Abstract
Transdermal drug delivery systems (TDDS) have many advantages and represent an excellent alternative to oral delivery and hypodermic injections. TDDS are more convenient and less invasive tools for disease and viral infection treatment, prevention, detection, and surveillance. The emerging development of microneedles for TDDS has facilitated improved skin barrier penetration for the delivery of macromolecules or hydrophilic drugs. Microneedle TDDS patches can be fabricated to deliver virus vaccines and potentially provide a viable alternative vaccine modality that offers improved immunogenicity, thermostability, simplicity, safety, and compliance as well as sharp-waste reduction, increased cost-effectiveness, and the capacity for self-administration, which could improve vaccine distribution. These advantages make TDDS-based vaccine delivery an especially well-suited option for treatment of widespread viral infectious diseases including pandemics. Because microneedle-based bioassays employ transdermal extraction of interstitial fluid or blood, they can be used as a minimally invasive approach for surveying disease markers and providing point-of-care (POC) diagnostics. For cutaneous viral infections, TDDS can provide localized treatment with high specificity and less systemic toxicity. In summary, TDDS, especially those that employ microneedles, possess special attributes that can be leveraged to reduce morbidity and mortality from viral infectious diseases. In this regard, they may have considerable positive impact as a modality for improving global health. In this article, we introduce the possible role and summarize the current literature regarding TDDS applications for fighting common cutaneous or systemic viral infectious diseases, including herpes simplex, varicella or herpes zoster, warts, influenza, measles, and COVID-19.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Skin as a route for drug delivery: strengths and weaknesses
The three anatomical layers of skin are the epidermis, dermis, and subcutis. The outermost epidermal layer is composed of four or five layers from superficial to deep, including the stratum corneum, stratum lucidum (only in palms and soles), stratum granulosum, stratum spinosum, and the stratum basale. The stratum corneum, the outermost layer of the epidermis, is only 10 to 20 μm thick. Nevertheless, it is the most important barrier for protecting underlying tissue from water loss, infection, chemical or mechanical irritation, and drug absorption. The stratum corneum allows primarily passive diffusion by drugs with specific physicochemical properties (molecular weight < 500 Da, high lipophilicity, and low melting point) [1, 2].
Transdermal drug delivery systems (TDDS) are an excellent alternative to oral delivery and hypodermic injections. Because of the delivery route, they naturally avoid potentially detrimental digestive/metabolic effects associated with oral drug delivery including the effluence of enzymatic digestion, gastric emptying time, gastrointestinal tract pH, and the first-pass effect, which occurs in the liver [1, 3]. They are also superior to oral delivery because they can be administered on unconscious or nauseated patients [4]. Compared to injection delivery, TDDS avoid pain, bruising, and bleeding, which improves patient acceptance and compliance. They also eliminate the risk of needle-associated disease transmission and accidental needle injury, and reduce the generation of dangerous medical waste sharps [5]. The advantages of TDDS are not just safety-based; they have been shown to reduce overall healthcare treatment costs [6]. Furthermore, TDDS can provide prolonged and controlled drug release [7], minimize the peak of drug concentration, and reduce associated systemic toxicity [4]. They have unique administrative flexibility—they are easy to apply and to remove, which not only means that drug delivery can be easily terminated if it causes localized or systemic side effects, but also means that they are well suited for self-administration. TDDS are especially well suited for treatment of dermatological diseases. Direct application of a drug to a target skin site can maximize drug efficacy and minimize side effects (Table 1).
The disadvantages of most concern regarding TDDS are possible skin irritation and sensitization (Table 1). Drugs, adhesives, or patch formulations might cause skin irritation or allergic contact dermatitis. Although skin tests are performed for all TDDS products before marketing, skin reaction differs from person to person. Skin irritant contact dermatitis (ICD) may be caused by chemical or physical irritants and manifested as itchy or painful erythematous patches or plaques. ICD activates the innate immune response by inducing proinflammatory mediators that directly recruit and activate T lymphocytes, without induction of antigen-specific memory T cells. In contrast to ICD, allergic contact dermatitis (ACD) is a response of the adaptive immune system, in which there is a delayed T-cell-mediated (type 4) allergic reaction. The delayed hypersensitivity reaction follows a two-step mechanism, comprising an induction phase and an elicitation phase. This results in a delayed appearance of skin rashes following continuous allergen contact. In some instances, both ICD and ACD may be present [8].
As mentioned previously, the stratum corneum allows primarily passive diffusion by drugs with low molecular weight (< 500 Da) and high lipophilicity[1, 2]. Without special techniques or drug modification for improving skin penetration, drugs with a hydrophilic structure or ionic drugs are not able to adequately penetrate skin and achieve high drug levels in systemic circulation. Because of the impermeable nature of skin, potent drugs must usually be used with traditional patches to achieve penetration. However, because the skin barrier function varies by body site and age, pharmacokinetics can be unpredictable. Additionally, patches may not adhere well to all types of skin, so adhesion performance is another concern [9] (Table 1).
What are the classifications of TDDS?
TDDS have primarily been used for topical formulations, transdermal patches, and integration with microneedles [1, 10]. To improve the penetration of drugs through the skin via TDDS, a wide range of physical and chemical techniques have been developed including chemical enhancers [11] or prodrugs [12], biochemical enhancers such as some cell-penetrating peptides [13], iontophoresis [14], non-cavitational or cavitational ultrasound [15, 16], electroporation[17], thermal ablation [18], and microdermabrasion [19]. Recently, nanocarriers have been developed that increase drug penetration and provide controlled and targeted drug release [20, 21].
