Objective Arm Swing Analysis in Early-Stage Parkinson’s Disease Using an RGB-D Camera (Kinect®)1
Abstract
Background:
Arm swing changes are common even in the early stages of Parkinson’s disease (PD). We hypothesized that arm swing changes decrease with age and can be detected using a low-cost, RGB-D depth-sensing camera.
Objective:
This study aimed to assess the differences in arm swing between PD patients and healthy participants and to investigate the possible effects of aging on these differences.
Methods:
Twenty-five PD patients (aged 45–87 years) and 25 age-matched, healthy subjects (aged 46–88 years) were included. Clinical variables were evaluated using a descriptive analysis. No spatiotemporal variables were normally distributed; therefore, we used a Mann–Whitney U test to compare the continuous variables between groups and to perform age-stratified analysis. A receiver operating characteristic analysis was generated to evaluate the discrimination activity of arm swing asymmetry (ASA).
Results:
The PD group showed significant reductions in arm swing magnitude (left, p = 0.002; right, p = 0.006) and arm swing speed (left, p = 0.002; right, p = 0.004) and significantly greater ASA (p < 0.001). The age-stratified analysis showed significant differences in ASA in the 40–59-year group (p = 0.001) and bilateral arm swing magnitude in the 60–66-year group. No differences were found in those aged >67 years.
Conclusions:
The camera detected differences in ASA, arm swing speed, and arm swing magnitude between PD patients and healthy individuals. Analysis of arm swing variables should be stratified by age, and the validity of the analysis may be questionable in patients aged >67 years.
INTRODUCTION
Parkinson’s disease (PD) is the second most common neurodegenerative disorder. Nearly 6.2 million individuals are currently affected worldwide, and some studies have estimated that its incidence is sharply increasing [1]. This increase will pose a greater public health challenge as global populations continue to age, thus requiring new strategies for early and accurate diagnosis of PD and for its proper monitoring and management.
Gait kinematic changes and spatiotemporal characteristics are hallmarks of PD and are used in its diagnosis [2]; in addition, identification of bradykinesia, resting tremor, postural instability, and rigidity are fundamental to the neurological evaluation of patients with a suspected parkinsonian syndrome. Motor changes in PD patients primarily affect the lower limbs and are associated with an increased risk of falls. However, recent studies have shown that changes in arm movements are also frequently reported, even in early stages of the disease [3]. These changes are usually characterized by a diminished arm swing, particularly on the side of the body that is more affected by the disease [4], and by a reduction in bilateral arm coordination, which increases the asymmetry between the upper limbs [5, 6].
Motor asymmetry can be assessed and described qualitatively using clinical tests such as the Movement Disorder Society’s Unified PD Rating Scale (MDS-UPDRS) score. However, changes in arm swing can be subtle and are influenced by age [7], which makes their assessment challenging, particularly for non-experienced care providers. Recent technological advances have resulted in specific devices that quantitatively assess gait, and objective gait evaluation is becoming increasingly common. However, analysis of upper limb movements is intricate and generally requires a gait and biomechanics laboratory. These complex systems are rarely available for direct use in medical consultations.
Based on previous studies using sagittal plane shoulder views to characterize arm movements [8], we hypothesized that arm swing changes in early-stage PD patients could be identified using a portable, low-cost, RGB-D depth-sensing camera [9]. In this study, we used this low-cost method to address the following two aims: to assess the differences in arm swing between PD patients and healthy participants and to investigate the possible effects of aging on these arm swing differences.
METHODS
Design
This observational, single-center study evaluated gait-linked arm swing changes in early-stage PD patients using an RGB-D camera coupled to a signal processing software [9]. This study was conducted between June and December, 2016, by the Movement Disorders Research Team of the neurology service at the Fundación Valle del Lili Academic Hospital and by the Informatics and Telecommunications Research Group i2t from Icesi University, Cali, Colombia. Institutional review board approval was obtained prior to commencing the study, and all participants provided written informed consent before participation.
