Abstract
Several worlds in our solar system are thought to hold oceans of liquid water beneath their frozen surfaces. These subsurface ice and ocean environments are promising targets in the search for life beyond Earth, but they also present significant new technical challenges to planetary exploration. With a focus on Jupiter's moon Europa, here we (1) identify major benefits and challenges to subsurface ocean world science, (2) provide a multidisciplinary survey of relevant sample handling and life detection technologies, and (3) integrate those perspectives into the Subsurface Science and Search for Life in Ocean Worlds (SSSLOW) concept payload. We discuss scientific goals across three complementary categories: (1) search for life, (2) assess habitability, and (3) investigate geological processes. Major mission challenges considered include submerged operation in high-pressure environments, the need to sample fluids with a range of possible chemical conditions, and detection of biosignatures at low concentrations. The SSSLOW addresses these issues by tightly integrated instrumentation and sample handling systems to enable sequential, complementary measurements while prioritizing preservation of sample context. In this work, we leverage techniques and technologies across several fields to demonstrate a path toward future subsurface exploration and life detection in ice and ocean worlds.

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The search for life beyond Earth is a primary goal of NASA (National Research Council 2011), and in our solar system, extant or relict ice and ocean worlds such as Europa, Enceladus, and Mars are among the most promising targets (Nimmo & Pappalardo 2016; Hendrix et al. 2019; Hand et al. 2020). In particular, investment in exploring Jupiter's moon Europa remains high, since the Voyager and Galileo NASA missions identified geologic (Pappalardo et al. 1999) and magnetic (Kivelson et al. 2000) evidence for a saline water ocean and silicate interior below an icy shell. The potential for the basic building blocks of life and chemical energy sources in Europa's subsurface ocean suggests an environment that may have given rise to life and remain habitable today (e.g., Chyba 2000; Hand et al. 2009; Russell et al. 2017; Hand & German 2018; Schmidt 2020). NASA's Europa Clipper (Howell & Pappalardo 2020) and ESA's Jupiter Icy Moons Explorer (JUICE; Grasset et al. 2013) multiple flyby missions are anticipated to reach the Jovian system in the early 2030s and further inform our understanding of Europa's habitability.
A landed mission is a likely next step, and detailed concept studies have already investigated how to directly search for evidence of life in the upper tens of centimeters of Europa's icy regolith (Korablev et al. 2011; Pappalardo et al. 2013; Hand et al. 2017; Blanc et al. 2020). However, it is the subsurface reservoirs of liquid water within the ice shell and the ocean below that are the most likely regions to maintain presently habitable conditions for extant life (e.g., Schmidt et al. 2011; Michaut & Manga 2014). Accessing these compelling environments will require a subsurface probe capable of operating in a high-pressure submerged environment (see companion article, Schmidt et al. 2023). Here we consider the scientific opportunities and challenges of such a mission and then present a conceptual payload that builds on existing technologies from both Earth and space science to meet many of them.
Europa Clipper and JUICE will provide subsurface reconnaissance of Europa through surface imaging and radar-based structural mapping in the ice shell. Subsequent landed missions would further bound Europa's interior with seismic sounding (Pappalardo et al. 2013; Hand et al. 2017) or electromagnetic (EM) methods (Pappalardo et al. 2013; Grimm et al. 2021). A lander may additionally be augmented with a small-scale (class 1; <10 m) penetrator (e.g., Biele et al. 2011) to sample the upper meters of the ice shell or demonstrate preliminary subsurface access technology, as advocated for in Schmidt et al. (2021a). The development of deeper class 2 (0.01–1 km) and class 3 (<1 km; i.e., Barr et al. 2004) subsurface mission architectures, access technologies, and vehicles has seen increasing support and advancement (Carsey et al. 1999; Zimmerman et al. 2001; Powell et al. 2005; Gowen et al. 2011; Allen et al. 2013; Kimball et al. 2018; Stone et al. 2018a, 2018b; Zacny et al. 2018; Cwik et al. 2019; Oleson et al. 2019; Stamenković et al. 2019; Aguzzi et al. 2020; Bryson et al. 2020; Dachwald et al. 2020; Hand et al. 2020; Stone et al. 2020; Schmidt et al. 2021a, 2021b, 2023). Most recently, from 2019 to 2021, NASA's Scientific Exploration Subsurface Access Mechanism for Europa (SESAME) program supported the formulation and maturation of mission elements required for a 3 yr mission capable of reaching 15 km into the Europan ice shell. Initial findings suggest that this may be achievable within the coming decades through concerted development efforts (Schmidt et al. 2021a, 2023), and there is growing community support for an interdisciplinary subsurface science definition team to be convened within this decade (Howell et al. 2021; Schmidt et al. 2021a).
A subsurface probe, or cryobot (i.e., French et al. 2001), will require a robust environmental characterization and life detection instrument package (Outer Planets Assessment Group (OPAG) Roadmaps to Ocean Worlds (ROW) 2018; Moore et al. 2020; Willis et al. 2021) designed to meet the challenges of operating within Europa's interior ice and water environments (Schmidt et al. 2021a, 2020b). This instrument suite must be able to withstand space flight, operate for years while submerged at high pressures, tolerate potentially corrosive chemical compositions, and, ideally, analyze liquid samples. Fortunately, there are many relevant technologies from orbital or landed planetary missions, as well as terrestrial ocean and ice exploration, that can be leveraged for subsurface ocean world science.
Examples of recent multisensor life detection payloads in development for landed surface missions to ocean worlds include the Europan Molecular Indicators of Life Investigation (EMILI; Brinckerhoff et al. 2019), the Ocean Worlds Life Surveyor (OWLS) system (Creamer et al. 2020; Willis et al. 2019), and the Complex Molecules Detector (Fairen et al. 2020). High-fidelity surface mission and instrument suite concepts include the Europa Lander (Pappalardo et al. 2013; Hand et al. 2017) and Enceladus Orbilander (MacKenzie et al. 2021). NASA's COLDTech, ICEE, and ICEE 2 programs are also funding the development of instruments addressing the specific challenges of ocean worlds. Community research and white papers (e.g., Neveu et al. 2018; Hendrix et al. 2019; Howell et al. 2021; Schmidt et al. 2021a, 2021b; Willis et al. 2021) provide additional guidance for life detection payload development priorities.
On Earth, oceanographic platforms and samplers are commonly designed to operate at pressure ranges encompassing those expected for Europa's ice and upper ocean. Examples of vehicles designed for operation under ice (Aguzzi et al. 2019, 2020; Barker et al. 2020; Dachwald et al. 2020) include profiling floats (Roemmich et al. 2009; Toole et al. 2011; Girton et al. 2019; Roemmich et al. 2019), autonomous gliders (Lee et al. 2010), and remote or autonomous underwater vehicles (ROV/AUVs), including the Icefin ROVs (Spears et al. 2016; Meister et al. 2018) and the hybrid ROV/AUV Nereid Under-Ice (Jakuba et al. 2018b). Relevant seafloor platforms include benthic rovers (Henthorn et al. 2010; Flögel et al. 2018; Lemburg et al. 2018) and landers (Orcutt et al. 2017; Girguis et al. 2018; Peoples et al. 2019). Examples of autonomous or semiautonomous deep-sea-water sampling and processing systems include the Deep Sea Environmental Sample Processor (D-ESP; Ussler et al. 2013; Scholin et al. 2017), Remote Access Sampler (McLane Research Laboratories, Inc.), GeoMICROBE sled (Cowen et al. 2012), and Clio water column sampler (Jakuba et al. 2018a), among additional systems (Dodd et al. 2006; Taylor et al. 2006; Girguis et al. 2008; Wulff et al. 2010; Taylor et al. 2015). Ocean world analog missions are also presently working to integrate subsurface access drills with scientific payloads for operation in glacial ice (e.g., Winebrenner et al. 2013; Wirtz & Hildebrandt 2016; Clark et al. 2018; Stone et al. 2018a; Malaska et al. 2020). Ongoing and increasingly collaborative instrument development efforts between the astrobiology, oceanography, and glaciology communities are already advancing the technology needed for future subsurface exploration.
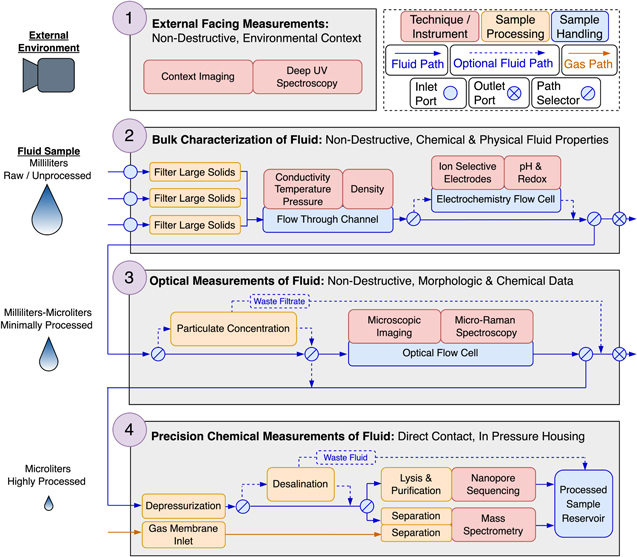
In this work, we first identify the scientific priorities for a subsurface life detection mission by distinguishing potentially habitable regions within Europa's ice shell, defining a set of possible science objectives (Table 1), and discussing the associated challenges (Section 2). We then present the Subsurface Science and Search for Life in Ocean Worlds (SSSLOW) payload concept (Figure 1) and detail the architecture of a sequential, multi-instrument approach to environmental characterization and detection of extant or past microbial life (Section 3). Finally, we summarize a review of relevant technologies across several fields (Table 2) that informed this work and describe additional considerations that could benefit future subsurface ocean world missions (Section 4).
Figure 1. Conceptual diagram of the SSSLOW payload. Phase 1, External Facing Instruments: A context imager and DUV spectrometer collect high-frequency observations of the borehole sidewall. Fluid sampling can be triggered if features of interest are detected, such as organics or pigmented inclusions. Phase 2, Bulk Physical Characterization of Fluid: Liquid from the melt pocket is drawn into the vehicle through one of multiple inlet ports. A low-power flow-through oceanographic package enables near continuous measurement of temperature, conductivity, pressure, and density. Sample aliquots can be passed to an electrochemistry cell for measurements of pH, redox potential, and ionic strength. Measurements here are used to investigate the surrounding environment, assess habitability, and inform downstream processing and analysis prioritization. Phase 3, Optical Measurements of Fluid: With optional preconcentration, sample aliquots enter an optical flow cell, where they are analyzed by a microscope for life detection and Raman spectrometer. These instruments provide correlated morphologic and chemical observations of minimally processed samples. Phase 4, Precision Chemical Measurements of Fluid: Discrete fluid sample packets are mechanically depressurized before entering a sealed scientific housing and can be desalted if needed. The sample is then divided for analysis by separation MS and nanopore sequencing. These precision chemical measurements detect nucleic acids and proteins and characterize amino acids, gases, ions, and other organic compounds of interest.
Download figure:
Standard image High-resolution imageTable 1. Science Opportunities Matrix
| Goal | Objective | Observables | Discussion in Community Documents | Measurement Discussion in This Work |
|---|---|---|---|---|
| Search for life | Search for active microbial life | Motility; growth; concurrent life stages; metabolic responses to stimuli or substrate addition; presence of unstable organics (e.g., RNA, ATP) | LOLD | 3.3–4 |
| Search for morphological signatures of present or past life | Cell-like morphologies; biomineral structures; biofabrics | LOLD, ELR 1B[1–3], ROW 4A[4] | 3.1, 3.3–4 | |
| Search for organic chemical signatures of present or past life | Relative abundance; distribution; complexity (mass); chirality of organic molecules (e.g., lipids; amino acids); informational polymers; pigments or fluorescence | LOLD, ELR 1A[1–3] | 3.3–4 | |
| Search for inorganic chemical signatures of present or past life | Isotope fractionations; biominerals; elemental distribution disequilibrium/ia | LOLD, ELR 1C[1], ROW 3B[2], 4A[4–5] | 3.1, 3.3–4 | |
| Investigate the source, processing history, and preservation potential of samples | Integrated context measurements (e.g., sample depth, ice type, temperature, salinity) | ELR 1D[1–2], ROW 4B[2, 4] | 3.1–4 | |
| Assess habitability | Map connection between subsurface water pockets and surface geology | Temperature; pressure; density; porosity; liquid fraction; acoustic events | ELR 2B[1, 4], 3A[4] | 3.1–2, 4.2 |
| Constrain the habitability of subsurface liquids, relative to known limits | Temperature; pressure; pH; salinity (dissolved solids); water activity; ionic strength and composition | LOLD, ∼ELR 2A[1–2], 3A[3], ROW 2A[2] | 3.1–2, 3.4 | |
| Map the abundance of non-ice material within the shell and ocean | Organic carbon; inorganic nutrients; relative oxidant and reductant abundances | ELR 1D[2], 2A[1], 3A[3], ROW 1C[2], 2A[1–2], 2B[2], 3A[1], 3B[1–2] | 3.1–2, 3.4 | |
| Salts; exogenic materials; minerals | ELR 1C[1], 1D[2], 2A[1], 3A[3], ROW 1C[2], 2A[1–2], 2B[2], 3A[1], 3B[1–2] | 3.1–2, 3.4 | ||
| Inorganic and organic volatiles | ELR 1D[2], 2A[1], 3A[3], ROW 1C[2], 2A[1–2], 2B[2], 3A[1], 3B[1–2] | 3.1, 3.4 | ||
| Investigate thermal and chemical signatures of seafloor activity | Seafloor heat flux; sediment thermal conductivity; seismic activity | ROW 1A[1] | 3.2, 4.1 | |
| Hydrothermal alteration signatures (volatiles; mineral precipitates) | ROW 3A[1], 3B | 3.1, 3.4 | ||
| Profile ocean structure to inform circulation | Temperature; pressure; density; sound speed; dissolved solids (salinity); current velocities | ROW 2A[2, 3], 2B[1]; ELR 2B[4], 3A[4], ROW 2A[2] | 3.2, 4.1 | |
| Investigate geologic processes | Investigate the frequency and magnitude of intrashell or seafloor seismicity | Passive acoustic monitoring | ∼ELR 2B[4], 3A[4], 3B[4], ROW 1B | 4.1–2 |
| Profile ice shell structure to understand transport processes | Thermal gradient and conductivity; salinity; density (seismic wave velocities); porosity; viscosity; liquid fraction; permittivity; ice fabric | ∼ELR 3B[3], ROW 1C[1–5], 2A[1] | 3.1–2, 4.2 | |
| Determine the relative magnitude of tidal dissipation in the ice shell | Thermal gradient; viscosity; tidal deformation (relative displacement) | ∼ELR 3B[4], ROW 1C[3–4] | 2.2.3 | |
Note. List of potential subsurface science objectives categorized into three primary mission goals. Referenced documents include the Ladder of Life Detection (LOLD; Neveu et al. 2018), 2017 Europa Lander Report (ELR; Hand et al. 2017), and Roadmaps to Ocean Worlds (ROW; Hendrix et al. 2019). Technical discussions of specific instrumentation related to each observable included in this work are indicated in the last column. The authors note that the SOM is intended as a menu, or trade space, of high-level subsurface opportunities, rather than a prescriptive set of requirements for any single mission.