Transdermal patches can employ reservoir systems, matrix systems, and micro-reservoir systems to delivery drugs [22]. During development, transdermal patches are tested to examine and optimize adhesion properties, physicochemical properties, in vitro drug release delivery, in vitro skin permeation, stability, and reduce skin irritation. Various drug-delivery transdermal patches are commercially available that have been optimized in accordance with therapy duration [22].
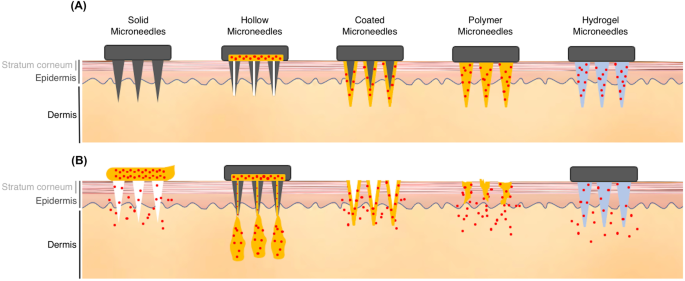
More recently, microneedles have become increasingly popular for incorporation into transdermal drug delivery systems, especially for macromolecules or hydrophilic substance. Microneedles can penetrate through the stratum corneum, the primary skin barrier, and into the deeper levels of epidermis. This dramatically improves skin permeability. Unlike hypodermic injections, microneedles are small enough to avoid contact with nerve fibers and blood vessels in the dermis [2]. Different types of microneedles with unique properties have been fabricated for a variety of drug delivery applications. These include solid, hollow, coated, polymer, and hydrogel microneedles (Fig. 1) [23, 24]. As illustrated in Fig. 1, solid microneedles are well suited for penetration, and can be used to increase permeability before drug application. Hollow microneedles are used to create pathways for drug infusion into the skin. Coated microneedles have a drug coating on their surface that dissolves after insertion into the skin. Biocompatible and biodegradable polymer microneedles have been created to contain encapsulated drugs that fully dissolve in the skin to release their encapsulated reagents. There are also microneedles made of non-dissolving, liquid-absorbing hydrogels that can be used for fluid and materials diffusion [23, 24]. So, microneedles can be used for both drug delivery and fluid extraction, which is an intriguing prospect in terms of integration with patch-embedded biomarker systems that might be used for diagnostic purposes [25]. Hollow and hydrogel microneedles can be fabricated to an array and molded into a patch. Such a patch could then be pressed directly onto the skin to extract interstitial fluid via osmotic pressure difference or negative pressure. Patches could subsequently be removed and processed to collect sampled fluid [23].
Different types of microneedles and their characteristics. (A) The structures of solid, hollow, coated, polymer, and hydrogel microneedles. (B) Each of these microneedles has different drug delivery properties. Solid microneedles are well suited for penetration and increasing drug permeability. Hollow microneedles create pathways for drug infusion. Coated microneedles contain drugs on their surface that dissolve after insertion into the skin. Microneedles made with biocompatible and biodegradable polymers contain drugs that fully dissolve in the skin to release their encapsulated reagents. Hydrogel microneedles made with non-dissolving, liquid-absorbing materials can be used for fluid and materials diffusion
How is TDDS advantageous in fighting viral infection?
There were many advantages and possible applications of TDDS for viral infectious diseases (Table 1). TDDS is a more convenient and less invasive method for drug delivery, and a better choice for those afraid of hypodermal injections. They are especially well suited for treating cutaneous viral infection because they can be applied directly and specifically to diseased skin, where they would decrease the systemic absorption and associated side effects. For example, buccal mucoadhesive or moisture-activated patches of acyclovir and acyclovir-loaded dissolving microneedle arrays have been used to treat herpes labialis while avoiding the side effects associated with systemic acyclovir treatment [36, 38, 39].
Regarding disease prevention, TDDS, especially in combination with microneedles, can be developed for vaccine distribution. The advantages of TDDS make the new-form microneedle vaccines an excellent, even superior alternative to conventional needle-and-syringe immunization. Dissolving microneedles have already been used to vaccinate against a variety of viruses including influenza [26], measles, and COVID-19 [27]. In fact, studies have demonstrated that microneedle-based vaccines provide improved immunity compared to subcutaneous injection [28], especially for those microneedles with antigen coating on the tips, which can be leveraged to target Langerhans cells that reside in the suprabasal layers of the epidermis [29]. Langerhans cells, the major dendritic cells of the skin for antigen-presenting, are capable of cross-priming antigen-specific CD8+ T cells. In terms of priming CD4+ T cells, Langerhans cells are largely responsible for orchestrating the differentiation of CD4+ IFN-γ- and IL-17-producing effectors [30]. By providing antigen delivery directly to dendritic cells in the skin, immunogenicity is more efficient compared to conventional delivery route methods [30]. In conclusion, microneedle vaccines may improve immunogenicity, delivery simplicity, acceptability, safety, cost-effectiveness, and most important of all, the capacity for at-home, self-administration [26]. These technological advances reduce cost, increase patient access, and increase vaccination coverage, thus improving morbidity and mortality rates from highly prevalent viral infections.