Participants
PD was diagnosed by a movement disorders specialist according to published guidelines. Early-stage PD was defined as Hoehn and Yahr stage I or II. All participants in the PD group were treated with a dopaminergic agonist and were evaluated while in the “on” state. An expert neurologist confirmed the absence of dementia and any other neurological conditions or severe comorbidities that could affect gait. No participant had a history of disease or surgery in the upper extremities, and all could walk independently without a walking aid. The inclusion criteria for the control group were the absence of any neurological disease or any severe comorbidity likely to affect movement, absence of dementia, and independent mobility without a walking aid.
Clinical evaluation of gait
Instruments
Assessing patients’ disease severity was important because it could affect the motor manifestations that were the primary objective of this study. Disease severity was evaluated using the MDS-UPDRS part III, and the risk of falls and postural instability were assessed using the Freezing of Gait Questionnaire (FOGQ) and Dynamic Gait Index (DGI), respectively.
Definition of clinical asymmetry
Effects of PD are asymmetrical during its onset, which is associated with the asymmetrical degeneration of the substantia nigra [4, 5] and the nigrostriatal circuits. To evaluate the asymmetry in patients and differences in motor involvement between limbs, clinical asymmetry was defined as the difference between the summed UPDRS scores of the left and right sides of the body, considering only the upper limbs (i.e., UPDRS scores 3.3–3.6 and 3.15–3.17). The side with the higher UPDRS score was defined as the more-affected [10].
Arm swing analysis
All participants underwent a single gait analysis session to assess their arm movements during gait while they walked along an indoor corridor 4 m long and 1.5 m wide. The corridor was free from interference, and light and air movement conditions were controlled. Body segments were tracked with an e-Motion capture system, which used an RGB-D camera (Kinecttrademark Version 1) as a sensing device with software developed by the i2t research group at Icesi University. Participants were instructed to start at the beginning of the corridor and walk at a normal pace toward the camera. Each participant performed three walking trials.
Data management and processing
The acceptable capture area was restricted to a distance of 1.5–3.5 m from the camera (Fig. 1A), which was able to record at least one full gait cycle per limb during each walking trial. The data were captured and then processed using digital signal processing techniques, with the hip joint center used as a reference point to obtain a time/distance representation. Based on the wrist joint movements, we calculated the following variables: arm swing magnitude, defined as the distance traveled by the wrist in the anterior/posterior direction with respect to the hip joint center [5] (Fig. 1B); arm swing time, defined as the time the wrist took to travel the distance between the maximum anterior and maximum posterior points during an arm swing cycle; arm swing speed, calculated as the distance traveled by the arm (arm swing magnitude) per unit of time (arm swing time); and arm swing asymmetry (ASA), calculated using the method proposed by Zifchock et al. and used by Lewek et al. [11] that defines ASA as the relationship between the arm swing magnitudes using the following equation:
Fig.1
General setting and results obtained with e-Motion system. A) Capture area. B) Arm movement in the sagittal plane.

ASA represents the asymmetry in arm swing magnitude between the arms, with a value of 0 indicating that both arms display exactly the same arm swing magnitude [5].
