Abstract
The corrosion protection of pure aluminium and its alloys AA2024-T3 and AA7075-T6 has been studied in 0.1 M NaCl with and without the addition of Ce(III) chloride, Ce(III) nitrate, Ce(III) acetate and Ce(IV) sulfate. The study of Ce(III) acetate has been scarce but is here shown to be the most effective of the cerium salts studied, especially for AA7075-T6. The inhibition effectiveness of cerium salts is: Ce(III) acetate > Ce(III) chloride > Ce(III) nitrate for the metals studied. Ce(IV) sulfate does not inhibit corrosion. Electrochemical potentiodynamic measurements were used to investigate the mechanism of inhibition by cerium salts. Immersion tests in 0.1 M NaCl, with and without the addition of Ce(III) chloride and Ce(III) acetate, were performed to determine the effects of the inhibitors on the extent of corrosion damage at the surface of all three metals. For pure aluminium the inhibitory effectiveness was 78.5%, 82.6% and 84.0% in the presence of 3 mM Ce(III) nitrate, chloride and acetate, respectively. For AA7075-T6 the values of 92.1%, 99.1% and 99.6% were obtained. The inhibitory effectiveness of cerium salts is shown to depend on a variety of factors: type of substrate, type of anion and salt concentration.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Aluminium and its alloys 2024-T3 (AA2024-T3) and 7075-T6 (AA7075-T6) generally have good resistance to corrosion under atmospheric conditions. They are used as construction materials, primarily in aircraft applications, because of their physical characteristics such as rigidity and high strength-to-weight ratio.1,2 The main differences between aluminium and its alloys lie in their mechanical and corrosion properties. Aluminium is cold worked, whereas AA2024-T3 and AA7075-T6 are heat treated and cold worked (T3) or artificially aged (T6). The reason for alloying aluminium is to increase strength by forming intermetallic particles, IMs.1,2
The good corrosion resistance of Al is due to the effective passivity produced by a protective oxide film composed of Al2O3, Al(OH)3 and AlO(OH).2 However, the presence of IMs in the alloys reduces their corrosion resistance. AA2024-T3 has an inhomogeneous distribution of Cu in IMs. Alloying of Al with other metals leads to the creation of local galvanic cells that can act as either anodic or cathode sites with respect to the aluminium matrix.3 The situation is similar for AA7075-T6 in which Zn is the main alloying element.3 Aluminium is thus more resistant to corrosion than 2024-T3 or 7075-T6 alloys.2,4 Aggressive ions such as chloride result in extensive, localized attack on Al alloys, i.e., pitting, crevice or galvanic corrosion, since they facilitate the breakdown of the aluminium oxide film.
Various coating systems are employed to protect aluminium and its alloys under service conditions.2,4–6 For many decades, chromate conversion coatings have been used, for which the chromate concentration is typically 250 g/L. Cr(III) oxide provides a barrier effect, while Cr(VI) results in a "self-repairing" effect.5,7 Hexavalent chromium Cr(VI) is both toxic and carcinogenic and it is thus essential to search for more suitable alternatives. Various corrosion inhibition strategies have been tested by traditional and rapid screening multi electrode methods.5,8–10 Among these, salts of rare earths – lanthanide and, especially, cerium salts – have been identified as being effective and environmentally acceptable.11–14 Ce(III) salts are more soluble in water than Ce(IV) salts, which have rather low solubility. For most of these inhibitors, the optimal concentration was around 1000 ppm (≈2.68 mM).11,13
It is well accepted that the inhibition of corrosion of aluminium and its alloys by Ce(III) is achieved by diminishing the cathodic reaction of oxygen reduction by precipitation of cerium hydroxide salts on the cathodic sites.15 The presence of Ce3+ and OH− in aqueous solution allows the formation of Ce(OH)3 and, later, oxidation of cerium hydroxy complexes to Ce(OH)4.16 The Ce-rich deposits are thus composed of Ce3+ and Ce4+ ions.13
Since the initial studies by Hinton et al. on AA7075-T6 in chloride solution containing CeCl3,11–13 the majority of studies have been on AA2024-T316–26 or other alloys like AA5052,15 AA5083,27–29 series AA6xxx,30–33 AA809034 and Al metal.35 The focus has been on the mechanism of inhibition, mainly by CeCl315,17,18,20,21,26–29,35,36 and Ce(NO3)316,19,22,25,37 which both act as efficient inhibitors on aluminium alloys. Combinations of Ce(NO3)3 and CeCl330–33 or of Ce(NO3)3 and Ce(CO3)2 were also tested.19 The effect of anions such as SO42−, has only rarely been considered24,38 and that of acetate anion are scarce.8,33,39–41 Mansfeld and Wang33 used immersion in hot cerium acetate as a first step of the surface modification process of AA2024. Sugama modified aminopropylsilane triol by using Ce acetate and as a dopant in the concentration range from 0.2 to 10 wt%.39 At 3 wt% Ce the maximum performance was achieved in terms of significantly decreased corrosion rate and increased hydrophobicity of the surface which was ascribed to precipitation of Ce3+ oxide within the organic coating.39 Catubig et al. used rare earth mercaptoacetate inhibitors on AA2024.40 The praseodymium mercaptoacetate was found to reduce the average corrosion current density to a greater extent that cerium mercaptoacetate.40 In addition to acetate, other organic cerium salts, namely cerium dibuthyl phosphate, was investigated.42 Compared to CeCl3, cerium dibuthyl phosphate offered a combined effect due to cerium oxide formation on Cu-rich areas and dibuthylphosphate on the overall surface with no preferential deposition.42
The main purpose of this study was to compare the inhibition performances of Ce(III) salts – chloride, nitrate and acetate – and of Ce(IV) sulfate on Al, AA2024-T3 and AA7075-T6. The aim was to seek answers to open questions regarding the effect of the type of Ce anion and of substrate on inhibition effectiveness. In particular, Ce(III) acetate is introduced as a potential inhibitor. Electrochemical potentiodynamic polarization measurements and immersion tests have been carried out. The effect of concentration was studied in the range from 1 to 5 mM but the majority of experiments were performed at 3 mM concentration of cerium salts.
Experimental
Metal substrates and chemicals
Aluminium (Al > 99.0%) as 1.0 mm thick sheet was supplied by GoodFellow, Cambridge Ltd., UK. The aluminium alloys AA2024-T3 and AA7075-T6, in the form of 0.5 mm thick sheets and distributed by Kaiser Aluminum, USA, were used as substrates. The compositions of the aluminium and its alloys are given in Table I. Discs of diameter 14 mm were cut from the sheets, then water-ground successively up to 4000-grit SiC emery papers (Struers, Denmark), rinsed thoroughly with deionized water, cleaned ultrasonically in ethanol for 10 minutes and dried with a stream of nitrogen.
Table I. The composition of aluminium, AA2024-T3 and AA7075-T6 substrates*.
| Nominal composition / wt. % | Si | Fe | Cu | Mg | Mn | Zn | Other | Al |
|---|---|---|---|---|---|---|---|---|
| Aluminium | - | - | - | - | - | - | 1.0 | 99.0 |
| AA2024-T3 | 0.09 | 0.25 | 4.35 | 1.33 | 0.53 | 0.10 | 0.07 | 93.28 |
| AA7075-T6 | 0.08 | 0.21 | 1.67 | 2.55 | - | 5.81 | - | 89.68 |
Aluminium was pre-treated in distilled water for one hour to obtain a thicker oxide layer, which improves the corrosion resistance and assists the incorporation of cerium ions into the oxide layer.