Download table as: ASCIITypeset image
Table 2. Survey of Relevant Instruments and Sampling Technologies Informing SSSLOW Payload Design
| Setting | Technique | Relevant Examples | Qualitative Description |
|---|---|---|---|
| Context Imaging | |||
| Planetary | Context imager | MAHLI, MSL (Edgett et al. 2012); CLUPI, ExoMars (Josset et al. 2017) | High-resolution imaging, autofocusing, and focal stacking |
| Context imager, cosighted with spectrometer | SHERLOC, Mars 2020 (Beegle et al. 2015); PIXL, Mars 2020 (Allwood et al. 2015) | High-resolution imaging cosighted with scanning spectroscopy for chemical analysis | |
| Context imager, hyperspectral | MicrOmega/IR, ExoMars (Bibring et al. 2017; Leroi et al. 2009); CIVA-M, Rosetta (Bibring et al. 2007) | Hyperspectral imaging for chemical analysis, coupled with microscopy | |
| Ocean | Context imagers | Underwater imaging review (Jaffe 2014; Armstrong et al. 2019) | Diverse underwater imaging technologies |
| Fluorescence imagers | PLIF (Franks & Jaffe 2008; Jaffe et al. 2013) | Laser-based techniques to detect and study planktonic life | |
| Hyperspectral imagers | Underwater hyperspectral review (Liu et al. 2020) | Spectral techniques for studying seafloor life and geology | |
| Glacier | Optical televiewing | (Hubbard et al. 2008) | Map glacial boreholes, characterize materials in ice |
| Prototype | Europa Lander prototypes | ELSSIE (Murchie et al. 2020); C-LIFE (Cook et al. 2020) | Multispectral imaging, polarized imaging to detect chirality |
| DUV Spectroscopy | |||
| Planetary | Scanning DUV spectroscopy | SHERLOC, Mars 2020 (Beegle et al. 2015) | Fluorescence and Raman with integrated context imaging |
| Ocean | DUV fluorescence | DEBI-t (Salas et al. 2015) | Detection of microbes and organics in deep-sea borehole |
| Glacier | Scanning DUV fluorescence | WATSON (Eshelman et al. 2019; Malaska et al. 2020) | Mapping of organics and microbes in glacial borehole |
| Prototype | Flow cytometry | UV fluorescence flow cytometry (Lambert et al. 2010) | Sorting biogenic particles based on native UV fluorescence |
| Lab | DUV fluorescence | (Bhartia et al. 2010) | Detection of microbes based on native DUV fluorescence |
| ISEs | |||
| Electrochemistry for life detection discussed by Thomson et al. (2019). Environmental ISEs reviewed by De Marco et al. (2007). Ocean microsensors reviewed by Kühl & Revsbech (2001) and Crespo (2017). | |||
| Planetary | Hydrogel ISE | MECA-WCL, Phoenix (Hecht et al. 2009; Kounaves et al. 2009, 2010) | Wet chemistry analysis of Martian surface, discovery of perchlorates |
| Ocean | High-pressure SC-ISE | High pressure SC-ISE (Weber et al. 2017) | Submersible SC-ISE tested under pressure |
| Other chemical microsensors | (Kühl & Revsbech 2001) | Additional microsensors with ocean heritage | |
| Ocean | ISFET | Biogeochemical-Argo (BGC-Argo; Claustre et al. 2020) | Autonomous biogeochemical global ocean profiling floats |
| Glaciology | ISFET | (Bagshaw et al. 2021) | pH measurements in glacial meltwater |
| Prototype | Microfluidic SC-ISE | MICA (Noell et al. 2019); SPLice (Chinn et al. 2017) | Microfluidic implementation and advancement of WCL capabilities |
| Miniature SC-ISE | Miniature SC-ISE (Jaramillo & Noell 2020) | Description and characterization of miniaturized SC-ISEs | |
| Microscope for Life Detection | |||
| Planetary microscopes reviewed by Nadeau et al. (2018). Ocean microscopes reviewed by Lombard et al. (2019) and Spanbauer et al. (2020). Ocean holography reviewed by Nayak et al. (2021). | |||
| Planetary (geological investigation) | Optical microscope | MECA-OM, Phoenix (Hecht et al. 2008); Beagle 2 (Thomas et al. 2004); CIVA-M, Rosetta (Bibring et al. 2007); MicrOmega/Vis, ExoMars (Leroi et al. 2009) | Microscopic imaging for geologic studies, multiwavelength illumination, max. resolution of several microns (∼4 μm pixel–1) |
| Atomic force microscope | MECA-AFM, Phoenix (Hecht et al. 2008); MIDAS, Rosetta (Riedler et al. 2007) | Mechanical scanning at high resolutions (<10 μm), slow sampling speeds | |
| Ocean (plankton imaging) | Digital holographic microscope | In-line (Bochdansky et al. 2013; Jericho et al. 2010); Twin beam off axis (Mullen et al. 2019) | Volumetric imaging of plankton, near micron resolution, deep-sea deployments |
| Bright and dark field imaging | Scripps Plankton Camera (Orenstein et al. 2020); Benthic Underwater Microscope (Mullen et al. 2016) | Imaging of undisturbed plankton and seafloor specimen, near micron resolution, fluorescence imaging and focal stacking | |
| Imaging flow cytometry | Imaging FlowCytobot (Olson & Sosik 2007) | High throughput plankton imaging, long-duration deployments | |
| Atomic force microscope | (Nishida et al. 2016) | Deep-sea implementation of AFM | |
| Field Tested (life detection) | Digital holographic microscope | Twin beam off axis (Wallace et al. 2015; Lindensmith et al. 2016); lensless (Serabyn et al. 2018); cytometry (Göröcs et al. 2018) | Volumetric imaging of microbes, motility tracking, submicron resolution |
| Fluorescence light field microscope | ELVIS (Kim et al. 2020) | Coupled volumetric light field fluorescence imaging and holographic microscopy | |
| Prototype (life detection) | Fourier ptychography | Portable Ptychographic Microscope (Nguyen et al. 2020) | Computational imaging, large field of view, submicron resolution |
| Multispectral/fluorescence | Europa Luminescence Microscope (Quinn et al. 2019) | Fluorescence using chemical stains, CubeSat heritage | |
| Atomic force microscope | ANTONIE (Murray et al. 2019) | AFM integrated with optical microscope, designed for life detection | |
| Raman Spectroscopy | |||
| Astrobiology applications of Raman spectroscopy discussed by Foucher (2019). Ocean Raman spectroscopy review by Du et al. (2020) and Brewer & Kirkwood (2013). | |||
| Planetary | UV Raman | SHERLOC (Beegle et al. 2015; Bhartia et al. 2021) | Scanning UV spectroscopy with integrated context imaging |
| Standoff Raman | SuperCam, Mars 2020 (Perez et al. 2017) | Standoff Raman integrated with multiple types of spectroscopy | |
| Raman spectroscopy | RLS (Rull et al. 2017) | Component of package for analysis of crushed rock samples | |
| Ocean | Raman spectroscopy | DORISS (Brewer et al. 2004; White 2009); RiP (Zhang et al. 2017b) | In situ geochemistry of hydrothermal vent fluids, cold seep fluids, and seafloor minerals, including deep-sea operations |
| Lab | UV resonance Raman | (Sapers et al. 2019); (Tarcea et al. 2007) | Investigations of UV Raman for life detection applications |
| Single-cell Raman | Single cell analysis (Harz et al. 2009; Serrano et al. 2014); flow cytometry (Zhang et al. 2017a; Suzuki et al. 2019) | Raman analysis of single cells using advanced lab techniques, including rapid flow cytometry analysis | |
| Prototype | SSE Raman | CIRS (Lambert & Wang 2019) | SSE separation of Raman and fluorescence, light field camera |
| Raman and holography | (Takahashi et al. 2020) | Integrated holographic and Raman for underwater applications | |
| Raman with flow cell | (Thomson et al. 2017) | Raman integrated with flow cell for life detection applications | |
| Nanopore Sequencing and Genomics | |||
| Potential astrobiology applications of sequencing technology discussed by Rezzonico (2014) and Carr et al. (2017). Ocean genomic instruments and sampling review by McQuillan & Robidart (2017) and Spanbauer et al. (2020). | |||
| Space | Nanopore | (Castro-Wallace et al. 2017) | Sequencing on board the International Space Station |
| Ocean | Informational biopolymer analysis | ESP (Scholin et al. 2017); IISA-Gene (Fukuba et al. 2011); D-ESP (Ussler et al. 2013) | Autonomous underwater DNA probe and protein arrays, as well as qPCR, preservation of samples for lab analysis |
| Genomic collection and preservation | ESP G3 (Yamahara et al. 2019) | In situ seawater filtration and chemical preservation for lab analysis, applications on board autonomous vehicles | |
| Prototype | Nanopore | Martian life detection (SETG; Carr et al. 2019) | DNA extraction and sequencing suite for Mars |
| In development | Solid-state nanopore | Planetary exploration (Bywaters et al. 2017; Carr et al. 2020) | Solid-state nanopore sequencing instruments for long-duration and deep space applications |
| Nanopore | Low-Earth orbit sequencing (Saboda et al. 2019) | CubeSat incubation, DNA extraction, and sequencing payload designed for low-Earth orbit | |
| MS and Separations | |||
| Solar system MS review by Arevalo et al. (2020). Searching for biosignatures on ocean worlds discussed by Willis et al. (2021). ME and CE for astrobiology reviewed by Willis et al. (2015) and Mora et al. (2012). Ocean MS review by Chua et al. (2016). | |||
| Planetary | GC/LD-Ion Trap MS | MOMA, ExoMars (Goesmann et al. 2017) | Analyze volatile and refractory organics in Martian sediment |
| GC-Quadrupole MS | SAM, MSL (Mahaffy et al. 2012) | Analyze volatiles from Martian atmosphere or sediments | |
| GC-Quadrupole MS | NMS, Huygens (Niemann et al. 2002) | Analyze atmosphere of Titan | |
| Ocean | LC | ISLC (Beckler et al. 2014) | Single-step marine an-ion detection |
| GC-MS | (Matz & Kibelka 2000) | Submerged implementation of GC-MS | |
| Cycloidal-MS | TETHYS (Camilli & Duryea 2009) | Track underwater hydrocarbon plumes | |
| Q-MS | ISMS (Wankel et al. 2010, 2011) | Investigate hydrothermal vents and biogeochemical fluxes | |
| Quadrupole/ART-MS | DOMS/Mini-DOMS (McMurtry et al. 2011) | 4000 m depths, less than 30 W consumption | |
| Quadrupole MS | MIMS MS (Short et al. 2001) | AUV-based hydrocarbon detection | |
| Prototype (targeting ocean worlds) | CE-MS/LIF/C4D | OCEANS (Willis et al. 2019; Creamer et al. 2020) | Analyze liquid samples, use multiple detection methods |
| GC/LD-Ion Trap MS | EMILI (Brinckerhoff et al. 2018, 2019) | Building on heritage from MOMA, targeting Europa | |
| LC-TOF-MS | OASIS (Getty et al. 2013) | Analyzing liquid samples, searching for biosignatures | |
| GCxGC-MS | MASPEX-ORCA (Blase et al. 2020) | Analyze semivolatiles and chemically derivatized biomolecules | |
| Field Tested | ME-LIF | Chemical Laptop (Mora et al. 2020) | ME tested in field |
| Prototype | CE-LIF | MOA (Stockton et al. 2010); MOAB (Mathies et al. 2017); (Mora et al. 2013; Creamer et al. 2017) | CE-LIF, microfluidic implementation, high-sensitivity detection of amino acids |
| Fluidic Systems and Flow Control | |||
| Satellite fluidic systems for microbiology review by Zea et al. (2021). Oceanographic millifluidic/microfluidic sensor systems by Nightingale et al. (2015), Fukuba & Fujii (2021), and McQuillan & Robidart (2017). | |||
| Planetary | Dispense reagents and calibrants | Mars Viking Lander (Klein 1977); WCL, Phoenix (Kounaves et al. 2009, 2010) | Dispense and stir small volumes of solution for analyzing Martian surface regolith |
| Space | Microfluidic and millifluidic packages for space and landed missions | Space biology (Zea et al. 2021) | Fluidic systems integrating manifolds, pumps, valves, and processing; capable of biological and chemical analyses; achieving miniaturized form factors |
| Field Tested | ME (Mora et al. 2020); organic molecule detection via immunoassays (Parro et al. 2011; de Diego-Castilla et al. 2011); subcritical water extraction (Kehl et al. 2019) | ||
| Prototype | OWLS/OCEAN (Willis et al. 2019; Creamer et al. 2020); MICA (Noell et al. 2019); SPLice (Chinn et al. 2017); MOAB/EOA (Mathies et al. 2017; Mora et al. 2011) | ||
| Ocean | Low-pressure, fluidic system in sealed housings | ESP (Scholin et al. 2017); Lab-On-Chip (Beaton et al. 2012); flow cytometry (Olson & Sosik 2007) | Fluidic systems within pressure-sealed housings,integrated with diverse sensors (genomic, chemical, imaging), including microfluidic Lab-On-Chip systems |
| Pressure-balanced water collection and filtration | (Breier et al. 2012; Okamura et al. 2013; Mullen et al. 2020) | Fluidic system for water sampling and filtration, pumping components held in pressure-balanced oil-filled housing | |
| Pressure-balanced chemical and biological sensors | IISA-Gene (Fukuba et al. 2011); nutrients (Thouron et al. 2003); CHEMINI (Vuillemin et al. 2009); SAMI (Lai et al. 2018) | Fluidic system for chemical and biological analyses, fluidic elements all in pressure-balanced oil-filled housing | |
