Abstract
Objective. Before peripheral nerve electrodes can be used for the restoration of sensory and motor functions in patients with neurological disorders, the behavioral and histological consequences of these devices must be investigated. These indices of biocompatibility can be defined in terms of desired functional outcomes; for example, a device may be considered for use as a therapeutic intervention if the implanted subject retains functional neurons post-implantation even in the presence of a foreign body response. The consequences of an indwelling device may remain localized to cellular responses at the device-tissue interface, such as fibrotic encapsulation of the device, or they may affect the animal more globally, such as impacting behavioral or sensorimotor functions. The objective of this study was to investigate the overall consequences of implantation of high-electrode count intrafascicular peripheral nerve arrays, High Density Utah Slanted Electrode Arrays (HD-USEAs; 25 electrodes mm−2). Approach. HD-USEAs were implanted in rat sciatic nerves for one and two month periods. We monitored wheel running, noxious sensory paw withdrawal reflexes, footprints, nerve morphology and macrophage presence at the tissue-device interface. In addition, we used a novel approach to contain the arrays in actively behaving animals that consisted of an organic nerve wrap. A total of 500 electrodes were implanted across all ten animals. Main results. The results demonstrated that chronic implantation (⩽8 weeks) of HD-USEAs into peripheral nerves can evoke behavioral deficits that recover over time. Morphology of the nerve distal to the implantation site showed variable signs of nerve fiber degeneration and regeneration. Cytology adjacent to the device-tissue interface also showed a variable response, with some electrodes having many macrophages surrounding the electrodes, while other electrodes had few or no macrophages present. This variability was also seen along the length of the electrodes. Axons remained within the proximity of the electrode tips at the distances required for theoretically effective stimulation and recording (⩽100 μm). Significance. We conclude from these studies that HD-USEAs do not cause overall global effects on the animals, at least up to the two-month period investigated here. These results demonstrate for the first time that the consequences of high-electrode count intrafascicular arrays compare with other peripheral nerve electrodes currently available for clinical or investigational neuromodulation.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Abbreviations
1. Introduction
The use of peripheral nerve electrodes offers new therapeutic approaches for the restoration of sensory and motor function following nerve injury or disease [1]. Assessment of the behavioral and histological consequences of implantation of such neural interfaces is an important milestone in the development of this technology as it progresses from acute animal experimentation to clinical applications. For a device to be considered for use as a potential therapeutic intervention, it must meet a predetermined set of criteria after implantation into living tissue.
Nerve electrode arrays have recently been developed that contain a large number of electrodes, allowing for more information to be transferred to and received from the nervous system for neuromodulation studies and applications [2]. To date, larger peripheral nerves (>3 mm diameter) have been implanted with devices that have between 16 [3] and 96 [4] electrodes. For smaller peripheral nerves (⩽1 mm), three different multi-electrode neural interfaces have been manufactured and chronically implanted (⩽3 months), including: high channel-count regenerative sieve electrodes (19 electrodes [5]); 27 electrodes [6]), transverse intrafascicular multichannel electrodes (TIMEs; 5 electrodes [7]), and thin-film longitudinal intrafascicular electrodes (tfLIFEs; eight electrodes [8]). The behavioral and cellular consequences of chronic implantation of these devices have been investigated [6–8]. However, no studies have been carried out that investigate the consequences of chronically implanted peripheral nerve high-electrode count penetrating microelectrode arrays.
A new high electrode-count neural interface, the High Density Utah Slanted Electrode Array (HD-USEA; 25 electrodes mm−2), has recently been fabricated and tested in acute experiments (<12 h) for its use in small peripheral nerves (<2 mm) [2, 9]. The motivation for developing such a high electrode-count array was to increase the selective intrafascicular access to nerve fibers in millimeter and sub-millimeter nervous system structures. In order to expand application of HD-USEAs to chronic preparations, we assessed the behavioral outcomes and cytology at the tissue-device interface in rats that had non-wired HD-USEAs implanted into their sciatic nerves. This study was motivated by the concern that implantation of such a high spatial density of penetrating microelectrodes could severely damage or crush the nerve, negating their use in short-term chronic experimentation. The behavioral and histological criteria we used in this study to assess the use of such high-electrode density arrays in chronic experimentation included: (1) that the devices must have remained implanted in actively behaving animals, (2) that the devices did not permanently interfere with overall animal behavior, and (3) that axons remained within 100 μm of the electrode tips. Non-wired devices were used in this study in order to assess the effects of the implanted electrodes alone, without confounding factors that may arise from wire bundles or a percutaneous connector.
The rat model was chosen for these studies as it has frequently been used in chronic peripheral nerve injury models [10–12], and for standard histological [13–16] and behavioral [10, 15, 17–20] quantification studies. Because functional recovery in the rat has been observed within 3–4 weeks after nerve crush injury and within 6–8 weeks after nerve transection repair, we implanted two cohorts of rats for durations of four and eight weeks to focus on assessing damage and recovery during these early phases of nerve injury [10, 20].
We found that HD-USEAs caused mild behavioral changes that recovered within days to weeks post-implantation and that there was a mild chronic inflammatory response characterized by a non-uniform distribution of macrophages surrounding the tissue-device interface. The novel containment system (an organic nerve wrap) that was used in these studies successfully protected the arrays, and nine of the arrays remained completely implanted in the nerve at the end of the two study periods. Morphology of the nerves was assessed both proximally and distally to the implant site, and signs of degeneration and regeneration were seen. Quantification of these sections showed no significant difference in the myelin thickness, while there was a significant difference the area and diameter of the nerve fibers or axons. Morphology was also assessed at the device tissue interface, and axons remained in close proximity (⩽100 μm) to the tips of the intrafascicular electrodes in both study cohorts. Future studies are now warranted to assess the capabilities of HD-USEAs that are implanted for >8 weeks and for devices that are wired for electrophysiological experimentation.
