Abstract
We have found that a small number of purified Th2-biased Aβ-specific T cells are sufficient to provide profound cognitive and pathological benefits in an APP+PS1 mouse model for Alzheimer’s disease. Six weeks after receiving T cell infusions, cognitively-impaired mice performed significantly better in working memory tasks, which correlated with higher plasma levels of soluble Aβ. Pathological analysis of the hippocampus revealed a 30% decrease of plaque- associated microglia and less vascular amyloidosis in T cell treated mice. The infusion of Aβ-specific Th2 cells also reduced plasma levels of IFN-γ, TNF-α, GM-CSF, IL-2 and IL-4, which are elevated in untreated APP+PS1 mice. No significant immune cell infiltration and no anti-Abeta antibody titers occurred in the T cell treated mice. These results demonstrate that Aβ-specific Th2 cells are sufficient to reverse cognitive impairment and provide multiple pathological benefits in an Alzheimer’s mouse model.
Introduction
The elevation of immune responses to amyloid-beta (Aβ) underlies several promising therapeutic strategies for the treatment of Alzheimer’s disease (AD). Vaccinations with Aβ plus adjuvant trigger adaptive immune responses that improve cognitive performance and lessen brain pathology in mouse models of AD (Schenk et al., 1999; Morgan et al., 2000). A clinical trial that vaccinated patients with Aβ1–42 (AN1792) was initially promising, but was halted after several patients developed aseptic meningoencephalitis. Subsequent analysis revealed that brain inflammation in those patients may have resulted from robust inflammatory T helper type-1 (Th1) responses due to the inclusion of polysorbate-80 in the vaccine formula (Orgogozo et al., 2003). Since then, many immunotherapeutic strategies have tried to limit T cell responses by employing anti-Aβ-specific monoclonal antibodies to provide passive immunity (Bayer et al., 2005). Although large doses of these antibodies are used, beneficial effects result from an ill-defined peripheral mechanism as antibodies are largely excluded from the brain parenchyma by the blood brain barrier (BBB). Recently, we have reported that non-inflammatory T cell responses to Aβ may provide long-term beneficial effects without encephalitogenic side-effects (Ethell et al., 2006).
Within the brain of Alzheimer’s patients, inflammatory responses associated with amyloid plaques consist of local innate immune mediators such as reactive astrocytes and activated microglia. The absence of activated T cells, neutrophils, and immunoglobulins within the parenchyma suggest that chronic AD pathology has minimal involvement by adaptive immune responders such as inflammatory (Th1) or allergy (Th2) mediating T cells. We previously reported that Aβ-specific T cells can reverse cognitive impairment and provide multiple pathological benefits when transferred into the APP+PS1 mouse model, without significant infiltration of the CNS (Ethell et al., 2006). These beneficial effects include the restoration of near normal working memory and fewer plaque-associated microglia in the hippocampus. Infusions containing both Th1 and Th2 cells were beneficial, while those biased toward Th1 or depleted of CD4+ T cells were not. We also found that infusions from mice that were vaccinated with an exogenous antigen (i.e. chicken ovalbumin) did not provide any cognitive benefits, indicating the effects were antigen-specific. APP+PS1 mice treated with Aβ-specific T cells did not show any signs of immune cell infiltration within the CNS as seen in the AN1792 trial. These findings suggest that Aβ-specific effector T cells can improve cognitive performance and slow pathological progression in a mouse model for AD, and underscore that allergy-mediating Th2 cells may be more relevant than inflammation-mediating Th1 cells for these benefits.
Here we report that purified Aβ-specific Th2 cells are necessary and sufficient to provide cognitive and pathological benefits in the APP+PS1 mouse model. Primary lymphocytes and splenocytes were isolated from Aβ-vaccinated donors, re-stimulated in vitro to bias a Th2 profile, and then T cells were purified with magnetic beads. Cognitively-impaired APP+PS1 mice received only 1.5% as many cells as in our previous studies, yet the beneficial effects were at least as significant. Mice were behaviorally tested starting 6 weeks after infusion, with extensive biochemistry and histopathology done thereafter.
