Abstract
Free full text

Latent activity rhythm disturbance sub-groups and longitudinal change in depression symptoms among older men
Abstract
Activity rhythms disturbances and depression often co-occur among older adults. However, little is known about how activity rhythm disturbances themselves co-occur, or how disturbances to multiple aspects of the activity rhythm relate to depression over time. In the current study, we performed a Latent Class Analysis to derive sub-groups of older men (total n=2933, mean age=76.28, SD=5.48) who shared similar patterns of activity rhythm disturbances (defined as extreme values of modeled activity rhythm parameters). We found eight sub-groups with distinct combinations of activity rhythm disturbances: one had all normative activity rhythm parameters (32.09%), one had only lower activity (10.06%), three had earlier activity (totaling 26.96%), and three had later activity (totaling 30.89%). Groups with similar timing were distinguished depending on whether the relative length of the active period was shorter and/or if the activity rhythm had lesser amplitude/robustness. We next examined whether the derived activity rhythm sub-groups were associated with different rates of change in depression symptom levels over an average of 5.5 (0.52 SD) follow-up years. The sub-group with lower activity only had faster increases in depressive symptoms over time (compared to the group with normative rhythm parameters), but this association was accounted for by adjustments for concurrently assessed health status covariates. Independent of these covariates, we found four activity rhythm disturbance sub-groups that experienced faster depressive symptom increases (compared with the normative sub-group): These included all three sub-groups that had later activity timing, and one sub-group that had earlier activity timing plus a shorter active period and a dampened rhythm. Low activity rhythm height/robustness with normal timing therefore may mark depression risk that is attributable to co-occurring disease processes; in contrast, having late or combined early/compressed/dampened activity rhythms may independently contribute to depression symptom development. Our findings suggest that activity rhythm related depression risk is heterogeneous, and may be detected when multiple aspects of rhythm timing are delayed or when early timing is accompanied by compressed/dampened activity rhythms. Future studies should consider how distinct combinations of altered activity rhythm timing and height/robustness develop and conjointly determine health risks. Future research is also needed to determine whether/how activity rhythms can be modified to improve depression outcomes.
Activity rhythm disturbances are associated with depressed mood (Luik, Zuurbier et al., 2013; Maglione, Ancoli-Israel et al., 2013; Robillard, Naismith et al., 2014; Luik, Zuurbier et al., 2015). These prior studies have identified particular activity rhythm characteristics (such as rhythm height, robustness, or timing) linked to depression. However, like disease (Marengoni, Rizzuto et al., 2009), activity rhythm disturbances may co-segregate in predictable and nuanced patterns. Currently, it is unknown whether, and if so how, activity rhythm disturbances tend to co-occur. If patterns of co-occurring activity rhythm disturbances do exist, their impact on future depression has not yet been established.
To our knowledge, only one study to date has examined the longitudinal relationship between activity rhythms and future depression risk (Smagula, Ancoli-Israel et al., 2014b). This prior study was limited by a relatively short follow-up period (an average of 1.2 years) and by investigating activity rhythm characteristics separately. Therefore, the nature of activity rhythm associated depression risk over longer time periods remains unclear, and it remains unknown whether specific combinations of activity rhythms disturbances synergistically elevate depression risk.
The current study therefore first aimed to determine whether activity rhythm disturbances tend to co-segregate in specific patterns or combinations occurring within particular sub-groups. To do so, we applied a person-centered, data-driven approach (Latent Class Analysis or LCA) to identify sub-groups that share similar patterns of co-occurring activity rhythm disturbances among a large sample of older men. Although this was a secondary data analysis, investigating activity rhythms in this group is important because both variability and disturbances are expected to be high. Aging is associated with increased activity rhythm fragmentation (Huang, Liu et al., 2002; Robillard, Naismith et al., 2014), and compared with younger men and older women, circadian rhythms in behaviors and subjective ratings are most poorly entrained to the internal circadian rhythm (as referenced by rectal temperature) (Monk & Kupfer, 2000). To further clarify the relationship between activity rhythm disturbances and the course of depression among older men, we tested whether any of the data-derived sub-groups experienced greater depressive symptom increases over five years. This novel approach was designed to assess the patterns of co-occurring activity rhythm disturbances prevalent within a population-based sample of older men, and to assess whether specific activity rhythm disturbances, or their combinations, are associated with depression risk over time.
Materials and Methods
Participants
The Osteoporotic Fractures in Men (MrOS) Sleep Study recruited 3,135 participants at six study sites in the United States (Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Monongahela Valley, Pennsylvania; Portland, Oregon; and San Diego, California) (Blank, Cawthon et al., 2005; Orwoll, Blank et al., 2005) between December 2003 and March 2005. The parent MrOS study included community-dwelling men ≥65 years who could walk without assistance and were without bilateral hip replacements. Men were excluded from the Sleep Study if they regularly used overnight nocturnal oxygen therapy, positive pressure, or oral appliances for treatment of sleep apnea (n=150). Other reasons for non-participation in the Sleep Study were: death (n=349), terminated study participation (n=39), declined Sleep Study (n=1997), or because MrOS Sleep Study recruitment goals had already been met (n=324). All men provided written informed consent, and the study was approved by the Institutional Review Board at each site. For consistency with prior work (Paudel, Taylor et al., 2011) and because 72 hours of recording is considered the minimum period for assessing sleep-wake patterns with actigraph (Littner, Kushida et al., 2003; Ancoli-Israel, Martin et al., 2015), participants were required to have ≥3, 24-hour periods of technically adequate actigraph data (excluded n=134); therefore, the cross-sectional LCA was conducted with 3001 men (mean age=76.35, SD=5.52). To be included in the longitudinal analysis, participants were required to have complete outcome data from baseline and at least one other time-point; 68 of the men failed to meet this criteria and were excluded from the longitudinal analysis (which included n=2933 men).
Activity Rhythm Disturbances
Objective estimates of sleep/wake activity were obtained using the Octagonal Sleep Watch actigraphy, or SleepWatch-O, (Ambulatory Monitoring, Inc, Ardsley, NY). Participants were asked to wear actigraphs on the non-dominant wrist for a minimum of 5 consecutive 24-hour periods except when bathing or during water sports. Movement was measured using a piezoelectric biomorph-ceramic cantilevered beam, which generates a voltage each time the actigraph is moved. These voltages are gathered continuously and stored in one minute epochs. Data collected in digital integration mode were used for this analysis. A five-parameter extended sigmoidally transformed cosine model with an antilogistic function was used to model activity data; this model fits activity rhythm data better than a standard cosine curve as humans typically exhibits a more “squared” activity rhythm (Marler, Gehrman et al., 2006).
