Abstract
Aims
The onset of the COVID-19 pandemic saw the suspension of centre-based cardiac rehabilitation (CBCR) and has underscored the need for home-based cardiac telerehabilitation (HBCTR) as a feasible alternative rehabilitation delivery model. Yet, the effectiveness of HBCTR as an alternative to Phase 2 CBCR is unknown. We aimed to conduct a meta-analysis to quantitatively appraise the effectiveness of HBCTR.Methods and results
PubMed, EMBASE, CENTRAL, CINAHL, Scopus, and PsycINFO were searched from inception to January 2021. We included randomized controlled trials (RCTs) comparing HBCTR to Phase 2 CBCR or usual care in patients with coronary heart disease (CHD). Out of 1588 studies, 14 RCTs involving 2869 CHD patients were included in this review. When compared with usual care, participation in HBCTR showed significant improvement in functional capacity {6-min walking test distance [mean difference (MD) 25.58 m, 95% confidence interval (CI) 14.74-36.42]}; daily step count (MD 1.05 K, 95% CI 0.36-1.75) and exercise habits [odds ratio (OR) 2.28, 95% CI 1.30-4.00)]; depression scores (standardized MD -0.16, 95% CI -0.32 to 0.01) and quality of life [Short-Form mental component summary (MD 2.63, 95% CI 0.06-5.20) and physical component summary (MD 1.99, 95% CI 0.83-3.16)]. Effects on medication adherence were synthesized narratively. HBCTR and CBCR were comparably effective.Conclusion
In patients with CHD, HBCTR was associated with an increase in functional capacity, physical activity (PA) behaviour, and depression when compared with UC. When HBCTR was compared to CBCR, an equivalent effect on functional capacity, PA behaviour, QoL, medication adherence, smoking behaviour, physiological risk factors, depression, and cardiac-related hospitalization was observed.Free full text

Effectiveness of home-based cardiac telerehabilitation as an alternative to Phase 2 cardiac rehabilitation of coronary heart disease: a systematic review and meta-analysis
Associated Data
Abstract
Aims
The onset of the COVID-19 pandemic saw the suspension of centre-based cardiac rehabilitation (CBCR) and has underscored the need for home-based cardiac telerehabilitation (HBCTR) as a feasible alternative rehabilitation delivery model. Yet, the effectiveness of HBCTR as an alternative to Phase 2 CBCR is unknown. We aimed to conduct a meta-analysis to quantitatively appraise the effectiveness of HBCTR.
Methods and results
PubMed, EMBASE, CENTRAL, CINAHL, Scopus, and PsycINFO were searched from inception to January 2021. We included randomized controlled trials (RCTs) comparing HBCTR to Phase 2 CBCR or usual care in patients with coronary heart disease (CHD). Out of 1588 studies, 14 RCTs involving 2869 CHD patients were included in this review. When compared with usual care, participation in HBCTR showed significant improvement in functional capacity {6-min walking test distance [mean difference (MD) 25.58 m, 95% confidence interval (CI) 14.74–36.42]}; daily step count (MD 1.05
m, 95% confidence interval (CI) 14.74–36.42]}; daily step count (MD 1.05 K, 95% CI 0.36–1.75) and exercise habits [odds ratio (OR) 2.28, 95% CI 1.30–4.00)]; depression scores (standardized MD −0.16, 95% CI −0.32 to 0.01) and quality of life [Short-Form mental component summary (MD 2.63, 95% CI 0.06–5.20) and physical component summary (MD 1.99, 95% CI 0.83–3.16)]. Effects on medication adherence were synthesized narratively. HBCTR and CBCR were comparably effective.
K, 95% CI 0.36–1.75) and exercise habits [odds ratio (OR) 2.28, 95% CI 1.30–4.00)]; depression scores (standardized MD −0.16, 95% CI −0.32 to 0.01) and quality of life [Short-Form mental component summary (MD 2.63, 95% CI 0.06–5.20) and physical component summary (MD 1.99, 95% CI 0.83–3.16)]. Effects on medication adherence were synthesized narratively. HBCTR and CBCR were comparably effective.
Conclusion
In patients with CHD, HBCTR was associated with an increase in functional capacity, physical activity (PA) behaviour, and depression when compared with UC. When HBCTR was compared to CBCR, an equivalent effect on functional capacity, PA behaviour, QoL, medication adherence, smoking behaviour, physiological risk factors, depression, and cardiac-related hospitalization was observed.
Introduction
Cardiac rehabilitation (CR) is a widely accepted treatment modality in the secondary prevention of coronary heart disease (CHD) and is differentiated into three main phases—Phase 1 (early mobilization during acute in-patient hospitalization); Phase 2 (rehabilitation services traditionally delivered in an outpatient setting that focuses on health behaviour change, risk factor modification, and psychosocial well-being.); and Phase 3 (long-term maintenance of lifestyle changes).1,2 While international guidelines have repeatedly recommended the provision of comprehensive CR to ensure optimized and cost-effective outcomes,2 exercise training remains a cornerstone of CR to improve functional capacity. Functional capacity, which represents cardiorespiratory fitness levels, is a powerful and independent predictor of cardiac and all-cause mortality in patients with CHD.3,4 Research has confirmed that with every metabolic increase in functional capacity, survival rates are improved by 13%.5 Yet, centre-based CR (CBCR) participation rates among eligible patients remain low at 10–30% worldwide, reportedly due to challenges surrounding accessibility, conflicting commitments, low socioeconomic status, and cost.6
While alternative models using technology to deliver home-based cardiac telerehabilitation (HBCTR) have received increased interest the past decade, the onset of the COVID-19 pandemic has underscored the importance of HBCTR, defined herein, as the use of information and communication technologies (ICTs) (e.g. web- and mobile-based platforms, wearable sensor devices) to deliver patient education, behavioural change counselling, remote exercise supervision, cardiovascular risk factor modification, and psychosocial support that are delivered completely outside of the conventional CBCR setting.7 The massive strain on healthcare systems due to rising COVID-19 cases has resulted in the partial or complete closures of Phase 2 CBCR programmes due to the re-deployment of manpower, resources, and infrastructure,8 while efforts to curb the spread of COVID-19 infections through safe distancing measures have rendered group exercise and therapy sessions nearly impossible.9 While this phenomenon is of concern as poorer patient outcomes have been associated with delays in CR,10 the rapid proliferation and ubiquitous use of ICTs in the area of telehealth have provided a fertile ground for the delivery of HBCTR.11,12 Importantly, this new delivery model encourages a shift in patient’s mentality of CR being a time-limited intervention supervised in a hospital setting and presents a greater emphasis on personal accountability on the part of the patients to self-regulate their daily lifestyle behaviours.7
To date, efforts have been made to ascertain the effectiveness of telehealth interventions in the delivery of CR for patients with CHD. Although earlier reviews by Neubeck et al.,13 Huang et al.,14 and Rawstorn et al.15 observed findings in support of the use of telehealth, included interventions were predominantly limited to land-based telephone calls. Recent systematic reviews investigated the use of internet-based and mobile applications, however, neither were specific to populations of CHD and included patients with heart failure and other cardiovascular diseases16–18 and one study did not attempt a meta-analysis due to heterogeneity of included studies.19 Furthermore, all of the aforementioned systematic reviews13,14,16–19 included studies where the majority delivered telehealth interventions as an adjunct to Phase 2 CBCR or to deliver Phase 3 maintenance programmes. Therefore, an updated systematic review that explores the technologies that support greater Phase 2 CR programme flexibility and is specific to the population of CHD is justified.