Methods and Materials
Mice
All mice were obtained from a 5th generation cross between heterozygous APPK670N,M671L and heterozygous PS1 transgenic mice (line 6.2 bearing the M146L mutation). The backgrounds of all offspring were an identical mix of C57/B6/SJL/Swiss Webster. Mice were genotyped and singly housed prior to the beginning of the study. All mice were maintained on a 12-h light-dark cycle, with free access to rodent chow and water. Behavioral testing was performed during the light period, by a researcher blind to animal genotypes or treatment. All animal procedures were performed in AAALAC-certified facilities under protocols approved by Institutional Animal Care and Use Committees at USF and UCR.
General Protocol
A total of 18 double transgenic APP+PS1 mice and 10 non-transgenic (NT) littermates, aged ~11 months, were pre-tested in the radial arm water maze (RAWM) test of working memory for 14 days. APP+PS1 mice were then divided into two groups, equally balanced in working memory performance, with one group receiving a single infusion of Aβ-sensitized Th2 cell and the other group receiving vehicle infusion (as did all NT mice). At 6 weeks following infusion, all mice were re-evaluated in the RAWM task for 9 days (5 trials/day, 3 days/block, 3 different blocks) and tested in platform recognition for an additional 4 days, after which they were euthanized and the brains processed for histological and neurochemical analysis.
Isolation and purification of Aβ-specific Th2 cells from donors
Congenic donor mice were vaccinated with 150 μg Aβ1–42 in 100 μL of complete Freund’s adjuvant over 3 sites on the pre-shaved back. Ten days after injection, donor mice were sacrificed, with the harvesting of spleen and lymph nodes done quickly. Primary splenocytes and lymphocytes were prepared and cultured as previously described (Ethell et al., 2006). Mixed cultures were re-stimulated for 4 days in vitro with 20 μg/ml Aβ1–42, and 10 ng/mL IL-2 and 5 ng/mL IL-4. This step expands the antigen-specific population and is a classic method of forcing CD4+ T cells to assume a Th2 phenotype (Ansel et al., 2006). T cells were purified using a pan-T-cell separation kit (#130-090-861, Miltenyi, Auburn, CA), LS-columns (#130-042-461, Miltenyi) and a Midi-MACS magnetic separator (Miltenyi). For each 20×106 viable cells, we isolated 3×105 pure T cells, which were visually checked for morphological homogeneity. Once isolated, T cells were placed in fresh media with 10 ng/mL IL-2 overnight. The next day, T cells were concentrated, re-suspended in PBS (3×105/mL), and injected into cognitively-impaired APP+PS1 mice. Mice were injected twice, one week apart. Each injection contained 1.5×105 purified T cells, so each mouse received a total of 3×105 T cells. Cells were injected into the tail vein of recipient mice, with each infusion consisting of 0.2 mL total volume. Control Tg and NT mice received equal volumes of PBS.
Behavioral Testing
Mice were tested for hippocampus-dependent memory using the RAWM, as previously described (Ethell et al., 2006; Arendash et al., 2006). Briefly, a 6-arm insert is placed into a 100 cm diameter circular pool filled with water. The maze contains a hidden (submerged) platform in one arm and the mice are placed into a different arm. Each time the mouse enters a wrong arm it is an error and the mice are taken back to the starting arm for that trial. Using visual cues from the room, mice learn which arm contains the hidden platform such that the number of wrong arm entries (i.e. errors) made on the 4th and 5th trials (T4 and T5) directly index cognitive performance. Each day of testing consists of 5 trials (4 consecutive trials and a 5th trial following a 30 minute delay) in which the location of the hidden platform remains the same, but the starting arm is changed with each trial. A different goal arm and sequence of start arms are semi-randomly determined each day. During each trial (60 s maximum), the mouse was returned to that trial’s start arm upon swimming into an incorrect arm and the number of seconds required to locate the submerged platform was recorded. If the mouse did not find the platform within a 60-s trial, it was guided to the platform for the 30-s stay. Since mice do not know the new location of the platform for the first trial of any given day, trial 1 is a “naïve” trial. By contrast, the numbers of errors during trials 4 and 5 are both considered indices of working memory and are temporally similar to the standard registration/recall testing of specific items used clinically in evaluating AD patients. Thus, T1, T4, and T5 were statistically evaluated (see statistical analysis). Mice were tested for 14 consecutive days prior to injection of PBS or Aβ-specific T cells and for 9 consecutive days (three sets of 3-day blocks) 1.5 months following injection. There were no group differences in swim speed during RAWM testing.