Not limited to drug and vaccine delivery, TDDS, especially when combined with microneedles, can be used for transdermal sampling to extract molecules of interstitial fluid, saliva, sweat, or blood [25]. These microneedle-based bioassays have been used to measure and monitor glucose, cholesterol, electrolytes, medicine concentrations, alcohol, cytokines, tumor makers, oligonucleotides, antibodies or other small proteins, and even T cells [31]. They can also be used for detecting viral infectious disease by sampling for and detecting viral antigens or antibodies, and more importantly, they do so in a less painful and more convenient way that facilitates at-home, self-examination. When microneedle devices were examined as alternative approaches for extracting disease markers for dengue virus NS1 protein, and surface-modified microneedle arrays were used to capture influenza antigen-specific IgG from the skin, the conclusions were that they demonstrated great promise for single-array, point-of-care (POC) diagnostics [32]. The most important prospective application for TDDS sampling is for diagnosing cutaneous diseases. For some cutaneous viral infections, viral antigens or antibodies may be detected locally at the skin lesion level via TDDS/microneedle extraction that may not present in blood. In such situations, TDDS are the most powerful approach for examining cutaneous diseases. For example, microneedle-based bioassays could be used for virus antigen capture in order to facilitate herpes simplex, or varicella/herpes zoster diagnosis and differentiation (methods in development). In this article, we introduce the possible role and summarize the current literature regarding TDDS application for fighting common cutaneous or systemic viral infectious diseases, including herpes simplex, varicella/herpes zoster, warts, influenza, measles, and COVID-19 (Table 2).
Herpes simplex
Herpes simplex is a viral infection caused by the herpes simplex virus (HSV). Categorized by infection site, the most common types are herpes labialis and genital herpes. Herpes labialis, also called cold sores, present with vesicles, ulcers, and burning pain on the lips or peri-oral area. It is usually caused by herpes simplex virus type 1 (HSV-1), and occasionally herpes simplex virus type 2 (HSV-2). In contrast, genital herpes, which occurs in or around the genital area, is usually caused by HSV-2, and less frequently by HSV-1 [33]. After the initial attack, HSV is transported by retrograde flow along axons that connect the point of entry into the body to nuclei of sensory neurons[34], and the virus may periodically reactivate to create another outbreak of painful vesicles or ulcers [35]. The standard therapy for HSV infection is the small-molecule drug, acyclovir. Acyclovir is a synthetic acyclic purine-nucleoside analogue [33]; it can inhibit HSV DNA replication at the stratum basale [36].
Systemic acyclovir has possible side effects including nausea, vomiting, diarrhea, headaches, malaise, nephrotoxicity, and neurotoxicity including agitation, hallucinations, disorientation, tremors, and myoclonus [33, 37, 38]. However, topical acyclovir requires frequent application to achieve only moderate efficacy, primarily because of its high hydrophilicity and poor skin permeation [39].
A TDDS-based buccal mucoadhesive system has been designed for systemic delivery of acyclovir. These patches can release drug into the oral cavity at a predetermined rate and present distinct advantages over traditional dosage forms [40]. This trans-buccal delivery system, which uses a mucoadhesive, copolymers of acrylic acid, and poly(ethylene glycol), has demonstrated feasibility for controlled oral mucosal acyclovir delivery according to in vitro permeation studies [41]. Anther buccal delivering film based on chitosan hydrochloride and polyacrylic acid sodium salt with a 1/1.3 weight ratio also represents a promising formulation for buccal delivery of acyclovir [42]. Moisture-activated patches, which are fabricated with gel intermediates for film-type patches with mucoadhesive polymer, viscosity builders, enhancers, and acyclovir, also demonstrated feasibility for acyclovir delivery through the skin or gingival mucosa [43].
To solve the limitations of poor skin permeation for topical acyclovir formulations, dissolving polymeric microneedle arrays loaded with acyclovir have been developed that break the skin barrier and improve acyclovir delivery to the stratum basale, thus achieving enhanced site-specific acyclovir delivery [44]. In vitro studies have shown that the percentage of total acyclovir loading inside the skin delivered by microneedle arrays was 45 times higher than that of commercially available acyclovir cream after 24 h. The accumulation of acyclovir at the stratum basale was also 5 times greater than the dose required for treating HSV infections. In one in vivo study, intradermal acyclovir delivery via microneedles arrays was also much higher than that for a commercially available cream formulation [44]. These studies indicate that acyclovir-loaded dissolving microneedle arrays are a promising approach for fighting topical HSV infections and they are especially useful for circumventing serious drug-related side effects associated with systemic drug delivery following oral or intravenous drug administration.
Varicella and herpes zoster
Varicella and herpes zosters are caused by the varicella-zoster virus (VZV). Primary VZV infection results in varicella, or chickenpox, which presents with diffuse papules, vesicles, pustules, and crusts. The endogenous reactivation of latent VZV typically results in painful grouped vesicles distributed along one or adjacent dermatomes, known as herpes zosters. Disease complications can develop in immunocompromised patients, including disseminated infection, myelitis, cranial nerve palsies, meningitis, pneumonia, and hepatitis. The most bothersome problem associated with herpes zosters is that the VZV reactivation often causes a postherpetic neuralgia, and some patients may suffer from long-term moderate to severe pain. This chronic pain may be attributable to inflammation from the viral infection and the death of primary neurons [45]. The site of pain is usually localized to the site of the herpes zosters. In addition to traditional oral systemic analgesic drugs, which may generate intolerable side effects, localized pain may be treated by transdermal lidocaine patches that have been developed for postherpetic neuralgia. A double-blind, randomized controlled study demonstrated that a 5% lidocaine patch applied twice daily was well-tolerated and significantly effective for relieving moderate to severe postherpetic neuralgia due to the pharmacological action of lidocaine and the physical barrier effect of the patch on sensitized skin [46].
Microneedles offer several benefits: they are painless, easy to use, and they eliminate the risk for cross-contamination associated with conventional needles. Further, microneedle-based vaccination can provide comparable or even higher immune response compared to conventional intramuscular injection approaches.
A herpes zoster vaccine has been developing using a microneedle coated with recombinant glycoprotein E (gE) of VZV with some different adjuvants. GE is important for VZV replication and cell-to-cell spread. The added adjuvants increased overall gE-specific antibody titers compared to results from non-adjuvant added tests, and different adjuvants demonstrated various potential for inducing humoral response or cell-mediated immune response. Generally speaking, microneedle-based herpes zosters subunit vaccines may be useful for providing a potent immune response against reactivation of VZV [47].
