Abstract
Importance
After the Food and Drug Administration (FDA) approved computer-aided detection (CAD) for mammography in 1998, and Centers for Medicare and Medicaid Services (CMS) provided increased payment in 2002, CAD technology disseminated rapidly. Despite sparse evidence that CAD improves accuracy of mammographic interpretations, and costs over $400 million dollars a year, CAD is currently used for the majority of screening mammograms in the U.S.
Objective
To measure performance of digital screening mammography with and without computer-aided detection in U.S. community practice.
Design, Setting and Participants
We compared the accuracy of digital screening mammography interpreted with (N=495,818) vs. without (N=129,807) computer-aided detection from 2003 through 2009 in 323,973 women. Mammograms were interpreted by 271 radiologists from 66 facilities in the Breast Cancer Surveillance Consortium. Linkage with tumor registries identified 3,159 breast cancers in 323,973 women within one year of the screening.
Main Outcomes and Measures
Mammography performance (sensitivity, specificity, and screen detected and interval cancers per 1,000 women) was modeled using logistic regression with radiologist-specific random effects to account for correlation among examinations interpreted by the same radiologist, adjusting for patient age, race/ethnicity, time since prior mammogram, exam year, and registry. Conditional logistic regression was used to compare performance among 107 radiologists who interpreted mammograms both with and without computer-aided detection.
Results
Screening performance was not improved with computer-aided detection on any metric assessed. Mammography sensitivity was 85.3% (95% confidence interval [CI]=83.6–86.9) with and 87.3% (95% CI 84.5–89.7) without computer-aided detection. Specificity was 91.6% (95% CI=91.0–92.2) with and 91.4% (95% CI=90.6–92.0) without computer-aided detection. There was no difference in cancer detection rate (4.1/1000 women screened with and without computer-aided detection). Computer-aided detection did not improve intra-radiologist performance. Sensitivity was significantly decreased for mammograms interpreted with versus without computer-aided detection in the subset of radiologists who interpreted both with and without computer-aided detection (OR 0.53, 95%CI=0.29–0.97).
Conclusions and Relevance
CAD does not improve diagnostic accuracy of mammography and may result in missed cancers. These results suggest that insurers pay more for computer-aided detection with no established benefit to women.
Introduction
Computer-aided detection (CAD) for mammography is intended to assist radiologists in identifying subtle cancers that might otherwise be missed. CAD marks potential areas of concern on the mammogram and the radiologist determines whether the area warrants further evaluation. Although CAD for mammography was approved by the Food and Drug Administration (FDA) in 1998 (1), by 2001, less than 5% of screening mammograms were interpreted with CAD in the United States. However, in 2002, the Centers for Medicare and Medicaid Services (CMS) increased reimbursement for CAD, and by 2008, 74% of all screening mammograms in the Medicare population were interpreted with CAD (2,3).
Measuring the true impact of CAD on the accuracy of mammographic interpretation has proved challenging. Findings on potential benefits and harms are inconsistent and contradictory (4–19). Study designs include reader studies of enriched case sets; (4–7) prospective “sequential reading” clinical studies in which a radiologist records a mammogram interpretation without CAD assistance, then immediately reviews and records an interpretation with CAD assistance; (8–12) and retrospective observational studies using historical controls (13–16). One large European trial used a randomized clinical trial design to compare mammographic interpretations by a single reader with CAD compared to double readings without CAD (17).
Comparisons of mammography interpretations with versus without CAD in U.S. community practice have not supported improved performance with CAD (18,19). However, these studies were limited by relatively small numbers and a focus on older women. Another limitation was that CAD technology was studied relatively early in its adoption, so examinations were interpreted during the early part of radiologists’ learning curves and included examinations with outdated film screen mammography. Our study addresses these limitations by using a large database of more than 495,000 full-field digital screening mammograms interpreted with CAD, accounting for radiologists’ early learning curves, and adjusting for patient and radiologist variables. We also assessed performance within a subset of radiologists who interpreted with and without CAD during the study period.
Methods
Data Source
Data were pooled from five mammography registries that participate in the Breast Cancer Surveillance Consortium (BCSC) (20) funded by the National Cancer Institute: (1) San Francisco Mammography Registry; (2) New Mexico Mammography Advocacy Project; (3) Vermont Breast Cancer Surveillance System; (4) New Hampshire Mammography Network; and (5) Carolina Mammography Registry. Each mammography registry links women to a state tumor registry or regional Surveillance Epidemiology and End Results program that collects population-based cancer data. Each registry and the BCSC Statistical Coordinating Center have institutional review board approval for either active or passive consenting processes or a waiver of consent to enroll participants, link data, and perform analytic studies. All procedures are Health Insurance Portability and Accountability Act compliant and all registries and the Statistical Coordinating Center have received a Federal Certificate of Confidentiality and other protection for the identities of women, physicians, and facilities that participate in this research.
Participants
We included digital screening mammography examinations interpreted by 271 radiologists with (N=495,818) or without CAD (N=129,807) between January 1, 2003 and December 31, 2009 among 323,973 women aged 40 to 89 years with information on race, ethnicity, and time since last mammogram. Of the radiologists, 82 never used CAD, 82 always used CAD, and 107 sometimes used CAD. The latter 107 radiologists contributed 45,990 exams interpreted without using CAD and 337,572 interpreted using CAD. The median percentage of exams interpreted using CAD among the 107 radiologists was 93% and the interquartile range was 31%.
Data Collection
Methods used to identify and assess screening mammograms, patient characteristics, and outcomes have been described previously (20,21). Briefly, screening mammograms were defined as bilateral mammograms designated as “routine screening” by the radiologist. Mammographic assessments followed the Breast Imaging Reporting and Data System (BI-RADS) of 0=additional imaging, 1=negative, 2=benign finding, 3=probably benign findings, 4=suspicious abnormality, or 5=abnormality highly suspicious for malignancy (22).
Woman-level characteristics including menopausal status, race/ethnicity, and first-degree family history were captured through self-administered questionnaires at each examination. Breast density was recorded by the radiologist at the time of the mammogram using the BI-RADS standard terminology of almost entirely fat, scattered fibroglandular densities, heterogeneously dense, and extremely dense (23).
