Abstract
Free full text

Feasibility of a Smartphone-Enabled Cardiac Rehabilitation Program in Male Veterans With Previous Clinical Evidence of Coronary Heart Disease
Associated Data
Abstract
Cardiac rehabilitation (CR) is recommended for patients with coronary heart disease, however participation among veterans remains poor. Smartphones may facilitate data transfer and communication between patients and providers, among other benefits. We evaluated the feasibility of a smartphone-enabled CR program in a population of veterans. Qualifying veterans were prospectively enrolled in a single-arm, non-randomized feasibility study of a smartphone-enabled, home-based CR program featuring an app with daily reminders to exercise, log vitals, and review educational materials. A coach remotely monitored patients through an online dashboard and scheduled telephone visits. Clinical endpoints were assessed as an exploratory aim. After 21 veterans provided informed consent, 18 were enrolled and successfully completed at least 30 days of the program; 13 completed the entire 12-week intervention. Mean (SD) age was 62 (7) years and 96% were male. Program completers logged a mean (SD) of 3.5 (1.4) exercise sessions and 150 (86) exercise minutes per week. The majority (84%) of program completers reported being satisfied overall with the program. Mean functional capacity improved by 1.0 metabolic equivalents (5.3 to 6.3, 95% CI, 0.3 to 1.7; P=0.008) and mean resting systolic blood pressure (SBP) improved by 9.6 mmHg (MD 9.6, 95% CI −19.0 to −0.7; P=0.049) among completers. Smartphone-enabled, home-based CR is feasible in veterans with heart disease and is associated with moderate to high levels of engagement and patient satisfaction.
INTRODUCTION
Cardiac rehabilitation (CR) is recommended for patients with coronary heart disease (CHD) as it reduces the risk of cardiovascular death and hospital readmission following acute MI.1,2 Despite the benefits, fewer than 10% of eligible veterans enroll in center-based CR.3 Commonly cited barriers include geographic distance, cost and low availability of on-site CR programs among Veterans Affairs (VA) hospitals.4–6 Home-based CR has been proposed as a safe and effective alternative to center-based programs predominantly in low-risk, elderly patients,7–10 however availability and widespread adoption has remained limited. Several studies have suggested that smartphones can enhance delivery of home-based CR to non-veterans,11,12 although the feasibility of such approaches among veterans, who have limited experience with technology,13 remains unknown. In this study, we evaluated the feasibility and acceptability of a smartphone-enabled, home-based CR among veterans with CHD. Additionally, we evaluated the safety and clinical efficacy of this approach as exploratory secondary outcomes.
METHODS
We report data from a prospective, single-arm feasibility study. Eligible participants were 18 years of age and older with a diagnosis of CHD and a qualifying indication for CR per established guidelines at the time the study was performed (acute myocardial infarction [AMI], percutaneous coronary intervention, and coronary artery bypass grafting). Exclusion criteria were the presence of any unstable cardiac condition (e.g. unstable angina, symptomatic aortic stenosis, decompensated heart failure, uncontrolled tachyarrhythmias, or high-grade atrioventricular block without a pacemaker), acute systemic illness, or congenital heart disease. Patients were also excluded if they had ischemic ST depression, a fall in systolic blood pressure, or non-sustained ventricular tachycardia on a pre-enrollment exercise treadmill test (ETT), or inability to exercise because of an orthopedic or vascular condition. Concurrent enrollment in a center-based CR program during was not a cause for exclusion.
Participants were enrolled as part of a convenience sample from patients who were referred to outpatient CR at the Atlanta VAMC from May through December 2016. All patients underwent baseline and follow-up functional assessment with either symptom-limited ETT or 6-minute walk test (6MWT). The choice of the specific protocol (e.g. Bruce or Naughton) was left to the discretion of the supervising practitioner. The study protocol was reviewed by the university institutional review board. Written informed consent was obtained from all patients.
