Proliferating cell nuclear antigen
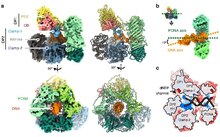
Proliferating cell nuclear antigen (PCNA) is a DNA clamp that acts as a processivity factor for DNA polymerase δ in eukaryotic cells and is essential for replication. PCNA is a homotrimer and achieves its processivity by encircling the DNA, where it acts as a scaffold to recruit proteins involved in DNA replication, DNA repair, chromatin remodeling and epigenetics.[5]
Many proteins interact with PCNA via the two known PCNA-interacting motifs PCNA-interacting peptide (PIP) box[6] and AlkB homologue 2 PCNA interacting motif (APIM).[7] Proteins binding to PCNA via the PIP-box are mainly involved in DNA replication whereas proteins binding to PCNA via APIM are mainly important in the context of genotoxic stress.[8]
Function
[edit]The protein encoded by this gene is found in the nucleus and is a cofactor of DNA polymerase delta. The encoded protein acts as a homotrimer and helps increase the processivity of leading strand synthesis during DNA replication. In response to DNA damage, this protein is ubiquitinated and is involved in the RAD6-dependent DNA repair pathway. Two transcript variants encoding the same protein have been found for this gene. Pseudogenes of this gene have been described on chromosome 4 and on the X chromosome.[9]
PCNA is also found in archaea, as a processivity factor of polD, the single multi-functional DNA polymerase in this domain of life.[10]
Expression in the nucleus during DNA synthesis
[edit]PCNA was originally identified as an antigen that is expressed in the nuclei of cells during the DNA synthesis phase of the cell cycle.[11] Part of the protein was sequenced and that sequence was used to allow isolation of a cDNA clone.[12] PCNA helps hold DNA polymerase delta (Pol δ) to DNA. PCNA is clamped[13] to DNA through the action of replication factor C (RFC),[14] which is a heteropentameric member of the AAA+ class of ATPases. Expression of PCNA is under the control of E2F transcription factor-containing complexes.[15] [16]
Role in DNA repair
[edit]Since DNA polymerase epsilon is involved in resynthesis of excised damaged DNA strands during DNA repair, PCNA is important for both DNA synthesis and DNA repair.[17][18]
PCNA is also involved in the DNA damage tolerance pathway known as post-replication repair (PRR).[19] In PRR, there are two sub-pathways: (1) a translesion synthesis pathway, which is carried out by specialised DNA polymerases that are able to incorporate damaged DNA bases into their active sites (unlike the normal replicative polymerase, which stall), and hence bypass the damage, and (2) a proposed "template switch" pathway that is thought to involve damage bypass by recruitment of the homologous recombination machinery. PCNA is pivotal to the activation of these pathways and the choice as to which pathway is utilised by the cell. PCNA becomes post-translationally modified by ubiquitin.[20] Mono-ubiquitin of lysine number 164 on PCNA activates the translesion synthesis pathway. Extension of this mono-ubiquitin by a non-canonical lysine-63-linked poly-ubiquitin chain on PCNA[20] is thought to activate the template switch pathway. Furthermore, sumoylation (by small ubiquitin-like modifier, SUMO) of PCNA lysine-164 (and to a lesser extent, lysine-127) inhibits the template switch pathway.[20] This antagonistic effect occurs because sumoylated PCNA recruits a DNA helicase called Srs2,[21] which has a role in disrupting Rad51 nucleoprotein filaments fundamental for initiation of homologous recombination.
PCNA-binding proteins
[edit]PCNA interacts with many proteins.[22]
- Apoptotic factors
- ATPases
- Base excision repair enzymes
- Cell-cycle regulators
- Chromatin remodeling factor
- Clamp loader
- Cohesin
- DNA ligase
- DNA methyltransferase
- DNA polymerases
- E2 SUMO-conjugating enzyme
- E3 ubiquitin ligases
- Flap endonuclease
- Helicases
- Histone acetyltransferase
- Histone chaperone
- Histone deacetylase
- Mismatch repair enzymes
- Licensing factor
- NKp44 receptor
- Nucleotide excision repair enzyme
- Poly ADP ribose polymerase
- Procaspases[23]
- Protein kinases
- TCP protein domain
- Topoisomerase
Interactions
[edit]PCNA has been shown to interact with:
- Annexin A2[24]
- CAF-1[25][26][27]
- CDC25C[28]
- CHTF18[24]
- Cyclin D1[29][30]
- Cyclin O[24][31]
- Cyclin-dependent kinase 4[30][32]
- Cyclin-dependent kinase inhibitor 1C[33]
- DNMT1[34][35][36]
- EP300[37]
- Establishment of Sister Chromatid Cohesion 2[38]
- Flap structure-specific endonuclease 1[39][40][41][42][43][44][45]
- GADD45A[46][47][48][49][50]
- GADD45G[51][52]
- HDAC1[53]
- HUS1[54]
- ING1[55]
- KCTD13[56]
- KIAA0101[45]
- Ku70[24][57]
- Ku80[24][57][58]
- MCL1[59]
- MSH3[24][60][61]
- MSH6[24][60][61]
- MUTYH[62]
- P21[33][41][45][63][64][65][66][67]
- POLD2[68]
- POLD3[24][69]
- POLDIP2[70]
- POLH[71]
- POLL[72][73][74]
- RFC1[24][63][75][76][77]
- RFC2[24][78][79]
- RFC3[24][80]
- RFC4[24][78]
- RFC5[24][76][78]
- Ubiquitin C[81][82][83]
- Werner syndrome ATP-dependent helicase[84][85]
- XRCC1[86]
- Y box binding protein 1[87]
Proteins interacting with PCNA via APIM include human AlkB homologue 2, TFIIS-L, TFII-I, Rad51B,[7] XPA,[88] ZRANB3,[89] and FBH1.[90]
Uses
[edit]Antibodies against proliferating cell nuclear antigen (PCNA) or monoclonal antibody termed Ki-67 can be used for grading of different neoplasms, e.g. astrocytoma. They can be of diagnostic and prognostic value. Imaging of the nuclear distribution of PCNA (via antibody labeling) can be used to distinguish between early, mid and late S phase of the cell cycle.[91] However, an important limitation of antibodies is that cells need to be fixed leading to potential artifacts.