Outcomes
We calculated sensitivity, specificity, cancer detection rates, and interval cancer rates. We defined positive mammograms as those with BI-RADS assessments of 0, 4 or 5, or 3 with a recommendation for immediate follow-up. Negative mammograms were defined as BI-RADS 1 or 2, or 3 without a recommendation for immediate follow-up. All women were followed for breast cancer from their mammogram up until their next screening mammogram or 12 months, whichever came first. Breast cancer diagnoses included ductal carcinoma in situ (DCIS) or invasive breast cancer within this follow-up period.
False-negative examinations were defined as mammograms with a negative assessment but a breast cancer diagnosis within the follow-up period. True-positive examinations were defined as those with a positive examination and breast cancer diagnosis. False-positive examinations were examinations with a positive assessment but no cancer diagnosis. True-negative examinations had a negative assessment and no cancer diagnosis. Sensitivity was calculated as the number of true-positive mammograms over the total number of breast cancers. For calculations of sensitivity, radiologists who interpreted no mammograms associated with cancer during the study period (n=136) were excluded. Specificity was calculated as the number of true-negative mammograms over the total number of mammograms without a breast cancer diagnosis. Cancer detection rate was defined as the number of true-positive exams over the total number of mammograms, and interval cancer rate was the number of false-negative examinations over the total number of mammograms, reported per 1000 mammograms (24).
Statistical Analysis
All analyses were conducted using the screening examination as the unit of analysis and allowing women to contribute multiple examinations during the study period; however, only one screening examination was associated with a breast cancer diagnosis. Distributions of breast cancer risk factors, demographic characteristics of exams, and mammographic density and assessments were computed separately by CAD use versus no use.
We evaluated the diffusion of digital screening mammography with and without CAD in the larger BCSC population from 2002–2012 including 5.2 million screening mammograms.
Mammography performance measures were modeled using logistic regression including normally distributed, radiologist-specific random effects to account for the correlation among exams read by the same radiologist. Random effects were allowed to vary by CAD use or non-use during the reading. Performance measures were estimated at the median of the random effects distribution. Adjusted, radiologist-specific relative performance was measured by an odds ratio (OR) with 95% confidence intervals (CI) comparing CAD use to no CAD, adjusting for patient age at diagnosis, time since last mammogram and year of exam, and the BCSC registry.
Receiver operating characteristic (ROC) curves were estimated from 135 radiologists who interpreted at least one mammogram associated with a cancer diagnosis using a hierarchical logistic regression model that allowed the threshold and accuracy parameters to depend on whether CAD was used during exam interpretation. We assumed a constant accuracy among radiologists for exams interpreted under the same condition (with or without CAD) and allowed the threshold for recall to vary across radiologists through normally distributed, radiologist-specific random effects that varied by whether the radiologist used CAD or not during the reading (25). We estimated the normalized partial area under the summary ROC curves across the observed range of false-positive rates from this model (26). We plotted the true-positive rate versus the false-positive rate and superimposed the estimated ROC curves.
Two separate main sensitivity analyses were conducted in subsets of total exams: 1) To account for a possible learning curve for using CAD, we excluded the first year of each radiologist’s CAD use; and 2) To estimate the within-radiologist effect of CAD, we limited analysis to the 107 radiologists who interpreted mammograms during the study period with and without CAD, using conditional logistic regression and adjusting for patient age, time since last mammogram, and race/ethnicity.
Two-sided statistical tests were used with P values less than 0.050 considered statistically significant. All analyses were conducted by R.W. using SAS® software v9.2 (SAS Institute Inc., Cary, NC, USA) for Windows 7.
Results
Digital screening mammography and CAD use increased from 2000 to 2012. In 2003, only 5% of all screening mammograms in the BCSC were digital with CAD; by 2012, 83% of all screening mammograms were acquired digitally and interpreted with CAD assistance (Figure 1).
Figure 1. Screening Mammography Patterns from 2000–2012 in U.S. Community Practices in the Breast Cancer Surveillance Consortium (5.2 million mammograms).

Data are provided from the larger BCSC population including all screening mammograms for the indicated time period.
Among 323,973 women aged 40 to 89, 625,625 digital screening mammography exams were performed (495,818 interpreted with CAD and 129,807 without CAD) between 2003 and 2009 by 271 radiologists. Breast cancer was diagnosed in 3,159 women within 12 months of the screening mammogram and prior to the next screening mammogram. Women undergoing screening mammography with and without CAD assistance were similar in age, menopausal status, family history of breast cancer, time since last mammogram, and breast density. Women undergoing screening mammography with CAD were more likely to be non-Hispanic white than women whose mammograms were interpreted without CAD (Table 1).
Table 1.
