which each occur exactly twice in each block of eight entries. With the assignments made in this way, the size of each study arm's membership within each stratum was guaranteed never to differ by more than 2.
6.2 Study 2 randomization
Patients with a positive FOBT or flexible sigmoidoscopy are automatically randomized to Group A (usual care) or to Group B (usual care and nurse care management) using a pre-set computer generated random number sequence as soon as the positive test appears in the database. Allocation is thus concealed to both patients and the study personnel. Randomization is stratified on medical center only, and assignments within each medical center stratum are blocked groups of 4. In this procedure, stratum-specific treatment assignment lists are comprised of random permutations of “Group A” and “Group B”, each occurring twice in every block of four entries. Study 1 intervention group is not a stratifying factor in the randomization for Study 2, but will be adjusted for as a regression covariate in the analysis of Study 2 data.
7. Blinding
Investigators are blinded to study group assignment until the end of Study 1 and Study 2 interventions and the completion of data collection (except statisticians who ascertain the quality of data collection). Because of the nature of the intervention, it is not possible to blind the MAs or RNs to the patient's assignment group.
8. Statistical Analysis
8.1 General Considerations
Given the large sample size, we expect that differences in age, sex, race, ethnicity, and education between groups at baseline will be small. Primary analyses will compare the unadjusted effect of treatment assignment on screening receipt. While we do not expect confounding, estimates of treatment effects from secondary adjusted analyses may benefit from improvements in precision, and correct for chance imbalances which may exist the distributions of baseline covariates. Secondary analyses will repeat the primary comparison of treatment effect across randomization group after adjustment for medical center, self-reported age, sex, race, ethnicity, and education. All analyses will be based on the “intent to treat” principle. Regardless of actual level of participation in the intervention by those in the intervention groups, outcomes will be analyzed using the groups participants were randomly assigned. We will also attempt to obtain follow-up data from all study participants, regardless of their levels of participation in the interventions.
8.2. Analysis of primary outcomes
Specific aim #1
To compare the effectiveness of each Study 1 intervention condition on increasing CRC screening. Being current for CRC screening in year 1 (T1-T2), year 2 (T2-T3), and for both years (T1-T3) will be treated as binary outcomes.
We will first perform an overall chi-square test to look for differences in the proportion screened across treatment groups. If this overall test is significant at P=.05, we will then compare the proportion screened in Group 1 with the proportions in each of the three intervention groups using chi-square tests. We will also test for differences between Groups 2, 3, and 4, adjusting our significance threshold to 0.5 ÷ 6 to account for the six possible pair-wise comparisons of the four intervention groups. Because data on FOBT, flexible sigmoidoscopy, colonoscopy, and barium enema are obtained from automated databases, the main reason for incomplete data will be disenrollment from the health plan. Based on prior analyses, we expect less than 12% of participants will leave the health plan during the study period. The data will be analyzed by using a modified intent-to-treat principle and the intent-to-treat principle but with sensitivity analysis for the loss to follow-up data. In the modified intent-to-treat analysis, participants who are lost to follow-up before undergoing screening will be excluded from the analysis. In the intent-to-treat analysis, we will conduct sensitivity analyses under various assumptions for the mechanism underlying loss to follow-up. In addition, we will apply inverse selection probability weighting and multiple imputation methods to adjust for the loss to follow-up data.[23]
We will use administrative data alone to measure our primary outcome (receipt of CRC screening). Administrative and claims data (FOBT, flexible sigmoidoscopy, and colonoscopys) is periodically reviewed comparing codes to chart audit and is nearly 100% accurate and complete (and validated by formal review for other studies). We do not have codes to directly distinguish screening and diagnostic tests. Therefore, if a patient completes a FOBT, flexible sigmoidoscopy, or colonoscopy because of symptoms, they are henceforth considered screened. However, for patients diagnosed with CRC, we will censor CRC screening tests occurring after the diagnosis.
