Summary
Exercise training benefits many organ systems and offers protection against metabolic disorders such as obesity and diabetes. Using the recently identified isoform of PGC1-α (PGC1-α4) as a discovery tool, we report the identification of meteorin-like (Metrnl), a circulating factor that is induced in muscle after exercise and in adipose tissue upon cold exposure. Increasing circulating levels of Metrnl stimulates energy expenditure, improves glucose tolerance and the expression of genes associated with beige fat thermogenesis and anti-inflammatory cytokines. Metrnl stimulates an eosinophil-dependent increase in IL-4 expression and promotes alternative activation of adipose tissue macrophages, which are required for the increased expression of the thermogenic and anti-inflammatory gene programs in fat. Importantly, blocking Metrnl actions in-vivo significantly attenuates chronic cold exposure-induced alternative macrophage activation and thermogenic gene responses. Thus, Metrnl links host adaptive responses to the regulation of energy homeostasis and tissue inflammation, and has therapeutic potential for metabolic and inflammatory diseases.
Introduction
The incidence of obesity has reached epidemic proportions worldwide, leading to a concomitant increase in associated disorders such as type II diabetes, cardiovascular disease and cancer. As a consequence, there is now great interest in brown fat, a tissue specialized for the dissipation of chemical energy in the form of heat. Brown fat defends mammals against hypothermia, obesity and type II diabetes. The dissipation of energy by brown fat cells is dependent on their high mitochondrial content and the mitochondrial protein UCP-1. This protein catalyzes a proton leak across the inner mitochondrial membrane, thereby uncoupling respiration from ATP synthesis. Recent studies have demonstrated that there are two distinct types of brown adipocytes. The classical brown fat, as exemplified by the interscapular depot of rodents and infant humans, contains cells from a muscle-like myf5+, pax7+ cell lineage (Seale et al., 2008). Many white adipose tissues (WAT) also contain a subset of cells that can express high levels of UCP-1 upon chronic exposure to cold and β-adrenergic stimulation (Cousin et al., 1992; Xue et al., 2005). These cells, called beige or brite fat cells, do not come from a myf5+ lineage (Seale et al., 2008) but are capable of elevated fuel oxidation and thermogenesis (Wu et al., 2012). Recent data demonstrate that ablation of beige adipose cells selectively makes mice more prone to obesity and metabolic dysfunction, particularly hepatic insulin resistance (Cohen et al., 2014). The molecular determinants of both types of adipocytes have been studied in detail and key transcriptional regulators include the key cell fat regulator PRDM16 (Seale et al., 2008), as well as PGC-1α (Puigserver et al., 1998), C/EBPβ (Karamanlidis et al., 2007) and FOXC2 (Cederberg et al., 2001).
Exercise training is a robust means to increase energy expenditure and is also an excellent primary intervention to combat obesity and associated metabolic disorders (Hawley, 2004; Pacy et al., 1986). Exercise mediates an increase in the circulating levels of certain hormones released from muscle (myokines), which are known to mediate some of the beneficial effects of exercise. In addition to increasing energy expenditure, exercise training also reduces adipose tissue inflammation (Baynard et al., 2012) (Gleeson et al., 2011), which may be a mechanism by which exercise training reduces insulin resistance and improves glucose homeostasis. Interestingly, certain forms of chronic exercise have been found to elevate thermogenic activity of beige/brown fat in rodents (Bostrom et al., 2012; Xu et al., 2011), perhaps providing an additional pathway for some of the chronic benefits of exercise training on glucose and lipid metabolism.
Many of the best known benefits of endurance training, such as fiber type switching, mitochondrial biogenesis and resistance to muscle atrophy, can be stimulated by expression of the transcriptional coactivator PGC1α in the skeletal muscle (Lin et al., 2002). PGC1α (now termed PGC1α1) itself is induced by endurance exercise in mice and humans (Akimoto et al., 2005) (Mathai et al., 2008). We recently identified a PGC1α1 dependent membrane protein, Fndc5, which is proteolytically cleaved into a novel secreted polypeptide termed irisin, which preferentially induces UCP-1 positive cells or “browning” of the white adipose tissue in cell culture and in vivo (Bostrom et al., 2012; Wu et al., 2012; Zhang et al., 2013). It also induces a neuro-protective gene program in the hippocampi of treated animals (Wrann et al., 2013).
A novel splice isoform of the PGC-1α gene has been recently identified that is induced upon resistance exercise and promotes muscle hypertrophy and strength (Ruas et al., 2012). The encoded protein, termed PGC-1α4, does not regulate the mitochondrial and oxidative metabolism programs induced by PGC-1α1, but rather alters expression of a distinct gene set, including IGF1 and myostatin. These are well-known regulators of skeletal muscle hypertrophy and strength. Muscle-specific transgenics expressing PGC-1α4 display increased muscle size and strength, and are resistant to the muscle wasting of cancer cachexia. Muscle-specific expression of PGC-1α4 also produces significant increases in whole-body energy expenditure (Ruas et al., 2012); however the underlying mechanisms have not been explored.
Here we report that expression of PGC-1α4 in skeletal muscle stimulates increased mRNA and secretion of a hormone called meteorin-like (Metrnl). Metrnl is induced after exercise and cold exposure in the skeletal muscle and adipose tissue, respectively, and is present in the circulation. Increases in circulating Metrnl cause an increase in whole-body energy expenditure associated with the browning of the white fat depots, and improves glucose tolerance in obese/diabetic mice. Interestingly, Metrnl does not appear to promote an increase in a thermogenic gene program through a direct action on adipocytes; rather it stimulates several immune cell subtypes to enter the adipose tissue and activate their pro-thermogenic actions. Finally, Metrnl is required for a significant portion of cold-induced thermogenic responses, thereby implicating a key physiological role for Metrnl in metabolic adaptations to cold temperatures.
RESULTS
Muscle-specific transgenic mice expressing PGC-1α4 are lean and display browning of white adipose tissues
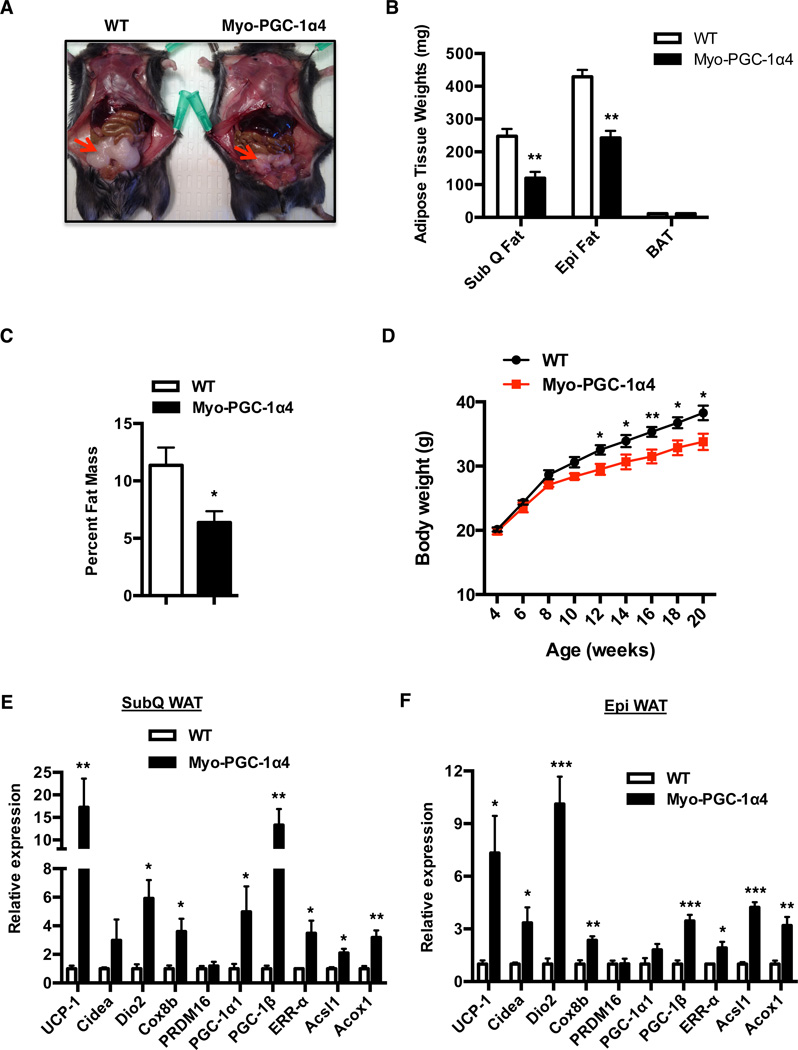
We recently reported that the muscle-specific PGC-1α4 transgenic mice (Myo-PGC-1α4) demonstrate muscle hypertrophy and increased basal energy expenditure, without any changes in food intake or movement (Ruas et al., 2012). The molecular basis for the increased energy expenditure was not explored. As shown in Figure 1A, the Myo-PGC-1α4 mice have an obvious reduction in the size of epididymal (epi) fat depot and demonstrate ~30–40% reduction in the weights of the epi and subcutaneous adipose tissue (inguinal) (SubQ WAT), compared to those of controls (Figure 1B). In addition, the Myo-PGC-1α4 mice demonstrated an overall lean phenotype, as evidenced by a 43% reduction in whole body fat content, when assessed by magnetic resonance imaging (MRI) (Figure 1C). Interestingly, the Myo-PGC-1α4 mice gain significantly less weight after 12 wk on a high fat diet (HFD) compared to their wild-type (WT) littermate controls. No significant weight differences were observed prior to the HFD challenge at 4 wk of age (Figure 1D).