The intervention was a 12-week home-based CR program delivered via a commercially available smartphone platform (Moving Analytics, Los Angeles, California) that included a patient-facing iOS and Android compatible smartphone app and an integrated hospital-facing online dashboard for remote patient monitoring and care coordination by a trained coach. The coach was a cardiology physician assistant. The platform (Figure 1) delivered an exercise-based CR program using clinical protocols based on MULTIFIT, a case-management system for secondary prevention in patients with CAD developed by Stanford University investigators.14 MULTIFIT has been previously shown to significantly improve low-density lipoprotein-cholesterol levels, functional capacity, and rates of smoking cessation over usual care.14,15
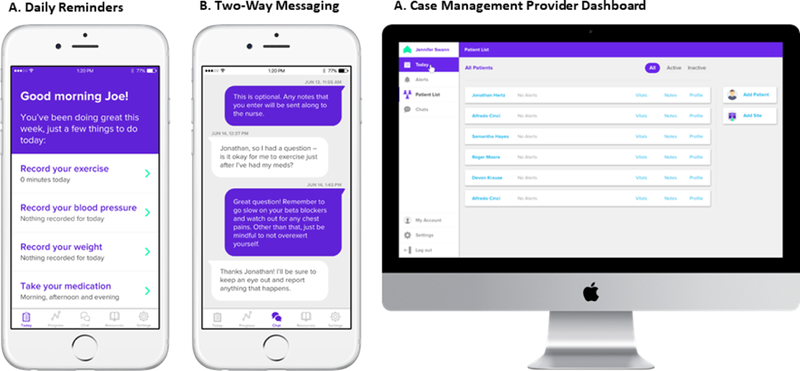
Smartphone-Enabled Platform with screenshots of (A) smartphone app with daily reminders for exercise, logging vitals and taking medications; (B) smartphone app 2-way messaging; and (C) online dashboard.
The app featured daily reminders to exercise, a virtual diary to document exercise sessions (including exercise type, length, and peak heart rate [HR] achieved) and vital signs, videos on heart conditions and risk factor modification, and 2-way messaging with the coach (Figure 1A). The dashboard allowed the coach to monitor patient performance remotely by reviewing the patient-entered app data, develop tailored exercise plans for each participant, and review patient messages and symptom alerts generated by patients via the app (Figure 1B).Patients were invited to use their own smartphone if it met basic technical requirements, otherwise a compatible device (Samsung Galaxy S4 or comparable) was provided.
Each participant received an exercise prescription based on a target range for HR derived from baseline functional testing that corresponded to 70–85% of peak HR achieved. The rate of perceived exertion (RPE), based on the Borg scale,16 was used as a secondary measure of exercise intensity. Participants were encouraged to perform 30 minutes of light to moderate exercise of their choosing (i.e. walking, jogging or bicycling) at least 5 times per week. Patients were instructed to perform a 5-minute warm-up followed by 20 minutes of vigorous exercise at or near their target HR or RPE goal and conclude with a 5-minute cool-down period. After 2 weeks, the coach could increase to target HR to 85% of maximum age predicted HR for the participant (220 minus the patient age in years) or 100% of their peak HR on baseline testing if there were no complications. Participants were instructed to stop or slow down and check their HR every 5 minutes during vigorous exercise by direct palpation of the radial or carotid artery.
The program was delivered by a trained coach who was experienced in both CR delivery and supervising ETTs. The coach performed all baseline exercise testing and developed the exercise prescription for each patient according to MULTIFIT guidelines, and also performed weekly or bi-weekly telephone-based coaching sessions with each participant to review their progress. During these calls, the coach delivered structured health-related education and, if necessary, made changes to the exercise plan.
