Abstract
Designing deep learning based methods with medical images has always been an attractive area of research to assist clinicians in rapid examination and accurate diagnosis. Those methods need a large number of datasets including all variations in their training stages. On the other hand, medical images are always scarce due to several reasons, such as not enough patients for some diseases, patients do not want to allow their images to be used, lack of medical equipment or equipment, inability to obtain images that meet the desired criteria. This issue leads to bias in datasets, overfitting, and inaccurate results. Data augmentation is a common solution to overcome this issue and various augmentation techniques have been applied to different types of images in the literature. However, it is not clear which data augmentation technique provides more efficient results for which image type since different diseases are handled, different network architectures are used, and these architectures are trained and tested with different numbers of data sets in the literature. Therefore, in this work, the augmentation techniques used to improve performances of deep learning based diagnosis of the diseases in different organs (brain, lung, breast, and eye) from different imaging modalities (MR, CT, mammography, and fundoscopy) have been examined. Also, the most commonly used augmentation methods have been implemented, and their effectiveness in classifications with a deep network has been discussed based on quantitative performance evaluations. Experiments indicated that augmentation techniques should be chosen carefully according to image types.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Medical image interpretations are mostly performed by medical professionals like clinicians and radiologists. However, the variations among different experts and complexities of medical images make it very difficult for the experts to diagnose diseases accurately all the time. Thanks to computerized techniques, the tedious image analysis task can be performed by semi-/fully-automatically, and they help the experts to make objective, rapid and accurate diagnoses. Therefore, designing deep learning based methods with medical images has always been an attractive area of research (Tsuneki 2022; van der Velden et al. 2022; Chen et al. 2022a). Particularly, deep Convolutional Neural Network (CNN) architectures have been used for several processes (e.g., image segmentation, classification, registration, content-based image retrieval, identification of disease severity, and lesion/tumor/tissue detection, etc.) in some medical diagnosis fields like eye, breast, brain, and lung (Yu et al. 2021). Although CNNs can produce results with higher performance compared to the results of traditional machine learning based methods, improving their generalization abilities and developing robust models are still significant challenging issues. Because training data sets should be constructed with a large number of images including all variations (i.e., heterogeneous images) to provide the high generalization ability and robustness of those architectures. However, the number of medical images is usually limited. The data scarcity problem can be due to not enough patients for some diseases, patients do not want to allow their images to be used, lack of medical equipment or equipment, inability to obtain images that meet the desired criteria, inability to access images of patients living in different geographical regions or of different races.
Another important issue is that, even if enough images are acquired, labeling them (unlike natural image labeling which is relatively easy) is not easy, requires certain domain knowledge of medical professionals, and is time-consuming. Also, legal issues and privacy are other concerns in the labeling of medical images. Therefore, imbalanced data causing overfitting, biased and inaccurate results is a significant challenge that should be handled while developing deep network based methods.
To overcome these challenges by using balanced number of images and expanding training datasets automatically, image augmentation methods are used. Thanks to the augmentation methods, deep network architectures’ learning and generalization ability can be improved, and the desired robustness property of the networks can be provided (Sect. 2). However, various augmentation techniques have been applied with different types of images in the literature. It is not clear which data augmentation technique provides more efficient results for which image type since different diseases are handled, different network architectures are used, and these architectures are trained and tested with different numbers of data sets in the literature (Sect. 2). According to the segmentation or classification results presented in the literature, it is not possible to understand or compare the impacts or contributions of the used augmentation approaches on the results, and to decide the most appropriate augmentation method.
In the literature, researchers have presented some surveys about augmentation methods. However, they have focused on the augmentation methods applied either;
-
(i)
with a specific type of images like natural images (Khosla and Saini 2020), mammography images (Oza et al. 2022), Computed Tomography (CT), and Magnetic Resonance (MR) images (Chlap et al. 2021) acquired with different imaging techniques and have different properties.
-
(ii)
to improve the performance of specific operations like polyp segmentation (Dorizza 2021) and text classification (Bayer et al. 2021).
-
(iii)
with a specific technique, such as Generative Adversarial Network (GAN) (Chen et al. 2022b), erasing and mixing (Naveed 2021).
In this work, a more comprehensive range of image types has been handled. Also, a more in-depth analysis has been performed through the implementation of common augmentation methods with the same data sets for objective comparisons, and evaluations based on quantitative results. According to our knowledge, there is no (i) comprehensive review that covers a greater range of medical imaging modalities and applications than articles in the literature, and (ii) any work on quantitative evaluations of the augmentation methods applied with different medical images to determine the most appropriate augmentation method.
To fill this gap in the literature, in this paper, the augmentation techniques that have been applied to improve performances of deep learning-based diagnosis of diseases in different organs (brain, lung, breast, and eye) using different imaging modalities (MR, CT, mammography, and fundoscopy) have been examined. The chosen imaging modalities are widely used in medicine for many applications such as classification of brain tumors, lung nodules, and breast lesions (Meijering 2020). Also, in this study, the most commonly used augmentation methods have been implemented using the same datasets. Additionally, to evaluate the effectiveness of those augmentation methods in classifications from the four types of images, a classifier model has been designed and classifications with the augmented images for each augmentation method have been performed with the same classifier model. The effectiveness of the augmentation methods in the classifications has been discussed based on quantitative results. This paper presents a novel comprehensive insight into the augmentation methods proposed for diverse medical images by considering popular medical research problems altogether.
The key features of this paper are as follows:
-
1.
The techniques used for augmentation of brain MR images, lung CT images, breast mammography images, and eye fundus images have been reviewed.
-
2.
The papers published between 2020 and 2022 and mainly collected by Springer, IEEE Xplore, and ELSEVIER, have been reviewed, and a systematic and comprehensive review and analyses of the augmentation methods have been carried out.
-
3.
Commonly used augmentations in the literature have been implemented with four different medical image modalities.
-
4.
For comparisons of the effectiveness of the augmentation methods in deep learning-based classifications, the same network architecture and expanded data sets with the augmented images from each augmentation methods have been used.
-
5.
Five evaluation metrics have been used, and the results provided from each classification have been evaluated with the same evaluation metrics.
-
6.
The best augmentation method for different imaging modalities showing different organs has been determined according to quantitative values.
We believe that the results and conclusions presented in this work will be helpful for researchers to choose the most appropriate augmentation technique in developing deep learning-based approaches to diagnose diseases with a limited number of image data. Also, this work can be helpful for researchers interested in developing new augmentation methods for different types of images. This manuscript has been structured as follows: A detailed review of the augmentation approaches applied with the medical images handled in this work between 2020 and 2022 is presented in the second section. The data sets and methods implemented in this work are explained in the third section. Evaluation metrics and quantitative results are given in the fourth section. Discussions are given in the fifth section, and conclusions are presented in the sixth section.
2 Related works
In this section, the methods used in the literature for augmentation of brain MR images, lung CT images, breast mammography images, and eye fundus images are presented.
2.1 Augmentation of brain MR images
In the literature, augmentation of brain MR images have been performed with various methods such as rotation, noise addition, shearing, and translation to increase number of images and improve performances of several tasks (Table 1). For example, augmentation has been provided by noise addition and sharpening to increase the accuracy of tumor segmentation and classification in a study (Khan et al. 2021). In another study, augmented images have been obtained by translation, rotation, random cropping, blurring, and noise addition to obtain high performance from the method applied for age prediction, schizophrenia diagnosis, and sex classification (Dufumier et al. 2021). In different studies, random scaling, rotation, and elastic deformation have been applied to increase tumor segmentation accuracy (Isensee et al. 2020; Fidon et al. 2020). Although mostly those techniques have been preferred in the literature, augmentation has also been provided by producing synthetic images. To generate synthetic images, Mixup method, which combines two randomly selected images and their labels, or modified Mixup, or GAN structures have been used (Table 1).
For instance, a modified Mixup is a voxel-based approach called TensorMixup, which combines two image patches using a tensor, and has been applied (Wang et al. 2022) to boost the accuracy of tumor segmentation. In a recent study, a GAN based augmentation has been used for cerebrovascular segmentation (Kossen et al. 2021).
All methods applied for augmentation of brain MR images in the publications reviewed in this work and the details in those publications are presented in Table 1. Those augmentation methods have been used in the applications developed for different purposes. In those applications, different network architectures, datasets, and numbers of images have been used, and also their performances have been evaluated with different metrics. High performances have been obtained from those applications according to the reported results. However, the most effective and appropriate augmentation method used in them with the brain MR images is not clear due to those differences. Also, it is not possible to determine augmentation methods’ contributions to those performances according to the presented results in those publications.