Table 1
General characteristics of the sample
| Variables | PD patients (n = 25) | Healthy subjects (n = 25) | p-value |
| Age* | |||
| Years (Median, IQR) | 67 (IQR 63 –75) | 67 (IQR 63 –75) | |
| 40–59 | 5 (20%) | 6 (24%) | 0.98 |
| 60–66 | 6 (24%) | 6 (24%) | |
| 67–75 | 8 (32%) | 7 (28%) | |
| 76–88 | 6 (24%) | 6 (24%) | |
| Gender* | |||
| Male | 13 (52%) | 15 (60%) | 0.59 |
| Female | 12 (48%) | 10 (40%) | |
| Education* | |||
| Elementary school | 8 (32%) | 4 (16%) | 0.28 |
| High school | 8 (32%) | 7 (28%) | |
| Graduate | 9 (36%) | 14 (56%) | |
| Occupation* | |||
| Employee | 7 (28%) | 11 (44%) | 0.77 |
| Housewife | 6 (24%) | 4 (16%) | |
| Retired | 12 (48%) | 10 (40%) | |
| Test | |||
| MoCA test** | 22 (IQR 16–26) | 22.5 (IQR 21–24) | 0.55 |
| GDI** | 21 (IQR 19–23) | 23 (IQR 21–24) | 0.29 |
| MDS-UPDRS | 36.8 (±13.41) | – | – |
| FOGQ | 6.16 (±4.74) | – | – |
*n (%), Chi-2 test. **Median (IQR: Interquartile range), Mann-Whitney test. Bold values, p < 0.05. MoCA, Montreal Cognitive Assessment; GDI, Gait Dynamic Index; MDS-UPDRS, Movement Disorder Society Unified Parkinson’s Disease Rating Scale; FOGQ, Freezing of Gait Questionnaire.
Statistical analysis
The participants were divided into age group quartiles. Continuous variables were reported as the mean and standard deviation if they were normally distributed or as the median and interquartile range (IQR) if they were not normally distributed. Baseline characteristics were compared between the groups. Chi-square test was used to compare categorical variables, and gait parameters were compared using the Mann–Whitney U test based on data distribution. Finally, we conducted a receiver operating characteristic (ROC) analysis to identify the cut-off points for the arm swing parameters that optimally discriminated between the PD patients and healthy participants. A statistically significant difference was indicated by a p-value of ≤0.05. Statistical analyses were performed using STATA® 13.0 software.
RESULTS
Sociodemographic characteristics and clinical evaluation
Fifty participants (25 with PD aged 45–87 years and 25 age-matched healthy subjects aged 46–88 years) were recruited for this study. The sociodemographic characteristics of the participants are shown in Table 1. Both groups had a median age of 67 years (IQR 63–75 years). Participants with PD were stratified by age to assess the effects of age-related gait changes. No significant differences (p < 0.05) were found when comparing the groups by sex or age.
The median disease duration from onset was 6 years (IQR 1–7 years). Of the PD patients, 16% were classified as Hoehn and Yahr stage I, and the remaining 84% were classified as stage II. According to Stebbins’s criteria [12], 18 patients displayed the postural instability/gait disorder PD subtype and 7 patients displayed the tremor-dominant PD subtype. The mean MDS-UPDRS score was 36.88 (±13.41), the median DGI was 21 (IQR 19–23), and the mean FOGQ score was 6.16 (±4.74). According to the MDS-UPDRS scores for each upper limb, 52% of the PD group was classified as having left-sided PD and 40% as having right-sided PD, with the remaining 8% classified as symmetrical.
In the age stratification analysis of PD patients, those aged 60–66 years had high MDS-UPDRS (43±13.8) and FOGQ (7.1±5.2) scores and low DGI scores (20.5, IQR 19–21) (Supplementary Table 1). Similarly, those aged 76–88 years had high MDS-UPDRS (43.5±8.8) and FOGQ (7.1±5.2) scores and low DGI scores (21.5, IQR 12–24). These results suggested that those aged 60–66 and 76–88 years had greater motor compromise and postural instability. They had also experienced at least one freezing of gait episode in the previous week, which could be related to a higher risk of falls.
Patients aged 40–59 years had the lowest FOGQ, the highest DGI, and the second lowest MDS-UPDRS scores, suggesting that this group was less affected by PD. These findings could be explained by the shorter disease duration (1 year, IQR 0–2) compared with that of the older age groups. Although the median PD duration for patients aged 67–75 years was 6 years, their mean MDS-UPDRS score was 30.3±8.1, which was lower than that of the other groups.
Arm swing evaluation
A comparison of the arm swing parameters, measured with the e-Motion capture system, is shown in Table 2. Compared with the control group, the PD group showed significant reductions in arm swing magnitude (left, p = 0.002; right, p = 0.006) and arm swing speed (left, p = 0.002; right, p = 0.004) and significantly greater ASA (p < 0.001).