Hence, the aim of this systematic review and meta-analysis was to evaluate if HBCTR is at least as effective as CBCR, or more effective than usual care (UC), in improving functional capacity, physical activity (PA), smoking, medication adherence, physiological risk factor control, health-related QOL and reducing depression, mortality, and cardiac-related hospitalizations for CHD patients.
Methods
Study design
This is a systematic review and meta-analysis of randomized controlled trials (RCTs) and is written in accordance with the guidelines from the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA)20 to improve transparency, accuracy, and completeness.
Study inclusion criteria
Studies were selected if they were RCTs which assessed an HBCTR programme in patients with CHD. The detailed inclusion criteria included: (i) population: patients aged ≥18 years with a medical diagnosis of CHD, myocardial infarction (MI), acute coronary syndrome (ACS), angina pectoris, and/or those who have undergone revascularization (i.e. percutaneous coronary intervention or coronary artery bypass grafting) who have not previously received CR; (ii) intervention: any website-based and/or mobile health (mHealth) application used either as a stand-alone or supplemented with other delivery modes, such as text message, telephone or video calls, email or tele-monitoring to deliver Phase 2 CR or secondary prevention exclusively in the home setting; (iii) the HBCTR programme should target at least one of the following lifestyle behaviours—PA, healthy diet, smoking cessation, medication adherence, and stress management; (iv) control: supervised CBCR or UC (standard medical care that does not include any supervised or structured exercise training); (v) primary outcome: functional capacity (as measured by maximal or submaximal exercise testing to assess cardiorespiratory fitness); (vi) secondary outcome: behavioural (i.e. PA, smoking, medication management), physiological (i.e. cardiovascular risk factor control), and clinical (i.e. quality of life, depression, cardiac-related hospitalization, mortality). Studies were excluded if: (i) population: heart failure patients regardless of left ventricular ejection fraction; (ii) intervention: delivered in tandem with CBCR (i.e. hybrid CR) or after participants had completed Phase 2 CR (i.e. Phase 3 CR); (iii) control group: received components of web-based and or mHealth CR; and (iv) qualitative studies, book chapter reviews, abstracts-only journals, editorials, discussions papers, conference proceedings, and letters. We also excluded studies where the intervention was only text messaging, telephone calls, video conferencing, or telemonitoring and uploading measurements alone. While we did not restrict studies based on sample size or follow-up duration, we only included studies published in English.
years with a medical diagnosis of CHD, myocardial infarction (MI), acute coronary syndrome (ACS), angina pectoris, and/or those who have undergone revascularization (i.e. percutaneous coronary intervention or coronary artery bypass grafting) who have not previously received CR; (ii) intervention: any website-based and/or mobile health (mHealth) application used either as a stand-alone or supplemented with other delivery modes, such as text message, telephone or video calls, email or tele-monitoring to deliver Phase 2 CR or secondary prevention exclusively in the home setting; (iii) the HBCTR programme should target at least one of the following lifestyle behaviours—PA, healthy diet, smoking cessation, medication adherence, and stress management; (iv) control: supervised CBCR or UC (standard medical care that does not include any supervised or structured exercise training); (v) primary outcome: functional capacity (as measured by maximal or submaximal exercise testing to assess cardiorespiratory fitness); (vi) secondary outcome: behavioural (i.e. PA, smoking, medication management), physiological (i.e. cardiovascular risk factor control), and clinical (i.e. quality of life, depression, cardiac-related hospitalization, mortality). Studies were excluded if: (i) population: heart failure patients regardless of left ventricular ejection fraction; (ii) intervention: delivered in tandem with CBCR (i.e. hybrid CR) or after participants had completed Phase 2 CR (i.e. Phase 3 CR); (iii) control group: received components of web-based and or mHealth CR; and (iv) qualitative studies, book chapter reviews, abstracts-only journals, editorials, discussions papers, conference proceedings, and letters. We also excluded studies where the intervention was only text messaging, telephone calls, video conferencing, or telemonitoring and uploading measurements alone. While we did not restrict studies based on sample size or follow-up duration, we only included studies published in English.
Search strategy
A systematic electronic search was performed in the following databases—PubMed, EMBASE, CENTRAL, CINAHL, Scopus, and PsycINFO up to January 2021. No limits on publication status and date were imposed on the search. The reference lists of relevant reviews and key literature were manually searched to supplement our database search. Details of our database search are documented in Supplementary material online, Table S1. Our search strategy was peer-reviewed by a university resource librarian with expertise in systematic review searching.
Study selection process
Search results were managed using EndNote X9. After the removal of duplicates, two reviewers independently screened the title and abstract of studies against the eligibility criteria. The full texts of all relevant studies were downloaded and further evaluated for compliance with the eligibility criteria. Disagreements between the two reviewers regarding inclusion were resolved by consultation with a third reviewer.
Risk of bias (quality) assessment
The Cochrane Risk of Bias tool21 for randomized trials was used to guide the quality assessment of each included study and consists of the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias (e.g. whether study groups were comparable at baseline). Two independent reviewers conducted the quality appraisal separately. Discrepancies were resolved through discussion.
Data extraction
Two reviewers used standardized forms to independently extract study data, any discrepancies were resolved between the two reviewers to reach a consensus. Two authors of the included studies were contacted through email to obtain missing data. Both replied and provided the required information.
Statistical analysis
For studies that measured outcomes at multiple timepoints, a decision was made to include outcomes immediately after intervention in the meta-analysis due to insufficient data and wide variation in timepoints across studies. Continuous data were analysed using mean differences (MD) as the effect measure; standardized mean difference (SMD) was used when varying outcome measurement instruments were used. Dichotomous data were analysed using odds ratio (OR). Heterogeneity between studies was explored by Cochran’s Q statistic and I2 index. I2 values of 25%, 50%, and 75% were considered low, moderate, and high heterogeneity, respectively.22 We adopted a random-effects model to perform the meta-analysis if moderate heterogeneity was present (P <
< 0.01 or I2 > 50%); otherwise, a fixed-effects model was assumed.23 Neither subgroup analysis nor meta-regression were performed for our primary outcome as the meta-analysis did not contain a minimum of 10 studies. Meta-analyses were stratified by type of comparison group to differentiate effects and results are presented in forest plots with 95% confidence interval (CI), with an alpha level of 0.05 considered as statistically significant. All computations for the meta-analyses were conducted using Review Manager 5.4. In studies where median and interquartile ranges were reported, mean and standard deviations were estimated by methods recommended by Wan et al.24 Studies that could not be pooled for meta-analysis were combined using narrative synthesis.
0.01 or I2 > 50%); otherwise, a fixed-effects model was assumed.23 Neither subgroup analysis nor meta-regression were performed for our primary outcome as the meta-analysis did not contain a minimum of 10 studies. Meta-analyses were stratified by type of comparison group to differentiate effects and results are presented in forest plots with 95% confidence interval (CI), with an alpha level of 0.05 considered as statistically significant. All computations for the meta-analyses were conducted using Review Manager 5.4. In studies where median and interquartile ranges were reported, mean and standard deviations were estimated by methods recommended by Wan et al.24 Studies that could not be pooled for meta-analysis were combined using narrative synthesis.