Figure 1. Muscle-specific PGC1α4 transgenic mice are lean and display increased thermogenesis in the adipose tissue.
See also Figure S1 (A) Representative images of epi fat from wild-type (WT) or Myo-PGC1α4 mice. (B) Determination of SubQ, Epi and BAT weights from WT (N=5) and Myo-PGC1α4 mice (N=7). (C) Determination of percent fat mass between WT and Myo-PGC1α4 mice using MRI (N=6). (D) Body weights of WT and Myo-PGC1α4 mice upon challenging with HFD starting at 4 weeks (N=10). (E, F) Real-time PCR (qPCR) analysis of markers associated with thermogenic, mitochondrial and β-oxidation genes in (E) subQ, and (F) epi adipose tissue of WT and Myo-PGC1α4 (N=6). *p<0.05, **p<0.001, ***p<0.0001. All data are presented as mean +/− s.e.m.
Increase in adipose thermogenesis can augment whole-body energy expenditure, independent of physical activity. We therefore analyzed the adipose tissues from Myo-PGC-1α4 mice for expression of genes related to thermogenesis, and/or genes involved in imparting a brown fat-like program (browning). As shown in Figure 1E, quantitative PCR (qPCR) analyses of mRNA revealed a robust increase in thermogenic, β-oxidation and mitochondrial gene programs, including UCP-1, Acsl1, PGC-1α and ERR-α in the subQ WAT. In addition, we also observed significant increases in expression of these same genes in the epi WAT (Figure 1F), a tissue that has a much lower capacity to induce activity of brown or beige fat thermogenic gene programs. However, no significant changes in these gene expression events were noted in the interscapular “classical” brown adipose tissue (BAT) (Figure S1A). Notably, PGC-1α4 transgene expression is localized to the skeletal muscle in the Myo-PGC-1α4 mice and is not expressed in the adipose tissues (Figure S1B). Taken together, these results indicate that muscle-specific expression of PGC-1α4 promotes browning of the WAT (both subQ and epi), which might contribute to the lean phenotype of the Myo-PGC-1α4 mice. These observations also point towards the existence of a PGC1α4-dependent myokine that mediates muscle-fat cross talk to promote expression of a broad beige thermogenic gene program.
Identification of Meteorin-like (METRNL) as a key PGC-1α4 target gene in skeletal muscle
We utilized two independent and unbiased approaches to identify novel secreted factors controlled by PGC1α4 that might contribute to the browning of white fat: gene expression analyses combined with bioinformatics algorithms, and quantitative Mass Spectrometry of secreted proteins. First, we screened affymetrix data obtained upon PGC1α4 over-expression in primary myotubes (Ruas et al., 2012), to identify potential candidates that satisfied all of the following criteria (i) >2-fold change in mRNA expression (ii) presence of an N-terminal signal peptide, and (iii) absence of a trans-membrane domain. The fold change of short-listed genes was independently confirmed in the quadriceps muscle from Myo-PGC-1α4 mice by qPCR (list of the short-listed genes, Table S1). Second, we performed quantitative protein mass spectrometric analysis to identify secreted factors that were up regulated (>2-fold) in serum-free culture supernatants of primary myotubes after forced expression of PGC-1α4 (Table S2). The list of short-listed candidates from both these approaches were then cross-referenced and only those gene candidates that were increased with both of these approaches were selected (Table S3). Potential candidates were further selected based on their mRNA abundance in skeletal muscle from WT mice, as quantified by absolute levels of expression by qPCR (Table S3).
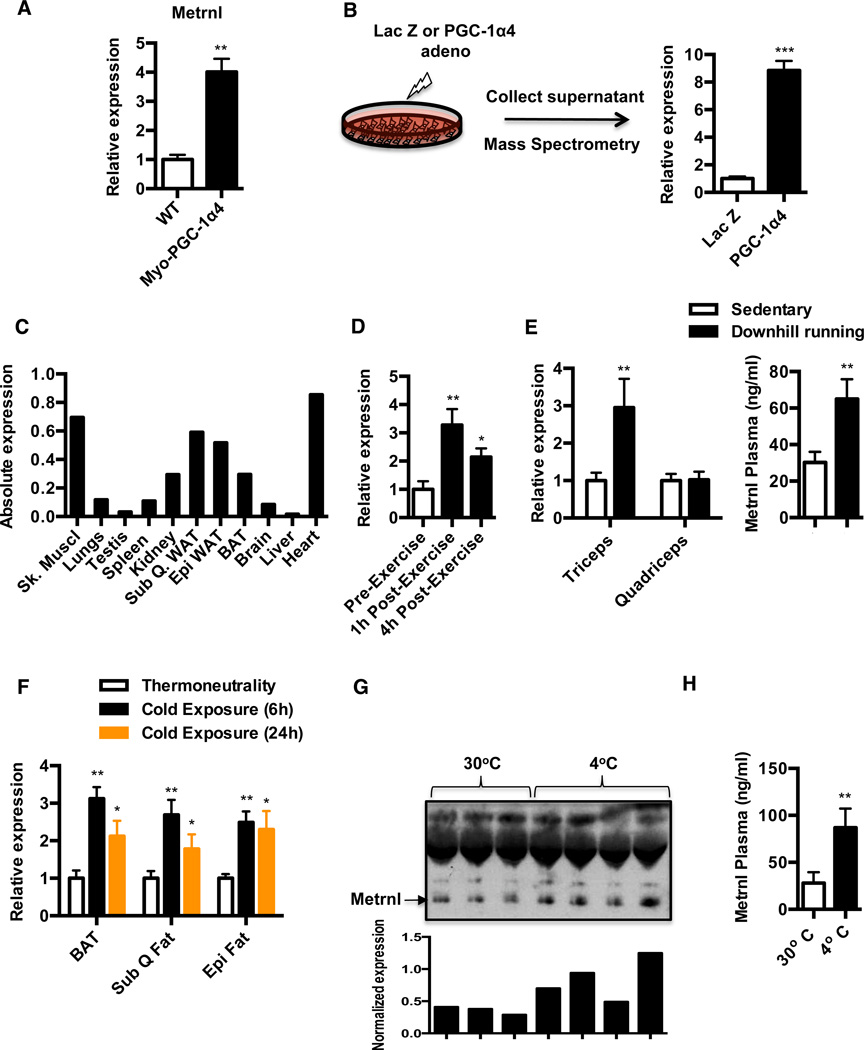
Based on the above criteria, we focused on a protein called meteorin-like (Metrnl). Metrnl mRNA was up-regulated about 4 fold in the mRNA isolated from quadriceps muscle from Myo-PGC-1α4 mice (Figure 2A) and the protein was increased approximately 8-fold in the mass spectrometric analysis from supernatants of cultured myotubes with forced PGC-1α4 expression (Figure 2B). To further confirm the identity of Metrnl as a bona fide secreted factor, we expressed Metrnl in Cos7 cell line, using adenoviral vectors expressing this protein fused to a C-terminal V5-His tag (Metrnl-Ad) or a Lac Z (Lac Z Ad) control. Ectopic Metrnl expression produced robust increases in V5-tagged Metrnl protein in the culture supernatants (Figure S2A). Next, we analyzed abundance of Metrnl expression across various tissues. In addition to its abundance in the skeletal muscle, it is also expressed at comparable levels in the subQ and epi WAT depots and heart, followed by BAT and kidney (Figure 2C).
Figure 2. Identification of Metrnl as a PGC1α4 target gene.