Table 2
Arm swing differences between PD patients and the healthy subject group
| Arm swing variables | Left wrist (n:50) | p-value | Right wrist (n:50) | p-value | ||
| PD patients | Healthy subjects | PD patients | Healthy subjects | |||
| Left wrist | Right wrist | |||||
| Arm swing magnitude | 0.16 | 0.26 | 0.002* | 0.16 | 0.26 | 0.006* |
| (IQR 0.08–0.2) | (IQR 0.17–0.33) | (IQR 0.09–0.24) | (IQR 0.20–0.34) | |||
| Arm swing time | 0.99 | 1.09 | 0.17 | 0.98 | 1.05 | 0.17 |
| (IQR 0.93–1.12) | (IQR 0.94–1.15) | (IQR 0.90–1.03) | (IQR 0.96–1.12) | |||
| Arm swing speed | 0.16 | 0.25 | 0.002* | 0.14 | 0.26 | 0.004* |
| (IQR 0.08–0.2) | (IQR 0.18–0.29) | (IQR 0.09–0.21) | (IQR 0.18–0.31) | |||
| Variables | PD patients | Healthy subjects | p-value | |||
| Arm swing asymmetry | 0.16 (IQR 0.09–0.23) | 0.063 (IQR 0.03–0.08) | <0.001* | |||
Median (IQR: Interquartile range), Mann-Whitney test. Bold values, p < 0.05.
Table 3
Arm swing differences between PD patients according to the clinical asymmetry distribution
| Variables | Left-sided PD (n:26) | p-value | Right-sided PD (n:22) | p-value | ||
| PD patients | Healthy subjects | PD patients | Healthy subjects | |||
| Arm swing magnitude left | 0.10 | 0.26 | 0.001* | 0.20 | 0.27 | 0.32 |
| Arm swing magnitude right | 0.21 | 0.24 | 0.42 | 0.11 | 0.25 | 0.01* |
| Arm swing time left | 0.93 | 1.08 | 0.06 | 1.07 | 1.12 | 0.87 |
| Arm swing time right | 0.99 | 1.05 | 0.85 | 0.96 | 1.04 | 0.06 |
| Arm swing speed left | 0.10 | 0.23 | 0.001* | 0.19 | 0.26 | 0.49 |
| Arm swing speed right | 0.19 | 0.26 | 0.29 | 0.12 | 0.23 | 0.02* |
| Arm swing asymmetry | 0.16 | 0.06 | 0.01* | 0.25 | 0.08 | 0.002* |
Median, Mann-Whitney test. Bold values*, p < 0.05.
The age stratification analysis (Table 4) reported significant differences for ASA in the 40–59-year group (p = 0.001); this group had the highest percentage of Hoehn and Yahr stage I patients (60%) and a shorter disease duration. In the 60–66-year group, significant differences were found for bilateral arm swing magnitude (left, p = 0.037; right, p = 0.001) and right arm speed (p = 0.01); differences in left arm speed almost reached statistical significance (p = 0.054). No significant differences were found between the 67–75-year and 76–88-year groups.