Results
Study selection
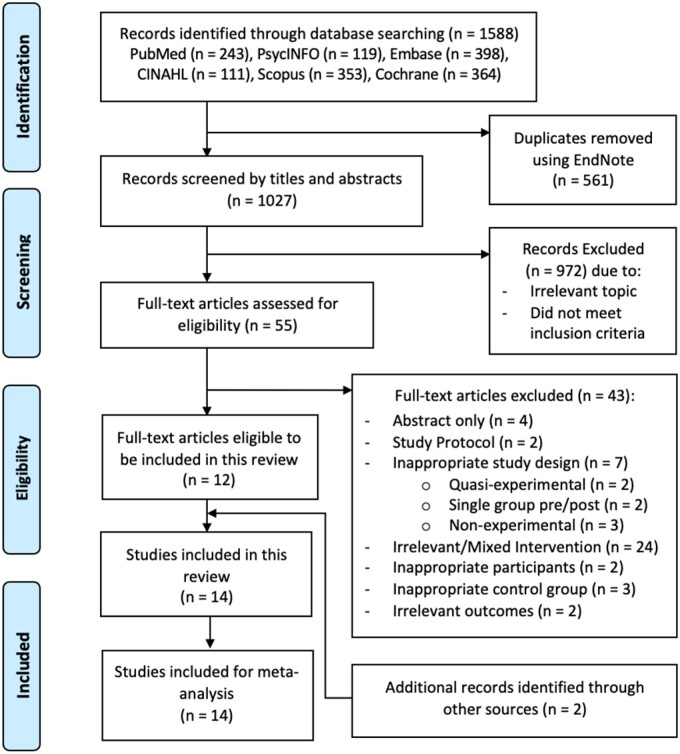
The initial search from the six electronic databases identified 1588 records, of which 561 duplicates were removed. After screening the title and abstracts of 1027 records, 973 were excluded for irrelevant topic and not meeting the inclusion criteria. Fifty-five remaining records were eligible for further full-text review for compliance with the eligibility criteria. The exclusion of 43 records with reasons is documented in Supplementary material online, TableS2. Additional searching of the reference lists of relevant studies identified two studies for inclusion. Finally, 14 studies were included in this review (Figure 1).
Study characteristics
A summary of the characteristics of included studies is presented in Table 1.25–38 All 14 studies were two-arm RCTs involving a total of 2869 participants (sample size ranging between 15 and 1000). Six studies were conducted in China,26–28,32,34,38 three in Canada,29,30,36 two in Australia,33,35 one in the New Zealand,37 one in the UK,25 and another was a multi-centre study across Europe.31 Participants included in this review were diagnosed with the following: angina,25,26,37 MI,26,33,36,37 ACS,29–31 CHD,27,32,35,37 or had undergone coronary revascularization.29,31,34,36–38 The mean age of participants ranged from 45.8 to 73.6.36 Females accounted for 21.8% of the overall sample. Control groups included three supervised exercise and health education in a CBCR setting, while the remaining majority were UC that involved outpatient visits to a physician or nurse25–28 and regular CHD health education.26–30,34,36,38 One of these studies had a waitlist control group that received UC.27
Table 1
Characteristics of included studies
| Author (year)/country | Study design | Population (P): a. Number of participants (N) b. Diagnosis c. Age (mean ± SD) d. Gender (female%) | Intervention (I): a. Number (n) b. Intervention development c. Theoretical framework d. Duration/frequency (per week) e. Intervention outline f. PA prescription g. Intervention compliance | Control (C): a. Number (n) b. Outline | Outcome (O): a. Behavioural b. Physiological c. Clinical | Remarks: a. Attrition b. ITT c. MDM d. Protocol e. Funding |
|---|---|---|---|---|---|---|
| Devi et al. (2014)/UK | Two-arm RCT |
|
|
|
|
|
| Dorje et al. (2019)/China | Two-arm RCT |
|
|
|
|
|
| Duan et al. (2018)/China | Two-arm RCT |
|
|
|
|
|
| Fang et al. (2019)/China | Two-arm RCT |
|
|
|
|
|
| Lear et al. (2014)/Canada | Two-arm RCT |
|
|
|
|
|
| Maddison et al. (2019)/New Zealand | Two-arm RCT |
|
|
|
|
|
| Reid et al. (2012)/Canada | Two-arm RCT |
|
|
|
|
|
| Snoek et al. (2020)/Europe+ | Two-arm RCT |
|
|
|
|
|
| Song et al. (2020)/China | Two-arm RCT |
|
|
|
|
|
| Varnfield et al. (2014)/Australia | Two-arm RCT |
|
|
|
|
|
| Wang et al. (2020)/China | Two-arm RCT |
|
|
|
|
|
| Yu et al. (2020)/China | Two-arm RCT |
|
|
|
|
|
| Yudi et al. (2020)/Australia | Two-arm RCT |
|
|
|
|
|
| Zutz et al. (2007)/Canada | Two-arm RCT |
|
|
|
|
|
6MWT, 6-min walk test; ACS, acute coronary syndrome; BCT, behaviour change theory; BG, blood glucose; BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass graft; CAP-CR, care assessment platform-cardiac rehabilitation; CBCR, centre-based cardiac rehabilitation; CBT, cognitive behavioural therapy; CG, control group; CHD, coronary heart disease; CPET, cardiopulmonary exercise testing; CR, cardiac rehabilitation; CRV, coronary revascularization; DBP, diastolic blood pressure; HBCTR, home-based cardiac telerehabilitation; HCP, healthcare professional; HDL, high-density lipoprotein; HR, heart rate; IG, intervention group; ITT, intention-to-treat; LDL, low-density lipoprotein; MDM, missing data management; MI, myocardial infarction; NR, not reported; PA, physical activity; PCI, percutaneous coronary intervention; QoL, quality of life; RCT, randomized controlled trial; REMOTE-CR, remotely monitored exercise-based cardiac rehabilitation; RF, risk factor; SBP, systolic blood pressure; SCT, social cognitive theory; SD, standard deviation; SDT, self-determination theory; SMART-CR/SP, smartphone-based-cardiac rehabilitation/secondary prevention; TC, total cholesterol; TG, triglycerides; TMX, treadmill exercise testing; vCRP, virtual cardiac rehabilitation programme; VO2, oxygen consumption; Yes*, only for primary outcome; Europe+, Netherlands, Switzerland, Denmark, France, Spain.
Intervention characteristics
HBCTR was delivered largely via web-based platforms in five studies25,27,29,30,36 and smartphone applications in nine studies.26,28,31–35,37,38 Ten studies used telemonitoring devices to support PA self-monitoring including pedometers,26,30,33 accelerometers,25 and heart-rate monitors.28,29,31,32,36,37 Supplementary forms of communication, such as text messages, phone calls, video calls, and emails were utilized between the participants and intervention team, and contact varied from a weekly to monthly basis. Eight studies mentioned the use of secured web servers,31,37 data portals,26,38 and password-protected websites25,29,30,36 for the transmission and storage of patient data.
Five studies27,32,35,37,38 reported the use of a theoretical framework. With the exception of one study,38 all included studies had a PA component as part of their intervention. Five studies26,28,30,33,34 had comprehensive HBCTR that included all the core components of secondary prevention (CHD risk factors management, PA, smoking cessation, medication adherence, and stress management), while three studies31,32,37 focused solely on PA. Stress management was the least addressed area and was covered in only six studies.