Modeled parameters included measures of rhythm height, timing, and robustness. For all activity rhythm parameters except timing variables, the lowest quartile was compared with the others to represent deviations the sample’s normative values thus indicating activity rhythm disturbances. For timing parameters, the lowest and the highest quartiles were both compared with the others (in a single variable) to represent both phase advances and delays. Using quartiles to represent non-normative or disturbed activity rhythm parameters is consistent with prior work (Paudel, Taylor et al., 2010; Tranah, Blackwell et al., 2010; Tranah, Blackwell et al., 2011; Smagula, Ancoli-Israel et al., 2014b) and, in the context of LCA, is designed to identify subgroups with specific patterns of activity rhythm parameters outside the normative range (disturbances).
Rhythm height parameters were amplitude (peak-nadir difference) and mesor (middle of the fitted curve). To assess whether low rhythm height is a consequence of activity levels overall, rather than relative peak-nadir differences, standardized amplitude was computed (as amplitude divided by mesor) with lower values indicating a dampened rhythm.
Rhythm timing measures were acrophase (time of day of peak activity level), up-mesor (time of day when activity passes up through mesor, approximating the time the participant “gets going” in the morning), and down-mesor (time of day when activity passes down through mesor, approximating the time of day the participant “settles down” for the night).
The extended cosine model also provides a measure known as the pseudo-F statistic which reflects of how well the modeled rhythm fits the observed data (Marler, Gehrman et al., 2006). Values indicate how robustly patterned activity is over the assessment period, with lower values indicating poorer model fit and suggesting an erratic and/or variable rhythm.
A parameter called alpha reflects the relative width of the activity peak compared with nadir; higher values indicate a relative narrowness of the active compared with rest period; the highest quartile was contrasted with the others to reflect a shorter activity period.
Outcome
The Geriatric Depression Scale-15 (GDS; (Sheikh & Yesavage, 1986)) was administered at baseline and three follow-up visits (conducted March 2005–May 2006, March 2007–March 2009, and March 2009–April 2011). Retention was high at the follow-up visits: of the men included in the longitudinal analysis, 99.5%, 90.1%, and 81.2% contributed complete GDS data at these subsequent time points; in addition, note that 80.7% contributed GDS data at all four time points. The GDS contains 15 items with binary response options indicating whether the symptom was present or absent over the “last week.” The GDS, a self-reported questionnaire, is a reliable and valid measure of depression among older adults. This version of the GDS indicates depression severity (compared with International Classification of Disease Version 10) and can therefore detect changes in depression over time (Almeida & Almeida, 1999). In the present study, depression severity was examined over time as reflected by repeated total GDS score (range 0–15) measurements.
Covariates
Demographics and lifestyle
Age, study site, race, weekly alcohol use, daily caffeine intake, educational attainment, smoking status, and BMI (measured with a standard balance beam or digital scale and wall-mounted stadiometer) were examined as covariates. The Physical Activity Scale for the Elderly (a validated self-report physical activity measure (Washburn, Smith et al., 1993; Schuit, Schouten et al., 1997)) was expressed as a continuous variable.
Mental health and cognitive impairment
Cognitive impairment was measured using the 3MS (Teng & Chui, 1987) which was expressed as a continuous variable. Anxiety symptoms were measured using the anxiety portion of the Goldberg Depression and Anxiety Scale (GDAS; (Goldberg, Bridges et al., 1988) as a continuous score reflecting symptom counts (range: 0–9).
Subjective sleep
The Pittsburgh Sleep Quality Index (PSQI) (Buysse, Reynolds et al., 1989) (range 0–21) was expressed continuously to reflect global sleep quality. The Epworth Sleepiness Scale (ESS) was also expressed as a continuous measure of excessive daytime sleepiness ranging from 0–24 (Johns, 1991).
Actigraph assessed sleep
ActionW-2 software (Ambulatory Monitoring, Inc., Ardsley, NY) was used to score the actigraphy data; for scoring algorithms details see (Jean-Louis, Kripke et al., 2001; Blackwell, Ancoli-Israel et al., 2005). This method produces reliable estimate of sleep-wake patterns (Pollak, Tryon et al., 2001; Ancoli-Israel, Cole et al., 2003). Participants were also asked to keep a sleep log which was used to edit the actigraphy data. Inter-scorer reliability has been previously found to be high in sleep studies performed by the MrOS Sleep Study team (intra-class coefficient=0.95) (Blackwell, Ancoli-Israel et al., 2005).
Actigraphy derived sleep parameters (covariates) were: total sleep time (TST; hours per night spent sleeping in bed after “lights off”), sleep latency (SL; amount of time until onset of sleep defined when participant achieved sleep for 20 continuous minutes in bed) and wake after sleep onset (WASO; minutes scored awake during the interval after sleep onset). Actigraphy measured sleep parameters were dichotomized to represent clinically significant disturbances: (1) short sleep <5 and long sleep >8 hours (contrasted in a single variable with 5–8 hour sleepers); (3) SL≥60 minutes, and (4) WASO≥90 minutes.
Polysomnography (PSG) assessed sleep
In-home sleep studies were conducted using one night of unattended PSG (Safiro, Compumedics, Inc., Melbourne, Australia) as described previously (Mehra, Stone et al., 2007). Centrally trained and certified staff members performed home visits for setup of the sleep study units; methods were similar to those in the Sleep Health Heart Study (Redline, Sanders et al., 1998). Polysomnography data quality was generally excellent, with a failure rate of less than 4% and more than 70% of studies graded as being of excellent or outstanding quality. The apnea hypopnea index (AHI) was computed as the average number of apneas and hypopneas per hour of recorded sleep using standard definitions of apneas and hypopnea (Quan, Howard et al., 1997). Rescoring studies over time indicates that inter- and intra- scorer reliability (ICC) for the AHI was high (ICC>0.95). The AHI was dichotomized at ≥30 to reflect severe sleep disordered breathing.