Table 4
Arm swing differences between PD patients according to the age group distribution
| Variables | 40–59 years (n:10) | p-value | 60–66 years (n:12) | p-value | 67–75 years (n:16) | p-value | 76–88 years (n:12) | p-value | ||||
| PD patients | Healthy subjects | PD patients | Healthy subjects | PD patients | Healthy subjects | PD patients | Healthy subjects | |||||
| Arm swing magnitude left | 0.17 | 0.30 | 0.14 | 0.07 | 0.24 | 0.03* | 0.19 | 0.25 | 0.24 | 0.12 | 0.26 | 0.33 |
| Arm swing magnitude right | 0.19 | 0.25 | 0.36 | 0.14 | 0.32 | 0.001* | 0.17 | 0.23 | 0.35 | 0.19 | 0.27 | 0.33 |
| Arm swing time left | 1.01 | 1.04 | 0.46 | 0.98 | 1.14 | 0.07 | 1.01 | 1.08 | 0.72 | 1.00 | 1.07 | 1.00 |
| Arm swing time right | 0.99 | 1.10 | 0.58 | 0.99 | 1.03 | 0.74 | 0.99 | 1.05 | 0.72 | 0.97 | 1.04 | 0.26 |
| Arm swing speed left | 0.17 | 0.27 | 0.14 | 0.07 | 0.24 | 0.05 | 0.18 | 0.27 | 0.20 | 0.13 | 0.21 | 0.33 |
| Arm swing speed right | 0.19 | 0.24 | 0.36 | 0.14 | 0.31 | 0.01* | 0.16 | 0.24 | 0.34 | 0.18 | 0.24 | 0.26 |
| Arm swing asymmetry | 0.23 | 0.04 | 0.001* | 0.18 | 0.07 | 0.26 | 0.12 | 0.07 | 0.16 | 0.16 | 0.06 | 0.05 |
Median, Mann-Whitney test. Bold values*, p < 0.05.
Clinical asymmetry analysis
For the clinical asymmetry analysis, we compared the more-affected side of the PD patients with the same side of the matched control group participants (Table 3). In the left-sided PD group, we found significant differences between the participants and controls in arm swing magnitude, arm swing speed, and ASA of the left wrist; in the right-sided PD group, we found significant differences between the participants and controls in arm swing magnitude, arm swing speed, and ASA of the right wrist. We did not compare the symmetrical group because of the limited sample size. We compared the more-affected limb detected by the motion capture system with that identified by the MDS-UPDRS scores; the results showed that our device recognized the more-affected side in 80% of cases.
ROC analysis
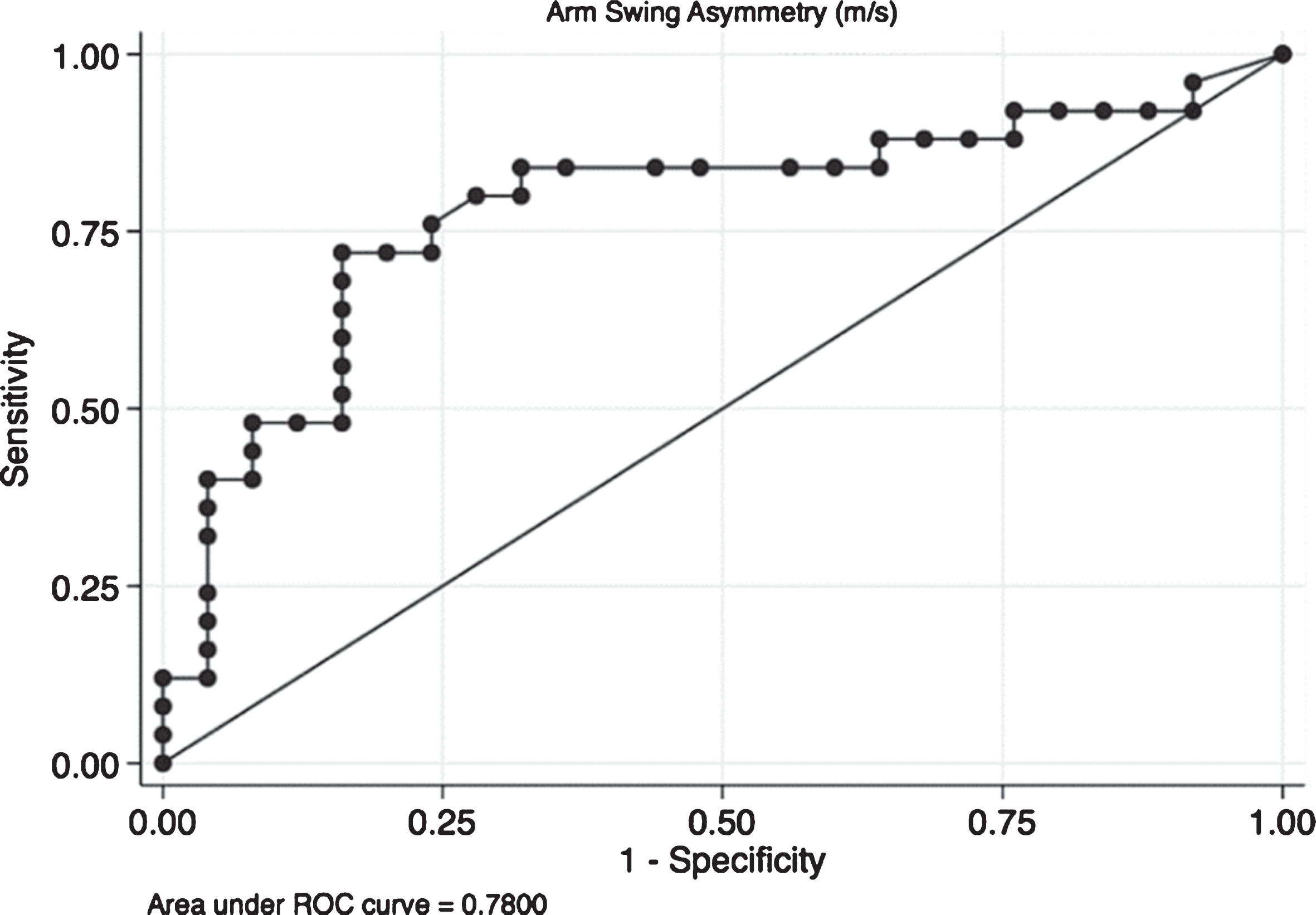
Finally, an ROC analysis was performed for ASA (Fig. 2). The optimal ASA cut-off value for detecting a participant with PD was ≥0.11. This cut-off provided a sensitivity of 72%, a specificity of 84%, and an area under the curve of 0.78 (0.64–0.91), which indicates that ASA could correctly differentiate controls from PD patients in 78% of cases.
Fig.2