The corrosive medium, 0.1 M NaCl, was prepared with analytical grade NaCl (Carlo Erba 99.5 – 100.5%, pH = 5.5) and Milli-Q Direct water with a resistivity of 18.2 MΩ cm at 25°C (Millipore, Billerica, MA). Inhibitory cerium salts were dissolved in this solution at 1 to 5 mM.
The following cerium(III) and cerium(IV) salts were used as corrosion inhibitors: cerium(III) chloride, (CeCl3 × 7H2O, 99.9%, Aldrich), cerium(III) nitrate, (Ce(NO3)3 × 6H2O, 99.5%, Fluka), cerium(IV) sulfate (Ce(SO4)2 × 2H2O, 99.9%, Carlo Erba) and cerium(III) acetate, (Ce(CH3COO)3 × nH2O, 99.9%, Aldrich). For the latter the amount of crystal-bound water was calculated from thermogravimetric analysis to give Ce(CH3COO)3 × 2H2O.
Surface analysis
Field emission scanning electron microscopy (SEM), in combination with energy dispersive X-ray spectrometry (EDS) was used to analyze the microstructure and composition of aluminium alloys. AA2024-T3 alloy was analyzed using a JEOL JSM-7600F SEM equipped with an Oxford Instruments Inca 400 EDS. AA7075-T6 was analyzed using a Carl Zeiss SUPRA 35VP SEM and an Oxford Instruments Inca 400 EDS. SEM analysis was performed in a secondary electron (SE) and back-scattered (BSE) mode with energy 5 kV.
Electrochemical measurements
Electrochemical measurements were performed in a three-electrode standard corrosion cell (Parstat Corrosion Cell Kit model K0047, volume 1 L) at 25°C. Substrate discs embedded in a Teflon holder (model K0105 Flat Specimen Holder Kit), leaving an area of 0.95 cm2 exposed to the corroding solution, served as a working electrode. A saturated calomel electrode (SCE, Hg/Hg2Cl2, 0.242 V vs. saturated hydrogen electrode) placed in a Luggin capillary salt bridge was used as a reference electrode. Carbon rods served as a counter electrode. All potentials in the text refer to the SCE scale. Electrochemical experiments were carried out with an Autolab PGSTAT 12 (Metrohm Autolab, Utrecht, The Netherlands) potentiostat/galvanostat and controlled by Nova 1.10 software.
Prior to measurements, samples were allowed to stabilize under open circuit conditions for approximately 1 hour. During that time the open circuit potential was measured every 2 seconds. The stable, quasi-steady state potential reached at the end of the stabilization period is denoted as the open circuit potential, Eoc. Electrochemical measurements were carried out following stabilization. Linear polarization was measured over a potential range of ± 10 mV vs. Eoc, using a potential scan rate of 0.1 mV/s. Values of polarization resistance, Rp, were deduced from the slope of fitted current density vs. potential lines, using Nova software. Potentiodynamic measurements were performed using a 1 mV/s potential scan rate, from −250 mV to Eoc. The potential was then increased in the anodic direction. This type of measurement is appropriate for comparison between different inhibitors; for a more accurate assessment of anodic and cathodic kinetics, separate anodic and cathodic scans starting at the Eoc should be performed.
For each sample, measurements were performed in at least triplicate. A representative measurement was chosen and presented in figures. In tables, the results are presented as mean value ± standard deviation. The following electrochemical parameters were determined: corrosion current density (jcorr), corrosion potential (Ecorr), pitting potential (Epit) and the difference ΔE = Epit – Ecorr.
The corrosion inhibition effectiveness, IE, was calculated according to Equation 1
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/163/3/C85/revision1/d0001.gif)
where Rp* is the polarization resistance for the inhibited, and Rp that for the non-inhibited system. The values are given in %.
Immersion test and confocal microscopy
Short-term (24 h) immersion tests were carried out in 0.1 M NaCl solution, with and without the addition of 3 mM CeCl3 and Ce(Ac)3. Tests were performed in 100 mL polyethylene vials at room temperature. After immersion test the samples were analyzed using a confocal microscope (Axio, CDM 700, Zeiss, Gottingen, Germany) with 50 × objective zoom.
Results and Discussion
Immersion of Al, AA2024-T3 and AA7075-T6 in 0.1 M NaCl
The heterogeneous microstructure of aluminium alloys results in a variety of types and a range of numbers of intermetallic particles (IMs). The IMs also differ in their electrochemical activity with respect to the aluminium matrix.3 The AA2024-T3 from 2xxx series contains the following main IMs: Al-Cu-Mg (S-phase), binary Al-Cu (θ phase) and Al-Cu-Fe-Mn-Si.43–48 The SEM/EDS analysis of AA2024-T3 is presented in Fig. 1. The Mg-containing IMs are spherical and several micrometres in diameter. Binary Al-Cu particles are also spherical and small (1-2 μm). Larger, Fe-rich, Al-Cu-Fe-Mn IMs are usually irregular in shape and are noble (more positive corrosion potential relative to matrix) (Fig. 1). Hughes et al. recently analyzed 18,000 IMs in AA2024-T3 and identified nine different compositions, including matrix and a periphery composition around S-phase/θ-phase composites as well as around other IMs containing Al, Cu, Fe, Mn and Si.48,49 The most common compositional domains were those containing Al, Cu, Fe, Mn and Si, constituting 47% of the total domain count. The S-phase and θ-phase constitute around 39% of the total. The remainder of the particles contained predominantly Al, Cu, Mn and Fe. 77% of the IMs domains were designated noble character.48
Figure 1. (a) SEM image recorded in the BSE mode of AA2024-T3 substrate and (b) composition, in atom %, deduced from EDS spectra recorded at the locations on the surface denoted in (a).
