Abstract
Previous research has indicated that anxious individuals are more prone to evaluate ambiguous information as negative compared to non-anxious individuals. The feedback-related negativity (FRN) component of event-related brain potential (ERP) has been shown to be sensitive to outcome evaluation. The current ERP study aimed to test the hypothesis that the FRNs associated with ambiguous outcomes and negative outcomes are different between high trait-anxiety (HTA) and low trait-anxiety (LTA) individuals. The FRN was measured as a difference wave created across conditions. We found significantly different FRN responses between high-anxious and low-anxious participants in ambiguous outcome condition, as well as in negative outcome condition. Moreover, the HTA group’s FRN responses under the ambiguous outcome condition were larger than the negative outcome condition. Nevertheless, the FRN following neutral outcome did not show any difference between the two groups. The present results support the idea that there is link between individual differences in anxiety and ambiguous outcome evaluation, which possibly reflects the adaptive function of anxiety. Additionally, the results indicate that the mechanisms underlying the evaluation of neutral outcomes and ambiguous outcomes might be different from each other.
Keywords: Anxiety, Decision-making, Outcome Evaluation, Feedback-Related Negativity (FRN), Ambiguous Outcome, Neutral Outcome, Guessing Activity
The evaluation of an outcome is an important step towards decision-making (Paulus, 2005), since adaptive decision-making requires encoding the outcome, either good or bad, of prior action (Platt, 2002). During the stage of outcome evaluation, neural signatures were found to be associated with the difference between expected and actual outcomes. This kind of signatures is a key to adjust the assessment of preferences among possible options of the subsequent decision-making. In this way, past outcomes could affect the future assessment of options available (Paulus, 2005).
It has been suggested that study of event-related potential (ERP) could provide important insights into the nature of outcome evaluation (Yeung, Holroyd, & Cohen, 2005). Feedback-Related Negativity (FRN), a negative-going component of the ERP, is related with processing of the outcome value and motivational significance of ongoing events (Yeung et al., 2005). The FRN peaks between 200 and 300 ms following the presentation of feedback stimuli, being larger after losses than after gains in simple tasks (Bellebaum & Daum, 2008; Gehring & Willoughby, 2002). In addition, the FRN has been demonstrated to be larger for unexpected negative outcomes than expected ones (Hajcak, Moser, Holroyd, & Simons, 2007; Holroyd, Hajcak, & Larsen, 2006). Taken together, the amplitude of FRN is influenced by both the valence and the expectation of the outcome (Cohen & Ranganath, 2007). According to reinforcement learning theory, the FRN might reflect a reward ‘prediction error’, a signal elicited when the monitoring system has to revise its reward expectations for the worse (Holroyd et al., 2006; Nieuwenhuis, Holroyd, Mol, & Coles, 2004). Emerging evidence has shown that the amplitude of FRN could be impacted by individual differences in affective processing (e.g., depression, stress and neuroticism), indicating the influence of emotion on outcome evaluation (Foti & Hajcak, 2009; Hirsh & Inzlicht, 2008; Sato et al., 2005; Simons, 2010).
Information regarding outcome valence plays a critical role in many aspects of decision-making (Lipshitz, Gilad, & Suleiman, 2001). Nevertheless, the outcomes received are often ambiguous or uninformative in our daily life. When clarity is lacking, the meaning of an ambiguous outcome would be influenced by perceiver’s own bias. Blanchette and Richards (2003) pointed out that ‘the process of resolving ambiguity is both pervasive and central for everyday cognition’.
Uncertainty may create situations that allow many factors (such as emotion) to bias judgment (Blader, 2007). It has been widely accepted that emotional states could affect the way in which events are interpreted (Blanchette & Richards, 2003). Previous studies have shown that high-level anxiety is associated with an increased likelihood of negative interpretation of ambiguous stimuli, such as facial expression and lexical information (Bensi & Giusberti, 2007; Blanchette & Richards, 2003; Kverno, 2000; Richards et al., 2002). This tendency is not surprising, since anxiety is linked to attentional bias toward threat-related information (Bar-Haim, Lamy, & Glickman, 2005; Blanchette & Richards, 2003; Li, Li, & Luo, 2005). Ambiguous stimuli could be more threatening than familiar ones, since the potential danger is not known clearly (Hirsh & Inzlicht, 2008). Thus, the relationship between anxiety and negative interpretation of ambiguous information might imply the adaptive function of anxiety. Hence, it would also be important to determine whether anxiety plays a role in the evaluation of ambiguous outcomes during decision-making.
Previous ERP research has demonstrated that neutral or ambiguous feedback could elicit an FRN as large as those elicited by negative feedback (Holroyd et al., 2006). Thus far, it is still not clear whether the FRN following ambiguous outcomes can be influenced by anxiety. Recently, we investigated the FRN during a simple monetary gambling task (Gu, Huang, & Luo, in press). In this task, participants were asked to gamble for rewards, and their choices were followed by feedback events. There were three kinds of outcome valence: positive, negative and ambiguous. Under the ambiguous outcome condition, participants either gained or lost money in the trials, but the feedback did not indicate the actual outcome (Holroyd et al., 2006). Before the experiment, the participants were encouraged to guess whether they won or lost when they received ambiguous outcomes.