Receiver operating characteristic (ROC) curve for arm swing asymmetry. The ROC curve it is a plot of the true positive rate against the false positive rate for the different possible cut points of a diagnostic test. In this case we present the plot for Arm Swing Asymmetry (ASA). The overall result of the Area Under the Curve (AUC) of 0.78 means that ASA is capable to adequately differentiate patients from controls in the 78% of cases which means that the accuracy of the diagnostic test is fair.
DISCUSSION
The main objective of this study was to characterize arm swing in PD patients and healthy participants using a portable RGB-D camera. Our results confirmed that arm swing kinematics differed between the groups with and without PD: patients with PD moved their arms more slowly, more stiffly, and more asymmetrically than the controls [13, 14]. Their global bradykinesia and upper limb rigidity limited their arm mobility, thus reducing wrist magnitude and speed during gait. However, there were no significant differences between the groups in the time traveled by each wrist during the gait cycle (arm swing time); we believe this can be explained by the PD patients’ wrists traveling shorter distances, thereby compensating for their slower arm movements.
Some degree of asymmetry in arm movement is physiological, with significant differences between left and right arm swing magnitudes demonstrated in healthy subjects. However, the asymmetry can be much larger in pathological conditions such as PD [15, 16]. It can also be difficult to assess in some groups because age is an important factor affecting gait kinematics. Our results confirmed that age influenced arm movement. In our experience, ASA can be used to differentiate patients in the early stages of PD (Hoehn and Yahr stages I and II) from controls aged <59 years. We believe that significant differences were observed in this younger age group because most of the patients had asymmetric motor involvement, whereas the controls did not present significant gait changes associated with aging. Although some authors have proposed that clinical asymmetry is maintained throughout the time course of PD [7], there is evidence suggesting that PD patients cannot compensate by increasing their arm swing on the less-affected side, and thus, their movement tends to become more symmetric as the disease progresses. This could explain why ASA differences were observed to be significant only in the early stages of PD, particularly in patients with a recent diagnosis, such as those in the youngest age group in the present study.