Immediately following RAWM testing, animals were tested for search and identification ability in the platform recognition task. Time required (latency) to find an elevated platform (9 cm diameter), which has a prominent ensign, was determined over 4 daily trials, with the platform moved to a different location (one of four possible) for each trial (60 sec max). Averaged latencies for the first and last (4th) day of testing were compared for each group.
Pathological Processing
Immediately after the final day of behavioral testing, each mouse was deeply anesthetized and blood taken from the heart. The mouse was intracardially-perfused with heparinized saline, and the brain excised. Half of the brain was placed into 4% paraformaldehyde in PBS, and the other half dissected into hippocampus, frontal lobe and parietal lobe for biochemical analysis, and then frozen at −80°C until use.
Blood and Hippocampal Neurochemistry
After extraction, the blood was immediately placed in a vial with EDTA and fractioned into plasma and cells by centrifugation. Plasma samples were frozen until use. Plasma aliquots were assayed for soluble Aβ1–40 and Aβ1–42, using an established ELISA assay (Invitrogen, CA). Plasma cytokine levels were assessed from this sample, using Luminex assay of Bio-plex for mouse IL-2, IL-4, IL-5, IL-10, IL12p70, GM-CSF, INF-γ, and TNF-α (Bio-Rad, CA). ELISA-based determination of plasma anti-Aβ antibody levels from blood taken at euthanasia was performed according to our established methodology (Ethell et al., 2006). Hippocampus tissue was homogenized, extracted, and then assayed for soluble and insoluble Aβ1–40 and Aβ1–42, as previously described (Ethell et al., 2006).
Histopathology
Paraformaldehyde fixed half-brains were cryo-protected in serial sucrose solutions of 10% and 30%. The frontal cortex was removed, dehydrated and embedded with resin for thin sectioning (6 μm) using a rotary microtome and processed for hematoxylin and eosin staining. The remaining half-brain was cut into 25 μm coronal sections with a cryostat. Coronal sections from the mid-thalamus were used for all immunostaining and thioflavin-S staining.
Thioflavin-S staining of dense-core deposits and CAA
Mounted sections were stained by immersion in 0.25% thioflavin-S (50% EtOH/50% PBS) for 60 s and then destained with 50% EtOH/50% PBS. Coverglass was mounted over the stained sections using vectashield with DAPI (Vector Labs), and Cytoseal (Company). Hippocampal areas in stained sections from each mouse were imaged using a Nikon fluorescence microscope fitted with a 20x Fluor objective, and a Hamamatsu cooled CCD camera running on Image-Pro software. Dense core deposits were identified using NIH-ImageJ, excluding vascular deposits, in 3–5 sections from each mouse and calculated as a percentage of the hippocampus area in that section.
Vascular deposits (cerebral amyloid angiopathy; CAA) were scored for thioflavin-S staining using these same sections. Small arteries and arterioles were identified by the arrangement of DAPI-stained nuclei (running parallel with Schaffer’s collaterals) and each vessel was scored for the presence or absence of thioflavin-S staining. Percentages were obtained in 5 sections from each mouse and averaged with the percentages of all other mice from that group.
