The Impact of Operating Conditions on the Performance of a CH4 Dry Reforming Membrane Reactor for H2 Production
Akira Nishimura 1, *![]() , Satoshi Ohata 2
, Satoshi Ohata 2![]() , Kaito Okukura 3
, Kaito Okukura 3![]() , Eric Hu 4
, Eric Hu 4![]()
- Division of Mechanical Engineering, Graduate School of Engineering, Mie University , 1577 Kurimamachiya-cho, Tsu, Mie, 514-8507 Japan
- Division of Mechanical Engineering, Graduate School of Engineering, Mie University , 1577 Kurimamachiya-cho, Tsu, Mie, 514-8507 Japan
- Division of Mechanical Engineering, Graduate School of Engineering, Mie University , 1577 Kurimamachiya-cho, Tsu, Mie, 514-8507 Japan
- School of Engineering, the University of Adelaide, Adelaide, SA 5005, Australia; E- M ail: eric.hu@adelaide.edu.au
* Correspondence: Akira Nishimura ![]()
Academic Editor: Alfonso Chinnici
Special Issue: Hydrogen Energy: Sustainable Production, Storage and Utilisation
Received: March 25, 2020 | Accepted: May 09, 2020 | Published: May 14, 2020
Journal of Energy and Power Technology 2020, Volume 2, Issue 2, doi:10.21926/jept.2002008
Recommended citation: Nishimura A, Ohata S, Okukura K, Hu E. The Impact of Operating Conditions on the Performance of a CH4 Dry Reforming Membrane Reactor for H2 Production. Journal of Energy and Power Technology 2020; 2(2): 008; doi:10.21926/jept.2002008.
© 2020 by the authors. This is an open access article distributed under the conditions of the Creative Commons by Attribution License, which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is correctly cited.
Abstract
Biogas is a promising resource for the production of H2 since it liberates energy by recycling waste, along with the reduction of CO2. In this paper, the biogas dry reforming membrane reactor is proposed to produce H2 for use in fuel cells. Pd/Cu alloy membrane is used to enhance the performance of the biogas dry reforming reactor. This study aims at understanding the effect of operating parameters such as feed ratio of sweep gas, pressure in the reactor, and reaction temperature on the performance of the biogas dry reforming membrane reactor. The effect of the molar ratio of the supplied CH4:CO2, feed ratio of the sweep gas, and the valve located at the outlet of the reaction chamber on the performance of biogas dry reforming are investigated. Besides, the thermal efficiency of the proposed reactor is also evaluated. The results show that the concentration of H2 in the closed valve condition is higher than that of the open valve, and the optimum feed ratio of the sweep gas to produce H2 is 1, irrespective of the molar ratio of supplied CH4:CO2. Also, H2 selectivity and CO selectivity increases and decreases respectively when the reaction temperature increases, irrespective of the molar ratio of supplied CH4:CO2. Therefore, the thermal efficiency of the closed valve is higher than that of the opened valve. Also, the thermal efficiency is the maximum when the feed ratio of the sweep gas is 1 due to high H2 production performance.
Keywords
CH4 dry reforming; membrane reactor; Ni catalyst; operation condition; selectivity; thermal efficiency
1. Introduction
The fuel cell is a promising power generation technology as environmentally toxic gases such as NOx, SOx, and CO2 are not emitted. H2 is fuel for the fuel cell, produced from different resources. However, CO2 would be emitted upon the production of H2 from fossil fuels such as coal, oil, and natural gas. Therefore, H2 production technology without CO2 emission is important in a sustainable world.
In this study, H2 is produced from biogas. Biogas is a gaseous fuel consisting of CH4 (55–75 vol%) and CO2 (25–45 vol%) [1], mainly produced upon fermentation of CH4 by the action of anaerobic microorganisms on raw materials such as garbage, livestock excretion, and sewage sludge. Besides, the conversion of biogas to H2 is carbon neutral, thus reduces carbon footprint. From a recent report by the World Bioenergy Association based on the data from the International Energy Agency (IEA) [2], the domestic supply of biogas globally reached 1.33 EJ in 2017 from 0.28 EJ in 2000. Therefore, biogas is a promising energy resource for the future.