2. Materials and methods
2.1. Animal groups and behavioral protocols
All studies were approved by the University of Utah Institutional Animal Care and Use Committee. Ten Wistar-Kyoto rats (Charles-River Laboratories International, USA) were randomly assigned to either four or eight-week implant cohorts (n = 5 for each cohort). Wistar–Kyoto rats were chosen as they have been shown to have increased voluntary wheel running over other rat strains [21]. Rats (age ≈ 40 days post-natal at the start of the study) were placed in separate cages each night and provided with food, water, and an exercise wheel ad libitum. Each wheel was equipped with a cyclometer that measured running distance (km), maximum speed (km h−1), average speed (km h−1), and total elapsed running time. Rats were returned to shared housing (3–4 rats/cage) with the same litter mates each morning. All rats were allowed to run voluntarily for nine weeks prior to implantation of HD-USEAs in order to establish pre-implant peak and average running distances [22]. The distances ran nightly were averaged for a weekly distance. For statistical analysis, average weekly distances were compared using t-test statistics for nine weeks of pre-implant data and for either four or eight weeks of post-implant data. Data analysis was carried out using MATLAB (The MathWorks, MA, USA) and Microsoft Excel (Microsoft, WA, USA).
2.2. Surgical procedures
Unwired HD-USEAs were cut to form a 5 × 10 grid of electrodes (lengths ranged from 200 to 800 μm with 200 μm spacing) and then coated in paralyene-C [2]. Prior to implantation, electrodes were cleaned and sterilized. HD-USEAs were implanted into the left sciatic nerve using previously described methodology [2, 9]. In brief, anesthesia was induced and maintained with Isoflurane (5.0% and 0.2% gas, respectively) and vital signs were monitored. The sciatic nerves were exposed and HD-USEAs were pneumatically implanted [23]. The implantation site was then wrapped with a commercially available nerve wrap (AxoGen, Alachua, FL, USA). Nerve wraps were trimmed to fit around the nerves and arrays, with approximately 5 mm of wrap extending both proximally and distally from the HD-USEA. The wraps were closed with sutures (8-0 Nylon, Ethicon) to minimize gaps between the wrap and the nerve. The ends of the wraps were then sutured to the epineurium in order to prevent slippage of the wrap along the length of the nerve. Rats were given minocycline (100 mg L−1) dissolved in water ad libitum for two days before and for five days after implantation of the HD-USEAs [24]. Dexamethasone (200 μg kg−1) was administered by a subcutaneous injection on the day of surgery [25].
2.3. Assessment of sensorimotor reflexes
Assessment was evaluated using the paw withdrawal reflex latency (WRL) test, defined as the time it took for the animals to withdraw their paw from a hot plate set to ≈50 °C (with a cut-off time of 12 s) [10, 19]. Tests were performed with rest intervals >1 min to prevent sensitization and average weekly WRLs were calculated from >3 tests on each hindlimb [10]. Our methodology differed from previous methods only in that we tested the WRL for both hindlimbs in order to control for any small differences in hot plate temperature that may have occurred between testing times. The ratio of WRLs for implanted and control hindlimbs were calculated and expressed as percentages [((Implanted-Control)/Implanted) × 100]. The sensorimotor reflexes were assessed one week before surgery, but not weekly during the control period when peak running distances were obtained. Footprint analysis was carried out using the ratio of the toe-spread length from the first to the fifth digit in the implanted versus the control limb, which has been shown to be an efficient and sensitive test for footprint deficits [18]. Briefly, the rats were placed in a box with a clear bottom and photographs were taken of the standing rats. In order to decrease the chance of any postural tone which could affect footprint stance, the testing room was free of startling noises and the animals were allowed to acclimate in the box before photos were taken [18].
2.4. Histological procedures
Animals were sacrificed by injection of pentobarbital and perfused with 4% paraformaldehyde. The nerves around the implant site and nerve wrap were excised, the nerve wrap was dissected from the nerve, and the HD-USEAs were explanted under a dissecting microscope. The nerves were then transferred to 0.01 M phosphate buffer solution (PBS) with 0.1% sodium azide. Each rat nerve was assigned to a morphological and/or immunohistochemistry processing group, which included: light microscopy cross- and longitudinal sections through the implanted array site; light microscopy cross-sections proximal and distal to the implanted array site; and cross- and longitudinal sections of the array site for immunohistochemical assessment of macrophages. The light microscopy designated samples were incubated for 1 h in 2% OsO4 and further processed for embedding in Epon 812 resin. Semithin sections (0.5 μm thick) were stained with toluidine blue and examined by light microscopy. Images of the whole sciatic nerve were acquired at various magnifications with a microscope (Olympus AX70) using a high-resolution digital camera (Olympus DP-11).
Images were analyzed using the Image J software (National Institutes of Health, Bethesda, MD) and a custom-modified version of software designed to study axonal morphometry (Cavalieri 3.0 macros by G MacDonald, Virginia Merrill Bloedel Hearing Research Center, University of Washington, Seattle, WA). Morphometrical evaluation was performed in sets of images chosen by systematic random sampling of squares representing at least 20–30% of the nerve cross-sectional area. Because it was difficult to automatically select the boundary between the nerve fibers and the surrounding background for small axons and thinly-myelinated fibers using standard image analysis techniques, each digitized image was analyzed using a semiautomatic method. The axonal contour and the external contour of the myelin sheath (fiber contour) were manually traced on enlarged images. A set of custom macros allowed the calculation of the lengths of the major and minor axes of the best fitting ellipse and the cross-sectional area. Fiber diameters and axonal diameters were deduced from the fiber and axonal perimeters assuming a cylindrical shape of axons. These data were used to derive the form factor, roundness of fiber, and myelin sheath thickness. Fibers were defined as the outer diameter of the neuron including the myelin. Axons were defined as the inner diameter of a neuron, not including the myelin. These diameters were calculated from the area of each assuming a circular geometry. Because many of the fibers had elliptical geometry, the form factor, roundness and mean diameter (major axis diameter + minor axis diameter/2) were calculated to quantify differences that could be due to changes in elongation. The ratio of the axon diameter to the fiber diameter (g-ratio) was calculated using two different methods: (1) using the diameters derived from area of the axon and fiber, assuming circular geometry, and (2) using the mean diameter of the major and minor axes.