| Lab | High-pressure microfluidics | (Hjort 2015; Andersson 2018) | High-pressure microfluidics for HPLC and other applications |
| Particulate Concentration | |||
| Review of laboratory techniques for cell and particle sorting by Shields et al. (2015) | |||
| Ocean | In situ membrane filtration | SUPR (Breier et al. 2012; Govindarajan et al. 2015); ESP 3G (Yamahara et al. 2019); HFS (Ver Eecke et al. 2012) | In situ seawater filtration (>0.2 μm) and fixation on robotic vehicles allows lab microscopic and genomic analysis |
| In situ membrane filtration and sample analysis | D-ESP (Olins et al. 2017; Scholin et al. 2017); GeoMICROBE sled (Cowen et al. 2012) | In situ seawater filtration (>0.2 μm) and genomic analysis (qPCR, DNA. and protein probe arrays) within underwater "lab in a can" | |
| Lab | Tangential flow filtration | (Petrusevski et al. 1995), (Giovannoni et al. 1990), (Benner et al. 1997) | Shipboard TFF, concentration of microbes from seawater, concentration for DNA analyses, 0.1 μm filtering (10 μm prefilter) |
| Sorting flow cytometry | UV fluorescence (Lambert et al. 2010), flow cytometry review (Vembadi et al. 2019) | Techniques using sheath flow, optical measurements, and active sorting to select particles of interest from larger volume | |
The SSSLOW is designed to search for microbial life in ice shell and ocean environments. Observations are made across relevant size scales (macroscopic microbial aggregates, cellular, molecular) of a variety of sample materials (sidewall ice matrix, melted ice, intrashell or ocean liquids) with multiple measurement techniques (imaging, electrochemistry, spectroscopy, spectrometry, molecular sequencing). The payload consists of four sequential phases ordered from least to most destructive such that a sample undergoes multiple complementary measurements and is modified only to the degree required for each subsequent investigation. A phased approach gradually builds contextual understanding of the sample and enables methods for autonomous prioritization of high-interest samples for more intensive downstream analysis or rejection of aliquots with potentially hazardous properties. The SSSLOW is compiled from a multidisciplinary review of observational and analytical instruments and specifically designed to enable the science objectives of the Vertical Entry Robot for Navigating Europa (VERNE) subsurface probe concept (see Schmidt et al. 2023). Many of the considerations presented here are also more broadly extensible to subsurface life detection at other worlds, such as Saturn's moon Enceladus or the Martian ice caps.
2. Subsurface Science
Observations and modeling indicate that Europa's ice shell is likely between 3 and 30 km thick (Billings & Kattenhorn 2005; Howell 2021) with an underlying ocean up to 100 km deep (Vance et al. 2018). Accessing the ocean is an unprecedented engineering challenge; however, there are commensurate scientific benefits relative to orbital or landed investigations. Below the surface, evidence of life would be shielded from the destructive effects of space weathering and irradiation (Nordheim et al. 2018). A thermomechanical probe would generate abundant liquid water in deeper regions of the ice shell, enabling high sample throughput and particulate concentration techniques to reduce the chance of a false-negative life detection result. Perhaps most significantly, if the ocean or liquid reservoirs within the ice shell prove habitable, a subsurface mission could directly search for extant life.
2.1. Potentially Habitable Europan Ice Shell Environments
A body of previous work has considered the habitability of Europa's ocean and seafloor (Reynolds et al. 1983; Chyba & Phillips 2002; Figueredo et al. 2003; Marion et al. 2003; Hand et al. 2009; Cockell et al. 2011; Pasek & Greenberg 2012; Russell et al. 2014; Vance et al. 2016; Russell et al. 2017; Schmidt 2020; Soderlund et al. 2020). With ice shell thickness estimates of tens of kilometers, a cryobot may spend the majority of the mission in ice, so we additionally highlight processes that could generate or maintain habitable liquid volumes in ice (Lipps & Rieboldt 2005; Zolotov & Kargel 2009; Schmidt 2020).
2.1.1. Terrestrial Ice Analogs
A growing understanding of the complex distribution of life and its signatures in ice on Earth informs the range of potentially habitable environments in Europa's ice shell. We consider two relevant types of terrestrial ice: (1) meteoric ice formed as snow accumulates and compresses under its own weight and (2) saline ice formed from the freezing of seawater.
Meteoric ice contains abundant chemical evidence of life, dormant cells, and potentially even metabolically active englacial life (Priscu & Christner 2004; Cockell et al. 2011; Anesio & Laybourn-Parry 2012). Habitable environments include microliter fluid inclusions between ice grains (Priscu & Christner 2004) and water films at the surfaces of mineral grains (Tung et al. 2006; Price 2007). Preserved bacteria have also been recovered from ancient glacial ice (Christner et al. 2003) up to 8 Ma in age (Bidle et al. 2007). These examples demonstrate the potential for the long-term maintenance of liquid reservoirs and organic materials in relatively pure ice relevant to low salinity predictions for Europa's ice shell (Wolfenbarger et al. 2022).
Saline ice on Earth can form as seawater freezes due to conductive heat loss to the atmosphere or an overlying ice mass or by accumulation of buoyant ice crystals generated in supercooled water (e.g., Lewis & Perkin 1986; Buffo et al. 2018; Haumann et al. 2020; Hoppmann et al. 2020; Lawrence 2020; Wolfenbarger et al. 2022). Different freezing processes and rates result in a variety of ice morphologies, but rejection of salt during freezing commonly forms porous ice with interstitial brines (Buffo et al. 2020). Nascent modeling of solute entrainment during Europan ice shell accretion suggests that brines and bulk salt concentrations are important to ice shell dynamics (Buffo et al. 2020, 2021a, 2021b; Wolfenbarger et al. 2022), and two terrestrial examples of solute-bearing ice, subshelf congelation ice and marine ice, may be particularly relevant to the Europan system (see Wolfenbarger et al. 2022 for a more complete discussion).
Congelation ice formation beneath ice shelves is driven by low thermal gradients. As the freezing front advances, ions are excluded from the growing crystalline matrix, and a multiphase, or "mushy," layer of ice forms with an interstitial network of liquid brine channels and pockets among solid ice (Feltham et al. 2006; Buffo et al. 2018). Limited observations or samples of subshelf congelation ice exist, but cores from a basal layer of congelation ice below Ross Ice Shelf exhibited this porous structure and were colored brown by materials entrained from the ocean (Zotikov et al. 1980). Thermal gradients, basal temperatures, and pressures at the ice–ocean interface below Antarctica's thickest ice shelves are expected to be of similar order below Europa's ice shell, where congelation ice growth may be a similarly important process (Buffo et al. 2021b; Wolfenbarger et al. 2022).
Marine ice formation occurs beneath ice shelves as supercooled water nucleates buoyant ice crystals that rise, accumulate, and compact against an overhead ice ceiling (Lewis & Perkin 1986; Hoppmann et al. 2020). Supercooling can occur below ice due to the pressure dependence of the seawater freezing point. When a parcel of seawater melts submerged ice, it is cooled toward the pressure-depressed (colder) freezing point and also made relatively buoyant by the fresh meltwater addition. Upon subsequent adiabatic upwelling to lower pressures and commensurately warmer freezing points, the seawater can become supercooled. This process is known as the ice pump and operates to smooth topographic disequilibria in submerged ice (Lewis & Perkin 1986). On Earth, ice pumping can generate layers of porous ice up to hundreds of meters thick with organics entrained throughout (Craven et al. 2009).
Brine pockets and channels in both congelation and marine ice constitute a significant habitat for algae and bacteria in sea ice (Junge et al. 2004; Boetius et al. 2015; Bowman & Colwell 2015; Deming et al. 2017). Bulk cellular abundances range from 103 ml−1 in colder upper sea ice to 107 ml−1 near the base and can reach 108 cells ml−1 within brine pockets (Deming et al. 2017). These concentrations often exceed those of ambient seawater with typical abundances of 105–106 (e.g., Vick-Majors et al. 2016). Experimental work has similarly found preferential entrainment of cells, independent of cellular viability, into lake ice (Santibáñez et al. 2019).
2.1.2. Englacial Europan Environments
As Europa's ice shell accreted, it likely entrained salts (Buffo et al. 2020, 2021a; Wolfenbarger et al. 2022). Depending on their abundance and composition, salts can impact ice structure, suppress freezing temperatures, and maintain liquid pockets within the ice. As collated by Schmidt (2020) and Vance et al. (2021), fluid-associated processes and regions within the ice shell could include frictional melt at subducting slab interfaces (Kattenhorn & Prockter 2014), impact-induced brine migration (Steinbrügge et al. 2020), near-surface brine reservoirs (Schmidt et al. 2011; Michaut & Manga 2014; Walker & Schmidt 2015; Manga & Michaut 2017; Chivers et al. 2021), brine percolation (Kalousová et al. 2014; Kalousova et al. 2016; Hesse et al. 2020), refreezing in basal crevasses (Buffo et al. 2020; Walker et al. 2021), basal ice mushy layers (Buffo et al. 2020, 2021a, 2021b), and gravity drainage-induced brinicles (Vance et al. 2019; Buffo et al. 2021a).
Analog terrestrial processes suggest that the rejection and concentration of salts into intergranular liquid pockets may generate and maintain habitable oases within Europa's ice shell. Porous brine networks may be an active habitat near the base of the ice shell, and further cooling as the basal freezing front advances could isolate brine pockets (around −5°C or 5% porosity in sea ice; i.e., Golden et al. 1998) and act to entomb organics within the ice shell. Basal crevasses in Europa's ice shell (Buffo et al. 2020; Walker et al. 2021) or locally thinned ice represent regions where ice pumping could preferentially accrete marine ice (Soderlund et al. 2014; Schmidt 2020; Wolfenbarger et al. 2022), akin to observations of ice accreting within basal crevasses below Ross Ice Shelf on Earth (Lawrence 2020).
The broad distribution of life and biosignatures within terrestrial ices suggests that Europa's shell could contain ocean-derived materials throughout, and potentially significant volumes of habitable liquids (Lipps & Rieboldt 2005; Hand et al. 2009; Buffo et al. 2021a; Wolfenbarger et al. 2022). The ice represents an opportunity, not an impediment, to life detection in advance of reaching the ocean (Schmidt 2020), and a subsurface mission should equally weight sampling throughout.
2.2. Science Opportunities
Subsurface access to Europa's icy shell would enable a wide range of new possible scientific investigations. Here we describe three primary mission goals in a science opportunities matrix (SOM; Table 1): (1) search for life, (2) assess habitability, and (3) investigate geologic processes. Each goal has multiple subobjectives and supporting observables. The SOM is informed by ocean world exploration priorities from the planetary science and astrobiology communities (e.g., Hand et al. 2017; Neveu et al. 2018; Hendrix et al. 2019) and extends these goals into the subsurface.
2.2.1. Search for Life
The search for life is a core motivation for subsurface exploration of Europa. Potential biosignatures include markers of biological activity (metabolism, growth, motility), morphological features (cell structure, biofabrics), and chemical signatures (complex organic molecules, distributions of simple organics, biominerals). Assiduous consideration of life detection strategies through biosignatures can be found within Europa Lander studies (Europa Study Team 2012; Hand et al. 2017), the Ladder of Life Detection (LOLD) framework (Neveu et al. 2018), and the growing, community-based Life Detection Knowledge Base (ldfknowledgebase.com). Below, we consider the relevant biosignatures for subsurface exploration.