Biogas is usually used as a fuel in the gas engine or micro gas turbine [3]. Since biogas contains CO2 of approximately 40 vol%, the heating value is low compared to natural gas, which results in a decrease in the efficiency of power generation. Therefore, in this study, biogas is used in the dry reforming process to produce H2, which is used as fuel for the fuel cells. Biogas dry reforming to produce H2 was previously studied by several researchers [4–17]. The catalyst used in the process is important in evaluating the performance of biogas dry reforming. Though some unique catalysts such as Fe/Al2O3 [4], Rh/Al2O3 [5], Co-Ce/ZrO2 [6], and Fe2O3/zeolite [7] are used, Ni-based catalyst is the most common one for the biogas dry reforming process [8–17], which is also used in this study. This study adopts a membrane reactor to improve the efficiency of biogas dry reforming. Generally, a membrane reactor is used to improve the CH4 steam reforming by separating H2 from the reaction space [18–20] as soon as it is produced. The synergetic effect of the membrane reactor on performing the reaction and separation in the same unit, their simplicity, and the possibility of advanced levels of automation and control offer an attractive opportunity to redesign industrial processes [21]. Generally, Pd based membrane is used to separate H2 due to its high efficiency [21–23]. Some studies used the membrane reactor for CH4 dry reforming [21–29]. The coupled effect of the membrane and catalyst on CH4 conversion was discussed earlier [21]. It was reported that the dense Pd/Ag/Cu alloy membrane and Ni/CeZrO2 catalyst together had the highest CH4 conversion of 65.2% [22] among the investigated combinations. The Pd/Au alloy membrane used in the two-zone fluidized bed reactor [23,29] showed the CH4 conversion and H2 selectivity to be higher compared to the conventional fluidized bed reactor. The highest CH4 conversion and H2/CO ratio were approximately 80% and 1.8, respectively. Ni was not only used in the membrane reactor but also as a catalyst to promote the CH4 dry reforming process in the same reactor [24]. Though the CH4 conversion and H2 recovery achieved up to 85% and 70%, respectively, the equilibrium conversion was not achieved due to the limited resistance time in the reactor. The numerical simulation using the double tube reactor model investigated the percentage of CH4 conversion, H2 concentration, and temperature in the CH4 dry reforming membrane reactor [27]. It was found that high reformer temperature in the membrane reactor was not favorable for the high production of H2. Besides, the impact of the number of layers of the membrane on H2 separation, as well as CH4 and CO2 conversion in the dry reforming process was investigated by numerical simulation [30]. The CH4 and CO2 conversion decreased as the number of layers increased, although the inclination slightly changed after six layers of the membrane. The permeation of H2 into the membrane increased as the number of layers increased. The gas separation membrane was also applied to the steam reforming of CH4 [31] and C2H5OH [32]. CO2 capture by dual-phase ceramic-carbonate was effective in obtaining highly pure H2, and achieved higher CO2 recovery compared to the conventional fixed bed reactor. The Pd/Cu membrane reactor with Ir/CeO2 catalyst provided a high H2 yield of 98.1% at 550 °C and a reforming pressure of 1300 kPa [32]. This membrane was used with Ru or Rh catalyst [33], and evaluated at 550 °C, resulting in the CH4 and CO2 conversion of 8% to 40%. These values were slightly higher than those obtained for a fixed bed reactor with the same amount of catalyst.
However, the impact of gas separation conditions such as feed ratio of the sweep gas, reactor pressure, and varying reaction temperature on the performance of biogas dry reforming reactor is currently unknown.
Therefore, this study aims to understand the effect of operating conditions such as feed ratio of the sweep gas, pressure in the reactor, and reaction temperature on the performance of biogas dry reforming. Since a pure Pd membrane has a relatively high solubility for carbon, it causes membrane degradation leading to the loss of permeability [34]. Therefore, the Pd/Cu alloy membrane was used in this study. The reaction scheme of CH4 dry reforming is as follow:
CH4 + CO2 → 2CO + 2H2 △H298o = 247 kJ/mol (1)
2. Experiment
2.1 Experiment Set-Up
Figure 1 illustrates the experimental set-up used in this study. It consists of a gas cylinder, mass flow controller (S48-32; produced by HORIBA METRON INC.), pressure sensor (KM31; by NAGANO KEIKI), valve, reactor, i.e., a combination of the reaction chamber and sweep chamber, and gas sampling tap. The reactor is in the furnace where the temperature is controlled by the far-infrared heater (MCHNNS1; produced by MISUMI). CH4 gas with purity over 99.4 vol% and CO2 gas with purity over 99.9 vol% are controlled by mass flow controller and mixed before passing through the reactor. The pressure of the mixed gas is measured by the pressure sensor. Ar gas having a purity over 99.99 vol% is controlled by the mass flow controller, and measured by the pressure sensor. This is fed as a sweep gas. The exhausted gas at the outlet of the reactor is suctioned by a gas syringe via the gas sampling tap, and its concentration is measured by FID gas chromatography and a methanizer. The minimum resolution of both the FID gas chromatograph and methanizer is 1 ppmV. The gas pressure at the outlet of the reactor is measured using the pressure sensor. The gas concentration and pressure are measured at the outlet of the reaction chamber and sweep chamber, respectively. A valve is installed next to the pressure gauge at the outlet of the reaction chamber to investigate the effect of pressure on the performance of the dry reforming of CH4. The valve is placed 65 cm away from the outlet of the reaction chamber. Though its position would better be near the outlet, this distance is kept due to the heat resistance of the pressure sensor installed before the valve. When the valve is closed, the gas in the reaction chamber flows to the sweep chamber preferentially.
Figure 1 Schematic drawing of the experimental set-up.
Figure 2 illustrates the details of the reactor. The reactor consists of the reaction chamber, sweep chamber, and H2 separation membrane. The reaction chamber and sweep chamber are made of stainless steel, having dimensions 40 mm × 100 mm × 40 mm. The volume of the reaction space is 16 × 10–5 m3. Pure Ni catalyst having a porous structure is placed in the reaction chamber, which means the catalyst is 100 wt% of Ni. The average pore diameter of this catalyst is 1.9 mm. The weight of the Ni catalyst is 63.1 g. The Pd/Cu alloy membrane (Cu of 40 wt%; produced by Tanaka Kikinzoku Kogyo), which is installed between the reaction and separation chambers, causes H2 separation. The thickness of the Pd/Cu alloy membrane is 20 mm. The temperatures at the inlet, middle, and outlet of the reaction and sweep chambers are measured by K-type thermocouples. The measured temperature and pressure are collected by the data logger (GL240; by Graphtec Corporation).
Figure 2 Schematic drawing of reactor.