Duration of HBCTR ranged from 6 weeks to 6
weeks to 6 months. Eight studies monitored participants’ intervention compliance via website or mobile application logins,25,29,35,36,38 exercise and monitoring data uploading,29,33,35,36 number of chat sessions attended,29,36 and progress of education modules and reading materials.26,34 Details of intervention characteristics can be found in Table 1 and Supplementary material online, Table S3.
months. Eight studies monitored participants’ intervention compliance via website or mobile application logins,25,29,35,36,38 exercise and monitoring data uploading,29,33,35,36 number of chat sessions attended,29,36 and progress of education modules and reading materials.26,34 Details of intervention characteristics can be found in Table 1 and Supplementary material online, Table S3.
Risk of bias of included studies
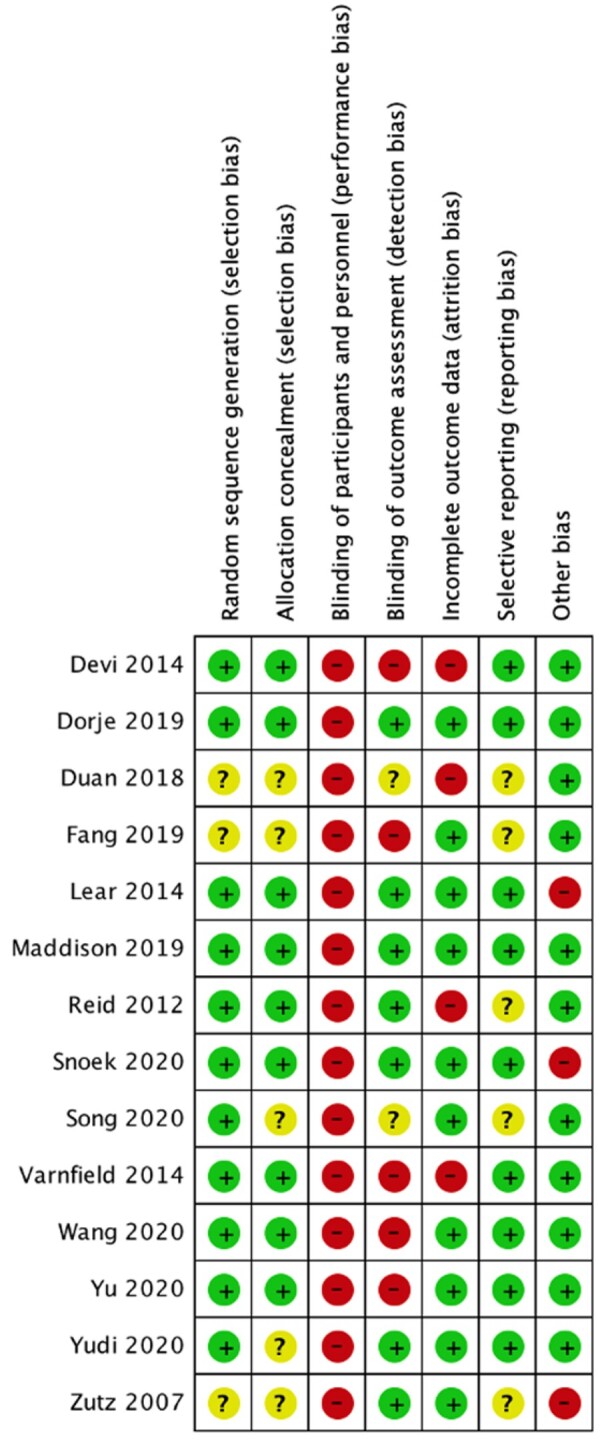
Of the 14 included RCTs, random sequence generation was clearly detailed in 11 studies.25,26,29–35,37,38 Six studies27,28,32,35,36 had an unclear risk of selection bias due to failure to describe how allocation concealment was undertaken. Due to the nature of the intervention, blinding of participants and personnel was not possible in all studies,25–38 rendering a high risk of performance bias. Detection bias was avoided in seven studies26,29–31,35–37 through blinding of outcome assessors; however, two studies were rated as unclear risk of bias as one study’s27 contactless online data collection precluded any direct involvement of outcome assessors and most of the outcomes in both studies27,32 were unlikely to be influenced by a lack of blinding. Ten studies26,28,29,31,32,34–38 had a low risk of bias as attrition rates were <20% and dropouts did not differ largely between treatment groups. Nine studies25,26,29–31,33,35,37,38 reported having a study protocol and were deemed low risk of reporting bias. Three studies29,31,36 had risk of bias due to imbalances in baseline characteristics between treatment groups, including type II diabetes, hypertension, total cholesterol, prior revascularization, family history of cardiovascular, self-reported PA, and total treadmill time. A summary of the risk of bias is provided in Figure 2.
Outcomes
Primary: functional capacity
Nine studies investigated outcomes on functional capacity at 6 weeks to 6
weeks to 6 months of follow-up. Four studies26,28,33,35 reported effects on 6MWT distance. Five other studies reported symptom-limited treadmill cardiopulmonary exercise testing (CPET)31,32,37 and treadmill exercise test (TMX) using the Bruce protocol and were combined using standardized mean difference.29,36 There was a statistically significant difference in 6MWT between the HBCTR vs. UC group in favour of the intervention group (MD 25.95
months of follow-up. Four studies26,28,33,35 reported effects on 6MWT distance. Five other studies reported symptom-limited treadmill cardiopulmonary exercise testing (CPET)31,32,37 and treadmill exercise test (TMX) using the Bruce protocol and were combined using standardized mean difference.29,36 There was a statistically significant difference in 6MWT between the HBCTR vs. UC group in favour of the intervention group (MD 25.95 m, 95% CI 12.67 to 39.22; P
m, 95% CI 12.67 to 39.22; P <
< 0.00001; I2 = 32%; Figure 3A) and but not in symptom-limited treadmill exercise testing between the HBCTR vs. UC group (SMD 0.30, 95% CI −0.07 to 0.68; P
0.00001; I2 = 32%; Figure 3A) and but not in symptom-limited treadmill exercise testing between the HBCTR vs. UC group (SMD 0.30, 95% CI −0.07 to 0.68; P =
= 0.11; I2 = 60%; Figure 3B). However, there was high heterogeneity across studies. Leave-one-out sensitivity analysis showed that when we removed the study by Snoek et al.,31 the overall effect changed into a small but significant difference in favour of the HB exercise group (SMD 0.44, 95% CI 0.14 to 0.74; P
0.11; I2 = 60%; Figure 3B). However, there was high heterogeneity across studies. Leave-one-out sensitivity analysis showed that when we removed the study by Snoek et al.,31 the overall effect changed into a small but significant difference in favour of the HB exercise group (SMD 0.44, 95% CI 0.14 to 0.74; P =
= 0.004; I2 = 42%). We expected this as all studies except Snoek et al.31 reported a significant effect or a trend in favour of HBCTR.
0.004; I2 = 42%). We expected this as all studies except Snoek et al.31 reported a significant effect or a trend in favour of HBCTR.

Forest plots of the effects of home-based cardiac telerehabilitation on functional capacity—(A) 6-min walk test; (B) Symptom-limited exercise testing. CBCR, centre-based cardiac rehabilitation; HBCTR, home-based cardiac telerehabilitation; UC, usual care.
We observed no statistically significant difference in 6MWT between the HBCTR vs. CBCR group (MD 10.60 m, 95% CI −32.22 to 53.41; P
m, 95% CI −32.22 to 53.41; P =
= 0.63; I2 = 60%; Figure 3A). Only one study by Maddison et al.37 compared symptom-limited CPET between HBCTR to CBCR but found no statistically significant difference (Figure 3B).