On the other hand, the study of the dynamics of replication and repair in living cells can be done by introducing translational fusions of PCNA. To eliminate the need for transfection and bypass the problem of difficult to transfect and/or short lived cells, cell permeable replication and/or repair markers can be used. These peptides offer the distinct advantage that they can be used in situ in living tissue and even distinguish cells undergoing replication from cells undergoing repair.[92]
caPCNA, a post-translationally modified isoform of PCNA common in cancer cells, is a potential therapeutic target in cancer therapy.[93][94] In 2023 City of Hope National Medical Center published preclinical research on a targeted chemotherapy using AOH1996 that appears to suppress tumor growth without causing discernable side effects.[95]
See also
[edit]- Ki-67 – cellular marker for proliferation
- Transcription
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000132646 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000027342 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Moldovan GL, Pfander B, Jentsch S (May 2007). "PCNA, the maestro of the replication fork". Cell. 129 (4): 665–679. doi:10.1016/j.cell.2007.05.003. PMID 17512402. S2CID 3547069.
- ^ Warbrick E (March 1998). "PCNA binding through a conserved motif". BioEssays. 20 (3): 195–199. doi:10.1002/(sici)1521-1878(199803)20:3<195::aid-bies2>3.0.co;2-r. PMID 9631646.
- ^ a b Gilljam KM, Feyzi E, Aas PA, Sousa MM, Müller R, Vågbø CB, et al. (September 2009). "Identification of a novel, widespread, and functionally important PCNA-binding motif". The Journal of Cell Biology. 186 (5): 645–654. doi:10.1083/jcb.200903138. PMC 2742182. PMID 19736315.
- ^ Mailand N, Gibbs-Seymour I, Bekker-Jensen S (May 2013). "Regulation of PCNA-protein interactions for genome stability". Nature Reviews. Molecular Cell Biology. 14 (5): 269–282. doi:10.1038/nrm3562. PMID 23594953. S2CID 25952152.
- ^ "Entrez Gene: PCNA proliferating cell nuclear antigen".
- ^ Madru C, Henneke G, Raia P, Hugonneau-Beaufet I, Pehau-Arnaudet G, England P, et al. (March 2020). "Structural basis for the increased processivity of D-family DNA polymerases in complex with PCNA". Nature Communications. 11 (1): 1591. Bibcode:2020NatCo..11.1591M. doi:10.1038/s41467-020-15392-9. PMC 7101311. PMID 32221299.
- ^ Leonardi E, Girlando S, Serio G, Mauri FA, Perrone G, Scampini S, et al. (May 1992). "PCNA and Ki67 expression in breast carcinoma: correlations with clinical and biological variables". Journal of Clinical Pathology. 45 (5): 416–419. doi:10.1136/jcp.45.5.416. PMC 495304. PMID 1350788.
- ^ Matsumoto K, Moriuchi T, Koji T, Nakane PK (March 1987). "Molecular cloning of cDNA coding for rat proliferating cell nuclear antigen (PCNA)/cyclin". The EMBO Journal. 6 (3): 637–642. doi:10.1002/j.1460-2075.1987.tb04802.x. PMC 553445. PMID 2884104.
- ^ Bowman GD, O'Donnell M, Kuriyan J (June 2004). "Structural analysis of a eukaryotic sliding DNA clamp-clamp loader complex". Nature. 429 (6993): 724–730. Bibcode:2004Natur.429..724B. doi:10.1038/nature02585. PMID 15201901. S2CID 4346799.
- ^ Zhang G, Gibbs E, Kelman Z, O'Donnell M, Hurwitz J (March 1999). "Studies on the interactions between human replication factor C and human proliferating cell nuclear antigen". Proceedings of the National Academy of Sciences of the United States of America. 96 (5): 1869–1874. Bibcode:1999PNAS...96.1869Z. doi:10.1073/pnas.96.5.1869. PMC 26703. PMID 10051561.
- ^ Egelkrout EM, Mariconti L, Settlage SB, Cella R, Robertson D, Hanley-Bowdoin L (December 2002). "Two E2F elements regulate the proliferating cell nuclear antigen promoter differently during leaf development". The Plant Cell. 14 (12): 3225–3236. doi:10.1105/tpc.006403. PMC 151214. PMID 12468739.
- ^ Nikolai BC, Lanz RB, York B, Dasgupta S, Mitsiades N, Creighton CJ, et al. (March 2016). "HER2 Signaling Drives DNA Anabolism and Proliferation through SRC-3 Phosphorylation and E2F1-Regulated Genes". Cancer Research. 76 (6): 1463–1475. doi:10.1158/0008-5472.CAN-15-2383. PMC 4794399. PMID 26833126.
- ^ Shivji KK, Kenny MK, Wood RD (April 1992). "Proliferating cell nuclear antigen is required for DNA excision repair". Cell. 69 (2): 367–374. doi:10.1016/0092-8674(92)90416-A. PMID 1348971. S2CID 12260457.
- ^ Essers J, Theil AF, Baldeyron C, van Cappellen WA, Houtsmuller AB, Kanaar R, Vermeulen W (November 2005). "Nuclear dynamics of PCNA in DNA replication and repair". Molecular and Cellular Biology. 25 (21): 9350–9359. doi:10.1128/MCB.25.21.9350-9359.2005. PMC 1265825. PMID 16227586.