Specific Aim #2
To compare the effectiveness of each Study 2 intervention condition, usual care or nurse care management, on follow-up after a positive screening FOBT or flexible sigmoidoscopy. We will compare the proportion of participants who followed-up within 6 months of the test result date across the two intervention conditions using a chi square test of proportions. To allow for covariate adjustment, we will use a logistic regression model to compare the adjusted odds of follow-up visit receipt. We will also compare time elapsed from receipt of a positive test result until post-test follow-up visits across the two intervention conditions. We will construct Kaplan-Meier survival curves [24] for each intervention condition and compare with log-rank tests that are both unstratified and stratified by original SOS treatment group assignment.
8.3. Analysis of secondary outcomes
Specific Aim #3
Those receiving the long survey at baseline (approximately 25% of those participating) will also be surveyed at the end of the intervention, 24 months, in order to determine the effects of each intervention condition on patient self-efficacy, cons, pros, social influences, and cancer worries related to CRC screening. We will summarize the frequency distributions of individual survey items, and compute means and standard deviations of psychosocial scales which are derived from individual measures. Additionally, we will assess the reliability of computed scales using Cronbach's alpha. Multiple survey items will be used to derive scales for self-efficacy (10 items), cons (12 items), pros (7 items), social influences (3 items), and cancer worries (3 items). Each scale is derived by summing the individual survey items which comprise the scale, and dividing by the number of items for which each respondent provided valid data. The resulting scores will range from 1 to 5, with higher scores associated with greater endorsement of self-efficacy, screening cons, screening pros, social influences, and cancer worries. Items comprising the cancer worries scale were transformed to standardize the valence and range of the possible responses prior to deriving the scale. We will use linear regression to estimate the association between treatment assignment and each of the five continuous psychosocial scales mentioned above. We will conduct each analysis both with and without adjustment for age, sex, clinic, ethnicity, race, and education. We will use two-sided Wald tests of regression coefficients to assess statistical significance, with an alpha of 0.05.
Specific Aim #4
To assess the incremental cost effectiveness of each intervention condition.
We will conduct a cost-effectiveness analysis (CEA) of Group 2, 3, and 4 on the outcomes of CRC screening and follow-up for a minimum of 30 months following randomization (T4). The first level of analysis will provide estimates of the direct medical care costs per patient of implementing each intervention. Next, we will conduct an incremental CEA from the health plan perspective to determine the costs of CRC screening and to what degree the costs change by intervention.
Relevant costs from a health plan/health system perspective are the resources necessary to deliver the intervention as well as the change in all medical care costs incurred by the plan or system derived from the intervention. Capital and hardware costs of the intervention (e.g. FOBT cards) will be tracked through invoices and Group Health's decision support system (DISC): a validated tool [25] that collect costs of care from multiple data feeds (clinic visits, lab tests, procedures) and Group Health ledgers and converts total care cost (nurses salaries, facility costs, supplies, overhead) into a unit of service. Each department uses one statistic (unit of service) as a proxy for cost behavior in that department. All costs will be expressed in local market terms rather than using Group Health specific costs to avoid idiosyncrasies of Group Health's purchasing or human resource policies. Evaluation costs incurred to satisfy research objectives will not be included, as these would not be incurred in a real world application of the CRC screening. All costs will be inflation-adjusted and reported in current year dollars.
To conduct a CEA from a health plan perspective, we will estimate the incremental cost effectiveness ratios (ICER) [26] associated with CRC screening. ICER yields the additional cost incurred through one intervention to improve on the outcomes obtained from a reference strategy given by the following equation: ICER = [Ci - Cj] /[Ei - Ej], where Ci,j and Ei,j are the costs and effectiveness associated with the iTH and jTH strategies. We will perform sensitivity analyses to examine the influence of uncertainty in the variables and assumptions used in analyses [27, 28]. We will initially perform univariate and bivariate sensitivity analyses. As results warrant, we will then estimate model performance through confidence intervals estimated in a probabilistic manner using Monte Carlo simulation [26, 29]. Through this approach, all model parameters are allowed to take a range of values described by specified distributions that represent the uncertainty in their estimation. In this way, probabilistic sensitivity analyses allow for the effects of joint uncertainty across all parameters in the model under consideration. We will report results of the probabilistic sensitivity analysis as cost-effectiveness acceptability curves (CEAccs). When comparing more than two strategies, a CEAcc illustrates the probability that a particular strategy is optimal (meaning, it produces the greatest excess of benefits over cost and therefore, should be implemented based on current information) over a range of possible amounts (λ) that the relevant decision maker might be willing to pay for an additional outcome unit. The probability that a treatment is optimal at a given λ is proxied by the proportion of simulation iterations for which that treatment is optimal.