See also Figure S2 and Supplementary Tables S1–S3 (A) qPCR analysis against Metrnl in quadriceps from Myo-PGC1α4 transgenic mice or WT littermate controls (N=4 each group). (B) Expression of Metrnl in culture supernatants from primary myotubes transduced with Lac Z or PGC1α4 expressing adenovirus using mass spectrometry (N=3 each group). (C) Tissue specific Metrnl expression patterns by qPCR. Bar graphs represent RNA samples from three independent mice pooled together. (D) Analysis of Metrnl gene expression in skeletal muscle biopsies from human volunteers. Vastus lateralis biopsies were obtained prior to commencement, 1hr and 4hr post-exercise. Gene expression was analyzed by qPCR. (E) Metrnl mRNA expression in skeletal muscle and plasma levels after an acute bout of downhill running exercise. C57/BL6 mice were divided into groups: sedentary (N=9) and run (N=10). The quadriceps and triceps muscles were harvested 6 hours after run and processed for gene expression by qPCR. Plasma was collected 24h after the run and Metrnl levels were measured by ELISA (F) Analysis of Metrnl mRNA expression in subQ, epi and brown adipose tissue of mice chronically housed at 30°C or acutely subjected to a 4°C cold challenge for the indicated time points (N=5 per group). (G–H) Under the same experimental setting as in (F), plasma from mice housed at 30°C or exposed to 4°C for 24 hrs were subjected to (G) western blot against Metrnl and Metrnl band is normalized to an invariant nonspecific band, and (H) ELISA against Metrnl. *p<0.05, **p<0.001, ***p<0.0001. All data are presented as mean +/− s.e.m.
Given that PGC-1α4 mRNA is expressed in the skeletal muscle of mice and humans upon resistance training (Ruas et al., 2012), we investigated the regulation of Metrnl in human skeletal muscle following an acute bout of concurrent exercise (resistance-followed by endurance exercise; concurrent training, see Experimental Procedures). Skeletal muscle biopsies from the vastus lateralis were obtained at rest, 1 h and 4 h following the completion of the exercise session. Figure 2D shows an increase in Metrnl mRNA expression at both the time-points, with maximal induction at 1h post-exercise. In addition, we also noted increases in PGC-1α4 (both time points) and PGC-1α1 (1 hour only) mRNA expression (Figure S2B). Next, we investigated the regulation of Metrnl in a mouse model of eccentric exercise that promotes muscle strength and hypertrophy. A single bout of downhill treadmill running exercise increases Metrnl mRNA expression in triceps, but not in the quadriceps muscle (Figure 2E). Concomitantly, we also observe an approximately 2 fold increase in circulating Metrnl at day 1 post-exercise, as assessed by ELISA (Figure 2E). The specificity of the antibody against Metrnl was demonstrated by antigen-antibody neutralization experiments using ELISA (Figure S2C). In addition, late in the manuscript review process, we obtained access to total body KO mice for Metrnl; the specificity of the antibody used here was shown definitively with these animals (Figure S2D). We did not observe any changes in Metrnl expression upon a program of endurance exercise training (free wheel running) (data not shown).
Because Metrnl is regulated by exercise, we also explored other physiological stimuli that might regulate its expression. Given the abundance of Metrnl mRNA in the adipose tissues (Figure 2C), we investigated its regulation by thermogenic stimuli, specifically acute and chronic exposure to cold. Metrnl expression was measured in BAT, subQ and epi fat depots of mice chronically housed at 30°C (thermoneutrality) or after an acute (6h, 24h) and chronic challenge (2 wk) at 4°C. Acute cold exposure significantly elevated Metrnl gene expression in all three adipose tissues, although with different kinetics of expression (Figure 2F). Notably, Metrnl mRNA was increased in a transient manner, with maximal induction observed at 6 h post-challenge. In addition, chronic cold exposure failed to maintain elevated Metrnl expression in all three adipose tissues examined (Figure S2E). Importantly, cold exposure for 24 h increased circulating levels of Metrnl, as assessed by western blotting and ELISA assay (Figure 2G and 2H). It is interesting to note that acute cold exposure does not induce Metrnl expression in the skeletal muscle (Figure S2F), and downhill running exercise specifically induces Metrnl expression in the skeletal muscle but not in the adipose tissue (Figure S2G). Overall these results identify Metrnl as a hormone can be selectively induced in different tissues, depending upon the physiologic stimulus. These data further suggest a potential role for this protein in the physiological adaptations to exercise and cold.
Metrnl promotes thermogenic and anti-inflammatory gene programs in adipose tissues in vivo
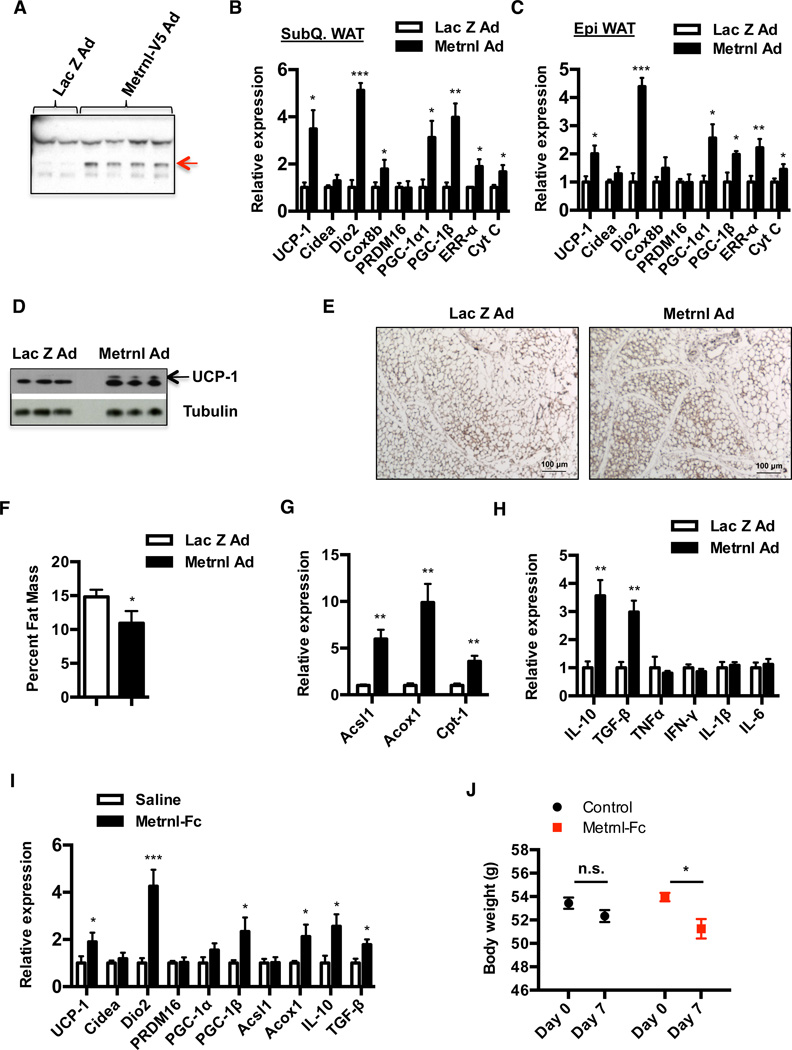
To determine whether Metrnl can promote browning of adipose tissues, we performed intravenous injections of adenoviral vectors to deliver full-length Metrnl constructs to the liver. This method generally results in robust expression of proteins in the liver and potential secretion to the plasma (Wei et al., 2008). Notably, serum AST levels were well within the normal range and showed no differences between the control and Metrnl injected mice. (Figure S3A). At day 3 post-injection, we observed a ~20-fold increase in liver Metrnl mRNA (Figure S3B) and a 5–6 fold increase in plasma Metrnl levels, as detected by western blotting with an anti-Metrnl antibody (Figure 3A). Strikingly, the increase in circulating Metrnl produced remarkable increases in broad brown/beige fat thermogenic and mitochondrial gene program in the subQ and epi WAT, including UCP-1, DIO2, PGC-1α, and ERR-α (Figure 3B and 3C). The increase in UCP-1 mRNA (~3.5 fold) was also accompanied by a robust increase in the UCP-1 protein expression in the subQ WAT (Figure 3D and 3E). In addition, we also observe moderate increases in thermogenic gene programs in the BAT (Figure S3C). Notably, changes in thermogenic gene expression were observed only between days 5 and 7 post-injection and returns to baseline expression by day 10 (Figure S3D), even though we detect increases in plasma Metrnl levels as early as day 3. Interestingly, we observe an overall lean phenotype in Metrnl injected mice, as evidenced by a 25% reduction in whole body fat content, when assessed by MRI (Figure 3F). Metrnl expression also stimulated elevated mRNA levels for genes associated with β-oxidation such as Acsl1, Acox1 and Cpt1 (Figure 3G). Finally, the increases in expression of thermogenic and β-oxidation gene programs due to Metrnl expression were also observed in a different strain of mice, the Balb/c strain (Figure S3E).
Figure 3. Increase in circulating Metrnl promotes increase in thermogenic and anti-inflammatory gene programs in the adipose tissue.