- ^ Lehmann AR, Fuchs RP (December 2006). "Gaps and forks in DNA replication: Rediscovering old models" (PDF). DNA Repair. 5 (12): 1495–1498. doi:10.1016/j.dnarep.2006.07.002. PMID 16956796.
- ^ a b c Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S (September 2002). "RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO". Nature. 419 (6903): 135–141. Bibcode:2002Natur.419..135H. doi:10.1038/nature00991. PMID 12226657. S2CID 205209495.
- ^ Pfander B, Moldovan GL, Sacher M, Hoege C, Jentsch S (July 2005). "SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase". Nature. 436 (7049): 428–433. Bibcode:2005Natur.436..428P. doi:10.1038/nature03665. PMID 15931174. S2CID 4316517.
- ^ Moldovan GL, Pfander B, Jentsch S (May 2007). "PCNA, the maestro of the replication fork". Cell. 129 (4): 665–679. doi:10.1016/j.cell.2007.05.003. PMID 17512402. S2CID 3547069.
- ^ Witko-Sarsat V, Mocek J, Bouayad D, Tamassia N, Ribeil JA, Candalh C, et al. (November 2010). "Proliferating cell nuclear antigen acts as a cytoplasmic platform controlling human neutrophil survival". The Journal of Experimental Medicine. 207 (12): 2631–2645. doi:10.1084/jem.20092241. PMC 2989777. PMID 20975039.
- ^ a b c d e f g h i j k l m Ohta S, Shiomi Y, Sugimoto K, Obuse C, Tsurimoto T (October 2002). "A proteomics approach to identify proliferating cell nuclear antigen (PCNA)-binding proteins in human cell lysates. Identification of the human CHL12/RFCs2-5 complex as a novel PCNA-binding protein". J. Biol. Chem. 277 (43): 40362–7. doi:10.1074/jbc.M206194200. PMID 12171929.
- ^ Zhang K, Gao Y, Li J, Burgess R, Han J, Liang H, et al. (June 2016). "A DNA binding winged helix domain in CAF-1 functions with PCNA to stabilize CAF-1 at replication forks". Nucleic Acids Research. 44 (11): 5083–5094. doi:10.1093/nar/gkw106. PMC 4914081. PMID 26908650.
- ^ Moggs JG, Grandi P, Quivy JP, Jónsson ZO, Hübscher U, Becker PB, Almouzni G (February 2000). "A CAF-1-PCNA-mediated chromatin assembly pathway triggered by sensing DNA damage". Molecular and Cellular Biology. 20 (4): 1206–1218. doi:10.1128/mcb.20.4.1206-1218.2000. PMC 85246. PMID 10648606.
- ^ Rolef Ben-Shahar T, Castillo AG, Osborne MJ, Borden KL, Kornblatt J, Verreault A (December 2009). "Two fundamentally distinct PCNA interaction peptides contribute to chromatin assembly factor 1 function". Molecular and Cellular Biology. 29 (24): 6353–6365. doi:10.1128/MCB.01051-09. PMC 2786881. PMID 19822659.
- ^ Kawabe T, Suganuma M, Ando T, Kimura M, Hori H, Okamoto T (March 2002). "Cdc25C interacts with PCNA at G2/M transition". Oncogene. 21 (11): 1717–1726. doi:10.1038/sj.onc.1205229. PMID 11896603.
- ^ Matsuoka S, Yamaguchi M, Matsukage A (April 1994). "D-type cyclin-binding regions of proliferating cell nuclear antigen". The Journal of Biological Chemistry. 269 (15): 11030–11036. doi:10.1016/S0021-9258(19)78087-9. PMID 7908906.
- ^ a b Xiong Y, Zhang H, Beach D (August 1993). "Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation". Genes & Development. 7 (8): 1572–1583. doi:10.1101/gad.7.8.1572. PMID 8101826.
- ^ Otterlei M, Warbrick E, Nagelhus TA, Haug T, Slupphaug G, Akbari M, et al. (July 1999). "Post-replicative base excision repair in replication foci". The EMBO Journal. 18 (13): 3834–3844. doi:10.1093/emboj/18.13.3834. PMC 1171460. PMID 10393198.
- ^ Serrano M, Hannon GJ, Beach D (December 1993). "A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4". Nature. 366 (6456): 704–707. Bibcode:1993Natur.366..704S. doi:10.1038/366704a0. PMID 8259215. S2CID 4368128.
- ^ a b Watanabe H, Pan ZQ, Schreiber-Agus N, DePinho RA, Hurwitz J, Xiong Y (February 1998). "Suppression of cell transformation by the cyclin-dependent kinase inhibitor p57KIP2 requires binding to proliferating cell nuclear antigen". Proceedings of the National Academy of Sciences of the United States of America. 95 (4): 1392–1397. Bibcode:1998PNAS...95.1392W. doi:10.1073/pnas.95.4.1392. PMC 19016. PMID 9465025.
- ^ Rountree MR, Bachman KE, Baylin SB (July 2000). "DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci". Nature Genetics. 25 (3): 269–277. doi:10.1038/77023. PMID 10888872. S2CID 26149386.
- ^ Iida T, Suetake I, Tajima S, Morioka H, Ohta S, Obuse C, Tsurimoto T (October 2002). "PCNA clamp facilitates action of DNA cytosine methyltransferase 1 on hemimethylated DNA". Genes to Cells. 7 (10): 997–1007. doi:10.1046/j.1365-2443.2002.00584.x. PMID 12354094. S2CID 25310911.