2.2 Augmentation of lung CT images
In computerized methods being developed to assist medical professionals in various tasks [e.g., nodule or lung parenchyma segmentation, nodule classification, prediction of malignancy level, disease diagnosis like coronavirus (covid-19), etc.], augmentations of CT lung images are commonly provided by traditional techniques like rotation, flipping, and scaling (Table 2). For example, image augmentation has been provided using flipping, translation, rotation, and brightness changing to improve automated diagnosis of covid-19 by Hu et al. (2020). Similarly, a set of augmentations (Gaussian noise addition, cropping, flipping, blurring, brightness changing, shearing, and rotation) has been applied to increase the performance of covid-19 diagnosis by Alshazly et al. (2021). Also, several GAN models have been used to augment lung CT images by generating new synthetic images (Table 2). While doing a GAN based augmentation of lung CT scans, researchers usually tend to generate the area showing lung nodules in the whole image. Because the lung nodules are small and therefore it is more cost-effective than generating a larger whole image or background area. For instance, guided GAN structures have been implemented to produce synthetic images showing the area with lung nodules by Wang et al. (2021). Nodules’ size information and guided GANs have been used to produce images with diverse sizes of lung nodules by Nishio et al. (2020).
All methods applied for augmentation of breast mammography images in the publications reviewed in this study and the details in those publications are presented in Table 2. Those augmentation methods have been applied in the applications developed for different purposes. In those applications, different network models, data sets and number of images have been used, and also their performances have been evaluated with different metrics. High performances have been obtained from those applications according to the reported results. However, the most effective and appropriate augmentation method used in them with the lung CT images is not clear due to those differences. Also, it is not possible to determine augmentation methods’ contributions to those performances according to the presented results in those publications.
2.3 Augmentation of breast mammography images
Generally, augmentation methods with mammography images have been applied to improve automated recognition, segmentation, and detection of breast lesions. Because failures in the detection or identification of the lesions lead to unnecessary biopsies or inaccurate diagnoses. In some works, in the literature, augmentation methods have been applied on extracted positive and negative patches instead of the whole image (Karthiga et al. 2022; Zeiser et al. 2020; Miller et al. 2022; Kim and Kim 2022; Wu et al. 2020b). Positive patches are extracted based on radiologist marks while negative patches are extracted as random regions from the same image. Also, refinement and segmentation of the lesion regions before the application of an augmentation method is the most preferred way to provide good performance from the detection and classification of the lesions. For instance, image refinement has been provided by noise reduction and resizing steps, and then segmentation and augmentation steps have been applied (Zeiser et al. 2020). Similarly, noise and artifacts have been eliminated before the segmentation of the images into non-overlapping parts and augmentation (Karthiga et al. 2022). Augmentation of patches or whole mammography images have usually been performed by scaling, noise addition, mirroring, shearing, and rotation techniques or using several combinations of them (Table 3). For instance, a set of augmentation methods (namely mirroring, zooming, and resizing) has been applied to improve automated classification of images into three categories (as normal, malignant, and benign) (Zeiser et al. 2020). Also, those techniques have been used together with GANs to provide augmentations with new synthetic images. For instance, flipping and deep convolutional GAN has been combined for binary classifications of images (as normal and with a mass) by Alyafi et al. (2020). Only GAN based augmentations have also been used. For instance, contextual GAN, which generates new images by synthesizing lesions according to the context of surrounding tissue, has been applied to improve binary classification (as normal and malignant) by Wu et al. (2020b). In another work (Shen et al. 2021), both contextual and deep convolutional GAN have been used to generate synthetic lesion images with margin and texture information and achieve improvement in the detection and segmentation of lesions.
All methods applied for augmentation of breast mammography images in the publications reviewed in this work and the details in those publications are presented in Table 3. Those augmentation methods have been applied in the applications developed for different purposes. In those applications, different network architectures, datasets and number of images have been used, and also their performances have been evaluated with different metrics. High performances have been obtained from those applications according to the reported results. However, the most effective and appropriate augmentation method used in them with breast mammography images is not clear due to those differences. Also, it is not possible to determine augmentation methods’ contributions to those performances according to the presented results in those publications.
2.4 Augmentation of eye fundus images
Eye fundus images show various important features, which are symptoms of eye diseases, such as abnormal blood veins (neovascularization), red/reddish spots (hemorrhages, microaneurysms), and bright lesions (soft or hard exudates). Those features are used in deep learning based methods being developed for different purposes like glaucoma identification, classification of diabetic retinopathy (DR), and identification of DR severity. Commonly used augmentation techniques with eye fundus images to boost the performance of deep learning based approaches are rotation, shearing, flipping, and translation (Table 4). For example, in a study (Shyamalee and Meedeniya 2022), a combined method including rotation, shearing, zooming, flipping, and shifting has been applied for glaucoma identification. Another combined augmentation including shifting and blurring has been used by Tufail et al. (2021) before binary classification (as healthy and nonhealthy) and multi-class (5 classes) classification of images to differentiate different stages of DR. In a different study (Kurup et al. 2020), combination of rotation, translation, mirroring, and zooming has been used to achieve automated detection of malaria retinopathy. Also, pixel intensity values have been used in some augmentation techniques since fundus images are colored images. For instance, to achieve robust segmentation of retinal vessels, augmentation has been provided by a channel-wise random gamma correction (Sun et al. 2021). The authors have also applied a channel-wise vessel augmentation using morphological transformations. In another work (Agustin et al. 2020), to improve the robustness of DR detection performance, image augmentation has been performed by zooming and a contrast enhancement process known as Contrast Limited Adaptive Histogram Equalization (CLAHE). Augmentations have also been provided by generating synthetic images using GAN models. For instance, conditional GAN (Zhou et al. 2020) and deep convolutional GAN (Balasubramanian et al. 2020) based augmentations have been used to increase the performance of the methods developed for automatic classifications of DR according to its severity level. In some works, retinal features (such as lesion and vascular features) have been developed and added to new images. For instance, NeoVessel (NV)-like structures have been synthesized in a heuristic image augmentation (Ara´ujo et al. 2020) to improve detection of proliferative DR which is an advanced DR stage characterized by neovascularization. In this augmentation, different NV kinds (trees, wheels, brooms) have been generated depending on the expected shape and location of NVs to synthesize new images.
All methods applied for augmentation of eye fundus images in the publications reviewed in this study and the details in those publications are presented in Table 4. Those augmentation methods have been applied in the applications developed for different purposes. In those applications, different network structures, data sets and number of images have been used, and also their performances have been evaluated with different metrics. High performances have been obtained from those applications according to the reported results. However, the most effective and appropriate augmentation method used in them with eye fundus images is not clear due to those differences. Also, it is not possible to determine augmentation methods’ contributions to those performances according to the presented results in those publications.
2.5 Commonly applied augmentation methods
2.5.1 Rotation
Rotation-based image augmentations are provided by rotating an image by concerning its original position. The rotation uses a new coordinate system and retains the same relative positions of the pixels of an image. It can be rightward or leftward across an axis within the range of [1°, 359°]. Image labels may not always be preserved if the degree of rotation increases. Therefore, the safety of this augmentation technique depends on the rotation degree. Although rotation transformation is not safe on images showing 6 and 9 in digit recognition applications, it is generally safe on medical images.
2.5.2 Flipping
Flipping is a technique generating a mirror image from an image. The pixel’s positions are inverted by concerning one of the two axes (for a two-dimensional image). Although it can be applied with vertical or/and horizontal axes, vertical flipping is rarely preferred since the bottom and top regions of an image may not always be interchangeable (Nalepa et al. 2019).
2.5.3 Translation
Images are translated along an axis using a translation vector. This technique preserves the relative positions between pixels. Therefore, translated images provide prevention of positional bias (Shorten and Khoshgoftaar 2019) and the models do not focus on properties in a single spatial location (Nalepa et al. 2019).
2.5.4 Scaling
Images are scaled along different axes with a scaling factor, which can be different or the same for each axis. Especially, scaling changings can be interpreted as zoom out (when the scaling factor is less than 1) or zoom in (when the scaling factor is greater than 1).
2.5.5 Shearing
This transformation slides one edge of an image along the vertical or horizontal axis, creating a parallelogram. A vertical direction shear slides an edge along the vertical axis, while a horizontal direction shear slides an edge along the horizontal axis. The amount of the shear is controlled by a shear angle.
2.5.6 Augmentation using intensities
This augmentation is provided by modification of contrast or brightness, blurring, intensity normalization, histogram equalization, sharpening, and addition of noise. If the preferred type of noise is Gaussian, intensity values are modified by sampling a Gaussian distribution randomly. If it is salt-and-pepper type of noise, pixel values are set randomly to white and black. Uniform noise addition is performed by modification of pixel values using randomly sampling a uniform distribution.
2.5.7 Random cropping and random erasing
Augmentation by random cropping is applied by taking a small region from an original image and resizing it to match the dimensions of the original image. Therefore, this augmentation can also be called scaling or zooming. Augmentation by random erasing is performed by randomly eliminating image regions.