The ERP results revealed that the amplitude of the FRN following negative outcomes was inversely related with trait anxiety level. In our opinion, this result reflected a difference in expected rather than actual outcome evaluation. Since anxious people are more likely to expect a negative outcome in risk-taking tasks (Eisenberg, Baron, & Seligman, 1998; Mitte, 2007; Shepperd, Grace, Cole, & Klein, 2005), we hypothesize that the ‘prediction error’ is smaller in anxious subjects than in non-anxious subjects when the outcome is negative.
However, no group difference was found in the FRN following ambiguous outcomes, which was contrary to our expectation. We suspected that when we encouraged participants to guess the valence of ambiguous outcomes, the FRN in the ambiguous condition might be intensely influenced by the activity of guessing, and the impact of anxiety on outcome evaluation would be harder to observe in this situation (Gu et al., in press).
This idea is supported by a study focus on neuroticism. Recently, Hirsh & Inzlicht (2008) reported that uncertain feedback elicited a larger FRN in neurotic individuals compared to non-neurotic individuals. It is worth noting that they did not ask the participants to guess the meaning of uncertain outcomes. Neuroticism is defined as a predisposition toward many kinds of negative affective states, including depression, anxiety, anger, and shame (Miller & Pilkonis, 2006). In light of this discovery, we speculate that the influence of anxiety on ambiguous outcome evaluation should be reflected on the amplitude of FRN, if we could reduce the impact of guessing activity.
In the current study, we aimed to use the FRN as an objective measure in exploring the relationship between anxiety and ambiguous outcome evaluation with limited interference of guessing activity. A version of Gehring & Willoughby’s (2002) monetary gambling task was applied while under EEG recording. The current paradigm was similar to our previous experiment (Gu et al., in press), except with the modification of reducing the influence of guessing activity. We hypothesized that the amplitude of the FRN would be significantly different between high-anxious participants and low-anxious participants in ambiguous outcome condition, as well as in negative outcome condition.
1. Methods
1.1 Participants
253 undergraduate students (All Chinese) participated in a mass screening with the Chinese version of Spielberger’s Trait Anxiety Inventory (STAI). This scale has demonstrated good internal consistency, as well as convergent and discriminant validity (Shek, 1993; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). Subsequently, students who scored high in trait anxiety (in the upper 25% of the distribution) were considered as high-trait anxious people, while the students who scored low (in the lower 25% of the distribution) were considered as low-trait anxious (LTA) people. From those who were suitable for this criteria, we randomly chose 34 students (18 females; mean age 20.53 years [S.D. = 2.15]) and invited them to participate in the experiment. 17 of them were assigned to the high-trait anxiety (HTA) group (6 females), while 17 were assigned to the low-trait anxiety (LTA) group (8 females). An independent-samples T test revealed that the two groups differed significantly in trait-anxious score (56.59 vs. 35.29, p < 0.001), but not in age (20.82 vs. 20.24, p = 0.433). All participants denied regular use of medication or other nonmedical substances with the potential to affect the central nervous system. All had normal vision (with correction), and none had a history of neurological disease. All of them were right-handed.
In order to control the effect of depression during analysis, the Chinese version of Zung self-rating depression scale (SDS) was used to assess self-reported symptoms of depression. Excellent reliability and validity of the SDS in both clinical and non-clinical samples has been previously established (Shu, 1993; Zung, Richards, & Short, 1965). An independent-samples T test revealed that the two groups differed significantly in SDS score (36.65 vs. 28.82, p = 0.002).
1.2 Procedure
Before the experiment, each participant was told that he/she would participant in a gambling game. He/she was instructed about the rules, as well as the meaning of symbols in the task, and was asked to respond in a way that would maximize the total score amount. The higher the score a participant earned, the more bonus money he/she would receive at the end of the experiment.
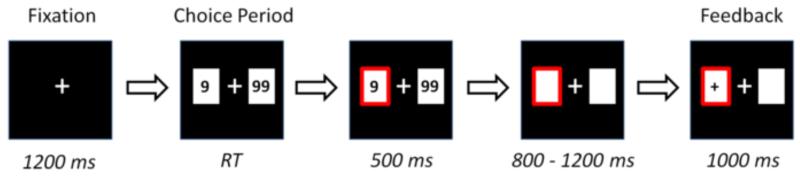
During the formal task, the participant sat comfortably in an electrically shielded room, approximately 100 cm in front of a computer screen. Each trial began with the presentation of a central fixation point (white against a black background). After 1200 ms, two white rectangles (2.5 degree × 2.5 degree) appeared on either side of the fixation point, in either of which individually presented the numbers ‘9’ or ‘99’ (indicating the score). The participant then selected one of the two alternatives by pressing the ‘F’ or ‘J’ keys on the keyboard with his/her left or right index finger (‘F’ for the alternative on the left, and ‘J’ for the one on the right). The alternatives remained on the screen until the participant made a choice, which was then highlighted by a thickening of the red outline of the chosen rectangle for 500 ms. Then the numbers disappeared for a short interval, of random duration between 800 and 1200 ms. Finally, the result of the participant’s choice was presented in the chosen rectangle for 1000 ms, indicating the valence of the feedback (see Fig. 1). The formal task consisted of four blocks of 160 trials each.