Samples designated for immunohistochemical processing were washed in PBS, cryoprotected in 30% sucrose at 4 °C and mounted in optimal cutting temperature compound. Longitudinal and traversal sections were cut at 16 μm with a minus 20 °C cryostat (Micron HM505E) and mounted directly onto Superfrost Plus (Thermo Fisher Scientific) slides. Slides were removed from the cryostat, air-dried, blocked for 30 min (0.1 M PBS, 10% normal donkey serum, and 0.5% Triton X-100) and incubated overnight at room temperature in the following primary antibodies (in 0.1 M PBS, pH 7.4, plus 0.5% Triton X-100): mouse anti-rat monocytes/macrophages clone ectodermal dysplasia-1 (ED1; Millipore 1 : 100); and rabbit anti-rat neurofilament-200 (NF-200; Sigma 1 : 300). After three 10 min washes in PBS, the corresponding secondary antibodies to IgG (Invitrogen) conjugated to Alexa Fluor 488 (green), 555 (red), 633 (far red) or 647 (far red) were applied at a 1:100 dilution for 1 h at room temperature. Finally slides were washed three times (10 min each) in PBS before being cover-slipped in fluorescent mounting medium. All fluorescent images were captured with a confocal laser-scanning microscope (TCS SPE, Leica Microsystems, Wetzlar, Germany). Mosaic images were created using Photoshop (Adobe Photoshop CS5, Adobe Systems USA).
3. Results
3.1. Behavioral assessment of rats implanted with HD-USEAs
3.1.1. Footprints
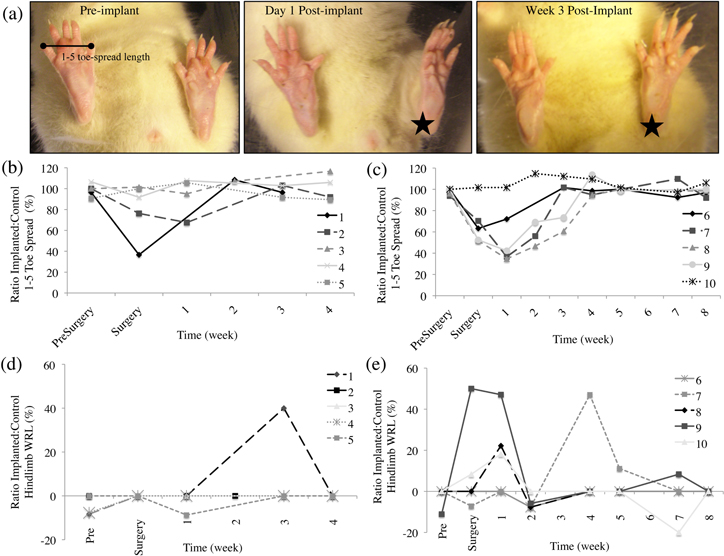
Rat toe-spread lengths were measured for each hindlimb between the first and fifth digits (denoted as '1–5 toe-spread'). An example of one rat's static footprint is shown in figure 1(a) (rat #7, eight-week cohort). This rat showed a decreased toe-spread post-implantation (middle photograph; star indicates implanted side). By the third week after surgery there was a full recovery of the 1–5 toe-spread length. For the four (figure 1(b)) and eight-week (figure 1(c)) cohorts, a total of four rats manifested no decreases in their 1–5 toe-spread lengths for the duration of their implants. The remaining six rats had decreased initial toe-spread lengths in their implanted hindlimbs, but these returned to normal lengths compared to the contralateral foot (mean time for full recovery = 3 weeks; range = 1–4 weeks).
Figure 1. Functional assessment of sensorimotor pathways in rats with HD-USEAs chronically implanted into their sciatic nerves. (a) Footprint analysis of a rat (#7, eight-week cohort) before and after HD-USEA implantation. The pre-implant photograph shows an example of the 1–5 toe-spread length measurement taken from a standing rat. The left sciatic nerve was implanted with an HD-USEA (indicated by a star in post-implant photographs). In this rat, the 1–5 toe-spread length decreased in the implanted leg for two weeks, then returned to control lengths. (b), (c) The 1–5 toe-spread lengths are plotted for rats implanted for four (b) and eight-weeks (c). Six rats had decreased 1–5 toe-spread lengths that returned to normal lengths within 2–4 weeks. (d), (e) The sensorimotor reflexes were assessed in rats implanted with HD-USEAs for (d) four and (e) eight-weeks by measuring the paw withdrawal reflex latency (WRL) times. Five rats had transient increases in WRL times in their implanted hindlimb, which recovered within three weeks (mean = 1.5 weeks). Across all rats, there was no correlation between decreased toe-spread lengths and increased WRL times.
Download figure:
Standard image High-resolution image3.1.2. Withdrawal reflex latencies
Paw WRLs were measured weekly for each rat beginning the week prior to surgery and weekly thereafter. The pre-surgical value for the WRL manifests no differences in the latency times between left and right hindlimbs as they reflexively withdrew from the hot plate. Figures 1(d), (e) show the mean value (computed from three measurements) of the ratio of WRLs in the implanted versus the control leg for the four and eight-week cohorts. A total of three rats had WRL ratios that increased above baseline within the first week after HD-USEA implantation, with latencies returning to normal by the second week. Two rats had increases in WRL times in their implanted leg 3–4 weeks after surgery, with times returning to baseline within 1–2 weeks. The mean recovery time for the five rats with increased WRL in their implanted leg was 1.5 weeks.