2.5.8 Color modification
Image augmentation by color modification is performed in several ways. For example, an RGB-colored image is stored as arrays, which correspond to 3 color channels representing levels of red, green, and blue intensity values. Because of this, the color of the image can be changed without losing its spatial properties. This operation is called color space shifting, and it is possible to perform the operation in any number of spaces because the original color channels are combined in different ratios in every space. Therefore, a way to augment an RGB-colored image by color modification is to put the pixel values in the color channels of the image into a histogram and then manipulate them using filters to generate new images by changing the color space characteristics. Another way is to take 3 shifts (integer numbers) from 3 RGB filters and add each shift into a channel in the original image. Also, image augmentation can be provided by updating values of hue, saturation, and value components, isolating a channel (such as a blue, red, or green color channel), and converting color spaces into one another to augment images. However, converting a color image to its grayscale version should be performed carefully because this conversion reduces performance by up 3% (Chatfield et al. 2014).
2.5.9 GAN based augmentation
GANs are generative architectures constructed with a discriminator to separate synthetic and true images and a generator to generate synthetic realistic images. The main challenging issues of GAN based image augmentations are generating images with high quality (i.e., high-resolution, clear) and maintaining training stability. To overcome those issues, several GAN variants were developed such as conditional, deep convolutional, and cycle GAN. Conditional GAN adds parameters (e.g., class labels) to the input of the generator to control generated images and allows conditional image generation from the generator. Deep convolutional GAN uses deep convolution networks in the GAN structure. Cycle GAN is a conditional GAN and translates images from one domain to another (i.e., image-to-image translation), which modifies input images into novel synthetic images that meet specific conditions. Wasserstein GAN is the structure that is constructed with the loss function designed with the earth-mover distance to increase the stability of the model. Style GAN and progressively trained GAN add coarse to fine details and learn features from training images to synthesize new images.
3 Materials and methods
3.1 Materials
In this work, publicly available brain MR, lung CT, breast mammography, and eye fundus images have been used.
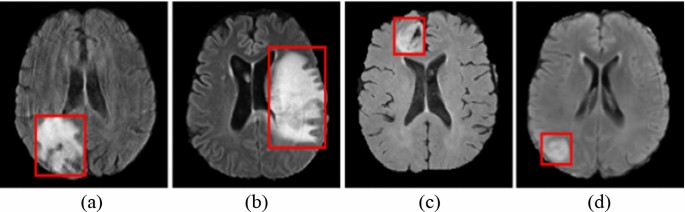
Brain MR images have been provided from Multimodal Brain Tumor Image Segmentation Challenge (BRaTS) (Bakas et al. 2018). The data for each patient includes four diverse models of MR images which are Fluid-Attenuated Inversion Recovery (FLAIR) images, T2-weighted MR images, T1-weighted MR images with Contrast Enhancement (T1-CE), and T1-weighted MR images. Different modal images provide different information about tumors. In this work, FLAIR images have been used. Example FLAIR images showing High-Grade Glioma (HGG) (Fig. 1a, b) and Low-Grade Glioma (Fig. 1c, d) (LGG) are presented in Fig. 1. The BRaTS database contains 285 images including HGG (210 images) and LGG (75 images) cases.
Brain MR scans showing HGG (a, b) and LGG (c, d) (Bakas et al. 2018)
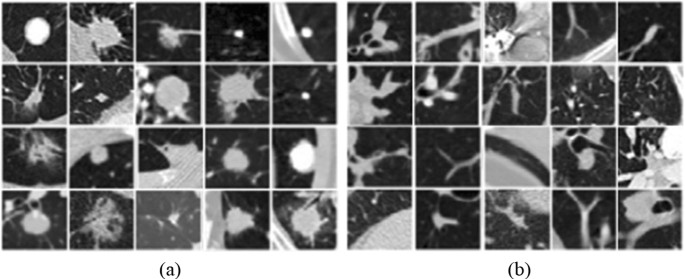
Lung images have been provided from Lung Image Database Consortium and Image Database Resource Initiative (LIDC-IDRI) database (Armato et al. 2011; McNitt-Gray et al. 2007; Wang et al. 2015) by the LUNA16 challenge (Setio et al. 2017). In the database, there are 1018 CT scans which belong to 1010 people. Those scans were annotated by four experienced radiologists. The nodules that were marked by those radiologists were divided into three groups which are “non-nodule ≥ 3 millimeters”, “nodule < 3 millimeters”, and “nodule ≥ 3 millimeters” (Armato et al. 2011). The images having a slice thickness greater than 3 mm were removed due to their usefulness according to the suggestions (Manos et al. 2014; Naidich et al. 2013). Also, the images having missing slices or not consistent slice spacing were discarded. Therefore, there are a total of 888 images extracted from 1018 cases. Example images with nodules (Fig. 2a) and without nodules (Fig. 2b) have been presented in Fig. 2.
Example images showing nodules (a) and non-nodule regions (b) (Armato et al. 2011)
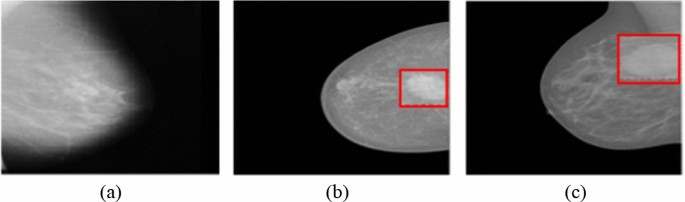
Breast mammography images have been provided from the INbreast database (Moreira et al. 2012). It contains 410 images, of which only 107 images show breast masses, and the total number of mass lesions is 115 since an image may contain more than one lesion. The number of benign and malignant masses is 52 and 63, respectively. Example mammography images without any mass (Fig. 3a), with a benign mass (Fig. 3b), and malignant mass (Fig. 3c) lesions have been presented in Fig. 3.
Mammography images without mass (a), a benign mass (b), and a malignant mass (c) (Moreira et al. 2012)
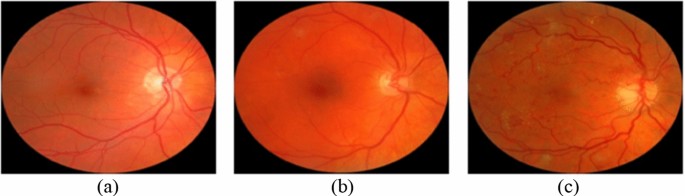
Eye fundus images have been provided from the Messidor database (Decencière et al. 2014) including 1200 images categorized into three classes (0-risk, 1-risk, and 2-risk) according to the diabetic macular edema risk level. The number of images in the categories is 974, 75, and 151, respectively. Example images showing macular edema with 0-risk (Fig. 4a), 1-risk (Fig. 4b), and 2-risk (Fig. 4c) have been presented in Fig. 4.
Eye fundus images showing macular edema with 0-risk (a), 1-risk (b), and 2-risk (c) (Decencière et al. 2014)
3.2 Implemented augmentation methods
In this work, commonly used augmentation methods based on transformations and intensity modifications have been implemented to see and compare their effects on the performance of deep learning based classifications from MR, CT, and mammography images. Also, a method based on a color modification to augment colored eye fundus images has been applied. Those methods are explained below.
-
1st Method In this method, a shearing angle, which is selected randomly within the range [− 15°, 15°], is used and a shearing operation is applied within the x and y-axis. This operation is repeated 10 times with 10 different shearing angles. By this method, 10 images are generated from an original image.
-
2nd Method In this method, translation operation is applied on the x and y-axis. This operation is repeated 10 times by using different values sampled randomly within the range [− 15, 15]. By this method, 10 images are generated from an original image.
-
3rd Method Rotation angles are selected randomly within the range [− 25°, 25°] and clockwise rotations are applied using 10 different angle values. 10 images are generated from an original image by this method.
-
4th Method In this method, noise addition is applied by I = I + (rand(size(I)) − FV) × N, where the term FV refers to a fixed value, N refers to noise and I refers to an input image. The noise to be added is generated using a Gaussian distribution. The variance of the distribution is computed from the input images while the mean value is set to zero. In this study, three different fixed values (0.3, 0.4, and 0.5) are used and 3 images are generated from an input image.
-
5th Method In this method, noise is added to each image with 3 density values (0.01, 0.02, and 0.03) and a salt-and-pepper type noise is used. Therefore, 3 images are generated from an input image by this method.
-
6th Method In this method, augmentation is provided by salt-and-pepper type noise addition (5th method) followed by shearing (1st method). 30 images are generated from an input image by this method.
-
7th Method In this method, augmentation is provided by Gaussian noise addition (4th method) followed by rotation (3rd method). 30 images are generated from an input image by this method.
-
8th Method In this augmentation method, clockwise rotation and then translation operations are applied. Rotation angles are selected randomly within the range [− 25°, 25°] and translation is applied on the x and y-axis by using different values sampled randomly within the range [− 15, 15]. This successive operation is applied 10 times, and 10 images are generated from an input image by this method.
-
9th Method In this method, translation is applied followed by the shearing. The translation step is applied on the x and y-axis with different values sampled randomly within the range [− 15, 15]. In the shearing step, an angle is selected randomly within the range [− 15°, 15°], and shearing is applied within the x and y-axis. The subsequent operations are applied 10 times and 10 images are generated from an input image by this method.