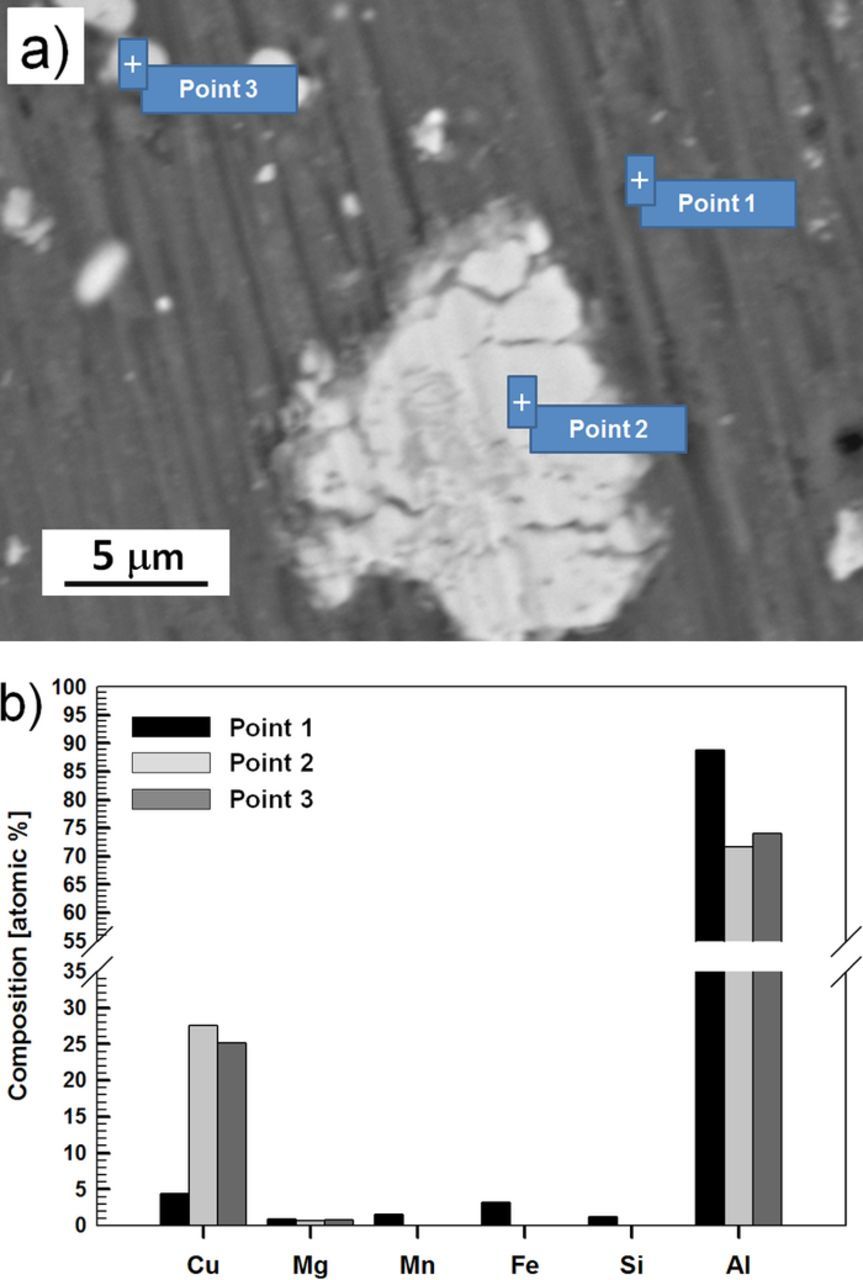
The main IMs in AA7075-T6 from 7xxx series are active (less positive corrosion potential) (Al-Cu-Mg-Zn) or noble (Al-Fe-Cu-Mn-Zn) relative to matrix.43,50–54 SEM/EDS analysis of AA7075-T6 is presented in Fig. 2. The most common particles are Al2CuFe, Al7Cu2Fe, Al2CuMg and Mg2Si particles; the Mg2Si particles are active in respect to matrix and dissolve preferentially.43,50 AA7075-T6 also contains precipitate particles of MgZn2 that are formed by nucleation and growth from a supersaturated solid solution. These particles are dispersed within the matrix.
Figure 2. (a) SEM image recorded in the SE mode of AA7075-T6 substrate and (b) composition in atomic % deduced from EDS spectra recorded at different locations on the surface as denoted in (a).
It is noteworthy that sample preparation process, namely grinding and polishing, can create an altered surface layer on Al alloys that has been shown to influence corrosion properties.44,45,55,56
Ground metal and alloy substrates have different appearances because of the presence of secondary phases, i.e. intermetallic particles of various shapes and sizes (Fig. 3, upper row), as described above. After 24 h immersion in chloride medium aluminium corroded slowly and the corrosion products were observed as grey-brown spots over an area more than 50 μm in diameter (Fig. 3, lower row). The corrosion was initiated at some weak points such as impurities, e.g. Cu, Fe, Mn, Si, Zn, present in aluminium, which may act as initiation sites for corrosion within the aluminium oxide surface.35 In contrast, corrosion on AA2024-T3 and AA7075-T6 surfaces was localized on intermetallic particles. The corrosion process was more pronounced on AA7075-T6 where almost all the IM particles were corroded, corroded areas coalesced and corrosion spread around the dark areas around inclusions. The localized corrosion of different types of IMs in these two alloys was described in details and includes the preferential dissolution of Mg-rich particles leaving behind copper-rich particles, and dissolution of matrix around primarily noble Al-Cu-Fe-Mn particles leading to so called trenching.44–46,48,51,53
Figure 3. Surfaces, imaged by confocal microscopy, of ground aluminium, AA2024-T3 and AA7075-T6 before (upper row) and after (lower row) immersion in 0.1 M NaCl for 24 hours.
Electrochemical measurements in 0.1 M NaCl
Following stabilization at the Eoc, values of polarization resistance, Rp, were measured in 0.1 M NaCl solution (Table II). The values suggest that AA7075-T6 exhibits the lowest corrosion resistance in chloride solution. Al and AA2024-T3 have more than 10-fold larger values of Rp. However, all three materials are subjected to corrosion and pitting in chloride solution (Fig. 3), and further protection is required.
Table II. Electrochemical parameters, deduced from potentiodynamic measurements, for aluminium, AA2024-T3 and AA7075-T6 in non-inhibited 0.1 M NaCl solution and in 0.1 M NaCl containing inhibitory Ce salts. Rp – polarization resistance determined from linear polarization measurements, jcorr – corrosion current density, Ecorr – corrosion potential, Epit – pitting potential and ΔE = Ecorr − Epit determined from potentiodynamic polarization curves (Figs. 4–6). IE: inhibition effectiveness.