Characteristics of Women Undergoing Digital Screening Mammography with and without CAD
| CAD | No CAD | Overall | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | (%) | N | (%) | N | (%) | |
|
|
||||||
| Age | ||||||
| 40–49 | 147,486 | (29.8) | 36,503 | (28.1) | 183,989 | (29.4) |
| 50–59 | 158,780 | (32.0) | 44,766 | (34.5) | 203,546 | (32.5) |
| 60–69 | 108,329 | (21.9) | 29,914 | (23.0) | 138,243 | (22.1) |
| 70–79 | 60,545 | (12.2) | 14,656 | (11.3) | 75,201 | (12.0) |
| 80–89 | 20,678 | (4.2) | 3968 | (3.1) | 24,646 | (3.9) |
| Menopausal status | ||||||
| Premenopausal | 120,559 | (30.3) | 34,688 | (32.9) | 155,247 | (30.9) |
| Postmenopausal, currently on hormone therapy | 33,764 | (8.5) | 6338 | (6.0) | 40,102 | (8.0) |
| Postmenopausal, not currently on hormone therapy | 243,105 | (61.2) | 64,335 | (61.1) | 307,440 | (61.2) |
| Missing | 98,390 | (19.8) | 24,446 | (18.8) | 122,836 | (19.6) |
| Race/ethnicity | ||||||
| White, non-Hispanic | 410,385 | (82.8) | 63,306 | (48.8) | 473,691 | (75.7) |
| Black, non-Hispanic | 28,533 | (5.8) | 7985 | (6.2) | 36,518 | (5.8) |
| Asian/Pacific Islander | 35,081 | (7.1) | 43,991 | (33.9) | 79,072 | (12.6) |
| American Indian or Alaska Native | 1194 | (0.2) | 590 | (0.5) | 1784 | (0.3) |
| Other | 7228 | (1.5) | 2497 | (1.9) | 9725 | (1.6) |
| Hispanic | 13,397 | (2.7) | 11,438 | (8.8) | 24,835 | (4.0) |
| First-degree family history of breast cancer | ||||||
| No | 412,071 | (83.6) | 110,544 | (87.4) | 522,615 | (84.4) |
| Yes | 80,800 | (16.4) | 15,902 | (12.6) | 96,702 | (15.6) |
| Missing | 2947 | (0.6) | 3361 | (2.6) | 6308 | (1.0) |
| Time since last mammogram | ||||||
| No prior mammogram | 12,518 | (2.5) | 6750 | (5.2) | 19,268 | (3.1) |
| 1 year | 361,842 | (73.0) | 74,687 | (57.5) | 436,529 | (69.8) |
| 2 years | 68,905 | (13.9) | 31,131 | (24.0) | 100,036 | (16.0) |
| 3 or more years | 52,553 | (10.6) | 17,239 | (13.3) | 69,792 | (11.2) |
| BI-RADS density | ||||||
| Almost entirely fat | 52,875 | (12.4) | 8833 | (11.4) | 61,708 | (12.2) |
| Scattered fibroglandular densities | 175,579 | (41.1) | 33,473 | (43.1) | 209,052 | (41.4) |
| Heterogeneously dense | 167,506 | (39.2) | 30,104 | (38.7) | 197,610 | (39.1) |
| Extremely dense | 31,252 | (7.3) | 5305 | (6.8) | 36,557 | (7.2) |
| Missing | 68,606 | (13.8) | 52,092 | (40.1) | 120,698 | (19.3) |
CAD, computer-aided detection; BI-RADS, Breast Imaging Reporting and Data System
Performance Measures for Mammography Interpreted with and without CAD
Overall
Diagnostic accuracy was not improved with CAD on any performance metric assessed. Sensitivity of mammography was 85.3% (95% confidence interval [CI]=83.6–86.9) with and 87.3% (95% CI=84.5–89.7) without CAD. Sensitivity of mammography for invasive cancer was 82.1% (95% CI=80.0–84.0) with and 85.0% (95% CI=81.5–87.9) without CAD; for DCIS, sensitivity was 93.2% (95% CI=91.1–94.9) with and 94.3% (95% CI=89.4–97.1) without CAD. Specificity of mammography was 91.6% (95% CI=91.0–92.2) with and 91.4% (95% CI=90.6–92.0) without CAD. There was no difference in overall cancer detection rate (4.1 cancers per 1000 women screened with CAD and without CAD) or in invasive cancer detection rate (2.9 vs 3.0 per 1000 women screened with CAD and without CAD). However, DCIS detection rate was higher in patients whose mammograms were assessed with CAD compared to those whose mammograms were assessed without CAD (1.2 vs 0.9 per 1000; 95% CI=1.0–1.9; p<0.03) (Table 2).
Table 2.
Performance Measures of Digital Screening Mammography with and without CAD
| Performance Measure | CAD | No CAD | CAD | No CAD | Adjusted OR* | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Events | Exams | Events | Exams | Mean | 95% CI | Mean | 95% CI | OR | 95% CI | ||
|
|
|||||||||||
| Cancers detected per 1000 exams | |||||||||||
| Total | 2145 | 495,818 | 558 | 129,807 | 4.06 | (3.8, 4.4) | 4.10 | (3.6, 4.6) | 0.99 | (0.84, 1.15) | 0.86 |
| Invasive | 1485 | 495,818 | 408 | 129,807 | 2.91 | (2.7, 3.1) | 3.05 | (2.7, 3.5) | 0.92 | (0.77, 1.08) | 0.30 |
| DCIS | 660 | 495,818 | 150 | 129,807 | 1.19 | (1.0, 1.3) | 0.95 | (0.7, 1.2) | 1.39 | (1.03, 1.87) | 0.031 |
| Interval cancers per 1000 exams | |||||||||||
| Total | 375 | 495,818 | 81 | 129,807 | 0.76 | (0.68, 0.84) | 0.62 | (0.50, 0.78) | 1.14 | (0.87, 1.50) | 0.33 |
| Invasive | 327 | 495,818 | 72 | 129,807 | 0.65 | (0.57, 0.74) | 0.55 | (0.42, 0.71) | 1.09 | (0.82, 1.46) | 0.54 |
| DCIS | 48 | 495,818 | 9 | 129,807 | 0.10 | (0.07, 0.13) | 0.03 | (0.00, 0.24) | 1.59 | (0.72, 3.51) | 0.25 |
| Sensitivity | |||||||||||
| Total | 2145 | 2520 | 558 | 639 | 85.3% | (83.6, 86.9) | 87.3% | (84.5, 89.7) | 0.81 | (0.60, 1.10) | 0.18 |
| Invasive | 1485 | 1812 | 408 | 480 | 82.1% | (80.0, 84.0) | 85.0% | (81.5, 87.9) | 0.83 | (0.59, 1.17) | 0.28 |
| DCIS | 660 | 708 | 150 | 159 | 93.2% | (91.1, 94.9) | 94.3% | (89.4, 97.1) | 0.88 | (0.37, 2.07) | 0.76 |
| Specificity | |||||||||||
| Total | 444,356 | 493,298 | 118,025 | 129,168 | 91.6% | (91.0, 92.2) | 91.4% | (90.6, 92.0) | 1.02 | (0.94, 1.11) | 0.58 |
| Invasive | 444,404 | 494,006 | 118,034 | 129,327 | 91.5% | (90.9, 92.1) | 91.3% | (90.5, 91.9) | 1.02 | (0.94, 1.11) | 0.58 |
| DCIS | 444,683 | 495,110 | 118,097 | 129,648 | 91.4% | (90.7, 92.0) | 91.0% | (90.3, 91.7) | 1.04 | (0.96, 1.13) | 0.36 |
| Recall rate per 100 exams | 51,087 | 495,818 | 11,701 | 129,807 | 8.7 | (8.1, 9.4) | 9.1 | (8.4, 9.8) | 0.96 | (0.89, 1.04) | 0.35 |
CAD, computer-aided detection; OR, odds ratio; CI, confidence interval; DCIS, ductal carcinoma in situ
Odds ratio for CAD vs . No CAD adjusted for site, age group, race/ethnicity, time since prior mammogram and calendar year of the exam using mixed effects model with random effect for exam reader and varying with CAD use.