The primary study endpoint was to assess the feasibility and acceptability of smartphone-enabled CR among Veterans. Feasibility was defined as the extent to which participants remained active and engaged with the program. Participants were considered active if they opened the app and logged at least 1 exercise session per week. Engagement was measured by the degree to which participants logged exercise, vital sign, and weight data into the app, reviewed educational modules, sent messages via the app to the coach, and participated in telephone-based coaching sessions. Acceptability was determined by the degree of patient satisfaction with the program and measured through a semi-qualitative survey (Online Figure 1). Clinical and functional endpoints including BP and functional capacity were evaluated as exploratory secondary outcomes given the feasibility nature of the study and as the rates of recruitment and participation were not known when planning the study. Safety was also evaluated as an exploratory outcome and was defined as the 30-day composite of major adverse outcomes including AMI, stroke, heart failure hospitalization, and death. All participants were invited for a follow-up visit at the completion of the program where a series of survey instruments were conducted, including for patient satisfaction, and measurements of the clinical and functional endpoints were performed.
Paired t tests were used to compare changes in the secondary outcomes measures over baseline. Change in functional capacity was assessed by comparing mean values of exercise exertion between baseline and follow-up visits. A value of P < 0.05 was considered statistically significant.
RESULTS
A total of 21 patients with clinical evidence of CHD were prospectively enrolled (15 following percutaneous coronary intervention and 6 following coronary artery bypass grafting), of which 18 patients (85.7%) successfully began the smartphone-based program and 13 (61.9%) completed the intervention including a follow-up visit and exit interview. A total of 8 patients (38.1%) enrolled but either withdrew prior to starting the program or were lost to follow-up procedures (3 withdrew prior to starting the program for uncited reasons, 1 patient withdrew for an inability to exercise due to non-cardiac reasons, 1 patient completed the program but decline to return for a follow-up visit due to distance, and 3 withdrew during the program for uncited reasons). The full consort diagram can be seen in Online Figure 2.
Demographic and clinical information for 18 patients who successfully began the smartphone program is shown in Table 1. The mean age was 65 ± 5 years and the entire cohort was male (100%). A majority of patients were African American (61.5%). The median time from qualifying event to enrollment was 113 days (range 13 – 294 days) across the study population. No participant reported completing another CR program prior to enrollment, however 4 patients reported participating in a center-based CR program at some point during the study period.
Table 1.
Clinical and Demographic Characteristics of the Study Population
| Patient | Age | Gender | Race | CR Indication | Days from Event | Completed Program | Traditional CR |
|---|---|---|---|---|---|---|---|
| 1 | 69 | M | AA | CABG | 125 | Yes | |
| 3 | 65 | M | AA | CABG | 113 | Yes | Yes |
| 4 | 63 | M | W | PCI | 272 | Yes | Yes |
| 8 | 68 | M | W | PCI | 217 | No | |
| 11 | 52 | M | AA | PCI | 240 | Yes | |
| 12 | 47 | M | W | CABG | 105 | No | |
| 13 | 65 | M | AA | CABG | 294 | Yes | |
| 14 | 67 | M | W | PCI | 113 | Yes | |
| 15 | 56 | M | AA | PCI | 331 | No | |
| 16 | 54 | M | W | PCI | No | ||
| 17 | 71 | M | AA | ACS/PCI | 84 | Yes | |
| 18 | 66 | M | W | ACS/PCI | 44 | Yes | Yes |
| 19 | 69 | M | W | PCI | 47 | Yes | |
| 20 | 67 | M | W | AMI/PCI | 280 | No | |
| 21 | 71 | M | W | PCI | 13 | Yes | |
| 26 | 66 | M | AA | CABG | 113 | Yes | Yes |
| 27 | 59 | M | AA | CABG | 195 | Yes | |
| 29 | 61 | M | AA | PCI | 46 | Yes |
Total N = 18. CR = cardiac rehabilitation; CABG = coronary artery bypass graft surgery
PCI = percutaneous coronary intervention; ACS = acute coronary syndrome
All 18 patients (100%) who began the program successfully accessed the app and logged an exercise session at least once and continued to remain active participants through the first 4 weeks of the program, which was defined as successfully accessing the app and logging an exercise session at least once per week on average. Among program completers, participants exercised (and reported meeting their pre-specified target HR) an average ± SD of 3.5 ± 1.4 times per week and performed an average ± SD of 150 ± 86 minutes of total weekly exercise over the first 4 weeks (Table 2). They also successfully logged their BP and weight at least 3 times per week on average (Table 2). They also reviewed a median of 5 education modules (interquartile range, 27) throughout the program. There was no significant trend towards decreased engagement with the app among program completers, as levels of engagement were consistent at both 4 and 12 weeks of the programs (Table 2). Program completers successfully participated in all the pre-scheduled phone sessions with the health coach as described in the methods. In addition, program completers sent an average ± SD of 26.3 ± 17 health-related messages to the coach via the app over the course of the program.