| Substrate / solution | Rp [kΩ cm2] | jcorr [nA/cm2] | Ecorr [V] | Epit [V] | ΔE [mV] | IE [%] |
|---|---|---|---|---|---|---|
| Al / NaCl | 43 ± 6 | 257 ± 22 | –0.68 ± 0.02 | –0.67 ± 0.01 | 10 | |
| 2024-T3 / NaCl | 16 ± 3 | 239 ± 30 | –0.70 ± 0.02 | –0.67 ± 0.01 | 30 | - |
| 7075-T6 / NaCl | 1.5 ± 0.2 | 1 896 ± 201 | –0.70 ± 0.01 | –0.69 ± 0.01 | 10 | - |
| Al / NaCl + CeCl3 | 80 ± 4 | 143± 11 | –0.71 ± 0.02 | –0.72 ± 0.01 | 20 | 82.6 |
| 2024-T3 / NaCl + CeCl3 | 35 ± 3 | 123 ± 17 | –0.62 ± 0.03 | –0.56 ± 0.01 | 50 | 52.9 |
| 7075-T6 / NaCl + CeCl3 | 162 ± 23 | 120 ± 9 | –0.91 ± 0.03 | –0.70 ± 0.01 | 210 | 99.1 |
| Al / NaCl + Ce(Ac)3 | 271 ± 16 | 110 ± 8 | –0.82 ± 0.01 | –0.61 ± 0.02 | 210 | 84.0 |
| 2024-T3 / NaCl + Ce(Ac)3 | 82 ± 14 | 185 ± 19 | –0.76 ± 0.01 | –0.58 ± 0.01 | 180 | 79.8 |
| 7075-T6 / NaCl + Ce(Ac)3 | 360 ± 25 | 62 ± 5 | –0.94 ± 0.03 | –0.71 ± 0.01 | 230 | 99.6 |
| Al / NaCl + Ce(NO3)3 | 63 ± 8 | 184 ± 10 | –0.69 ± 0.02 | –0.55 ± 0.01 | 140 | 78.5 |
| 2024-T3 / NaCl + Ce(NO3)3 | 26 ± 3 | 49 ± 8 | –0.57 ± 0.01 | –0.54 ± 0.01 | 30 | 37.8 |
| 7075-T6 / NaCl + Ce(NO3)3 | 18 ± 1 | 357 ± 32 | –0.65 ± 0.01 | –0.62 ± 0.01 | 30 | 92.1 |
| Al / NaCl + Ce(SO4)2 | 1 ± 0.4 | 10 375 ± 981 | –0.83 ± 0.02 | –0.64 ± 0.02 | 190 | - |
| 2024-T3 / NaCl + Ce(SO4)2 | 3 ± 0.3 | 3 195 ± 463 | –0.65 ± 0.02 | –0.55 ± 0.02 | 100 | - |
| 7075-T6/NaCl+ Ce(SO4)2 | 1 ± 0.2 | 2 964 ± 342 | –0.71 ± 0.02 | –0.71 ± 0.02 | 0 | - |
Polarization curves were recorded for Al, AA2024-T3 and AA7075-T6 in NaCl solution (Figs. 4–6), together with the corresponding electrochemical parameters (Table II). Cathodic curves are related to reduction of dissolved oxygen which can proceed through 2e− or 4e− reactions.17,57 Values of Ecorr were between −0.68 V and −0.70 V, while those of jcorr differed considerably between the substrates, AA2024-T3 and AA7075-T6. The rapid increase in anodic current density with increasing potential is associated with the pitting corrosion related to dissolution of IM particles.58 No passive region was established before pitting. At more positive potentials, one or two breakdown potentials may occur depending on the type of substrate, surface preparation and deaeration of solution.44,45,55,56,58 For AA7075 in deaerated solution the first breakdown is related to transient dissolution of surface layer enriched in Zn formed during polishing.55 The second breakdown is associated with combined intergranular and selective grain attack.56 For AA2024 two breakdowns are related to transient dissolution of S phase and initiation and initiation of intergranular corrosion.44,45,58
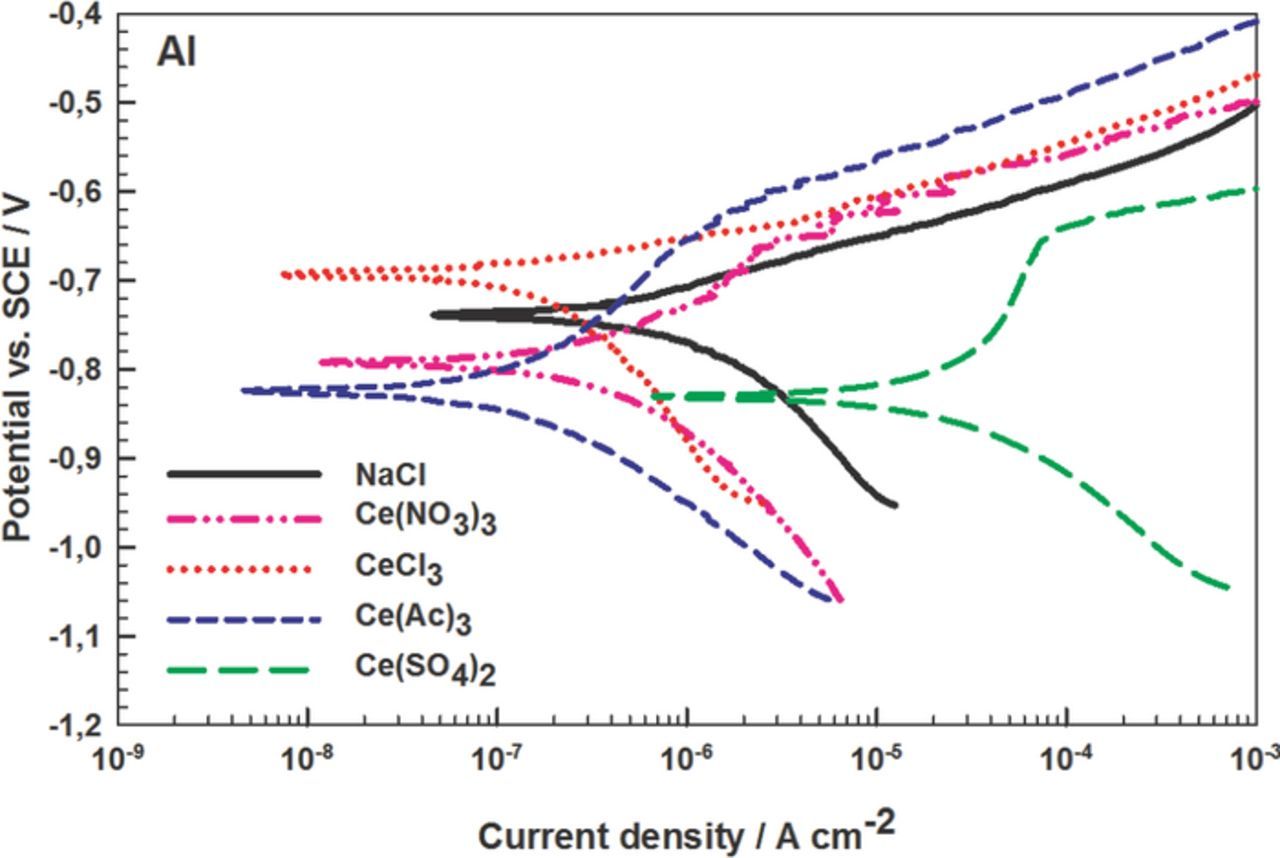
Figure 4. Potentiodynamic polarization measurements for aluminium in 0.1 M NaCl with or without 3 mM Ce(III) and Ce(IV) salts as corrosion inhibitors. Scan rate dE/dt = 1mV/s. The curves were recorded after 1 h stabilization at Eoc. Electrochemical parameters are given in Table II.
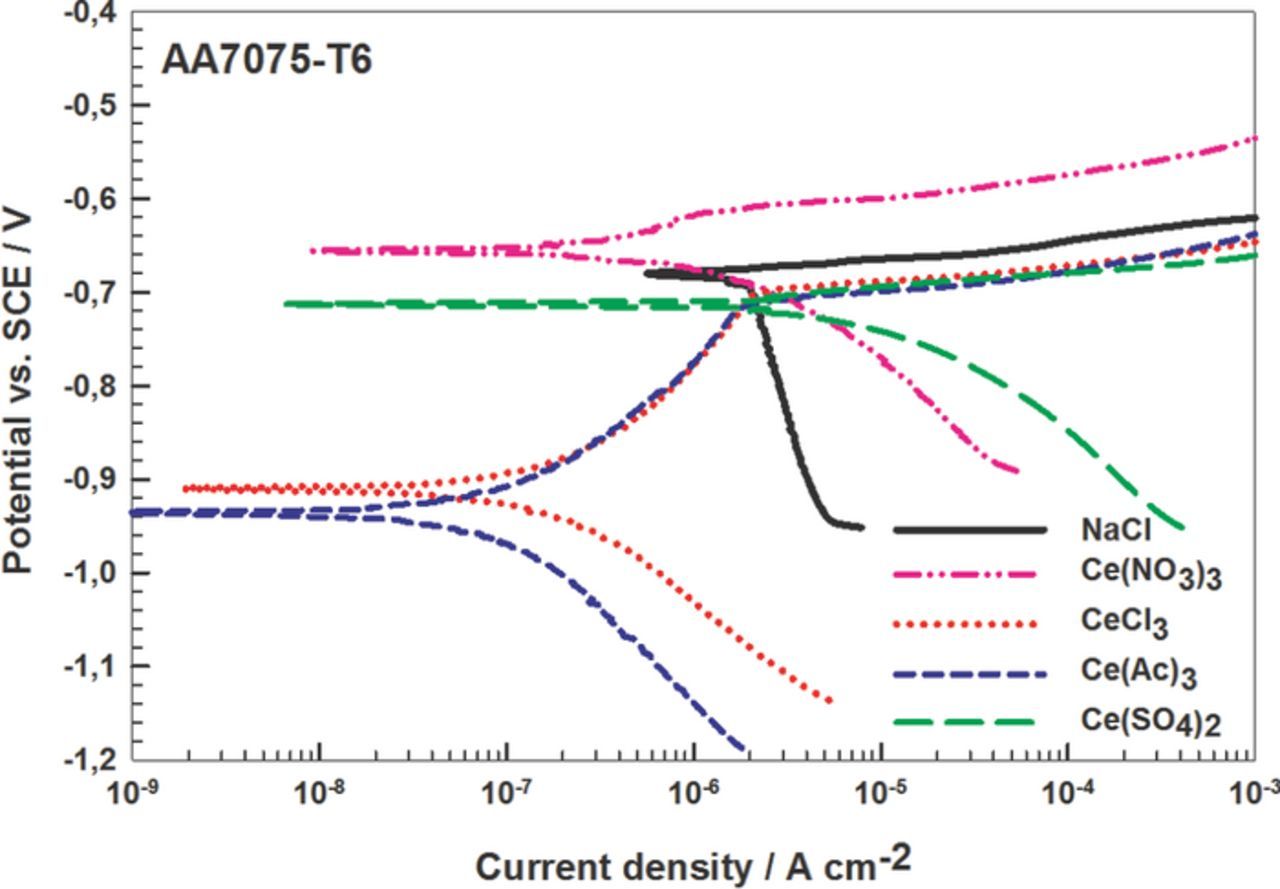
Figure 6. Potentiodynamic polarization measurements for AA7075-T6 in 0.1 M NaCl without or with addition of 3 mM Ce(III) or Ce(IV) salts as corrosion inhibitors. dE/dt = 1mV/s. The curves were recorded after 1 h stabilization at the Eoc. Electrochemical parameters are given in Table II.