9. Results
Baseline characteristics of the study population
A total of 4,675 patients have been randomized to the SOS trial. Baseline socio-demographic characteristics, general health status, and family history of CRC do not vary by group (table 4a). Over 85% are age 50 -64 in part, most likely, because there are more patients in this age group at Group Health, and because they have had less time to be exposed and current for CRC screening than those age 65 and over. Of those enrolled, 46% had not been previously screened for CRC, with no differences by group. (This was expected as patients were stratified by age and self-reported previous CRC screening prior to randomization.)
Table 4a
Participant characteristics at randomization (baseline) by treatment assignment
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| UC | UC + Auto | UC + Auto + Assisted | UC + Auto + Assisted + Care Mgt | |
| Characteristics | n=1167 | n=1173 | n=1161 | n=1174 |
| Age, mean (sd) | 58.0 (6.1) | 57.6 (5.9) | 57.9 (5.9) | 57.9 (5.9) |
| (missing) | 0 | 0 | 0 | 0 |
| Age group, n (%) | ||||
| 50 – 64 | 988 (85%) | 1000 (85%) | 983 (85%) | 992 (85%) |
| 65 – 75 | 179 (15%) | 173 (15%) | 178 (15%) | 182 (16%) |
| (missing) | 0 | 0 | 0 | 0 |
| Female, n (%) | 653 (56%) | 631 (54%) | 616 (53%) | 649 (55%) |
| (missing) | 0 | 0 | 0 | 0 |
| Race / Ethnicity, n (%) | ||||
| American Indian/Alaska Native | 40 (3%) | 50 (4%) | 47 (4%) | 52 (4%) |
| Asian | 64 (6%) | 62 (5%) | 53 (5%) | 58 (5%) |
| Black or African American | 44 (4%) | 54 (5%) | 64 (6%) | 67 (6%) |
| Hawaiian or Pacific Islander | 8 (1%) | 14 (1%) | 12 (1%) | 7 (1%) |
| Hispanic | 43 (4%) | 27 (2%) | 35 (3%) | 49 (4%) |
| White | 950 (82%) | 944 (81%) | 928 (80%) | 923 (79%) |
| Other | 7 (1%) | 18 (2%) | 14 (1%) | 14 (1%) |
| (missing) | 11 | 4 | 8 | 4 |
| General Health, n (%) | ||||
| Excellent | 259 (22%) | 261 (22%) | 247 (21%) | 266 (23%) |
| Very Good | 464 (40%) | 477 (41%) | 483 (42%) | 486 (41%) |
| Good | 338 (29%) | 366 (31%) | 354 (30%) | 335 (29%) |
| Fair | 97 (8%) | 62 (5%) | 70 (6%) | 73 (6%) |
| Poor | 7 (1%) | 7 (1%) | 4 (0%) | 13(1%) |
| (missing) | 2 | 0 | 3 | 1 |
| Married or living with partner, n (%) | 838 (72%) | 873 (74%) | 867 (75%) | 870 (74%) |
| (missing) | 3 | 0 | 2 | 2 |
| Highest education, n (%) | ||||
| High school grad, GED, or less | 191 (16%) | 170 (15%) | 174 (15%) | 168 (14%) |
| Some college, 2 year degree, or vocational training | 368 (32%) | 364 (31%) | 353 (30%) | 383 (33%) |
| Bachelor's degree or higher | 606 (52%) | 637 (54%) | 634 (55%) | 623 (53%) |
| (missing) | 2 | 2 | 0 | 0 |
| Employment, n (%) | ||||
| Full time or part time | 837 (72%) | 850 (72%) | 832 (72%) | 837 (71%) |
| Retired | 260 (22%) | 267 (23%) | 273 (24%) | 276 (24%) |
| Other* | 68 (6%) | 56 (5%) | 56 (5%) | 59 (5%) |
| (missing) | 2 | 0 | 0 | 2 |
| Never been screened for CRC, n (%) | 540 (46%) | 545 (46%) | 541 (47%) | 544 (46%) |
| (missing) | 0 | 0 | 0 | 0 |
| First degree relative with CRC > age 60, n (%) | 55 (5%) | 50 (4%) | 51 (4%) | 59 (5%) |
| (missing) | 22 | 16 | 17 | 18 |
| Other family or friend with CRC, n (%) | 201 (17%) | 217 (19%) | 213 (18%) | 211 (18%) |
| (missing) | 15 | 24 | 10 | 12 |
| Completed Long Survey | 321 (28%) | 349 (30%) | 285 (25%) | 297 (25%) |
| (missing) | 0 | 0 | 0 | 0 |
In general, participants were very educated, with 84% reporting having at least some education greater than a high school degree or GED. This may be, in part, due to the fact that most Group Health patients not enrolled in Medicare are insured by employee plans. Even in this educated population with health insurance, almost half reported they had never completed any type of CRC screening prior to enrollment in the study.