Rapid virological diagnosis of varicella and herpes zoster facilitates improved antiviral treatment. Polymerase chain reaction (PCR) for detecting VZV DNA and immunofluorescent VZV-specific antigen staining are two best diagnostic approaches in regard to sensitivity and specificity [48]. Also, swabs from the base and clear vesicular fluid of early vesicles provide the best sensitivity [48]. Recover from infectious VZV and varicella lesions is achievable in 2 to 3 days but can be isolated from herpes zoster lesions for a week or longer [45]. Before varicella or herpes zoster vesicles appear, there are earlier stages that present erythematous papules or edematous erythematous plaques. Microneedles may extract interstitial fluid with VZV from pre-vesicular skin lesions for virus antigen detection, providing even earlier diagnosis (methods in development).
Warts
Warts or verruca are cutaneous viral infections caused by human papillomavirus (HPV). They present papules or plaques of variable size, often with a rough scaly surface. Local spread of skin lesions is often found. According to anatomic location or morphology, warts are usually classified as common warts (verruca vulgaris), flat warts (verruca plana), plantar and palmar warts, and anogenital warts (condyloma acuminatum). Treatment of warts often requires physical or immune-mediated destruction of infected epithelial cells. Cryotherapy, using liquid nitrogen to freeze and destroy wart lesions, is the most often used method today. However, cryotherapy is quite painful and some patients may not tolerate the pain of repeated treatment.
Topical salicylic acid is also a common treatment for warts. Salicylic acid has a chemical peeling effect on the skin, and it can be used to partially remove epidermal tissue, as well as the warts, over time. Early, transdermal delivery of karaya gum patches containing salicylic acid have been used to treat verruca vulgaris, and have demonstrated good wart resolution rate. Karaya gum patches provide an occluded environment, which is an additional therapeutic advantage. This is a typical example of a simple TDDS application that can significantly improve topical drug penetration [49].
Microneedle drug delivery can also be used to treat warts. Intralesional bleomycin, a common off-label product for warts treatment, has side effects including burning, severe pain, and tissue necrosis. Additionally, the cure percentages for intralesional bleomycin wart treatment vary from 0 to 100%, probably because of variable drug distribution and poor drug infiltration into the wart lesion [50]. Microneedles that create a penetration route have been used with topical bleomycin to increase wart cure rates [51]. Intralesional 5-fluorouracin (5-FU) is also an off-label treatment product for warts that can cause intense pain when used. Microneedles used in combination with topical 5-FU solution also demonstrated comparable results to intralesional 5-fluorouracin, but with significantly less pain, less sessions required, and improved patient satisfaction [52].
Recently, a newly developed bleomycin microneedle patch has been developed to treat warts. The efficacy of the bleomycin microneedle patch was comparable to that of cryotherapy according to both physician’s and patient’s global assessment scores. However, treatment with the bleomycin microneedle patch was significantly less painful than cryotherapy as evidenced by reports of significantly lower visual analogue scale pain from patients in the microneedle group. A primary advantage of TDDS is decreased pain. Microneedles for encapsulated drug delivery are effective, convenient, and less painful than normal injection drug delivery, and it is especially well suited for treating cutaneous lesions [53].
HPV vaccine has recently drawn great public attention. It can prevent HPV infection and subsequent HPV-related diseases including genital warts, cervical dysplasia, and invasive cancer. Although the recommended population for HPV vaccine is women aged 9–26 years, women aged over 26 years can still benefit from HPV vaccination [54]. As HPV vaccination is more and more prevalent, and the major vaccine recipients are children and young women, a less painful and convenient vaccination modality would be beneficial. A recent in vivo study examined the use of a new-form HPV vaccine using HPV pseudovirus-encapsidated plasmids delivered by an microneedle array, and found that this approach elicited robust HPV-specific immune responses in mice [55]. This study suggested that microneedle HPV vaccine delivery demonstrated potential as a practical disease prevention modality.
Influenza
Influenza is a contagious respiratory illness caused by influenza viruses. People with influenza present variable severity from typical symptoms such as fever, muscle pain, headaches, cough, sore throat, and runny nose to more severe symptoms such as pneumonia that can lead to hospitalization or death. Immunocompromised or elderly patients are at high risk for developing serious complications and have demonstrated a greater mortality rate. Influenza vaccination is the most effective approach for preventing influenza and for preventing its spread among the population. In Taiwan, the government-funded seasonal influenza vaccination launches before influenza season, usually starts from December, and peaks in January to February of the following year [56]. A typical seasonal vaccine contain several antigens, influenza A subtypes H1N1 and H3N2 with one or two antigens from influenza type B, and is administered by intramuscular injection traditionally [57]. However, some studies have suggested that intradermal injection of a reduced, as low as one fifth, dose of influenza vaccine induces comparable levels of antibody compared with an intramuscular injection of full-dose influenza vaccine [58, 59].
Compared to needle-based intradermal injection, microneedle patch-based systems facilitate less painful and more simplified vaccine administration. Influenza vaccination using inactivated virus-coated microneedles in a murine model has been widely studied in recent 10 years [24, 60]. Microneedle vaccines incorporated with a stabilizer coating formulation, such as trehalose, prevent antigenicity loss of the influenza vaccine [61, 62]. Multiple studies suggest enhanced memory responses after microneedle vaccination compared to intramuscular injection or subcutaneous injection.[24, 62–64]. Microneedles coated with stabilized vaccine induced virus-neutralizing antibodies, enhanced humoral immunity, induced cellular recall responses, and provided superior protection against a lethal challenge compared to intramuscular injection, as evidenced by effective virus clearance from the lungs [24, 63]. One study demonstrated that microneedle influenza vaccine induced long-lived high functional immunity capable of complete survival against H1N1 lethal challenge even 6 months after a single vaccination [64].