To allow for the possibility that performance improved after the first year of CAD use by a radiologist, and to account for any possible learning curve, we excluded the first year of mammographic interpretations with CAD for individual radiologists and found no differences for any of our performance measurements (data not shown).
From the ROC analysis, the accuracy of mammographic interpretations with CAD was significantly lower than for those without CAD (P=0.0023). The normalized partial area under the summary ROC curve was 0.84 for interpretations with CAD and 0.88 for interpretations without CAD (Figure 2). In this subset of 135 radiologists who interpreted at least one mammogram associated with a cancer diagnosis, sensitivity of mammography was 84.9% (95% confidence interval [CI]=82.9–86.9) with and 89.3%% (95% CI=86.9–91.7) without CAD. Specificity of mammography was 91.1% (95% CI=90.4–91.8) with and 91.3% (95% CI=90.5–92.1) without CAD.
Figure 2.
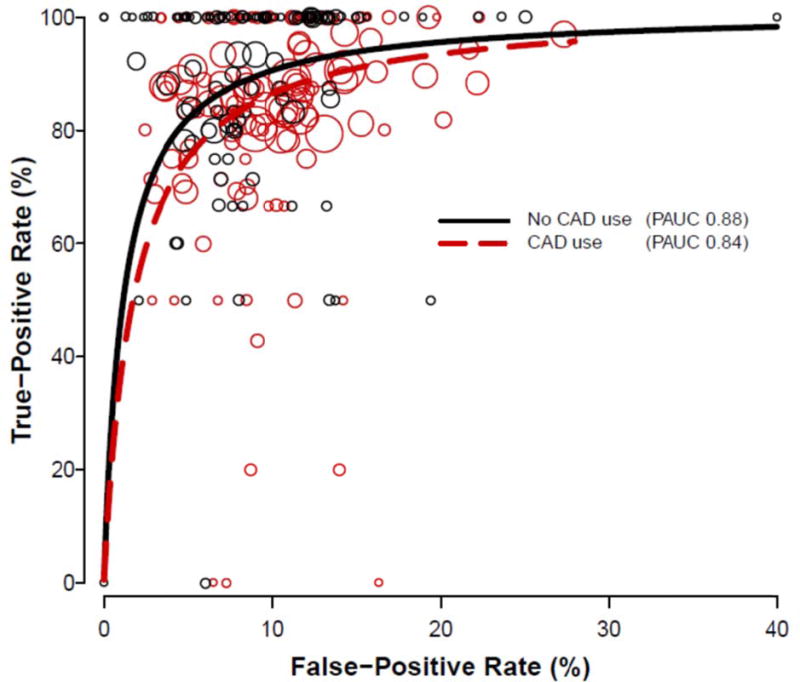
Receiver operating characteristic curves for digital screening mammography with and without the use of computer-aided detection (CAD), estimated from 135 radiologists who interpreted at least one exam associated with cancer. Each circle represents the true-positive or false-positive rate for a single radiologist, for exams interpreted with (red) or without (black) CAD. Circle size is proportional to the number of mammograms associated with cancer interpreted by that radiologist with or without CAD. PAUC = partial area under the curve.
By age, breast density, menopausal status, time since last mammogram
We found no differences in diagnostic accuracy of mammographic interpretations with and without CAD in any of the subgroups assessed, including patient age, breast density, menopausal status and time since last mammogram (Table 3).
Table 3.
Performance Measures of Digital Screening Mammography with and without CAD, by Exam-level Patient Characteristics
| CAD | No CAD | CAD | No CAD | Adjusted OR* | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| By age | Events | Exams | Events | Exams | Mean | 95% CI | Mean | 95% CI | OR | 95% CI | |
|
|
|||||||||||
| Cancers delected per 1000 examinations | |||||||||||
| 40–49 | 419 | 147,486 | 107 | 36,503 | 2.7 | (2.4, 3.1) | 2.6 | (2.0, 3.4) | 1.12 | (0.80, 1.57) | 0.50 |
| 50–73 | 1358 | 295,392 | 383 | 82,000 | 4.3 | (4.0, 4.7) | 4.5 | (4.0, 5.2) | 0.94 | (0.79, 1.11) | 0.46 |
| Sensitivity | |||||||||||
| 40–49 | 419 | 515 | 107 | 126 | 81.6 | (77.4, 85.2) | 89.9 | (74.2, 96.5) | 0.62 | (0.24, 1.61) | 0.32 |
| 50–73 | 1358 | 1581 | 383 | 437 | 85.9 | (84.1, 87.6) | 87.6 | (84.2, 90.4) | 0.87 | (0.57, 1.32) | 0.50 |
| Specificity | |||||||||||
| 40–49 | 127,519 | 146,971 | 32,228 | 36,377 | 88.7 | (87.8, 89.6) | 89.1 | (88.1, 90.0) | 0.99 | (0.90, 1.09) | 0.80 |
| 50–73 | 267,865 | 293,811 | 75,251 | 81,563 | 92.3 | (91.7, 92.9) | 92.2 | (91.5, 92.8) | 1.01 | (0.92, 1.10) | 0.88 |
| By BI-RADS breast density | |||||||||||
| Cancers detected per 1000 examinations | |||||||||||
| Almost entirely fat | 147 | 52,875 | 34 | 8833 | 2.8 | (2.4, 3.3) | 3.8 | (2.7, 5.4) | 0.58 | (033, 1.03) | 0.061 |
| Scattered fibroglandular dens it its | 717 | 175,579 | 135 | 33,473 | 3.8 | (3.3, 4 2) | 4.0 | (3.4,4.8) | 0.86 | (0.69, 1.07) | 0.17 |
| Heterogeneously dense | 783 | 167,506 | 123 | 30,104 | 4.5 | (4.1, 5.0) | 3.9 | (3.1, 4.9) | 1.11 | (0.86, 1.44) | 0.43 |
| Extremely dense | 102 | 31,252 | 20 | 5305 | 2.7 | (1.9, 3.7) | 1.7 | (0.5, 5.4) | 1.72 | (046, 6.34) | 0.42 |
| Sensitivity | |||||||||||
| Almost entirely fat | 147 | 163 | 34 | 36 | 90.2 | (84.4, 94.0) | 100.0 | (91.4, 100.0) | 1.26 | (1.01, 1.56) | 0.037 |
| Scattered fibroglandular densities | 717 | 810 | 135 | 151 | 89.0 | (86.1, 91.4) | 89.4 | (83.3, 93.4) | 0.73 | (0.37, 1.41) | 0.34 |
| Heterogeneously dense | 783 | 949 | 123 | 143 | 82.5 | (79.9, 84.8) | 86.0 | (79.2, 90.8) | 0 86 | (0.48, 1.56) | 0.62 |
| Extremely dense | 102 | 144 | 20 | 26 | 72.1 | (60.5, 81.4) | 77.8 | (51.4, 92.0) | 0.85 | (0.24, 3.04) | 0.80 |
| Specificity | |||||||||||
| Almost entirely fat | 49,864 | 52,712 | 8330 | 8797 | 95.8 | (95.2, 96.3) | 94.7 | (93.8, 95.4) | 0.77 | (0.63, 0.94) | 0.013 |
| Scattered fibroglandular densities | 158,575 | 174,769 | 30,230 | 33,322 | 92.1 | (91.4, 92.7) | 92.0 | (91.2, 92.7) | 1.01 | (0.92, 1.12) | 0.80 |
| Heterogeneously dense | 146,180 | 166.557 | 26,510 | 29.961 | 89.2 | (88.1, 90.2) | 892 | (88.1, 90.2) | 1.03 | (0.92, 1.15) | 0.63 |
| Extremely dense | 27,930 | 31,108 | 4724 | 5279 | 91.4 | (90.2, 92.4) | 89.5 | (88.0, 90.8) | 1.30 | (1.09, 1.55) | 0.0031 |
| By menopausal status | |||||||||||
| Cancers detected per 1000 examinations | |||||||||||