Group Health does not collect data on race or ethnicity. To maximize minority enrollment, we oversampled from clinics with higher proportions of minority groups. This resulted in 20% of our participants reporting a race or ethnicity other than non-Hispanic white. The U.S. Census bureau estimated that in 2008, 15.4% of the state of Washington's population was a race other than white [30]. To improve generalizability, we only excluded patients with end-stage or life-threatening diseases (for example, renal failure or patients undergoing chemotherapy for cancer). We were uncertain as to whether this would allow patients in poor health to be enrolled. However, most participants reported being in good to excellent health (93%). Only <1%, reported poor health.
Baseline scores for potential psychosocial mediators (self-efficacy, pros, cons, social influence, and cancer worries) for CRC screening are presented in table 4b for the 1,252 participants randomly pre-selected to answer the long survey (26.8% of the total sample).
Table 4b
Psychosocial Scale Scores among 1,252 study participants randomly selected to complete the supplementary SOS Long Survey
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| UC | UC + Auto | UC + Auto + Assisted | UC + Auto + Assisted + Care Mgt | |
| Characteristics | n=321 | n=349 | n=285 | n=297 |
| Self Efficacy Scale, mean (sd) | 4.3 (0.8) | 4.3 (0.8) | 4.2 (0.8) | 4.3 (0.8) |
| (missing) | 1 | 1 | 1 | 0 |
| CRC Screening Cons Scale, mean (sd) | 1.8 (0.7) | 1.7 (0.7) | 1.9 (0.8) | 1.7 (0.7) |
| (missing) | 1 | 1 | 1 | 0 |
| CRC Screening Pros Scale, mean (sd) | 4.5 (0.6) | 4.6 (0.5) | 4.4 (0.6) | 4.5 (0.6) |
| (missing) | 1 | 0 | 1 | 0 |
| Social Influence Scale, mean (sd) | 3.7 (1.1) | 3.9 (0.9) | 3.6 (1.1) | 3.6 (1.0) |
| (missing) | 5 | 8 | 10 | 8 |
| Cancer Worries Scale, mean (sd) | 2.3 (0.8) | 2.2 (0.8) | 2.2 (0.7) | 2.2 (0.7) |
| (missing) | 1 | 0 | 1 | 1 |
All scales range from 1 to 5, with higher scores associated with stronger endorsement of self-efficacy, screening cons, screening pros, social influence, and cancer worries.