Arm swing magnitude and right wrist speed were significantly reduced in the right-sided patients aged 60–66 years old. Although left wrist speed was not significantly slower in the left-sided PD patients, this difference almost reached significance (p = 0.054), possibly because most of the study patients had right-sided disease. Those aged >67 years displayed a slight tendency to have smaller arm swing magnitudes and lower speed values than the controls, although these differences were not significant. This could be explained by the physiological slowness of the arm swing movements in older patients.
Given PD’s asymmetry, it is important to identify the patient’s more-affected side to complement diagnosis and follow-up as well as to access the clinical effectiveness of dopaminergic therapy. Our device was able to correctly differentiate the more-affected side, according to the MDS-UPDRS scores, in 20 out of 25 (80%) cases. For the five patients in whom the two methods did not agree, only two had differences greater than two points in the MDS-UPDRS scores for each arm.
Regarding the ROC curve results, ASA was able to correctly differentiate PD patients from healthy subjects in 78% of the cases, with a specificity of 84%. This finding indicates that ASA calculated using the e-Motion system is a fair variable to help diagnose PD. We chose our cut-off value of 0.11 based on the optimal cut-off point provided by the ROC analysis for our system, which indicates the point with the highest number of correctly classified patients. Furthermore, it was chosen knowing that the e-Motion system and ASA could be used as a diagnostic aid and not as a diagnostic test in the context of neurological consultation. Moreover, our cut-off results are similar to those described by other researchers [16], but a larger sample is required to confirm our findings.
Limitations
The main objective of this study was to characterize the walking pattern using arm swing variables; the other reference points and measurements obtained by the Kinect system were not considered. Gait kinematics have been proposed to help differentiate between tremor-dominant and postural instability/gait difficulty PD subtypes [17], but the small number of patients with tremor-dominant subtype and clinical asymmetry of the disease has limited the analysis of some subgroups. Although the cut-off for ASA in the ROC analysis provided sufficient sensitivity and specificity to differentiate between patients in the early stages of PD and healthy participants, which could be useful to complement the diagnosis, future studies with more participants are necessary to confirm these findings. In addition, further advances in software and hardware are required to enhance the sensitivity of Kinect® for kinematic measurements. Despite these limitations, we believe that this study’s data collection strategy presents advantages in terms of cost, accessibility, and the small area required compared with gait laboratories.
Challenges and future research
Precision medicine is a growing new field that allows the objective characterization of patients. There is a need to develop new approaches using new, wearable sensor technology and big data analysis, which could help classify and manage PD even in the early and prodromal stages. In our case, the e-Motion system is a diagnostic aid that could be used, along with other complementary technologies, to improve and quantify gait assessment of patients with neurological diseases such as PD. Although the Kinect system is no longer in production, there are other RGB-D cameras that can implement the e-Motion software. The operation of these cameras does not require specialized training and can be placed in the clinical context without making significant adjustments to the test area; therefore, its adaptability is good. In future research, evaluating more subjects will establish cut-off points that could help differentiate between subjects with PD and controls, helping monitor the disease’s symptoms and severity.
Conclusions
The e-Motion system is portable and can be used in a clinical context to assess the arm movements of early-stage PD patients. ASA, arm swing speed, and arm swing magnitude are useful for differentiating PD patients from healthy individuals. The analysis of arm swing variables should be stratified by age, and the validity of the analysis may be questionable in patients aged >67 years.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
We thank our colleagues from the Centro de Investigaciones Clínicas, Cali, Colombia (CIC), who provided insight and expertise that greatly assisted this research.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: 10.3233/JPD-181401.