- ^ Chuang LS, Ian HI, Koh TW, Ng HH, Xu G, Li BF (September 1997). "Human DNA-(cytosine-5) methyltransferase-PCNA complex as a target for p21WAF1". Science. 277 (5334): 1996–2000. doi:10.1126/science.277.5334.1996. PMID 9302295.
- ^ Hasan S, Hassa PO, Imhof R, Hottiger MO (March 2001). "Transcription coactivator p300 binds PCNA and may have a role in DNA repair synthesis". Nature. 410 (6826): 387–391. Bibcode:2001Natur.410..387H. doi:10.1038/35066610. PMID 11268218. S2CID 2129847.
- ^ Bender D, De Silva E, Chen J, Poss A, Gawey L, Rulon Z, Rankin, S (December 2019). "Multivalent interaction of ESCO2 with replication machinery is required for sister chromatid cohesion in vertebrates". Proc. Natl. Acad. Sci. U.S.A. 117 (2): 1081–1089. doi:10.1073/pnas.1911936117. PMC 6969535. PMID 31879348.
- ^ Henneke G, Koundrioukoff S, Hübscher U (July 2003). "Phosphorylation of human Fen1 by cyclin-dependent kinase modulates its role in replication fork regulation". Oncogene. 22 (28): 4301–13. doi:10.1038/sj.onc.1206606. PMID 12853968.
- ^ Hasan S, Stucki M, Hassa PO, Imhof R, Gehrig P, Hunziker P, Hübscher U, Hottiger MO (June 2001). "Regulation of human flap endonuclease-1 activity by acetylation through the transcriptional coactivator p300". Mol. Cell. 7 (6): 1221–31. doi:10.1016/s1097-2765(01)00272-6. PMID 11430825.
- ^ a b Jónsson ZO, Hindges R, Hübscher U (April 1998). "Regulation of DNA replication and repair proteins through interaction with the front side of proliferating cell nuclear antigen". EMBO J. 17 (8): 2412–25. doi:10.1093/emboj/17.8.2412. PMC 1170584. PMID 9545252.
- ^ Gary R, Ludwig DL, Cornelius HL, MacInnes MA, Park MS (September 1997). "The DNA repair endonuclease XPG binds to proliferating cell nuclear antigen (PCNA) and shares sequence elements with the PCNA-binding regions of FEN-1 and cyclin-dependent kinase inhibitor p21". J. Biol. Chem. 272 (39): 24522–9. doi:10.1074/jbc.272.39.24522. PMID 9305916.
- ^ Chen U, Chen S, Saha P, Dutta A (October 1996). "p21Cip1/Waf1 disrupts the recruitment of human Fen1 by proliferating-cell nuclear antigen into the DNA replication complex". Proceedings of the National Academy of Sciences of the United States of America. 93 (21): 11597–11602. Bibcode:1996PNAS...9311597C. doi:10.1073/pnas.93.21.11597. PMC 38103. PMID 8876181.
- ^ Dianova II, Bohr VA, Dianov GL (October 2001). "Interaction of human AP endonuclease 1 with flap endonuclease 1 and proliferating cell nuclear antigen involved in long-patch base excision repair". Biochemistry. 40 (42): 12639–12644. doi:10.1021/bi011117i. PMID 11601988.
- ^ a b c Yu P, Huang B, Shen M, Lau C, Chan E, Michel J, et al. (January 2001). "p15(PAF), a novel PCNA associated factor with increased expression in tumor tissues". Oncogene. 20 (4): 484–489. doi:10.1038/sj.onc.1204113. PMID 11313979.
- ^ Smith ML, Chen IT, Zhan Q, Bae I, Chen CY, Gilmer TM, Kastan MB, O'Connor PM, Fornace AJ (November 1994). "Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen". Science (Submitted manuscript). 266 (5189): 1376–80. Bibcode:1994Sci...266.1376S. doi:10.1126/science.7973727. PMID 7973727.
- ^ Chen IT, Smith ML, O'Connor PM, Fornace AJ (November 1995). "Direct interaction of Gadd45 with PCNA and evidence for competitive interaction of Gadd45 and p21Waf1/Cip1 with PCNA". Oncogene. 11 (10): 1931–7. PMID 7478510.
- ^ Vairapandi M, Azam N, Balliet AG, Hoffman B, Liebermann DA (June 2000). "Characterization of MyD118, Gadd45, and proliferating cell nuclear antigen (PCNA) interacting domains. PCNA impedes MyD118 AND Gadd45-mediated negative growth control". J. Biol. Chem. 275 (22): 16810–9. doi:10.1074/jbc.275.22.16810. PMID 10828065.
- ^ Hall PA, Kearsey JM, Coates PJ, Norman DG, Warbrick E, Cox LS (June 1995). "Characterisation of the interaction between PCNA and Gadd45". Oncogene. 10 (12): 2427–33. PMID 7784094.
- ^ Yang Q, Manicone A, Coursen JD, Linke SP, Nagashima M, Forgues M, Wang XW (November 2000). "Identification of a functional domain in a GADD45-mediated G2/M checkpoint". J. Biol. Chem. 275 (47): 36892–8. doi:10.1074/jbc.M005319200. PMID 10973963.
- ^ Azam N, Vairapandi M, Zhang W, Hoffman B, Liebermann DA (January 2001). "Interaction of CR6 (GADD45gamma ) with proliferating cell nuclear antigen impedes negative growth control". J. Biol. Chem. 276 (4): 2766–74. doi:10.1074/jbc.M005626200. PMID 11022036.
- ^ Nakayama K, Hara T, Hibi M, Hirano T, Miyajima A (August 1999). "A novel oncostatin M-inducible gene OIG37 forms a gene family with MyD118 and GADD45 and negatively regulates cell growth". J. Biol. Chem. 274 (35): 24766–72. doi:10.1074/jbc.274.35.24766. PMID 10455148.