0.63; I2 = 60%; Figure 3A). Only one study by Maddison et al.37 compared symptom-limited CPET between HBCTR to CBCR but found no statistically significant difference (Figure 3B).
Secondary: behavioural outcomes
Physical activity
Nine studies reported PA behaviour by using accelerometers25 and pedometers30,37 to assess steps per day or daily minutes of moderate PA; self-reported days per week of moderate-vigorous PA,31 International Physical Activity Questionnaire (minutes/week),27 Minnesota Leisure time physical activity (LTPA) questionnaire (reported as the average weekly kilocalories kcal/week)29,36; and exercise habits (number of participants reporting 30 min of moderate activity performed 3–5 times/week).32,34 Due to variation in how studies defined PA behaviour, we performed separate meta-analysis and conducted a narrative synthesis where appropriate.
min of moderate activity performed 3–5 times/week).32,34 Due to variation in how studies defined PA behaviour, we performed separate meta-analysis and conducted a narrative synthesis where appropriate.
Between HBCTR vs. UC, a statistically significant difference was seen in steps per day (we used K to represent thousands) at 6 weeks to 6
weeks to 6 months (MD 1.05K, 95% CI 0.35 to 1.75; P
months (MD 1.05K, 95% CI 0.35 to 1.75; P =
= 0.003; I2 = 0%; Supplementary material online, Figure S1A) and exercise habits at 6–12
0.003; I2 = 0%; Supplementary material online, Figure S1A) and exercise habits at 6–12 months (OR 2.28, 95% CI 1.30 to 4.00; P
months (OR 2.28, 95% CI 1.30 to 4.00; P =
= 0.004; I2 = 19%; Supplementary material online, Figure S1B) favouring the intervention group. The effect of HBTR vs. UC on LTPA at 3–4
0.004; I2 = 19%; Supplementary material online, Figure S1B) favouring the intervention group. The effect of HBTR vs. UC on LTPA at 3–4 months, although favouring the intervention group, was not statistically significant (MD 0.46K kcal/week, 95% CI −0.74 to 1.65; P
months, although favouring the intervention group, was not statistically significant (MD 0.46K kcal/week, 95% CI −0.74 to 1.65; P =
= 0.45; I2 = 37%; Supplementary material online, Figure S1C).
0.45; I2 = 37%; Supplementary material online, Figure S1C).
A narrative synthesis for HBCTR on PA was conducted for three studies not included in the meta-analysis. Comparing HBCTR vs. UC, one study27 found a statistically significant increase (P <
< 0.01) in PA minutes/week using the International Physical Activity Questionnaire favouring the intervention group, but another did not find significant differences in self-reported days per week of moderate-vigorous PA.31 Maddison et al.37 also did not find significant difference in accelerometer-measured daily minutes of moderate PA between the HBCTR and CBCR group.
0.01) in PA minutes/week using the International Physical Activity Questionnaire favouring the intervention group, but another did not find significant differences in self-reported days per week of moderate-vigorous PA.31 Maddison et al.37 also did not find significant difference in accelerometer-measured daily minutes of moderate PA between the HBCTR and CBCR group.
Smoking
Five studies26,29,30,34,38 investigated the effects of HBCTR to UC on current smoking status of participants and were pooled for meta-analysis. There was no significant difference in the overall smoking event rate in the intervention group compared to the UC group at 8 weeks to 12
weeks to 12 months of follow-up (OR 0.88, 95% CI 0.59 to 1.33; P
months of follow-up (OR 0.88, 95% CI 0.59 to 1.33; P =
= 0.55; I2 = 0%; Supplementary material online, Figure S1D). One study35 compared the effects of HBCTR to CBCR and observed no statistical differences in current smoking status between groups (Figure 1D).
0.55; I2 = 0%; Supplementary material online, Figure S1D). One study35 compared the effects of HBCTR to CBCR and observed no statistical differences in current smoking status between groups (Figure 1D).
Medication adherence
Outcomes could not be pooled for meta-analysis due to variation in measurements of medication adherence, and hence a narrative synthesis was performed on three studies comparing HBCTR to UC. Two studies26,38 reported the percentage of participants adherent to each of the four cardioprotective medication classes [antiplatelets, angiotensin-converting-enzyme inhibitor (ACE-I) or angiotensin II receptor blockers (ARBs), beta-blockers, and statins] and found no statistically significant difference between treatment groups. One study34 reported adherence rates for all four cardioprotective medication classes (aspirin, ACE-I or ARB, β-blocker, and statin) and found that patients in the intervention group were more likely to be adherent than those in the control group (OR 1.79; 95% CI 1.76 to 1.87; P =
= 0.019). All study outcomes were collected at 6
0.019). All study outcomes were collected at 6 months.
months.
Secondary: physiological outcomes
At 6 weeks to 12
weeks to 12 months of follow-up, there was small significant effect on low-density lipoprotein favouring HBCTR compared to UC (MD −0.09, 95% CI −0.18 to −0.01; P
months of follow-up, there was small significant effect on low-density lipoprotein favouring HBCTR compared to UC (MD −0.09, 95% CI −0.18 to −0.01; P =
= 0.04; I2 = 36%; Figure 4D) but not when compared with CBCR. There were no statistically significant differences found for systolic blood pressure, diastolic blood pressure, total cholesterol, high-density lipoprotein, triglycerides, fasting blood glucose, and body mass index when HBCTR was compared to either UC or CBCR. Forest plots for all the physiological outcomes are found in Figure 4. Two studies31,35 reported HbA1c and could not be included in the meta-analysis. Both studies found no statistically significant difference in HbA1c between intervention and control groups.
0.04; I2 = 36%; Figure 4D) but not when compared with CBCR. There were no statistically significant differences found for systolic blood pressure, diastolic blood pressure, total cholesterol, high-density lipoprotein, triglycerides, fasting blood glucose, and body mass index when HBCTR was compared to either UC or CBCR. Forest plots for all the physiological outcomes are found in Figure 4. Two studies31,35 reported HbA1c and could not be included in the meta-analysis. Both studies found no statistically significant difference in HbA1c between intervention and control groups.