10. Challenges encountered
10.1 Preserving the fidelity of MA and RN roles
An explicit study aim is to test the incremental cost-effectiveness of the active interventions. Thus, we designed each intervention to be as efficient and low cost as possible. Prior to enrolling patients, we decided to have MA/LPNs make the initial phone call to Arm 4 patients who did not complete FOBT or request an alternative. MAs handle logistic concerns (reminders, resending cards). RN care management is reserved for those who request flexible sigmoidoscopy or colonoscopy, need further counseling to make a choice or those who have clinical concerns. Arm 4 RN care management is intended to be “centralized care,” with nurses using specific protocols to assess patient procedural risk, provide shared decision for screening test choice, notify the patient's physician when colonoscopy screening was chosen, but not requiring the physician to order the test. Group Health RNs, however, were not authorized to order referrals in the EMR. We tried using a paper system, but papers were misplaced and referrals needed to be re-requested. If there was a wait for a colonoscopy, the nurse would have to re-establish the patient's place in the queue. We subsequently decided the RNs would send an electronic order to the patient's physician (similar to the MAs). This requirement could potentially blur the differences between Arm 3 and 4 interventions, as physicians might choose not to approve or ignore the requested endoscopy order. There are still differences between Arm 3 MA assistance and Arm 4 RN care management. RNs provide motivational counseling and ongoing tracking of Arm 4 patients planning to do a flexible sigmoidoscopy or colonoscopy until completion and those who told the MA they were planning to do a FOBT but did not complete this after 3 weeks time. In contrast, MAs only provide brief assistance and do not track test completion. After our study was in the field, the Agency for Health Research and Quality recently [31] published a technology review of interventions to increase CRC screening. They concluded that “health systems” interventions that included “patient care navigators” (who navigated the health care system with the patient until screening was completed) led to increased FOBT, flexible sigmoidoscopy, and colonoscopy screening rates. It is not clear whether this type of provider needs to be a RN, but currently in our health care system MAs and LPNs do not provide care that involves clinical decision making such as risk assessment, motivational counseling, and ongoing tracking of care. We will be able to determine the effect of brief MA assistance versus ongoing RN care management on flexible sigmoidoscopy and colonoscopy ordering and completion rates, and the incremental cost-effectiveness of each.
10.2 Changes in national and local CRC screening guidelines
Over the course of the study, national and local CRC guidelines changed for patients at average risk for CRC. In 2008, 2 new sets of CRC guidelines were released. One set of guidelines was developed by the American Cancer Society, American College of Radiology, and American gastroenterology societies (the American College of Gastroenterology, The American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy) [32]. This guideline differentiates CRC screening tests into 2 groups: tests that identify early CRC (FOBT and stool DNA), and tests that identify both early CRC and premalignant adenomatous polyps (flexible sigmoidoscopy, optical colonoscopy, virtual CT colonoscopy, and double contrast barium enema). The second guideline was developed by the US Preventive Services Task Force, an independent panel of experts sponsored by the Agency for Health Care Research and Quality [33]. Based on their systematic review of the literature and economic modeling, they recommend CRC screening for adults age 50-75 either by “high sensitivity” FOBT annually (fecal immunochemical tests [FIT] or fecal guaiac Hemoccult SENSA©), flexible sigmoidoscopy every 5 years with a high sensitivity FOBT in between, or a colonoscopy every 10 years.
Prior to 2009, Group Health recommended screening for adults age 50-80 preferably by flexible sigmoidoscopy every 10 years combined with annual FOBT (Hemoccult SENSA©) or colonoscopy every 10 years as an alternative. In accordance with the USPSTF changes, beginning in the fall of 2009, Group Health's recommended CRC screening for average risk adults age 50-75 is annual FOBT as the preferred option, or combining this with flexible sigmoidoscopy every 5 years or colonoscopy alone every 10 years. Most of these changes have little impact on the SOS protocols, as we already limited eligibility to adults age 50-75 and send patients the “high sensitivity” SENSA FOBT annually, unless they request an alternative. However, MA/LPN/RNs found these differences confusing and needed additional training and monitoring to master these changes.
10.3 Quality improvements in follow-up of positive screening tests
After the grant was awarded, other investigators at Group Health's Research Institute discovered that complete diagnostic evaluation rates after a positive FOBT were increasing at Group Health. These results were published in Medical Care in 2008 [34]. Miglioretti et al. found that in 2005, 82% of patients with a positive FOBT had a complete diagnostic evaluation within 1 year (almost exclusively colonoscopy), compared to our estimates of a 65% completion rate at 6 months used for our power estimates (based on older data). Group Health has a remind system to notify physicians of patients not completing a CDE after a positive FOBT (section 2.6). However, Group Health's quality initiative does not include RN care management. We hypothesize that RN care management might further increase rates, diagnostic testing will occur more quickly, and if a CDE was not done, documentation will be improved.