- ^ Milutinovic S, Zhuang Q, Szyf M (June 2002). "Proliferating cell nuclear antigen associates with histone deacetylase activity, integrating DNA replication and chromatin modification". J. Biol. Chem. 277 (23): 20974–8. doi:10.1074/jbc.M202504200. PMID 11929879.
- ^ Komatsu K, Wharton W, Hang H, Wu C, Singh S, Lieberman HB, Pledger WJ, Wang HG (November 2000). "PCNA interacts with hHus1/hRad9 in response to DNA damage and replication inhibition". Oncogene. 19 (46): 5291–7. doi:10.1038/sj.onc.1203901. PMID 11077446.
- ^ Scott M, Bonnefin P, Vieyra D, Boisvert FM, Young D, Bazett-Jones DP, Riabowol K (October 2001). "UV-induced binding of ING1 to PCNA regulates the induction of apoptosis". J. Cell Sci. 114 (Pt 19): 3455–62. doi:10.1242/jcs.114.19.3455. PMID 11682605.
- ^ He H, Tan CK, Downey KM, So AG (October 2001). "A tumor necrosis factor alpha- and interleukin 6-inducible protein that interacts with the small subunit of DNA polymerase delta and proliferating cell nuclear antigen". Proc. Natl. Acad. Sci. U.S.A. 98 (21): 11979–84. Bibcode:2001PNAS...9811979H. doi:10.1073/pnas.221452098. PMC 59753. PMID 11593007.
- ^ a b Balajee AS, Geard CR (March 2001). "Chromatin-bound PCNA complex formation triggered by DNA damage occurs independent of the ATM gene product in human cells". Nucleic Acids Res. 29 (6): 1341–51. doi:10.1093/nar/29.6.1341. PMC 29758. PMID 11239001.
- ^ Matheos D, Ruiz MT, Price GB, Zannis-Hadjopoulos M (October 2002). "Ku antigen, an origin-specific binding protein that associates with replication proteins, is required for mammalian DNA replication". Biochim. Biophys. Acta. 1578 (1–3): 59–72. doi:10.1016/s0167-4781(02)00497-9. PMID 12393188.
- ^ Fujise K, Zhang D, Liu J, Yeh ET (December 2000). "Regulation of apoptosis and cell cycle progression by MCL1. Differential role of proliferating cell nuclear antigen". J. Biol. Chem. 275 (50): 39458–65. doi:10.1074/jbc.M006626200. PMID 10978339.
- ^ a b Kleczkowska HE, Marra G, Lettieri T, Jiricny J (March 2001). "hMSH3 and hMSH6 interact with PCNA and colocalize with it to replication foci". Genes Dev. 15 (6): 724–36. doi:10.1101/gad.191201. PMC 312660. PMID 11274057.
- ^ a b Clark AB, Valle F, Drotschmann K, Gary RK, Kunkel TA (November 2000). "Functional interaction of proliferating cell nuclear antigen with MSH2-MSH6 and MSH2-MSH3 complexes". J. Biol. Chem. 275 (47): 36498–501. doi:10.1074/jbc.C000513200. PMID 11005803.
- ^ Parker A, Gu Y, Mahoney W, Lee SH, Singh KK, Lu AL (February 2001). "Human homolog of the MutY repair protein (hMYH) physically interacts with proteins involved in long patch DNA base excision repair". J. Biol. Chem. 276 (8): 5547–55. doi:10.1074/jbc.M008463200. PMID 11092888.
- ^ a b Fotedar R, Mossi R, Fitzgerald P, Rousselle T, Maga G, Brickner H, Messier H, Kasibhatla S, Hübscher U, Fotedar A (August 1996). "A conserved domain of the large subunit of replication factor C binds PCNA and acts like a dominant negative inhibitor of DNA replication in mammalian cells". EMBO J. 15 (16): 4423–33. doi:10.1002/j.1460-2075.1996.tb00815.x. PMC 452166. PMID 8861969.
- ^ Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M (October 2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- ^ Frouin I, Maga G, Denegri M, Riva F, Savio M, Spadari S, Prosperi E, Scovassi AI (October 2003). "Human proliferating cell nuclear antigen, poly(ADP-ribose) polymerase-1, and p21waf1/cip1. A dynamic exchange of partners". J. Biol. Chem. 278 (41): 39265–8. doi:10.1074/jbc.C300098200. PMID 12930846.
- ^ Gulbis JM, Kelman Z, Hurwitz J, O'Donnell M, Kuriyan J (October 1996). "Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNA". Cell. 87 (2): 297–306. doi:10.1016/s0092-8674(00)81347-1. PMID 8861913. S2CID 17461501.
- ^ Touitou R, Richardson J, Bose S, Nakanishi M, Rivett J, Allday MJ (May 2001). "A degradation signal located in the C-terminus of p21WAF1/CIP1 is a binding site for the C8 alpha-subunit of the 20S proteasome". EMBO J. 20 (10): 2367–75. doi:10.1093/emboj/20.10.2367. PMC 125454. PMID 11350925.
- ^ Lu X, Tan CK, Zhou JQ, You M, Carastro LM, Downey KM, So AG (July 2002). "Direct interaction of proliferating cell nuclear antigen with the small subunit of DNA polymerase delta". J. Biol. Chem. 277 (27): 24340–5. doi:10.1074/jbc.M200065200. PMID 11986310.
- ^ Ducoux M, Urbach S, Baldacci G, Hübscher U, Koundrioukoff S, Christensen J, Hughes P (December 2001). "Mediation of proliferating cell nuclear antigen (PCNA)-dependent DNA replication through a conserved p21(Cip1)-like PCNA-binding motif present in the third subunit of human DNA polymerase delta". J. Biol. Chem. 276 (52): 49258–66. doi:10.1074/jbc.M106990200. PMID 11595739.