Table 2.
Participant Smartphone App Engagement and Activity Logging
| Activity | 4 Weeks | 12 Weeks |
|---|---|---|
| Exercise (sessions per week)1 | 3.5 ± 1.4 | 3.5 ± 1.1 |
| Blood pressure (recordings per week)1 | 3.6 ± 2.1 | 3.6 ± 1.9 |
| Weight (recordings per week)1 | 3.3 ± 2.2 | 3.4 ± 1.7 |
| Patient chats (total)2 | - | 26.3 ± 17.2 |
Values are mean ± SD. N=13.
Among program completers, 84% were satisfied overall, with 6 being “satisfied” and 5 being “very satisfied.” The other 2 cited technical difficulties with the app (N=1) and the smartphone hardware provided by the study (N=1) (Online Figure 3). At the end of the program, a majority also reported exercising more because of the intervention (85%), tracking their blood pressure and HR (92%), increased confidence in caring for themselves and completing a health action plan (77%), and exercising without making their symptoms worse (62%). Participants reported that the platform was ‘not hard to use and at times fun,’ ‘nice to have reminders,’ and it ‘helped me to set goals.’
Analysis of the available functional data demonstrated that 85% of program completers increased their exercise capacity on follow-up (Figure 2), with a significant improvement from 5.3 to 6.3 METS (MD 1, 95% CI, 0.3 to 1.7; P=0.008). Mean resting systolic blood pressure (SBP) was significantly reduced from 140.1 to 130.5 mmHg (MD 9.6, 95% CI −19.0 to −0.7; P=0.049). Diastolic blood pressure improved from 83.1 to 78.8 mmHg, however this was not statistically significant (MD 4.3; 95% CI, −12.2 to 3.6; P=0.256). No change in heart rate (HR) was observed (Figure 3). With regard to the exploratory aim of safety, there were no reported major adverse events related to the smartphone program over the course of 12-weeks.

Mean change in METS achieved during ETT, at baseline and at 12 weeks; (MD 1, 95% CI, 0.3 to 1.7; P=0.008). Box plots show data for the cohort as a whole (N=13). Line plots show changes for individual participants. METS = metabolic equivalents; ETT = exercise treadmill testing

Changes in resting vital signs at baseline and at 12 weeks for those that completed the intervention (n=16) with (A) mean change in resting HR (0 BPM, 95% CI, −5.4 to 3.7); (B) mean change in resting SBP (MD 9.6, 95% CI −19.0 to −0.7; P=0.049); and (C) mean change in resting DBP (MD 4.3; 95% CI, −12.2 to 3.6; P=0.256). HR = heart rate; BPM = beats per minute; SBP = systolic blood pressure; DBP = diastolic blood pressure
DISCUSSION
In this study, we present the initial feasibility data of a smartphone-enabled, home-based CR for secondary prevention in veterans with CHD. There was a > 90% participation rate at 4 weeks of enrollment, even among subjects who eventually withdrew, with moderate to high levels of engagement and perceived acceptability among program completers. We also report significant improvements in functional capacity and SBP, with no adverse events noted, however these findings should be carefully interpreted considering the small sample size and lack of a comparator group and should be considered exploratory. This is the first study of its kind in veterans, showing that newer mobile technologies can be successfully adopted in this population despite little precedent and the need for a certain level of technologic literacy. Nearly half of our participants were older than 65 years of age and/or smartphone-naïve, factors which may limit many patients from successfully adopting technology-based interventions.