Table 1 lists the experimental conditions. The molar ratio of the supplied CH4:CO2 varies at 1.5:1, 1:1, or 1:1.5 since this ratio simulates the biogas. The feed ratio of the sweep gas, which is defined as the flow rate of sweep gas divided by the flow rate of supply gas composed of CH4 and CO2, is set to 0.5, 1, or 2. The effects of these ratios, in the conditions when the valve is open or close and the reaction temperatures of 400 °C, 500 °C and 600 °C, were studied. The gas concentrations in the reaction and sweep chambers were evaluated by FID gas chromatograph, and methanizer (produced by GL Science). This study shows the average data of ten trials for different experimental conditions in the following figures. The distribution of each of the gas concentration was below 10%. H2 and CO selectivity were evaluated. The thermal efficiency of the proposed reactor was also evaluated.
Table 1 Experimental condition.

2.2 Evaluation Procedure of Performance of the Proposed Reactor
In this study, the performance of the proposed reactor is evaluated in terms of gas concentration at the outlet of the reaction and sweep chambers, pressure difference between the gas and sweep chambers, H2 and CO selectivity, and thermal efficiency. H2 and CO selectivities are defined as follows:
SH2 = CH2, out/(CH2, out + CCO, out) × 100 (2)
SCO = CCO, out/(CH2, out + CCO, out) × 100 (3)
where SH2 is the H2 selectivity (%), CH2, out is the concentration of H2 at the outlet of the reaction and sweep chambers (ppmV), CCO, out is the concentration of CO at the outlet of the reaction chamber (ppmV), and SCO is the CO selectivity (%). The thermal efficiency is defined as follows:
η = QH2/(Qreact + Qsweep) ×100 (4)
QH2 = {(mCH4 + mCO2)/(22.4×60}×(CH2, out/106)×(Hl×22.4×103) (5)
Qreact = QCH4 + QCO2 (6)
QCH4 = {(mCH4/(22.4×60)}×Cp, CH4×(Treact – Troom) (7)
QCO2 = {(mCO2/(22.4×60)}×Cp, CO2×(Treact – Troom) (8)
Qsweep = {(mAr/(22.4×60)}×Cp, Ar×(Treact – Troom) (9)
where η is the thermal efficiency (%), QH2 is the heating value of produced H2 (W), mCH4 is the flow rate of CH4 at the inlet of the reaction chamber (NL/min), mCO2 is the flow rate of CO2 at the inlet of the reaction chamber (NL/min), Hl is the low heating value of H2 (= 10.79) (MJ/Nm3), Qreact is the heat required for the reaction gases (W), QCH4 is the heat needed to preheat the supplied CH4 (W), QCO2 is the heat needed to preheat the supplied CO2 (W), Cp, CH4 is the specific heat of CH4 (kJ/(kmol · K)), Treact is the reaction temperature (K), Troom is the room temperature (K), Cp, CO2 is the specific heat of CO2 (kJ/(kmol · K)), Qsweep is the heat needed to preheat sweep gas (W), mAr is the flow rate of Ar at the inlet of the sweep chamber (NL/min), and Cp, Ar is the specific heat of CO2 (kJ/(kmol · K)).
3. Results and Discussion