-
10th Method In this method, three operations (translation, shearing, and rotation) are applied subsequently. Implementation of the translation and shearing operations are performed as explained in the 9th method. In the rotation step, a random angle is selected within the range [− 25°, 25°] clockwise rotation is applied. The successive operations are applied 10 times, and 10 images are generated from an input image by this method.
-
11th Method Color shifting, sharpening, and contrast changing have been combined in this method. Color shifting has been applied by taking 3 shifts (integer numbers) from 3 RGB filters and adding each shift into one of the 3 color channels in the original images. Sharpening has been changed by blurring the original images and subtracting the blurred images from the original images. A Gaussian filter, whose variance value is 1, has been used for the blurring process. Contrast changing has been applied by scaling the original images linearly between i1 and i2 (i1 < i2), which will be the maximum and minimum intensities in the augmented images, and then mapping the original images’ pixels with intensity values higher than i2 (or lower than i1) to 255 (or 0). Therefore, 3 images are generated from an input image by this method.
3.3 Classification
In this work, to compare the effectiveness of the augmentation approaches in classifications, a CNN-based classifier has been used. In this step, the original images taken from the public databases (Sect. 3.1) have been used in construction of training and testing datasets. However, the databases have imbalanced class distributions. Therefore, to obtain unbiased classification results, an equal number of images have been taken from the databases for the classes. Those balanced numbers of images have been used with the augmentation methods and the generated images from them have been added into the data sets. 80% of the total data has been used in the training stage, the rest 20% of the data has been used in the testing stage. It should be reminded here that the goal in this study is to evaluate the augmentation techniques used with different types of images in improving the classification rather than evaluations of classifier models. Therefore, ResNet101 architecture has been chosen and implemented in this work. The reason to choose this architecture is to utilize its advantage in addressing the gradient vanishing issue and high learning ability (He et al. 2016). Pre-training of networks by using large datasets [such as ImageNet (Russakovsky et al. 2015)] and then fine-tuning the networks for target tasks having less training data has been commonly applied in recent years. Pre-training has provided superior results on many tasks, such as action recognition (Simonyan and Zisserman 2014; Carreira and Zisserman 2017), image segmentation (He et al. 2017; Long et al. 2015), and object detection (Ren et al. 2015; Girshick 2015; Girshick et al. 2014). Therefore, transfer learning from a model trained using ImageNet has been used in this work for fine-tuning of the network. Optimization has been provided by Adam, and activation has been provided by ReLU. The initial learning rate has been chosen as 0.0003, and cross-entropy loss has been computed to update the weights in the training phase. The number of epochs and mini-batch size have been set as 6 and 10, respectively.
4 Results
Classification results have been evaluated by using these five measurements: specificity, accuracy, sensitivity, Matthew Correlation Coefficient (MCC), and F1-score computed by:
where FP, FN, TN, and TP refer to false positives, false negatives, true negatives, and true positives, respectively. The number of images obtained from each augmentation method for each augmentation approach is given in Table 5.
The augmentation methods and quantitative results obtained by classifications of HGG and LGG cases from brain MR images have been presented in Table 6.
Lung CT scans have been classified as a pulmonary nodule or a non-nodule with the same classifier. 1004 lung nodules of which 450 positive candidates provided by the LIDC-IDRI have been used. After generation more positive candidate nodules with the augmentation methods, equal number of negative and positive candidates have been used in the classifications. The augmentation methods and quantitative results of classifications have been presented in Table 7.
The breast mammography images have been classified into three groups as malignant, benign, and normal. The augmentation methods and quantitative results of classifications have been presented in Table 8.
Unlike from MR, CT, and mammography images, the eye fundus images are colored. Therefore, in addition to those 10 augmentation methods applied for grayscale images, the 11th method has also been used for augmentation of this images. The augmentation methods and quantitative results of the classifications into three classes according to macular edema risk level have been presented in Table 9.
5 Discussions
To overcome the imbalance distribution and data scarcity problems in deep learning based approaches with medical images, data augmentations are commonly applied with various techniques in the literature (Sect. 2). In those approaches, different network architectures, different types of images, parameters, and functions have been used. Although they achieved good performances with augmented images (Tables 1, 2, 3 and 4), the results presented in the publications do not indicate on which aspects of the approaches contributed the most. Although augmentation methods are applied as pre-processing steps and therefore have significant impacts on the remaining steps and overall performance, their contributions are not clear. Because of this, it is not clear which data augmentation technique provides more efficient results for which image type. Therefore, in this study, the effects of augmentation methods have been investigated to determine the most appropriate method for automated diagnosis from different types of medical images. This has been performed by these steps: (i) The augmentation techniques used to improve the performance of deep learning based diagnosis of diseases in different organs (brain, lung, breast, and eye) have been reviewed. (ii) The most commonly used augmentation methods have been implemented with four types of images (MR, CT, mammography, and fundoscopy). (iii) To evaluate the effectiveness of those augmentation methods in classifications, a classifier model has been designed. (iv) Classifications using the augmented images for each augmentation method have been performed with the same classifier and datasets for fair comparisons of those augmentation methods. (v) The effectiveness of the augmentation methods in classifications have been discussed based on the quantitative results.
It has been observed from the experiments in this work that transformation-based augmentation methods are easy to implement with medical images. Also, their effectiveness depends on the characteristics (e.g., color, intensity, etc.) of the images, and whether all significant visual features according to medical conditions exist in the training sets. If most of the images in the training sets have similar features, then there is a risk of constructing a classifier model with overfitting and less generalization capability. In general, the pros and cons of the widely used traditional augmentation approaches are presented in Table 10.
Generating synthetic images by GAN based methods can increase diversity. However, GANs have their own challenging problems. A significant problem is vanishing gradient and mode collapsing (Yi et al. 2019). Since the collapse of the mode restricts the capacity of GAN architecture to be varied, this interconnection is detrimental for augmentations of medical images (Zhang 2021). Another significant problem is that it is hard to acquire satisfactory training results if the training procedure does not assure the symmetry and alignment of both generator and discriminator networks. Artifacts can be added while synthesizing images, and therefore the quality of the produced images can be very low. Also, the trained model can be unpredictable since GANs are complex structures and managing the coordination of the generator and discriminator is difficult. Furthermore, they share similar flaws with neural networks (such as, poor interpretability). Additionally, they require strong computer resources, a long processing time, and hamper the quality of the generated images in case of running on computers having low computational power with constrained resources.
Quality and diversity are two critical criteria in the evaluation of synthetically generated images from GANs. Quality criterion indicates the level of similarity between the synthetic and real images. In other words, it shows how representative synthetic images are of that class. A synthetic image’s quality is characterized by a low level of distortions, fuzziness, noise (Thung and Raveendran 2009), and its feature distribution matching with the class label (Zhou et al. 2020; Costa et al. 2017; Yu et al. 2019).
Diversity criteria indicates the level of dissimilarity of the synthetic and real images. In other words, it shows how uniform or wide the feature distribution of the synthetic image is (Shmelkov et al. 2018). When the training datasets are expanded with the images lacking diversity, the datasets can provide only limited coverage of the target domain and cause low classification performance due to incorrect classifications of the images containing features that belong to the less represented regions. When the training datasets are expanded by adding synthetic images with low quality, the classifier cannot learn the features representing different classes which leads to low classification performance. Therefore, if synthetic images are used in the training sets, they should be sufficiently diverse to represent features of each class and have high quality.
The diversity and quality of the new images produced by GANs are evaluated manually by a physician or quantitatively by using similarity evaluation measurements, which are generally FID, structural similarity index, and peak signal-to-noise ratio (Borji 2019; Xu et al. 2018). The validity of these measurements for medical image data sets is still under investigation and there is no accepted consensus. Manual evaluations are subjective as well as time-consuming (Xu et al. 2018) since they are based on the domain knowledge of the physician. Also, there is no commonly accepted metric for evaluating the diversity and quality of synthesized images.
About Brain MR Image Augmentation techniques The methods used for brain MR image augmentation have some limitations or drawbacks. For instance, in a study (Isensee et al. 2020), the augmentation approach uses elastic deformations, which add shape variations. However, the deformations can bring lots of damage and noise when the deformation field is varied seriously. Also, the generated images seem not to be realistic and natural. It has been shown in the literature that widely used elastic deformations produce unrealistic brain MR images (Mok and Chung 2018). If the simulated tumors are not in realistic locations, then the classifier model can focus on the lesion’s appearance features and be invariant to contextual information. In another study (Kossen et al. 2021), although the generated images yielded a high dice score in the transfer learning approach, performance only slightly improved when training the network with real images and additional augmented images according to the results presented by the authors. This might be because of the less blurry and noisy appearance of the images produced by the used augmentation. The discriminator observed results of the generator network at intermediate levels in the augmentation with multi-scale gradient GAN (Deepak and Ameer 2020). Because the proposed GAN structure included a single discriminator and generator with multiple connections between them. Although augmentation with TensorMixup improved the diversity of the dataset (Wang et al. 2022), its performance should be further verified with more datasets including medical images with complicated patterns as samples generated with this augmentation may not satisfy the clinical characterizations of the images. Li et al. (2020) has observed that an unseen tumor label cannot be provided with the augmentation method and therefore the virtual semantic labels’ diversity is limited.