In addition to vaccines using inactivated influenza virus, some microneedle coated vaccine using virus-like particle (VLP) have been performed. Microneedles coated with influenza VLPs using an unstabilized formulation demonstrated decreased haemagglutinin activity, but the inclusion of trehalose disaccharide was shown to preserve influenza VLP vaccine haemagglutinin activity; therapeutic results demonstrated stronger immunity compared to intramuscular injection [24, 65, 66]. Recombinant subunit vaccine consisting of trimeric influenza hemagglutinin protein has also been administrated via microneedles, and showed improved immunity compared to subcutaneous injection in mice [28]. Vaccines with selective antigen coating have been applied to the tips of microneedles in order to improve vaccine delivery to target Langerhans cells that are primarily in the suprabasal layers of the epidermis [29], and efficiently prime antigen-specific CD8+ T cells and facilitate the differentiation of CD4+ cells [30]. This microneedle design targeting Langerhans cells thus significantly reduced the amount of vaccine required for immunization. The results of this study revealed that most of the coated vaccine material was released from the tips of the microneedle projections to the target locations within 2 min of skin application and that a strong immune response against influenza was subsequently demonstrated in a murine model [67].
Recently, a randomized, partly blinded, placebo-controlled, phase 1 trial for an inactivated influenza microneedle vaccine was conducted [26]. The microneedle patch in this trial was applied directly onto the wrist, and the microneedles coated with inactivated influenza vaccine dissolved and released the vaccine into the dermis. The residual patch was disposed of as non-sharps waste. The microneedle vaccine was stable and could be stored for at least one year at 40 °C. Results showed that these dissolvable microneedle patches for influenza vaccination were well-tolerated, resulted in robust antibody responses, and could be reliably self-administered. Microneedle vaccination was preferred over conventional influenza vaccination using needles and syringes [26].
Surface-modified microneedle arrays have been developed to capture circulating influenza biomarkers from the skin. Previous studies have investigated the capacity for microneedle arrays to detect influenza antigen-specific IgG. One animal-model study inserted microneedles covalently linked with influenza antigens into laser-treated skin of animals previously treated for influence to increase circulating biomarkers in the upper dermis by increasing vascular permeability. Results indicated that microneedle detection provided comparable sensitivity, specificity, and accuracy to results attained by standard blood sample immunoassays [32].
Measles
Measles is a contagious disease caused by the measles virus. It characteristically presents fever, cough, coryza, conjunctivitis, and morbilliform skin rashes [68]. Measles is highly infectious and transmitted by aerosol or respiratory droplet aspirated into the respiratory route. It remains one of the most devastating causes of worldwide morbidity and mortality in children despite the availability of a safe and efficacious vaccine [69, 70]. Two vaccines have been developed and licensed: a formalin-inactivated whole virus vaccine and a live attenuated vaccine [69]. In Taiwan, nationwide immunization programs evolved from 2-dose measles vaccine in 1978 to 2-dose measles-mumps-rubella (MMR) vaccine in 1992, and the coverage rate of MMR was over 95% in 2001.[71, 72]. Following the rise in routine immunization coverage for children, the global coverage for the first dose of measles vaccine increased to 85% in 2017, and measles incidence decreased significantly [73, 74].
Simplifying measles vaccination with a less invasive approach that would be more acceptable among children would provide greater coverage and impact. Vaccination using a microneedle patch was first studied in 2013 [75]. Edens et al. fabricated microneedles coated with live-attenuated measles vaccine that was stabilized by trehalose. Vaccination using microneedles in a rat model generated a considerable immune response, equivalent to that generated by subcutaneous injection [75].
Later, a polymeric microneedle patch was formulated to encapsulate the standard dose of measles vaccine and test immunogenicity in rhesus macaques [76]. Compared with subcutaneous injection, the microneedle vaccine generated equivalent neutralizing antibody titers. In addition, the microneedle vaccines showed better thermostability compared to standard lyophilized vaccine, which improved storage and facilitated an increase in vaccination coverage [76].
A dissolving microneedle patch was developed for delivering both measles and rubella vaccine, and immunogenicity was evaluated in rhesus macaques [77]. The results showed that protective titers of measles neutralizing antibodies were detected in 100% of macaques in the microneedle group, compared to only 75% of macaques in the subcutaneous injection groups [77]. Rubella-neutralizing antibody titers were adequate for all groups. However, maternal antibodies can interfere with infant immune response to the vaccination. Vaccination by both routes was unable to generate protective immune responses to measles in infant macaques pretreated with measles immunoglobulin to simulate maternal antibody production [77].
For global elimination of measles, very high vaccination coverage is required, especially for those in resource-limited regions. Microneedle measles vaccines can simplify vaccine delivery logistics, provide thermostability outside of the cold chain, enable vaccination by minimally trained personnel, leave no biohazardous sharps waste, reduce total cost, and could, as a result, increase vaccination coverage. The development of microneedle patch delivery systems for measles vaccination has great potential benefit for eliminating measles.
COVID-19
Coronavirus disease 2019 (COVID-19), a newly emerged contagious respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a pandemic outbreak. Patients with COVID-19 mostly present with mild to moderate symptoms. However, approximately 15% of patients progress to severe pneumonia, and about 5% develop acute respiratory distress syndrome, septic shock, or multiple organ failure finally [78, 79]. Although COVID-19 is, fortunately, not especially prevalent in Taiwan, it has had devastating impact globally and measures to combat viral spread are still critically important. Herein, we summarize the role of TDDS to help fight this international public health emergency.