Immunostaining for CD3
Coronal brain sections were immunostained for T cells using an anti-CD3 antibody. Sections were mounted and dried on charged glass slides, then rehydrated with PBS. Antigen recovery was done by immersing mounted sections in 10 mM sodium citrate (pH=8.3) at 80°C for 30 min, then allowed to cool slowly. Sections were rinsed with PBS, treated with 3% H2O2 for 10 min to remove residual peroxidase activity, and then rinsed again with PBS. Sections were permeabilized with 1% NP-40/0.1% Triton X-100 for 10 min, rinsed 3x with PBS, then blocked with 10% normal goat serum, and 10 μg/ml human Fc. Samples were incubated in a humid chamber at 4°C overnight with 1:1000 anti-CD3 (C7930, Sigma) in PBS + 1% normal goat serum. Sections were rinsed 3x with PBST (0.05% tween20), then incubated with biotinylated anti-mouse antibody and ABC reagent, as per the manufacturers protocols (Vector Labs). CD3 immunostaining was developed using diaminobenzidine for 20 min.
Microglia staining and analysis
Plaque-associated microglia were assessed as previously described (Ethell et al., 2006). Briefly, coronal sections through the hippocampus were mounted and dried onto charged glass slides, incubated overnight in 10 μg/mL biotinylated tomato lectin, and then developed with ABC reagent and developed with diaminobenzidine for 10 min.
Statistical analysis
For statistical analysis of RAWM data, the 14-days of pre-treatment testing or the 9-days of post-treatment testing were evaluated over all days of testing, as well as for the last 3-day block of testing, using one-way ANOVAs. Thereafter, post hoc pair-by-pair differences between groups were resolved with the Fisher LSD (least significant difference) test. Pre- vs. post-treatment RAWM performance for each group, as well as first vs. last day performance for each group in both RAWM and platform recognition testing, was evaluated using paired student’s t-test. Although only error data is presented and analyzed for RAWM performance, escape latency data agreed closely with the error day for individual animals and groups. To avoid redundancy in data presentation, only the error data are presented. All group comparisons involving neuropathologic and neurochemical measures involved one-way ANOVAs with post hoc Fisher’s LSD test or student’s t-tests.
Results
Purified Aβ-specific Th2 cells improve the cognitive performance of APP+PS1 mice
Hippocampus-dependent working memory is required for mice to locate a hidden platform in the RAWM, such that a high number of errors in the 4th and 5th trials (T4, T5) indicate cognitive impairment. Pre-infusion testing of APP+PS1 and non-transgenic (NT) mice confirmed significant (p<0.05) cognitive impairment in 11 month old transgenic mice, as shown by more errors in T4+T5 and T5 alone (Fig. 1A). Post-infusion testing (1½–2 months later) showed further declines in the performance of APP+PS1 mice that received vehicle alone (Tg), compared with NT controls (Fig. 1B). By contrast, APP+PS1 mice that received Th2 cells (Tg/Th2) showed working memory performance that was similar to NT controls by block 2, and significantly better than Tg controls by block 3. The marked improvement of Tg/Th2 mice was underscored by significantly fewer errors in T4 and T5 than Tg mice over all days of post-infusion testing (Fig. 1C). Thus, Tg/Th2 mice progressively improved their working memory performance during RAWM testing, while Tg controls could not do so. This progressive improvement of Tg/Th2 mice was underscored by 1) significantly fewer T4 and T5 errors than Tg mice over all days of post-infusion testing (Fig. 1C) and 2) overall differences in Tg/Th2 vs. NT group performances due to block 1, wherein both Tg groups were impaired (Fig. 1B,C). Nonetheless, Tg mice did not reduce their overall number of T5 errors in pre- versus post-injection RAWM testing (Fig. 1D), while Tg/Th2 mice showed significantly better post-injection performance, as did NT controls.
Figure 1. Adoptive transfer of Aβ-specific Th2 cells improves the cognitive performance of APP+PS1 mice in several tasks at 1½-2 M following infusion.