See also Figure S3 (A–H) C57/BL6 mice were injected with adenoviral vectors (Ad) expressing Lac Z or Metrnl intravenously, via tail vein injections and (A) Plasma from Lac Z or Metrnl injected mice was subjected to western blotting against Metrnl at day 5 post-injection, (B–C) qPCR analysis of markers associated with thermogenesis and mitochondrial gene programs in (B) subQ, (C) Epi WAT at day 7 (N=6) (D) Western blotting against UCP-1 (N=3), and (E) immunohistochemistry against UCP-1 in subQ WAT at day 7 (N=2). (F) Determination of percent fat mass between Lac Z and Metrnl-injected mice using MRI at day 7 (N=6) (G–H) qPCR analysis of markers associated with β-oxidation and pro/anti-inflammatory gene programs in the subQ WAT at day 7 (N=6). (I–J) C57/BL6 mice fed a HFD for 20 weeks (N=8) were injected daily with saline or Metrnl-Fc protein (10mg/kg) i.p. for 7 days, and (I) 6 hours after the last injection, animals were sacrificed and subQ WAT was analyzed for changes in thermogenic, β-oxidation and pro/anti-inflammatory gene programs, (J) Body weights of mice. *p<0.05, **p<0.001, ***p<0.0001. All data are presented as mean +/− s.e.m.
Increases in brown and beige fat thermogenesis are typically inversely correlated with changes in the expression of inflammatory genes (Chiang et al., 2009); we therefore analyzed whether Metrnl also regulated expression of inflammation-linked genes. As shown in Figure 3H, Metrnl expression promotes increases in expression of anti-inflammatory genes, such as IL-10, TGF-β. We did not detect any changes in expression of pro-inflammatory genes such as TNF-α and IL-1β. Since mice rendered obese by HFD display higher basal expression of pro-inflammatory gene markers in adipose tissues (Hotamisligil, 2006; Hotamisligil et al., 1993), we next analyzed whether Metrnl can act to suppress expression of pro-inflammatory genes in these mice. Indeed, expression of Metrnl promotes modest decreases in expression of pro-inflammatory cytokines, such as TNF-α, IFN-γ and IL-1β (Figure S3F).
To further study the ability of Metrnl to positively regulate browning and thermogenesis, we generated and purified a recombinant protein fused to the Fc portion of IgG to the C-terminus of Metrnl (Metrnl-Fc); this was then injected into mice rendered obese by feeding a HFD. This Metrnl protein had a relatively short half-life (Figure S3G), so we injected mice daily with a dose of 10mg/kg for 7 days. Consistent with the adenoviral experiments, recombinant Metrnl protein increased adipose expression of thermogenic, β-oxidation and anti-inflammatory genes, including UCP-1, DIO2, Acox1 and IL-10 (Figure 3I). The magnitude of these changes were weaker than those observed with the viral-mediated expression, presumably reflecting the suboptimal pharmacokinetics seen with this Metrnl fusion protein. Nevertheless, these increases in thermogenic gene expression were accompanied by a small but significant reduction in body weight, compared to controls (Figure 3J). Overall, these results identify Metrnl as a hormone that can promote an increase in a broad beige/brown fat thermogenic gene program in vivo.
Metrnl increases whole-body energy expenditure and improves glucose tolerance
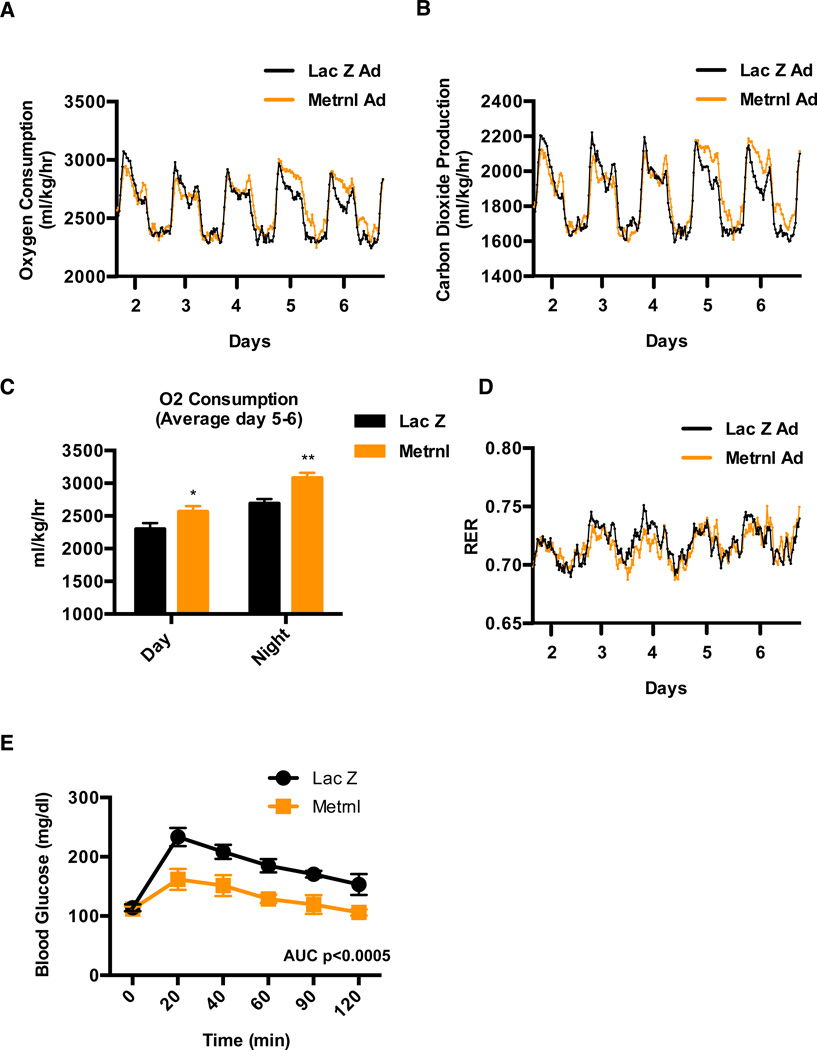
Increase in adipose tissue thermogenesis or browning of white fat can be accompanied by increases in whole-body energy expenditure and improved glucose homeostasis in vivo (Bostrom et al., 2012; Cederberg et al., 2001). We therefore studied these metabolic parameters after delivering Metrnl-expressing adenoviral vectors to mice. Viral vectors were used for these experiments since they require less frequent handling of the mice than protein injections. We first measured energy expenditure using a comprehensive laboratory animal monitoring system (CLAMS) and observed a highly significant increase in energy expenditure in Metrnl injected mice, compared to Lac Z controls (Figure 4A–C). Notably, the increase in oxygen consumption and carbon dioxide production was observed 5 days post-injection, consistent with the time-course of thermogenic gene expression. This suggests that the action of Metrnl may not directly regulate thermogenesis, but might regulate various biological processes that promote remodeling of the adipose tissue in a way conducive for increased browning of the white fat. Importantly, there was no change in respiratory exchange ratio (RER), indicating that Metrnl did not stimulate any substantial shift from carbohydrate to fat-based fuels (Figure 4D). Importantly, these changes in energy expenditure were independent of food intake or locomotor activity (Figure S4A and S4B).
Figure 4. Metrnl expression increases energy expenditure and improves glucose tolerance.
See also Figure S4 (A–E) C57/BL6 mice fed a HFD for 20 weeks were injected with Lac Z or Metrnl adenovirus (i.v.) and (A–D) energy expenditure was measured (N=7), (A) oxygen consumption, (B) Carbon dioxide production, (C) quantification of oxygen consumption between day 5 and 6 and (D) respiratory exchange ratio (RER), (E) IP-glucose tolerance test was performed at day 6 (N=8). *p<0.05, **p<0.001, ***p<0.0001. All data are presented as mean +/− s.e.m.
Next, intraperitoneal glucose tolerance test (GTT) were performed in obese mice; Metrnl expression significantly improved glucose tolerance when compared to control mice injected with Lac Z (Figure 4E). Collectively, these data illustrate that increases in circulating Metrnl causes an increase in energy expenditure and an improvement in glucose homeostasis in obese/diabetic mice.