Early etiological diagnosis plays a critical role in controlling the COVID-19 pandemic. Early diagnosis of COVID-19 usually relies on viral nucleic acid testing from oropharyngeal swabs. Limited sampling efficiency associated with conventional oropharyngeal swabs may lead to false-negative results. A study has been conducted using microneedle-based oropharyngeal swabs to reduce false negative COVID-19 testing rates [80]. This study used microneedles integrated with virus-specific antibodies in the swab to provide effective penetration and facilitate virus capture. This highly effective newly designed microneedle sampling significantly reduced false negative rates, and may contribute to the containment of the COVID-19 pandemic [80].
For preventing COVID-19 spread, safe vaccines that rapidly induce potent and long-lasting virus-specific immune responses against coronavirus are urgently needed. The coronavirus spike (S) protein, a characteristic structural component of the viral envelope, is considered a key target for vaccines. Microneedle arrays have been developed for COVID-19 vaccines delivery. Novel vaccines with dissolving microneedle arrays containing embedded SARS-CoV-2-S1 subunits have been fabricated [27]. This study demonstrated that SARS-CoV-2 S1 subunit vaccines delivered with microneedles provided effective immune responses observed 2 weeks after vaccination, and responses were significantly stronger than those administered by traditional subcutaneous needle injection, indicating the improved immunogenicity of skin-targeted delivery. Microneedle vaccines are a promising immunization strategy to combat coronavirus infection. They may even be more beneficial because they are easy to use, they reduce pain, and are noninvasive by nature, and the superior capacity for self-administration could increase vaccine coverage and help eliminate the disease [27].
Conclusion
TDDS represent an excellent alternative to oral delivery and hypodermic injections. To fight viral infections, TDDS, especially microneedles, provide a more convenient and less invasive way for disease treatment, prevention, and surveying. Microneedles improve skin barrier penetration to improve the delivery of macromolecules and hydrophilic drugs. Microneedles may also provide a viable, alternative approach for vaccination with improved immunogenicity, tolerance, and ease of use. Further, microneedle vaccines are thermostable and simplify storage needs, eliminate the need for vaccine reconstitution, eliminate sharps waste, reduce vaccine wastage, and are more cost-effective. The improved patient compliance and possibility for self-administration may increase vaccine coverage, which is especially desirable for treating pandemic and other highly prevalent viral infections such as influenzas, measles, and COVID-19 (Table 2). They could be an invaluable tool for eliminating infectious diseases globally. On the other hand, microneedle-based bioassays employing transdermal extraction of interstitial fluid or blood provide a minimally invasive approach for surveying disease markers and possible point-of-care diagnostics. And, for cutaneous viral infection, TDDS provide localized treatment with high specificity and reduced systemic toxicity. In summary, TDDS, especially those employing microneedles, are well suited for reducing the morbidity and mortality associated with viral infectious diseases, and could be of great potential value to global health.
References
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–8.
Dharadhar S, Majumdar A, Dhoble S, Patravale V. Microneedles for transdermal drug delivery: a systematic review. Drug Dev Ind Pharm. 2019;45(2):188–201.
Kalia YN, Merino V, Guy RH. Transdermal drug delivery: clinical aspects. Dermatol Clin. 1998;16(2):289–99.
Kornick CA, Santiago-Palma J, Moryl N, Payne R, Obbens EA. Benefit-risk assessment of transdermal fentanyl for the treatment of chronic pain. Drug Saf. 2003;26(13):951–73.
Ita K. Transdermal delivery of drugs with microneedles—potential and challenges. Pharmaceutics. 2015;7(3):90–105.
Frei A, Andersen S, Hole P, Jensen NH. A one year health economic model comparing transdermal fentanyl with sustained-release morphine in the treatment of chronic noncancer pain. J Pain Palliat Care Pharmacother. 2003;17(2):5–26.
Varvel J, Shafer S, Hwang S, Coen P, Stanski D. Absorption characteristics of transdermally administered fentanyl. The Journal of the American Society of Anesthesiologists. 1989;70(6):928–34.
Ale IS, Maibach HA. Diagnostic approach in allergic and irritant contact dermatitis. Expert Rev Clin Immunol. 2010;6(2):291–310.
Bala P, Jathar S, Kale S, Pal K. Transdermal drug delivery system (TDDS)—a multifaceted approach for drug delivery. J Pharm Res. 2014;8(12):1805–35.
Bariya SH, Gohel MC, Mehta TA, Sharma OP. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64(1):11–29.
Thong HY, Zhai H, Maibach HI. Percutaneous penetration enhancers: an overview. Skin pharmacology and physiology. 2007;20(6):272–82.
Sloan KB, Wasdo SC, Rautio J. Design for optimized topical delivery: prodrugs and a paradigm change. Pharm Res. 2006;23(12):2729–47.
Rothbard JB, Garlington S, Lin Q, Kirschberg T, Kreider E, McGrane PL, et al. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat Med. 2000;6(11):1253–7.
Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004;56(5):619–58.
Machet L, Boucaud A. Phonophoresis: efficiency, mechanisms and skin tolerance. Int J Pharm. 2002;243(1–2):1–15.
Ogura M, Paliwal S, Mitragotri S. Low-frequency sonophoresis: current status and future prospects. Adv Drug Deliv Rev. 2008;60(10):1218–23.
Zhao YL, Murthy SN, Manjili MH, Guan L, Sen A, Hui SW. Induction of cytotoxic T-lymphocytes by electroporation-enhanced needle-free skin immunization. Vaccine. 2006;24(9):1282–90.