3.1.3. Voluntary running behavior
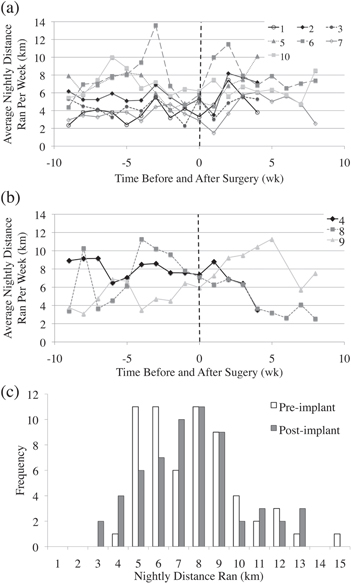
Average distances in kilometers ran per night over the course of one week were plotted for rats that had no significant (figure 2(a); two-tailed t-test; p > 0.05 for each rat) and significant (figure 2(b); two-tailed t-test; p < 0.05 for each rat) differences in running distances before and after HD-USEA implantation. Note that here the rats have been grouped according to significant (three rats) and non-significant (seven rats) running changes and not by implanted time periods. For the rats that had significant changes in running distances after surgery, two of these rats had decreased weekly running distances (rat #8, 8-week group, pre-implant average = 7.44 km/night, post-implant average = 4.45 km/night, p = 0.0294; rat #4, 4-week group, pre-implant average = 7.64 km/night, post-implant average = 2.87 km/night, p ≪ 0.0005), while one rat had increased average nightly distances ran (rat #9, pre-implant average = 4.94 km/night, post-implant average = 8.73 km/night, p = 0.0005). There were no significant differences (p = 0.70; two-tailed t-test;) in the mean and standard deviations of the weekly distances ran across all rats when comparing pre-implantation (6.24 ± 2.3 km/night; mean ± std) and post-implantation (6.07 ± 2.45 km/night) groups (figure 2(c)).
Figure 2. Voluntary running in rats implanted with HD-USEAs. Average nightly distance ran per week for (a) rats that had no significant changes (p > 0.05) and (b) rats with significant changes (p < 0.05) in the distances ran after implantation of HD-USEAs. Rat identifications are shown in the legends. Note for the rats in the four-week cohort, the last data points are four-weeks post-surgery. (c) No significant differences (p = 0.70; two-tailed t-test) were seen in the distribution of nightly distances ran by all the rats (both time period groups combined) for four weeks before (pre-implant; white bars; mean ± std = 6.24 ± 2.3 km/night) and four weeks after (post-implant; gray bars; 6.07 ± 2.45 km/night) HD-USEA implantation. Note that both groups are included in this distribution data, but only the first four weeks of the eight-week group are included in the data set for statistical comparison.
Download figure:
Standard image High-resolution image3.2. Tissue assessment of rats implanted with HD-USEAs
3.2.1. Macroscopic assessment of the containment system and implanted electrodes
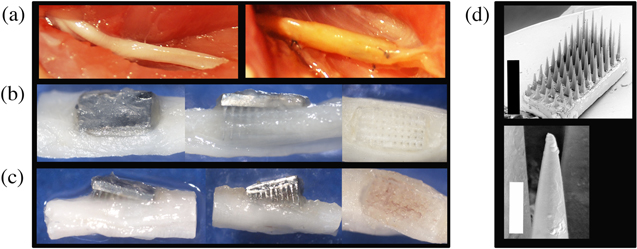
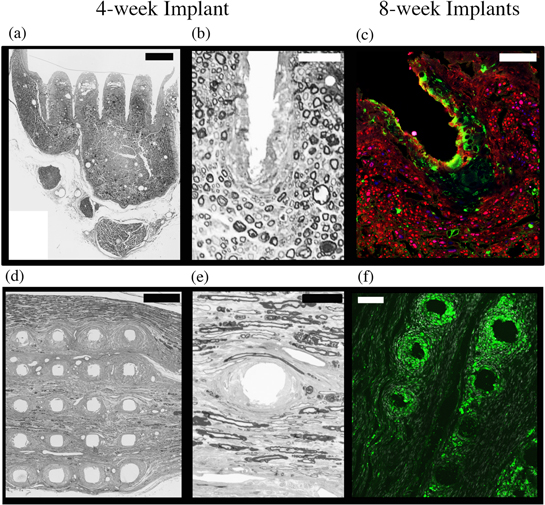
Nerves were dissected in post-perfused animals and the organic nerve wraps were subjectively assessed for the quality of the nerve tissue and the extent of fibrotic encapsulation surrounding the implant site. A representative example of a control nerve and the contralateral implanted nerve is shown in figure 3(a). In all of the rats, the organic nerve wraps remained in place and each of the non-wired arrays were still contained within the organic material at the end of the study period. Furthermore, no major adhesions between the wrap and the epineurium were formed. Each HD-USEA implantation was dissected under a microscope and assessed for the extent by which the arrays remained implanted in the sciatic nerve. Figures 3(b), (c) show two nerves with the HD-USEA exposed for the qualitative assessment of the extent of end-point implantation. An implant was categorized as 'complete' if the entire substrate of the array was located immediately adjacent to the epineurium (figure 3(b)). Implants were characterized as 'partial' if the substrate had backed away from the nerve with any portion of the electrode shanks visible outside the epineurium (figure 3(c)). Nine rats were found to have devices that were completely implanted post-sacrifice. After removal of the HD-USEAs, a waffle-like pattern produced by the electrodes was visible (third photograph figures 3(b), (c)). One rat had an HD-USEA that had partially migrated out of the nerve (figure 3(c); rat 4; four-week cohort). Note the waffle-like pattern from this device appeared to have an enhanced fibrotic response with suspected hemosiderin-laden macrophages or erythrocytes causing pigmentation on the epineurium. None of the implants had electrode arrays that had completely migrated out of the nerve. Scanning electrode microscopy (SEM) images were taken of several HD-USEAs after explantation and organic material adhering to the surface of the device was evident (figure 3(d)).
Figure 3. Macroscopic assessment of chronic HD-USEA implantations. (a) After 4–8 weeks of implantation in actively behaving animals, HD-USEAs all remained within the confines of the organic nerve wrap containment system (left image = control nerve; right image = implanted nerve). Black suture can be seen in the image, which was used to close the wrap around the implant site and to tack the wrap to the epineurium. The distal ends of the nerves are on the right sides of the images. (b), (c) Dissection of each implant site allowed for a qualitative characterization of implants as either: complete; partial; or explanted. Nerve wraps were completely dissected away from the nerve for the examples shown. (b) Example of an HD-USEA that remained completely implanted into the nerve (rat 2; four-week implant). The last image shows the waffle-like pattern seen after array explantation. Nine rats had implants characterized as complete at the end of the study period. (c) One rat had a partially implanted HD-USEA at the end of the study period and the waffle pattern after array explantation on these devices showed marked increases in fibrosis (rat 4; four-week cohort). (d) Scanning electron microscopy (SEM) image for an HD-USEA device that was implanted for eight weeks (top image). The image on the bottom shows a close-up of one of the electrode tips with sparse organic material adhering to the surface of the device. None of the electrodes were broken across all ten devices implanted. Scale bars = 1 mm top image; 50 μm bottom image.