Figure 1. The sequence of events within a single trial of the monetary gambling task.
On each trial, the fixation point would last for 1200 ms. Then the participant was presented with a choice of two alternatives, one of which he/she was asked to select using their left or right index finger. The alternatives would remain still until the participant made his/her choice. After that, his/her choice was highlighted for 500 ms. After a subsequent interval of 800-1200 ms, the participant received feedback, lasting 1000 ms, which indicated whether he/she gained or lost in this trial. RT: Response time.
There were four kinds of possible feedbacks: ‘+’, ‘−’, ‘0’, and ‘*’. The ‘+’ symbol indicated that the participant won as many points as he/she chose in this trial, while the ‘−’ symbol indicated the reverse. The ‘0’ indicated a neutral feedback, which means the participant neither won nor lost. The ‘*’ symbol indicated an ambiguous feedback. Prior to the experiment, the participant was told that the ambiguous feedback was an uninformative outcome, of which the valence could be positive, negative or neutral, but it was impossible for him/her to guess the real valence in any given trial. Unbeknownst to the participant, the feedbacks were provided according to a pre-determined pseudorandom sequence, and all participants received exactly 160 of each type of feedback.
At the end of the task, the participant was informed of the total score that he/she had earned. Then they were paid 50-70 Chinese yuan for their participation (the exact number is depended on their task performance). Stimulus display and behavioral data acquisition were conducted using E-Prime software (Version 1.1, Psychology Software Tools, Inc., Pittsburgh, PA).
1.3 Psychophysiological recording, data reduction, and analysis
The electroencephalogram (EEG) was recorded from 64 scalp sites using tin electrodes mounted in an elastic cap (NeuroScan Inc., Herndon, Virginia, USA), with an online reference to the right mastoid and off-line algebraic re-reference to the average of the left and right mastoids. Horizontal electrooculogram (HEOG) was recorded from electrodes placed at the outer canthi of both eyes. Vertical electrooculogram (VEOG) was recorded from electrodes placed above and below the left eye. All inter electrode impedance was maintained at < 5 kΩ. EEG and EOG signals were amplified with a 0.05–100 Hz bandpass filter and continuously sampled at 1000 Hz/channel.
During the offline analysis, ocular artifacts were removed from the EEG signal using a regression procedure implemented in the Neuroscan software (Semlitsch, Anderer, Schuster, & Presslich, 1986). Any trials in which EEG and EOG voltages exceeded a threshold of ±100 μV during the recording epoch were excluded from the analysis. The EEG data were digitally filtered with a 30-Hz low-pass filter and were baseline-corrected by subtracting from each sample the average activity of that channel during the baseline period.
For each feedback stimulus, a 1200 ms epoch of data was extracted from the continuous data files for analysis (from 200 ms before the feedback onset to 1000 ms after the feedback onset). To minimize overlap between the FRN and other ERP components, the amplitude of the FRN was measured as the maximum difference wave between different conditions in a window of 200-400 ms following the feedback presentation, relative to a 200 ms pre-stimulus baseline. We followed the methods of Holroyd & Krigolson (2007) and created three kinds of difference waves by subtracting the ERP on positive outcome trials from the ERP on negative outcome trials (called ‘negative – positive’ difference wave), from the ERP on neutral outcome trials (called ‘neutral – positive’ difference wave), and from the ERP on ambiguous outcome trials (called ‘ambiguous – positive’ difference wave). The difference waves were evaluated at the electrodes where maximal activity was reached (See 2.2 FRN for detail).
For the purpose of comparison, we also evaluated the amplitude of P3 by identifying its maximum voltage at electrode position Pz, where this ERP component typically reaches maximum amplitude (Holroyd, Baker, Kerns, & Muller, 2008), within a 300–600ms window.
2. Results
2.1 Behavioral results
We defined the choice of ‘9’ to be the risk-avoidant choice in our experiment, predicting that participants would make this choice to avoid the possibility of a large loss (‘−99’). However, by making this choice, they also gave up the opportunity to receive the larger reward (‘+99’). In contrast, the choice of ‘99’ was defined as the risky choice (high-risk or high-return).
To provide a basis for comparison with previous research, an independent-samples T test was used to compare the average level of risk-avoidant choice made by each participant, as well as the average time each participant took to decide, and between groups. The group difference of the average number of risk-avoidant choices made by members of the HTA group (302.06 times [S.D. = 96.44]) and the LTA group (355.71 times [S.D. = 98.88]) were not statistically significant (t (34) = −1.60, p = 0.119). The average time taken for decision-making by the HTA group (763.16 ms [S.D. = 385.48]) was shorter than for the LTA group (838.45 ms [S.D. = 585.10]), but this result failed to reached significance (t (34) = −0.44, p = 0.661).