3.1 Effect of Feed Ratio of Sweep Gas and Pressure in Reaction Chamber on H2 Production
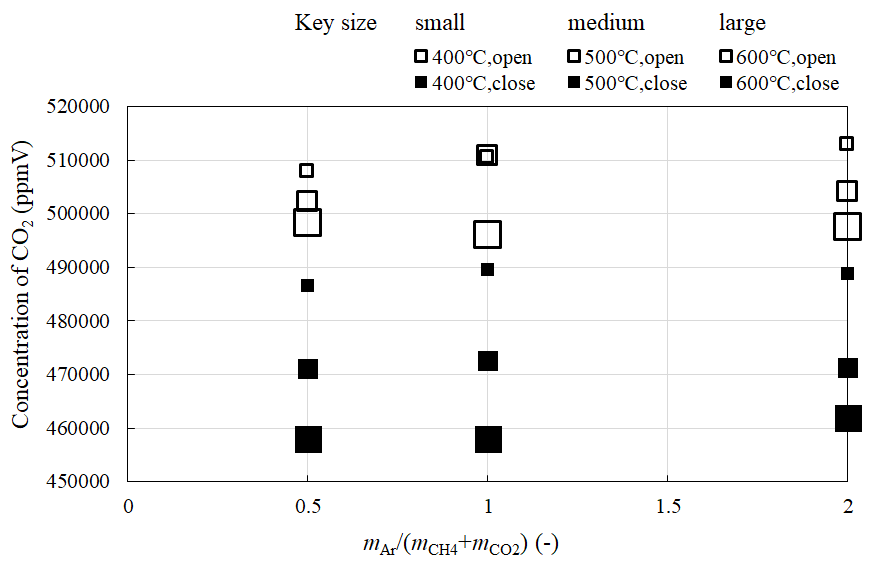
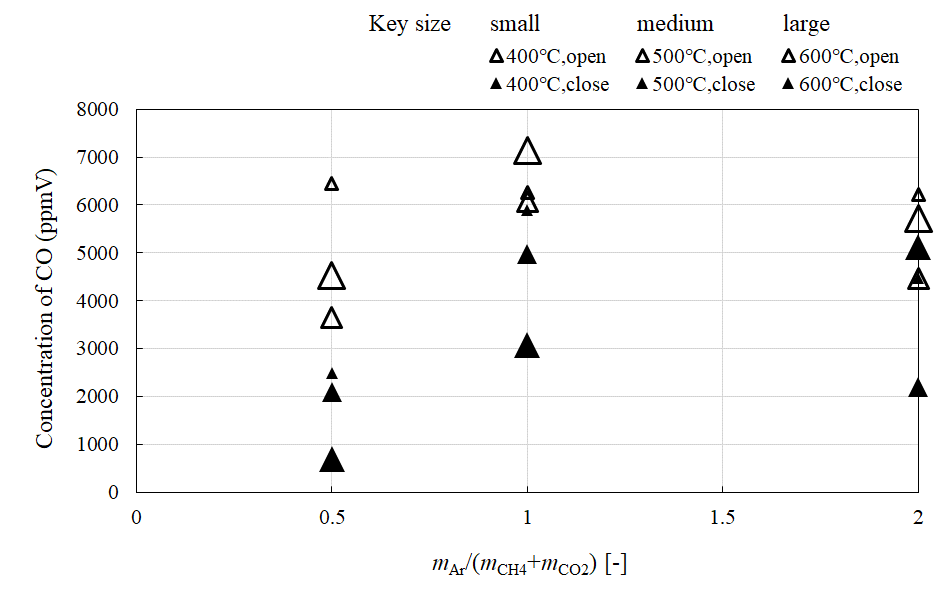
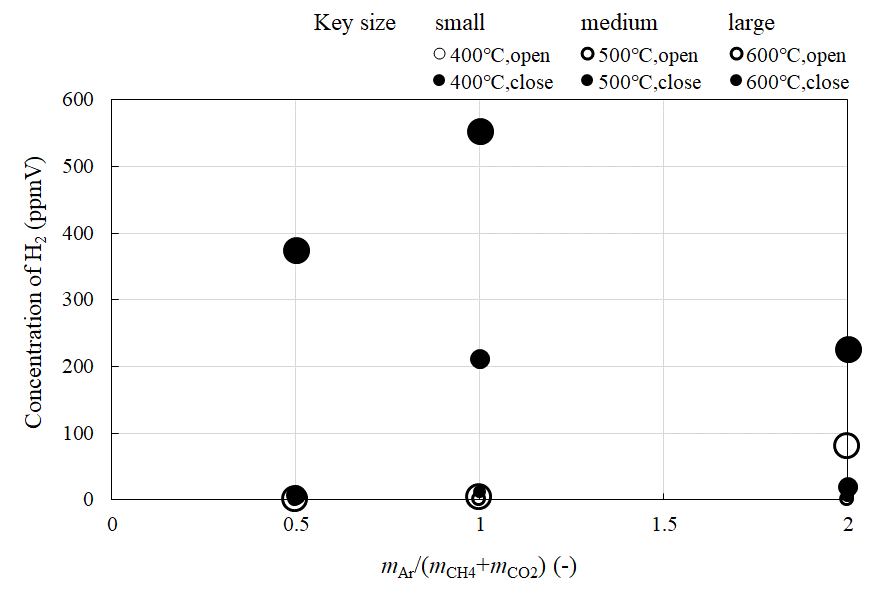
Figure 3, Figure 4, Figure 5 and Figure 6 compare the relationship between the concentration of CH4, CO2, H2, and CO at the outlet of the reaction chamber, and the feed ratio of sweep gas, when the valve installed at the outlet is open or close, respectively. Figure 7 shows the comparison of the relationship between the concentration of H2 at the outlet of the sweep chamber and feed ratio of sweep gas when the valve installed at the outlet of the reaction chamber is open or close, respectively. In these figures, the feed ratio of sweep gas is indicated by mAr/(mCH4+mCO2). The molar ratio of the supplied CH4:CO2 is 1.5:1. The reaction temperature is varied to 400 °C, 500 °C, or 600 °C.
According to Figure 4 and Figure 5, the concentration of CO2 and H2 in the reaction chamber are lower and higher, respectively, when the valve is close. Since the pressure difference between the reaction and sweep chambers is higher upon closing the valve, it is thought that H2 separation is promoted, increasing the concentration of H2. The increase in the H2 concentration in the sweep chamber is shown in Figure 7. Though the concentration of CO2 decreased, those of CH4 (Figure 3) and CO (Figure 6) increased, when the valve is close. The following reactions [8,12] would take place:
CO2 + H2 → CO + H2O △H298o = 41.2 kJ/mol (10)
CO2 + 4H2 → CH4 + 2H2O △H298o = -165.0 kJ/mol (11)
According to the reactions shown in Eq (10) and (11), CH4 and CO consumption increased upon consumption of CO2 and H2.
Figure 3 Comparison of the relationship between the concentration of CH4 at the outlet of the reaction chamber and feed ratio of sweep gas with the valve open or close (CH4 : CO2 = 1.5 : 1).
Figure 4 Comparison of the relationship between the concentration of CO2 at the outlet of the reaction chamber and feed ratio of sweep gas with the valve open or close (CH4 : CO2 = 1.5 : 1).
Besides, the highest concentration of H2 is obtained for mAr/(mCH4+mCO2) = 1, according to Figure 5, Figure 6 and Figure 7. It is revealed that the optimum mAr/(mCH4+mCO2) to produce H2 is 1. This result agrees with a previous study [22]. According to this [22], since the main CH4 dry reforming reaction, i.e., Eq (1) is reversible, introducing excess amounts of reactants could drive the reaction forward, resulting in the generation of more H2. However, the produced H2 could also react with the unreacted CO2 in the reversible water-gas shift reactions, i.e., Eqs. (10) and (11). Therefore, higher CO2 consumption was observed compared to that of CH4. As a result, the highest concentration of H2 was obtained when mAr/(mCH4+mCO2) = 1, as the reactions are balanced.