Sleep stages (rapid eye movement (REM), stages 1, 2, and slow wave sleep (N3 and N4 combined) were scored using standard criteria (Rechtschaffen A, 1968) and were expressed as the percentage of sleep time spent in these states. Also included was REM latency, which was defined as the number of minutes from sleep onset to the first REM period.
Instrumental Activity of Daily Living (IADL) impairment
The number of 5 IADL impairments was expressed as a continuous variable. The five IADLs were: heavy housework, preparing own meals, shopping for groceries or clothing, walking 2–3 blocks, and climbing ten stairs (Pincus, Summey et al., 1983; Fitti & Kovar, 1987).
Chronic diseases and falls
Participants were asked if they had a fall in the past 12 months. They also reported whether they had ever received a physician diagnosis of peripheral vascular disease, osteoarthritis, rheumatoid arthritis, hypertension, stroke, angina, congestive heart failure, myocardial infarction, diabetes, chronic obstructive pulmonary disease, Parkinson’s disease, renal disease, cataracts, or liver disease.
Inflammatory markers
Serum was collected during morning clinic visits after an overnight fast. A natural log transformation was applied to normalize their distributions which were initially skewed. CRP was measured using the ELISA assay kit from ALPCO (CRP sensitive ELISA). IL-6, TNF-α, and IFN-γ were assayed using the Human ProInflammatory I 4-Plex Ultra-Sensitive Kit by MSD (catalog #K15009C-4). TNF-αsRII was measured with an ELISA from R&D Systems (Minneapolis, MN; catalog #DRT200). Inter-assay CVs for these markers have been published previously (Smagula, Ancoli-Israel et al., 2014a).
Medications
Participants were asked to bring all medications used within the last 30 days to the sleep examination. Medications were entered into an electronic database and matched to their ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) (Pahor, Chrischilles et al., 1994). Psychoactive medications (antidepressants, benzodiazepines, and non-benzodiazepine non-barbiturate sedatives/hypnotics) were considered based on expected clinical relationships between these drugs and the primary predictors and outcomes. Other medications considered were NSAIDs and corticosteroids due to their relations with inflammatory markers and potentially the outcomes.
Psychosocial factors
During the visit occurring March 2005–May 2006 (approximately 1.2 years after the Sleep Visit), participants were asked whether, over the past 12 months, they had experienced any stressful event including “Serious illness or accident of wife or partner,” “Death of other close relative or close friend,” “Separation from child, close friend, or other relative who participant depends on,” “Loss of a pet,” “Moved or changed in residence,” “Serious financial trouble,” and “Anything else important.” At this time, participants were also administered a questionnaire (Michael, Berkman et al., 2001) providing measures of their social networks size and levels of social participation; responses to the individual questions from this measure were analyzed as covariates.
Statistical Methods
Latent Class Analysis
LCA postulates the presence of an unmeasured categorical latent variable that directly causes an observed pattern of indicators (single variable items) within a given set. LCA assumes local independence, in other words conditional independence of the indicators given latent class assignment. In the present analysis, indicators were dichotomous, except for timing variables which had three categories (i.e. early acrophase vs. normative vs. late acrophase representing the earliest quartile of activity peaks vs. the middle two quartiles vs. the latest quartile of activity peaks).
An add-in SAS procedure (PROC LCA) was utilized to conduct the LCA (Lanza, Collins et al., 2007). The number of latent groups was selected based on both interpretability and model fit statistics. The Bayesian Information Criterion (BIC) was used to compare models with a different number of latent classes (sub-groups). A smaller value is favored although BIC may indicate improvements in model fit when additional classes that do not reflect distinct or clinically relevant sub-groups. Therefore, while BIC was used to select the optimal number of latent classes, the number of latent groups was only increased when doing so captured a distinct and clinically relevant sub-group. Solutions with small latent classes (<5% of the sample) were rejected.
Growth Curve Modeling
The outcome was total GDS score reflecting overall depression symptoms levels over time. Growth curve modeling was implemented using SAS PROC MIXED with and random slopes and intercepts, with time expressed as a continuous variable (in years from baseline). First, separate base models (adjusted for age and study site) assessed crude associations between latent activity rhythm sub-groups and the level or rate of change in the outcome over time (associations with the rate of change were examined as the interactions between activity rhythm sub-group and time). Associations between all covariates and the level or rate of change in the outcome were similarly assessed. From these separate models, a maximum multivariable was constructed including all associations that achieved at least p<0.10 with the level (intercept) or rate of change (slope) in depression symptoms over time. To achieve a parsimonious final model, variables which were not significantly associated with the outcome in the maximum model (p<0.10) were removed. The covariates included in the final model are listed at the bottom of Table 3.
Table 3
Crude and adjusted associations of individual predictors with the rate of change in depressive symptoms
| Crude Model1 (n=2933) | Adjusted Model2 (n=2700) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Circadian Activity Rhythm Latent Class | Predictor * Time
| Predictor * Time
| ||||
| β | SE | p | β | SE | p | |
|
|
| |||||
| Normal rhythm | Reference | Reference | ||||
| Low activity | 0.10 | 0.03 | 0.0004 | 0.04 | 0.03 | 0.21 |
| Early activity | −0.01 | 0.03 | 0.66 | −0.02 | 0.03 | 0.47 |
| Early/short active period | 0.03 | 0.03 | 0.34 | 0.01 | 0.03 | 0.65 |
| Early/short active period/dampened | 0.13 | 0.03 | <0.0001 | 0.09 | 0.03 | 0.01 |
| Late activity | 0.05 | 0.02 | 0.02 | 0.05 | 0.02 | 0.03 |
| Late/short active period | 0.12 | 0.03 | <0.0001 | 0.06 | 0.03 | 0.03 |
| Late with low activity | 0.13 | 0.03 | <0.0001 | 0.07 | 0.03 | 0.02 |
Results
Latent Class Analysis
Activity rhythm variables were best modeled using eight groups (Supplementary Table 1). Groups were assigned names based on examining conditional item-response probabilities (Table 1). For descriptive purpose, activity data with the modeled rhythm are presented (Figure 1) from single subjects representing each of the derived sub-groups.