Bramson J, Dayball K, Evelegh C, Wan Y, Page D, Smith A. Enabling topical immunization via microporation: a novel method for pain-free and needle-free delivery of adenovirus-based vaccines. Gene Ther. 2003;10(3):251–60.
Herndon TO, Gonzalez S, Gowrishankar T, Anderson RR, Weaver JC. Transdermal microconduits by microscission for drug delivery and sample acquisition. BMC Med. 2004;2(1):1–11.
Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Coll Interface Sci. 2006;123:369–85.
Palmer BC, DeLouise LA. Nanoparticle-Enabled Transdermal Drug Delivery Systems for Enhanced Dose Control and Tissue Targeting. Molecules. 2016;21(12). https://doi.org/10.3390/molecules21121719.
Patel AV, Shah BN. Transadermal drug delivery system: a review. Pharma Science Monitor. 2018;9(1).
Waghule T, Singhvi G, Dubey SK, Pandey MM, Gupta G, Singh M, et al. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–58.
Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–68.
Tasca F, Tortolini C, Bollella P, Antiochia R. Microneedle-based electrochemical devices for transdermal biosensing: a review. Curr Opin Electrochem. 2019;16:42–9.
Rouphael NG, Paine M, Mosley R, Henry S, McAllister DV, Kalluri H, et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet. 2017;390(10095):649–58. https://doi.org/10.1016/s0140-6736(17)30575-5.
Kim E, Erdos G, Huang S, Kenniston TW, Balmert SC, Carey CD, et al. Microneedle array delivered recombinant coronavirus vaccines: immunogenicity and rapid translational development. EBioMedicine. 2020;55:102743.
Weldon WC, Martin MP, Zarnitsyn V, Wang B, Koutsonanos D, Skountzou I, et al. Microneedle vaccination with stabilized recombinant influenza virus hemagglutinin induces improved protective immunity. Clin Vaccine Immunol. 2011;18(4):647–54.
Romani N, Holzmann S, Tripp CH, Koch F, Stoitzner P. Langerhans cells–dendritic cells of the epidermis. APMIS. 2003;111(7–8):725–40.
Zaric M, Lyubomska O, Poux C, Hanna ML, McCrudden MT, Malissen B, et al. Dissolving microneedle delivery of nanoparticle-encapsulated antigen elicits efficient cross-priming and Th1 immune responses by murine Langerhans cells. J Investig Dermatol. 2015;135(2):425–34.
Liu GS, Kong Y, Wang Y, Luo Y, Fan X, Xie X, et al. Microneedles for transdermal diagnostics: recent advances and new horizons. Biomaterials. 2020;232:119740.
Li B, Wang J, Yang SY, Zhou C, Wu MX. Sample-free quantification of blood biomarkers via laser-treated skin. Biomaterials. 2015;59:30–8.
Whitley RJ, Roizman B. Herpes simplex virus infections. The lancet. 2001;357(9267):1513–8.
Stevens JG, Cook ML. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971;173(3999):843–5.
Opstelten W, Neven AK, Eekhof J. Treatment and prevention of herpes labialis. Can Fam Physician. 2008;54(12):1683–7.
Peira E, Trotta M, Carlotti ME, Gallarate M, Chirio D. Elastic positively-charged liposomes for topical administration of acyclovir. Journal of drug delivery science and technology. 2007;17(5):321–4.
Haefeli WE, Schoenenberger RA, Weiss P, Ritz RF. Acyclovir-induced neurotoxicity: concentration-side effect relationship in acyclovir overdose. Am J Med. 1993;94(2):212–5.
Sawyer MH, Webb DE, Balow JE, Straus SE. Acyclovir-induced renal failure: clinical course and histology. Am J Med. 1988;84(6):1067–71.
Spruance SL, Crumpacker CS. Topical 5 percent acyclovir in polyethylene glycol for herpes simplex labialis: antiviral effect without clinical benefit. Am J Med. 1982;73(1):315–9.
Saxena A, Tewari G, Saraf SA. Formulation and evaluation of mucoadhesive buccal patch of acyclovir utilizing inclusion phenomenon. Braz J Pharm Sci. 2011;47(4):887–97.
Shojaei AH, Zhuo S, Li X. Transbuccal delivery of acyclovir (II): feasibility, system design, and in vitro permeation studies. J Pharm Pharm Sci. 1998;1(2):66–73.
Rossi S, Sandri G, Ferrari F, Bonferoni MC, Caramella C. Buccal delivery of acyclovir from films based on chitosan and polyacrylic acid. Pharm Dev Technol. 2003;8(2):199–208.
Kim AM, Gwak HS, Chun IK. Formulation and evaluation of moisture-activated acyclovir patches. J Pharm Investig. 2006;36(6):393–9.
Pamornpathomkul B, Ngawhirunpat T, Tekko IA, Vora L, McCarthy HO, Donnelly RF. Dissolving polymeric microneedle arrays for enhanced site-specific acyclovir delivery. Eur J Pharm Sci. 2018;121:200–9. https://doi.org/10.1016/j.ejps.2018.05.009.
Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9(3):361–81.
Lin PL, Fan SZ, Huang CH, Huang HH, Tsai MC, Lin CJ, et al. Analgesic effect of lidocaine patch 5% in the treatment of acute herpes zoster: a double-blind and vehicle-controlled study. Reg Anesth Pain Med. 2008;33(4):320–5.
Cha HR, Shim DH, Lee J. A microneedle vaccination with glycoprotein E of Varicella Zoster virus elicits antibody production and polyfuctional T cells in mice. Am Assoc Immnol. 2020.
Sauerbrei A, Eichhorn U, Schacke M, Wutzler P. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14(1):31–6.