Active microbial life, as opposed to dormant (e.g., spores) or past life, may be detectable through observations of growth, reproduction, metabolism, and Darwinian evolution (Neveu et al. 2018). A subsurface mission could search for cellular motility or concurrent cellular life stages (i.e., actively dividing or budding cell structures) indicative of reproduction with microscopic imaging (Section 3.3.2). Recent biologic activity may be inferred from the presence of organics, which, given context measurements of host fluid composition, would not be expected to be stable for long durations (e.g., RNA, ATP). Their presence might therefore indicate ongoing organic synthesis or catabolic processes of life. The design of agnostic experiments to assess Darwinian evolution is challenging (Saboda et al. 2019); however, informational polymer sequencing (Section 3.4.4) could look for conserved regions exhibiting variability indicative of evolutionary processes, akin to variable sequences within the 16S rRNA gene conserved across bacteria and archaea (Woese & Fox 1977).
Morphological signatures of life also include macroscopic aggregates or biofabrics, such as stromatolites or microbial mats. Aggregate life may be suspended in the ocean, organized into biofilms at the base of the ice shell (Daly et al. 2013; Boetius et al. 2015), or in pockets within the ice shell. Macroimaging of the borehole sidewall (Section 3.1) and microscopic observations of sampled fluid (Sections 3.3.2–3) can enable morphological observations at multiple relevant scales.
Strong chemical evidence for life includes (1) the presence of complex, high molecular weight organic molecules, such as informational polymers (e.g., DNA, RNA, proteins), and (2) patterns of chirality, abundance, isotopic composition, and mass distribution of smaller molecules (e.g., amino acids and lipids; (Lovelock 1965; Hand et al. 2017; Carr et al. 2017; Chan et al. 2019; Neveu et al. 2020; MacKenzie et al. 2021; Willis et al. 2021). Spectroscopy (Section 3.3.3), spectrometry (Section 3.4.3), and polymer sequencing (Section 3.4.4) investigations may be used to test for the presence and distribution of these compounds. An additional novel biosignature to consider on icy worlds is extracellular polymeric substances (EPSs), complex organic molecules (predominantly carbohydrates) produced by ice-dwelling algae and bacteria for metabolic and ice-protective purposes. They are ubiquitous throughout sea ice on Earth and retained even in the absence of active cellular production (Deming et al. 2017). The presence of compounds serving an analogous purpose to EPSs in extraterrestrial ice habitats could be assessed with mass spectrometry (MS) techniques and may help to inform instrument sensitivity requirements (Rivaro et al. 2021).
Examples of inorganic mineral biosignatures include apatite, calcite, aragonite, and siliceous structures (e.g., diatom frustules). These are potentially detectable by microscopic imagers, chemical analysis, or vibrational spectroscopy. Other inorganic chemical biosignatures include isotopic fractionation indicative of metabolic processes (Hand et al. 2017; Chan et al.2019).
Environmental context is important for interpreting biosignature detections. Context includes habitability or preservation potential of the sample source, alternative abiotic sources, and the transport and processing history of the sample (Neveu et al. 2018; Schmidt 2020). At Europa, improved knowledge of ice shell and ocean transport processes from complementary subsurface science goals will help to determine sample provenance. For example, temperate ice devoid of oxidants might indicate a recent oceanic source, suggesting that any organics within the ice had limited or no exposure to cryogenic temperatures and radiolytic modification at the ice surface.
2.2.2. Assess Habitability
The habitability of an aqueous environment can be considered as a function of both immediate fluid properties and planetary scale processes that support continuous habitability over geologic timescales (Cockell et al. 2016; Hand et al. 2017). While habitability can only be defined at present for life as we know it, it is important contextual information to support suspected detections of extant life. Immediate fluid properties include physicochemical state (e.g., temperature, pressure) and concentrations of non-ice materials (e.g., salts, energy sources, substrates for biosynthesis). Continuous habitability considers the renewal of metabolic substrates and maintenance of energy sources through geologic processes. A subsurface mission can map the variety and vertical distribution of non-ice materials to explore both immediate fluid habitability and their influence on ice shell overturning in support of continuous habitability.
Europa's ice shell likely contains salts, inorganic nutrients, and volatiles (Chyba & Phillips 2002; Hand et al. 2006, 2007; Buffo et al. 2020). Endogenous source processes include entrainment of solutes and hydrothermal alteration products from the ocean during ice shell formation and radiolytic generation at the surface. Examples of exogenous sources include meteoritic input and delivery of sulfur species from Io. Immediate fluid properties can be characterized by physical sensors measuring temperature and pressure (Section 3.2.3), coupled with chemical sensors to bound the concentration of different ionic species (Section 3.2.4).
The presence of salts can strongly influence habitability by modulating water activity or the water not bound by solutes and available to organisms. Water activity (aW) ranges from zero to 1 (pure water), and while a low aW is not necessarily immediately deleterious (Barbosa-Cnovas & Juliano 2007), no microbial organisms are known to reproduce below the presently accepted limit of ∼0.6aW (Grant 2004). However, depending on the ionic composition, even saturated brines can be habitable (Hallsworth et al. 2007; Cray et al. 2013; Klempay et al. 2021), as evidenced by halophilic organisms living at low aW in salterns (Lee et al. 2018) and deep hypersaline anoxic brine pools (Merlino et al. 2018; Antunes et al. 2020; Fisher et al. 2021). Brines may also act to preserve organics for long periods of time (Perl & Baxter 2020; Klempay et al. 2021; Perl et al. 2021). To the authors' knowledge, it is not currently possible to directly measure aW while submerged, and a subsurface mission should observe the necessary parameters (temperature, pressure, pH, ion concentration; Sections 3.2.3–4) to reconstruct aW through modeling. Understanding the composition, formation, and distribution of brines in Europa's ice shell is important to bound subsurface habitability.
At larger scales, the hypothesis that Europa is habitable is in part predicated on the potential for circulation of material through the ice shell. Possible exchange mechanisms through the ice shell include impactors (Cox & Bauer 2015), ice subsumption or subduction (Kattenhorn & Prockter 2014), percolation along grain boundaries in temperate ice (e.g., terrestrial ice stream margins, Nye 1989; Fowler 2006; Schoof & Hewitt 2016), brine drainage (Kalousová et al. 2014; Hesse et al. 2020), and ice shell convection (Allu Peddinti & McNamara 2015). Regional ice overturning may be required to maintain chemical disequilibrium (Pappalardo et al. 1998; Showman & Han 2005; Russell et al. 2017; Howell & Pappalardo 2018; Vance et al. 2018; Schmidt 2020; Soderlund et al. 2020), analogous to the renewal of materials through plate tectonics on Earth (Falkowski et al. 2008). Cold, downwelling ice masses may deliver radiolytically generated oxidants from the surface into the ocean, acting to locally enhance oxidant availability in a reduced ocean (Hand et al. 2007; Vance et al. 2016). Upwelling ice diapirs may thin the ice and encourage marine ice infilling due to the ice pump (Lewis & Perkin 1986), entraining ocean-derived compounds (Soderlund et al. 2020; Wolfenbarger et al. 2022) and transporting them upward.
Ocean circulation could influence habitability by coupling the generation of reduced species at the seafloor to oxidants delivered through the ice shell (Hand et al. 2007; Soderlund et al. 2014, 2020). Circulation may be influenced by stratification, tidal forcing, and planetary rotation (Soderlund et al. 2014); orbital factors such as tides, libration, and precession (Soderlund et al. 2020); or electromagnetic (EM) pumping (Gissinger & Petitdemange 2019). Instrumentation deployed into the ocean by a subsurface probe could make in situ measurements of current speeds and density structure to bound some of these processes (Section 3.2.3). Ocean science objectives compel extension of subsurface probe objectives to include deeper profiling of ocean structure, as described in the VERNE mission study (Schmidt et al. 2023).
2.2.3. Investigate Geological Processes
Geological processes include ice strain, flow, fracture, and fluid flux within the ice shell, as well as basal ice–ocean interactions (melting and freezing). These transport processes may support continuous habitability in the ice and the ocean below. The critical Rayleigh number (Barr et al. 2004; Barr & Pappalardo 2005) delineates the onset of convection in ice and depends on the thermal gradient, ice temperature, thickness, density, and viscosity. Ice shell porosity and salt content have also been shown to influence transport and subduction processes (Johnson et al. 2017). In sum, these factors inform priority in situ measurements for an understanding of ice shell processes and the potential for maintenance of habitable environments over geologic timescales (Schmidt 2020).
Subsurface mission concept architectures (Cwik et al. 2019; Oleson et al. 2019; Schmidt et al. 2023) converge on a necessity for distributed communication hardware through the ice shell, and these elements could enable novel measurement strategies of intrashell processes. Vertical tidal strains of up to 30 m predicted within the ice shell (Moore & Schubert 2000) could be investigated from fiber optic tether strain measurements (Zumberge et al. 2002) and relative acoustic ranging between the vehicle, englacial communications relays, and the surface lander. Similar techniques could enable observation of glacial flow rates over long duration missions. Passive acoustic techniques have been demonstrated for subglacial water flux monitoring (Nanni et al. 2021) and may be similarly applied to observe subsurface hydrology in Europa's ice shell (Hand et al.2017).
While a cryobot will necessarily modify the temperature of the ice while melting or drilling, the initial ice temperature may be bounded from the descent rate, ambient melt pocket temperature (at the solute and pressure-dependent freezing point), thermal output of the probe, and the mechanical drill rate. Higher-fidelity observations may be possible through distributed temperature sensing via a vehicle's fiber optic communications tether (Stern et al. 2013; Tyler et al. 2013; Tulaczyk et al. 2014) or from sensors in distributed communication relays as the borehole returns to thermal equilibrium in the vehicle wake (Schmidt et al. 2023). Fiber optic systems are also used for distributed acoustic sensing and seismic monitoring in Earth's cryosphere (e.g., Winberry et al. 2009; Walter et al. 2020; Brisbourne et al. 2021; Olsen et al. 2021).
In addition to temperature and strain, ice viscosity also depends on grain size (Barr et al. 2004; Barr & McKinnon 2007). Terrestrial ice grain size observations have been made via in situ borehole imaging (Engelhardt & Kamb 2013) and imaging from an ROV below an ice shelf (Lawrence 2020). Grain size could be constrained using borehole macroimaging systems (Section 3.1). A subsurface probe may also provide direct observations of additional sidewall ice properties such as density and permittivity to further inform global models of ice circulation and support ice-penetrating radar observations (Section 4.2).
An additional priority goal of a subsurface Europan mission is to understand interactions between the ocean and ice shell (Schmidt 2020; Schmidt et al. 2023). This leads to objectives of measuring the water temperature relative to the pressure and salinity-dependent freezing point, current speeds, and ice interface properties (morphology, porosity, salinity). Small variations in these parameters drive large variability in terrestrial ocean circulation and sub-ice melt and freeze processes (Lewis & Perkin 1986; Corr et al. 2010; Lawrence 2020; Wolfenbarger et al. 2022). At Europa, the balance of these processes has implications for the regional distribution of redox species available for metabolism and informs the overall habitability in the ice shell and ocean (Schmidt 2020; Soderlund et al. 2020; Schmidt et al. 2023).
2.3. Mission Challenges
A subsurface mission represents a significant departure from previous robotic planetary exploration. Unique challenges include submerged operation at high pressures, extended exposure to potentially corrosive fluids, and data transmission through kilometers of ice. The life detection payload must be able to intake liquids or solid ice and search for low concentrations of microbial life that could differ significantly from that on Earth. Finally, operation in a potentially habitable aqueous environment will incur significant planetary protection requirements to prevent forward contamination.
2.3.1. Environmental Challenges
Pressures at the base of a hypothetical 35 km thick Europan ice shell would be ∼40 MPa. While these pressures are magnitudes greater than those encountered in previous space exploration (typically ≤0.15 MPa, except for short-lived atmospheric probes or Venus landers at ∼9 MPa), they are equivalent to the average depth of Earth's oceans (∼4000 m). Electronic components deployed to these depths are typically held within high-strength housings made of aluminum, steel, titanium, ceramic, fiber composites, or glass. In situ scientific sensors and fluidic elements generally either employ a voidless solid-state design (e.g., pressure-tolerant electronics), make measurements through an optical viewport, depressurize water samples prior to intake into a low-pressure housing for analysis, or use a pressure-balanced housing filled with nonelectrically conductive oil (Fukuba & Fujii 2021).
High concentrations of non-ice materials within the shell may also present challenges. Possibilities include salts (Buffo et al. 2020), sulfuric acid (Carlson et al. 1999b), strong oxidants (Carlson et al. 1999a; Johnson et al. 2003), and debris or dust. These have the potential to corrode components, clog intakes, accumulate in the borehole, saturate sensors, or oxidize and degrade target organics upon mixing as ice is melted. A probe traversing the shell will also experience temperatures ranging from 100 K at the surface (Spencer et al. 1999) to ∼273 at the ice shell base (depending on pressure and salinity; e.g., 267.9 K for seawater at 40 MPa). Gases trapped in the ice (Hand et al. 2006) could also be destabilized by a thermal probe, and samples depressurized by the vehicle after intake may exsolve large volumes of gas. In the brittle upper ice (above ∼3 km), the vehicle may encounter voids, fractures, liquid lenses tens of meters thick, or salt layers that are meters thick (Chivers et al. 2021). Salt deposits could saturate the melt pocket around the vehicle, modify the stability of organic compounds, increase fluid viscosity and corrosion potential, induce solid salt precipitation, and necessitate desalting for analyses inhibited by high ionic strengths. While one hazard mitigation strategy might be to avoid intaking samples above a threshold solute concentration, brines are also target fluids for life detection given their potential to preserve target biosignatures (Tuovila et al. 1987; Borin et al. 2008).