Figure 5 Comparison of the relationship between the concentration of H2 at the outlet of the reaction chamber and feed ratio of sweep gas with the valve open or close (CH4 : CO2 = 1.5 : 1).
Figure 6 Comparison of the relationship between the concentration of CO at the outlet of the reaction chamber and feed ratio of sweep gas with the valve open or close (CH4 : CO2 = 1.5 : 1).
Figure 7 Comparison of the relationship between the concentration of H2 at the outlet of sweep chamber and feed ratio of sweep gas with the valve open or close (CH4 : CO2 = 1.5 : 1).
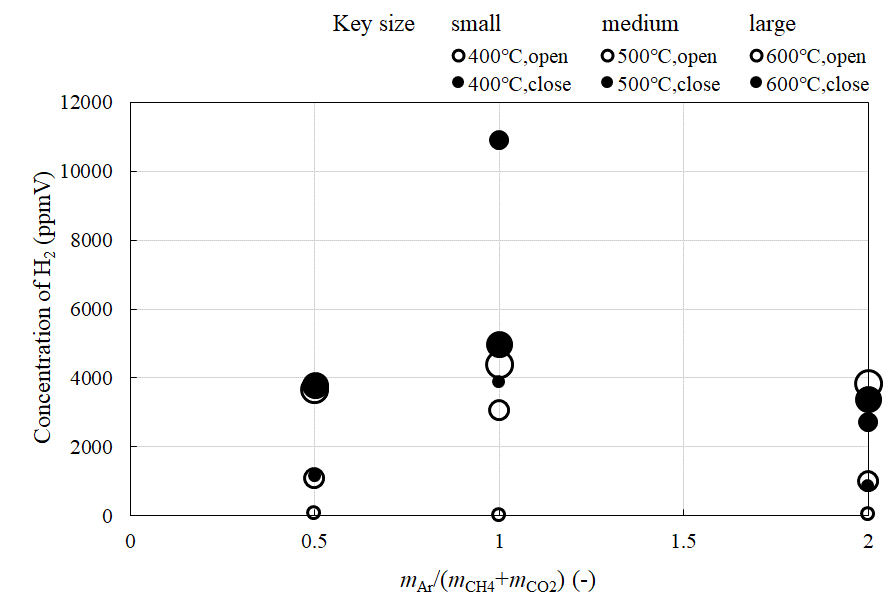
Figure 8 and Figure 9 show the comparison of the relationship between the concentration of H2 and CO at the outlet of the reaction chamber and mAr/(mCH4+mCO2) when the valve installed at the outlet of the reaction chamber is open or close, respectively. Figure 10 shows the comparison of the relationship between the concentration of H2 at the outlet of the sweep chamber and mAr/(mCH4+mCO2) when the valve is open or close, respectively. The molar ratio of the supplied CH4:CO2 is 1:1. The reaction temperature is changed to 400 °C, 500 °C, and 600 °C.
Figure 8 Comparison of the relationship between the concentration of H2 at the outlet of the reaction chamber and feed ratio of sweep gas with the valve is open or close (CH4 : CO2 = 1 : 1).
Figure 9 Comparison of the relationship between the concentration of CO at the outlet of the reaction chamber and feed ratio of sweep gas with the valve is open or close (CH4 : CO2 = 1 : 1).
According to Figures 8 and 10, the concentration of H2 is higher when the valve is close. Since the pressure difference between the reaction and sweep chambers is higher upon closing the valve, it is thought that the H2 separation is promoted, resulting in an increase in the concentration of H2. Also, it is known from Figure 9 that the concentration of CO is higher when the valve is close. Since the molar ratio of the supplied CH4:CO2 = 1:1 is the same as the theoretical value as shown in Eq. (1), it is believed that the dry reforming progressed well. Besides, it is seen from Figures 8 and 10 that the highest concentration of H2 is obtained for mAr/(mCH4+mCO2) = 1. It is revealed that the optimum mAr/(mCH4+mCO2) to produce H2 is 1, and shows the same tendency when the molar ratio of supplied CH4:CO2 = 1.5:1.
Figure 10 Comparison of the relationship between the concentration of H2 at the outlet of sweep chamber and feed ratio of sweep gas with the valve is open or close (CH4 : CO2 = 1 : 1).
Figure 11 and Figure 12 compare the relationship between the concentration of H2 and CO at the outlet of the reaction chamber and mAr/(mCH4+mCO2), when the valve installed at the outlet of the reaction chamber is open or close, respectively. Figure 13 shows the comparison of the relationship between the concentration of H2 at the outlet of the sweep chamber and mAr/(mCH4+mCO2) when the valve installed at the outlet of the reaction chamber is open or close, respectively. The molar ratio of the supplied CH4:CO2 is 1:1.5. The reaction temperature is changed to 400 °C, 500 °C or 600 °C.
According to Figures 11 and 13, the concentration of H2 is higher when the valve is close. Since the pressure difference between the reaction and sweep chambers is higher when the valve is close, it is thought that H2 separation is promoted, increasing the concentration of H2. Additionally, it is seen from Figures 11 and 13 that the highest concentration of H2 is obtained for mAr/(mCH4+mCO2) = 1. It is revealed that the optimum mAr/(mCH4+mCO2) to produce H2 is 1, which is also the case when the molar ratio of the supplied CH4:CO2 = 1.5:1 and 1:1. It is obvious that mAr/(mCH4+mCO2) = 1 is the optimum ratio irrespective of the molar ratio of the supplied CH4 : CO2, where the reactions are balanced as described above.