For the purpose of investigating the potential influence of outcome valence to ongoing decision-making, we also calculated the ratio of risk-avoidant choice associated with each kind of outcome. To accomplish this, we divided the number of risk-avoidant choices following each kind of outcome by the total number of choices following the corresponding outcome. The result was entered into a 4 (outcome valence) × 2 (outcome magnitude) ANOVA test, using the trait anxiety group as the between-subject factor. The main effects of valence (F (3, 96) = 27.68, p < 0.001) and magnitude (F (1, 32) = 16.43, p < 0.001), as well as the valence × magnitude (F (3, 96) = 22.82, p < 0.001) interaction were significant. This result indicated that the participants were more prone to be risk-avoidant after they received positive outcomes (59.6%) than other kinds of outcomes (negative: 42.5%, neutral: 52.1%, ambiguous: 51.7%). This finding is consistent with previous research that decision makers would become more risk-avoidant after receiving rewards (Isen, Nygen, & Ashby, 1988; Loewenstein, Weber, Hsee, & Welch, 2001). However, we did not find significance in the main effect of group (F (1, 32) = 1.87, p = 0.181), the valence × group interaction (F (1, 32) = 1.39, p = 0.256), the magnitude × group interaction (F (1, 32) = 0.09, p = 0.767), or the valence × magnitude × group interaction (F (1, 32) = 2.50, p = 0.082). These results indicated that there was no difference in the behavioral performance between HTA group and LTA group.
2.2 FRN
Consistent with our previous study (Gu et al., in press), the scalp distribution of the difference waves was maximal at frontal-central areas of the scalp, at electrode position FCz (−6.51 μV), 299.25 ms following the onset of the feedback.
To confirm that the amplitude of the FRN was not confounded by overlap with the P3, we followed the method of Holroyd & Krigolson (2007) and Hirsh & Inzlicht (2008) to carry out paired-samples T tests on the amplitude of difference wave at FCz and Pz (where the peak of P3 is localized in our study). The result indicated that the difference waves were significantly larger at FCz than Pz (−5.99 μV vs. −4.98 μV, t (34) = −5.60, p <0.001).
ERP amplitude of difference waves at electrode FCz were entered into a 3 (outcome valence: ‘negative – positive’, ‘neutral – positive’, ‘ambiguous – positive’) × 2 (outcome magnitude: ‘9’ vs. ‘99’) × 2 (trait anxiety group: HTA vs. LTA) ANOVA test, using the trait anxiety group as the between-subject factor. The Greenhouse-Geisser correction for repeated measures was applied to within-subject comparison.
The main effect of outcome valence was not significant (F (2, 64) = 2.30, p = 0.116), indicating that there was no significantly difference in the amplitude when we compare three kinds of ERP difference waves. The main effect of outcome magnitude (F (1, 32) = 8.06, p = 0.008) was significant. The valence × group interaction (F (2, 64) = 13.92, p < 0.001) was also significant. However, the interactions were not significant, i.e. the magnitude × group interaction (F (1, 32) = 0.60, p = 0.444), the valence × magnitude interaction (F (2, 64) = 3.314, p = 0.058), nor the valence × magnitude × group interaction (F (2, 64) = 1.40, p = 0.253).
Consequently, simple effect analysis was conducted to investigate the valence × group interaction. The result showed a reliable group effect (F (1, 32) = 5.20, p = 0.024) on the ‘negative - positive’ difference wave, indicating that the FRN was significantly higher in LTA group (−7.04 μV) than HTA group (−5.10 μV). In contrast with this effect, we found that the ‘ambiguous - positive’ difference wave was significantly lower in LTA group than HTA group (−5.91 μV vs. −7.83 μV; F (1, 32) = 5.10, p = 0.025). Nevertheless, the group factor failed to reach significance on the amplitude of the ‘neutral - positive’ difference wave (−6.20 μV vs. −6.96 μV; F (1, 32) = 0.81, p = 0.369).
Moreover, we separated the sample into HTA group and LTA group, and used paired-samples T test to compare the ‘negative - positive’ and ‘ambiguous - positive’ difference wave in both groups. In HTA group, the ‘negative - positive’ difference wave was significantly smaller than ‘ambiguous - positive’ one (−4.4 μV vs. −7.5 μV; t (16) = 4.91, p < 0.001). However, the opposite was true in LTA group: the ‘negative - positive’ difference wave was larger than ‘ambiguous - positive’ one (−6.7 μV vs. −5.3 μV; t (16) = −3.33, p = 0.004).
Since depression has also been linked to modulation of the FRN amplitude (Foti & Hajcak, 2009), we measured the possible effect of depression in two different ways. First, the SDS score was entered into the ANOVA test described above as a covariate. Although the main effect of outcome magnitude (F (1, 31) = 1.58, p = 0.218) was not significant, the valence × group interaction (F (2, 62) = 9.43, p < 0.001) remained significant, indicating that there is a unique influence of anxiety on FRN amplitude. The main effect of SDS score itself (F (1, 31) = 1.20, p = 0.281) failed to reach significance.