The span of ΔE (Ecorr−Epit) is taken as the measure of initiation of pit nucleation.11 Values of ΔE ranged only from 10 to 30 mV, indicating the high susceptibility these alloys to pit nucleation (Table II). From the appearance of the surface (Fig. 3) and the electrochemical parameters (Figs. 3–6, Table II) it is evident that, of the three substrates, AA7075-T6 is the most susceptible to both uniform and localized corrosion.
Electrochemical measurements in 0.1 M NaCl containing cerium salts
In general, immersion in solutions containing Ce(III) salts improved corrosion properties, resulting in increased Rp, reduced jcorr, a shift in Ecorr and the appearance of a passive region (Table II). The Ce(IV) salt, Ce(SO4)2, did not act as a corrosion inhibitor. As presented below, the polarization behavior in the presence of various Ce salts is dependent on their type and also on the type of substrate.
Ce(NO3)3 and Ce(Ac)3 shifted the Ecorr of Al to more negative values, indicating their effect on the blocking of cathodic sites and the reduction of oxygen (Fig. 4, Table II). On the anodic side a quasi-passive range was established in the presence of both salts, indicating that aluminium oxide formation/thickening is stimulated. The higher value of ΔE was noticed in the presence of Ce(Ac)3 (210 mV) than that of Ce(NO3)3 (20 mV). On addition of CeCl3 the shift of Ecorr was more positive. It acted as a mixed inhibitor; however, no passive range was established as in the case of nitrate and acetate. Ce(SO4)2 accelerated the corrosion of Al.
The effect of Ce salts on AA2024-T3 is presented in Fig. 5. Ce(Ac)3 acted in a similar way to its effect on Al, causing a negative shift of Ecorr. It affected both the cathodic and anodic reactions, with a passive region established up to −0.58 V. The action of Ce(NO3)3 on AA2024-T3 however, differed from that on Al, with Ecorr shifted ∼100 mV more positive. The established passive range is narrower than for Al but lower current densities were achieved. The action of CeCl3 was similar to that on Al. Ce(SO4)2 increased the cathodic current density by 30 times.
Figure 5. Potentiodynamic polarization measurements for AA2024-T3 in 0.1 M NaCl without or with addition of 3 mM Ce(III) and Ce(IV) salts as corrosion inhibitors. dE/dt = 1mV/s. The curves were recorded after 1 h stabilization at the Eoc. Electrochemical parameters are given in Table II.
Polarization curves were determined for AA7075-T6 in the presence of various Ce salts in chloride solution (Fig. 6). Here CeCl3 and Ce(Ac)3 acted as classical cathodic inhibitors, shifting Ecorr to more negative values by more than 200 mV. The cathodic current density was significantly reduced. At E>Ecorr the increase in current density slowed down, however the abrupt increase in current density was similar as that in uninhibited solution. This behavior was described by Hinton et al.11 for AA7075-T6 in the presence of CeCl3. The absence of pitting was ascribed to displacement of Ecorr away from Epit in the negative direction. The value of ΔE can be considered as a relevant indicator of pitting resistance.11 The values of ΔE of 210 mV and 230 mV for CeCl3 and Ce(Ac)3 indicate that these two salts act as effective cathodic inhibitors for AA7075-T6. The effect of Ce(NO3)3 was similar to that on AA2024-T3, i.e. it changed the value of Ecorr to more positive and slowed down the dissolution, due to the stimulation of oxide formation.24 Ce(SO4)2 acted as a corrosion accelerator owing to increases in both the cathodic and anodic current density.
From the values of electrochemical parameters and of inhibition effectiveness, IE, the most effective inhibitor for all three materials is seen to be Ce(Ac)3 as it shifted values of Ecorr to more negative values but affected both cathodic and anodic reactions (Table II). The passivation region appeared independently on the metal substrate. The broad ΔE region accounts for the good protection against pitting corrosion. CeCl3 was less effective (Table II). It acted as a typical cathodic inhibitor on AA7075-T6, with values of Ecorr displaced negatively but, for Al and AA2024-T3, the shift was positive. The related value of IE was less for AA7075-T6 than for Al (Table II). Ce(NO3)3 caused a positive shift of Ecorr for AA2024-T3 and AA7075-T6 and affected the anodic reaction, as is typical for nitrate as a passivating inhibitor. In terms of IE, it is less effective than Ce(Ac)3 and CeCl3. The value of IE is highest for AA7075-T6 (Table II). CeCl3 and Ce(NO3)3 acted similarly for AA2024-T3 but not for AA7075-T6. For Al, CeCl3 and Ce(Ac)3 acted similarly while nitrate showed an intermediate behavior.
The most effective cerium salts, Ce(Ac)3 and CeCl3, have been investigated in more detail by making electrochemical measurements as a function of concentration and by the immersion testing.
Influence of concentration on the effectiveness of corrosion inhibition
It has been reported that the inhibition effectiveness of various cerium salts on AA7075-T611,12 and on AA2024-T316,23,24,26,34,35 is concentration dependent. For AA7075-T6 the optimal concentration of CeCl3 is 1000 ppm (0.0027 M), while, for AA2024-T3, lower concentrations (e.g. 10−5 M) are more effective corrosion inhibitors than higher concentrations (10−2 M).16,24 Decreased inhibition effectiveness with increase in concentration is related to the decrease in solution pH with increasing salt concentration.16,24 If the pH drops to values far below those at which Al2O3 is stable the corrosion protection is disabled. Herein, we present the behavior of Al and AA7075-T6 in the presence of various concentrations of CeCl3 and Ce(Ac)3 (Figs. 7–9). Different concentrations were studied to optimize the inhibition performance but all were in the lower range of 1–5 mM in order to avoid the dissolution of Al oxide at low pH.