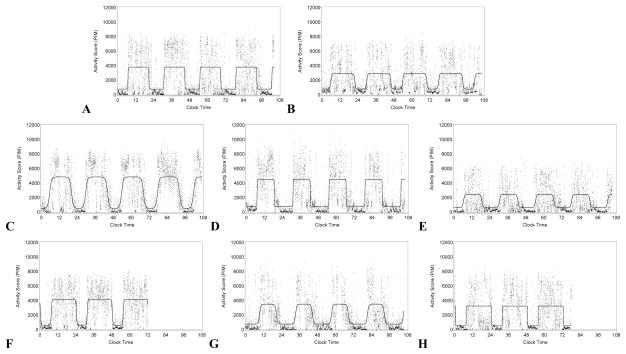
Activity data is plotted over time and the modeled rhythm is plotted in red. The eight men belonged to the following groups: Panel A, Normal rhythm; Panel B, Low activity; Panel C, Early activity; Panel D, Early/short active period; Panel E, Early/short active period/dampened; Panel F, Late activity; Panel G, Late/short active period; Panel H, Late/low activity
Table 1
Item-response probabilities conditional on latent group membership (n=3001)
| Group Name | Early acrophase | Early up- mesor | Early down- mesor | Late acrophase | Late up- mesor | Late down- mesor | Low st. amp. | Low mesor | Low amp. | Low psuedo-F | High alpha |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
| ||||||||||
| Normal rhythm | 0.01 | 0.26 | 0.01 | 0.01 | 0.04 | 0.12 | 0.07 | 0.11 | 0.01 | 0.05 | 0.01 |
| Low activity | 0.10 | 0.37 | 0.01 | 0.01 | 0.02 | 0.15 | 0.38 | 0.78 | 0.86 | 0.62 | 0.02 |
| Early activity | 0.99 | 0.88 | 0.48 | 0.01 | 0.01 | 0.01 | 0.16 | 0.13 | 0.05 | 0.13 | 0.01 |
| Early/short active period | 0.76 | 0.15 | 0.99 | 0.01 | 0.20 | 0.01 | 0.31 | 0.02 | 0.01 | 0.12 | 0.96 |
| Early/short active period/dampened | 0.88 | 0.35 | 0.99 | 0.01 | 0.17 | 0.01 | 0.88 | 0.44 | 0.99 | 0.73 | 0.82 |
| Late activity | 0.01 | 0.01 | 0.01 | 0.99 | 0.50 | 0.85 | 0.08 | 0.10 | 0.01 | 0.08 | 0.02 |
| Late/short active period | 0.01 | 0.01 | 0.34 | 0.32 | 0.95 | 0.01 | 0.29 | 0.18 | 0.24 | 0.28 | 0.99 |
| Late with low activity | 0.01 | 0.01 | 0.01 | 0.99 | 0.64 | 0.88 | 0.50 | 0.77 | 0.87 | 0.73 | 0.06 |
Acrophase, time of day of peak activity level; Up-mesor, time of day when activity passes up through mesor (approximating the time the participant “gets going” in the morning); Down-mesor, time of day when activity passes down through mesor (approximating the time of day the participant “settles down” for the night); Amplitude, peak-nadir difference (rhythm height); Mesor, estimated middle of the fitted curve; Standardized amplitude, amplitude divided by mesor (lower values indicate a dampened rhythm); Pseudo-F, robustness of the activity pattern (lower values indicating poorer model fit); Alpha, the relative width of the activity peak compared with nadir (higher values indicate a relative narrowness of the active compared with rest period)
The largest group had normative activity rhythms (as indicated by low probabilities of being in any extreme quartile) and were labeled the “normal rhythm” group (32.09% of the sample; Panel A in Figure 1).
One group had indications of low rhythm height (high probability of low mesor and amplitude with an intermediate probability of low robustness) and was labeled “low activity” (10.06%; Panel B in Figure 1); note that this group had lower probabilities of having standardized amplitude and low probabilities of non-normative timing.
Two groups appeared to have non-normative timing but no other indication of abnormal activity rhythms: one group had earlier activity peaks and up-mesor plus an intermediate probability of an earlier down-mesor (“early activity,” 10.53%; Panel C in Figure 1). Another group had later activity peaks and down-mesors with an intermediate probability of a later up-mesor (“late activity,” 14.46%; Panel F in Figure 1).
Two groups had altered timing and a shorter active period. Of these groups, one had an earlier peak and down-mesor combined with a high alpha (“early/short active period,” 9.63%; Panel D in Figure 1). Another group had a later up-mesor with a short active period (“late/short active period”; 8.23%; Panel G in Figure 1).
The final two groups had later or earlier activity similar to groups described above, except that these last groups also had indication of lower rhythm height (high probabilities of low rhythm height/robustness). One group “early/short active period/dampened,” 6.80%; Panel E in Figure 1) had high probabilities of having an earlier activity timing, plus a high alpha (indicating a shorter active period) and low rhythm height (both standardized amplitude and robustness). The remaining group had later activity plus a high probability of low height/robustness (“late with low activity,” 8.20%; Panel H in Figure 1).
To further examine between-group differences indicated by the item-response probabilities, we examined activity rhythm characteristics expressed continuously and stratified by LCA derived sub-group (see bottom of Table 2). Substantial differences in mean activity rhythm characteristics by group were apparent and consistent with the conditional item response probabilities. For descriptive purposes, health characteristics that were entered in the final longitudinal model are also shown by activity rhythm sub-group (Table 2).