10.4. Lower than expected positive FOBT rates
An additional concern is that our positive FOBT rate has been lower than we predicted (section 4). In our trial, only 3% of the completed FOBT tests have been positive. These rates are lower than Group Health historically and studies with Hemoccult SENSA© [21, 35, 36]. The Group Health lab has confirmed compliance with quality procedures and data reporting is complete. We hypothesize that study participants are younger and healthier than the overall population receiving FOBT at clinic visits and that our pictorial instructions may be leading to higher dietary compliance. Ongoing positive flexible sigmoidoscopy rates are similar to our estimates (approximately 12% require follow-up testing). Lower than expected positive FOBT rates may compromise our ability to find significant differences between Study 2 usual care and RN care management interventions.
11. Discussion
Systems of Support to Increase Colorectal Cancer Screening and Follow-up (SOS) is a 2-part randomized controlled trial based on the Chronic Care Model for the organization of care and Preventive Health Model for behavioral strategies to increase CRC screening and follow-up. We have successfully enrolled and randomized 4,675 patients to one of four intervention groups. Patients receive increasing levels of systems of support and behavioral support to complete screening and diagnostic evaluation after a positive test. Our primary outcome is receipt of CRC screening. Our secondary aims include change in complete diagnostic evaluation rates after a positive screening test, moderators and mediators related to screening, and the incremental cost-effectiveness of the 3 different interventions. This is the first study we know of to use the Chronic Care Model to organize care to increase CRC screening rates. Our study is also unique in that we include an intervention to improve follow-up after a positive test. Additionally, SOS study interventions last 2 years, allowing us to have an opportunity to study whether patients are willing to repeat FOBT screening annually.
Even though we have encountered a few challenges, we believe these are outweighed by the trial's strengths. We have successfully recruited 4,675 patients over 15 months. We have created a simple database registry that efficiently tracks a patient's screening status, automatically generates mailings, and informs MAs and RNs of needed interventions. Interventions are being successfully delivered by 1 research specialist for automated Arm 2 interventions, 3 part-time MAs for assisted Arm 3 interventions, and 2 part-time RNs for care managed Arm 4 and Study 2 interventions. Our initial results demonstrate that baseline characteristics of the study participants are balanced by group. A-priori planned sub-analyses by age group (50-64 and 65 -75 and prior CRC screening yes/no) will be possible. We will also be able to assess the comparative effectiveness and cost-effectiveness of automated and stepped levels of assisted and arranged care on improving CRC screening rates relative to usual care.
Acknowledgments
This research is funded by a grant from the National Cancer Institute: Grant R01 CA121125 ClinicalTrials.gov Identifier: NCT00697047
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.cct.2010.07.012
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2956868?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.cct.2010.07.012
Article citations
Patterns of Care Following a Positive Fecal Blood Test for Colorectal Cancer: A Mixed Methods Study.
J Gen Intern Med, 21 May 2024
Cited by: 0 articles | PMID: 38771535
Applying evidence-based intervention (EBI) mapping to identify the components and logic of colorectal cancer screening interventions.
Transl Behav Med, 12(2):304-323, 01 Feb 2022
Cited by: 2 articles | PMID: 34730181 | PMCID: PMC8848996
A Centralized Program with Stepped Support Increases Adherence to Colorectal Cancer Screening Over 9 Years: a Randomized Trial.
J Gen Intern Med, 37(5):1073-1080, 28 May 2021
Cited by: 3 articles | PMID: 34047921 | PMCID: PMC8162159
First-year implementation of mailed FIT colorectal cancer screening programs in two Medicaid/Medicare health insurance plans: qualitative learnings from health plan quality improvement staff and leaders.
BMC Health Serv Res, 20(1):132, 21 Feb 2020
Cited by: 6 articles | PMID: 32085767 | PMCID: PMC7035739
Using a continuum of hybrid effectiveness-implementation studies to put research-tested colorectal screening interventions into practice.
Implement Sci, 14(1):53, 29 May 2019
Cited by: 13 articles | PMID: 31142348 | PMCID: PMC6542058
Go to all (28) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT00697047
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.