Abstract
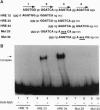
Alu repeats are interspersed repetitive DNA elements specific to primates that are present in 500,000 to 1 million copies. We show here that an Alu sequence encodes functional binding sites for retinoic acid receptors, which are members of the nuclear receptor family of transcription factors. The consensus sequences for the evolutionarily recent Alu subclasses contain three hexamer half sites, related to the consensus AGGTCA, arranged as direct repeats with a spacing of 2 bp, which is consistent with the binding specificities of retinoic acid receptors. An analysis was made of the DNA binding and transactivation potential of these sites from an Alu sequence that has been previously implicated in the regulation of the keratin K18 gene. These Alu double half sites are shown to bind bacterially synthesized retinoic acid receptors as assayed by electrophoretic mobility shift assays. These sites are further shown to function as a retinoic acid response element in transiently transfected CV-1 cells, increasing transcription of a reporter gene by a factor of approximately 35-fold. This transactivation requires cotransfection with vectors expressing retinoic acid receptors, as well as the presence of all-trans-retinoic acid, which is consistent with the known function of retinoic acid receptors as ligand-inducible transcription factors. The random insertion of potentially thousands of Alu repeats containing retinoic acid response elements throughout the primate genome is likely to have altered the expression of numerous genes, thereby contributing to evolutionary potential.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brini A. T., Lee G. M., Kinet J. P. Involvement of Alu sequences in the cell-specific regulation of transcription of the gamma chain of Fc and T cell receptors. J Biol Chem. 1993 Jan 15;268(2):1355–1361. [PubMed] [Google Scholar]
- Britten R. J., Baron W. F., Stout D. B., Davidson E. H. Sources and evolution of human Alu repeated sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4770–4774. doi: 10.1073/pnas.85.13.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Britten R. J. Evidence that most human Alu sequences were inserted in a process that ceased about 30 million years ago. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6148–6150. doi: 10.1073/pnas.91.13.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J. Evolutionary selection against change in many Alu repeat sequences interspersed through primate genomes. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5992–5996. doi: 10.1073/pnas.91.13.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D. P., Ross J. Human beta-globin promoter and coding sequences transcribed by RNA polymerase III. Cell. 1983 Oct;34(3):857–864. doi: 10.1016/0092-8674(83)90543-3. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Batzer M. A., Hutchison C. A., 3rd, Edgell M. H. Master genes in mammalian repetitive DNA amplification. Trends Genet. 1992 Sep;8(9):307–311. doi: 10.1016/0168-9525(92)90262-3. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Devary O. V., Rosenfeld M. G. Multiple cell type-specific proteins differentially regulate target sequence recognition by the alpha retinoic acid receptor. Cell. 1990 Nov 16;63(4):729–738. doi: 10.1016/0092-8674(90)90139-6. [DOI] [PubMed] [Google Scholar]
- Hambor J. E., Mennone J., Coon M. E., Hanke J. H., Kavathas P. Identification and characterization of an Alu-containing, T-cell-specific enhancer located in the last intron of the human CD8 alpha gene. Mol Cell Biol. 1993 Nov;13(11):7056–7070. doi: 10.1128/mcb.13.11.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. H., Sakamoto K. Alu interspersed repeats: selfish DNA or a functional gene family? New Biol. 1990 Sep;2(9):759–770. [PubMed] [Google Scholar]