It has been observed that usage of the augmented images obtained by the combination of rotation with shearing, and translation provides higher performance than the other augmentation techniques in the classifications of HGG and LGG cases from FLAIR types of brain images. On the other hand, augmentation with the combination of shearing and salt-and-pepper noise addition is the least efficient approach for augmentation in improving classification performance (Table 6).
About Lung CT Image Augmentation techniques Further studies on augmentation techniques for lung CT images are still needed since current methods still suffer from some issues. For instance, in a study (Onishi et al. 2020), although performance in the classification of isolated nodules and nodules having pleural tails increased, it did not increase in the classification of nodules connected to blood vessels or pleural. The reason might be due to the heavy usage of isolated nodule images (rather than the images with the nodules adjacent to other wide tissues like the pleural) for the training of the GAN. Also, the Wasserstein GAN structure leads to gradient vanishing problems due to small weight clipping and a long time to connect because of huge clipping. In another study (Nishio et al. 2020), the quality of the generated 3-dimensional CT images that show nodules is not low. On the other hand, in some of those images, the lung parenchyma surrounding the nodules does not seem natural. For instance, the CT values around the lung parenchyma in the generated images are relatively higher than the CT values around the lung parenchyma in the real images. Also, lung vessels, chest walls, and bronchi in some of those generated images are not regular. The radiologists easily distinguish those generated images according to the irregular and unnatural structures. The augmentation method in a different work (Nishio et al. 2020) generate only lung nodules’ 3-dimensional CT images. However, there exist various radiological findings (e.g., ground glass, consolidation, and cavity) and it is not clear whether those findings are generated or not. Also, the application can classify nodules only according to their sizes rather than other properties (e.g., absence or presence of spicules, margin characteristics, etc.). Therefore, further evaluation should be performed to see whether nodule classification performance can be increased with the new lung nodules for other classifications, such as malignant or benign cases. A constant coefficient was used in the loss function to synthesize lung nodules by Wang et al. (2021). It affects the training performance and should be chosen carefully. Therefore, the performance of the augmentation method should be evaluated with increased a number of images. Although the method proposed by Toda et al. (2021) has the potential to generate images, spicula-like features are obscure in the generated images and it does not include the distribution of true images. Also, the generation of some features contained in the images (e.g., cavities around or inside the tumor, prominent pleural tail signs, non-circular shapes) are difficult with the proposed augmentation. Therefore, images with those features tend to be misclassified. In a study, augmentation with elastic deformations, which add shape variations, brings noise and damage when the deformation field is varied seriously (Müller et al. 2021). In a different approach, as it is identified by the authors, the predicted tumor regions are prone to imperfections (Farheen et al. 2022).
It has been observed that usage of the augmented images obtained by the combination of translation and shearing techniques in the classification of images with nodules and without nodules provides the highest performance than the other augmentation methods. On the other hand, augmentation only by shearing is the least efficient approach (Table 7).
About breast Mammography Image Augmentation techniques Augmentation of breast mammography images is one of the significant and fundamental directions that need to be focused on for further investigations and future research efforts. Although scaling, translation, rotation, and flipping are widely used, they are not suitable enough for augmentation since the additional information provided by them is not sufficient to make variations in the images, and so the diversity of the resulting dataset is limited. For instance, in a study performed by Zeiser et al. (2020), although the classifier classifies pixels having high intensity values as masses as it is desired, the network ends up producing FPs in dense breasts. Therefore, the augmentation technique applied as a pre-processing step should be modified to expand the datasets more efficiently and to increase the generalization skill of the proposed classifier. Besides, the performance of the applications should be tested with not only virtual images but also real-world images. Although the results of the applications that use generated images from a GAN based augmentation are promising (Table 3), their performances should be evaluated with increased numbers and variations of images. Since augmentations by synthesizing existing mammography images are difficult because of the variations of masses in terms of shape and texture as well as the presence of diverse and intricate breast tissues around the masses. The common problems in those GAN based augmentations are mode collapsing and saddle point optimization (Yadav et al. 2017). In the optimization problem, there exists almost no guarantee of equilibrium between the training of the discriminator and generator functions, causing one network to inevitably become stronger than the other network, which is generally the discriminator function. In the collapsing mode problem, the generator part focuses on limited data distribution modes and causes the generation of images with limited diversity.
Experiments in this study indicated that the combined technique consisting of translation, shearing, and rotation is the most appropriate approach for the augmentation of breast mammography images in order to improve the classification of the images as normal, benign, and malignant. On the other hand, the combined technique consisting of salt and pepper noise addition and shearing is the least appropriate approach (Table 8).
About Eye Fundus Image Augmentation techniques Further research on the augmentation techniques for fundus images is still needed to improve the reliability and robustness of computer-assisted applications. Because the tone qualities of fundus images are affected by the properties of fundus cameras (Tyler et al. 2009) and the images used in the literature have been acquired from different types of fundus cameras. Therefore, the presented applications fitting well to images obtained from a fundus camera may not generalize images from other kinds of fundus camera systems. Also, images can be affected by pathological alterations. Because of this reason, the applications should be robust on those alterations, particularly on the pathological changes that do not exist in the images used in the training steps. Therefore, although the results of the applications in the current literature indicated high performances (Table 4), their robustness should be evaluated using other data sets with increased numbers and variations of images and taken from different types of fundus cameras. Also, the contributions of the applied augmentation techniques to the presented performances are not clear. In a study performed by Zhou et al. (2020), although the GAN structure is able to synthesize high-quality images in most cases, the lesion and structural masks used as inputs are not real ground truth images. Therefore, the generator’s performance depends on the quality of those masks. Also, the applied GAN architecture fails to synthesize some lesions, such as microaneurysms. In another study performed by Ju et al. (2021), the GAN based augmentation might lead to biased results because of matching the generated images to the distribution of the target domain.
Experiments in this study indicated that usage of the augmented images obtained by the combination of color shifting with sharpening, and contrast changing provides higher performance than the other augmentation techniques in the classifications of eye fundus images. On the other hand, augmentation with translation is the least efficient approach for augmentation to improve classification performance (Table 9).
6 Conclusion
Transformation-based augmentation methods are easy to implement with medical images. Also, their effectiveness depends on the characteristics (e.g., color, intensity, etc.) of the images, and whether all significant visual features according to medical conditions exist in the training sets.
GAN based augmentation methods can increase diversity. On the other hand, GANs have vanishing gradient and mode collapsing problems. Also, obtaining satisfactory training results is not easy if the training procedure does not assure the symmetry and alignment of both generator and discriminator networks. Besides, GANs are complex structures and managing the coordination of the generator and discriminator is difficult.
Combination of rotation with shearing, and translation provides higher performance than the other augmentation techniques in the classifications of HGG and LGG cases from FLAIR types of brain images. However, augmentation with the combination of shearing and salt-and-pepper noise addition is the least efficient approach for augmentation in improving classification performance.
Combination of translation and shearing techniques in the classification of lung CT images with nodules and without nodules provides the highest performance than the other augmentation methods. However, augmentation only by shearing is the least efficient approach.
Combination of translation, shearing, and rotation is the most appropriate approach for the augmentation of breast mammography images in order to improve the classification of the images as normal, benign, and malignant. On the other hand, the combined technique consisting of salt and pepper noise addition and shearing is the least appropriate approach.
Combination of color shifting with sharpening, and contrast changing provides higher performance than the other augmentation techniques in the classifications of eye fundus images. On the other hand, augmentation with translation is the least efficient approach for augmentation to improve classification performance.
As an extension of this work, the effectiveness of the augmentation methods will be evaluated in the diagnosis of diseases from positron emission tomography, ultrasonography images, other types of MR sequences (e.g., T2, T1, and proton density-weighted), and also in the classification of other types of images such as satellite or natural images. Also, GAN based augmentations will be applied, quantitative and qualitative analyses of the generated images will be performed to ensure their diversity and realness. In this study, ResNet101 has been used due to its advantage based on residual connections and efficiency in classification. Therefore, the implementation of other convolutional network models will be performed in our future works.