Communication through kilometers of ice is another fundamental challenge. Subsurface probes may utilize fiber optic tethers with lower-bandwidth acoustic or EM contingency relays (Oleson et al. 2019; Schmidt et al. 2023). In any case,data transmission will be delayed and severely bandwidth-limited. The scientific payload therefore needs to operate autonomously to meet scientific objectives while returning only a small subset of data. The vehicle will need to be able to determine when to sample, how to optimize power and consumable resources (such as reagents and filters) during sample processing, and prioritize the return of the most relevant data products.
2.3.2. Sampling Challenges
Liquid water samples within the ice shell can be obtained either by drilling and melting discrete ice cores or directly sampling ambient borehole meltwater. An ambient melt sample strategy has several advantages relative to coring: it eliminates the need for vehicle station keeping in the borehole, samples can be acquired at higher temporal and spatial frequencies, and large water volumes can be acquired to enable filter concentration strategies (Section 3.3.1). Preliminary thermal modeling suggests that a pressurized melt pocket may form around the probe within tens of meters of the ∼110 K surface ice if the melt probe maintains a 300 K surface temperature, enabling liquid intake from the earliest phases of the mission (see Schmidt et al. 2023).
However, direct fluid sampling from the borehole presents challenges as well, including dilution and mixing of non-ice materials from intergranular brines upon melting. Dilution may be ameliorated with concentration techniques (Section 3.3.1), but mixing in the vehicle's melt wake (potentially tens of meters long; see Schmidt et al. 2023) would limit the ability to bound the depth origin of a sample. However, this could be mitigated if the fluid intake rate does not exceed the melt production rate, such that the sample can be bound to a depth bin based on ingestion time (e.g., Clark et al. 2018).
For example, traversing a 15 km ice shell in 3 yr requires an average descent rate of 0.016 cm s−1, which for a hypothetical 5.5 m long, 25 cm diameter vehicle (Meister et al. 2018; Cwik et al. 2019; Oleson et al. 2019; Schmidt et al. 2023) represents a melt rate of ∼8 ml s−1. Because the bulk salinity in the majority of the ice shell is expected to be less than 24 ppt (Buffo et al. 2020; such that at pressures less than ~28 MPa the temperature of maximum density is 4°C), the warmer water generated at the drilling head will be most dense. As water is displaced aft, or upward, along the skin of the vehicle, it will become increasingly buoyant as it cools back down toward the freezing point which may act to stably stratify the melt pocket. Assuming a 1 cm melt skin thickness around the vehicle, the 8 ml s−1 of melt flushes ∼0.2 cm of the vehicle with fresh melt each second, or the full vehicle length every 0.5 m of descent. The melt production rate then places an upper bound on the sample intake rate to avoid entraining mixed fluid from the upper melt pocket aft of the vehicle. These assumptions suggest that profiling of ice shell properties (e.g., bulk salinity) at ∼meter vertical resolutions may be possible through direct melt sampling.
By comparison, pumped sensors measuring conductivity, temperature, and depth at up to 32 Hz (collectively referred to as CTDs in oceanographic profiling; Section 3.2.3) are designed to be flushed at similar rates of ∼10–40 ml s−1 and commonly operate autonomously for years on battery-powered profiling floats and gliders on Earth (Jayne et al. 2017). These temporal and spatial sampling resolutions may be attainable by the analogous flow-through package included in the SSSLOW payload (Sections 3.2.1–3). Modeling of fluid mixing within the melt pocket for different initial ice temperatures, drilling methods, and bulk salinities could further inform liquid sampling strategies and placement of sample intakes, akin to fluid models for downhole mud circulation in petroleum exploration wells (Epelle & Gerogiorgis 2020).
For SSSLOW, we employ a direct liquid intake approach for both scientific and operational benefits. However, liquid sampling could be augmented by discrete ice core collection within the shell (e.g., Oleson et al. 2019) with benefits including targeted coring of sidewall features observed by context imaging (e.g., Malaska et al. 2020). Sidewall ice coring has been demonstrated in Antarctica (Talalay 2014) and is conducted in commercial petroleum exploration drilling. Additionally, modeling with colder mean vehicle skin temperatures below 280 K suggests that it may be difficult to generate melt in the cryogenic temperatures in the upper kilometers of the ice shell (Schmidt et al. 2023), and in this case without a coring mechanism the SSSLOW concept payload would be limited to context imaging until a melt pocket formed in deeper, warmer ice.
Ice coring does share some challenges with liquid sampling, however, including the potential for brine dilution and cellular lysis upon melting (i.e., Deming et al. 2017). Sidewall ice will also be warmed by drilling ahead of the opportunity to collect a core, so ice structure and composition may still be altered from a pristine state, although to a lesser extent than complete melting. Further, ice cores may be limited by vehicle dimensions and mass to tens of milliliters, which reduces the benefit of sample concentration techniques.
2.3.3. Life Detection Challenges
If present, extant Europan life may vary significantly from that on Earth, be present at low abundances, or exist only in a dormant state. Life detection payloads should strive to combine multiple types of measurements for independent and complementary assessments, and be sensitive to a wide variety of biosignatures to reduce the potential for ambiguous or false-positive results.
Models of cellular abundances in Europa's ocean based on estimated chemical energy availability range from 0.1 to 100 cells ml−1 (Chyba & Phillips 2002; Hand et al. 2017), orders of magnitude less than the range of 10–1000 cells ml−1 observed in low-biomass accreted ice above subglacial lake Vostok (Priscu et al. 1999; Christner et al. 2006). Furthermore, life is not likely to be homogeneously distributed in the ocean and ice shell. For example, the high thermal and compositional gradients expected in the bottom meters of an accreting ice base may contain elevated concentrations of biota akin to distributions in terrestrial sea ice (Buffo et al. 2021b) and thus represent an important yet challenging region to sample.
Europan life may also have unique characteristics distinct from life as we know it. It is thus important to take an agnostic life detection approach using multiple independent observations to search for different types of biosignatures (e.g., organic, inorganic, morphological). Complementary and sequential measurements of the same water sample can also provide context and increased confidence in the observations (Neveu et al. 2018). Ideally, liquid sample acquisition and handling should also minimally modify materials to preserve context and prevent destruction of target materials.
2.3.4. Planetary Protection Consideration
At present, subsurface probes are not explicitly considered in planetary protection guidelines but missions involving direct contact (e.g., landers) with a body of significant astrobiological interest such as Europa are categorized by the Committee on Space Research (COSPAR) as Category IV. The Office of Planetary Protection (OPP), in accordance with COSPAR guidelines, requires that missions of this nature (1) include a bioassay to enumerate bioburden, (2) conduct a probability of contamination analysis, and (3) inventory bulk constituent organics (Coustenis et al. 2019). Full OPP requirements are typically issued on a case-by-case basis, and potential requirements for a subsurface mission would likely involve extensive sterilization and bioburden reduction, hardware bioshielding, and novel end-of-mission protocols. Clean access drilling for subglacial exploration on Earth (Dachwald et al. 2013; Rack 2016; Makinson et al. 2021) provides case studies for planetary exploration, and including nucleic acid sequencing instruments in payloads (Section 3.4.4) enables monitoring for terrestrial or forward microbial contamination. The development of consensus planetary protection guidelines for subsurface science operations and end-of-mission procedures is an important next step to develop requirements to guide the design of increasingly higher-fidelity payloads and mission architectures.
3. SSSLOW Payload Concept
To meet many of the opportunities and challenges of subsurface life detection, SSSLOW tightly couples instrumentation and sampling handling concepts into four sequential observational phases. Phase 1 collects external observations of the ice borehole with a context imager and deep-UV (DUV) spectrometer. Phase 2 performs bulk physical characterization of fluids using an oceanographic flow-through suite with conductivity, temperature, pressure, and density sensors. Samples can optionally route to an electrochemistry suite of solid-contact ion-selective electrodes (SC-ISEs), pH sensors, and a redox electrode. In phase 3, samples can be concentrated before passing into an optical flow cell with a microscope and spectrometer capable of Raman and fluorescence observations. In phase 4, discrete fluid samples are mechanically depressurized and desalted by electrodialysis (when required as informed by phase 2 characterization) and then split between a separation-mass spectrometer and an informational polymer sequencer.
3.1. Phase 1: External-facing Instruments
The first payload phase consists of a co-boresighted context imager and DUV spectrometer used to collect overlapping observations of the borehole sidewall as the vehicle descends through the ice shell. Data products from the paired optical systems can be merged to correlate the morphologic and spectroscopic features of materials embedded in the ice. This enables persistent external context measurements through a pressure-rated viewport and the ability to trigger more resource-intensive fluid sampling in specific regions of interest. Finally, optical measurements complement the microscale imaging and spectroscopy performed in phase 3, allowing similar data to be compared across spatial scales.
3.1.1. Context Imager
A high-resolution context imager is included in the payload to characterize the size, texture, and color of materials embedded in the ice. These data allow detection of macro-scale biosignatures such as layering of pigments, biofilms, biofabrics, or biominerals in the ice, features known to occur in analog ice environments on Earth (Boetius et al. 2015).
High-resolution imaging is frequently used across planetary and Earth exploration. Similar instruments on previous planetary missions have had resolutions on the order of 10 μm and fields of view of 10–1000 mm2 (see Table 2). These planetary instruments have employed a variety of specialized optical techniques, including focal stacking, fluorescence imaging, multispectral imaging, and integration of additional spectroscopic instruments. There is significant underwater microscopic imaging heritage in oceanographic applications (Jaffe 2014), including near micron-resolution benthic imagers (Mullen et al. 2016). In ice boreholes, macroscopic optical televiewing is used to investigate stratigraphy, fractures, bubbles, salt inclusions, debris content, and reconstructed ice density (Hubbard et al. 2008).
Persistent borehole observations provided by external imaging can allow intelligent sampling guided by compositional or morphological changes in adjacent ice or melt pockets. The context imager should ideally have sufficient resolution to investigate ice grains, which may vary from less than 10 μm to over a millimeter in size (Barr & McKinnon 2007), while observing as large an area as possible. We recommend integrating the optical path of the context imager with the spectrometer described below for correlation of morphological and chemical information (Beegle et al. 2015). Incorporating fluorescence or multispectral imaging capabilities may also be considered as a means of biosignature detection. Observations of a large water pocket or ocean would require an additional imaging system; however, this would open new opportunities, such as incorporation of a laser sheet for detecting plankton fluorescence (Jaffe et al. 2013).
3.1.2. DUV Spectrometer
A scanning laser DUV spectrometer (<250 nm) is included to analyze the chemical properties of the borehole sidewall and operate in tandem with the context imager. The DUV spectroscopy is contact-free, does not require sample preparation, and can detect organics embedded within the ice matrix and observe their spatial variation at fine scales. The resulting information can be correlated with morphologic features detected by the context imager such as pigmented layers, and used to trigger fluid sampling.
Lab and field tests have demonstrated the use of DUV laser-induced native fluorescence spectra to detect organics and microbes, as well as distinguish microbes from other organic compounds (Bhartia et al. 2019). This technique has been applied in situ within boreholes in glacial ice and igneous ocean crust (Salas et al. 2015; Eshelman et al. 2019). Scanning measurements by the WATSON instrument were used to map two-dimensional (75 × 25 mm) distributions of organics in a glacial borehole wall, identify organic hot spots, and detect organic chemicals at depths greater than 10 mm within the ice (Eshelman et al. 2019; Malaska et al. 2020). DUV fluorescence has also been used to optimize autonomous in situ sampling of glacial meltwater (Clark et al. 2018). The SHERLOC instrument on NASA's Perseverance Mars rover uses DUV spectrometry to analyze small rock samples, scanning a 50 μm diameter laser spot over a 7 × 7 mm area (Beegle et al. 2015). SHERLOC is able to simultaneously measure both native fluorescence and Raman scattering (discussed in more detail in Section 3.3.3), two distinct light–matter interactions that provide complementary information about the sample's molecular composition.
The DUV spectrometry is a promising contact-free technology for biosignature detection with relevant field heritage that complements context imaging. In addition to DUV fluorescence, with appropriate spectrometer resolution and range it should be possible to retrieve Raman spectra that can provide a specific chemical signature of organics and inorganics. Here it will be important to consider that the vehicle may have a variable descent rate and distance from the borehole wall. It may also be possible to share the excitation laser and/or spectrometer with the phase 3 spectroscopy system. Alternative contact-free approaches for chemical analysis with relevant heritage include laser-induced breakdown spectroscopy (LIBS), infrared (IR) spectroscopy, and X-ray fluorescence.
3.2. Phase 2: Bulk Characterization of Fluid
The second payload phase measures the bulk chemical and physical properties of liquid water samples. Water is first drawn into the payload from the surrounding melt pocket or ocean through coarse filters to reduce risk of clogging. Samples then pass through a millifluidic flow-through channel with an oceanographic and electrochemical sensor package. Together, these data are used to profile the ice shell and ocean structure and composition, assess the environment's habitability, inform downstream processing requirements (e.g., desalting or pH adjustment), and provide context for interpreting biosignatures if detected by later instrumentation. Phase 2 measurements can additionally inform payload sampling routines and vehicle drill or melt systems. Throughout this phase, the sampled fluid remains at ambient pressure and is minimally processed.