Table 2
Descriptive characteristics by activity rhythm sub-groups (total n=3001), mean (SD) shown unless otherwise noted
| Normal rhythm | Low activity | Early activity | Early/short active period | Early/short active period/dampened | Late activity | Late/short active period | Late with low activity | |
|---|---|---|---|---|---|---|---|---|
| 32.09 (963) | 10.06 (302) | 10.53 (316) | 9.63 (289) | 6.80 (204) | 14.46 (434) | 8.23 (247) | 8.20 (246) | |
| Age | 75.76 (5.26) | 78.31 (5.88) | 75.32 (5.07) | 75.92 (5.14) | 77.55 (5.6) | 75.71 (5.33) | 76.48 (5.39) | 78.13 (6.3) |
| BMI | 26.65 (3.51) | 27.46 (4.16) | 27.02 (3.61) | 27.4 (3.41) | 28.73 (4.45) | 26.93 (3.92) | 27.85 (3.99) | 27.78 (4.18) |
| Cognitive function | 93.35 (5.42) | 91.87 (7.14) | 93.1 (5.22) | 92.66 (5.55) | 91.06 (7.84) | 93.56 (5.13) | 91.6 (6.55) | 92.05 (6.36) |
| Anxiety symptoms | 0.87 (1.78) | 1.05 (1.93) | 0.85 (1.72) | 1.04 (1.88) | 0.74 (1.65) | 0.88 (1.85) | 1.39 (2.28) | 1.43 (2.31) |
| PSQI | 5.23 (2.96) | 5.97 (3.43) | 5.35 (3.35) | 5.63 (3.22) | 5.98 (3.62) | 5.43 (2.96) | 6.32 (3.71) | 6.46 (3.74) |
| Physical Activity | 159.02 (70.38) | 121.61 (69.07) | 167.34 (78.8) | 154.89 (66.42) | 123.76 (65.77) | 145.14 (65.59) | 143.51 (72.29) | 107.73 (62.83) |
| IADL impairment | 0.18 (0.58) | 0.54 (0.96) | 0.29 (0.73) | 0.24 (0.6) | 0.56 (1.07) | 0.3 (0.76) | 0.56 (1.09) | 0.86 (1.22) |
| COPD, % (n) | 3.32 (32) | 8.94 (27) | 6.33 (20) | 3.81 (11) | 6.40 (13) | 4.84 (21) | 6.48 (16) | 6.91 (17) |
| Fall in past 12 months, % (n) | 26.38 (254) | 34.11 (103) | 26.90 (85) | 25.26 (73) | 30.54 (62) | 32.95 (143) | 36.03 (89) | 38.62 (95) |
| Diabetes, % (n) | 11.63 (112) | 16.56 (50) | 8.86 (28) | 13.15 (38) | 18.23 (37) | 12.44 (54) | 15.38 (38) | 17.89 (44) |
| Parkinson’s, % (n) | 0.73 (7) | 2.32 (7) | 0 (0) | 0 (0) | 3.45 (7) | 0.46 (2) | 2.02 (5) | 3.25 (8) |
| Severe SDB, % (n) | 14.21 (128) | 21.91 (7) | 11.74 (35) | 16.55 (46) | 24.37 (48) | 16.96 (68) | 20.51 (48) | 25.99 (59) |
| Activity Rhythm Variables
| ||||||||
| Acrophase | 14.29 (0.39) | 14.19 (0.53) | 12.97 (0.55) | 12.99 (0.65) | 12.71 (0.95) | 15.66 (0.65) | 14.66 (0.72) | 15.99 (1.16) |
| Up-mesor | 6.71 (0.61) | 6.5 (0.8) | 5.54 (0.79) | 7.08 (0.83) | 6.82 (1.21) | 7.88 (0.85) | 8.83 (1.15) | 8.3 (1.47) |
| Down-mesor | 21.86 (0.68) | 21.88 (0.79) | 20.4 (0.73) | 18.89 (1.16) | 18.6 (1.54) | 23.44 (0.85) | 20.49 (1.12) | 23.68 (1.23) |
| Amp. | 3953.75 (658.03) | 2529.92 (450.64) | 3839.14 (703.04) | 4290.21 (1241.03) | 2296.71 (466.29) | 3868.43 (642.66) | 4275.28 (1738.7) | 2429.83 (537.53) |
| Mesor | 2234.51 (329.52) | 1688.58 (322.48) | 2237.79 (368.08) | 2606.41 (609.99) | 1917.22 (398.34) | 2213.97 (304.36) | 2511.47 (760.59) | 1682.3 (315.54) |
| St. Amp. | 1.78 (0.19) | 1.53 (0.29) | 1.73 (0.23) | 1.65 (0.26) | 1.22 (0.25) | 1.75 (0.18) | 1.68 (0.33) | 1.46 (0.30) |
| Pseudo-f | 1282.27 (495.09) | 628.59 (227.38) | 1163.29 (511.24) | 1200.96 (501.88) | 576.59 (223.78) | 1173.9 (411.5) | 1003.51 (515.46) | 584.5 (216.95) |
| Alpha | −0.40 (0.12) | −0.42 (0.14) | −0.36 (0.13) | 0.07 (0.29) | 0.03 (0.25) | −0.44 (0.14) | 0.13 (0.34) | −0.41 (0.20) |
Note: Selected health characteristic are those that entered the final model predicting depression severity over time;
SD, standard deviation; Acrophase, time of day of peak activity level; Up-mesor, time of day when activity passes up through mesor (approximating the time the participant “gets going” in the morning); Down-mesor, time of day when activity passes down through mesor (approximating the time of day the participant “settles down” for the night); Amplitude, peak-nadir difference (rhythm height); Mesor, estimated middle of the fitted curve; Standardized amplitude, amplitude divided by mesor (lower values indicate a dampened rhythm); Pseudo-F, robustness of the activity pattern (lower values indicating poorer model fit); Alpha, the relative width of the activity peak compared with nadir (higher values indicate a relative narrowness of the active compared with rest period)
Growth Curve Model
The final follow-up assessment was conducted an average of 5.5 years (0.52 SD) after baseline. In the crude model (Table 3), the following sub-groups had faster increases in depressive symptoms over time (this and all subsequent comparisons are with the “normal rhythm” group which was set as the reference sub-group): “low activity,” “late activity,” “late/short active period,” “early/short active period/dampened” and “late with low activity.” In other words, before covariate adjustment, compared with the “normal rhythm” group, all groups with non-normative parameters except for “early activity” and “early/short active period” had significantly greater longitudinal increases in average depression symptoms levels when compared with the normative rhythm group.
The final model included all covariate associations with the level or rate of change that retained significance in the maximum model. After covariate adjustment, the association of “low activity” with faster depression symptoms increases was attenuated and no longer statistically significant (Table 3). All other associations between activity rhythm sub-groups retained significance after adjustment. Expressing actigraph and PSG sleep variables as continuous variables did not alter these results. Being in any group with late activity (“late activity [only],” “late/short active period” and “late with low activity”) was associated with significantly higher annual increases in overall depressive symptomology (Table 3). Being in the “early/short active period/dampened” sub-group also remained significantly associated with faster symptom increases over time. These interactions are illustrated (Figure 2) as adjusted mean predicted depression symptom scores shown by activity rhythm sub-group over time. Mean GDS scores in each group by time point are also included for descriptive purposes (Supplementary Table 2).

Note the scale of the y-axis was modified from that of the GDS (range: 0–15) to visually illustrate the significant interactions wherein four CAR sub-groups (marked with a *) have greater average subject-specific slopes by CAR sub-group compared with the normal rhythm group.