Metrnl induces IL-4/IL-13 cytokine expression and promotes alternative macrophage activation in adipose tissue in vivo
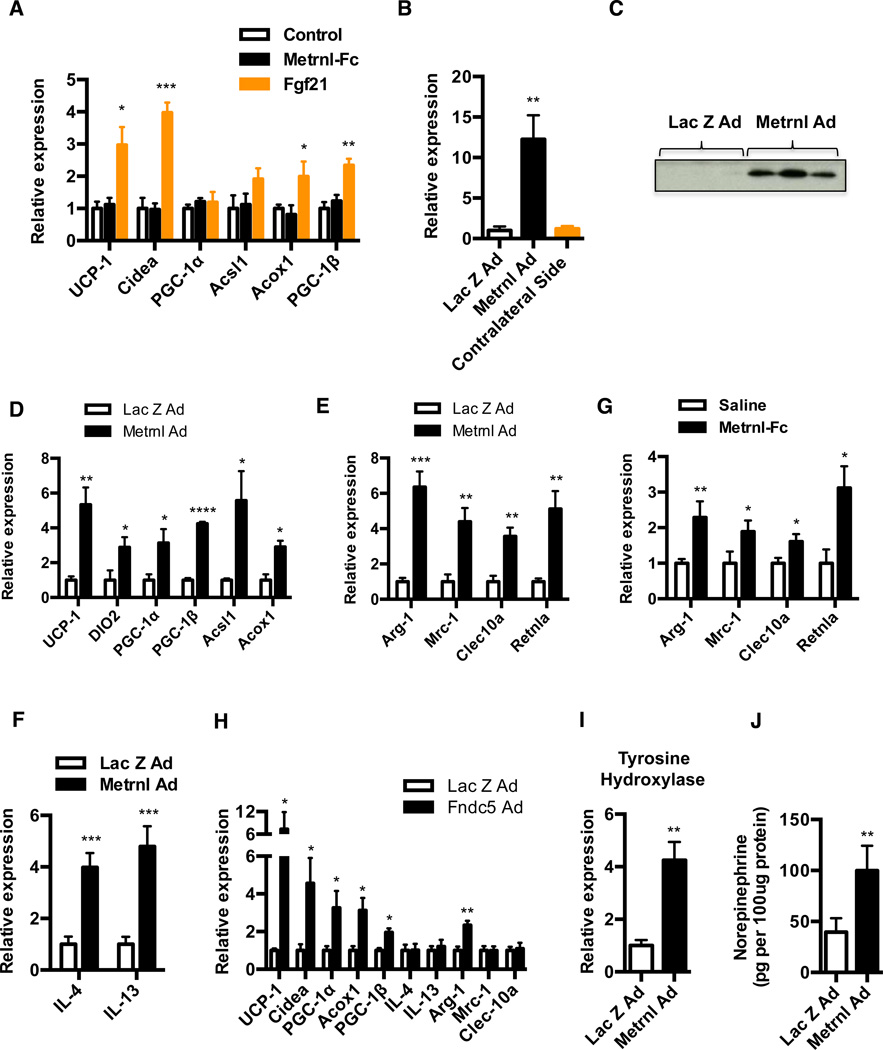
The mechanisms underpinning these effects of Metrnl were first studied by applying recombinant Metrnl-Fc protein directly to the stromal vascular fraction (SVF) of subcutaneous white adipocytes during differentiation in vitro. Interestingly, there was no detectable effect on the regulation of thermogenic or β-oxidation genes such as UCP-1, DIO2, and Acsl1 etc, at varying doses and duration of treatments tested. (Figure 5A and data not shown). We used Fgf21 as a positive control, as it has been previously demonstrated to induce expression of thermogenic genes such as UCP-1 and Cidea, in adipose cultures (Fisher et al., 2012). Notably, the inability of Metrnl to induce changes in gene expression was not due to inactivity of the Metrnl-Fc protein, in that the same preparations of protein caused adipose tissue browning in vivo (Figure 3I) and were able to induce STAT3 phosphorylation in a dose-dependent manner in cultured primary cortical neuron cells (Figure S5A). In addition, we tested a commercially available recombinant Metrnl protein and adenoviral mediated transduction of these same SVF cultures, and got similar negative results (Figure S5B). These results suggest that Metrnl may induce adipose tissue thermogenesis in vivo independent of a simple, direct action on adipocytes.
Figure 5. Metrnl expression induces an increase in alternative activation of adipose tissue macrophages.
See also Figure S5 (A) SVF from the inguinal fat depot was differentiated into adipocytes for 6 days and treated with either saline, recombinant Metrnl-Fc (5ug/ml) or Fgf21 (100ng/ml), during last two days of differentiation. qPCR analysis was performed for indicated genes, 48h post-treatment (N=4). (B–D) SubQ WAT (left pad) of C57/BL6 mice was injected with either Lac Z and Metrnl adenovirus (N=5) and the injected and contralateral WAT (right, un-injected) were harvested (B–C) at day 3 post-injection to analyze for increase in Metrnl expression (B) by qPCR (C) western blotting, and (D) at day 5 post-injection to assess for changes in thermogenic and β-oxidation genes by qPCR. (E–F) C57/BL6 mice were injected with adenoviral vectors expressing Lac Z or Metrnl (i.v.) (N=6), and (E) analyzed for markers associated with alternative macrophage activation in the subQ WAT at day 7, and (F) IL4/IL13 cytokine expression at day 5. (G) C57/BL6 mice fed a HFD for 20 weeks (N=8) were injected daily with saline or Metrnl-Fc protein (10mg/kg) (i.p.) for 7 days and analyzed for changes in markers of M2 macrophage activation in the subQ WAT. (H) BALB/c mice were injected with Lac Z or Fndc5-Ad (i.v.). Animals were sacrificed 7 days later and subQ WAT was assessed for indicated genes by qPCR (I–J) Under the same experimental setting as in (E–F), (I) tyrosine hydroxylase mRNA expression, and (J) norepinephrine content of subQ adipose tissue at day 7. *p<0.05, **p<0.001, ***p<0.0001, ****p<0.00001. All data are presented as mean +/− s.e.m.
We therefore considered the possibility that this increase in adipose tissue thermogenesis caused by Metrnl in vivo could require actions on non-adipose cell types. To explore this further, we first investigated whether Metrnl can induce a thermogenic phenotype when expressed locally in adipose tissues in vivo. Adenoviral injections were performed directly into the subQ fat pad using Metrnl or control Lac Z adenovirus and analyzed for changes in gene expression 5 days post-injection. This method results in robust and localized Metrnl expression (mRNA and protein) only at the site of injection as compared to the uninjected contralateral side on the same mouse (Figure 5B and 5C). In addition, we did not detect any increase in Metrnl expression in the liver upon fat pad injections (data not shown). Importantly, this forced expression of Metrnl produces significant increases in both thermogenic and β-oxidation genes, when compared to mice injected with Lac Z control (Figure 5D). These results demonstrate that when expressed at the adipose tissue level, Metrnl can act to induce expression of thermogenic and β-oxidation genes, despite its inability to do so in primary adipocyte cultures in vitro.
While the characteristic cell type of adipose tissue is the adipocyte, this tissue consists of a heterogeneous population of multiple different cell types, such as preadipocytes and many immune cells, including macrophages, eosinophils, T cells, B-cells and mast cells. In addition to changes in the expression of thermogenic and β-oxidation genes, we also observed an increase in anti-inflammatory genes (e.g. IL-10) in the adipose tissue (Figure 3H). A major source of IL-10 in the adipose tissue is known to be alternatively activated macrophages (M2) that protect adipocytes from inflammation and improve glucose homeostasis (Odegaard and Chawla, 2011). To investigate whether Metrnl induces a phenotypic switch in adipose tissue macrophages, we examined mRNA from the subQ WAT of Metrnl-treated animals (adenoviral-mediated expression) and observed significant increases in several genes associated with alternative macrophage activation, including Arg1, Mrc-1, Clec10a and Retnla (Figure 5E). Notably, these changes in gene expression associated with alternative macrophage are robust with ~5–6 fold increase in mRNA for Arg1 and Retnla, and ~3–4 fold increase in Mrc-1 and Clec10a. These changes were also observed in epi WAT and BAT, albeit with different magnitude of gene expression changes (Figure S5C). In addition to the switch in phenotype, Metrnl caused a significant increase in the number of CD11b+ F4/80+ macrophages (~ 2.2 fold) in the subQ WAT, as assessed by flow cytometry (Figure S5D) In contrast, gene expression for markers of classical (M1) macrophage activation, such as TNF-α, Nos2 and CD274 was unchanged (Figure S5E). More importantly, Metrnl expression also increased expression of cytokines IL-4 and IL-13 in the adipose tissue; these are the cytokines that are dominant regulators of the macrophage alternative activation program (Figure 5F). The changes in IL4/IL13 gene expression were consistently observed at early time-points (~days 4–5) after Metrnl expression and had returned to baseline at day 7, when we observe the increases in expression of thermogenic and alternative macrophage activation genes. We also observed increases in expression of genes associated with alternative macrophage activation and cytokines IL4/IL13 in the adipose tissue upon infusion of the recombinant Metrnl-Fc fusion protein in vivo, and with localized Metrnl expression in the fat pad using adenoviral vectors (Figure 5G, S5F and S5G). Notably, these increases in IL4/IL13 gene expression and alternative macrophage activation in vivo were not observed with Irisin, a secreted form of Fndc5 that has been previously shown to stimulate adipose tissue thermogenesis via a direct action on adipocytes (Figure 5H) (Bostrom et al., 2012).