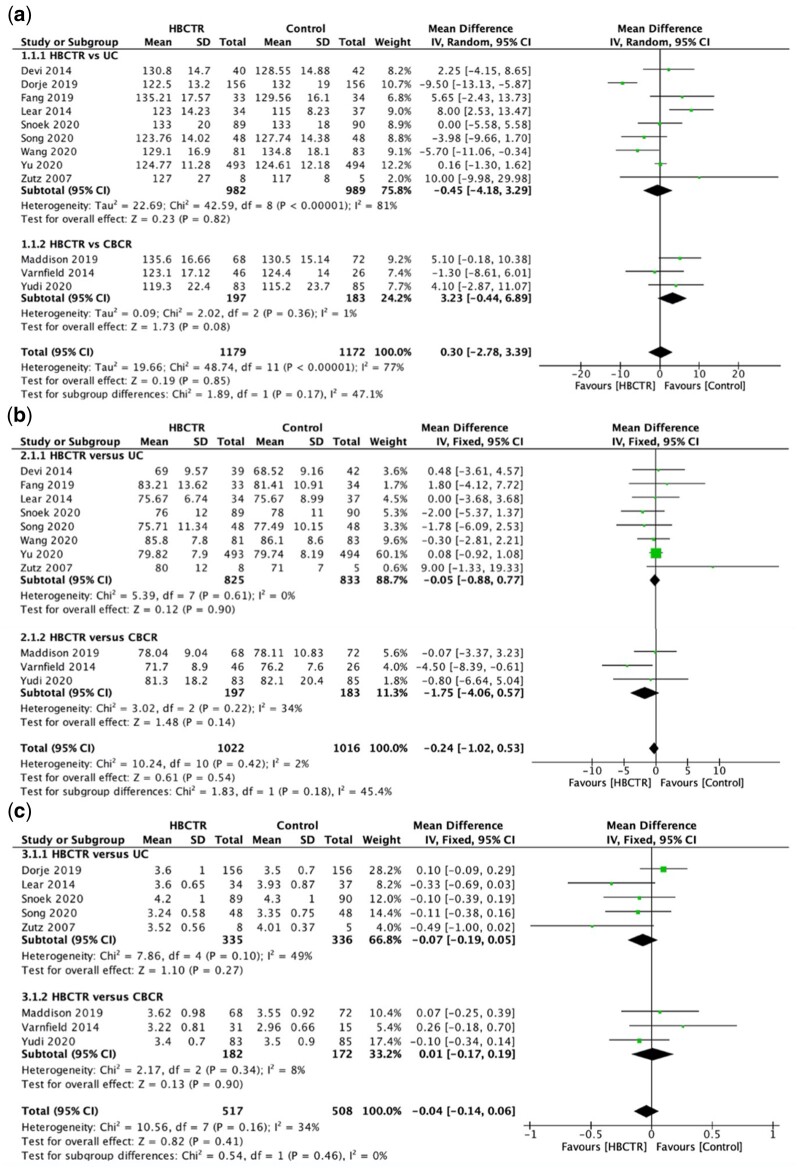
Forest plots of the effect of home-based cardiac telerehabilitation on physiological outcomes (A) systolic blood pressure, (B) diastolic blood pressure, (C) total cholesterol, (D) low-density lipoprotein, (E) high-density lipoprotein, (F) triglycerides, (G) blood glucose, and (H) body mass index. CBCR, centre-based cardiac rehabilitation; HBCTR, home-based cardiac telerehabilitation; UC, usual care.
Secondary: clinical outcomes
Quality of life
QoL was evaluated in nine studies at 6 weeks to 6
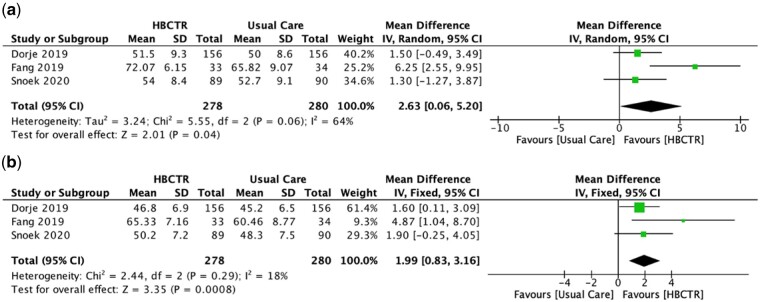
weeks to 6 months of follow-up using a variety of instruments: MacNew heart disease QoL,25,30 Medical Outcomes Study Short Form (SF) 1226 & 36,28,31,35 EuroQoL-5D (EQ5D),33,37 and World Health Organization’s QoL (WHOQoL).27 Separate meta-analyses were performed due to differences in scoring and unit of data. Of the four studies reporting SF-12 & 36, one study35 reported individual domain scores instead of the mental component summary (MCS) and physical component summary (PCS) score and therefore could not be pooled for meta-analysis. There was a statistically significant increase in QoL for participants in the HBCTR intervention group compared to the UC group for SF-MCS (MD 2.63, 95% CI 0.06 to 5.20; P
months of follow-up using a variety of instruments: MacNew heart disease QoL,25,30 Medical Outcomes Study Short Form (SF) 1226 & 36,28,31,35 EuroQoL-5D (EQ5D),33,37 and World Health Organization’s QoL (WHOQoL).27 Separate meta-analyses were performed due to differences in scoring and unit of data. Of the four studies reporting SF-12 & 36, one study35 reported individual domain scores instead of the mental component summary (MCS) and physical component summary (PCS) score and therefore could not be pooled for meta-analysis. There was a statistically significant increase in QoL for participants in the HBCTR intervention group compared to the UC group for SF-MCS (MD 2.63, 95% CI 0.06 to 5.20; P =
= 0.04; I2 = 64%; Figure 5A) and SF-PCS (MD 1.99, 95% CI 0.83 to 3.16; P
0.04; I2 = 64%; Figure 5A) and SF-PCS (MD 1.99, 95% CI 0.83 to 3.16; P =
= 0.0008; I2 = 18%; Figure 5B); higher scores represent higher QoL. Meta-analysis of the remaining studies showed significant heterogeneity (I2 = 90%; P
0.0008; I2 = 18%; Figure 5B); higher scores represent higher QoL. Meta-analysis of the remaining studies showed significant heterogeneity (I2 = 90%; P <
< 0.001). Hence, a narrative synthesis was conducted instead on the remaining six studies. With the exception of one study27 that found a statistically significant difference in WHOQoL scores favouring the HBCTR group compared to UC (P
0.001). Hence, a narrative synthesis was conducted instead on the remaining six studies. With the exception of one study27 that found a statistically significant difference in WHOQoL scores favouring the HBCTR group compared to UC (P <
< 0.00001), no difference in QoL scores between HBCTR compared to UC and CBCR in the remaining studies.25,30,33,35,37
0.00001), no difference in QoL scores between HBCTR compared to UC and CBCR in the remaining studies.25,30,33,35,37
Depression
Six studies evaluated depression using the Patient Health Questionnaire (PHQ-9),26,31 Cardiac Depression Scale,28,35 Center for Epidemiological Studies—Depression (CES-D),27 and the Depression Anxiety Stress Scales (DASS).33 At 6 weeks to 6
weeks to 6 months of follow-up, pooling of data on depression showed statistical significant differences between the HBCTR and UC favouring the HBCTR group (SMD −0.16, 95% CI −0.32 to −0.01; P
months of follow-up, pooling of data on depression showed statistical significant differences between the HBCTR and UC favouring the HBCTR group (SMD −0.16, 95% CI −0.32 to −0.01; P =
= 0.04; I2 = 31%; Supplementary material online, Figure S1E) but this difference was insignificant in the HBCTR vs. CBCR groups (SMD 0.02, 95% CI −0.24 to 0.28; P
0.04; I2 = 31%; Supplementary material online, Figure S1E) but this difference was insignificant in the HBCTR vs. CBCR groups (SMD 0.02, 95% CI −0.24 to 0.28; P =
= 0.87; I2 = 48%; Supplementary material online, Figure S1E).
0.87; I2 = 48%; Supplementary material online, Figure S1E).
Mortality
Meta-analysis of four studies29–31,38 did not show a statistically significant difference between HBCTR and UC at 4 to 6 months of follow-up on mortality events rates (OR 0.85, 95% CI 0.27 to 2.64; P
months of follow-up on mortality events rates (OR 0.85, 95% CI 0.27 to 2.64; P =
= 0.77; I2 = 0%; Supplementary material online, Figure S1F).
0.77; I2 = 0%; Supplementary material online, Figure S1F).
Cardiac-related hospitalization
Meta-analysis of three studies30,31,35,38 showed no statistically significant difference between HBCTR and UC at 8 weeks to 12
weeks to 12 months of follow-up on cardiac-related hospitalization rates (OR 0.77, 95% CI 0.50 to 1.18; P
months of follow-up on cardiac-related hospitalization rates (OR 0.77, 95% CI 0.50 to 1.18; P =
= 0.23; I2 = 10%; Supplementary material online, Figure S1G). Similarly, the study by Yudi et al.35 compared the effects of HBCTR to CBCR and observed no statistical differences in cardiac-related hospitalization rates.