In addition, we calculated the Pearson’s correlation between SDS score and the amplitude of FRN at position FCz. The results revealed that the correlation between SDS score and the ‘ambiguous - positive’ difference wave (r = −0.46, p = 0.006) turn out to be significant. However, the correlation between SDS score and the ‘negative - positive’ (r = −0.01, p = 0.967) or the ‘neutral - positive’ (r = −0.08, p = 0.649) difference wave did not reach significance.
2.3 P3
ERP amplitude of the P3 at electrode Pz was analyzed in a 4 (outcome valence: positive, negative, neutral, ambiguous) × 2 (outcome magnitude: ‘9’ vs. ‘99’) × 2 (trait anxiety group: HTA vs. LTA) ANOVA test, with trait anxiety group as the between-subject factor. The Greenhouse-Geisser correction for repeated measures was applied to within-subject comparison.
The main effects of valence (F (3, 96) = 19.37, p < 0.001) and magnitude (F (1, 32) = 53.71, p < 0.001) were significant. According to these results, the P3 was larger in the positive outcome condition (17.23 μV) than the other three conditions (negative: 15.85 μV, neutral: 14.73 μV, ambiguous: 14.15 μV). In addition, it was larger in the risky condition (18.44 μV) than risk-avoidant condition (12.55 μV). However, the main effect of group factor (F (1, 32) = 1.00, p = 0.324) was not significant. Also note that neither the valence × group interaction (F (3, 96) = 1.86, p = 0.165) nor the magnitude × group interaction (F (1, 32) = 3.57, p = 0.068) reached significance. The valence × magnitude interaction (F (3, 96) = 4.09, p = 0.010) was significant. The valence × magnitude × group interaction (F (3, 96) = 0.43, p = 0.723) was not significant. To sum up, the anxiety group factor did not affect the amplitude of P3 significantly.
3. Discussion
The present study used classic negative deflection FRN as an indicator of responses to various feedback among high and low anxiety individuals in a decision-making task. Consistent with our hypotheses, the FRN revealed group differences in negative outcome condition and ambiguous outcome condition. Compared to the LTA group, the HTA group exhibited a smaller FRN following negative feedback and a larger FRN following ambiguous feedback. Nevertheless, the FRN following neutral feedback did not show any difference between HTA and LTA participants. The amplitude of P3 did not reveal any group difference. Therefore, the possibility that the impact of anxiety on the FRN was confounded by the P3 could be excluded.
3.1 Negative outcome vs. ambiguous outcome
This study replicated our previous finding (Gu et al., in press) that the FRN elicited by negative outcome was significantly smaller for HTA than for LTA participants. In our opinion, this result reflects the impact of anxiety on outcome expectation. According to reinforcement learning theory, the system which generates the FRN encodes the difference between expectations and actual outcomes as a reward prediction error (Cohen, Elger, & Ranganath, 2007). Since the anxious people are more likely to hold pessimistic outcome expectation (Eisenberg et al., 1998; Mitte, 2007; Shepperd et al., 2005), their expectation would be much closer to negative outcome compared with non-anxious people. As a result, the FRN signaling the difference between expectations and negative outcomes would be smaller for HTA people than for LTA people.
But why did the HTA group showed larger FRN than LTA group in the ambiguous condition? It has been pointed out that humans always expect to receive feedback with certainty (Polezzi, Lotto, Daum, Sartori, & Rumiati, 2008). In other words, individuals with different anxiety levels may have similar outcome expectation to ambiguous outcome. Thus, outcome expectation may not count for the ERP difference in this condition. According to many researchers, anxiety is linked with intolerance to uncertainty (Krain et al., 2008; Maner & Schmidt, 2006). Consequently, anxious people might think the ambiguous outcome is very intolerable and has negative feeling to it, although it can’t be worse than negative outcome actually. Consistent with this idea, the FRN was larger following ambiguous outcomes than following negative outcomes in the HTA group, suggesting that the ambiguous outcomes were even worse than negative outcomes in the HTA participants’ minds.
As an alternative, the group difference in the FRN following ambiguous outcome may be better accounted for by the relationship between anxiety and attention, since a lot of research on this relationship have revealed that anxiety is associated with systematic biases in the interpretation of ambiguous information (Blanchette & Richards, 2003). Previous research suggested that the FRN indicates the motivational, or affective significance of ongoing events (Yeung & Sanfey, 2004). It is reasonable to assume that the HTA participants paid more attention to ambiguous outcomes, and be more likely to be affected by ambiguity compared with the LTA participants. That is to say, the affective influence of ambiguous outcome on HTA participants was larger than on LTA participants.
Both of these explanations indicate that the anxious people are more likely to have negative feeling about the ambiguous outcomes. Whereas exposure to familiar negative stimuli produces a well-defined threat, ambiguous feedback can be even more threatening because the potential danger is not yet understood (Hirsh & Inzlicht, 2008). Thus, the interpretation bias toward ambiguous feedback could help us detecting the potential danger or harm. In evolutionary terms, the main function of anxiety is keeping us away from cues that indicate the presence of a threat (Nesse, 2005), possibly including potential threat. It’s not surprising to see neural responses to ambiguous feedback are also related to the personality trait of neuroticism (Hirsh & Inzlicht, 2008), which is closely linked with anxiety (Miller & Pilkonis, 2006).