Catecholamine production by alternatively activated macrophages has been shown to be important for induction of thermogenic and β-oxidation genes in WAT and BAT of cold exposed mice (Nguyen et al., 2011). Because Metrnl promotes alternative activation of adipose tissue macrophages, we tested whether it promotes an increase in catecholamine production in the adipose tissue. An experimental increase in circulating Metrnl causes a significant increase in adipose expression of tyrosine hydroxylase (Th), the rate-limiting step in the synthesis of catecholamines (Figure 5I). Consistent with this, Metrnl expression also increased the levels of norepinephrine content in the adipose tissue by approximately 2.5 fold, when compared with the Lac Z control (Figure 5J). Together, these results strongly suggest that Metrnl induces a phenotypic switch in adipose tissue macrophages in vivo, along with production of pro-thermogenic catecholamines, possibly via inducing expression of the M2-regulatory cytokines IL-4 and IL-13.
Disruption of IL4/IL13 signaling abrogates the browning response induced by Metrnl
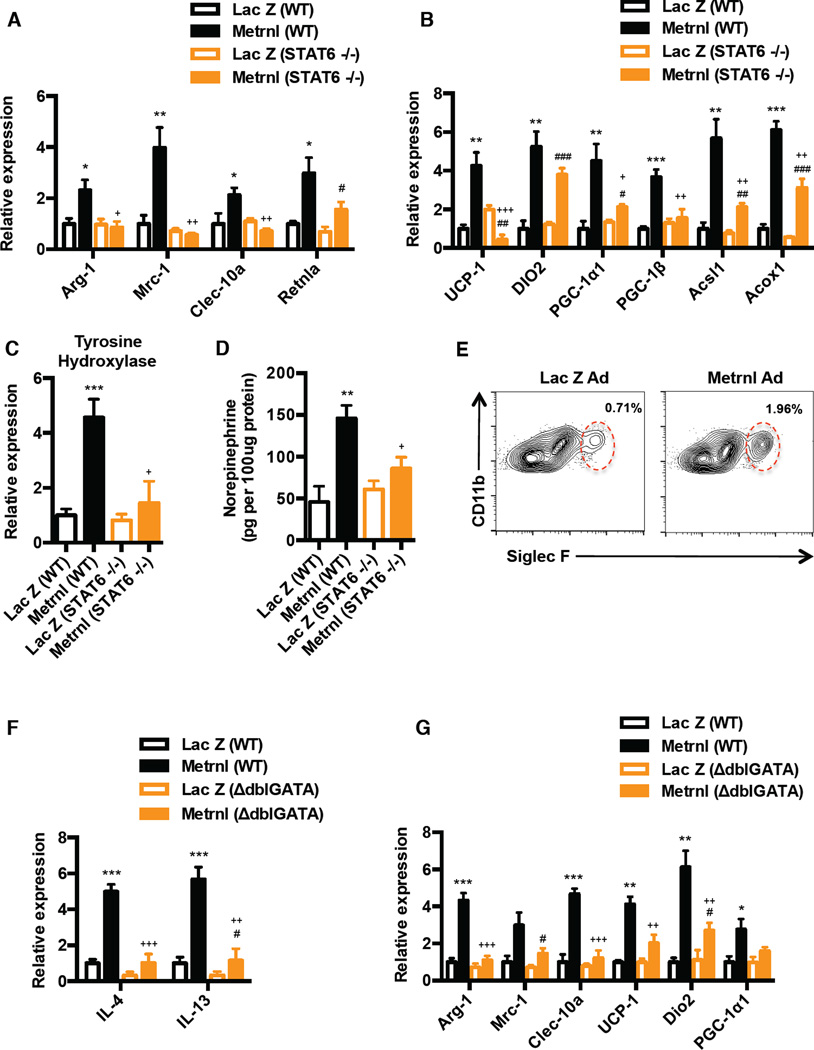
To investigate the requirement of IL4/IL13 signaling and alternative macrophage activation in the Metrnl-induced browning response, we utilized two independent approaches. STAT6 is a key transcription factor downstream of IL4/IL13 signaling, so we first elevated Metrnl expression in STAT6 KO mice. Disruption of IL4/IL-13 signaling through the use of STAT6−/− mice (Martinez et al., 2009) completely abrogated the Metrnl-induced increase in alternative activation of subQ WAT macrophages (Figure 6A). This was a specific defect in M2 activation, as STAT6 deficiency did not cause a preferential increase in classical activation markers (Figure S6A). Most importantly, STAT6 deficiency significantly attenuated the expression of the broad range of beige fat thermogenic genes (UCP-1, PGC-1α) and the β-oxidation genes (Acsl1, Acox1) in the STAT6 KOs, compared to the wild-type mice (Figure 6B). Next, in an independent approach, we abrogated IL4/IL13 signaling using a neutralizing antibody against the IL-4 receptor α–chain (IL4Rα). This receptor subunit is utilized by both IL-4 and IL-13 to promote alternative activation in macrophages (Martinez et al., 2009). Consistent with the results obtained with the genetic STAT6 deficiency, blockade of IL-4Rα completely abrogated the Metrnl-induced increase in genes characteristic of alternative macrophage activation, and blunted the expression of the beige fat thermogenic and β-oxidation gene programs. (Figure S6B and S6C).
Figure 6. Metrnl induced browning response requires IL4/IL13 signaling.
See also Figure S6 (A–B) Wild-type (WT) and STAT6−/− mice (N=5) were injected with Lac Z or Metrnl-Ad (i.v.) and analyzed at day 7 post-injection for markers associated with (A) M2 macrophage activation, and (B) thermogenic and β-oxidation genes. (C–D) Under the same experimental setting as in (A–B) (C) tyrosine hydroxylase mRNA expression and (D) norepinephrine content of subQ WAT was assessed at day 7. (E) C57/BL6 mice were injected with Lac Z or Metrnl-Ad (i.v.) (N=4) and flow cytometric analysis of eosinophils (defined as CD11b+ and Siglec F+; gating strategy in Figure S6F) in the SVF from subQ WAT at day 4. Numbers represent percentage of CD11b+/Siglec F+ cells in total SVF. (F–G) Wild-type (WT) and ΔdblGATA mice (N=5) were injected with Lac Z or Metrnl-Ad (i.v.) and analyzed for (F) IL-4/IL13 mRNA expression at day 4, and (G) M2 macrophage and thermogenic genes at day 7. *p<0.05, **p<0.001, ***p<0.0001 comparison between WT mice injected with Lac Z and Metrnl-Ad. #p<0.05, ##p<0.001, ###p<0.0001 comparison between STAT6−/− or ΔdblGATA mice injected with Lac Z and Metrnl-Ad. +p<0.05, ++p<0.001, +++p<0.0001 comparison between WT or STAT6−/− / ΔdblGATA mice injected with Metrnl-Ad. All data are presented as mean +/− s.e.m
Next, we investigated whether the increase in catecholamines driven by Metrnl is dependent on IL4/IL13 signaling and/or increase in alternative macrophage activation. Metrnl expression caused significant increases in mRNA expression for tyrosine hydroxylase and stimulated catecholamine secretion from adipose tissues derived from wild-type mice (Figure 6C and 6D). However, loss of IL4/IL13 signaling in STAT6−/− mice abrogated Metrnl-induced increase Th and reduced norepinephrine content in the subQ WAT by ~ 60–70% in STAT6−/− mice, compared to those seen in the wild-type mice (Figure 6C and 6D). Consistent with the results obtained with STAT6−/− mice, blockade of IL-4Rα significantly reduced norepinephrine content in the subQ WAT (Figure S6D). These observations demonstrate that induction of IL4/IL13 expression and alternative macrophage activation by Metrnl is essential to promote production of catecholamines and to regulate of expression of genes associated with brown/beige adipose thermogenesis and β-oxidation gene programs.
Metrnl stimulates an eosinophil-dependent increase in IL4/IL13 expression and alternative macrophage activation in the adipose tissue
These observations strongly suggest that the ability of Metrnl to induce IL4/IL13 expression is key to the cascade of events leading to increases in expression of UCP-1 and other thermogenic genes in fat. Interestingly, primary bone marrow derived macrophages did not induce IL4/IL13 and were unable to undergo alternative activation when treated with recombinant Metrnl-Fc protein in vitro (Figure S6E).