Download figure:
Standard image High-resolution image3.2.2. Proximal and distal nerve morphology
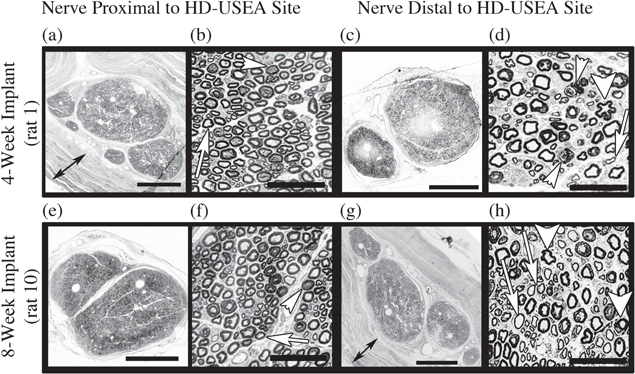
Representative proximal and distal nerve cross-sections are shown in figure 4 for a rat in each of the implant cohorts. For each of the samples processed for proximal and distal morphology (n = seven rats; four-week cohort rats #1–4; eight-week cohort rats #6, 7 and 10), the distal nerve showed more signs of nerve degeneration and regeneration, consistent with local damage to the axons at the implant site. Such axonal degeneration was characterized by encroachment of axoplasm, redundant myelin (including collapse of the myelin into the axoplasm; thick arrowheads figures 4(d) and (h)), and presumptive presence of phagocytic immune cells (thin arrowheads figures 4(d) and (h)). These injuries left the endoneurium and perineurial tubes intact, which presumably facilitated the regeneration of damaged axons. In the nerve distal to the implant, regenerating axons are seen with their characteristically thin myelin (arrows in figures 4(d) and (h)). There was an overall decrease in fiber packing in the distal section (rat 1distal = 723 and rat 10distal = 951 fibers mm−2) compared to the proximal sections (rat 1proximal = 1357 and rat 10proximal = 1146 fibers mm−2). An example of the organic nerve wrap material used to contain the arrays can be seen in the light microscopy images for each rat (double headed arrow figures 4(a) and (g)).
Figure 4. Nerve morphology of the tissue proximal and distal to the HD-USEA implant site. Assessment of the whole-nerve shows intact epineurium and perineurium adjacent to (<5 mm) the site of device implantation (4-week implant (a) and (c); 8-week implant (e) and (g). These rats were chosen as representative samples that included some deficits in their sensorimotor testing. The organic nerve wrap used to contain the HD-USEAs can be seen as multiple layers surrounding the nerve ((a) and (g); double-headed arrows). Magnification of the neuronal fibers (proximal- (b) and (f); distal- (d) and (h)) within the largest fascicle showed an overall decrease in fiber packing in the distal section (rat 1distal = 723 and rat 10distal = 951 fibers mm−2) compared to the proximal sections (rat 1proximal = 1357 and rat 10proximal = 1146 fibers mm−2). (b) and (f) The proximal nerve had normal myelinated fibers (arrows) and presumptive regenerating/degenerating fibers indicated by dilation of the Schmidt–Lanterman incisures and/or myelin collapse (arrowheads). (d) and (h) The distal nerve showed normal myelinated axons, presumptive regenerating axons (thinly myelinated; arrows), degenerating axons (myelin collapse; thick arrowheads) and phagocytic macrophages (thin arrowheads in (d)). Scale bars (a), (c), (e), (g) = 500 μm; (b), (d), (f), (h) = 50 μm.
Download figure:
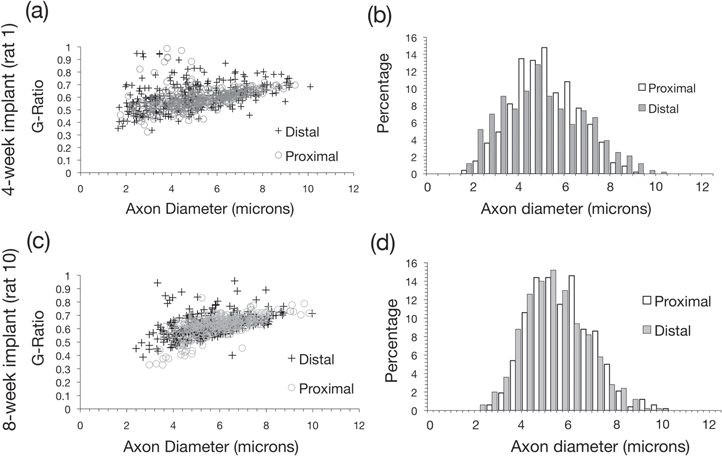
Standard image High-resolution imageNerve morphology was assessed in nerve sections taken proximal and distal to the HD-USEA implant site for two rats representative of each group (number of fibers: four-week proximal = 534, distal = 483; eight-week proximal = 520, distal = 499). There was no significant difference (two-tailed homoscedastic t-test) found in the thickness of myelin (prat 1 = 0.64; prat 10 = 0.17) or the g-ratio (figures 5(a) and (c); prat 1 = 0.24; prat 10 = 0.25) between proximal and distal sections for both rats. We calculated g-ratios using two different methods to obtain the fiber and axon diameters. The first method used diameter measurements derived from the fiber and axon areas, assuming a circular geometry, and there was no significant difference in the g-ratios for both rats (figures 5(a) and (c); prat 1 = 0.24; prat 10 = 0.25). The second method used diameter measurements derived by measuring the mean diameter from both the major and minor axes for a given fiber and axon, and there was a significant different in the g-ratio of the distal tissue for the eight-week implant (p = 0.02; proximal = 0.62 ± 0.07; distal = 0.63 ± 0.08), but there was no significant different for the 4-week implant (p = 0.63; proximal = 0.61 ± 0.07; distal = 0.60 ± 0.10).