| Premenopausal | 401 | 120,559 | 117 | 34,688 | 3.3 | (3.0, 3.7) | 2.9 | (2.1, 3.9) | 1.16 | (0.85, 1.57) | 0.36 |
| Postmenopausal, currently on hormone therapy | 204 | 33,764 | 44 | 6338 | 6.0 | (5.3, 6.9) | 6..2 | (3.9, 10.0) | 0.88 | (0.47, 1.65) | 0.69 |
| Postmenopausal, not currently on hormone therapy | 1217 | 243,105 | 304 | 64,335 | 4.7 | (4.3, 5.1) | 4.6 | (4.0, 5.3) | 0.96 | (0.80, 1.15) | 0.66 |
| Sensitivity | |||||||||||
| Premenopausal | 401 | 484 | 117 | 141 | 83.0 | (79.0, 86.4) | 84.2 | (74.5, 90.7) | 1.06 | (0.52, 2.17) | 0.87 |
| Postmenopausal, currently on hormone therapy | 204 | 243 | 44 | 51 | 84.0 | (78.7, 88.1) | 86.3 | (73.6, 93.4) | 0.93 | (0.33, 2.62) | 0.89 |
| Postmenopausal, not currently on hormone therapy | 1217 | 1408 | 304 | 343 | 86.4 | (84.5, 88.1) | 90.3 | (84.7, 94.0) | 0.72 | (0.41, 1.27) | 0.26 |
| Specificity | |||||||||||
| Premenopausal | 102,940 | 120,075 | 30,505 | 34,547 | 87.9 | (86.9, 88.8) | 88.3 | (87.4, 89.2) | 0.99 | (0.90, 1.09) | 0.84 |
| Postmenopausal, currently on hormone therapy | 30,129 | 33,521 | 5701 | 6287 | 90.7 | (89.8, 91.6) | 91.3 | (90.1, 92.3) | 0.88 | (0.75, 1.04) | 0.13 |
| Postmenopausal, not currently on hormone therapy | 222,887 | 241,697 | 59,263 | 63,992 | 93.2 | (92.6, 93.7) | 92.7 | (92.1, 93.3) | 1.05 | (0.96, 1.16) | 0.27 |
| By time since prior mammogram | |||||||||||
| Cancers delected per 1000 examinations | |||||||||||
| No prior mammogram | 78 | 12,518 | 28 | 6750 | 6.0 | (4.5, 8.1) | 4.1 | (2.8, 6.0) | 1.53 | (0.90, 2.59) | 0.11 |
| 1 year | 1354 | 361,842 | 278 | 74,687 | 3.5 | (3.2, 3.8) | 3.5 | (3.0, 4.1) | 0.98 | (0.82, 1.18) | 0.84 |
| 2 years | 308 | 68,905 | 142 | 31,131 | 4.1 | (3.5, 4.8) | 4.4 | (3.5, 5.5) | 0.75 | (0.55, 1.04) | 0.084 |
| 3 or more years | 405 | 52,553 | 110 | 17,239 | 7.5 | (6.7, 8.5) | 6.0 | (4.7, 7.6) | 1.17 | (0.87, 1.57) | 0.31 |
| Sensitivity | |||||||||||
| No prior mammogram | 78 | 86 | 28 | 35 | 100.0 | (95.9, 100.0) | 83.2 | (47.4, 96.5) | Could not be estimated | ||
| 1 year | 1354 | 1636 | 278 | 331 | 83.2 | (80.7, 85.4) | 84.0 | (79.6, 87.6) | 0.93 | (0.64, 1.35) | 0.69 |
| 2 years | 308 | 350 | 142 | 156 | 88.0 | (84.1, 91.0) | 95.2 | (80.5, 99.0) | 0.45 | (0.20, 1.00) | 0.049 |
| 3 or more years | 405 | 448 | 110 | 117 | 90.4 | (87.3, 92.8) | 100.0 | (96.9, 100.0) | 0.95 | (0.83, 1.08) | 0.42 |
| Specificity | |||||||||||
| No prior mammogram | 9333 | 12,432 | 5638 | 6715 | 75.9 | (73.3, 78.4) | 81.6 | (78.3, 84.5) | 0.89 | (0.73, 1.09) | 0.25 |
| 1 year | 328,519 | 360,206 | 68,463 | 74,356 | 92.7 | (92.1, 93.2) | 92.4 | (91.7, 93.0) | 1.05 | (0.96, 1.14) | 0.27 |
| 2 years | 61,616 | 68,555 | 28,710 | 30,975 | 91.1 | (90.2, 91.8) | 92.2 | (91.4, 93.0) | 0.94 | (0.83, 1.07) | 0.35 |
| 3 or more years | 44,888 | 52,105 | 15,214 | 17,122 | 88.0 | (86.9, 89.0) | 89.1 | (87.9, 90.3) | 1.06 | (0.93, 1.20) | 0.42 |
CAD, computer-aided detection: OR odds ratio: BI-RADS Breast Imaging Reporting and Data System
Odds ratio for CAD vs. No CAD adjusted for BCSC registry, age group, race/ethnicity, time since prior mammogram and calendar year of the exam using mixed effects model with random effect for exam reader
Intra-radiologist Performance Measures for Mammography with and without CAD
Among 107 radiologists who interpreted mammograms both with and without CAD, intra-radiologist performance was not improved with CAD, and CAD was associated with decreased sensitivity. Sensitivity of mammography was 83.3 (95% confidence interval [CI]=81.0–85.6) with and 89.6%% (95% CI=86.0–93.1) without CAD. Specificity of mammography was 90.7% (95% CI=89.8–91.7) with and 89.6% (95% CI=88.6–91.1) without CAD. The OR for specificity between mammograms interpreted with CAD and those interpreted without CAD by the same radiologist was 1.02 (95% CI=0.99–1.05). Sensitivity was significantly decreased for mammograms interpreted with versus without CAD in the subset of radiologists who interpreted both with and without CAD assistance (OR 0.53, 95%CI=0.29–0.97).
Discussion
We found no evidence that CAD applied to digital mammography in U.S. community practice improves screening mammography performance on any performance measure or in any subgroup of women. In fact, mammography sensitivity was decreased in the subset of radiologists who interpreted mammograms with and without CAD. This study builds on prior studies (18–19) by demonstrating that radiologists’ early learning curve and patient characteristics do not account for the lack of benefit from CAD.