How does Metrnl increase expression of IL4/IL13 in the adipose tissue? It has been previously demonstrated that eosinophils are the major IL-4 producing cells within white adipose tissue (Wu et al., 2011) and their presence is required for the maintenance of alternatively activated macrophages. Using sialic acid-binding immunoglobulin receptor, Siglec-F and Ccr3 as molecular markers for eosinophils, we next investigated whether circulating Metrnl causes an increase in the number or trafficking of eosinophils into the adipose tissues (the eosinophil gating strategy is shown in Figure S6F). Systemic Metrnl expression through the use of adenoviral vectors caused a significant increase in adipose mRNA levels of both Siglec-F and Ccr3 (approximately 6.5 fold) (Figure S6G) and in the number of eosinophils in the subQ WAT by 2.7 fold, as assessed by flow cytometry (Figure 6E). The kinetics of expression of Siglec-F mRNA was comparable to those of IL-4 in the subQ WAT (Figure S6J). Notably, Siglec-F and IL-4 expression is induced by day 3, peaks at day 4 and returns to baseline expression by day 7 (Figure S6J). The increased expression of these molecular eosinophil markers was also observed with recombinant Metrnl-Fc protein (Figure S6H), and with localized Metrnl expression in the fat pad using adenoviral vectors (Figure S6I). However, Metrnl did not cause an increase in other IL-4 expressing cells types, such as T cells and basophils (Figure S6K and S6L), whereas mast cells were not detected in the SVF from subQ WAT. These results indicate that systemic administration of Metrnl causes increases in adipose tissue eosinophils and strongly suggests this eosinophil recruitment is a likely source of much of the IL-4 induced by Metrnl in the adipose tissue.
To determine whether eosinophils are the primary source of IL4/IL13 upon Metrnl treatment, we used mice with a Gata1 promoter mutation that lack eosinophils (ΔdblGATA mice) (Yu et al., 2002). The absence of eosinophils completely abrogated the Metrnl-induced increase in IL4/IL13 expression in the subQ WAT at day 4 post-injection (Figure 6F). Notably, and consistent with our observation with disruption of IL4/IL13 signaling, Metrnl was not able to induce expression of genes associated with alternative macrophage activation in ΔdblGATA mice (Figure 6G). Finally, the induction of thermogenic genes (UCP-1, Dio2 and PGC-1α1) induced by Metrnl was significantly blunted (~ 50–60%) in the ΔdblGATA mice at day 7 (Figure 6G). These results demonstrate that eosinophils are the primary source of Metrnl-induced IL4/IL13 in the adipose tissue and they are required for increased alternative macrophage activation and induction of thermogenic gene responses.
Metrnl is required for cold-induced adaptive thermogenesis
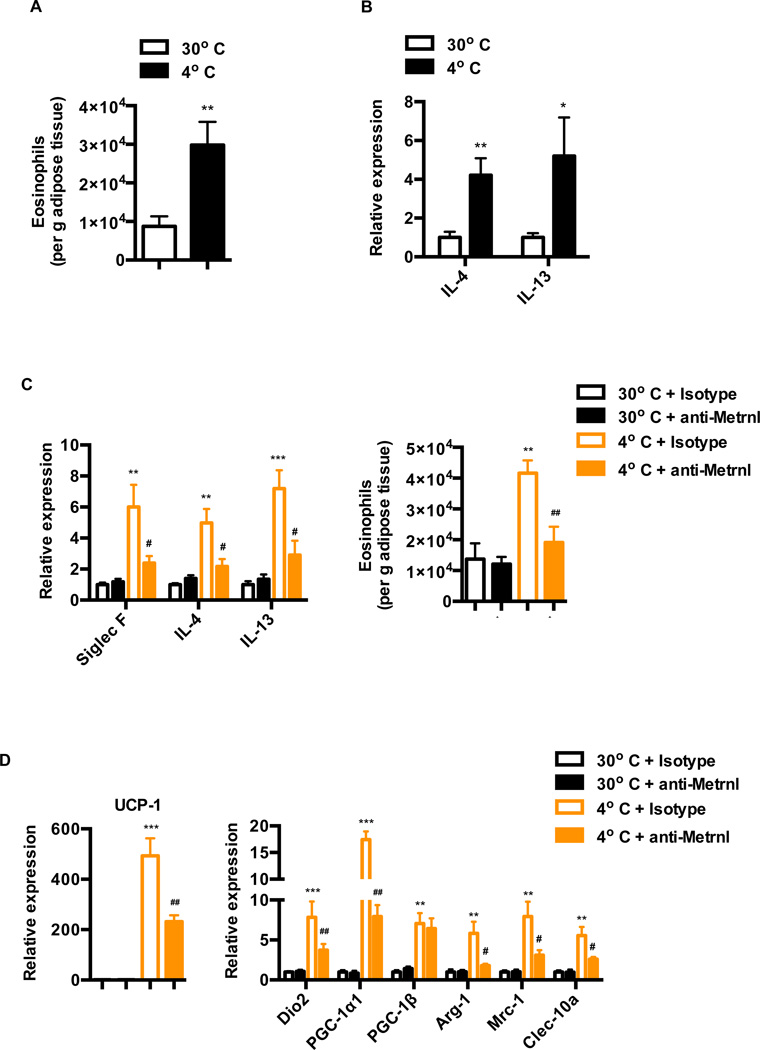
Metrnl expression is induced upon cold challenge in the adipose tissue, resulting in increases in circulating Metrnl levels (Figure 2F–2H). Given that Metrnl induces eosinophil-dependent IL4/IL13 expression in the adipose tissue, we investigated whether cold challenge also promotes an increase in eosinophil numbers and IL4/IL13 expression. Acute exposure (24h) of mice to cold, after being chronically housed at thermo neutral temperatures, promotes a significant increase (~ 3 fold) in the number of eosinophils and a concomitant increase in IL4/IL13 expression in the subQ WAT (Figure 7A and 7B). The increase in adipose tissue eosinophils combined with the previously established role of IL4/IL13 signaling and M2 macrophages in cold-induced thermogenesis (Nguyen et al., 2011), suggests a potential role for Metrnl in cold-induced increases in IL4/IL13 expression. This, in turn, could lead to M2 macrophage activation and induction of thermogenic genes.
Figure 7. Metrnl is required for physiologic adaptation to cold temperatures.
See also Figure S7 (A–B) Mice chronically housed at 30°C were subjected to a 4°C cold challenge for 24 h and (A) number of adipose eosinophils (gated as described in Figure S7F) is shown per g adipose tissue (B) mRNA expression of IL4/IL13 in the subQ WAT. (C–D) Mice chronically housed at 30°C were injected with isotype or Metrnl antibody (i.p.) and moved to cold 6 hrs later. Sub Q WAT was harvested and analyzed for (C) mRNA expression of the indicated genes and number of eosinophils per g adipose tissue at 24hrs post cold-challenge, and (D) markers of genes associated with thermogenic and M2 macrophage activation at 72hrs post cold-challenge. **p<0.001, ***p<0.0001 comparison between mice at 30°C and 4°C or isotype Ab treatment of mice at 30°C and 4°C. #p<0.05, ##p<0.001 comparison between Metrnl Ab treatment of mice at 30°C and 4°C. All data are presented as mean +/− s.e.m
To directly examine the role of Metrnl in cold adaptation, mice housed at thermoneutrality were injected with anti-Metrnl antibody to neutralize Metrnl in vivo, and then exposed to cold 6 hours later. As shown in Figure 7C, the anti-Metrnl antibody dramatically reduced the mRNA expression of Siglec F and IL4/IL13, and the number of eosinophils induced upon cold challenge (acute, 24h), when compared to an isotype or control antibody.
We next investigated the role of endogenous Metrnl in regulating expression of thermogenic genes. With 72 hrs of cold-challenge, antibody neutralization of Metrnl significantly inhibited the expression of genes characteristic of M2 macrophages (Arg-1, Mrc-1, Clec-10a) and adipose thermogenesis (UCP-1, Dio2, PGC-1α) (Figure 7D). For example, Arg-1 mRNA levels are decreased by 70%, and UCP1 mRNA levels are decreased by 55% (Figure 7D). Moderate but significant attenuation of thermogenic and M2 macrophage genes were also observed at 48h post cold-challenge, as evidenced by a 40% reduction in Arg-1 mRNA levels and 30% reduction in UCP-1 mRNA levels (Figure S7B). However, with 24 hr of cold exposure, the induction of thermogenic genes (UCP-1 and PGC-1α) was not altered with anti-Metrnl treatment, although we do observe consistent and significant reduction in expression of Dio2 (Figure S7A). In addition, this cold exposure period did not induce expression M2 macrophage-associated genes (Figure S7A), although we do observe an increase in eosinophil numbers and IL4/IL13 expression at this time-point (Figure 7A and 7B). Taken together, these results indicate that Metrnl is required for a substantial part of host’s adaptive thermogenesis in response to cold challenge. Importantly, these results implicate a physiological role for Metrnl in coordinating an eosinophil-dependent increase in IL4/IL13 expression, alternative macrophage activation and expression of thermogenic genes upon chronic exposure to cold.