Figure 5. Quantification of the nerve morphology of the tissue proximal and distal to the HD-USEA implant site. The top two graphs represent data from a four-week implant (rat 1; (a), (b) and the bottom graphs represent data from an eight-week implant (rat 10; (c)–(d)). (a), (c) The g-ratios (back-calculated from area, assuming circular geometry) are plotted against the axon diameters measured in nerve sections proximal and distal to the implants. No significant difference in the g-ratio was measured (p > 0.2 both rats; rat 1proximal = 0.59 ± 0.08; rat1distal = 0.59 ± 0.1; rat 10proximal = 0.61 ± 0.07; rat10distal = 0.62 ± 0.08; mean ± std). There was a significant difference (p = 0.02) in the g-ratios for rat 10 when they were calculated from mean major and minor axes. (b) There was no significant difference in the proximal and distal axon diameters (p = 0.18; proximal = 5.25 ± 1.39; distal = 5.11 ± 1.86) in rat 1, although there was a significant difference in axon diameters for rat 10 (p < 0.005; proximal = 5.73 ± 1.31; distal = 5.38 ± 1.33). The smaller diameter axons seen in rat 10 may reflect more regenerating axons in an eight-week nerve. Note these are the same rats shown in figure 4.
Download figure:
Standard image High-resolution imageIn the tissue implanted for four-weeks, no significant difference between the proximal and distal morphology was found for axon diameter (figure 5(b); p = 0.18; proximal = 5.25 ± 1.39; distal = 5.11 ± 1.86 μm), fiber area (p = 0.47; proximal = 65 ± 26, distal = 64 ± 37 μm2; mean ± std) or axon area (p = 0.94; proximal = 23 ± 12; distal = 23 ± 17 μm2). In the tissue implanted for eight-weeks, there were significant differences in the axon diameter (figure 5(d); p < 0.005; proximal = 5.73 ± 1.31; distal = 5.38 ± 1.33 μm), fiber area (p < 0.005; proximal = 70 ± 24; distal = 62 ± 26 μm2), axon area (p < 0.005; proximal = 27 ± 13; distal = 24 ± 12 μm2), and fiber dimeter (p < 0.005; proximal = 9.3 ± 1.56; distal 8.68 ± 1.85 μm). Morphometric parameters with significant differences in proximal and distal segments for tissue implanted for four or eight weeks included: form factor (increased for both groups indicating elongation; p ≪ 0.001), mean fiber and axon diameter (decreased for both fiber and axon measurements in both groups indicating smaller fibers; p < 0.05), and roundness of the fiber (decreased for both groups; p ≪ 0.001).
3.2.3. HD-USEA site nerve morphology and cytology
Figure 6(a) shows a representative cross-section through a row of electrodes from a rat that was implanted for four weeks (rat #1). Magnification of the electrode tracks shows myelin-stained axons that were still intact and within 50 μm of the electrode tracks (figure 6(b)). Antibodies were used to identify the presence of ED1 positive macrophages at the device-tissue interface. Macrophages and neurons were found along the electrode tracks (figure 6(c); ED1 positive cells = green; Neurofilament positive cells = red; rat #8; eight-week cohort). Figures 6(d), (e) shows a longitudinal section taken from the same rat shown in figures 6(a), (b) (rat #1) and the osmium-stained myelin again shows that many of the axons are within 100 μm of the electrode tracks. A longitudinal section that was exposed to antibodies for ED1 positive macrophages demonstrates again the presence of macrophages at the electrode-tissue interface (figure 6(f); rat #7; eight-week cohort). The presence of macrophages around the electrode shafts was found to be non-uniform, with some electrode tracks having a greater number of macrophages then others.
Figure 6. Morphometric and immunological assessment of the tissue adjacent to the electrode tracks. Transverse (a), (b) and longitudinal sections (d), (e) of the implant sites were stained for myelin and show axons within 25 μm of the electrode tracks (rat #1; four-week cohort). (c) An electrode track from a rat implanted for eight-weeks (rat #8) was stained for macrophages (ED1 = green) and neurons (NF200 = red). The macrophages lined some of the electrode tracks (as shown in this example); however, despite the presence of these immune cells, axons were still found within 50 μm of the electrode track, with some axons as close as 25 μm. (f) A representative longitudinal section was stained for macrophages (ED1 = green) of a rat sciatic nerve that was implanted for eight weeks (rat #7). The distribution of macrophages around the HD-USEA microelectrodes varied across the electrode tracks, and in this image two rows of electrodes are shown that each have varying degrees of macrophage infiltration. Note that electrode diameters differ along the length of an electrode, such that near the tip the diameter is smaller (smaller holes in the tissue) while closer to the substrate the diameters are larger. Scale bars are as follows: (a) and (d) = 200 μm; (b), (c) and (e) = 50 μm; (f) = 100 μm.
Download figure:
Standard image High-resolution image3.3. Overall assessment of the functional and histological consequences of implanted HD-USEAs
Table 1 summarizes the overall results for each implanted rat. Grades were given for the following categories: sensorimotor tests ('Sensorimotor'), running distance ('Running'), macroscopic assessment of the depth of implantation of the electrode arrays at the end of the study period ('Implant'), and an assessment of the proximity of axons to electrode tracks ('Axons'). None of the rats had deficits across all of the categories. Overall scores were given with one point for each severity of deficit so that a total of seven points would indicate major deficits in each category (major biocompatibility issues) and a score of zero indicated no deficits across all the categories (no biocompatibility issues). The mean scores were 1.2 and 1.8 for the four and eight-week cohorts, respectively.