According to Figures 3 to 13, the concentrations of produced H2 and CO are low. This low conversion is thought to be caused by the use of pure Ni as the catalyst. In this study, pure Ni was used to evaluate the basic characteristics of the proposed membrane reactor as the first step to develop the reactor. According to the literature, noble metals such as Pd, Pt [21], and Ru, as well as the alloy of Ni and the other metals, e.g., Co, Cu, Fe, and Ce [28] showed better catalytic activity for the conversion of CH4 and CO2 compared to pure Ni. Therefore, this study used other catalysts, as mentioned above, to promote the CH4 and CO2 conversion in the proposed membrane reactor as the next step.
Figure 11 Comparison of the relationship between the concentration of H2 at the outlet of the reaction chamber and feed ratio of sweep gas with the valve is open or close (CH4 : CO2 = 1 : 1.5).
Figure 12 Comparison of the relationship between the concentration of CO at the outlet of the reaction chamber and feed ratio of sweep gas with the valve is open or close (CH4 : CO2 = 1 : 1.5).
Figure 13 Comparison of the relationship between the concentration of H2 at the outlet of sweep chamber and feed ratio of sweep gas with the valve is open or close (CH4 : CO2 = 1 : 1.5).
3.2 Evaluation on Performance of Proposed Reactor
Table 2 and Table 3 list the pressure differences between the reaction and sweep chambers when the molar ratio of CH4:CO2 = 1.5:1, 1:1, 1:1.5, and the valve at the outlet of the reaction chamber is open or close, respectively. It is seen from the data that the pressure differences are 0 kPa when the valve is open, irrespective of the molar ratio. Since the valve is open, the gases in the reaction chamber might flow out without H2 separation by the Pd/Cu alloy membrane. This is further confirmed by the concentration of H2 at the outlet of the sweep chamber, which is almost 0 ppmV, as shown in Figures 7, 10, and 13. When the valve is close, the pressure difference and concentration of H2 at the outlet of the sweep chamber increase with the rise in the reaction temperature. In other words, closing the valve, i.e., increasing the pressure difference and reaction temperature, promotes the CH4 dry reforming reaction.
Table 2 Pressure difference between the reaction chamber and the sweep chamber in the case of the molar ratio of CH4 : CO2 = 1.5 : 1, 1 : 1, 1 : 1.5, with the valve installed at the outlet of the reaction chamber is open (unit: kPa).

Table 3 Pressure difference between the reaction chamber and the sweep chamber in the case of the molar ratio of CH4 : CO2 = 1.5 : 1, 1 : 1, 1 : 1.5, without the valve installed at the outlet of the reaction chamber is open (unit: kPa).

Figure 14, Figure 15 and Figure 16 compare the relationship between H2 or CO selectivity and mAr/(mCH4+mCO2) when the molar ratio of supplied CH4:CO2 = 1.5:1, 1:1, and 1:1.5, respectively with a closed valve. The reaction temperature is set to 400 °C, 500 °C, and 600 °C, respectively. The figures indicate that H2 selectivity increases and CO selectivity decreases with an increase in the reaction temperature as the CH4 dry reforming is an endothermic reaction [9,35]. According to the previous study [36], the H2/CO ratio increases with increased reaction temperature because CH4 dry reforming is an endothermic reaction, which is thermodynamically and kinetically favored at high temperatures.
Besides, these figures show that the CO selectivity is higher than that of H2 at mAr/(mCH4+mCO2) = 2. If mAr/(mCH4+mCO2) is higher than 2, the biogas dry reforming in the reaction chamber requires longer reaction time.
Figure 14 Comparison of the relationship between H2 or CO selectivity and feed ratio of sweep gas with the closed valve (CH4 : CO2 = 1.5 : 1).
Figure 15 Comparison of the relationship between H2 or CO selectivity and feed ratio of sweep gas with the closed valve (CH4 : CO2 = 1 : 1).
Figure 16 Comparison of the relationship between H2 or CO selectivity and feed ratio of sweep gas with the closed valve (CH4 : CO2 = 1 : 1.5).
Table 4 and Table 5 give a comparison of thermal efficiency under different operating conditions when the valve is open and close, respectively. The data show that the thermal efficiency with a closed valve is higher than that when it is open, irrespective of the reaction temperature, mAr/(mCH4+mCO2), and the molar ratio of the supplied CH4:CO2. Also, it is revealed that the thermal efficiency at mAr/(mCH4+mCO2) = 1 is higher than any other conditions.
Table 4 Comparison of thermal efficiency among different operation conditions with the open valve (unit: %).

Table 5 Comparison of thermal efficiency among different operation conditions with the close valve (unit: %).

In this study, the thermal efficiencies achieved were not high under different conditions. It is thought that effective H2 separation was not obtained since the pressure difference between the reaction and sweep chambers was not high enough to extract H2. As a result, there was no displacement of the thermodynamic equilibrium of the reaction, causing less production of H2. It is necessary to improve the reaction performance of biogas dry reforming and H2 separation. Since this study investigated pure Ni catalyst and Ni-based alloy catalyst such as Ni/Ce [10], Ni/La [17], Ni/ZnO-Al2O3 [37], the latter was found to have better performance and can be preferentially used for further studies. Also, the characterization of the H2 separation membrane, such as composition, thickness, and metal type, is important to improve the efficiency of H2 separation. Since this study investigated one type of H2 separation membrane only, i.e., Pd/Cu alloy membrane (Cu of 40 wt%), the composition and thickness of the Pd/Cu alloy membrane will also be investigated in the future.