(A) Pre-testing of ~11 month old APP+PS1 in the RAWM task confirmed working memory impairment in comparison to NT control mice. Tg mice made a higher number of averaged Trial 4 plus Trial 5 (T4+T5) errors on the last block of testing, as well as a greater number of T5 errors over all 14 days of pre-testing. *p<0.05; **p<0.005. (B) Over three 3-day blocks of post-treatment RAWM testing, NT mice quickly learned to reduce their number of errors between the first naïve trial (T1) and the two working memory trials (T4, T5). Tg/Th2 mice became as proficient as NT mice by Blocks 2 and 3, while Tg controls remained impaired across all 3 Blocks. † = both Tg and Tg/Th2 groups impaired versus NT at p<0.02; * = Tg impaired versus NT at p<0.05; ** = Tg impaired versus both other groups at p<0.05 or higher level of significance. (C) Overall T4 and T5 errors during RAWM post-treatment testing. Tg mice were markedly impaired for both trials, while Tg/Th2 mice showed significantly better working memory for both trials. *p<0.001 versus NT; **p<0.05 versus both other groups. (D) A comparison of “overall” T5 performance pre- vs. post-injection for each group. Only NT and Th2 groups showed significantly improved working memory. *p<0.05 for pre- versus post-injection T5 errors. (E) Platform recognition testing revealed that only NT and Tg/Th2 mice could improve their search/identification skills, as evidenced by a significantly shorter escape latencies between the first and last day of testing. *p<0.05 or higher significance versus first day performance.
In the platform recognition task, administered following RAWM testing, animals must switch from the spatial strategy of RAWM testing to a search/identification strategy. Tg/Th2 and NT mice located the escape platform more quickly subsequent to the initial day of testing, while the impairment of Tg mice was evident by similar escape latencies on the first and last days of testing (Fig. 1E).
Adoptive transfer lowers plaque-associated microglia in the hippocampus, but does not decrease dense-core Aβ deposits
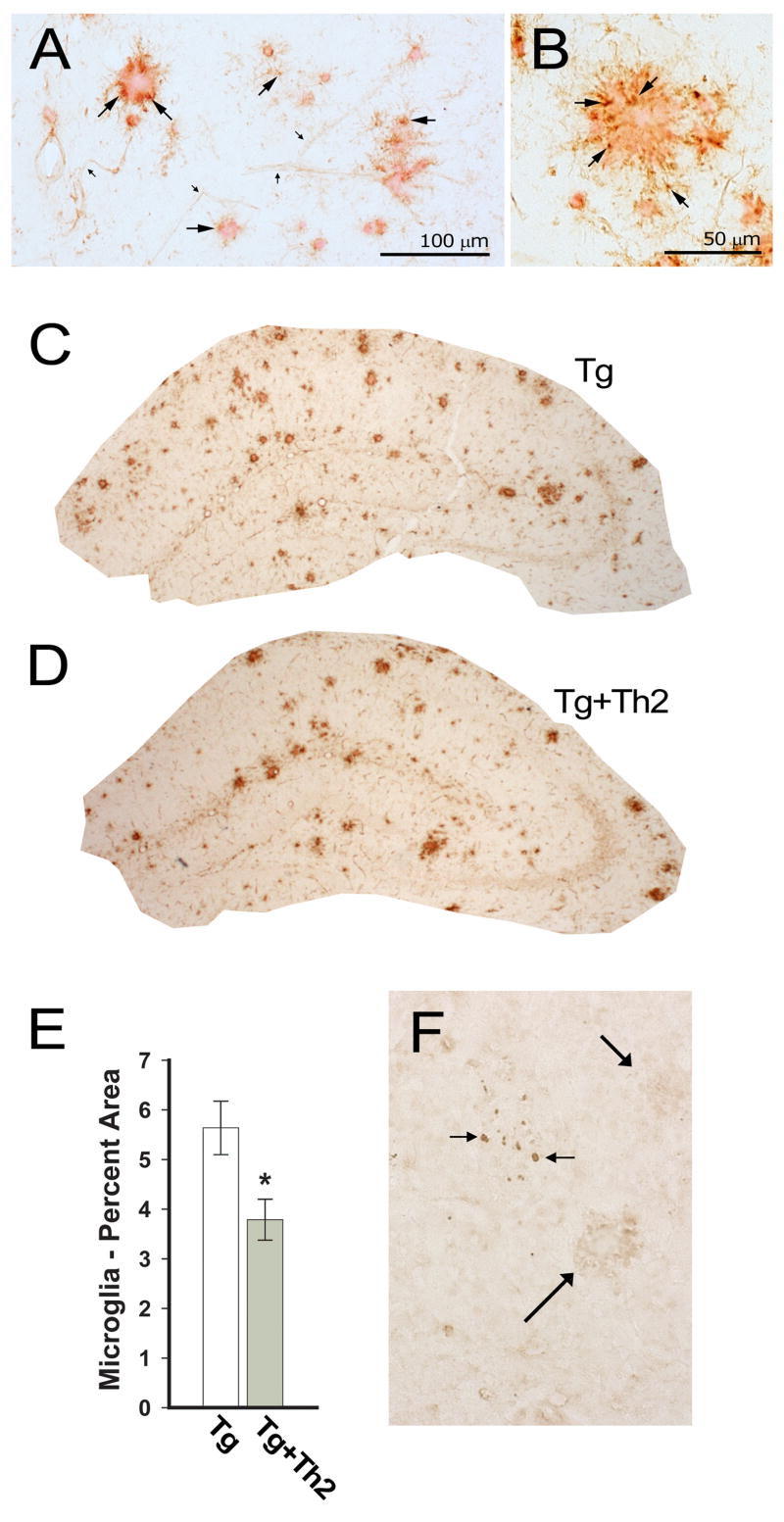
We previously reported that plaque-associated microglia are reduced by the adoptive transfer of Aβ-specific splenocytes/lymphocytes, containing both Th1 and Th2 cells. In this study we also used tomato lectin to stain for microglia (cell bodies and processes) and visualize the shells they tend make around dense core deposits of Aβ (Fig. 2A, B, Suppl. fig. 1). We found that infusions of purified Aβ-specific Th2 cells (Tg/Th2) reduced plaque-associated microglia in