However, we could not rule out the possibility that the depression selectively influenced the evaluation of ambiguous outcomes, since there is a significant correlation between the SDS score and the amplitude of FRN associated with ambiguous outcomes, but not between SDS and the other two kinds of FRN. Similar with the effect of anxiety, depression is linked with an increased tendency to impose negative interpretations on ambiguous information (Lawson & MacLeod, 1999). Nevertheless, this result is not completely consistent with previous research, since it has been demonstrated that the FRN to non-rewards relative to rewards was inversely related to depression (Foti & Hajcak, 2009). Further proof is still needed.
3.2 Ambiguous outcome vs. neutral outcome
According to the reinforcement learning theory, the FRN reflects the outcome evaluation based on a binary classification of good versus bad outcomes (Hajcak, Moser, Holroyd, & Simons, 2006). Both neutral feedback and uninformative feedback were considered to be ‘bad’ outcomes, since the FRN associated with these two kinds of outcomes were both as large as which associated with negative outcome (Holroyd et al., 2006). However, we found that the FRN elicited by neutral outcomes did not show a significant group difference, while the FRN elicited by ambiguous outcomes did. These results indicate that the mechanisms underlying the evaluation of neutral outcomes and ambiguous outcomes might be different from each other. While the neutral outcome only contains certain information, the ambiguous outcome is an ill-defined feedback. Interpreting ambiguous information is not a purely data-driven process but can be biased by top-down influence (Voss, Rothermund, & Brandtstadter, 2008). Thus, emotional states could have a wide-ranging influence on many aspects of this kind of information processing (Voss et al., 2008). According to our results, we suggest that the level of anxiety is correlated with the evaluation of ambiguous outcomes, but not correlated with the evaluation of neutral outcomes. Nevertheless, this study is inconclusive. Further work will be needed to explore the underlying mechanism of these two kinds of outcome evaluation.
3.3 Behavioral performance
Supposing the process of outcome evaluation is indeed different between HTA and LTA individuals, it might be surprising to see there was not any intergroup difference in behavioral performance in our experiment. However, it is worth noting that the behavioral results of many previous studies are similar with our work (Gu et al., in press; Hockey, Maule, Clough, & Bdzola, 2000; Mitte, 2007; Paulus, Feinstein, Simmons, & Stein, 2004). Previous research suggests that the interaction between anxiety and performance in a stressful task does not always have a negative effect, since additional cognitive resources may be allocated to cope with the stressful temporary condition (Righi, Mecacci, & Viggiano, 2009). It’s possible that HTA participants did try to control the effect of emotion during the task. That is, they might hold a form of compensation strategy to help them with decision making, as well as in other cognitive tasks (Cumming & Harris, 2001).
3.4 The role of guessing activity
In our previous experiment (Gu et al., in press), the ‘FRN’ elicited by ambiguous outcomes was apparently larger than the FRN elicited by negative outcomes. In addition, the FRN did not show any relationship with anxiety level. We suspected that when we encouraged participants to guess the valence of ambiguous outcomes, the FRN would be overlapped by another negative wave associated with guessing activity (Gu et al., in press). This kind of negative wave is indifferent to level of anxiety. In this study, we did not ask the participants to guess the meaning of ambiguous outcomes by themselves. Moreover, we told the participants before the experiment that it was impossible to guess the real valence in any given trial, so as to reduce the possible guessing activity. Consequentially, the FRN following ambiguous outcomes revealed a group difference, and it was no longer significantly larger than the FRN following negative outcomes. In our opinion, the differences in the results reflect the disappearance of the negative wave associated with guessing activity in the current study.
As an alternative interpretation, it is possible that the LTA participants only orient to the ambiguous outcome when explicitly told to do so. In contrast, the HTA participants orient to the ambiguous outcome regardless of whether they were told to or not. Since we don’t have enough evidence to support that our method had successfully controlled the influence of guessing in the current study, a conclusion could not be reached. Nevertheless, the possibility that guessing (or other mental activity) may interfere with anxiety-related responses definitely deserves future investigation.
To sum up, the current ERP results provide evidence that the FRN following ambiguous and negative outcomes is significantly different between HTA and LTA individuals. Additionally, HTA group’s FRN responses following ambiguous outcomes were larger than those following negative outcomes. Our results indicate that the high-anxious people are more likely to assign very negative value to the ambiguous outcomes, compared to low-anxious people. Taken together with our previous study (Gu et al., 2009), guessing or other activity might modulate such negative effect under ambiguity or uncertainty among high-anxious people. These investigations may prove beneficial to the understanding of the underlying features of anxiety and clinical application.
Highlights.
-
➢
The feedback-related negativity in response to negative outcome is smaller in high-anxiety group than low-anxiety group.
-
➢
The feedback-related negativity in response to ambiguous outcome is larger in high-anxiety group than low-anxiety group.