- Hull M. W., Erickson J., Johnston M., Engelke D. R. tRNA genes as transcriptional repressor elements. Mol Cell Biol. 1994 Feb;14(2):1266–1277. doi: 10.1128/mcb.14.2.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J., Smith T. A fundamental division in the Alu family of repeated sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4775–4778. doi: 10.1073/pnas.85.13.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa R., Yu V. C., När A., Kyakumoto S., Han Z., Silverman S., Rosenfeld M. G., Glass C. K. Differential orientations of the DNA-binding domain and carboxy-terminal dimerization interface regulate binding site selection by nuclear receptor heterodimers. Genes Dev. 1993 Jul;7(7B):1423–1435. doi: 10.1101/gad.7.7b.1423. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992 Oct;17(10):427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- Neznanov N. S., Oshima R. G. cis regulation of the keratin 18 gene in transgenic mice. Mol Cell Biol. 1993 Mar;13(3):1815–1823. doi: 10.1128/mcb.13.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel L. E., Crick F. H. Selfish DNA: the ultimate parasite. Nature. 1980 Apr 17;284(5757):604–607. doi: 10.1038/284604a0. [DOI] [PubMed] [Google Scholar]
- Oshima R. G., Abrams L., Kulesh D. Activation of an intron enhancer within the keratin 18 gene by expression of c-fos and c-jun in undifferentiated F9 embryonal carcinoma cells. Genes Dev. 1990 May;4(5):835–848. doi: 10.1101/gad.4.5.835. [DOI] [PubMed] [Google Scholar]
- Oshima R. G., Trevor K., Shevinsky L. H., Ryder O. A., Ceceña G. Identification of the gene coding for the Endo B murine cytokeratin and its methylated, stable inactive state in mouse nonepithelial cells. Genes Dev. 1988 May;2(5):505–516. doi: 10.1101/gad.2.5.505. [DOI] [PubMed] [Google Scholar]
- Pfahl M. Nuclear receptor/AP-1 interaction. Endocr Rev. 1993 Oct;14(5):651–658. doi: 10.1210/edrv-14-5-651. [DOI] [PubMed] [Google Scholar]
- Pfahl M., Tzukerman M., Zhang X. K., Lehmann J. M., Hermann T., Wills K. N., Graupner G. Nuclear retinoic acid receptors: cloning, analysis, and function. Methods Enzymol. 1990;189:256–270. doi: 10.1016/0076-6879(90)89297-u. [DOI] [PubMed] [Google Scholar]
- Saffer J. D., Thurston S. J. A negative regulatory element with properties similar to those of enhancers is contained within an Alu sequence. Mol Cell Biol. 1989 Feb;9(2):355–364. doi: 10.1128/mcb.9.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C., Maraia R. Transcriptional regulation and transpositional selection of active SINE sequences. Curr Opin Genet Dev. 1992 Dec;2(6):874–882. doi: 10.1016/s0959-437x(05)80110-8. [DOI] [PubMed] [Google Scholar]
- Slagel V. K., Deininger P. L. In vivo transcription of a cloned prosimian primate SINE sequence. Nucleic Acids Res. 1989 Nov 11;17(21):8669–8682. doi: 10.1093/nar/17.21.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G., Milner R. J., Gottesfeld J. M., Reynolds W. Control of neuronal gene expression. Science. 1984 Sep 21;225(4668):1308–1315. doi: 10.1126/science.6474179. [DOI] [PubMed] [Google Scholar]
- Thorey I. S., Ceceña G., Reynolds W., Oshima R. G. Alu sequence involvement in transcriptional insulation of the keratin 18 gene in transgenic mice. Mol Cell Biol. 1993 Nov;13(11):6742–6751. doi: 10.1128/mcb.13.11.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomilin N. V., Iguchi-Ariga S. M., Ariga H. Transcription and replication silencer element is present within conserved region of human Alu repeats interacting with nuclear protein. FEBS Lett. 1990 Apr 9;263(1):69–72. doi: 10.1016/0014-5793(90)80707-p. [DOI] [PubMed] [Google Scholar]
- Tran P., Zhang X. K., Salbert G., Hermann T., Lehmann J. M., Pfahl M. COUP orphan receptors are negative regulators of retinoic acid response pathways. Mol Cell Biol. 1992 Oct;12(10):4666–4676. doi: 10.1128/mcb.12.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullu E., Weiner A. M. Upstream sequences modulate the internal promoter of the human 7SL RNA gene. 1985 Nov 28-Dec 4Nature. 318(6044):371–374. doi: 10.1038/318371a0. [DOI] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- White R. J., Stott D., Rigby P. W. Regulation of RNA polymerase III transcription in response to F9 embryonal carcinoma stem cell differentiation. Cell. 1989 Dec 22;59(6):1081–1092. doi: 10.1016/0092-8674(89)90764-2. [DOI] [PubMed] [Google Scholar]
- Wu J., Grindlay G. J., Bushel P., Mendelsohn L., Allan M. Negative regulation of the human epsilon-globin gene by transcriptional interference: role of an Alu repetitive element. Mol Cell Biol. 1990 Mar;10(3):1209–1216. doi: 10.1128/mcb.10.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]