the hippocampus by >30%, compared with Tg controls (Fig. 2C–E). Although large and small plaques shells were still present, the proportion of hippocampus covered by them is lower in Tg/Th2 mice (Fig. 2D). Interestingly, this reduction in microglial staining (p<0.05) was not accompanied by any significant difference in thioflavin S-staining of dense-core Aβ, within the hippocampus of Tg or Tg/Th2 mice (not shown). These findings reveal that plaque-associated microglia are reduced by the adoptive transfer of Aβ-responsive Th2 cells, but this reduction is not dependent on changes in dense-core Aβ loads.
Figure 2. Th2-treated APP+PS1 mice show fewer plaque-associated microglia and no significant infiltration of CD3+ T cells.
(A, B) Tomato lectin staining (brown) of microglia surrounding Congo red stained deposits of Aβ. Several microglial cell bodies are designated, with large arrows indicating microglia cell bodies. Small arrows indicate tomato lectin stained blood vessels, which are distinctive from microglia. Scale bars as indicated. (C) Tomato-lectin staining of microglia and blood vessels in the hippocampus of representative control Tg, and (D) Tg/Th2 mice. (E) The area of the hippocampus stained by tomato-lectin, excluding blood vessels, was significantly lower in Tg/Th2 mice, compared with Tg controls (* p<0.05). (F) CD3 immunostaining of the hippocampus revealed a small number of sporadic cell clusters (small arrows) in each group of mice. A cluster of these cells are shown in the hippocampus of a Tg/Th2 mouse; note that they do not cluster around two plaques (large arrows).
Adoptive therapy does not lead to T cell infiltration of the brain
To investigate if reductions in microglia staining may have been caused by infiltrating Aβ-specific Th2 cells, we immunostained brain sections with anti-CD3 to identify all T cells in the parenchyma. A few small clusters of CD3+ T cells were found in the hippocampus of mice in each group, but the numbers were relatively low (3–10 cells/cluster) and they were not seen in all sections nor in all mice of any group (Fig. 2F). Notably, these few small groups of CD3+ cells were not associated with plaques in either Tg or Tg/Th2 mice (Fig. 2F). Likewise, we did not observe any CD3+ cell clustering around the vasculature of any mice, which would have been expected to occur with an encephalitogenic response. Consistent with these findings, hematoxylin and eosin staining of frontal cortex did not show vascular cuffing or any signs of mononuclear cell infiltration in NT, Tg or Tg/Th2 mice (not shown).