Discussion
We used a person-centered, data-driven clustering approach (LCA) to identify sub-groups of older men who had similar patterns of co-occurring activity rhythm disturbances. This approach produced intuitive and novel insights into the types of activity rhythms disturbances that are common among older men. Although replication in other samples is necessary, we identified eight sub-groups which had distinct activity rhythm characteristics, and the depression risk associated with these groups differed.
In our sample, timing variables had a role distinguishing activity rhythm sub-groups (i.e. into “larks” or “owls”). Having a narrow active period and/or low rhythm height/robustness appeared to further distinguish the derived sub-groups. Note that rhythm robustness (measured with the pseudo-F statistic) and the unstandardized rhythm height measures separated sub-groups in the same pattern. That is, sub-groups with a lack of rhythm robustness appeared to also have less activity overall, and vice versa. Future longitudinal research is needed to determine if low rhythm robustness (regularity) precedes or results from activity reductions.
In the current study, we found that having only low activity height/robustness was associated with an increased burden of depressive symptoms, but that this association was accounted for by covariate adjustments including lifestyle and health factors. Note that this finding is consistent with our prior study which found that an association between low rhythm amplitude and future depression was accounted for by concurrent health status covariates (Smagula, Ancoli-Israel et al., 2014b). The present work also found that all three sub-groups with indication of more delayed activity rhythms experienced significantly faster increases in depressive symptoms over time, independent of including chronic diseases, night-time sleep characteristics, and self-reported physical activity; when later activity timing was accompanied by either a compressed active period or low activity levels, depressive symptom increases appeared somewhat faster (compared with having later activity timing alone). Note that having delayed timing was related to future depressive symptoms even in the sub-group with a normative active period length; this suggests that it is not necessarily an excessive build-up of homeostatic drive, but rather, potentially a mismatch between the sleep-wake cycle and the internal clock/exogenous cues that is depressogenic in these men.
In addition, we found that having earlier timing, only when combined with a dampened rhythm and short active period, was independently associated with an increasing burden of depressive symptoms. This group most clearly underscores the utility of our data-driven approach: early activity timing and rhythm height reductions were only independently associated with faster depressive symptom accumulation when co-occurring (Table 3). Early activity in this group is consistent with the hypothesis that depression is associated with a phase advance in cortisol and norepinephrine secretion (Koenigsberg, Teicher et al., 2004). Noting that early timing itself was not related to depression, advances in circadian physiological arousal may be associated with depression only when accompanied by a dampened activity rhythm amplitude and a shorter active period. These reductions in rhythm height and the duration of the active period may reflect an inability to maintain wakefulness at appropriate due to a lack of exogenous timing cues (such as daytime light and social interaction), an insensitivity to them, or a loss of endogenous clock control over behavior.
The current study has several key advantages over our prior investigation (Smagula, Ancoli-Israel et al., 2014b), which was limited to a short term follow-up (an average of 1.2 years), and only examined activity rhythm disturbances separately. In our prior investigation, rhythm robustness (measured with the pseudo-F statistics) was the only activity rhythm parameter that independently predicted depressive symptom increases. Our current work now clarifies that low rhythm robustness is independently associated with faster depressive symptom increases only when accompanied by other alterations, specifically as in the “Late with low activity” or “Early/short active period/dampened” groups. Our earlier study may have thus detected a sub-group at risk due to low rhythm height/robustness that heterogeneously combined with delayed or advanced activity timing.
Our prior study also failed to detect an association between activity timing delays and future depressive symptoms. Again, the current findings clarify by suggesting depression risk is only elevated when older men have multiple measures of their modeled activity rhythm affected (i.e. the groups with indication of delay had both later acrophase and either a later up- or down-mesor); because our prior study only investigated timing variables (up-mesor, acrophase, and down-mesor) separately, this may have adversely affected the available signal and added noise (e.g. by including individuals in each delay parameter’s disturbance group, when they had normal activity timing on 2/3 measures). Alternatively, it is possible discrepancies between the current and past findings reflect differences in the nature of activity rhythm related depression risk over short (1.2 years in our prior study) and longer (over five years in the current study) terms. Although future research is needed to conclusively establish the roles of activity rhythm disturbances in relation to depression risk, our current findings suggest examining multiple activity rhythm characteristics is more informative than examining individual activity rhythm characteristics alone.
Several limitations should be highlighted, including the current need to confirm the existence, prevalence, and health-relevance of the identified activity rhythm disturbances combinations in independent samples. Actigraph-recorded activity rhythms reflect the activity of the master biological keeper in the suprachiasmic nucleus, but should not be interpreted as a direct indicator of circadian biology (Ancoli-Israel, Cole et al., 2003). The MrOS Sleep Study consists of older men who were mostly white, and these findings cannot necessarily generalize to other populations. The derivation of sub-groups via LCA and subsequent use of these sub-groups to predict change in a longitudinal mixed model introduces measurement error. However, any such classification error would potentially reduce the available signal (potency of the true activity rhythm configurations) and bias our results to the null. Residual confounding may have influenced our results, in particular, our analysis did not account for a potential role of past depressive episodes in relation to current activity rhythms and future depression symptoms. The average magnitude of depression severity and changes in severity associated with these activity rhythm groups was not large (Figure 2). However, it is worth noting that the increased rate of symptom change independently associated with four activity rhythm sub-groups (discussed above) was comparable to that of traditional depression risk factors identified in our sample (and included in the final model (i.e. diabetes, past year fall, and a lack of participation in social groups). Our analysis examined differences in the average subject-specific slope of change in overall depression symptom severity and did not test whether activity rhythm sub-groups were related to distinct patterns of symptom accumulation (i.e. if the sample was composed of mixtures of slope parameter distributions) or the incidence of clinical depression.
Strengths of our study include the large, population-based sample that was comprehensively characterized at baseline and followed-up over a substantial time period (of more than five years). Our innovative application of LCA adds substantively to past literature which has focused on each aspect of the activity rhythm separately. This novel data-driven approach demonstrated that, among older men, activity rhythm disturbances are highly co-morbid and tend to co-occur in specific and objectively measurable patterns. Our longitudinal analysis revealed that later timing or early/compressed/dampened activity rhythms were independently related to depression risk. Sub-groups with lower rhythm height/robustness, and/or shorter active periods may have resulted from losses in the integrity or effectiveness of the central circadian output to regulate behaviors (Hofman, 2000; Monk & Kupfer, 2000; Münch, Knoblauch et al., 2005). However, little is known regarding how activity rhythms disturbances develop, and future research is also needed determine how different combinations activity rhythm disturbances might synergistically affect depression and other aspects of health.