0.23; I2 = 10%; Supplementary material online, Figure S1G). Similarly, the study by Yudi et al.35 compared the effects of HBCTR to CBCR and observed no statistical differences in cardiac-related hospitalization rates.
Discussion
Fourteen RCTs (n =
= 2869) were included in this systematic review and meta-analysis that examined the use of HBCTR as a Phase 2 CR programme in a CHD population. The key findings were that HBCTR appeared to be at least as effective as CBCR, and in some cases more effective than UC, for improving functional capacity, PA, QoL, and depression scores.
2869) were included in this systematic review and meta-analysis that examined the use of HBCTR as a Phase 2 CR programme in a CHD population. The key findings were that HBCTR appeared to be at least as effective as CBCR, and in some cases more effective than UC, for improving functional capacity, PA, QoL, and depression scores.
HBCTR vs. UC
Our finding of a statistically significant mean difference of 25.95 m in the 6MWT distance between HBCTR and UC is clinically relevant, as it reached the minimal clinically importance difference of 25
m in the 6MWT distance between HBCTR and UC is clinically relevant, as it reached the minimal clinically importance difference of 25 m for the 6MWT in CHD patients undergoing CR.39 Unexpectedly, we did not observe a significant difference in symptom-limited exercising testing between HBCTR and UC. The European Society of Cardiology recommends the evaluation of personal preferences and goals in the prescription of individualized approaches to exercise training to achieve long-term adherence and optimized health benefits.2 Closer evaluation of our analysis revealed that all but one study31 showed a trend of improved functional capacity in favour of HBCTR. Subsequent omission of this study by leave-out sensitivity analysis changed the results into a significant difference in favour of HBCTR. A possible explanation for this is that all the HBCTR participants in Snoek et al.31 received a standardized exercise training plan of 30
m for the 6MWT in CHD patients undergoing CR.39 Unexpectedly, we did not observe a significant difference in symptom-limited exercising testing between HBCTR and UC. The European Society of Cardiology recommends the evaluation of personal preferences and goals in the prescription of individualized approaches to exercise training to achieve long-term adherence and optimized health benefits.2 Closer evaluation of our analysis revealed that all but one study31 showed a trend of improved functional capacity in favour of HBCTR. Subsequent omission of this study by leave-out sensitivity analysis changed the results into a significant difference in favour of HBCTR. A possible explanation for this is that all the HBCTR participants in Snoek et al.31 received a standardized exercise training plan of 30 min of moderate activity for five times/week, whereas participants in the other three studies29,32,36 included in the meta-analysis provided tailored exercise prescriptions. This suggests that if patients are not given alternatives to their training plan that align with their preferences, they may not adhere sufficiently to the minimum PA levels required to obtain significant health benefits of improved functional capacity. However, as there were only four studies in this meta-analysis, more research is required for firmer conclusions.
min of moderate activity for five times/week, whereas participants in the other three studies29,32,36 included in the meta-analysis provided tailored exercise prescriptions. This suggests that if patients are not given alternatives to their training plan that align with their preferences, they may not adhere sufficiently to the minimum PA levels required to obtain significant health benefits of improved functional capacity. However, as there were only four studies in this meta-analysis, more research is required for firmer conclusions.
HBCTR also showed some evidence of improved PA behaviour compared with UC in objective (step count/day) and subjective (proportion of patients categorized as physically active) measures, but not in another subjective measure (energy expenditure kcal/week). Similarly, narrative synthesis of three included trials27,31,37 produced polarizing results on the effects of HBCTR on PA behaviour. Our observation of positive PA improvements in some studies and not others are due to a few reasons. First, as included studies were inconsistent in their PA definition and measurements, we were limited in our ability to reasonably pool all available data into a meta-analysis. Second, the use of subjective PA measures and the classification of continuous data into categories of PA could have resulted in measurement recall bias and a loss of data sensitivity, respectively.40 Therefore, in order to evaluate the potential superiority of HBCTR in improving PA behaviours, we recommend that future studies rely on objective measures of PA [such as percentage of peak heart rate, heart rate reserve and peak VO2 or metabolic equivalent of task (MET) via wearable accelerometers, and heart-rate monitors], and report outcomes of PA intensity, duration, and frequency that are paralleled with recommended national guidelines.2,41 Meta-analysis by Rawstorn et al.15 found significant improvement in PA behaviour but not in functional capacity when comparing telehealth exercise-based CR with UC. The following may offer possible explanations for this inconsistency: (i) our review focused on interactive web or smartphone HBCTR as the major delivery platform, whereas Rawstorn et al.15 included land-based telephone services; and (ii) variation in PA intervention intensity, duration, frequency, and engagement. It is likely that differences in these programme characteristics have an influence on intervention delivery and effectiveness and may have contributed to this variation in findings.
With respect to the effect of HBCTR vs. UC on QoL, our review revealed varied results. Meta-analysis of the effects on QoL measured by Short-Form questionnaire revealed significant results in favour of HBCTR, but this was not echoed in our narrative synthesis. While the reason for our discrepancy in findings is unclear, it warrants exploration in future studies as QoL has been known to be significantly impaired in patients with CHD, and improvements in QoL have been associated with a reduction in rehospitalization,42 re-cardiac events, and mortality.43 Hence, improvements in QoL remains an important therapeutic goal in CHD management, although the mechanism of this prognostic effect is not completely understood.44
Our finding of significant effects on depression scores in favour of HBCTR is encouraging, as depression is common in the CHD population and has been associated with re-cardiac event, cardiac-related mortality, and reduced participation in CR programmes.45 Favourable effects were also seen in medication adherence but given the insufficiency of data and employment of distinctive methodology, this outcome effect was difficult to quantify and could not be confirmed with a meta-analysis.
While it was unexpected that we did not find statistically significant effects of HBCTR on physiological cardiovascular risk factor outcomes, smoking, mortality, and cardiac-related hospitalization when compared with UC, our findings are similar to a previous meta-analysis.15 Advancements in early diagnostic and therapeutic coronary revascularizations coupled with the introduction of statins and the systematic use of cardio-protective pharmacotherapy have led to substantial improvements in risk factor control and overall patient outcomes in this modern era of cardiology.46 Hence, against this backdrop of reduced risk profile, any impact derived from participation in secondary prevention programmes like CR on long-term physiological and clinical outcomes could be potentially attenuated.46 Evidently, this was observed in our current review, where all our included trials belonged to this modern era. Furthermore, given the paucity of adverse events like current smoking status, mortality, and hospitalization and our low number of included studies with relatively short follow-up duration, we are prevented from detecting a difference in outcome effect between groups and are unable to confidently arrive at an evidenced-based conclusion on the effect of HBCTR vs. UC for these outcomes.
HBCTR vs. CBCR
Meta-analysis of the three included studies that compared HBCTR to CBCR found equivalent effects on functional capacity, PA behaviour, smoking, physiological risk factors, QoL, depression, and cardiac-related hospitalization. While our small number of included CBCR-controlled studies may have been insufficient to detect intervention effects, our findings are consistent with previous reviews.14,15 This suggests that HBCTR may serve as a suitable alternative to Phase 2 CBCR, especially when the need for CBCR cannot be met due to existing restrictions and resource limitations brought about by the COVID-19 pandemic.