- ^ Liu L, Rodriguez-Belmonte EM, Mazloum N, Xie B, Lee MY (March 2003). "Identification of a novel protein, PDIP38, that interacts with the p50 subunit of DNA polymerase delta and proliferating cell nuclear antigen". J. Biol. Chem. 278 (12): 10041–7. doi:10.1074/jbc.M208694200. PMID 12522211.
- ^ Haracska L, Johnson RE, Unk I, Phillips B, Hurwitz J, Prakash L, Prakash S (November 2001). "Physical and functional interactions of human DNA polymerase eta with PCNA". Mol. Cell. Biol. 21 (21): 7199–206. doi:10.1128/MCB.21.21.7199-7206.2001. PMC 99895. PMID 11585903.
- ^ Haracska L, Unk I, Johnson RE, Phillips BB, Hurwitz J, Prakash L, Prakash S (February 2002). "Stimulation of DNA synthesis activity of human DNA polymerase kappa by PCNA". Mol. Cell. Biol. 22 (3): 784–91. doi:10.1128/mcb.22.3.784-791.2002. PMC 133560. PMID 11784855.
- ^ Maga G, Villani G, Ramadan K, Shevelev I, Tanguy Le Gac N, Blanco L, Blanca G, Spadari S, Hübscher U (December 2002). "Human DNA polymerase lambda functionally and physically interacts with proliferating cell nuclear antigen in normal and translesion DNA synthesis". J. Biol. Chem. 277 (50): 48434–40. doi:10.1074/jbc.M206889200. hdl:10261/338844. PMID 12368291.
- ^ Shimazaki N, Yoshida K, Kobayashi T, Toji S, Tamai K, Koiwai O (July 2002). "Over-expression of human DNA polymerase lambda in E. coli and characterization of the recombinant enzyme". Genes Cells. 7 (7): 639–51. doi:10.1046/j.1365-2443.2002.00547.x. PMID 12081642. S2CID 29714829.
- ^ Maruyama T, Farina A, Dey A, Cheong J, Bermudez VP, Tamura T, Sciortino S, Shuman J, Hurwitz J, Ozato K (September 2002). "A Mammalian bromodomain protein, brd4, interacts with replication factor C and inhibits progression to S phase". Mol. Cell. Biol. 22 (18): 6509–20. doi:10.1128/mcb.22.18.6509-6520.2002. PMC 135621. PMID 12192049.
- ^ a b Mossi R, Jónsson ZO, Allen BL, Hardin SH, Hübscher U (January 1997). "Replication factor C interacts with the C-terminal side of proliferating cell nuclear antigen". J. Biol. Chem. 272 (3): 1769–76. doi:10.1074/jbc.272.3.1769. PMID 8999859.
- ^ van der Kuip H, Carius B, Haque SJ, Williams BR, Huber C, Fischer T (April 1999). "The DNA-binding subunit p140 of replication factor C is upregulated in cycling cells and associates with G1 phase cell cycle regulatory proteins". J. Mol. Med. 77 (4): 386–92. doi:10.1007/s001090050365. PMID 10353443. S2CID 22183443.
- ^ a b c Cai J, Gibbs E, Uhlmann F, Phillips B, Yao N, O'Donnell M, Hurwitz J (July 1997). "A complex consisting of human replication factor C p40, p37, and p36 subunits is a DNA-dependent ATPase and an intermediate in the assembly of the holoenzyme". The Journal of Biological Chemistry. 272 (30): 18974–18981. doi:10.1074/jbc.272.30.18974. PMID 9228079.
- ^ Pan ZQ, Chen M, Hurwitz J (January 1993). "The subunits of activator 1 (replication factor C) carry out multiple functions essential for proliferating-cell nuclear antigen-dependent DNA synthesis". Proceedings of the National Academy of Sciences of the United States of America. 90 (1): 6–10. Bibcode:1993PNAS...90....6P. doi:10.1073/pnas.90.1.6. PMC 45588. PMID 8093561.
- ^ Merkle CJ, Karnitz LM, Henry-Sánchez JT, Chen J (August 2003). "Cloning and characterization of hCTF18, hCTF8, and hDCC1. Human homologs of a Saccharomyces cerevisiae complex involved in sister chromatid cohesion establishment". The Journal of Biological Chemistry. 278 (32): 30051–30056. doi:10.1074/jbc.M211591200. PMID 12766176.
- ^ Motegi A, Liaw HJ, Lee KY, Roest HP, Maas A, Wu X, et al. (August 2008). "Polyubiquitination of proliferating cell nuclear antigen by HLTF and SHPRH prevents genomic instability from stalled replication forks". Proceedings of the National Academy of Sciences of the United States of America. 105 (34): 12411–12416. Bibcode:2008PNAS..10512411M. doi:10.1073/pnas.0805685105. PMC 2518831. PMID 18719106.
- ^ Unk I, Hajdú I, Fátyol K, Hurwitz J, Yoon JH, Prakash L, et al. (March 2008). "Human HLTF functions as a ubiquitin ligase for proliferating cell nuclear antigen polyubiquitination". Proceedings of the National Academy of Sciences of the United States of America. 105 (10): 3768–3773. Bibcode:2008PNAS..105.3768U. doi:10.1073/pnas.0800563105. PMC 2268824. PMID 18316726.
- ^ Brun J, Chiu R, Lockhart K, Xiao W, Wouters BG, Gray DA (February 2008). "hMMS2 serves a redundant role in human PCNA polyubiquitination". BMC Molecular Biology. 9: 24. doi:10.1186/1471-2199-9-24. PMC 2263069. PMID 18284681.