-
➢
The feedback-related negativity in response to neutral outcome is insensitive to level of anxiety.
-
➢
The level of depression is selectively correlated with the feedback-related negativity in response to ambiguous outcome.
Figure 2. Grand-average event-related potentials evoked by the presentation of feedback at the FCz recording site.
Positive, negative, neutral, ambiguous: valence of the outcome.
Figure 3. Three types of ERP difference waves of HTA and LTA groups at the FCz site (left panel), as well as the corresponding scalp topographies (right panel).
The gray shaded areas indicate the 200-400 ms analysis window in which the FRN was quantified.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (30930031), Key Technologies R & D Program (2009BAI77B01), Ministry of Education (PCSIRT, IRT0710), Global Research Initiative Program, United States National Institute of Health (1R01TW007897), and Scientific Research Foundation of Beijing Normal University (2009SAP-8). We sincerely thank JT Smith for assistance in manuscript editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Bar-Haim Y, Lamy D, Glickman S. Attentional bias in anxiety: A behavioral and ERP study. Brain and Cognition. 2005;59(1):11–22. doi: 10.1016/j.bandc.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Daum I. Learning-related changes in reward expectancy are reflected in the feedback-related negativity. European Journal of Neuroscience. 2008;27(7):1823–1835. doi: 10.1111/j.1460-9568.2008.06138.x. [DOI] [PubMed] [Google Scholar]
- Bensi L, Giusberti F. Trait anxiety and reasoning under uncertainty. Personality and Individual Differences. 2007;43(4):827–838. [Google Scholar]
- Blader SL. What determines people’s fairness judgments? Identification and outcomes influence procedural justice evaluations under uncertainty. Journal of Experimental Social Psychology. 2007;43(6):986–994. [Google Scholar]
- Blanchette I, Richards A. Anxiety and the interpretation of ambiguous information: Beyond the emotion-congruent effect. Journal of Experimental Psychology-General. 2003;132(2):294–309. doi: 10.1037/0096-3445.132.2.294. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage. 2007;35(2):968–978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Ranganath C. Reinforcement learning signals predict future decisions. Journal of Neuroscience. 2007;27(2):371–378. doi: 10.1523/JNEUROSCI.4421-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming SR, Harris LM. The impact of anxiety on the accuracy of diagnostic decision-making. Stress and Health. 2001;17(5):281–286. [Google Scholar]
- Eisenberg AE, Baron J, Seligman MEP. Individual difference in risk aversion and anxiety. Psychological Bulletin. 1998;87:245–251. [Google Scholar]
- Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology. 2009;81(1):1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. Medial prefrontal cortex and error potentials - Response. Science. 2002;296(5573):1611–1611. doi: 10.1126/science.296.5573.1610. [DOI] [PubMed] [Google Scholar]
- Gu R, Huang Y.-x., Luo Y.-j. Anxiety and feedback negativity. Pschopsysiology. doi: 10.1111/j.1469-8986.2010.00997.x. in press. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biological Psychology. 2006;71(2):148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. It’s worse than you thought: The feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology. 2007;44(6):905–912. doi: 10.1111/j.1469-8986.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Hirsh JB, Inzlicht M. The Devil You Know: Neuroticism Predicts Neural Response to Uncertainty. Psychological Science. 2008;19(10):962–967. doi: 10.1111/j.1467-9280.2008.02183.x. [DOI] [PubMed] [Google Scholar]
- Hockey GRJ, Maule AJ, Clough PJ, Bdzola L. Effects of negative mood states on risk in everyday decision making. Cognition & Emotion. 2000;14(6):823–855. [Google Scholar]
- Holroyd CB, Baker TE, Kerns KA, Muller U. Electrophysiological evidence of atypical motivation and reward processing in children with attention-deficit hyperactivity disorder. Neuropsychologia. 2008;46(8):2234–2242. doi: 10.1016/j.neuropsychologia.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Hajcak G, Larsen JT. The good, the bad and the neutral: Electrophysiological responses to feedback stimuli. Brain Research. 2006;1105:93–101. doi: 10.1016/j.brainres.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Krigolson OE. Reward prediction error signals associated with a modified time estimation task. Psychophysiology. 2007;44(6):913–917. doi: 10.1111/j.1469-8986.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- Isen AM, Nygen TE, Ashby FG. Influence of positive affect on the subjective utility of gains and losses: It is just not worth the risk. Journal of Personality and Social Psychology. 1988;55(5):710–717. doi: 10.1037//0022-3514.55.5.710. [DOI] [PubMed] [Google Scholar]
- Krain AL, Gotimer K, Hefton S, Ernst M, Castellanos FX, Pine DS, et al. A functional magnetic resonance imaging investigation of uncertainty in adolescents with anxiety disorders. Biological Psychiatry. 2008;63(6):563–568. doi: 10.1016/j.biopsych.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Kverno KS. Trait anxiety influences on judgments of frequency and recall. Personality and Individual Differences. 2000;29(3):395–404. [Google Scholar]