Whether CAD provides added value to women undergoing screening mammography is a topic of strong debate (27–36). The lack of consensus may be partly explained by wide variation in CAD use and inherent biases in the methods used to study the impact of CAD on screening mammography. Early studies supporting the efficacy of CAD were laboratory based and measured the ability of CAD programs to mark cancers on selected mammograms. The reported “high sensitivities” of CAD from these studies did not translate to higher cancer detection in clinical practice. In clinical practice, the vast majority of positive marks by CAD must be reviewed and discounted by a radiologist to avoid unacceptably high rates of false-positive exams and unnecessary biopsies, and to practice within acceptable performance parameters recommended by the American College of Radiology (24). The most optimistic view of CAD is that it improves mammography sensitivity by 20% (8,28,30,32). If this were true, cancer detection rates of 4–5 per 1000 without CAD would increase to 5–6 per 1000 with CAD. In other words, for every 1000 women whose screening mammograms were interpreted with CAD, one cancer would be identified that was missed by the unassisted radiologist interpretation. To achieve that single true-positive CAD marking in 1000 women, CAD would render between 2000 and 4000 false-positive marks. Thus, under this scenario, a radiologist would need to recommend diagnostic evaluation for the single CAD mark of the otherwise missed cancer, while discounting thousands of false-positive CAD marks.
Consistent with reports of a prior BCSC cohort study (18) and SEER-Medicare data (2) which primarily evaluated film-screen mammography, we found higher rates of DCIS lesions detected with CAD on digital mammography, but no differences in sensitivity for cancer (whether for DCIS or invasive) and no differences in rates of invasive cancers detected. A meta-analysis in 2008 of 10 studies of CAD applied to screening mammography concluded that CAD significantly increased recall rate with no significant improvement in cancer detection rates compared to readings without CAD (37). The largest recent reader study of digital mammography obtained during the Digital Mammography Imaging Screening Trial (DMIST) found no impact of CAD on radiologist interpretations of mammograms (5). In that report, the authors concluded that radiologists overall were not influenced by CAD markings and CAD had no impact, either beneficial or detrimental, on mammography interpretations.
Our study had sufficiently large numbers to compare interpretations of mammograms read by radiologists who practiced at some sites with CAD and at other sites without CAD. We are concerned that, in these comparisons, sensitivity was lower in CAD-assisted mammograms. Prior reports have confirmed that not all cancers are marked by CAD, and that cancers are overlooked more often if CAD fails to mark a visible lesion. In a large reader study, Taplin et al. (7) reported that visible, non-calcified lesions that went unmarked by CAD were significantly less likely to be assessed as abnormal by radiologists. However, our finding of lower sensitivity with CAD was in a subgroup analysis and should be interpreted with caution.
Given the observational methods of our study, we could not compare mammography performance among women who had their mammograms interpreted both with and without CAD. It is possible that CAD was used preferentially in women whose mammograms were more challenging. However, given the large sample size we were able to control for multiple key factors known to influence mammography performance including patient age, breast density, menopausal status and time since last mammogram. We also were not able to control for radiologist characteristics, such as experience, and thus compared performance with and without CAD in the same radiologists, to address across-radiologist variability.
Our study found no beneficial impact of CAD on mammography interpretation. However, CAD may offer advantages beyond interpretation, such as improved workflow or reduced search time for faint calcifications. Future research on potential applications of CAD may emphasize the contribution of CAD to guide decision-making about management of a radiologist-detected lesion, with the worthy goals of reducing unnecessary biopsy of a mammography lesion with specific benign features or supporting biopsy of a lesion with specific malignant features. Finally, CAD might improve mammography performance when appropriate training is provided on how to use it to enhance performance. Nevertheless, given that the evidence of the current application of CAD in community practice does not show an improvement in diagnostic accuracy, we question the policy of continuing to charge for a technology that provides no established benefits to women.