- ^ Rodríguez-López AM, Jackson DA, Nehlin JO, Iborra F, Warren AV, Cox LS (February 2003). "Characterisation of the interaction between WRN, the helicase/exonuclease defective in progeroid Werner's syndrome, and an essential replication factor, PCNA". Mechanisms of Ageing and Development. 124 (2): 167–174. doi:10.1016/s0047-6374(02)00131-8. PMID 12633936. S2CID 37287691.
- ^ Huang S, Beresten S, Li B, Oshima J, Ellis NA, Campisi J (June 2000). "Characterization of the human and mouse WRN 3'→5' exonuclease". Nucleic Acids Research. 28 (12): 2396–2405. doi:10.1093/nar/28.12.2396. PMC 102739. PMID 10871373.
- ^ Fan J, Otterlei M, Wong HK, Tomkinson AE, Wilson DM (2004). "XRCC1 co-localizes and physically interacts with PCNA". Nucleic Acids Res. 32 (7): 2193–201. doi:10.1093/nar/gkh556. PMC 407833. PMID 15107487.
- ^ Ise T, Nagatani G, Imamura T, Kato K, Takano H, Nomoto M, et al. (January 1999). "Transcription factor Y-box binding protein 1 binds preferentially to cisplatin-modified DNA and interacts with proliferating cell nuclear antigen". Cancer Research. 59 (2): 342–346. PMID 9927044.
- ^ Gilljam KM, Müller R, Liabakk NB, Otterlei M (2012). "Nucleotide excision repair is associated with the replisome and its efficiency depends on a direct interaction between XPA and PCNA". PLOS ONE. 7 (11): e49199. Bibcode:2012PLoSO...749199G. doi:10.1371/journal.pone.0049199. PMC 3496702. PMID 23152873.
- ^ Ciccia A, Nimonkar AV, Hu Y, Hajdu I, Achar YJ, Izhar L, et al. (August 2012). "Polyubiquitinated PCNA recruits the ZRANB3 translocase to maintain genomic integrity after replication stress". Molecular Cell. 47 (3): 396–409. doi:10.1016/j.molcel.2012.05.024. PMC 3613862. PMID 22704558.
- ^ Bacquin A, Pouvelle C, Siaud N, Perderiset M, Salomé-Desnoulez S, Tellier-Lebegue C, et al. (July 2013). "The helicase FBH1 is tightly regulated by PCNA via CRL4(Cdt2)-mediated proteolysis in human cells". Nucleic Acids Research. 41 (13): 6501–6513. doi:10.1093/nar/gkt397. PMC 3711418. PMID 23677613.
- ^ Schönenberger F, Deutzmann A, Ferrando-May E, Merhof D (29 May 2015). "Discrimination of cell cycle phases in PCNA-immunolabeled cells". BMC Bioinform. 16 (180): 180. doi:10.1186/s12859-015-0618-9. PMC 4448323. PMID 26022740.
- ^ Herce HD, Rajan M, Lättig-Tünnemann G, Fillies M, Cardoso MC (3 September 2014). "A novel cell permeable DNA replication and repair marker". Nucleus (Austin, Tex.). 5 (6): 590–600. doi:10.4161/nucl.36290. PMC 4615156. PMID 25484186.
- ^ Wang SC (April 2014). "PCNA: a silent housekeeper or a potential therapeutic target?". Trends in Pharmacological Sciences. 35 (4): 178–186. doi:10.1016/j.tips.2014.02.004. PMID 24655521.
- ^ Gu L, Lingeman R, Yakushijin F, Sun E, Cui Q, Chao J, et al. (December 2018). "The Anticancer Activity of a First-in-class Small-molecule Targeting PCNA". Clinical Cancer Research. 24 (23): 6053–6065. doi:10.1158/1078-0432.CCR-18-0592. PMC 6279569. PMID 29967249.
- ^ Gu L, Li M, Li CM, Haratipour P, Lingeman R, Jossart J, et al. (July 2023). "Small molecule targeting of transcription-replication conflict for selective chemotherapy". Cell Chemical Biology. 30 (10): 1235–1247.e6. doi:10.1016/j.chembiol.2023.07.001. PMC 10592352. PMID 37531956.
Further reading
[edit]- Almendral JM, Huebsch D, Blundell PA, Macdonald-Bravo H, Bravo R (March 1987). "Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins". Proceedings of the National Academy of Sciences of the United States of America. 84 (6): 1575–1579. Bibcode:1987PNAS...84.1575A. doi:10.1073/pnas.84.6.1575. PMC 304478. PMID 2882507.
- Chen IT, Smith ML, O'Connor PM, Fornace AJ (November 1995). "Direct interaction of Gadd45 with PCNA and evidence for competitive interaction of Gadd45 and p21Waf1/Cip1 with PCNA". Oncogene. 11 (10): 1931–1937. PMID 7478510.
- Chen M, Pan ZQ, Hurwitz J (April 1992). "Sequence and expression in Escherichia coli of the 40-kDa subunit of activator 1 (replication factor C) of HeLa cells". Proceedings of the National Academy of Sciences of the United States of America. 89 (7): 2516–2520. Bibcode:1992PNAS...89.2516C. doi:10.1073/pnas.89.7.2516. PMC 48692. PMID 1313560.
- Fukuda K, Morioka H, Imajou S, Ikeda S, Ohtsuka E, Tsurimoto T (September 1995). "Structure-function relationship of the eukaryotic DNA replication factor, proliferating cell nuclear antigen". The Journal of Biological Chemistry. 270 (38): 22527–22534. doi:10.1074/jbc.270.38.22527. PMID 7673244.
- Hall PA, Kearsey JM, Coates PJ, Norman DG, Warbrick E, Cox LS (June 1995). "Characterisation of the interaction between PCNA and Gadd45". Oncogene. 10 (12): 2427–2433. PMID 7784094.