Figure 7. Potentiodynamic polarization measurements for aluminium in 0.1 M NaCl and inhibited with Ce(Ac)3 in the concentration range from 1 to 4 mM. dE/dt = 1mV/s. The curves were recorded after 1 h stabilization at the Eoc. Electrochemical parameters are given in Table III.
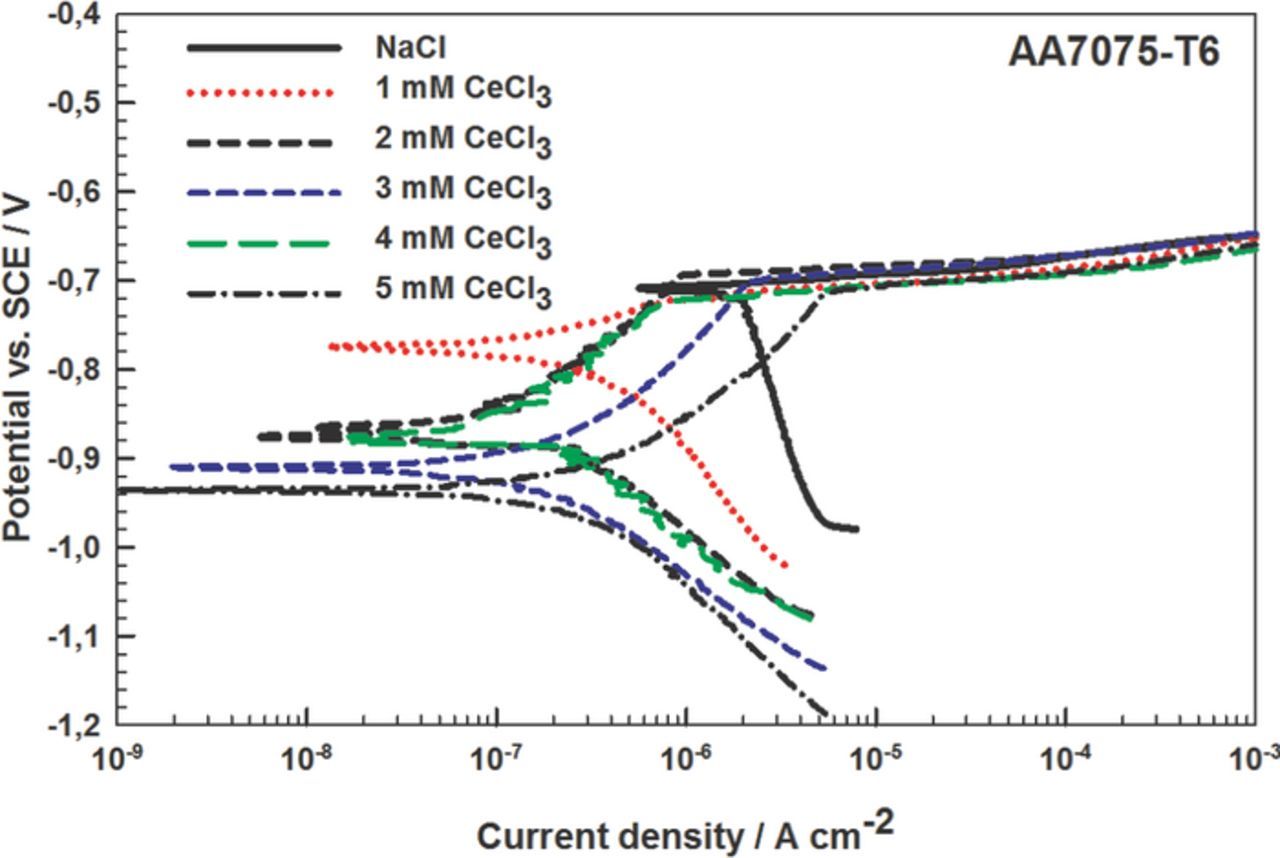
Figure 9. Potentiodynamic polarization measurements for alloy AA7075-T6 in 0.1 M NaCl and inhibited with CeCl3 in the concentration range from 1 to 5 mM CeCl3. dE/dt = 1mV/s. The curves were recorded after 1 h stabilization at the Eoc. Electrochemical parameters are given in Table V.
Rp values for Al in NaCl containing Ce(Ac)3 were, at all concentrations, higher than those in non-inhibiting solutions (Table III). However, at the lowest concentration of Ce(Ac)3 (1 mM) Rp was at its highest value (IE ∼92%) and decreased with increasing amount of cerium salt to an IE of 80%. At the same time Ecorr shifted to more negative values, causing ΔE to increase to 280 mV, thus affecting the susceptibility to pit nucleation. The quasi passive range was established as being broader at higher concentrations (Fig. 7). The results thus show that it is not possible to predict the resistance against pit nucleation judging from the Rp and jcorr values alone. Although the optimal value of IE was observed at the lowest concentration (1 mM), only at higher concentrations did the modified surface of aluminium exhibit a stronger effect on the cathodic part as well as the anodic part, indicating that Ce(III) acetate is capable of protecting Al against both general and localized corrosion.
Table III. Electrochemical parameters deduced for Al in non-inhibited 0.1 M NaCl and inhibited with Ce(Ac)3 in the concentration range from 1 to 4 mM (Fig. 7).