Th2 cell transfer recipients had fewer CAA-containing vessels in the hippocampal formation
With age, APP+PS1 mice develop dense-core Aβ deposits in the parenchyma (plaques) and around blood vessels (cerebral amyloid angiopathy/CAA) (Fig. 3A–C). We evaluated thioflavin S staining of microvessels between the dentate gyrus and the hippocampal proper (Fig. 3C). The percentage of these blood vessels that contained thioflavin-S stained deposits was significantly lower in Th2/Tg mice than Tg controls (Fig. 3D). Evaluating all Tg mice irrespective of treatment, showed that mice with lower CAA burdens had significantly lower levels of soluble Aβ1–42 in the contra-lateral hippocampus (Fig. 3E) and better working memory (Fig. 3F).
Figure 3. APP+PS1 mice treated with Th2 cells have a lower proportion of CAA-containing vessels in the hippocampal formation.
(A, B) Examples of thioflavin-S staining (green) reveals extensive CAA in large vessels at the ventral and medial periphery of the hippocampal formation. DAPI staining of all nuclei is blue. (C) CAA-containing microvessels (long arrows) between the dentate gyrus and the hippocampal proper are identifiable by DAPI-stained endothelial cell nuclei that are arranged as the endothelial lining of vessels (short arrows). (D) Percentage of these microvessels with thioflavin-S staining (i.e. CAA) was significantly lower in Tg/Th2 mice than Tg controls (**, p<0.01). (E) Categorizing all Tg and Tg/Th2 mice as having either high or low levels of CAA in the hippocampus revealed significantly more soluble Aβ1–42 in the brains of mice with high levels of CAA (*, p<0.05). (F) Mice with lower levels of CAA in microvessels of the hippocampal formation made fewer working memory errors in the RAWM task during the last block (T4) of post-infusion testing (*p<0.05). T5 results were similar, but not statistically significant.
Aβ-specific Th2 infusions increased plasma levels of Aβ, which correlated with better working memory
Significantly higher plasma levels of Aβ1–40 were seen in Tg/Th2 mice 2 months after transfer (Fig. 4A). Plasma levels of Aβ1–42 were also higher in Tg/Th2 mice than Tg controls (Fig. 4C), although this increase did not reach significance (p=0.06). Correlation analysis revealed highly significant correlations between better working memory and higher plasma levels of Aβ1–40 or Aβ1–42 (Fig. 4B, D). Although hippocampal levels of soluble and insoluble Aβ1–40 and Aβ1–42 trended lower in Tg/Th2 mice they were not significantly different from Tg controls (Fig. 4A, C).
Figure 4. Higher plasma Aβ correlates with superior working memory performance.
(A) Tg/Th2 mice had significantly higher levels of Aβ1–40 in plasma, although hippocampal levels of both soluble and insoluble Aβ1–40 were not significantly different. (B) Higher plasma levels of Aβ1–40 correlated with better working memory for both Tg and Tg/Th2 mice. (C) Plasma levels of Aβ1–42 levels were similarly increased by Th2 cell infusions, though not significant (p=0.06), and hippocampal levels of Aβ1–42 levels were not significantly reduced. (D) Highly significant correlations were present between increased plasma levels of Aβ1–42 and working memory performance in the RAWM task.
High plasma levels of key cytokines in APP+PS1 mice were reduced by Aβ-specific Th2 cell infusion
Compared to NT mice, Tg mice had higher plasma levels of IL-2, IL-4, GM-CSF, IFN-γ, and TNF-α (Fig. 5A). In sharp contrast, plasma levels of these cytokines were significantly reduced in Tg/Th2 mice to levels that were essentially identical to NT control mice. Lower plasma levels of GM-CSF, IFN-γ, TNF-αand IL-2 correlated with better working memory (Fig. 5C). There were significant correlations between higher plasma Aβ levels and lower levels of IL-2, and IFN-γ, (Fig. 5B) in APP+PS1 mice irrespective of treatment. Interestingly, Tg/Th2 mice had undetectably low anti-Aβ antibody titers, using a polyvalent method that detects IgA, IgG and IgM (not shown). The lower plasma cytokine levels of Tg/Th2 mice correlated with higher plasma Aβ levels and better working memory performance, suggesting the involvement of peripheral or vascular mechanisms.