Our findings raise important questions regarding how activity rhythms are mechanistically involved in the pathogenesis of depression. We observed that grossly different activity rhythm profiles (e.g. groups with earlier and later timing) were both associated with depression risk. It is possible these differences in the nature of activity rhythm disturbance associated with depression risk may signal heterogeneous etiological pathways which lead to similarly heterogeneous clinical presentations of major depressive disorder. Determining the relevance of activity rhythm disturbances to depression will requires resolving resolve how activity rhythms relate to the known biological, psychological, and social determinants of health. Research in this spirit is already underway (e.g. (Sherman, Mumford et al.; Oosterman, van Harten et al., 2008; Zuurbier, Ikram et al., 2015)), and in the future, holds the promise of establishing more precise pathways linking circadian disruption, activity rhythm patterns, and depression. Even without knowledge of precisely how activity rhythms relate to depression, because the activity rhythm appears to be a modifiable, independent contributor to the development of depression symptoms, future research targeting the activity rhythm for depression prevention is warranted.
Supplementary Material
Supplemental 1
Supplemental 2
Footnotes
Declaration of Interests
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. SFS has been supported by T32 AG000181 and T32 MH019986.
References
- Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. International journal of geriatric psychiatry. 1999;14:858–865. [Abstract] [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. [Abstract] [Google Scholar]
- Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L, Liu L, Meltzer LJ, Sadeh A, Spira AP, Taylor DJ. The SBSM Guide to Actigraphy Monitoring: Clinical and Research Applications. Behavioral sleep medicine. 2015;13(Suppl 1):S4–s38. [Abstract] [Google Scholar]
- Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28:1599–1605. [Abstract] [Google Scholar]
- Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemporary clinical trials. 2005;26:557–568. [Abstract] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28:193–213. [Abstract] [Google Scholar]
- Fitti JE, Kovar MG. The Supplement on Aging to the 1984 National Health Interview Survey. Vital and health statistics Ser 1, Programs and collection procedures. 1987:1–115. [Abstract] [Google Scholar]
- Goldberg D, Bridges K, Duncan-Jones P, Grayson D. Detecting anxiety and depression in general medical settings. BMJ (Clinical research ed) 1988;297:897–899. [Europe PMC free article] [Abstract] [Google Scholar]
- Hofman MA. The human circadian clock and aging. Chronobiology international. 2000;17:245–259. [Abstract] [Google Scholar]
- Huang YL, Liu RY, Wang QS, Van Someren EJ, Xu H, Zhou JN. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiology & behavior. 2002;76:597–603. [Abstract] [Google Scholar]
- Jean-Louis G, Kripke DF, Mason WJ, Elliott JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. Journal of neuroscience methods. 2001;105:185–191. [Abstract] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. [Abstract] [Google Scholar]
- Koenigsberg HW, Teicher MH, Mitropoulou V, Navalta C, New AS, Trestman R, Siever LJ. 24-h Monitoring of plasma norepinephrine, MHPG, cortisol, growth hormone and prolactin in depression. Journal of psychiatric research. 2004;38:503–511. [Abstract] [Google Scholar]
- Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: A SAS Procedure for Latent Class Analysis. Structural equation modeling : a multidisciplinary journal. 2007;14:671–694. [Europe PMC free article] [Abstract] [Google Scholar]
- Littner M, Kushida CA, Anderson WM, Bailey D, Berry RB, Davila DG, Hirshkowitz M, Kapen S, Kramer M, Loube D, Wise M, Johnson SF. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–341. [Abstract] [Google Scholar]
- Luik AI, Zuurbier LA, Direk N, Hofman A, Van Someren EJ, Tiemeier H. 24-hour activity rhythm and sleep disturbances in depression and anxiety: a population-based study of middle-aged and older persons. Depression and anxiety 2015 [Abstract] [Google Scholar]
- Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, Tiemeier H. Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiology international. 2013;30:1223–1230. [Abstract] [Google Scholar]
- Maglione JE, Ancoli-Israel S, Peters KW, Paudel ML, Yaffe K, Ensrud KE, Tranah GJ, Stone KL. Depressive Symptoms and Circadian Activity Rhythm Disturbances in Community-Dwelling Older Women. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 2013 [Europe PMC free article] [Abstract] [Google Scholar]
- Marengoni A, Rizzuto D, Wang HX, Winblad B, Fratiglioni L. Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc. 2009;57:225–230. [Abstract] [Google Scholar]
- Marler MR, Gehrman P, Martin JL, Ancoli-Israel S. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Statistics in medicine. 2006;25:3893–3904. [Abstract] [Google Scholar]
- Mehra R, Stone KL, Blackwell T, Ancoli Israel S, Dam TT, Stefanick ML, Redline S. Prevalence and correlates of sleep-disordered breathing in older men: osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2007;55:1356–1364. [Europe PMC free article] [Abstract] [Google Scholar]
- Michael YL, Berkman LF, Colditz GA, Kawachi I. Living arrangements, social integration, and change in functional health status. American journal of epidemiology. 2001;153:123–131. [Abstract] [Google Scholar]
- Monk TH, Kupfer DJ. Circadian rhythms in healthy aging--effects downstream from the pacemaker. Chronobiology international. 2000;17:355–368. [Abstract] [Google Scholar]
- Münch M, Knoblauch V, Blatter K, Schröder C, Schnitzler C, Kräuchi K, Wirz-Justice A, Cajochen C. Age-related attenuation of the evening circadian arousal signal in humans. Neurobiology of aging. 2005;26:1307–1319. [Abstract] [Google Scholar]
- Oosterman J, van Harten B, Vogels R, Gouw A, Weinstein H, Scheltens P, Scherder E. Distortions in rest-activity rhythm in aging relate to white matter hyperintensities. Neurobiology of aging. 2008;29:1265–1271. [Abstract] [Google Scholar]
- Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemporary clinical trials. 2005;26:569–585. [Abstract] [Google Scholar]
- Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. European journal of epidemiology. 1994;10:405–411. [Abstract] [Google Scholar]
- Paudel ML, Taylor BC, Ancoli-Israel S, Blackwell T, Stone KL, Tranah G, Redline S, Cummings SR, Ensrud KE. Rest/activity rhythms and mortality rates in older men: MrOS Sleep Study. Chronobiology international. 2010;27:363–377. [Europe PMC free article] [Abstract] [Google Scholar]
- Paudel ML, Taylor BC, Ancoli-Israel S, Stone KL, Tranah G, Redline S, Barrett-Connor E, Stefanick ML, Ensrud KE. Rest/activity rhythms and cardiovascular disease in older men. Chronobiology international. 2011;28:258–266. [Europe PMC free article] [Abstract] [Google Scholar]
- Pincus T, Summey JA, Soraci SA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis and rheumatism. 1983;26:1346–1353. [Abstract] [Google Scholar]
- Pollak CP, Tryon WW, Nagaraja H, Dzwonczyk R. How accurately does wrist actigraphy identify the states of sleep and wakefulness? Sleep. 2001;24:957–965. [Abstract] [Google Scholar]
- Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, Wahl PW. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [Abstract] [Google Scholar]
- Rechtschaffen AKA, editor. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington DC: National Institutes of Health; 1968. NIH Publication 204. [Abstract] [Google Scholar]
- Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, Bonekat WH, Rapoport DM, Smith PL, Kiley JP. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–767. [Abstract] [Google Scholar]
- Robillard R, Naismith SL, Smith KL, Rogers NL, White D, Terpening Z, Ip TKC, Hermens DF, Whitwell B, Scott EM, Hickie IB. Sleep-Wake Cycle in Young and Older Persons with a Lifetime History of Mood Disorders. PloS one. 2014;9:e87763. [Europe PMC free article] [Abstract] [Google Scholar]
- Schuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the Physical Activity Scale for the Elderly (PASE): according to energy expenditure assessed by the doubly labeled water method. Journal of clinical epidemiology. 1997;50:541–546. [Abstract] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontology. 1986;5:165–173. [Google Scholar]
- Sherman SM, Mumford JA, Schnyer DM. Hippocampal activity mediates the relationship between circadian activity rhythms and memory in older adults. Neuropsychologia [Abstract] [Google Scholar]
- Smagula SF, Ancoli-Israel S, Barrett-Connor E, Lane NE, Redline S, Stone KL, Cauley JA. Inflammation, sleep disturbances, and depressed mood among community-dwelling older men. Journal of psychosomatic research. 2014a;76:368–373. [Europe PMC free article] [Abstract] [Google Scholar]
- Smagula SF, Ancoli-Israel S, Blackwell T, Boudreau R, Stefanick ML, Paudel ML, Stone KL, Cauley JA. Circadian Rest-Activity Rhythms Predict Future Increases in Depressive Symptoms Among Community-Dwelling Older Men. Am J Geriatr Psychiatry 2014b [Europe PMC free article] [Abstract] [Google Scholar]
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. The Journal of clinical psychiatry. 1987;48:314–318. [Abstract] [Google Scholar]
- Tranah GJ, Blackwell T, Ancoli-Israel S, Paudel ML, Ensrud KE, Cauley JA, Redline S, Hillier TA, Cummings SR, Stone KL for the Study of Osteoporotic Fractures Research G. Circadian Activity Rhythms and Mortality: The Study of Osteoporotic Fractures. Journal of the American Geriatrics Society. 2010;58:282–291. [Europe PMC free article] [Abstract] [Google Scholar]
- Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, Cauley JA, Redline S, Hillier TA, Cummings SR, Yaffe K. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Annals of neurology. 2011;70:722–732. [Europe PMC free article] [Abstract] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. Journal of clinical epidemiology. 1993;46:153–162. [Abstract] [Google Scholar]
- Zuurbier LA, Ikram MA, Luik AI, Hofman A, Van Someren EJW, Vernooij MW, Tiemeier H. Cerebral small vessel disease is related to disturbed 24-h activity rhythms: a population-based study. European Journal of Neurology. 2015 n/a-n/a. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.3109/07420528.2015.1102925
Read article for free, from open access legal sources, via Unpaywall:
https://escholarship.org/content/qt7t56v3qp/qt7t56v3qp.pdf?t=o3cwlz
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.3109/07420528.2015.1102925
Article citations
Depressive symptom screening in elderly by passive sensing data of smartphones or smartwatches: A systematic review.
PLoS One, 19(6):e0304845, 27 Jun 2024
Cited by: 0 articles | PMID: 38935797 | PMCID: PMC11210876
Review Free full text in Europe PMC
Sleep-wake behavioral characteristics associated with depression symptoms: findings from the Multi-Ethnic Study of Atherosclerosis.
Sleep, 47(4):zsae045, 01 Apr 2024
Cited by: 0 articles | PMID: 38394355
Examining Human-Smartphone Interaction as a Proxy for Circadian Rhythm in Patients With Insomnia: Cross-Sectional Study.
J Med Internet Res, 25:e48044, 15 Dec 2023
Cited by: 3 articles | PMID: 38100195 | PMCID: PMC10757227
Actigraphic correlates of neuropsychiatric symptoms in adults with focal epilepsy.
Epilepsia, 64(6):1640-1652, 24 Apr 2023
Cited by: 2 articles | PMID: 37029747 | PMCID: PMC10251370
24-Hour Rest-Activity Rhythm in Middle-Aged and Older Persons with Depression.
Int J Environ Res Public Health, 20(7):5275, 27 Mar 2023
Cited by: 1 article | PMID: 37047891 | PMCID: PMC10094496
Go to all (21) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: UL1 TR000128
NHLBI NIH HHS (8)
Grant ID: R01 HL070847
Grant ID: R01 HL070841
Grant ID: R01 HL070838
Grant ID: R01 HL070839
Grant ID: R01 HL070848
Grant ID: R01 HL071194
Grant ID: R01 HL070837
Grant ID: R01 HL070842
NIA NIH HHS (8)
Grant ID: T32 AG000181
Grant ID: U01 AG042143
Grant ID: U01 AG027810
Grant ID: U01 AG042145
Grant ID: U01 AG042140
Grant ID: U01 AG042124
Grant ID: U01 AG042139
Grant ID: U01 AG042168
NIAMS NIH HHS (1)
Grant ID: U01 AR066160
NIMH NIH HHS (1)
Grant ID: T32 MH019986