Bart BJ, Biglow J, Vance JC, Neveaux JL. Salicylic acid in karaya gum patch as a treatment for verruca vulgaris. J Am Acad Dermatol. 1989;20(1):74–6.
Saitta P, Krishnamurthy K, Brown LH. Bleomycin in dermatology: a review of intralesional applications. Dermatol Surg. 2008;34(10):1299–313. https://doi.org/10.1111/j.1524-4725.2008.34281.x.
Konicke K, Olasz E. Successful treatment of recalcitrant plantar warts with bleomycin and microneedling. Dermatol Surg. 2016;42(8):1007–8. https://doi.org/10.1097/dss.0000000000000738.
Ghonemy S, Ibrahim Ali M, Ebrahim HM. The efficacy of microneedling alone vs its combination with 5-fluorouracil solution vs 5-fluorouracil intralesional injection in the treatment of plantar warts. Dermatol Ther. 2020;33(6):e14179.
Ryu HR, Jeong HR, Seon-Woo HS, Kim JS, Lee SK, Kim HJ, et al. Efficacy of a bleomycin microneedle patch for the treatment of warts. Drug Deliv Transl Res. 2018;8(1):273–80.
Basu P, Ngan HYS, Hseon TE, Board ACCPA. HPV vaccination in women over 25 years of age: Asian Cervical Cancer Prevention Advisory Board recommendations. J Obste Gynaecol Res. 2009;35(4):712–6.
Kines RC, Zarnitsyn V, Johnson TR, Pang YYS, Corbett KS, Nicewonger JD et al. Vaccination with human papillomavirus pseudovirus-encapsidated plasmids targeted to skin using microneedles. PloS One. 2015;10(3):e0120797.
Su CP, Tsou TP, Chen CH, Lin TY, Chang SC, Group IC et al. Seasonal influenza prevention and control in Taiwan—strategies revisited. J Formos Med Assoc. 2019;118(3):657–63.
Kommareddy S, Baudner B, Bonificio A, Gallorini S, Palladino G, Determan A, et al. Influenza subunit vaccine coated microneedle patches elicit comparable immune responses to intramuscular injection in guinea pigs. Vaccine. 2013;31(34):3435–41.
Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351(22):2286–94. https://doi.org/10.1056/NEJMoa043555.
Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351(22):2295–301.
Zhu Q, Zarnitsyn VG, Ye L, Wen Z, Gao Y, Pan L, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci. 2009;106(19):7968–73.
Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142(2):187–95.
Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS ONE. 2009;4(9):e7152.
Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine. 2009;27(49):6932–8.
Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, Jacob J, Prausnitz MR, Compans RW, et al. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. J Infect Dis. 2011;204(4):582–91.
Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, et al. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol. 2010;84(15):7760–9.
Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS PharmSciTech. 2010;11(3):1193–201.
Chen X, Corbett HJ, Yukiko SR, Raphael AP, Fairmaid EJ, Prow TW, et al. Site-selectively coated, densely-packed microprojection array patches for targeted delivery of vaccines to skin. Adv Func Mater. 2011;21(3):464–73.
Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis. 2004;189(Supplement_1):S4-S16.
Griffin DE. Measles vaccine. Viral Immunol. 2018;31(2):86–95.
Wolfson LJ, Grais RF, Luquero FJ, Birmingham ME, Strebel PM. Estimates of measles case fatality ratios: a comprehensive review of community-based studies. Int J Epidemiol. 2009;38(1):192–205.
Liu D, Wang E, Pan Y, Cheng S. Innovative applications of immunisation registration information systems: example of improved measles control in Taiwan. Eurosurveillance. 2014;19(50):20994.
Lee YC, Lee YH, Lu CW, Cheng SY, Yang KC, Huang KC. Measles immunity gaps in an era of high vaccination coverage: a serology study from Taiwan. Travel Med Infect Dis. 2020;36:101804.
Dabbagh A, Laws RL, Steulet C, Dumolard L, Mulders MN, Kretsinger K, et al. Progress toward regional measles elimination—worldwide, 2000–2017. Morb Mortal Wkly Rep. 2018;67(47):1323.
Prausnitz MR, Goodson JL, Rota PA, Orenstein WA. A microneedle patch for measles and rubella vaccination: a game changer for achieving elimination. Curr Opin Virol. 2020;41:68–76.
Edens C, Collins ML, Ayers J, Rota PA, Prausnitz MR. Measles vaccination using a microneedle patch. Vaccine. 2013;31(34):3403–9.
Edens C, Collins ML, Goodson JL, Rota PA, Prausnitz MR. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine. 2015;33(37):4712–8.
Joyce JC, Carroll TD, Collins ML, Chen MH, Fritts L, Dutra JC et al. A microneedle patch for measles and rubella vaccination is immunogenic and protective in infant rhesus macaques. J Infect Dis. 2018;218(1):124–32.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China The lancet. 2020;395(10223):497–506.
Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–70.
Chen W, Cai B, Geng Z, Chen F, Wang Z, Wang L, et al. Reducing false negatives in COVID-19 testing by using microneedle-based oropharyngeal swabs. Matter. 2020;3(5):1589–600.
Acknowledgements
The authors would like to thank all subjects who participated in this study, and the study CMRPGBL0021.
Funding
CMRPGBL0021
Author information
Authors and Affiliations
Contributions
F.-Y.W., Y.C., and Y.-Y.H. reviewed the articles from the journal databases, drafted, and revised the manuscript. C.-M.C. highlighted the concept of this review article, and revised the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Consent for publication
All authors have agreed with this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, FY., Chen, Y., Huang, YY. et al. Transdermal drug delivery systems for fighting common viral infectious diseases. Drug Deliv. and Transl. Res. 11, 1498–1508 (2021). https://doi.org/10.1007/s13346-021-01004-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01004-6