| Solution | Rp [kΩ cm2] | jcorr [nA/cm2] | Ecorr [V] | Epit [V] | ΔE [mV] | IE [%] |
|---|---|---|---|---|---|---|
| NaCl | 43 ± 6 | 257 ± 22 | –0.68 ± 0.02 | –0.67 ± 0.01 | 10 | - |
| + 1 mM Ce(Ac)3 | 520 ± 63 | 45 ± 6 | –0.66 ± 0.01 | –0.63 ± 0.01 | 30 | 91.7 |
| + 2 mM Ce(Ac)3 | 309 ± 40 | 66 ± 8 | –0.80 ± 0.02 | –0.64 ± 0.02 | 160 | 86.1 |
| + 3 mM Ce(Ac)3 | 270 ± 16 | 110 ± 8 | –0.82 ± 0.01 | –0.61 ± 0.02 | 210 | 84.0 |
| + 4 mM Ce(Ac)3 | 220 ± 14 | 163 ± 12 | –0.80 ± 0.02 | –0.52 ± 0.03 | 280 | 80.4 |
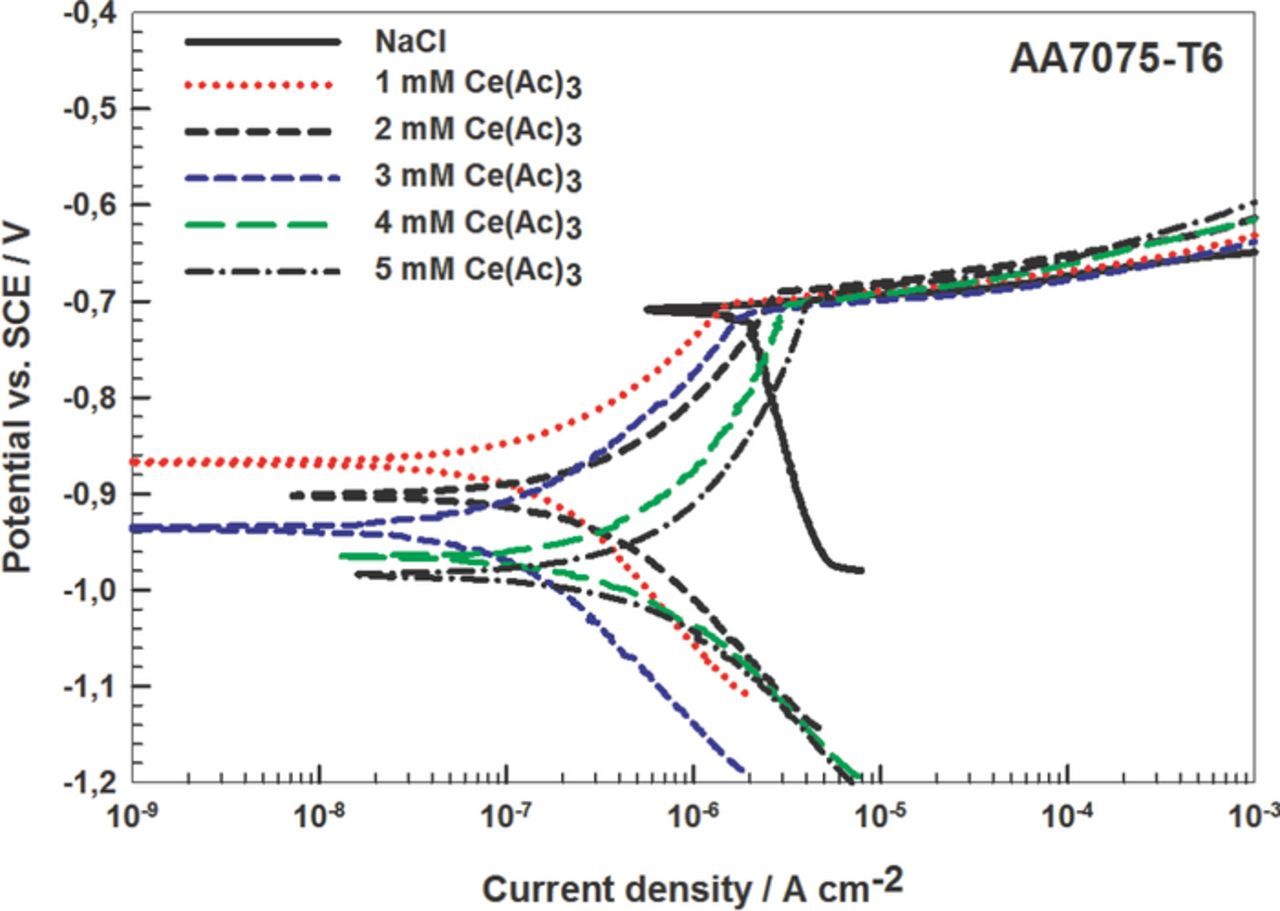
Potentiodynamic measurements were made on AA7075-T6 in 0.1 M NaCl containing Ce(Ac)3 in the concentration range of 1 mM to 5 mM (Table IV, Fig. 8). With increasing concentrations of Ce(Ac)3, Ecorr shifted to more negative values. The broadening of ΔE was related only to shift of Ecorr in the negative direction. Due to the larger negative shift of Ecorr, the values of ΔE were larger, indicating greater protection against pit nucleation. Optimal conditions, large Rp, small jcorr and broad ΔE, were reached at 3 mM Ce(Ac)3. At higher concentrations, ΔE broadened but values of jcorr increased and of Rp decreased. The higher IE for Ce(Ac)3 on AA7075-T6 than on Al can be attributed to the deposition of cerium hydroxide on noble IMs in the alloy, which additionally increases the protection. As IMs are the main pitting sites in the alloy, protection of the alloy is dependent on their protection.
Table IV. Electrochemical parameters deduced for AA7075-T6 in non-inhibited 0.1 M NaCl and inhibited with Ce(Ac)3 in the concentration range from 1 to 5 mM (Fig. 8).
| Solution | Rp [kΩ cm2] | jcorr [nA/cm2] | Ecorr [V] | Epit [V] | ΔE [mV] | IE [%] |
|---|---|---|---|---|---|---|
| NaCl | 1.5 ± 0.2 | 1 896 ± 201 | –0.70 ± 0.01 | –0.69 ± 0.01 | 10 | - |
| + 1 mM Ce(Ac)3 | 247 ± 30 | 77 ± 7 | –0.87 ± 0.02 | –0.70 ± 0.01 | 170 | 99.4 |
| + 2 mM Ce(Ac)3 | 142 ± 12 | 131 ± 11 | –0.90 ± 0.01 | –0.69 ± 0.01 | 210 | 98.9 |
| + 3 mM Ce(Ac)3 | 360 ± 25 | 62 ± 5 | –0.94 ± 0.03 | –0.71 ± 0.01 | 230 | 99.6 |
| + 4 mM Ce(Ac)3 | 109 ± 8 | 307 ± 25 | –0.96 ± 0.01 | –0.70 ± 0.01 | 260 | 98.6 |
| + 5 mM Ce(Ac)3 | 82 ± 10 | 376 ± 32 | –0.98 ± 0.03 | –0.69 ± 0.01 | 290 | 98.2 |
Figure 8. Potentiodynamic polarization measurements for AA7075-T6 in 0.1 M NaCl and inhibited with Ce(Ac)3 in the concentration range from 1 to 5 mM. dE/dt = 1mV/s. The curves were recorded after 1 h stabilization at the Eoc. Electrochemical parameters are given in Table IV.
Potentiodynamic measurements were made on AA7075-T6 in 0.1 M NaCl containing CeCl3 in the concentration range from 1 mM to 5 mM (Table V and Fig. 9). The parameters obtained show that the optimal CeCl3 concentration was 3 mM, resulting in the broadest ΔE, the largest Rp and the smallest jcorr. The protection mechanism is similar to that for Ce(Ac)3 (Fig. 8).
Table V. Electrochemical parameters deduced for AA7075-T6 in non-inhibited 0.1 M NaCl and inhibited with CeCl3 in the concentration range from 1 to 5 mM (Fig. 9).
| Solution | Rp [kΩ cm2] | jcorr [nA/cm2] | Ecorr [V] | Epit [V] | ΔE [mV] | IE [%] |
|---|---|---|---|---|---|---|
| NaCl | 1.5 ± 0.2 | 1 896 ± 201 | –0.70 ± 0.01 | –0.69 ± 0.01 | 10 | - |
| + 1 mM CeCl3 | 80 ± 9 | 251 ± 31 | –0.77 ± 0.02 | –0.72 ± 0.01 | 50 | 98.1 |
| + 2 mM CeCl3 | 142 ± 12 | 130 ± 12 | –0.86 ± 0.03 | –0.69 ± 0.01 | 170 | 98.9 |
| + 3 mM CeCl3 | 162 ± 23 | 120 ± 9 | –0.91 ± 0.03 | –0.70 ± 0.01 | 210 | 99.1 |
| + 4 mM CeCl3 | 109 ± 13 | 159 ± 16 | –0.88 ± 0.02 | –0.72 ± 0.01 | 16 | 98.6 |
| + 5 mM CeCl3 | 132 ± 13 | 186 ± 26 | –0.94 ± 0.03 | –0.71 ± 0.01 | 23 | 98.9 |
Immersion of Al, AA2024-T3 and AA7075-T6 in 0.1 M NaCl containing cerium salts
Surface images of Al, AA2024-T3 and AA7075-T6 were obtained following their immersion in NaCl containing the most effective cerium salts, CeCl3 and Ce(Ac)3 (Fig. 10) and are to be compared to those of ground samples and those immersed in NaCl (Fig. 3). Following immersion in either of the two Ce salts, there was no evidence of corrosion on Al, the surfaces appearing similar to those of ground samples (Figs. 10a, 10d). Only a few, less deep, dark pits were observed on AA2024-T3 in the presence of CeCl3 (Fig. 10b) while, in the presence of Ce(Ac)3, even the surface between IMs appeared to be covered by particles (Fig. 10e). The effect of Ce salts is most significant for AA7075-T6. In CeCl3 containing NaCl there were no dark areas of coalescent pits; IM particles appeared darker, probably due to deposition of Ce(III) hydroxide (Fig. 10c). In Ce(Ac)3-containing NaCl the appearance of IM particles was similar although there were small particles deposited on the surface surrounding IMs (Fig. 10f); the overall effect was more emphasized than that on AA2024-T3. It is assumed that these particles are related to the deposition of Ce-rich particles on the Al matrix. This effect was observed only in the presence of Ce(Ac)3.