DISCUSSION
Using unbiased genetic and proteomic approaches, we report here the identification Metrnl, a protein that is induced upon physiological stimuli such as exercise and cold exposure. This protein is unique in several respects. First, it utilizes a rather unconventional mechanism to stimulate thermogenesis by inducing immune cytokines (IL4/IL13) whose actions are necessary to induce expression of thermogenic genes. The role of M2 macrophages in potentiating cold-induced thermogenesis was recently illustrated by the important work of Chawla and colleagues (Nguyen et al., 2011). This mechanism distinguishes Metrnl from other known polypeptide inducers of thermogenesis, such as Irisin and Fgf-21, which both act directly on adipocytes to stimulate thermogenesis (Bostrom et al., 2012; Fisher et al., 2012; Zhang et al., 2013). Second, in addition to stimulating thermogenesis, Metrnl also promotes a macrophage phenotype associated with the suppression of inflammatory cytokines. Importantly, the ability of Metrnl to recruit and engage immune cell types to promote thermogenesis is demonstrated by three independent approaches used in the present study: use of adenoviral vectors to increase circulating Metrnl, use of localized Metrnl expression by direct injection of these viruses into fat pads, and the systemic injection of recombinant Metrnl-Fc fusion proteins.
Observation of the time course of events taking place after Metrnl expression has allowed delineation of the cascade of processes leading from Metrnl to increase in expression of UCP-1 and other thermogenic genes. It is clear that increases in circulating Metrnl recruits eosinophils into the adipose tissue, which are the major source of the cytokines IL-4 and IL-13. It is possible that Metrnl may induce expression of eosinophil-specific chemokines in the adipose cells, thereby giving circulating Metrnl highly localized actions. Abrogation of IL4/IL13 signaling abolishes the increases in alternative macrophage activation, catecholamine secretion and expression of beige fat thermogenic genes. These studies further illustrate a highly regulated pathway to brown/beige fat thermogenesis that is parallel to the classical SNS pathway. Importantly, the attenuation of cold-induced thermogenic response upon blocking Metrnl actions in vivo clearly attributes a physiological role for Metrnl in long-term adaptation to cold temperatures.
Given the ability of Metrnl to induce alternative macrophage activation and brown/beige fat thermogenesis, its therapeutic potential in metabolic diseases is obvious. The recombinant Metrnl protein used here hints at that potential but other proteins with better pharmacological properties will be required. The important role of eosinophils and M2 macrophages in other repair processes, including those in damaged muscle (Arnold et al., 2007; Heredia et al., 2013) and liver (Goh et al., 2013), suggests additional interesting therapeutic applications.
EXPERIMENTAL PROCEDURES
Animals and in vivo experiments
All animal experiments were performed according to procedures approved by Beth Israel Deaconess Medical Center (BIDMC) Institutional Animal Care and Use Committee (IACUC). Wild-type C57/BL6J, STAT6−/− mice (BL/6), BALB/cJ and ΔdblGATAmice on a BALB/cJ background were purchased from Jackson Laboratory. The Myo-PGC-1α4 transgenic mice have been previously described (Ruas et al., 2012). Mice heterozygous for Metrnl were purchased from Taconic and were bred in-house to obtain Metrnl KO animals. Additional information can be found in Extended Experimental Procedures.
Human and mouse exercise training program
The experimental procedures and possible risks with the study were explained to all subjects, who gave written informed consent before participation. The Human Research Ethics Committee of RMIT University approved the study. Human concurrent exercise protocol: Eight healthy male subjects [age 19.1 ± 1.4 yr, body mass 78.1 ± 15.6 kg, peak oxygen uptake (VO2peak) 46.7 ± 4.4 mL/kg/min, leg extension one repetition maximum (1-RM) 130 ± 14 kg; values are mean ± SD] completed a single bout of resistance exercise and cycling (concurrent exercise) after 48 h diet and exercise control. In brief, subjects performed eight sets of five repetitions leg extension at ~ 80% 1RM and then rested for 15 min before completing 30 min of continuous cycling at ~70% of individual VO2peak. Skeletal muscle biopsies from the vastus lateralis were obtained at rest, and 1 and 4 h post-exercise. Mouse downhill running exercise: mice were randomly placed into sedentary (N=9) or downhill exercise groups (N=10). Mice ran at -20 degrees at 15m/m for 60 minutes after a 5 min warm up at 10m/m. Mice were sacrificed 6 hours after the exercise bout. For mouse endurance exercise training, mice were housed and exercised as described previously (Chinsomboon et al., 2009).
Statistical analysis
All data are presented as mean +/− S.E.M. and analysed using Prism (Graphpad). Statistical significance was determined using the Student’s t-test. A P value of <0.05 was considered to be statistically significant, and is presented as * (P,0.05), ** (P,0.01), or *** (P,0.001).
Supplementary Material
Research Highlights.
Identification of Meteorin-like (Metrnl) as a PGC1α4 regulated hormone
Metrnl is induced upon exercise and cold exposure
Metrnl promotes browning by inducing cytokines IL4/IL13 and M2 macrophage activation
Metrnl is required for long-term adaptations to cold temperatures
ACKNOWLEDGEMENTS
We thank members of the Spiegelman laboratory for useful discussions and suggestions on the manuscript. This work was funded by National Institutes of Health grant (DK061562) grant to B.M.S. and a grant from the JPB foundation. R.R.R. is supported by Irvington Institute Fellowship Program of the Cancer Research Institute. J.Z.L. is supported by the American Diabetics Association/Canadian Diabetics Association (ADA/CDA) joint post-doctoral fellowship. B.M.S. is a consultant and shareholder in Ember Therapeutics, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. The Journal of biological chemistry. 2005;280:19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into anti-inflammatory macrophages to support myogenesis. The Journal of experimental medicine. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynard T, Vieira-Potter VJ, Valentine RJ, Woods JA. Exercise training effects on inflammatory gene expression in white adipose tissue of young mice. Mediators of inflammation. 2012;2012:767953. doi: 10.1155/2012/767953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, White NM, Ma JT, Zhou J, Qi N, Westcott D, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. Journal of cell science. 1992;103(Pt 4):931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nature reviews Immunology. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- Goh YP, Henderson NC, Heredia JE, Red Eagle A, Odegaard JI, Lehwald N, Nguyen KD, Sheppard D, Mukundan L, Locksley RM, et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9914–9919. doi: 10.1073/pnas.1304046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley JA. Exercise as a therapeutic intervention for the prevention and treatment of insulin resistance. Diabetes/metabolism research and reviews. 2004;20:383–393. doi: 10.1002/dmrr.505. [DOI] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K. FGF21 is an Akt-regulated myokine. FEBS letters. 2008;582:3805–3810. doi: 10.1016/j.febslet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanlidis G, Karamitri A, Docherty K, Hazlerigg DG, Lomax MA. C/EBPbeta reprograms white 3T3-L1 preadipocytes to a Brown adipocyte pattern of gene expression. The Journal of biological chemistry. 2007;282:24660–24669. doi: 10.1074/jbc.M703101200. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annual review of immunology. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Mathai AS, Bonen A, Benton CR, Robinson DL, Graham TE. Rapid exercise-induced changes in PGC-1alpha mRNA and protein in human skeletal muscle. Journal of applied physiology. 2008;105:1098–1105. doi: 10.1152/japplphysiol.00847.2007. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annual review of pathology. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacy PJ, Webster J, Garrow JS. Exercise and obesity. Sports medicine. 1986;3:89–113. doi: 10.2165/00007256-198603020-00002. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, et al. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Selman C. Physical activity and resting metabolic rate. The Proceedings of the Nutrition Society. 2003;62:621–634. doi: 10.1079/PNS2003282. [DOI] [PubMed] [Google Scholar]
- Wei K, Kuhnert F, Kuo CJ. Recombinant adenovirus as a methodology for exploration of physiologic functions of growth factor pathways. Journal of molecular medicine. 2008;86:161–169. doi: 10.1007/s00109-007-0261-7. [DOI] [PubMed] [Google Scholar]
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise Induces Hippocampal BDNF through a PGC-1alpha/FNDC5 Pathway. Cell metabolism. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY, Tzan K, Wang A, Parthasarathy S, He G, et al. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. American journal of physiology Regulatory, integrative and comparative physiology. 2011;300:R1115–R1125. doi: 10.1152/ajpregu.00806.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Coulter A, Rim JS, Koza RA, Kozak LP. Transcriptional synergy and the regulation of Ucp1 during brown adipocyte induction in white fat depots. Molecular and cellular biology. 2005;25:8311–8322. doi: 10.1128/MCB.25.18.8311-8322.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. The Journal of experimental medicine. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, Qi L, Zhang M, Wang X, Cui T, et al. Irisin Stimulates Browning of White Adipocytes through Mitogen-Activated Protein Kinase p38 MAP Kinase and ERK MAP Kinase Signaling. Diabetes. 2013 doi: 10.2337/db13-1106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.