4. Conclusions
In this paper, the effect of operating conditions such as the feed ratio of the sweep gas, pressure in the reactor, and reaction temperature on the performance of biogas dry reforming was studied. The effects of the molar ratio of the supplied CH4:CO2, as well as mAr/(mCH4+mCO2) on the performance of biogas dry reforming were also investigated. The effects were evaluated by comparing the conditions when the valve was open and close at the outlet of the reaction chamber. The reaction temperature was set to 400 °C, 500 °C, and 600 °C, respectively. The following conclusions are obtained from the study:
- 1)
- The final concentration of H2 in the reaction chamber with closed valve is higher than that with the open valve, irrespective of the molar ratio of supplied CH4:CO2, since H2 separation is promoted by the increase in the pressure difference between the reaction and sweep chambers.
- 2)
- It is confirmed that the optimum mAr/(mCH4+mCO2) to produce H2 is 1, irrespective of the molar ratio of supplied CH4:CO2.
- 3)
- The H2 selectivity increases and CO selectivity decreases with an increase in the reaction temperature, irrespective of the molar ratio of supplied CH4:CO2.
- 4)
- CO selectivity is likely to be higher than H2 selectivity at mAr/(mCH4+mCO2) = 2 since the separation speed would be higher compared to the reaction speed of biogas dry reforming.
- 5)
- The thermal efficiency of the reactor with a closed valve is higher than that with the open valve, irrespective of the reaction temperature, mAr/(mCH4+mCO2), and the molar ratio of supplied CH4:CO2.
- 6)
- It is revealed that the thermal efficiency at mAr/(mCH4+mCO2) = 1 is the highest due to maximum H2 production.
Author Contributions
Akira Nishimura managed this study and wrote the paper; Satoshi Ohata carried out the experiment and collected the data; Kaito Okukura carried out the experiment and collected the data; Eric Hu refined the paper from the view point of native English speaker.
Funding
This work was financially supported by the joint usage/research program of the Institute of Materials and Systems for Sustainability (IMaSS), Nagoya University.
Competing Interests
The authors have declared that no competing interests exist.
References
- Kalai DY, Stangeland K, Jin Y, Tucho WM, Yu Z. Biogas dry reforming for syngas production on La promoted hydrotalcite-derived Ni catalysts. Int J Hydrogen. 2018; 43: 19438-19450. [CrossRef]
- World Bioenergy Association. Global Bioenergy Statistics. Available from: https://worldbioenergy.org/global-bioenergy-statistics.
- The Japan Gas Association. Available from: https://www.gas.or.jp/gas-life/biomass/.
- Jabbour K, Saad A, Inaty L, Davidson A, Massiani P, Hassan NE. Ordered mesoporous Fe-Al2O3 based-catalysts synthesized via a direct “one-pot” method for the dry reforming of a model biogas mixture. Int J Hydrogen Energ. 2019; 44: 14889-14907. [CrossRef]
- Navarro-Puyuelo A, Reyero I, Moral A, Bimbela F, Banares MA, Gandia LM. Effect of oxygen addition, reaction temperature and thermal treatments on syngas production from biogas combined reforming using Rh/alumina catalysts. J Ind Eng Chem. 2019; 80: 21-226. [CrossRef]
- Paksoy AI, Akdag CY, Caglayan BS, Aksoylu AE. Kinetic and mechanistic features of carbon dioxide reforming of methane over Co-Ce/ZrO2 catalysts. Int J Chem Kinet. 2019; 51: 138-145. [CrossRef]
- Hernandez B, Martin M. Optimal production of syngas via super-dry reforming. Analysis for natural gas and biogas under different CO2 taxes. Chem Eng Res Des. 2019; 148: 375-392. [CrossRef]
- Price CAH, Arnold W, Pastor-Perez L, Amini-Horri B, Reina TR. Catalytic upgrading of a biogas model mixture via low temperature DRM using multicomponent catalysts. Top Catal. 2019. DOI: 10.1007/s11244-019-01216-8. [CrossRef]
- Chein R, Yang Z. Experimental study on dry reforming of biogas for syngas production over Ni-based catalysts. ACS Omega. 2019; 4: 20911-20922. [CrossRef]
- Kalai DY, Stangeland K, Tucho WM, Jin Y, Yu Z. Biogas reforming on hydrotalcite-derived Ni-Mg-Al catalysts: The effect of Ni loading and Ce promotion. J CO2 Util. 2019; 33: 189-200. [CrossRef]
- Schiaroli N, Lucarelli C, Luna GS, Fornasari G, Vaccari A. Ni-based catalysts to produce synthesis gas by combined reforming of clean biogas. Appl Catal A Gen. 2019; 582. DOI: 10.1016/j.apcata.2019.05.021. [CrossRef]
- Calgaro, CO, Perez-Lopez OW. Biogas dry reforming for hydrogen production over Ni-M-Al catalysts (M = Mg, Li, Ca, La, Cu, Co, Zn). Int J Hydrogen Energ. 2019; 44: 17750-17766. [CrossRef]
- Rosha P, Mohapatra SK, Mahla SK, Dhir A. Catalytic reforming of synthetic for hydrogen enrichment over Ni supported on ZnO-CeO2 mixed catalyst. Biomass Bioenerg. 2019; 125: 70-78. [CrossRef]