References
Agustin T, Utami E, Al Fatta H (2020) Implementation of data augmentation to improve performance cnn method for detecting diabetic retinopathy. In: 3rd International conference on information and communications technology (ICOIACT), Indonesia, Yogyakarta, pp 83–88
Alshazly H, Linse C, Barth E et al (2021) Explainable covid-19 detection using chest ct scans and deep learning. Sensors 21:1–22
Aly GH, Marey M, El-Sayed SA, Tolba MF (2021) Yolo based breast masses detection and classification in full-field digital mammograms. Comput Methods Programs Biomed 200:105823
Alyafi B, Diaz O, Marti R (2020) DCGANs for realistic breast mass augmentation in X-ray mammography. IN: Medical imaging 2020: computer-aided diagnosis, International Society for Optics and Photonics, pp 1–4. https://doi.org/10.1117/12.2543506
Araújo T, Aresta G, Mendonça L et al (2020) Data augmentation for improving proliferative diabetic retinopathy detection in eye fundus images. IEEE Access 8:462–474
Armato IIISG, McLennan G, Bidaut L, McNitt-Gray MF, Meyer CR, Reeves AP, Zhao B, Aberle DR, Henschke CI, Hoffman EA, Kazerooni EA (2011) The lung image database consortium (LIDC) and image database resource initiative (IDRI): a completed reference database of lung nodules on CT scans. Med Phys 38:915–931
Asia AO, Zhu CZ, Althubiti SA, Al-Alimi D, Xiao YL, Ouyang PB, Al-Qaness MA (2020) Detection of diabetic retinopathy in retinal fundus images using cnn classification models. Electronics 11:1–20
Aswathy AL, Vinod Chandra SS (2022) Cascaded 3D UNet architecture for segmenting the COVID-19 infection from lung CT volume. Sci Rep. https://doi.org/10.1038/s41598-022-06931-z
Ayana G, Park J, Choe SW (2022) Patchless multi-stage transfer learning for improved mammographic breast mass classification. Cancers. https://doi.org/10.3390/cancers14051280
Bakas S, Reyes M, Jakab A, Bauer S, Rempfler M, Crimi A, Shinohara RT, Berger C, Ha SM, Rozycki M, Prastawa M (2018) Identifying the best machine learning algorithms for brain tumor segmentation, progression assessment, and overall survival prediction in the brats challenge. arXiv preprint, pp 1–49. arXiv:1811.02629
Balasubramanian R, Sowmya V, Gopalakrishnan EA, Menon VK, Variyar VS, Soman KP (2020) Analysis of adversarial based augmentation for diabetic retinopathy disease grading. In: 11th International conference on computing, communication and networking technologies (ICCCNT), India, Kharagpur, pp 1–5
Barile B, Marzullo A, Stamile C, Durand-Dubief F, Sappey-Marinier D (2021) Data augmentation using generative adversarial neural networks on brain structural connectivity in multiple sclerosis. Comput Methods Programs Biomed 206:1–12
Basu A, Sheikh KH, Cuevas E, Sarkar R (2022) Covid-19 detection from CT scans using a two-stage framework. Expert Syst Appl 193:1–14
Bayer M, Kaufhold MA, Reuter C (2021) A survey on data augmentation for text classification. ACM-CSUR. https://doi.org/10.1145/3544558
Borji A (2019) Pros and cons of gan evaluation measures. Comput Vis Image Underst 179:41–65
Carreira J, Zisserman A (2017) Quo vadis, action recognition? a new model and the kinetics dataset. In: Conference on computer vision and pattern recognition, Hawaii, Honolulu, pp 6299–6308
Chaki J (2022) Two-fold brain tumor segmentation using fuzzy image enhancement and DeepBrainet2.0. Multimed Tools Appl 81:30705–30731
Chatfield K, Simonyan K, Vedaldi A, Zisserman A (2014) Return of the devil in the details: delving deep into convolutional nets, pp 1–11. arXiv preprint. https://doi.org/10.48550/arXiv.1405.3531
Chen X, Wang X, Zhang K, Fung KM, Thai TC, Moore K, Mannel RS, Liu H, Zheng B, Qiu Y (2022a) Recent advances and clinical applications of deep learning in medical image analysis. Med Image Anal. https://doi.org/10.1016/j.media.2022.102444
Chen Y, Yang X, Wei Z, Heidari AA et al (2022b) Generative adversarial networks in medical image augmentation: a review. Comput Biol Med. https://doi.org/10.1016/j.compbiomed.2022.105382
Chlap P, Min H, Vandenberg N et al (2021) A review of medical image data augmentation techniques for deep learning applications. Med Imaging Radiat Oncol 65:545–563
Costa P, Galdran A, Meyer MI, Niemeijer M, Abràmoff M, Mendonça AM, Campilho A (2017) End-to-end adversarial retinal image synthesis. IEEE Trans Med Imaging 37:781–791
Decencière E, Zhang X, Cazuguel G, Lay B, Cochener B, Trone C, Gain P, Ordonez R, Massin P, Erginay A et al (2014) Feedback on a publicly distributed image database: the messidor database. Image Anal Stereol 33:231–234
Deepak S, Ameer P (2020) MSG-GAN based synthesis of brain mri with meningioma for data augmentation. In: IEEE international conference on electronics, computing and communication technologies (CONECCT), India, Bangalore, pp 1–6
Desai SD, Giraddi S, Verma N, Gupta P, Ramya S (2020) Breast cancer detection using gan for limited labeled dataset. In: 12th International conference on computational intelligence and communication networks, India, Bhimtal, pp 34–39
Dodia S, Basava A, Padukudru Anand M (2022) A novel receptive field-regularized V‐net and nodule classification network for lung nodule detection. Int J Imaging Syst Technol 32:88–101. https://doi.org/10.1002/ima.22636
Dorizza A (2021) Data augmentation approaches for polyp segmentation. Dissertation, Universita Degli Studi Di Padova
Dufumier B, Gori P, Battaglia I, Victor J, Grigis A, Duchesnay E (2021) Benchmarking cnn on 3d anatomical brain mri: architectures, data augmentation and deep ensemble learning. arXiv preprint, pp 1–25. arXiv:2106.01132
Farheen F, Shamil MS, Ibtehaz N, Rahman MS (2022) Revisiting segmentation of lung tumors from CT images. Comput Biol Med 144:1–12
Fidon L, Ourselin S, Vercauteren T (2020) Generalized wasserstein dice score, distributionally robust deep learning, and ranger for brain tumor segmentation: brats 2020 challenge. in: International MICCAI brain lesion workshop, Lima, Peru, pp 200–214
Girshick R (2015) Fast R-CNN. In: IEEE international conference on computer vision, Santiago, USA, pp 1440–1448
Girshick R, Donahue J, Darrell T, Malik J (2014) Rich feature hierarchies for accurate object detection and semantic segmentation. In: IEEE conference on computer vision and pattern recognition, Columbus, USA, pp 580–587
Halder A, Datta B (2021) COVID-19 detection from lung CT-scan images using transfer learning approach. Mach Learn: Sci Technol 2:1–12
Haq AU, Li JP, Agbley BLY et al (2022) IIMFCBM: Intelligent integrated model for feature extraction and classification of brain tumors using mri clinical imaging data in IoT-Healthcare. IEEE J Biomed Health Inf 26:5004–5012
Hashemi N, Masoudnia S, Nejad A, Nazem-Zadeh MR (2022) A memory-efficient deep framework for multi-modal mri-based brain tumor segmentation. In: 2022 44th annual international conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Scotland, Glasgow, UK, pp 3749–3752
He K, Zhang X, Ren S, Sun J (2016) Deep residual learning for image recognition. In: IEEE conference on computer vision and pattern recognition (CVPR), Las Vegas, USA, pp 770–778
He K, Gkioxari G, Dollár P, Girshick R (2017) Mask R-CNN. In: Proceedings of the IEEE international conference on computer vision, Venice, Italy, pp 2961–2969
Hu R, Ruan G, Xiang S, Huang M, Liang Q, Li J (2020) Automated diagnosis of covid-19 using deep learning and data augmentation on chest CT. medRxiv, pp 1–11
Humayun M, Sujatha R, Almuayqil SN, Jhanjhi NZ (2022) A transfer learning approach with a convolutional neural network for the classification of lung carcinoma. Healthcare. https://doi.org/10.3390/healthcare10061058
Isensee F, J¨ager PF, Full PM et al (2020) nnu-Net for brain tumor segmentation. In: International MICCAI brainlesion workshop. Springer, Cham, pp 118–132
Islam MR, Abdulrazak LF, Nahiduzzaman M, Goni MO, Anower MS, Ahsan M, Haider J, Kowalski M (2022) Applying supervised contrastive learning for the detection of diabetic retinopathy and its severity levels from fundus images. Comput Biol Med. https://doi.org/10.1016/j.compbiomed.2022.105602
Jha M, Gupta R, Saxena R (2022) A framework for in-vivo human brain tumor detection using image augmentation and hybrid features. Health Inf Sci Syst 10:1–12