Table 1. Overall assessment of rats chronically implanted with electrode arrays.
| Animal # | Sensorimotor | Running | Implant | Axons | Overall |
|---|---|---|---|---|---|
| Four-week cohort: | |||||
| 1 | ++ | − | − | − | 2 |
| 2 | + | − | − | − | 1 |
| 3 | − | − | − | − | 0 |
| 4 | − | + | + | N/A | 2 |
| 5 | + | − | − | − | 1 |
| — | — | — | — | — | Mean = 1.2 |
| Eight-week cohort: | |||||
| 6 | + | − | − | − | 1 |
| 7 | ++ | − | − | − | 2 |
| 8 | ++ | + | − | N/A | 3 |
| 9 | ++ | * | − | − | 2 |
| 10 | + | − | − | − | 1 |
| — | — | — | — | — | Mean = 1.8 |
Sensorimotor grade:− No damage+ Transient deficit on one test++ Transient deficit on both tests+++ Permanent deficit on ⩾ one testSensorimotor tests = WRL and Toe-spread lengthRunning behavior grade:− No change in running* Increased running+ Decreased runningArray implant grade:− Array base adjacent to the nerve+ Array base partially explanted++ Array completely explantedAxon grade:− Axons ⩽ 100 μm of electrode tips+ Axons > 100 μm of electrode tipsOverall grade:One point was given for each '+'0 = no consequences overall7 = major behavioral and neuronal consequencesAbbreviations:N/A = data not available
4. Discussion
4.1. Effect on animal behavior
In order to quantify animal behavior and sensorimotor changes that may result as a consequence of electrode array implantation, we chose standard tests that have been previously reported to accurately reflect nerve crush or transection injuries (WRL and standing 1–5 toe-spread). In addition, we measured nightly running behavior in each rat in order to obtain a quantification of the more global effects the devices may have had on animal behavior. The results of these studies suggest each of the tests utilized here were useful for measuring the consequences of these devices on animal behavior and sensorimotor function.
The peak times of the deficits in the WRL and 1–5 toe-spread lengths did not correlate in time across all of the rats. One of the two rats in the four-week cohort (rat #1) and one in the eight-week cohort (rat #7) that showed impaired toe-spreads on their implanted side also had impaired WRL times in this limb. However, the times corresponding to each deficit did not overlap, suggesting that the sensorimotor fibers needed for stance were damaged or inhibited first, while impairment of the sensorimotor fibers needed for noxious withdrawal reflexes occurred subsequently. However, this trend was not consistent for rat 9, where both WRL and toe-spreads showed deficits within the first week post-implantation. The different assessment sensitivities of the WRL and toe-spread ratio suggest these two methods for assessing sensorimotor function are specific to different types of nerve injury. We speculate that the 1–5 toe-spread ratio reflects acute nerve injury resulting from HD-USEA implantation, as all deficits revealed by this test were seen immediately following implantation. However, the WRL test showed deficits that appeared at varying times throughout the study period, which could be reflective of different stages of inflammation, nerve compression or injury.
The two rats that had decreased weekly running distances (rat #8, eight-week cohort; rat #4, four-week cohort) did not show any major deficits in their sensorimotor tests towards the end of the study periods. Rat #8 had transient deficits in both its toe-spread and WRL, but both sensorimotor tests returned to normal while its average weekly distances continued to decline. Finally, the rat (#4) with the HD-USEA that had partially migrated out of the nerve by the end of the study also had decreased weekly running distances, but did not have deficits in the other sensorimotor tests.
The nightly enrichment cages used in these studies were larger than standard AAALAC holding cages and had wire walls to encourage rat-climbing activity. These enrichment cages allowed for substantially more activity both on the exercise wheel and during wall climbing than standard cages. One motivation for using these cages was to increase the possible mechanical stress on or around the implant system and to provide a challenging environment in which to test the ability of HD-USEAs to remain implanted in peripheral nerves, especially while using the novel organic nerve wrap containment system. However, forced treadmill running in rats has been shown to increase healing after peripheral nerve injury [26], and therefore, the time required by nerves to recover following HD-USEA implantation may be shortened in our rats compared to more sedentary animals.
4.2. Containment of the HD-USEAs using an organic nerve wrap
In previous studies using a lower electrode-density version of the HD-USEA (the USEA with 6.25 electrodes mm−2), chronic feline sciatic nerve implants were contained using a three-step containment system: silicone elastomer cuffs were wrapped around the implant and nerve, surrounded by a gold screen (to minimize EMG interference), and then the entire system was coated with surgical-grade adhesive [27, 28]. In order to investigate a new containment system that would have less foreign material surrounding the implant, and thus, a potentially decreased foreign body response, we used a de-cellularized organic nerve wrap that has been approved for use in human nerve repair or protection surgeries [29]. Because few or no adhesions between the organic nerve wrap and the epineurium were formed, the wrapping material could easily be dissected away from the nerve after being implanted for four or eight weeks. Such a containment system for HD-USEAs would be important for applications in semi-chronic human experiments where the implanted arrays would need to be explanted from the nerve at the end of the study.
4.3. Morphology and cytology at the tissue-device interface
Tissue damage after implantation and/or the formation of a large fibrotic scar can effectively displace the indwelling electrodes from the nerve axons, negating their use in chronic studies [7, 30–33]. Fibrotic scarring can also increase the distance from the tip of the electrodes to active neural tissue, potentially placing the electrode outside its effective recording distance (150 μm) [34]. Also, an extensive fibrotic or granulation response surrounding the device could require higher stimulation currents in order to evoke a given response over time, and thus, may lead to stimulation levels that would no longer be safe for the electrodes or the surrounding neural tissue [35]. The results of this study showed that the tips of the electrodes remained within distances that would be useful for stimulating and recording from these fibers on the time scales investigated herein. Although axons were found within such useful ranges, it is unknown whether these axons were functional; however, Rushton pointed out that g-ratios (of peripheral nerves) close to 0.6 provide fiber geometries that allow for the optimal spread of current between nodes of Ranvier [36]. The g-ratios reported herein were all between 0.59–0.63, and thus, we hypothesize these axons would be functional for electrophysiological recording or stimulation studies, and future studies using wired HD-USEAs are warranted to investigate this hypothesis.