A majority of participants were satisfied or very satisfied with the intervention, and many of them used the unique features of the app including the electronic health diary, secure app messaging with the coach, and the educational modules. There was no observed attrition in the use of the app over time with consistent participation from weeks 4 through 12. Participants also reported improved self-care behaviors including exercise, medication adherence and self-care.
Our results are comparable to prior studies of home-based CR.17–19 A systematic review of 18 trials by Jolly et al. demonstrated that home-based CR programs achieved a 4 mmHg greater reduction in SBP (95% CI 1.5 to 6.5) and a significant improvement in functional capacity (1.1 METS, 95% CI 0.2 to 2.1) over usual care. In comparison to center-based programs, there was no significant difference in SBP or functional capacity among patients performing home-based CR.19 Several additional studies have further demonstrated home-based CR to be as safe and efficacious as center-based CR for improving modifiable risk factors, clinical outcomes and self-reported measures of health status in low risk patients with ischemic heart disease.7,20 This was best demonstrated in a Cochrane meta-analysis of 17 randomized trials including 2172 participants undergoing CR following acute MI, following myocardial revascularization, or with congestive heart failure that showed no significant difference in mortality (RR = 0.79, 95% CI, 0.43 to 1.47, P = 0.46, fixed-effect), exercise capacity (SMD = −0.10, 95% CI, −0.29 to 0.08, P = 0.29, random-effects), health-related quality of life, and modifiable risk factors (including blood pressure, lipids, and smoking behavior) after up to 12 months of follow-up between home- and center-based CR.7
Our findings suggest that a smartphone-enabled home-based CR intervention may be an acceptable alternative for veterans who cannot enroll in center-based CR. This intervention provides incremental evidence among a backdrop of previous studies evaluating smartphones in the delivery of remote CR. Early studies were limited to the passive monitoring of physical activity in patients participating in home-based CR, through either smartphone-based activity questionnaires21 or the functionalities of the device itself, such as its motion sensor or integrated global positioning system (GPS).22 More recent studies have demonstrated the feasibility of smartphones in the direct delivery of exercise-based CR, but most have included limited mobile interventions (i.e. SMS messaging and structured mobile questionnaires),11 and were further limited by their short program duration (typically 6 to 8 weeks),23,24 or hybrid designs featuring both remote and center-based CR.25 In a recent study of 120 post-MI patients randomized to either smartphone-based or center-based CR, Varnfield et al. demonstrated that patients receiving a smartphone-based program had higher levels of CR utilization with significant improvements in weight and quality of life; however, the program was restricted to Nokia devices, as opposed to the Android system in our study.11 The app evaluated in our study offers several potential advantages over previously published programs. It provided an interconnected platform between the patient’s smartphone and the coach, allowing for easier data sharing and communication (2-way messaging using a HIPAA-compliant protocol more secure than SMS), and is commercially available, thereby making it easier for our approach to be translated clinically and tested at other facilities. Another potential advantage is the integrated web-based dashboard that allowed for remote monitoring of patients.
We report results from an early feasibility study, and the results should therefore be interpreted considering several limiting factors including the small sample size, absence of control or usual care group, and the short follow-up period. Four patients also participated in center-based CR which limits the interpretation the observed outcomes. Finally, the study was conducted from a single Veterans Affairs hospital and enrolled only male veterans, and thus the generalizability to other populations is limited.
Smartphone-enabled, home-based CR is feasible and acceptable among low or moderate risk, middle-aged and elderly veterans with CHD, and the preliminary results presented here are consistent with prior studies on home-based CR. Although center-based programs are the standard of care, mobile technology-enabled CR may represent a viable option when cost of attendance and geographic distance prohibit access to traditional CR programs. These preliminary results highlight the initial feasibility of a smartphone-based intervention as an acceptable approach for CR delivery, in particular among older and technology-naïve populations such as veterans.