Gross et al. reported that the costs of breast cancer screening exceed $1 billion annually in the Medicare fee-for-service population (38). Consistent with our findings, they found wide variation in CAD use and very limited effectiveness, and encouraged attention to more appropriate and evidence-based application of new technologies in breast cancer screening programs. Despite its overall lack of improvement on interpretive performance, CAD has become routine practice in mammography interpretations in the United States. Seventeen years have passed since the FDA approved the use of CAD in screening mammography and 14 years have passed since Congress mandated Medicare coverage of CAD. Ten years ago, the Institute of Medicine stated that more information on CAD applied to mammography was needed before making conclusions about its effect on interpretation (39). The U.S. FDA estimates that 38.8 million mammograms are performed each year in the United States. In the BCSC database, 80% of mammograms are performed for screening and by 2012, 83% of screening mammograms in the BCSC were digital examinations interpreted with CAD. Current CMS reimbursement for CAD is roughly $7 per exam and many private insurers pay over $20 per exam for CAD, translating to over $400 million per year in current U.S. healthcare expenditures, with no added value and in some cases decreased performance.
In the era of Choosing Wisely® and clear commitments to support technology that brings added value to the patient experience, while aggressively reducing waste and containing costs, (40) CAD is a technology that does not appear to warrant added compensation beyond coverage of the mammographic examination. The results of our comprehensive study lend no support for continued reimbursement for CAD as a method to increase mammography performance or improve patient outcomes.
Supplementary Material
Acknowledgments
Funding/Support: This original research was supported by the National Cancer Institute (PO1CA154292). Data collection was further supported by the National Cancer Institute-funded Breast Cancer Surveillance Consortium (BCSC) (HHSN261201100031C, P01CA154292) and PROSPR U54CA163303. The collection of cancer and vital status data used in this study was supported in part by several state public health departments and cancer registries throughout the United States. For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. We thank the participating women, mammography facilities, and radiologists for the data they have provided for this study. A list of the BCSC investigators and procedures for requesting BCSC data for research purposes are provided at: http://breastscreening.cancer.gov.
Footnotes
Conflict of Interest Disclosures: Dr. Lehman has received grant support from General Electric (GE) Healthcare and is a member of the Comparative Effectiveness Research Advisory Board for GE Healthcare. No other disclosures are reported.
Contributor Information
Constance D. Lehman, Department of Radiology and the Seattle Cancer Care Alliance, University of Washington Medical Center, Seattle, WA
Robert D. Wellman, Group Health Research Institute, Seattle, WA
Diana S.M. Buist, Group Health Research Institute, Seattle, WA
Karla Kerlikowske, Departments of Medicine and Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, CA
Anna N. A. Tosteson, Norris Cotton Cancer Center, Geisel School of Medicine at Dartmouth, Dartmouth College, Lebanon, NH
Diana L. Miglioretti, Group Health Research Institute, Seattle, WADepartment of Public Health Sciences, School of Medicine, University of California, Davis, CA
References
- 1.Summary of Safety and Effectiveness Data: R2 Technologies(P970058) U. S. Food and Drug Administration; 1998. p. 970058. http://www.fda.gov/ohrms/dockets/98fr/123098b.txt. [Google Scholar]
- 2.Fenton JJ, Xing G, Elmore JG, et al. Short-term outcomes of screening mammography using computer-aided detection: A population-based study of medicare enrollees. Ann Intern Med. 2013;158(8):580–87. doi: 10.7326/0003-4819-158-8-201304160-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao VM, Levin DC, Parker L, et al. How widely is computer-aided detection used in screening and diagnostic mammography? J Am Coll Radiol. 2010;7(10):802–5. doi: 10.1016/j.jacr.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert FJ, Astley SM, McGeeMA, et al. Single reading with computer-aided detection and double reading of screening mammograms in the United Kingdom National Breast Screening Program. Radiology. 2006;241(1):47–53. doi: 10.1148/radiol.2411051092. [DOI] [PubMed] [Google Scholar]
- 5.Cole EB, Zhang Z, Marques HS, et al. Impact of computer-aided detection systems on radiologist accuracy with digital mammography. AJR Am J Roentgenol. 2014;203(4):909–16. doi: 10.2214/AJR.12.10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciatto S, Del Turco MR, Risso G, et al. Comparison of standard reading and computer aided detection (CAD) on a national proficiency test of screening mammography. Eur J Radiol. 2003;45(2):135–8. doi: 10.1016/s0720-048x(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 7.Taplin SH, Rutter CM, Lehman CD. Testing the effect of computer- assisted detection on interpretive performance in screening mammography. AJR Am J Roentgenol. 2006;187(6):1475–82. doi: 10.2214/AJR.05.0940. [DOI] [PubMed] [Google Scholar]
- 8.Freer TW, Ulissey MJ. Screening mammography with computer-aided detection: Prospective study of 12,860 patients in a community breast center. Radiology. 2001;220(3):781–86. doi: 10.1148/radiol.2203001282. [DOI] [PubMed] [Google Scholar]
- 9.Birdwell RL, Bandodkar P, Ikeda DM. Computer-aided detection with screening mammography in a university hospital setting. Radiology. 2005;236(2):451–7. doi: 10.1148/radiol.2362040864. [DOI] [PubMed] [Google Scholar]
- 10.Ko JM, Nicholas MJ, Mendel JB, Slanetz PJ. Prospective assessment of computer-aided detection in interpretation of screening mammography. AJR Am J Roentgenol. 2006;187(6):1483–91. doi: 10.2214/AJR.05.1582. [DOI] [PubMed] [Google Scholar]