Quantification of the nerve morphology at an electrode-tissue interface has historically been carried out for neural interfaces with either a low number of electrodes, such as long intrafascicular electrodes, or with many electrodes along a single plane, such as the Michigan probe [37]. However, the electrode arrays here differed in that many electrodes are distributed along multiple planes. Our results—and other previous results from conventionally spaced Utah Arrays implanted in the peripheral and central nervous systems [28, 38]—indicate that much variability of the tissue adjacent to the electrode tracks is observed in both light microscopy and immunohistochemical samples. A total of 500 electrodes were implanted across ten animals in this study, and the tips of some of these electrodes penetrate into fascicular space, while others penetrate into endoneural space. This results in variability in the number of axons within distances needed for recording or stimulation, as well as variability in the number of macrophages at the electrode-tissue interface (both between electrodes and along the length of a single electrode). Some electrodes had many macrophages surrounding the shaft/tip of the electrode, while other electrodes had little or no macrophages present. With such variability, it was difficult to obtain quantification of the morphology or immunology that would be representative of all of the 500 implanted electrodes.
As with the immunohistochemistry, there was variability in the morphology of the distal nerve sections, depending on which section of the nerve was magnified. Some areas within the fascicles had nerve morphology that showed little to no signs of degenerating/regenerating fibers, while others showed marked differences. In figure 4 we did our best to show regions of the nerve that had the most marked signs of degeneration/regeneration, although we note that other areas of the nerve remained free from such changes. Quantification of the nerve tissue proximal and distal to the implanted devices supported such variability, with significant differences seen for some parameters (such as increases in form factor and decreases in mean axon diameters in the distal tissue), while there were no significant differences in other factors (such the thickness of the myelin). Thus, the quantified morphometric data did not support overall degeneration/regeneration throughout the entire section of the distal nerve.
The g-ratio has been traditionally used as a method for quantifying nerve morphology and is defined as the ratio of the axon to the fiber (axon and myelin) diameters [39]. Many of the axons in the proximal tissue closely approximated circular geometries, whereas many regenerating axons in the distal tissue approximated more elliptical geometries. When we calculated the g-ratio using diameters back-calculated by the area of the fiber/axon, assuming circular geometry, we did not find significant differences in the proximal and distal g-ratios for rats implanted for four or eight weeks. However, when we calculated the g-ratio using mean diameters by measuring the major and minor axes, we found a significant difference in the g-ratio of the distal tissue (eight-week implant). Future studies that utilize the g-ratio as a metric for quantification of nerve morphology following nerve injury or implantation of nerve electrodes should consider calculation of g-ratios using the mean of major and minor axes, which may better reflect geometric changes in regenerating axons.
Immunohistochemistry was performed to obtain additional information about the distribution of macrophages surrounding the device. The tissue reaction to HD-USEA implantation was characterized by a mild to moderate inflammatory response localized mainly around the device-tissue interface, an observation consistent with previously published results for electrode arrays implanted in the peripheral [6–8, 28] and central [32, 38, 40, 41] nervous systems. Macrophages were non-uniformly distributed at the device-tissue interface, a finding that may reflect localized impalement of blood vessels in the nerve. Macrophages are known to differentiate into different subsets that are either pro-inflammatory (referred to as M1 cells) or reparative (referred to as M2 cells) [42] and future studies are warranted to assess which subtype is found at the device-tissue interface and whether the subtype changes over different post-implantation times.
The variability seen in the immunohistochemistry and the morphometric analysis of the nerves is in agreement with previous studies that were carried out using Utah Arrays with 400 μm electrode spacing implanted for various time periods in the cat sciatic nerve [28]. Future studies are warranted to determine what causes this variability among arrays of electrodes (possibly a result of localized blood vessel impalement or positioning of the electrodes within the nerves either intrafascicularly or interfascicularly). Methods for automating such quantification for high electrode-count devices across three-dimensional space within the nerves would facilitate studying this question.
4.4. Overall assessment of the histological consequences of HD-USEAs
In this study, an overall subjective evaluation was made across the data collected using behavioral assays, assessment of the extent that the devices remained intrafascicularly implanted, and the distance of nerve axons from the electrode tips. Compilation of the data in table 1 resulted in an overall rating for the consequences of chronic implantation of HD-USEAs for four and eight weeks, and for each cohort, these devices were considered to have minor consequences. Future studies need to investigate not only longer duration implants, but also devices that are wired to a percutaneous connector for electrophysiological studies. Such wired electrode arrays may produce additional tethering forces that could exacerbate the immune response as seen for tethered devices in the central nervous system [32]. There may also be increased mechanical strain on the implant site for wired devices, which may result in differences in tissue damage and/or explantation of the devices.
4.5. Modifying the cellular response at device-tissue interface
Previous research has shown that the cellular response to chronically implanted brain electrodes can be attenuated by systemic or local delivery of anti-inflammatory drugs [24, 25, 43]. The rats in this study were given both oral minocycline and injected dexamethasone, and although it was not the aim of this study to investigate the effects of these drugs, the administration of these drugs was carried out to potentially provide a more neuroprotective environment in the face of indwelling HD-USEAs. Future studies are warranted to assess whether administration of these drugs can help modify the sensorimotor and/or cellular responses to chronically implanted peripheral nerve electrodes. Additionally, modifications to the surface chemistry [43–45] or surface area [37] of HD-USEAs could assist in altering the host response to these devices.
5. Conclusions
The present study was aimed at evaluating the consequences of HD-USEA implantations in actively behaving animals in terms of overall animal behavior and cytology at the tissue-device interface. Using a combination of behavioral assessment tools, the results showed that HD-USEAs evoke mild deficits that generally returned to normal within days to weeks. Cytological analysis showed that although macrophages were present at the device-tissue interface, nerve axons remained within distances needed for neural recording or stimulation. Signs of axonal damage were observed in all animals, with the distal nerve showing signs of regenerating neurons. Our results indicate that unwired high count (25 electrodes mm−2) microelectrode arrays passed the biocompatibility criteria defined for this study. Future studies are now warranted to investigate longer duration implants, devices that are wired to a connector, and histological analysis of the variability in morphometric and immune cell changes throughout the nerve and along the device-tissue interface.
Acknowledgements
This work was supported by the USA National Science Foundation (CBET-1134545), the Spanish National Organization of the Blind, and the Spanish Ministry of Economy and Competitiveness. We would also like to acknowledge Meredith Gibbons for her assistance with the perfusions and Rebecca Pfeiffer for her assistance with some of the histology.