- Lawson C, MacLeod C. Depression and the interpretation of ambiguity. Behav Res Ther. 1999;37(5):463–474. doi: 10.1016/s0005-7967(98)00131-4. [DOI] [PubMed] [Google Scholar]
- Li XY, Li XB, Luo YJ. Anxiety and attentional bias for threat: an event-related potential study. Neuroreport. 2005;16(13):1501–1505. doi: 10.1097/01.wnr.0000176522.26971.83. [DOI] [PubMed] [Google Scholar]
- Lipshitz R, Gilad Z, Suleiman R. The one-of-us effect in decision evaluation. Acta Psychologica. 2001;108(1):53–71. doi: 10.1016/s0001-6918(00)00072-x. [DOI] [PubMed] [Google Scholar]
- Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychological Bulletin. 2001;127(2):267–286. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- Maner JK, Schmidt NB. The role of risk avoidance in anxiety. Behavior Therapy. 2006;37(2):181–189. doi: 10.1016/j.beth.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Miller JD, Pilkonis PA. Neuroticism and affective instability: The same or different? American Journal of Psychiatry. 2006;163(5):839–845. doi: 10.1176/ajp.2006.163.5.839. [DOI] [PubMed] [Google Scholar]
- Mitte K. Anxiety and risk decision-making: The role of subjective probability and subjective cost of negative events. Personality and Individual Differences. 2007;43(2):243–253. [Google Scholar]
- Nesse RM. Natural selection and the regulation of defenses - A signal detection analysis of the smoke detector principle. Evolution and Human Behavior. 2005;26(1):88–105. [Google Scholar]
- Nieuwenhuis S, Holroyd CB, Mol N, Coles MGH. Reinforcement-related brain potentials from medial frontal cortex: origins and functional significance. Neuroscience and Biobehavioral Reviews. 2004;28(4):441–448. doi: 10.1016/j.neubiorev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Neurobiology of decision-making: Quo vadis? Cognitive Brain Research. 2005;23(1):2–10. doi: 10.1016/j.cogbrainres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Simmons A, Stein MB. Anterior cingulate activation in high trait anxious subjects is related to altered error processing during decision making. Biological Psychiatry. 2004;55(12):1179–1187. doi: 10.1016/j.biopsych.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Platt ML. Neural correlates of decisions. Current Opinion in Neurobiology. 2002;12(2):141–148. doi: 10.1016/s0959-4388(02)00302-1. [DOI] [PubMed] [Google Scholar]
- Polezzi D, Lotto L, Daum I, Sartori G, Rumiati R. Predicting outcome of decisions in the brain. Behavioural Brain Research. 2008;187:116–122. doi: 10.1016/j.bbr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Richards A, French CC, Calder AJ, Webb B, Fox R, Young AW. Anxiety-related bias in the classification of emotionally ambiguous facial expressions. Emotion. 2002;2(3):273–287. doi: 10.1037/1528-3542.2.3.273. [DOI] [PubMed] [Google Scholar]
- Righi S, Mecacci L, Viggiano MP. Anxiety, cognitive self-evaluation and performance: ERP correlates. Journal of Anxiety Disorders. 2009;23(8):1132–1138. doi: 10.1016/j.janxdis.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Sato A, Yasuda A, Ohira H, Miyawaki K, Nishikawa M, Kumano H, et al. Effects of value and reward magnitude on feedback negativity and P300. Neuroreport. 2005;16(4):407–411. doi: 10.1097/00001756-200503150-00020. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A Solution for Reliable and Valid Reduction of Ocular Artifacts, Applied to the P300 Erp. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shek DT. The Chinese version of the State-Trait Anxiety Inventory: its relationship to different measures of psychological well-being. J Clin Psychol. 1993;49(3):349–358. doi: 10.1002/1097-4679(199305)49:3<349::aid-jclp2270490308>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Shepperd JA, Grace J, Cole LJ, Klein C. Anxiety and outcome predictions. Personality and Social Psychology Bulletin. 2005;31(2):267–275. doi: 10.1177/0146167204271322. [DOI] [PubMed] [Google Scholar]
- Shu L. Self-rating depression scale and depression status inventory. Chinese Journal of Mental Health. 1993;7(Supplement):160–162. [Google Scholar]
- Simons RF. The way of our errors: Theme and variations. Psychophysiology. 2010;47(1):1–14. doi: 10.1111/j.1469-8986.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. Consulting Psychologist Press; Palo Alto, CA: 1983. [Google Scholar]
- Voss A, Rothermund K, Brandtstadter J. Interpreting ambiguous stimuli: Separating perceptual and judgmental biases. Journal of Experimental Social Psychology. 2008;44(4):1048–1056. [Google Scholar]
- Yeung N, Holroyd CB, Cohen JD. ERP correlates of feedback and reward processing in the presence and absence of response choice. Cerebral Cortex. 2005;15(5):535–544. doi: 10.1093/cercor/bhh153. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. Journal of Neuroscience. 2004;24(28):6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung WW, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch Gen Psychiatry. 1965;13(6):508–515. doi: 10.1001/archpsyc.1965.01730060026004. [DOI] [PubMed] [Google Scholar]