Ju L, Wang X, Zhao X, Bonnington P, Drummond T, Ge Z (2021) Leveraging regular fundus images for training UWF fundus diagnosis models via adversarial learning and pseudo-labeling. IEEE Trans Med Imaging 40:2911–2925
Karthiga R, Narasimhan K, Amirtharajan R (2022) Diagnosis of breast cancer for modern mammography using artificial intelligence. Math Comput Simul 202:316–330
Khan AR, Khan S, Harouni M, Abbasi R, Iqbal S, Mehmood Z (2021) Brain tumor segmentation using K-means clustering and deep learning with synthetic data augmentation for classification. Microsc Res Tech 84:1389–1399
Khosla C, Saini BS (2020) Enhancing performance of deep learning models with different data augmentation techniques: a survey. In: International conference on intelligent engineering and management (ICIEM), London, UK, pp 79–85
Kim YJ, Kim KG (2022) Detection and weak segmentation of masses in gray-scale breast mammogram images using deep learning. Yonsei Med J 63:S63
Kossen T, Subramaniam P, Madai VI, Hennemuth A, Hildebrand K, Hilbert A, Sobesky J, Livne M, Galinovic I, Khalil AA, Fiebach JB (2021) Synthesizing anonymized and labeled TOF-MRA patches for brain vessel segmentation using generative adversarial networks. Comput Biol Med 131:1–9
Kurup A, Soliz P, Nemeth S, Joshi V (2020) Automated detection of malarial retinopathy using transfer learning. In: IEEE southwest symposium on image analysis and interpretation (SSIAI), Albuquerque, USA, pp 18–21
Li Q, Yu Z, Wang Y et al (2020) Tumorgan: a multi-modal data augmentation framework for brain tumor segmentation. Sensors 20:1–16
Li H, Chen D, Nailon WH, Davies ME, Laurenson DI (2021) Dual convolutional neural networks for breast mass segmentation and diagnosis in mammography. IEEE Trans Med Imaging 41:3–13
Li Z, Guo C, Nie D, Lin D, Cui T, Zhu Y, Chen C, Zhao L, Zhang X, Dongye M, Wang D (2022) Automated detection of retinal exudates and drusen in ultra-widefield fundus images based on deep learning. Eye 36:1681–1686
Lim G, Thombre P, Lee ML, Hsu W (2020) Generative data augmentation for diabetic retinopathy classification. In: IEEE 32nd international conference on tools with artificial intelligence (ICTAI), Baltimore, USA, pp 1096–1103
Lin M, Hou B, Liu L, Gordon M, Kass M, Wang F, Van Tassel SH, Peng Y (2022) Automated diagnosing primary open-angle glaucoma from fundus image by simulating human’s grading with deep learning. Sci Rep. https://doi.org/10.1038/s41598-022-17753-4
Liu Y, Kwak HS, Oh IS (2022) Cerebrovascular segmentation model based on spatial attention-guided 3D inception U-Net with multi-directional MIPs. Appl Sci. https://doi.org/10.3390/app12052288
Long J, Shelhamer E, Darrell T (2015) Fully convolutional networks for semantic segmentation. In: Proceedings of the IEEE conference on computer vision and pattern recognition, Boston, USA, pp 3431–3440
Mahmood T, Li J, Pei Y, Akhtar F, Jia Y, Khand ZH (2021) Breast mass detection and classification using deep convolutional neural networks for radiologist diagnosis assistance. In: 45th Annual computers, software, and applications conf (COMPSAC), Madrid, Spain, pp 1918–1923
Mahmood T, Li J, Pei Y, Akhtar F, Rehman MU, Wasti SH (2022) Breast lesions classifications of mammographic images using a deep convolutional neural network-based approach. PLoS ONE. https://doi.org/10.1371/journal.pone.0263126
Manos D, Seely JM, Taylor J, Borgaonkar J, Roberts HC, Mayo JR (2014) The lung reporting and data system (LU-RADS): a proposal for computed tomography screening. Can Assoc Radiol J 65:121–134
Mayya V, Kulkarni U, Surya DK, Acharya UR (2022) An empirical study of preprocessing techniques with convolutional neural networks for accurate detection of chronic ocular diseases using fundus images. Appl Intell 1:1–19
McNitt-Gray MF, Armato SG III, Meyer CR, Reeves AP, McLennan G, Pais RC, Freymann J, Brown MS, Engelmann RM, Bland PH et al (2007) The Lung Image Database Consortium (LIDC) data collection process for nodule detection and annotation. Acad Radiol 14:1464–1474
Meijering E (2020) A bird’s-eye view of deep learning in bioimage analysis. Comput Struct Biotechnol J 18:2312–2325
Miller JD, Arasu VA, Pu AX, Margolies LR, Sieh W, Shen L (2022) Self-supervised deep learning to enhance breast cancer detection on screening mammography. arXiv preprint, pp 1–11. arXiv:2203.08812
Mok TC, Chung A (2018) Learning data augmentation for brain tumor segmentation with coarse-to-fine generative adversarial networks. In: International MICCAI brain lesion workshop, Granada, Spain, pp 70–80
Moreira IC, Amaral I, Domingues I, Cardoso A, Cardoso MJ, Cardoso JS (2012) Inbreast: toward a full-field digital mammographic database. Acad Radiol 19:236–248
Müller D, Soto-Rey I, Kramer F (2021) Robust chest CT image segmentation of COVID-19 lung infection based on limited data. Inf Med Unlocked 25:1–11
Naidich DP, Bankier AA, MacMahon H, Schaefer-Prokop CM, Pistolesi M, Goo JM, Macchiarini P, Crapo JD, Herold CJ, Austin JH, Travis WD (2013) Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 266:304–317
Nalepa J, Marcinkiewicz M, Kawulok M (2019) Data augmentation for brain-tumor segmentation: a review. Front Comput Neurosci 13:1–18
Naveed H (2021) Survey: Image mixing and deleting for data augmentation. arXiv preprint, pp 1–15. arXiv:2106.07085
Nayan AA, Mozumder AN, Haque M, Sifat FH, Mahmud KR, Azad AK, Kibria MG (2022) A deep learning approach for brain tumor detection using magnetic resonance imaging. arXiv preprint, pp 1–9. arXiv:2210.13882
Neelima G, Chigurukota DR, Maram B, Girirajan B (2022) Optimal DeepMRSeg based tumor segmentation with GAN for brain tumor classification. Biomed Signal Process Control. https://doi.org/10.1016/j.bspc.2022.103537
Nishio M, Muramatsu C, Noguchi S, Nakai H, Fujimoto K, Sakamoto R, Fujita H (2020) Attribute-guided image generation of three-dimensional computed tomography images of lung nodules using a generative adversarial network. Comput Biol Med. https://doi.org/10.1016/j.compbiomed.2020.104032
Onishi Y, Teramoto A, Tsujimoto M, Tsukamoto T, Saito K, Toyama H, Imaizumi K, Fujita H (2020) Multiplanar analysis for pulmonary nodule classification in CT images using deep convolutional neural network and generative adversarial networks. Int J Comput Assist Radiol Surg 15:173–178
Oza P, Sharma P, Patel S, Adedoyin F, Bruno A (2022) Image augmentation techniques for mammogram analysis. J Imaging. https://doi.org/10.3390/jimaging8050141
Padalia D, Vora K, Mehta D, Mehendale N (2022) EEF-Net: an enhanced efficientnet for breast tumor classification in mammograms. SSRN 4220435. https://doi.org/10.2139/ssrn.4220435
Quintana-Quintana OJ, De León-Cuevas A, Gonzalez-Gutierrez A, Gorrostieta-Hurtado E, Tovar-Arriaga S (2022) Dual U-Net-based conditional generative adversarial network for blood vessel segmentation with reduced cerebral MR training volumes. Micromachines. https://doi.org/10.3390/mi13060823
Ramya J, Rajakumar MP, Maheswari BU (2022) Deep cnn with hybrid binary local search and particle swarm optimizer for exudates classification from fundus images. J Digit Imaging 35:56–67
Razali NF, Isa IS, Sulaiman SN, Karim NK, Osman MK (2021) High-level features in deeper deep learning layers for breast cancer classification. In: 11th IEEE international conference on control system, computing and engineering (ICCSCE), Penang, Malaysia, pp 170–175
Ren S, He K, Girshick R, Sun J (2015) Faster R-CNN: towards real-time object detection with region proposal networks. Adv Neural Inf Process Syst 28:1–9
Russakovsky O, Deng J, Su H, Krause J, Satheesh S, Ma S, Huang Z, Karpathy A, Khosla A, Bernstein M, Berg AC (2015) Imagenet large scale visual recognition challenge. Int J Comput Vis 115:211–252
Sabani A, Landsmann A, Hejduk P, Schmidt C, Marcon M, Borkowski K, Rossi C, Ciritsis A, Boss A (2022) BI-RADS-based classification of mammographic soft tissue opacities using a deep convolutional neural network. Diagnostics. https://doi.org/10.3390/diagnostics12071564
Salama WM, Aly MH (2021) Deep learning in mammography images segmentation and classification: automated CNN approach. Int Alexandria Eng J 60:4701–4709. https://doi.org/10.1016/j.aej.2021.03.048