3.2.1. Filtered Inlet Ports
Minerals or other particles larger than ∼100 μm present clogging hazards to microfluidic systems (Dressaire & Sauret 2016), and fluid is drawn in through one of several redundant inlet ports with integrated mechanical filters. Underwater systems have used sintered stainless-steel filters with micron-scale pores to collect water from marine sediments (Zhang et al. 2010) and borehole melt in glaciers (Clark et al. 2018), and prefiltering is also demonstrated in a variety of open ocean sampling systems (McQuillan & Robidart 2017). Backflushing and the use of multiple inlet ports with separate filters can be used to mitigate clogging and provide redundancy.
3.2.2. Fluid Routing and Flow Control
An advanced fluid routing and flow control system is needed to precisely move liquid samples throughout all payload phases. This will require a combination of fluidic manifolds, valves, and pumps. Potential flow control components include solenoid, diaphragm, syringe, or peristaltic pumps (Nightingale et al. 2015; Fukuba & Fujii 2021). Considerations for pump selection include lifetime, flow rate, discharge pressure, precision, and low-temperature operation. Solid-state osmotic techniques may also be considered (Wheat et al. 2011). The fluidic system will need to be tightly integrated with the scientific instruments and capable of controlling the motion of milliliter to submicroliter liquid sample volumes.
Previous fluidic sample handling on planetary missions includes reagent and calibrant handling, for example, as on the Mars Viking Lander (Klein 1977) and Phoenix Lander Wet Chemistry Lab (WCL; Kounaves et al. 2009, 2010). Over the last two decades, several microfluidic science payloads have also successfully flown on small satellites (Zea et al. 2021), and similar packages are in development for future planetary missions (Mora et al. 2011; Noell et al. 2019). A variety of microfluidic and millifluidic systems have also been deployed in the ocean (Nightingale et al. 2015; Fukuba & Fujii 2021).
In the deep sea, water samples can be handled and analyzed within a sealed housing held at low pressure after fluid has been depressurized (Olson & Sosik 2007; Ussler et al. 2013). Alternatively, deep ocean instruments often place pumps, valves, and manifolds in pressure-compensated or pressure-balanced housings filled with inert nonelectrically conductive oil and match the pressure of the surrounding environment (see Fukuba & Fujii 2021, Figure 1). Many electrical and mechanical instruments can operate submerged in oil at full ocean pressure (Barnes & Gennari 1976; Bingham 2013). Pressure-balanced fluid handling systems are used for deep-sea-water sampling and filtration (Breier et al. 2012) and for more complex chemical and biological measurements (Fukuba et al. 2011; Lai et al. 2018). However, oil-filled designs may be less compatible with space systems due to complexities including added weight, off-gassing, and organic contamination potential. Microfluidic systems capable of withstanding high-pressure gradients may offer another solution, such as those developed for high-pressure liquid chromatography (Hjort 2015; Andersson 2018).
Handling of liquid samples on planetary missions is a critical area for continued technology development (Schmidt et al. 2021a, 2021b; Willis et al. 2021), and high-pressure submerged operations present additional challenges (Fukuba & Fujii 2021). A successful future planetary mission to an underwater environment must integrate and advance liquid handling technologies from space and ocean sciences.
3.2.3. Oceanography: CTD and Density
A CTD and densitometer are included in the payload to measure the physical ocean properties that control circulation and ice interactions. CTDs are fundamental tools in physical oceanography that pair colocated measurements of electrical conductivity, temperature, and pressure with an equation of state to determine important seawater properties, such as salinity, density, and freezing point (Talley 2011; Pawlocwicz et al. 2012). CTDs are commonly carried on autonomous profilers and vehicles in Earth's oceans, where their low power requirements and data volumes enable operation for multiple years (Roemmich et al. 2009; Jayne et al. 2017; Claustre et al. 2020). Efforts have also sought to develop miniaturized CTDs, such as the 30 × 15 mm chip CTD integrated into the DADU AUV (Jonsson et al. 2012) and other microfabricated devices (Asgari & Lee 2019; Hurwitz et al. 2020; Wu et al. 2020). These technologies are adapted in the SSSLOW payload where a flow-through oceanographic system would monitor the melt pocket fluid and ice shell properties during the downward traverse (akin to shipboard thermosalinographs) and then enable vertical profiling once the ocean is reached (see Schmidt et al. 2023).
In Earth's ocean, however, while density and salinity importantly describe ocean structure and circulation, neither is generally directly measured in situ. Standard practice is to instead relate CTD observations with the thermodynamic equation of seawater (Millero 2010; McDougall & Barker 2011) and applying a geographically-dependent correction to determine absolute salinity (SA; the mass fraction of dissolved solutes, g kg−1) from seawater conductivity and temperature. Density is then determined from empirical relationships with SA. However, this process is valid only for the typical temperature range of sodium chloride–dominated ocean water (∼2 to 42 ppt and −2°C to 35°C).
Neither the composition nor concentration of ions in Europa's ocean are yet well constrained (Zolotov & Shock 2001; Brown & Hand 2013; Hand & Carlson 2015; Trumbo et al. 2019), and they may also not be accurately represented in surface reconnaissance data due to solute fractionation within the ice shell (Buffo et al. 2020; Wolfenbarger et al. 2022). This limits the ability to develop an equation of state for the Europan ocean and motivates direct measurement of density in situ. Oscillating tube densitometers are used to develop the equations of state for Earth's oceans (Le Menn 2012) and have been employed to explore density and salinity relationships in seawater at high pressures (<50 MPa) in the lab (Schmidt et al. 2016, 2018). These low-power and sample volume (<5 ml) instruments may be adaptable for submerged use to supplement CTD observations and provide real-time density measurements and a conductivity-independent assessment inclusive of both the ionic and non-ionic total dissolved solids (salinity).
3.2.4. Electrochemistry: Ion-selective Electrodes, pH, and Redox Potential
Following the physical characterization of the fluid sample, liquid aliquots can be passed to a microfluidic electrochemistry cell to measure the sample's ionic composition, pH, and redox potential. A microfluidic manifold is used to reduce the required volume of calibrants and enable their retention if required for planetary protection concerns.
Ion-selective electrodes (ISEs) are compact, nondestructive sensors that measure the activity of soluble ionic species. These measurements support investigation of the ionic composition of the ice shell and ocean, the availability of redox couples that could support metabolism, environmental habitability, and the potential to preserve biosignatures. A vertical profile of ion concentrations through the ice shell can be used to investigate the ice formation history, including the ion exclusion processes during freezing. Ionic composition measurements are also important for the development of an equation of state needed to inform ocean and ice shell modeling. Finally, abundance measurements enable modeling of water activity, which is an important parameter when considering habitability (Cray et al. 2013; Stevens & Cockell 2020; Fisher et al. 2021).
The WCL on board the NASA 2007 Phoenix Mars Lander carried an array of ISEs which enabled the discovery of high levels of perchlorate on the Martian surface (Hecht et al. 2009; Kounaves et al. 2009). Recent work has evaluated and highlighted the potential of miniaturized SC-ISEs for future landed ocean world missions (Jaramillo & Noell 2020). In terrestrial applications, SC-ISEs have been demonstrated to work at pressures up to 10 MPa (Weber et al. 2017) in submerged environments, and microelectrodes have a significant history of deep-sea ocean use (Table 2).
Measurements of pH can be made with potentiometric (glass electrode), spectrophotometric, or solid-state instruments, but a limited tolerance of freezing and indicator requirements makes the former two techniques poorly suited for flight applications. The Phoenix WCL included H+ ISEs for pH and a robust iridium oxide sensor (Kounaves et al. 2009). Additionally promising solid-state instruments include low-power and drift ion-selective field effect transistors (ISFETs), which have been used to monitor glacial meltwater with demonstrated freeze–thaw tolerance (Bagshaw et al. 2021) and on multiyear deployments of terrestrial oceanographic floats and gliders (Martz et al. 2010; Hofmann et al. 2011; Claustre et al. 2020; see also Fukuba & Fujii 2021). In addition to providing sample context and habitability determination, pH monitoring is also important for sample handling considerations due to the potential for sulfuric acid in Europan ice (Carlson et al. 2005; Brown & Hand 2013).
3.3. Phase 3: Optical Measurements of Fluid
The third payload phase consists of a particulate concentration stage followed by sample analysis using paired microscopic imaging and Raman spectroscopy. Optical measurements are performed through pressure-rated viewports, with minimally processed water samples held in an optical flow cell (Zhang et al. 2017a; Lai et al. 2018) at ambient pressures. The microscope searches for cell-like morphologies and potential microbial motility, while the Raman spectrometer analyzes the sample's chemical composition. These two instruments collect complementary morphologic and compositional data, and their optical pathways can be combined such that they interrogate an overlapping volume (Beegle et al. 2015; Takahashi et al. 2020). Furthermore, phase 1 and 3 instrumentation both collect similar spectroscopic and morphologic data that can be compared across macro- and microscopic spatial scales.
3.3.1. Particulate Concentration
Biosignatures in Europa's ice shell or ocean, such as organic molecules or cells, may be present at very low concentrations (Section 2.3.3). Concentrating particles in the liquid sample prior to sample analysis may significantly increase the probability of life detection and science return of all downstream instruments. An array of different concentrating techniques are used in microbiology (Shields et al. 2015), and it is common practice in oceanography to concentrate liters of seawater through membrane filters before analyzing samples with techniques such as eDNA sequencing (Djurhuus et al. 2017; Yamahara et al. 2019) or microscopic imaging (e.g., Vick-Majors et al. 2016).
Membrane filtration is a simple and widely used technique in which sample fluid is directed through a filter substrate that passes water and collects particulates larger than its pore size. Several submerged ocean instruments perform membrane filtration in situ (as reviewed by McQuillan & Robidart 2017; Spanbauer et al. 2020). These systems can filter liters of seawater to collect small particulates (<0.2 μm) and have been deployed on a variety of underwater platforms, including robotic vehicles (see Table 2; Breier et al. 2012). The submersible ESP microbiology "laboratory in a can" system can concentrate seawater using membrane filters and then apply a range of molecular analyses, such as quantitative PCR and DNA probe arrays, all within an underwater pressure housing (Preston et al. 2011; Ussler et al. 2013; Scholin et al. 2017). However, while membrane filters are simple and robust, they typically have a short life span before saturating depending on pore size (commonly ∼0.2 μm for microbial sampling), and require an elution step to free concentrated materials.
An alternative approach is tangential flow filtration (TFF) in which fluid is circulated parallel to a membrane filter. This is less likely to clog and retains particulates in the fluid phase instead of on the filter. TFF has a history of use for concentrating plankton and organic particles from seawater (Giovannoni et al. 1990; Petrusevski et al. 1995; Benner et al. 1997). Alternative passive concentrating techniques use hydrodynamic and inertial forces (Shields et al. 2015). Active particle sorting could also be investigated, and flow cytometry (Olson & Sosik 2007; Vembadi et al. 2019) with cell sorting has been explored as an enabling tool for astrobiological investigations (Lambert et al. 2010).
Ocean world subsurface missions need a robust, nondestructive concentrating system capable of long-duration operations under a diverse range of salinity, temperature, and pressure conditions. Conventional membrane filtration followed by elution has been demonstrated within an underwater pressure housing (Scholin et al. 2009). This approach could be implemented at ambient pressure but would require significantly extending the system lifetime. Alternatively, implementation of TFF would provide longer life filters and eliminate the need for elution.
3.3.2. Microscope for Life Detection
An optical microscope for life detection is included in the payload to search for microbial morphology and motility. Optical microscopy is a classic contact-free and nondestructive technique that can be used to search for cell-like morphologies by analyzing the size, shape, and structure of particles (Nadeau et al. 2018). If active specimens are present, imaging can detect biological activity such as microbial motility, a potentially conclusive biosignature (Nadeau et al. 2016). Imaging can further provide environmental context information by characterizing the size distribution, concentration, and potential material properties of abiotic particles.
Microbes found in analog ocean and ice environments on Earth typically range from 0.2 to 3 μm in size (Hand et al. 2017). The maximum resolution of a conventional optical microscope is limited to hundreds of nanometers due to the diffraction of light (Nadeau et al. 2018). Achieving submicron resolution will be critical; however, microbes with sizes on the order of the resolution limit may still lack distinguishing morphological features. It will therefore be important to employ multiple approaches to distinguish between potentially biotic and abiotic particles (Serabyn et al. 2018). Material properties such as those retrieved from holography or fluorescence imaging may offer key observables (Nadeau et al. 2008), and active motility may offer a conclusive life detection (Nadeau et al. 2018). Finally, there are also important considerations regarding the volume sampling rate, as high-resolution systems typically result in shallow depths of field and very small interrogated volumes.
Microscopes have flown on several planetary missions; however, previous systems were designed primarily for geological investigations rather than microbe detection and accordingly used optical designs with lower maximum resolutions (∼4 μm pixel−1; Table 2). Currently, several submicron-resolution optical microscopes are being developed specifically for life detection on planetary missions (Table 2). Optical microscopes can be enhanced with a number of modalities that may benefit biosignature detection, including volumetric, computational, fluorescence, and multispectral imaging (Hand et al. 2017; Nadeau et al. 2018). Significant efforts have been focused on holographic systems to relieve the complications from optical stack alignment that affect microscope operation. In oceanography, underwater microscopic imaging through pressure-rated optical ports is widely used to study planktonic organisms in situ; similarly, a diverse range of techniques have been implemented, including on mobile platforms (Mullen et al. 2019) (Table 2).