Supplementary Material
1
2
3
ACKNOWLEDGEMENTS
The authors express their gratitude to Ms. Susan Hansen for her detailed review and thoughtful contributions to this manuscript. Ms. Hansen has provided the corresponding author with her permission to be named in this manuscript.
SOURCES OF FUNDING:
Funded by the Department of Veterans Affairs Center for Innovation (A.S.,A.M.Z), National Heart, Lung, and Blood Institute (A.S., 5K23HL127251), and by an in-kind project grant from Moving Analytics for the use of their software and support services (A.S., A.H.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration: ClinicalTrials.gov number, NCT02791685
DISCLOSURES
Dr. Harzand reports the following activities with Moving Analytics: science advisory board, speaker’s bureau (modest), and shareholder (modest, non-fiduciary). None of the remaining authors report any potential conflicts of interest.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.amjcard.2018.07.028
Read article for free, from open access legal sources, via Unpaywall:
http://manuscript.elsevier.com/S0002914918315005/pdf/S0002914918315005.pdf
Citations & impact
Impact metrics
Article citations
Technological Interventions to Implement Prevention and Health Promotion in Cardiovascular Patients.
Healthcare (Basel), 12(20):2055, 16 Oct 2024
Cited by: 0 articles | PMID: 39451470 | PMCID: PMC11507996
Review Free full text in Europe PMC
User Engagement With mHealth Interventions to Promote Treatment Adherence and Self-Management in People With Chronic Health Conditions: Systematic Review.
J Med Internet Res, 26:e50508, 24 Sep 2024
Cited by: 0 articles | PMID: 39316431 | PMCID: PMC11462107
Review Free full text in Europe PMC
Rationale and Design of the mTECH-Rehab Randomized Controlled Trial: Impact of a Mobile Technology Enabled Corrie Cardiac Rehabilitation Program on Functional Status and Cardiovascular Health.
J Am Heart Assoc, 13(2):e030654, 16 Jan 2024
Cited by: 1 article | PMID: 38226511 | PMCID: PMC10926786
Effects of a patient-centered digital health intervention in patients referred to cardiac rehabilitation: the Smart HEART clinical trial.
BMC Cardiovasc Disord, 23(1):453, 12 Sep 2023
Cited by: 1 article | PMID: 37700245 | PMCID: PMC10496208
Examining standardized tools used for the evaluation of mobile health applications for cardiovascular disease.
Front Public Health, 11:1155433, 14 Jun 2023
Cited by: 0 articles | PMID: 37388154 | PMCID: PMC10303135
Go to all (24) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT02791685
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Feasibility, Safety, and Effectiveness of a Mobile Application in Cardiac Rehabilitation.
Isr Med Assoc J, 22(6):357-363, 01 Jun 2020
Cited by: 13 articles | PMID: 32558441
SMARTphone and social media-based Cardiac Rehabilitation and Secondary Prevention (SMART-CR/SP) for patients with coronary heart disease in China: a randomised controlled trial protocol.
BMJ Open, 8(6):e021908, 30 Jun 2018
Cited by: 31 articles | PMID: 29961032 | PMCID: PMC6042601
Long-term follow-up with a smartphone application improves exercise capacity post cardiac rehabilitation: A randomized controlled trial.
Eur J Prev Cardiol, 27(16):1782-1792, 28 Feb 2020
Cited by: 48 articles | PMID: 32106713 | PMCID: PMC7564298
Smartphones in the secondary prevention of cardiovascular disease: a systematic review.
BMC Cardiovasc Disord, 18(1):25, 07 Feb 2018
Cited by: 52 articles | PMID: 29415680 | PMCID: PMC5803998
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: L30 HL124549
Grant ID: K23 HL127251