Setio AA, Traverso A, De Bel T, Berens MS, Van Den Bogaard C, Cerello P, Chen H, Dou Q, Fantacci ME, Geurts B, van der Gugten R (2017) Validation, comparison, and combination of algorithms for automatic detection of pulmonary nodules in computed tomography images: the luna16 challenge. Med Image Anal 42:1–3
Shmelkov K, Schmid C, Alahari K (2018) How good is my GAN? In: Proceedings of the European conference on computer vision (ECCV), Germany, Munich, pp 213–229
Shen T, Hao K, Gou C, Wang FY (2021) Mass image synthesis in mammogram with contextual information based on GANS. Comput Methods Programs Biomed. https://doi.org/10.1016/j.cmpb.2021.106019
Shi H, Lu J, Zhou Q (2020) A novel data augmentation method using style-based GAN for robust pulmonary nodule segmentation. In: IEEE Chinese control and decision conference (CCDC), Hefei, China, pp 2486–2491
Shorten C, Khoshgoftaar TM (2019) A survey on image data augmentation for deep learning. J Big Data 6:1–48
Shyamalee T, Meedeniya (2022) D CNN based fundus images classification for glaucoma identification. In: 2nd International conference on advanced research in computing (ICARC), Belihuloya, Sri Lanka, pp 200–205
Simonyan K, Zisserman A (2014) Two-stream convolutional networks for action recognition in videos. Adv Neural Inf Process Syst 27:1–9
Singh LK, Garg H, Khanna M (2022) Deep learning system applicability for rapid glaucoma prediction from fundus images across various data sets. Evol Syst 13:807–836
Soulami KB, Kaabouch N, Saidi MN (2022) Breast cancer: classification of suspicious regions in digital mammograms based on capsule network. Biomed Signal Process Control. https://doi.org/10.1016/j.bspc.2022.103696
Srinivas C, KS NP, Zakariah M, Alothaibi YA, Shaukat K, Partibane B, Awal H (2022) Deep transfer learning approaches in performance analysis of brain tumor classification using MRI images. J Healthc Eng. https://doi.org/10.1155/2022/3264367
Sun Y, Yuan P, Sun Y (2020) MM-GAN: 3D MRI data augmentation for medical image segmentation via generative adversarial networks. In: IEEE international conference on knowledge graph (ICKG), Nanjing, China, pp 227–234
Sun X, Fang H, Yang Y et al (2021) Robust retinal vessel segmentation from a data augmentation perspective. In: International workshop on ophthalmic medical image analysis, pp 189–198
Tandon R, Agrawal S, Raghuwanshi R, Rathore NP, Prasad L, Jain V (2022a) Automatic lung carcinoma identification and classification in CT images using CNN deep learning model. In: Augmented intelligence in healthcare: a pragmatic and integrated analysis. Springer, Singapore. https://doi.org/10.1007/978-981-19-1076-0_9
Tandon R, Agrawal S, Chang A, Band SS (2022b) VCNet: Hybrid deep learning model for detection and classification of lung carcinoma using chest radiographs. Front Public Health. https://doi.org/10.3389/fpubh.2022.894920
Tang N, Zhang R, Wei Z, Chen X, Li G, Song Q, Yi D, Wu Y (2021) Improving the performance of lung nodule classification by fusing structured and unstructured data. Inf Fusion 88:161–174
Thung KH, Raveendran P (2009) A survey of image quality measures. In: IEEE international conference for technical postgraduates (TECHPOS), Kuala Lumpur, Malaysia, pp 1–4
Toda R, Teramoto A, Tsujimoto M, Toyama H, Imaizumi K, Saito K, Fujita H (2021) Synthetic ct image generation of shape-controlled lung cancer using semi-conditional InfoGAN and its applicability for type classification. Int J Comput Assist Radiol Surg 16:241–251
Tsuneki M (2022) Deep learning models in medical image analysis. J Oral Biosci. https://doi.org/10.1016/j.job.2022.03.003
Tufail AB, Ullah I, Khan WU, Asif M, Ahmad I, Ma YK, Khan R, Ali M (2021) Diagnosis of diabetic retinopathy through retinal fundus images and 3D convolutional neural networks with limited number of samples. Wirel Commun Mob Comput 2021:1–15
Tyler ME, Hubbard LD, Boydston K, Pugliese AJ (2009) Characteristics of digital fundus camera systems affecting tonal resolution in color retinal images. J Ophthalmic Photogr 31:1–9
Ueda D, Yamamoto A, Onoda N, Takashima T, Noda S, Kashiwagi S, Morisaki T, Fukumoto S, Shiba M, Morimura M et al (2022) Development and validation of a deep learning model for detection of breast cancers in mammography from multi-institutional datasets. PLoS ONE 17:1–15
van der Velden BH, Kuijf HJ, Gilhuijs KG, Viergever MA (2022) Explainable artificial intelligence (XAI) in deep learning-based medical image analysis. Med Image Anal. https://doi.org/10.1016/j.media.2022.102470
Wang W, Luo J, Yang X, Lin H (2015) Data analysis of the lung imaging database consortium and image database resource initiative. Acad Radiol 22:488–495
Wang Q, Zhang X, Zhang W, Gao M, Huang S, Wang J, Zhang J, Yang D, Liu C (2021) Realistic lung nodule synthesis with multi-target co-guided adversarial mechanism. IEEE Trans Med Imaging 40:2343–2353
Wang Y, Ji Y, Xiao H (2022) A Data Augmentation Method for Fully Automatic Brain Tumor Segmentation. arXiv preprint, pp 1–15. arXiv:2202.06344
Woan SL, Lai KW, Chuah JH, Hasikin K, Khalil A, Qian P, Xia K, Jiang Y, Zhang Y, Dhanalakshmi S (2022) Multiclass convolution neural network for classification of COVID-19 CT images. Comput Intell Neurosci. https://doi.org/10.1155/2022/9167707
Wu W, Lu Y, Mane R, Society et al (2020a) EMBC, Canada, Montreal, pp 1516–1519
Wu E, Wu K, Lotter W (2020b) Synthesizing lesions using contextual gans improves breast cancer classification on mammograms. arXiv preprint, pp 1–10. arXiv:2006.00086
Xie L, Chen Z, Sheng X et al (2022) Semi-supervised region-connectivity-based cerebrovascular segmentation for time-of-flight magnetic resonance angiography image. Comput Biol Med. https://doi.org/10.1016/j.compbiomed.2022.105972
Xu Q, Huang G, Yuan Y, Guo C, Sun Y, Wu F, Weinberger K (2018) An empirical study on evaluation metrics of generative adversarial networks. arXiv preprint, pp 1–14. arXiv:1806.07755
Yadav JK, Yadav S (2022) Computer-aided diagnosis of cataract severity using retinal fundus images and deep learning. Comput Intell 38:1450–1473
Yadav A, Shah S, Xu Z, Jacobs D, Goldstein T (2017) Stabilizing adversarial nets with prediction methods. arXiv preprint, pp 1–21. arXiv:1705.07364
Yi X, Walia E, Babyn P (2019) Generative adversarial network in medical imaging: a review. Med Image Anal. https://doi.org/10.1016/j.media.2019.101552
Yu Z, Xiang Q, Meng J, Kou C, Ren Q, Lu Y (2019) Retinal image synthesis from multiple-landmarks input with generative adversarial networks. Biomed Eng Online. https://doi.org/10.1186/s12938-019-0682-x
Yu H, Yang LT, Zhang Q, Armstrong D, Deen MJ (2021) Convolutional neural networks for medical image analysis: state-of-the-art, comparisons, improvement and perspectives. Neurocomputing 444:92–110. https://doi.org/10.1016/j.neucom.2020.04.157
Yuan Y (2020) Automatic brain tumor segmentation with scale attention network. In: International MICCAI brain lesion workshop. Lecture notes in computer science. https://doi.org/10.1007/978-3-030-72084-1_26
Zahoor S, Shoaib U, Lali IU (2022) Breast Cancer Mammograms classification using deep neural network and entropy-controlled Whale optimization Algorithm. Diagnostics. https://doi.org/10.3390/diagnostics12020557
Zeiser FA, da Costa CA, Zonta T et al (2020) Segmentation of masses on mammograms using data augmentation and deep learning. J Digit Imaging 33:858–868
Zhang K (2021) On mode collapse in generative adversarial networks. In: 30th International conference on artificial neural networks, Bratislava, Slovakia, pp 563–574
Zhou Y, Wang B, He X, Cui S, Shao L (2020) DR-GAN: conditional generative adversarial network for fine-grained lesion synthesis on diabetic retinopathy images. IEEE J Biomedical Health Inf 26:56–66
Author information
Authors and Affiliations
Contributions
It is a single-authored article.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goceri, E. Medical image data augmentation: techniques, comparisons and interpretations. Artif Intell Rev 56, 12561–12605 (2023). https://doi.org/10.1007/s10462-023-10453-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10462-023-10453-z