A robust microscope that can image through a pressure-rated optical port and achieve submicron resolution is needed for subsurface missions. Such systems should also detect multiple biosignatures and provide a high-volume sampling rate and significant processing to reduce data volume. Digital holographic microscopy (DHM) is a strong candidate technology. The DHM records a light interference pattern, from which it can digitally reconstruct an image at multiple depths. This approach provides a larger imaged volume, allows motility tracking in three dimensions, and eliminates the need for mechanical focusing. Holography can additionally provide light-phase information and the material index of refraction. Multiple light-based techniques can be coupled with the same optical path, such as DHM and light field fluorescence microscopy (Kim 2020; Takahashi et al. 2020).
3.3.3. Micro-Raman Spectrometer
A Raman spectrometer is paired with the optical microscope to noninvasively analyze the sample's chemical composition. Raman spectroscopy measures inelastic light scattering, which occurs when incident photons gain or lose energy as a result of exciting specific vibrational modes in a molecule (e.g., atomic bond stretching and bending). The Raman spectrum records shifts in the illumination wavelength that provide a distinct signature or "fingerprint" of the sample's molecular structure. This technique is contact-free, does not require sample preparation, and can be performed on gas, liquid, or solid phases (Foucher 2019; Du et al. 2020). Depending on the sensitivity, Raman spectroscopy can detect and identify molecules including organics (DNA/RNA bases, amino acids, fatty acids, proteins), pigments (e.g., carotenoids), carbonaceous matter (e.g., kerogen), and minerals (Tarcea et al. 2008; Marshall et al. 2010; Foucher 2019).
The Perseverance Rover mission carries the first two planetary Raman spectrometers (Williford et al. 2018; Bhartia et al. 2021). The standoff Raman (532 nm) on the SuperCam package investigates surface features at a distance and identifies targets of interest for SHERLOC (Wiens et al. 2017). The DUV resonance Raman (248.6 nm) scans rock surfaces (7 × 7 mm area) to look for organic material on the surface of Mars (Beegle et al. 2015). Additionally, the ESA ExoMars mission will carry the Raman Laser Spectrometer (532 nm) to analyze crushed rock samples from a drill system. Underwater Raman systems on Earth (Brewer & Kirkwood 2013; Du et al. 2020; Sobron et al. 2021) have successfully been used for in situ geochemistry studies of the fluids from hydrothermal vents and cold seeps, as well as seafloor rocks and minerals. These systems often use 532 nm lasers and microscopic optics (Brewer et al. 2004; Zhang et al. 2017a; Xi et al. 2018; see Table 2). Raman spectroscopy is also widely used in biological sciences (Butler et al. 2016), including for detailed analysis of single cells (Table 2).
We propose a DUV Raman system (<250 nm) integrated with the optical path of the microscope for life detection (Tarcea et al. 2007; Beegle et al. 2015; Sapers et al. 2019). Raman scattering is a weak process that can be overwhelmed by fluorescence or other noise (Marshall et al. 2010). The signal strength of DUV Raman is enhanced by as much as 4 orders of magnitude due to (1) using a shorter wavelength that increases scattering and (2) having resonance with the absorption band of many relevant aromatic molecules. During submerged operations, the Raman spectral peaks from water can be separated from most chemicals of interest (Brewer et al. 2004). However, spectral peaks can shift slightly with temperature and pressure due to structural changes in the sample molecules (Brewer et al. 2004; Du et al. 2020). It will therefore be important to characterize the system at a range of relevant physical conditions on Earth and bring onboard calibration targets. Here chemical data from the Raman sensor will be correlated with morphological data from the imaging microscope (Beegle et al. 2015; Takahashi et al. 2020). It may also be possible to share the laser and/or spectrometer from phase 1.
3.4. Phase 4: Precision Chemical Measurements of Fluid
In the final payload phase, precision organic and inorganic chemical measurements are made on depressurized and optionally desalted samples. Samples are then split into two paths for analysis by either a nanopore sequencer or capillary electrophoresis (CE) and MS. The CE-MS system provides high-fidelity measurements of the sample's chemical composition, including the presence and distribution of critical organics. The nanopore sequencer operates in parallel and searches for informational biopolymers, which may be a universally essential feature of life. Finally, small volume-processed liquid samples are retained indefinitely within the housing to prevent potential forward contamination and eliminate the need to repressurize, sterilize, filter, and eject samples. In addition to the liquid handling, this phase also includes a membrane inlet that allows dissolved gases to pass from the external environment into the housing for subsequent analysis by MS (e.g., Wankel et al. 2013; Chua et al. 2016). This phase is positioned last in the system architecture, as it has the smallest volume throughput, requires reagents, and is destructive. Prior phases provide a means for sample prioritization to efficiently utilize phase 4 instrumentation.
3.4.1. Depressurization
Precision chemical analysis instruments in phase 4 require direct contact with samples at low pressures (0–10 kPa). A mechanical fluid depressurization stage and a gas membrane inlet introduce liquid and gas samples from a surrounding high-pressure melt pocket or ocean environment into the low-pressure instrument housing. Sample depressurization is potentially destructive to any cells and is energetically expensive; for these reasons, instruments requiring low-pressure samples are grouped into this last payload phase to minimize the possibility for altering sample materials and the volume of samples requiring depressurization.
Several deep ocean underwater MS instruments have been developed using membrane inlet MS (MIMS) to analyze gaseous species. MIMS systems use a semipermeable hydrophobic membrane to pass dissolved gases and small volatile organic molecules into the instrument housing while excluding water (Chua et al. 2016). This simple and effective depressurization method is appropriate for studies of gases, but the technique is not applicable for investigating larger organics like complex biomolecules because only small molecules can pass through such a membrane. Alternatively, liquid samples can be mechanically depressurized in situ. The Deep Environmental Sample Processor (D-ESP) (Pargett et al. 2013; Ussler et al. 2013) demonstrated the ability to depressurize large 10 L water volumes at 4000 m ocean depth prior to analysis within a pressure vessel. This system uses a flexible seawater bladder held in a rigid oil-filled chamber, and high-pressure pumps can move oil in and out of the rigid chamber to control the water bladder pressure.
Mechanical depressurization technology requires further development, particularly for milliliter-scale sample volumes. While available deep-sea depressurization is limited (Pargett et al. 2013; Ussler et al. 2013; Wilcock et al. 2021), other mechanical depressurization techniques can be envisioned, such as a sealed, screw-driven piston that mechanically expands the volume of the high-pressure water. MIMS technology for gaseous samples may require development to characterize membrane function under relevant temperature and chemical environments. Sample depressurization may induce other effects that may need to be considered, such as the precipitation of salts (Marion et al. 2005) and/or ice crystal formation via depressurization-induced supercooling. Dissolved gas exsolution, such as while drilling through clathrate ice (Hand et al. 2006), may also present a challenge. Laboratory tests can help assess whether these will be critical issues and if additional processing elements are needed, such as heaters or a separate pathway to compress and exhaust gases.
3.4.2. Desalting
The vehicle is likely to encounter high-salinity environments (Section 2.1) necessitating additional sample desalting before MS or nanopore analysis. Predictions for bulk salt content in Europa's ice shell and ocean range from brackish to saturation for NaCl or MgSO4, with a potential for meters-thick deposits of precipitates within the ice shell (Buffo et al. 2020; Chivers et al. 2021; Schmidt et al. 2023). High salt concentrations may inhibit downstream measurements, for example, by confounding sample ionization during MS or reducing DNA extraction efficiency during sample preparation for sequencing. Sample desalting is guided by phase 2 measurements to mitigate these challenges if salinity exceeds instrument requirements and enables future options for alternative instrumentation that may be intolerant of salts.
Salt separation techniques include electrodialysis; reverse osmosis; ion-exchange resins; gel filtration chromatography; micro-, ultra-, or nanofiltration; and reverse-phase column chromatography. While filtration techniques concentrate all solutes above the filter cutoff (including salts in some cases), electrodialysis instead selectively removes ions while retaining the majority of organics. Electrodialysis operates through application of an electric potential across a water volume, drawing anions and cations through ion-selective membranes. This technique is uniquely powerful for use in planetary applications, since it can be used to desalt large volumes of water (>1 L) while retaining organic matter (Chambers et al. 2016), and recent efforts have made progress in miniaturizing electrodialysis for milliliter or smaller volumes as required for SSSLOW (Bryson et al. 2020). These miniaturized systems will soon be demonstrated on marine concentrate samples but as yet are reliant on human-in-the-loop processing (Chen et al. 2011; Gumuscu et al. 2016).
Challenges for electrodialysis include the potential for filtration pressures and osmotic stress to be destructive to cellular structures, motivating desalting (Section 3.4.1) only when necessary and downstream of morphology investigations (Section 3.1–3). The choice of fluid into which to concentrate ions removed from the sample is also under investigation (Bryson et al. 2020), as inclusion of an ion-depleted solution (e.g., ultrapure water) would require mass and volume, but using an excess sample at the same initial salt concentration may require more energy or an additional onboard process to adjust temperature (ion solubility) or produce an ion-depleted solution (e.g., by reverse osmosis). An embedded conductivity sensor enables real-time monitoring of electrodialysis processing relative to the initial sample properties measured in phase 2 to determine when the sample has been sufficiently desalted to satisfy phase 4 instrument requirements.
3.4.3. Separation and MS
A combined chemical species separation and MS package will search for biosignatures, including the presence of large complex biomolecules not known to occur abiotically (e.g., DNA, proteins), and specific ordered distributions (e.g., relative abundance, size distributions, chirality) of smaller organics (e.g., amino acids, nucleic acids, fatty acids) that can occur abiotically (Lovelock 1965; Hand et al. 2017). Measurements of inorganic molecules can also be used to assess the environment's habitability and investigate geochemical processes. The MS is specifically selected as a chemical analyzer due to its broad capabilities and significant heritage in both space and ocean science (Chua et al. 2016; Arevalo et al. 2020). Chemical separation stages prior to MS are included for both gas and liquid phases to allow clear identification of a wide range of relevant chemicals. Gas-phase separation is well suited for smaller gases and volatile organics, while liquid-phase separation is better for larger biomolecules such as proteins and amino acids (Willis et al. 2021). Sensitive detection techniques can then follow the separation stage, as discussed below.
Prior planetary exploration has primarily used gas chromatography (GC) for chemical separation of samples (e.g., Niemann et al. 2002; Mahaffy et al. 2012). In pyrolysis GC, after extreme heating of the sample, separation occurs in the gas phase based on the analytes' affinity for the column (e.g., Goesmann et al. 2017). Following separation, gas-phase molecules can be ionized via simple and robust electron ionization (EI; Li et al. 2017; Brinckerhoff et al. 2018) and directed into MS. However, heating during GC can damage nonvolatile organics, and EI will further break up large molecules. Alternatively, liquid-phase separation techniques such as LC, CE, and microchip electrophoresis (ME; e.g., Stockton et al. 2010; Mora et al. 2012, 2020) typically leave large biomolecules intact, critical for astrobiology investigations. While not yet flown on planetary missions, efforts are developing LC-MS (Getty et al. 2013; Southard et al. 2014) and CE-MS (Willis et al. 2019; Creamer et al. 2020) for future missions. Other sensitive detection techniques benefiting from separation stages can be integrated prior to MS. Laser-induced fluorescence (LIF) coupled with CE can nondestructively detect fluorescently tagged amino acids with high sensitivity (5 nM) and measure their relative abundance and chirality (Creamer et al. 2017). Additionally, capacitively coupled contactless conductivity detection (C4D) can be used for identification of inorganic ions and also does not contact or modify the sample. The OCEANS payload under development at NASA JPL combines CE, LIF, C4D, and MS (Willis et al. 2019; Creamer et al. 2020).
Before mass analysis, the sample must be ionized. In benchtop systems, liquid samples are often ionized using electrospray ionization, which is a less destructive soft ionization technique that has not yet flown in space but is in development for surface operations with the OCEANS payload (Willis et al. 2019; Creamer et al. 2020). Following ionization, species are separated in a mass analyzer based on their mass-to-charge ratios (Arevalo et al. 2020). The ESA Rosalind Franklin Mars Rover will fly the first planetary linear ion trap (LIT) mass analyzer, which provides flexibility to utilize two different ionization modes. The rover will be equipped with a GC coupled to the mass spectrometer using EI for separation and detection of volatiles. The other end of the LIT uses laser desorption (LD) ionization coupled to the mass spectrometer, which provides a soft ionization method to preserve higher-order organics that are nonvolatile.
There are also a number of underwater mass spectrometers (reviewed by Chua et al. 2016, Table 2), including systems on robotic vehicles that have tracked hydrocarbon plumes (Camilli et al. 2010) and used integrated GC-MS (Chua et al. 2016). Long-duration deployments of underwater MS in Earth's oceans demonstrates the low-power capabilities and vacuum systems that will be required for subsurface planetary operations. However, to the best of our knowledge, all submersible MS systems to date have used membrane inlets (Table 2) that are only permeable to gases and volatile organics, not large biomolecules.