- Kato S, Sekine S, Oh SW, Kim NS, Umezawa Y, Abe N, et al. (December 1994). "Construction of a human full-length cDNA bank". Gene. 150 (2): 243–250. doi:10.1016/0378-1119(94)90433-2. PMID 7821789.
- Kemeny MM, Alava G, Oliver JM (November 1992). "Improving responses in hepatomas with circadian-patterned hepatic artery infusions of recombinant interleukin-2". Journal of Immunotherapy. 12 (4): 219–223. doi:10.1097/00002371-199211000-00001. PMID 1477073.
- Krotz D (28 July 2004). "Structure of a clamp–loader complex". ALSNews. Vol. 243. Lawrence Berkeley National Laboratory. Archived from the original on 11 October 2004. Retrieved 3 August 2023.
- Ku DH, Travali S, Calabretta B, Huebner K, Baserga R (July 1989). "Human gene for proliferating cell nuclear antigen has pseudogenes and localizes to chromosome 20". Somatic Cell and Molecular Genetics. 15 (4): 297–307. doi:10.1007/BF01534969. PMID 2569765. S2CID 27217843.
- Li X, Li J, Harrington J, Lieber MR, Burgers PM (September 1995). "Lagging strand DNA synthesis at the eukaryotic replication fork involves binding and stimulation of FEN-1 by proliferating cell nuclear antigen". The Journal of Biological Chemistry. 270 (38): 22109–22112. doi:10.1074/jbc.270.38.22109. PMID 7673186.
- Matsuoka S, Yamaguchi M, Matsukage A (April 1994). "D-type cyclin-binding regions of proliferating cell nuclear antigen". The Journal of Biological Chemistry. 269 (15): 11030–11036. doi:10.1016/S0021-9258(19)78087-9. PMID 7908906.
- Miura M (March 1999). "Detection of chromatin-bound PCNA in mammalian cells and its use to study DNA excision repair". Journal of Radiation Research. 40 (1): 1–12. Bibcode:1999JRadR..40....1M. doi:10.1269/jrr.40.1. PMID 10408173.
- Miyata T, Suzuki H, Oyama T, Mayanagi K, Ishino Y, Morikawa K (September 2005). "Open clamp structure in the clamp-loading complex visualized by electron microscopic image analysis". Proceedings of the National Academy of Sciences of the United States of America. 102 (39): 13795–13800. Bibcode:2005PNAS..10213795M. doi:10.1073/pnas.0506447102. PMC 1236569. PMID 16169902.
- Morris GF, Mathews MB (September 1990). "Analysis of the proliferating cell nuclear antigen promoter and its response to adenovirus early region 1". The Journal of Biological Chemistry. 265 (27): 16116–16125. doi:10.1016/S0021-9258(17)46196-5. PMID 1975809.
- Pan ZQ, Chen M, Hurwitz J (January 1993). "The subunits of activator 1 (replication factor C) carry out multiple functions essential for proliferating-cell nuclear antigen-dependent DNA synthesis". Proceedings of the National Academy of Sciences of the United States of America. 90 (1): 6–10. Bibcode:1993PNAS...90....6P. doi:10.1073/pnas.90.1.6. PMC 45588. PMID 8093561.
- Prelich G, Kostura M, Marshak DR, Mathews MB, Stillman B (1987). "The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro". Nature. 326 (6112): 471–475. Bibcode:1987Natur.326..471P. doi:10.1038/326471a0. PMID 2882422. S2CID 4336365.
- Prosperi E (1998). "Multiple roles of the proliferating cell nuclear antigen: DNA replication, repair and cell cycle control". Progress in Cell Cycle Research. Vol. 3. pp. 193–210. doi:10.1007/978-1-4615-5371-7_15. ISBN 978-1-4613-7451-0. PMID 9552415.
- Smith ML, Chen IT, Zhan Q, Bae I, Chen CY, Gilmer TM, et al. (November 1994). "Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen". Science (Submitted manuscript). 266 (5189): 1376–1380. Bibcode:1994Sci...266.1376S. doi:10.1126/science.7973727. PMID 7973727.
- Szepesi A, Gelfand EW, Lucas JJ (November 1994). "Association of proliferating cell nuclear antigen with cyclin-dependent kinases and cyclins in normal and transformed human T lymphocytes". Blood. 84 (10): 3413–3421. doi:10.1182/blood.V84.10.3413.3413. PMID 7949095.
- Travali S, Ku DH, Rizzo MG, Ottavio L, Baserga R, Calabretta B (May 1989). "Structure of the human gene for the proliferating cell nuclear antigen". The Journal of Biological Chemistry. 264 (13): 7466–7472. doi:10.1016/S0021-9258(18)83257-4. hdl:11380/811513. PMID 2565339.
- Warbrick E, Lane DP, Glover DM, Cox LS (March 1995). "A small peptide inhibitor of DNA replication defines the site of interaction between the cyclin-dependent kinase inhibitor p21WAF1 and proliferating cell nuclear antigen". Current Biology. 5 (3): 275–282. doi:10.1016/S0960-9822(95)00058-3. PMID 7780738. S2CID 1559243.
- Webb G, Parsons P, Chenevix-Trench G (November 1990). "Localization of the gene for human proliferating nuclear antigen/cyclin by in situ hybridization". Human Genetics. 86 (1): 84–86. doi:10.1007/bf00205180. PMID 1979311. S2CID 27107553.
External links
[edit]- PCNA at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ANA: Cell cycle related (Mitotic): PCNA type 1 and type 2 Antibody Patterns—Antibody Patterns.com
- Overview of all the structural information available in the PDB for UniProt: P12004 (Proliferating cell nuclear antigen) at the PDBe-KB.