REFERENCES
[1] | Dorsey ER , Bloem BR ((2018) ) The Parkinson pandemic-a call to action. JAMA Neurol 75: , 9–10. |
[2] | Gelb DJ , Oliver E , Gilman S ((1999) ) Diagnostic criteria for Parkinson disease. Arch Neurol 56: , 33–39. |
[3] | Schneider SA , Drude L , Kasten M , Klein C , Hagenah J ((2012) ) A study of subtle motor signs in early Parkinson’s disease. Mov Disord 27: , 1563–1566. |
[4] | Huang X , Mahoney JM , Lewis MM , Dunull Guangwei , Piazza SJ , Cusumano JP ((2012) ) Both coordination and symmetry of arm swing are reduced in Parkinson’s disease. Gait Posture 35: , 373–377. |
[5] | Lewek MD , Poole R , Johnson J , Halawa O , Huang X ((2010) ) Arm swing magnitude and asymmetry during gait in the early stages of Parkinson’s disease. Gait Posture 31: , 256–260. |
[6] | Roggendorf J , Chen S , Baudrexel S , van de Loo S , Seifried C , Hilker R ((2012) ) Arm swing asymmetry in Parkinson’s disease measured with ultrasound based motion analysis during treadmill gait. Gait Posture 35: , 116–120. |
[7] | Mirelman A , Bernad-Elazari H , Nobel T , Thaler A , Peruzzi A , Plotnik M , Giladi N , Hausdorff JM ((2015) ) Effects of aging on arm swing during gait: The role of gait speed and dual tasking. PLoS One 10: , e0136043. |
[8] | Behrman AL , Teitelbaum P , Cauraugh JH ((1998) ) Verbal instructional sets to normalise the temporal and spatial gait variables in Parkinson’s disease. J Neurol Neurosurg Psychiatry 65: , 580–582. |
[9] | Paredes JDA , Muñoz B , Agredo W , Ariza-Araújo Y , Orozco JL , Navarro A ((2015) ) A reliability assessment software using Kinect to complement the clinical evaluation of Parkinson’s disease. In 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp. 6860–6863. |
[10] | Boonstra TA , van Vugt JPP , van der Kooij H , Bloem BR ((2014) ) Balance asymmetry in Parkinson’s disease and its contribution to freezing of gait. PLoS One 9: , e102493. |
[11] | Zifchock RA , Davis I , Higginson J , Royer T ((2008) ) The symmetry angle: A novel, robust method of quantifying asymmetry. Gait Posture 27: , 622–627. |
[12] | Stebbins GT , Goetz CG , Burn DJ , Jankovic J , Khoo TK , Tilley BC ((2013) ) How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: Comparison with the unified Parkinson’s disease rating scale. Mov Disord 28: , 668–670. |
[13] | Isenberg C , Conrad B ((1994) ) Kinematic properties of slow arm movements in Parkinson’s disease. J Neurol 241: , 323–330. |
[14] | Mirelman A , Bernad-Elazari H , Thaler A , Giladi-Yacobi E , Gurevich T , Gana-Weisz M , Saunders-Pullman R , Raymond D , Doan N , Bressman SB , Marder KS , Alcalay RN , Rao AK , Berg D , Brockmann K , Aasly J , Waro BJ , Tolosa E , Vilas D , Pont-Sunyer C , Orr-Urtreger A , Hausdorff JM , Giladi N ((2016) ) Arm swing as a potential new prodromal marker of Parkinson’s disease. Mov Disord 31: , 1527–1534. |
[15] | Meyns P , Bruijn SM , Duysens J ((2013) ) The how and why of arm swing during human walking. Gait Posture 38: , 555–562. |
[16] | Kuhtz-Buschbeck JP , Brockmann K , Gilster R , Koch A , Stolze H ((2008) ) Asymmetry of arm-swing not related to handedness. Gait Posture 27: , 447–454. |
[17] | Herman T , Weiss A , Brozgol M , Giladi N , Hausdorff JM ((2014) ) Gait and balance in Parkinson’s disease subtypes: Objective measures and classification considerations. J Neurol 261: , 2401–2410. |