The SSSLOW needs to be able to analyze liquid and gas samples, detect a wide range of different potential biomolecules, and achieve high sensitivity in the case of low biosignature density. Chemical analysis systems such as OCEANS, EMILI, and others in development will achieve these capabilities for landed life detection missions to ocean worlds (e.g., Brinckerhoff et al. 2018; Willis et al. 2019; Brinckerhoff et al. 2019; Creamer et al. 2020). These systems may be adaptable for subsurface operation in a pressure vessel. For increased sensitivity, CE-LIF (Creamer et al. 2017) should be considered for integrating with the liquid separation phase, and C4D would give further insight into the ionic composition of Europa's ocean.
3.4.4. Nanopore Sequencing
A nanopore sequencer is included in the payload to search for chains of nucleic acids or other informational biopolymer molecules. These are a requisite feature of biology as we know it, and the detection of analogous molecules on another world would be strong evidence of life. Sequencing instruments can require as little as tens of microliters of fluid input, but for environmental samples, this is typically a purified extract concentrated from a much larger initial sample volume. In situ filtration of tens to hundreds of liters of water is a commonly employed concentration technique in Earth's ocean, for example, through boreholes under ice (e.g., Vick-Majors et al. 2016) or with vehicle-borne samplers (Table 2). The potential for extant life in Europa's subsurface makes sequencing an exciting investigation, further benefited by an abundance of liquid sample volume available for preconcentration. Technologies in development, such as solid-state nanopores and nanogaps, may enable near-reagent-free detection and more agnostic approaches.
Instruments such as the Oxford Nanopore Technologies MinIONTM are compact, low-mass (<100 g), low-power (1–2 W) tools commonly used to sequence DNA (Deamer et al. 2016) and RNA (Garalde et al. 2018), with ongoing efforts for protein (Restrepo-Perez et al. 2018), amino acid (Ouldali et al. 2020), and XNA detection (Carr 2016). Recent developments have attained DNA detection limits of <5 pg, theoretically requiring a 5%–10% extraction yield from ∼104 total cells (Carr et al. 2019). At Enceladus, MacKenzie et al. (2021) similarly found that sample volumes of ∼30 ml may be sufficient for a positive detection of life at abundances of at least 10 cells ml–1. During nanopore sequencing, target molecules are driven by a voltage potential through protein nanopores embedded in a high-resistance membrane, and small variations in the ionic current through each pore are associated with base sequences (basecalling). In addition to the presence and abundance of informational polymers, sequencing could enable a variety of astrobiological investigations, such as bounding the biochemical system complexity by the diversity of observed repeating bases (Carr et al. 2017), genome construction, and insight into possible evolution by detection of conserved patterns with variability (Saboda et al. 2019). In environments where low pH or other chemical conditions suggest short residence times, the detection of informational polymers may also suggest ongoing synthesis by life. Importantly, sequencing additionally provides a means to rule out possible forward contamination by comparing putative life to terrestrial life and clean-room databases (Carr et al. 2016; Pontefract et al. 2018).
In SSSLOW, following concentration (Section 3.3.1) and desalting (Section 3.4.2), cells are disrupted (lysed), and the target informational polymer is purified. Reagent-free mechanical lysis techniques (e.g., OmniLyse®) are effective for organisms with tough outer structures, such as spores with higher demonstrated yields relative to acoustic or chemical techniques (Carr et al. 2016; Craft et al. 2021). Subsequent DNA purification from the lysate can be accomplished by selective binding and elution, and for DNA, prior desalting reduces competitive binding by salts to improve yield (Carr et al. 2017; Mojarro et al. 2017, 2019). Low sample and waste volumes of <5 ml run–1 enable long-term retention of processed fluids and organic reagents for planetary protection.
Environmental genomics capabilities are rapidly advancing in Earth and ocean science (Thomsen & Willerslev 2015) with a push toward in situ capabilities. The submersible Environmental Sample Profiler (ESP) is capable of performing qPCR and array hybridization underwater (Scholin et al. 2017), and a number of systems can perform autonomous sample filtration and preservation for genomic analysis (Table 2). Beyond Earth, nanopore sequencing is in use on the International Space Station (Parra et al. 2017), and low-Earth orbit cellular evolution experiments are planned with incubation, sample prep, and sequencing hardware in a 4U CubeSat form factor (Saboda et al. 2019). A complete sample processing and nanopore sequencing payload is also in development for Martian surface sampling (e.g., Carr et al. 2016; Mojarro et al. 2017; Carr et al. 2017, 2020), and for deep space applications, efforts are ongoing to determine the radiation tolerance of both nanopores and lyophilized reagents (Carr et al. 2013; Pontefract et al. 2018; Sutton et al. 2019).
Other challenges include high data volumes—sequencing can generate raw data up to GB s-1 (Carr et al. 2016)—but the data are compressible to ∼200 MB hr–1 of sequencing (Saboda et al. 2019), and strategies to further reduce volumes in consideration for surface planetary missions (MacKenzie et al. 2021) will benefit low-bandwidth subsurface exploration. Synthetic or solid-state nanopores (Goto et al. 2020) are also in development for improved tolerance to freezing, radiation, and preflight sterilization to further enable long-duration astrobiology missions (Bywaters et al. 2017). Solid-state approaches have not yet achieved single base resolution even with high (MHz) bandwidth (e.g., Shekar et al. 2016), but solid-state alternatives using quantum electron tunneling nanogaps that can achieve base-level resolution (Ohshiro et al. 2012) and detect single amino acids (Ohshiro et al. 2014) are in development for space applications (Carr et al.2020).
4. Subsurface Science Augmentations and Broader Perspectives
4.1. Deep Ocean Profiling
There is significant trade space for science operations upon reaching the ocean. Options include vertical water column profiling (e.g., Toole et al. 2011; Schmidt et al. 2023), deployment of an instrumented under-ice mooring (e.g., Stevens et al. 2020), or the release of autonomous floats, gliders, or vehicles (Wirtz & Hildebrandt 2016; Cwik et al. 2019; Aguzzi et al. 2020; Dachwald et al. 2020). However, with limited payload volume and power, the challenges of underwater navigation and communication, and poorly constrained Europan ocean density and current speeds, it will be difficult to profile farther than a few hundred meters from the base of the ice shell with any of these techniques—potentially less than 1% of Europa's full ocean depth.
For deeper understanding, the subsurface vehicle could release expendable CTDs (XCTDs), small (<1 L volume) sondes that relay data over a thin electrical conductor as they freefall through the water column. The XCTDs are commonly deployed by ships and have been adapted for deployment from submarines working under Arctic ice, where they enable profiling to multiple km depths (Keigwin & Johnson 1992; Sambrotto et al. 2013).
An untethered sonde with a low-frequency acoustic link to the vehicle anchored in the ice base could provide even deeper profiling data. Desirable measurements through the water column in addition to CTD data include turbidity and redox to enable detection of possible hydrothermal plumes (e.g., the Miniature Autonomous Plume Recorder; Baker 1997; see also German et al. 1998). Long-range acoustic links demonstrated on Earth have reached several bits per second over horizontal distances in excess of 400 km (Freitag et al. 2015) and hundreds of bits per second down to full ocean depth (Singh et al. 2009; Roberts et al. 2012), but vertical ranges in the Europan ocean will depend strongly on water column structure. Should a sonde be able to communicate from the seafloor, a time series of benthic boundary layer properties and tidal (pressure) variation could be relayed until the battery was exhausted. If the sonde embedded in sediment, it might also be possible to make a direct seafloor heat flux measurement to bound geologic activity (e.g., Fisher et al. 2015). Acoustic tomography between the vehicle and sonde during data relay could also enable continued measurements of bulk water column properties. Passive acoustic monitoring, as detailed in Aguzzi et al. (2020) for application to Enceladus, could also be used to monitor for nearby seafloor vent and tectonic activity (Crone et al. 2006).
4.2. Relevant Geologic Drilling Technologies
The petroleum and mineral exploration industries have developed a variety of in situ measuring-while-drilling (MWD) borehole instruments for high pressures (<200 MPa), low pH (2–5), and high-vibration environments. MWD instruments can measure sidewall density, formation resistivity, seismic propagation, and a variety of fluid properties (Gooneratne et al. 2019). A continuous profile of sidewall properties through Europa's ice shell would contribute significantly to our understanding of ice shell structure, transport processes, and formation history.
Sidewall ice density can be mapped with active gamma-ray sources such as the hostile environment lithodensity sonde instrument, which determines bulk density from the energy of backscattered rays (Major et al. 1998; Gooneratne et al. 2019). Neutron scattering was first translated from geology to glaciology in the early 1950s (Leighty 1965), and advances in recent years permit continuous sidewall density logging at centimeter-scale vertical resolutions (Morris & Cooper 2003). Active EM propagation instruments can profile dielectric properties in an adjacent formation (Gooneratne et al. 2019), potentially enabling far-field observations in an ice borehole beyond the thermally modified sidewall surface. Active acoustic techniques map geologic formation structure and elastic properties (Gooneratne et al. 2019), and seismic investigations in ice have been used in combination with ice density profiles to determine elastic ice properties and crystal orientation since the early 1960s (Thiel & Ostenso 1961). In situ seismic logging tools are also used for investigation of ice fabric (Gusmeroli et al. 2012).
Downhole fluid analysis (DFA) packages for in situ sampling and flow-through chemical measurements are also employed in high-pressure petroleum exploration and production (Creek et al. 2010). Similar to the integrated sensor suite proposed in SSSLOW, DFA enables real-time determination of a variety of fluid properties, including temperature, pressure, pH, gas concentrations, density, and fluorescence (Creek et al. 2010; Fujisawa & Yamate 2013; Schlumberger In Situ Fluid Analyzer; Gooneratne et al. 2019). Fluids can also be extracted from porous formations directly through the sidewall (e.g., Schlumberger's Saturn Radial Probe). Logging instrumentation developed for detection of organics and microbial life in oceanic crust (Salas et al. 2015) has also been recently translated to ice (Eshelman et al. 2019). While MWD instruments have been developed for significantly different material and temperature regimes than the Europan ice shell, these technologies may inform the development of future subsurface planetary instruments.
4.3. Broader Ocean World Applications
Our focus on Europa's subsurface in this work reflects broader community priorities (e.g., Hendrix et al. 2019; Moore et al. 2020; Howell et al. 2021; Schmidt et al. 2021a), but the science objectives and conceptual life detection payload presented are extensible to other worlds with frozen or liquid water suspected to harbor past or extant life.
Additional targets for subsurface astrobiological investigation include Enceladus and the Martian ice caps. At Enceladus, NASA's Cassini spacecraft detected organics, salts, and hydrothermal alteration products in plume materials venting from subsurface reservoirs, suggesting a possibly habitable interior ocean (Postberg et al. 2011; Hsu et al. 2015; Waite et al. 2017). Subsequent mission concept studies have demonstrated the feasibility for orbital missions (Reh et al. 2016; Mitri et al. 2018), combination orbiter/lander missions (MacKenzie et al. 2021), ocean access via active plumes (Carpenter et al. 2021), and instrumented platforms (Aguzzi et al. 2020) to characterize the environment and search for life. Enceladus's unique plume dynamics may provide a natural means for preliminary sampling of subsurface materials without requiring a subsurface mission. However, the payload concepts presented here would support ensuing missions seeking less modified organic materials or extant life in the ice and ocean below.
Mars is a relict ocean world with kilometers-thick polar ice caps and subsurface ice deposits at lower latitudes. These reservoirs may preserve signatures of past life in layers of water ice (e.g., Westall et al. 2015; Hays et al. 2017; Perl & Baxter 2020; Perl et al. 2021). Orbital radars have operated at Mars for decades (Stamenković et al. 2019; Wray 2021), and some interpretations suggest the possibility of subglacial liquid water below the southern ice cap (Orosei et al. 2018) although a debate has ensued (Schroeder & Steinbrügge 2021). With these reconnaissance data and the additional benefits of an atmosphere, short cruise periods relative to the outer systems, and decades of contextual understanding, Mars is also a priority target for life detection and cryobot exploration and technology advancement. While NASA has presently prioritized near-surface drilling (<1 m) sample return (Beaty et al. 2019), kilometer-scale drills are under study (Stamenković et al. 2019) and the Martian subsurface remains a target for astrobiology (Elliot & Carsey 2004; Onstott et al. 2019; Albert & Koutnik 2021). The SSSLOW payload concept could also enable life detection and vertical compositional characterization of Martian ice and, if present, subglacial liquid water.
4.4. Closing
We have described a life detection payload for a future subsurface ocean world mission, highlighting the engineering and science trades unique to submerged science. We find that close integration between a suite of complementary instrumentation and sample handling technology is important to meet the challenges specific to the search for biosignatures at higher pressures in the subsurface of an ocean world, and that many enabling technologies are already in use or development for oceanographic and space science. Continued advancement of fluidic sample processing systems and life detection instrumentation together can make many of the subsurface science objectives discussed here possible at Europa. These concomitant goals support the establishment of formal working groups composed of a diverse group of Earth and space scientists and engineers to further define the scientific priorities for subsurface ocean world exploration. Continued SSSLOW and steady progress will enable the search for life beyond Earth in the ocean frontiers of our solar system.
This payload concept was developed by a broad, student-led team supported by NASA SESAME 80NSSC19K0615, PI: B. E. Schmidt. We acknowledge contributions and guidance from the full VERNE concept team and coinvestigators and helpful insights and suggestions from two anonymous reviewers. We are grateful for additional conversations and guidance from Mark Fox-Powell, Shannon MacKenzie, Peter Girguis, Aaron Noell, Heather Graham, Ziqin Ni, K. Marshall Seaton, and Morgan Cable during the evolution of this payload concept study.