- Rosha P, Mohapatra SK, Mahla SK, Dhir A. Hydrogen enrichment of biogas via dry and autothermal-dry reforming with pure nickel (Ni) nanoparticle. Energy. 2019; 172: 733-739. [CrossRef]
- Sache E, Johnson S, Pastor-Perez L, Horri BA, Reina TR. Biogas upgrading via dry reforming over a Ni-Sn/CeO2-Al2O3 catalyst: Influence of the biogas source. Energies. 2019; 12: 1-14. DOI: 10.3390/en12061007. [CrossRef]
- Rosha P, Mohapatra SK, Mahla SK, Dhir A. Biogas reforming for hydrogen enrichment by ceria decorated over nickel catalyst supported on titania and alumina. Int J Hydrogen Energ. 2018; 43: 21246-21255. [CrossRef]
- Kalai DY, Stangeland K, Jin Y, Tucho WM, Yu Z. Biogas dry reforming for syngas production on La promoted hydrotalcite-derived Ni catalysts. Int J Hydrogen Energ. 2018; 43: 19438-19450. [CrossRef]
- Anzelmo B, Wilcox J, Liguori S. Natural gas steam reforming reaction at low temperature and pressure conditions for hydrogen production via Pd/PSS membrane reactor. J Membrane Sci. 2017; 522: 343-350. [CrossRef]
- Yan Y, Cui Y, Zhang L, Li L, Zhang J, Chen Y, Tang Q, Lin C. Experimental investigation of methane auto-thermal reforming in hydrogen-permeable membrane reactor for pure hydrogen production. Int J Hydrogen Energ. 2016; 41: 13069-13076. [CrossRef]
- Dolan MD, Beath AC, Hla SS, Way JD, Hawa HWAE. An experimental and techno-economic assessment of solar reforming for H2 production. Int J Hydrogen Energ. 2016; 41: 14583-14595. [CrossRef]
- Brunetti A, Fontananova E. CO2 conversion by membrane reactors. J Nanosci Nanotechnol. 2019; 19: 3124-3134. [CrossRef]
- Sumrunronnasak S, Tantayanon S, Kiatgamolchai S, Sukonket T. Improved hydrogen production from dry reforming reaction using a catalytic packed-bed membrane reactor with Ni-based catalyst and dense PdAgCu alloy membrane. Int J Hydrogen Energ. 2016; 41: 2621-2630. [CrossRef]
- Duran P, Sanz-Martinez A, Soler J, Menendez M, Herguido J. Pure hydrogen from biogas: Intensified methane dry reforming in a two-zone fluidized bed reactor using permselective membranes. Chem Eng J. 2019; 370: 772-781. [CrossRef]
- Leimert JM, Karl J, Dillig M. Dry reforming of methane using a nickel membrane reactor. Processes. 2017; 5: 82. DOI: 10.3390/pr5040082. [CrossRef]
- Alexandrov AV, Gavrilova NN, Kislov VR, Skudin VV. Comparison of membrane and conventional reactors under dry methane reforming conditions. Petrol Chem. 2017; 57: 804-812. [CrossRef]
- Lee B, Lim H. Parametric studies for CO2 reforming of methane in a membrane reactor as a new CO2 utilization process. Korean J Chem Eng. 2017; 34: 199-205. [CrossRef]
- Lee B, Lee S, Lim H. Numerical modeling studies for a methane dry reforming in a membrane reactor. J Nat Gas Sci Eng. 2016; 34: 1251-1261. [CrossRef]
- Gao Y, Jiang J, Meng Y, Yan F, Aihemaiti A. A review of recent developments in hydrogen production via biogas dry reforming. Energ Convers Manage. 2018; 171: 133-155. [CrossRef]
- Ugarte P, Duran P, Lasobras J, Soler J, Menendez M, Herguido J. Dry reforming of biogas in fluidized bed: Process intensification. Int J Hydrogen Energ. 2017; 42: 13589-13597. [CrossRef]
- Sunggeun L, Hankwon L. The effect of changing the number of membranes in methane carbon dioxide reforming: A CFD study. J Ind Eng Chem. In press.
- Wu HC, Rui Z, Lin JYS. Hydrogen production with carbon dioxide capture by dual-phase ceramic-carbonate membrane reactor vis steam reforming of methane. J Membrane Sci. 2020; 598. DOI: 10.1016/j.memsci.2019.117780. [CrossRef]
- Jia H, Xu H, Sheng X, Yang X, Shen W, Goldbach A. High-temperature ethanol steam reforming in PdCu membrane reactor. J Membrane Sci. 2020; 605. DOI: 10.1016/j.memsci.2020.118083. [CrossRef]
- Fontana AD, Faroldi B, Cornaglia LM, Tarditi AM. Development of catalytic membranes over PdAu selective films for hydrogen production through the dry reforming of methane. Mol Catal. 2020; 481. DOI: 10.1016/j.mcat.2018.07.018. [CrossRef]
- Li H, Goldbach A, Li W, Xu H. PdC formation in ultra-thin Pd membrane during separation of H2/CO mixtures. Int J Hydrogen Energ. 2016; 41: 10193-10201.
- Karemore AL, Vaidya PD, Sinha R, Chugh P. On the dry and mixed reforming of methane over Ni/Al2O3 – Influence of reaction variables on syngas production. Int J Hydrogen Energ. 2016; 41: 22963-22975. [CrossRef]
- Chein RY, Fung WY. Syngas production via dry reforming of methane over CeO2 modified Ni/Al2O3 catalysts. Int J Hydrogen Energ. 2019; 44: 14303-14315. [CrossRef]
- Nataj SMM, Alavi SM, Mazloom G. Catalytic performance of Ni supported on ZnO-Al2O3 composites with different Zn content in methane dry reforming. Soc Chem Ind. 2018; 94: 1305-1314. [CrossRef]