Figure 10. Surface appearance, imaged by confocal microscopy, of ground aluminium, AA2024-T3 and AA7075-T6 alloys after immersion in 0.1 M NaCl containing 3 mM CeCl3 or Ce(Ac)3 for 24 hours. Compare with images of ground samples and samples immersed in 0.1 M NaCl only for 24 h (Fig. 3).
Factors affecting inhibition of corrosion
The above results indicate that several factors affect the inhibition effectiveness of a particular Ce salt for a particular substrate, viz. (i) the type of intermetallic particles, (ii) the particular Ce salt and (iii) its concentration. In all these cases the effect is pH dependent. The addition of 3 mM cerium salt to 0.1 M NaCl changed its pH from 5.5 to 6.8 for Ce(Ac)3, 5.5 for Ce(NO3)3, 5.2 for CeCl3 and 1.9 for Ce(SO4)2. The solution with acetate salt has the highest pH and that with sulfate salt the lowest. pH changes with the salt concentration. Addition of Ce(Ac)3 increases the pH from 6.5 for 1 mM to 6.9 for 5 mM. Increasing the concentration of CeCl3 and of Ce(NO3)3 in 0.1 M NaCl from 1 to 5 mM does not affect the pH significantly. Since the pH range of the stability of Al2O3 is between 4.5 and 8.5,1 it can be assumed, judging from the pH alone, that the oxide would be most stable in acetate solution. However, the stability of the alloy is not determined solely by that of Al oxide but also by those of the most common IMs.59,60 MgZn2 is unstable at any pH within the range of 2.5 to 12.5; Al2CuMg dissolves at lower pH but decreasingly so with increasing pH; Al7Cu2Fe is spontaneously passive at pH values between 2.5 and 6 while AlFe3 and Al2Cu show well-defined regions of passivity at higher pH as a result of the passivity of Fe.60 Local morphology is therefore dependent on the solution pH: stochastic pitting is observed at acid pH, deterministic-type pitting at near-neutral pH, and general corrosion at an alkaline pH.60 The Al-Cu-Mg IMs in AA2024-T3 are rapidly dissolved at Eoc, whereas for those in Al-Cu-Fe-Mn the dissolution is several orders of magnitude slower.59 The differences in performance of the Ce salts observed could be related to the dependence on pH of the behavior of the different IMs in the two alloys. In AA2024-T3, the concentration of Cu governs the behavior of the alloy while, in AA7075-T6, Cu concentration is lower and the alloy contains more Zn and Mg (Table I). As MgZn2 is not passivated at any pH,60 AA7075-T6 may be even more affected by lowering the pH in the presence of Ce salt, due to higher dissolution of IMs. The type of protection of AA7075-T6 by Ce salts is reported to be identical to that of AA2024-T3, i.e. precipitation of Ce oxide/hydroxides at the cathodic sites in the alloy.24 However, the type of salt affected the inhibition effectiveness as well as the mechanism of inhibition, as observed in the present work when using different substrates. Harvey et al. investigated a range of structurally-related organic compounds and noticed that the inhibition effectiveness is different depending whether AA2024 or AA7075 substrate was used, despite their corrosion rate in 0.1 M NaCl being similar.8 Furthermore, for AA2024 the addition of sodium acetate produced an acceleration effect, whilst for AA7075 the inhibition effect was only 15%.8 Considering the high inhibition effect produced by cerium(III) acetate in the present work it can be stated that cation (i.e. Ce3+) plays a major role in the inhibition process. The most effective inhibitors for AA7075-T6 are CeCl3 and Ce(Ac)3 which act by shifting Ecorr in the negative direction and by reducing the range not susceptible to pitting in the same direction. Different mechanisms of protection were observed for Al and AA2024-T3 as CeCl3 and Ce(Ac)3 affected the anodic process as well. Since Ecorr were shifted more positive, conditions were reached under which formation and thickening of aluminium oxide took place.
Enrichment of copper at Al-Cu-Mg precipitates occurs after selective dissolution of Mg and Al which leaves Cu-rich remnant particles. If copper dissolves, it can then redeposit as thin copper layer onto alloy matrix around the IMs.16,44,45,61 Redeposited copper increases the effective surface area of the cathodic zone.16,44,45,61 NO3− anions reduce to provide a higher cathodic current. In the presence of NO3− anions, the potential of the alloys shifts in the anodic direction, reaching the conditions for formation of an oxide film, which inhibits corrosion (Figs. 5, 6), as noticed previously.24 Nitrate ions can be reduced electrochemically from very dilute solutions with a sufficiently high rate in the presence of cuprous ions.62 This process enhances the passivation ability of nitrates in acidic media − higher concentrations would otherwise be required for reliable passivation.62 Ce(SO4)2, as a Ce(IV) salt, proved ineffective as a corrosion inhibitor due to its low solubility and to the low pH of its aqueous solution, as well as to the fact that the formation of precipitates requires the formation of Ce(III) ions.
Conclusions
The inhibitory actions on Al, AA2024-T3 and AA7075-T6 of various cerium salts in 0.1 M NaCl were compared. Although in all cases protection is achieved by deposition of cerium hydroxide on cathodic sites, the course of inhibition is dependent on the type of anion and on the substrate, i.e. the composition of intermetallic particles in the alloy. Full understanding of this phenomenon requires a detailed further study in order to correlate types of intermetallic particle inhibited by cerium ions as a function of counter-anion.
The inhibitory action of all cerium(III) salts was greatest on AA7075-T6, followed by Al, and the smallest on AA2024-T3. The reason for this is interpreted in terms of the different compositions of intermetallic particles in the particular alloy and of the dependence of corrosion protection on the type of anion and pH. Addition of the same concentration of different cerium(III) salts leads to different pHs, consequently affecting the stability, not only of the intermetallic particles but also of the surrounding aluminium oxide matrix. Ce(III) acetate was the most effective inhibitor, followed by Ce(III) nitrate and chloride.
Ce(III) acetate has been shown to be an effective inhibitor for all three materials studied. This is reflected, not only in the increase in polarization resistance and decrease in corrosion current density, but also in the increase in resistance to pit nucleation. Preliminary results show that acetate anions follow a course of protection that differs somewhat from that with chloride.
Acknowledgments
The Slovenian Research Agency is acknowledged for financial support (grants No. J1-6734 and P2-0393). The authors thank prof. R. H. Pain for proof reading the manuscript.