Challenges of HBCTR
Our review also identified distinct methodological and implementation challenges of HBCTR. Firstly, the very nature of HBCTR necessitates a greater responsibility on the patient to self-manage and change long-standing habits of unhealthy behaviours.7 Accordingly, HBCTR programmes should be developed to support patients with the necessary skills to successfully undertake this behaviour change. Only five of the included studies in this review stated the use of a behaviour change theory in their intervention development. However, with the exception of one study,27 the extent to which the theoretical framework informed the intervention components to effectively support behaviour change related to CHD is unclear. Secondly, as interventions like HBCTR are self-administered, methods to ensure patients receive an appropriate dose and complete the intervention are challenging but important.47 Information on levels of engagement and receipt of treatment could serve as an instructive adjunct to determine why an intervention outcome is effective or not.47 Attempts to measure intervention compliance (Table 1) varied and were not consistently reported across included studies. There is a need for future studies to monitor intervention compliance and investigate if HBCTR improves uptake and completion of CR programmes compared with CBCR. Additionally, quality assurance of HBCTR programmes should be considered if HBCTR is to see favourable and cost-effective outcomes, and available quality metrics outlined by Randal et al.7 may serve to guide future programmes in this regard. Lastly, CR providers should acknowledge the value that patients place on their privacy and its consequences on their willingness to engage in digital platforms like HBCTR.8,48 Less than half of our included studies specified the use of secure password-protected intervention platforms. Future programmes should ensure the provision of a transparent privacy policy and secure health information and clinical data storage systems to encourage patients to participate in technology-led healthcare interventions.
Limitations
We believe this is the first meta-analysis to evaluate the effect of HBCTR as an alternative to Phase 2 CBCR or usual care specific to CHD. While we employed a robust search strategy across six databases, our restriction to only studies in English may have left out some relevant trials. Lack of blinding of participants and outcome assessors were key issues raised in the risk of bias assessment, and we acknowledge the implications this has on the strength of evidence in our review. Additionally, the generalizability of our study findings is limited due to: (i) under-representation of female participants and (ii) considerable participant’s dropouts in a third of studies. Lastly, included studies were heterogenous with respect to study designs. Varied definitions of outcome measurements resulted in small number of studies per single meta-analysis and prevented the evaluation of potential publication bias by funnel plot. Heterogeneity of study duration and follow-up may mirror actual variations in routine clinical settings and practices across countries and healthcare systems where differences in clinical testing exist. Despite this, the similarity achieved in functional capacity and PA outcomes reflect the strength of the effect of HBCTR across heterogenous study designs.
Conclusion
Overall, the potential of technology-led HBCTR in relation to its effect as an alternative to Phase 2 CR is appealing. In patients with CHD, HBCTR was associated with an increase in functional capacity, PA behaviour, and depression when compared with UC. When HBCTR was compared to CBCR, an equivalent effect on functional capacity, PA behaviour, QoL, medication adherence, smoking behaviour, physiological risk factors, depression, and cardiac-related hospitalization was observed. There is a need for future studies to develop interventions that are centred on behaviour change theories and offer secured technology platforms that can monitor patient compliance and objectively assess PA.
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology online.
Supplementary Material
zwab106_Supplementary_Data
Acknowledgement
The authors acknowledge the assistance of Ms Annelissa Chin with the peer-review database search strategy.
Contributor Information
Hadassah Joann Ramachandran, Alice Lee Centre for Nursing Studies, Yong Loo Lin School of Medicine, National University of Singapore, Block MD 11, 10 Medical Drive, Singapore 117597, Singapore.
Ying Jiang, Alice Lee Centre for Nursing Studies, Yong Loo Lin School of Medicine, National University of Singapore, Block MD 11, 10 Medical Drive, Singapore 117597, Singapore.
Wilson Wai San Tam, Alice Lee Centre for Nursing Studies, Yong Loo Lin School of Medicine, National University of Singapore, Block MD 11, 10 Medical Drive, Singapore 117597, Singapore.
Tee Joo Yeo, Cardiac Rehabilitation, Department of Cardiology, National University Heart Centre, Singapore, Singapore.
Wenru Wang, Alice Lee Centre for Nursing Studies, Yong Loo Lin School of Medicine, National University of Singapore, Block MD 11, 10 Medical Drive, Singapore 117597, Singapore.
Funding
This project has received funding from the National Medical Research Council Health Services Research Grant (Project ID: MOH-000364), under the Ministry of Health Singapore.
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
Full text links
Read article at publisher's site: https://doi.org/10.1093/eurjpc/zwab106
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8344786
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/109308889
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/eurjpc/zwab106
Article citations
Improving transitional care after acute myocardial infarction: A scoping review.
Health Care Sci, 3(5):312-328, 13 Oct 2024
Cited by: 0 articles | PMID: 39479273 | PMCID: PMC11520247
Review Free full text in Europe PMC
Efficacy of Wearable Single-Lead ECG Monitoring during Exercise Stress Testing: A Comparative Study.
Sensors (Basel), 24(19):6394, 02 Oct 2024
Cited by: 0 articles | PMID: 39409434 | PMCID: PMC11479017
Technological Developments, Exercise Training Programs, and Clinical Outcomes in Cardiac Telerehabilitation in the Last Ten Years: A Systematic Review.
Healthcare (Basel), 12(15):1534, 02 Aug 2024
Cited by: 0 articles | PMID: 39120237 | PMCID: PMC11311841
Review Free full text in Europe PMC
The Accessibility and Effect of Cardiac Rehabilitation in COVID-19 Pandemic Era.
Ann Rehabil Med, 48(4):249-258, 30 Jul 2024
Cited by: 0 articles | PMID: 39074836 | PMCID: PMC11372283
Efficacy of a Mobile Health App (eMOTIVA) Regarding Compliance With Cardiac Rehabilitation Guidelines in Patients With Coronary Artery Disease: Randomized Controlled Clinical Trial.
JMIR Mhealth Uhealth, 12:e55421, 25 Jul 2024
Cited by: 0 articles | PMID: 39052330 | PMCID: PMC11310647
Go to all (63) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effects of home-based cardiac telerehabilitation programs in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis.
BMC Cardiovasc Disord, 23(1):101, 22 Feb 2023
Cited by: 8 articles | PMID: 36814188 | PMCID: PMC9945630
Review Free full text in Europe PMC
Innovative Application of a Home-Based and Remote Sensing Cardiac Rehabilitation Protocol in Chinese Patients After Percutaneous Coronary Intervention.
Telemed J E Health, 25(4):288-293, 07 Sep 2018
Cited by: 39 articles | PMID: 30192210
Home-Based Cardiac Rehabilitation Alone and Hybrid With Center-Based Cardiac Rehabilitation in Heart Failure: A Systematic Review and Meta-Analysis.
J Am Heart Assoc, 8(16):e012779, 17 Aug 2019
Cited by: 50 articles | PMID: 31423874 | PMCID: PMC6759908
Review Free full text in Europe PMC
The Potential of Cardiac Telerehabilitation as Delivery Rehabilitation Care Model in Heart Failure during COVID-19 and Transmissible Disease Outbreak: A Systematic Scoping Review of the Latest RCTs.
Medicina (Kaunas), 58(10):1321, 21 Sep 2022
Cited by: 6 articles | PMID: 36295482 | PMCID: PMC9609719
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Ministry of Health Singapore
National Medical Research Council Health Services Research Grant (1)
Grant ID: MOH-000364