- 11.Morton MJ, Whaley DH, Brandt KR, Amrami KK. Screening mammograms: Interpretation with computer-aided detection—prospective evaluation. Radiology. 2006;239(2):375–83. doi: 10.1148/radiol.2392042121. [DOI] [PubMed] [Google Scholar]
- 12.Georgian-Smith D, Moore RH, Halpern E, et al. Blinded comparison of computer-aided detection with human second reading in screening mammography. AJR Am J Roentgenol. 2007;189(5):1135–41. doi: 10.2214/AJR.07.2393. [DOI] [PubMed] [Google Scholar]
- 13.Gur D, Sumkin JH, Rockette HE, et al. Changes in breast cancer detection and mammography recall rates after the introduction of a computer-aided detection system. J Natl Cancer Inst. 2004;96(3):185–90. doi: 10.1093/jnci/djh067. [DOI] [PubMed] [Google Scholar]
- 14.Cupples TE, Cunningham JE, Reynolds JC. Impact of computer-aided detection in a regional screening mammography program. AJR Am J Roentgenol. 2005;185(4):944–50. doi: 10.2214/AJR.04.1300. [DOI] [PubMed] [Google Scholar]
- 15.Romero C, Varela C, Muñoz E, et al. Impact on breast cancer diagnosis in a multidisciplinary unit after the incorporation of mammography digitalization and computer-aided detection systems. AJR Am J Roentgenol. 2011;197(6):1492–7. doi: 10.2214/AJR.09.3408. [DOI] [PubMed] [Google Scholar]
- 16.Gromet M. Comparison of computer-aided detection to double reading of screening mammograms: review of 231,221 mammograms. AJR Am J Roentgenol. 2008;190(4):854–9. doi: 10.2214/AJR.07.2812. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert FJ, Astley SM, Gillan MG, et al. Single reading with computer-aided detection for screening mammography. N Engl J Med. 2008;359(16):1675–84. doi: 10.1056/NEJMoa0803545. [DOI] [PubMed] [Google Scholar]
- 18.Fenton JJ, Taplin SH, Carney PA, et al. Influence of computer-aided detection on performance of screening mammography. N Engl J Med. 2007;356(14):1399–409. doi: 10.1056/NEJMoa066099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenton JJ, Abraham L, Taplin SH, et al. Effectiveness of computer-aided detection in community mammography practice. J Natl Cancer Inst. 2011;103(15):1152–61. doi: 10.1093/jnci/djr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cancer Institute Breast Cancer Surveillance Consortium. http://breastscreening.cancer.gov/
- 21.Yankaskas BC, Taplin SH, Ichikawa L, et al. Association between mammography timing and measures of screening performance in the U.S. Radiology. 2005;234(2):363–373. doi: 10.1148/radiol.2342040048. [DOI] [PubMed] [Google Scholar]
- 22.D’Orsi CJ, Sickles EA, Mendelson EB, et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 23.Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS®, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. ACR BI-RADS® Mammography. [Google Scholar]
- 24.Sickles EA, D’Orsi CJ. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. ACR BI-RADS® Follow-up and Outcome Monitoring. [Google Scholar]
- 25.Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20(19):2865–84. doi: 10.1002/sim.942. [DOI] [PubMed] [Google Scholar]
- 26.Pepe MS, editor. The statistical evaluation of medical tests for classification and prediction. New York: Oxford University Press; 2003. [Google Scholar]
- 27.Elmore JG, Carney PA. Computer-aided detection of breast cancer: has promise outstripped performance? J Natl Cancer Inst. 2004;96(3):162–3. doi: 10.1093/jnci/djh049. [DOI] [PubMed] [Google Scholar]
- 28.Feig SA, Sickles EA, Evans WP, Linver MN. Letter to the Editor. Re: Changes in breast cancer detection and mammography recall rates after introduction of a computer-aided detection system. J Natl Cancer Inst. 2004;96(16):1260–1. doi: 10.1093/jnci/djh257. [DOI] [PubMed] [Google Scholar]
- 29.Hall FM. Breast imaging and computer-aided detection. N Engl J Med. 2007;356(14):1464–1466. doi: 10.1056/NEJMe078028. [DOI] [PubMed] [Google Scholar]
- 30.Feig SA, Birdwell RL, Linver MN. Computer-aided screening mammography. N Engl J Med. 2007;357(1):84. [PubMed] [Google Scholar]
- 31.Gur D. Computer-aided screening mammography. N Engl J Med. 2007;357(1):83–4. [PubMed] [Google Scholar]
- 32.Birdwell RL. The preponderance of evidence supports computer-aided detection for screening mammography. Radiology. 2009;253(1):9–16. doi: 10.1148/radiol.2531090611. [DOI] [PubMed] [Google Scholar]
- 33.Philpotts LE. Can computer-aided detection be detrimental to mammographic interpretation? Radiology. 2009;253(1):17–22. doi: 10.1148/radiol.2531090689. [DOI] [PubMed] [Google Scholar]
- 34.Berry DA. Computer-assisted detection and screening mammography: Where’s the Beef? J Natl Cancer Inst. 2011;103(15):1139–1141. doi: 10.1093/jnci/djr267. [DOI] [PubMed] [Google Scholar]
- 35.Nishikawa RM, Giger ML, Jiang Y, Metz CE. Re: Effectiveness of computer-aided detection in community mammography practice. J Natl Cancer Inst. 2012;104(1):77–8. doi: 10.1093/jnci/djr492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levman J. Re: effectiveness of computer-aided detection in community mammography practice. J Natl Cancer Inst. 2012;104(1):77–8. doi: 10.1093/jnci/djr491. [DOI] [PubMed] [Google Scholar]
- 37.Taylor P, Potts HW. Computer aids and human second reading as interventions in screening mammography: Two systematic reviews to compare effects on cancer detection and recall rate. Eur J Cancer. 2008;44(6):798–807. doi: 10.1016/j.ejca.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Gross CP, Long JB, Ross JS, et al. The cost of breast cancer screening in the Medicare population. JAMA Intern Med. 2013;173(3):220–6. doi: 10.1001/jamainternmed.2013.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nass S, Ball J, editors. Institute of Medicine and National Research Council of the National Academies. The National Academies Press; Washington DC: 2005. Improving Breast Imaging Quality Standards. [Google Scholar]
- 40.American Board of Internal Medicine Foundation, Choosing Wisely campaign. http://www.abimfoundation.org/Initiatives/Choosing-Wisely.aspx.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