Figure 5. APP+PS1 mice have higher plasma levels of key cytokines that are reduced by Aβ-specific Th2 cell infusion.
(A) Tg control mice exhibited elevated plasma cytokine levels, while Tg/Th2 mice had reduced plasma cytokine levels that were nearly identical to NT controls. **Significantly different from other two groups at p<0.05 or higher level of significance, *Significantly different from Tg/Th2 group at p<0.02. (B) Irrespective of treatment (Tg or Tg/Th2), lower plasma levels of IL-2 or IFN-γ correlated with higher plasma Aβ1–40 and Aβ1–42. (C) There were significant correlations between lower plasma levels of GM-CSF, IFN-γ, TNF-α or IL-2 (all pg/ml), and better working memory (overall/OA post-infusion T5).
Discussion
Immunogenic responses to Aβ are a promising therapeutic avenue for the treatment of AD. This study adds substantially to that knowledge by showing that a relatively small number of Aβ-responsive Th2 cells can reverse cognitive impairment and improve multiple pathological features in the APP+PS1 mouse model of AD. Specifically, Th2 cell infusion resulted in: 1) improved working memory and identification/recognition ability, 2) a reduction in plaque-associated microglia, 3) a decrease in cerebrovascular amyloid deposition with the hippocampus, 4) higher plasma soluble Aβ levels, and 5) a reduction of plasma cytokine levels down to normal. Indeed, by the end of testing, Tg mice that had received Th2 infusion exhibited working memory and identification/recognition ability that was no different from non-transgenic controls. These benefits occurred without inducing measurable anti-Aβ antibodies and without any signs of T cell infiltration into the brain. We conclude that Aβ-specific Th2 cells override an endogenous mechanism that chronically represses adaptive immune responses to Aβ, which may occur peripherally and/or in the cerebrovasculature.
These results support and extend our initial study (Ethell et al., 2006), which showed that a single injection of Aβ-specific Th1+Th2 cells was capable of reversing cognitive impairment for at least 2½months, while also suppressing hippocampal Aβ levels over the same extended time period, without inducing a measurable anti-Aβ antibody response. In that study we also showed antigen-specificity to those responses as infusates from donor mice vaccinated with an exogenous antigen (i.e. chicken ovalbumin) were ineffective. Further, the use of IL-12 during in vitro re-stimulation created a Th1 bias in the infusates and diminished the effectiveness of the transfers, suggesting that Aβ-specific Th1 cells were not the primary mediators of the beneficial effects observed. As predicted from that earlier study, our present study provides clear evidence that Aβ-specific Th2 cells are necessary and sufficient to provide cognitive and pathological benefits to APP+PS1 mice. This result is highly desirable in that Th1-mediated responses (cell immunity) are pro-inflammatory, while Th2 responses (humoral immunity) are anti-inflammatory. The ability of Aβ-responsive Th2 cells to bring the elevated plasma cytokine levels of Tg mice back down to normal underscores this anti-inflammatory action. The AN1792 clinical study involving direct vaccination was halted due to a Th1 driven encephalitogenic responses in some patients (Orgogozo et al., 2003), but our findings indicate that a Th2 response is beneficial and is not accompanied by extensive mononuclear cell infiltration of the CNS. Such anti-inflammatory effects may contribute to the cognitive benefits we have observed since lower plasma cytokine levels were consistently correlated with better working memory in APP+PS1 mice.
Aβ-sensitized Th2 cells reversed cognitive impairment and increased plasma levels of soluble Aβ in APP+PS1 mice. Indeed, higher levels of plasma Aβ strongly correlated with better cognitive performance and lower plasma levels of key cytokines. Thus, APP+PS1 mice with high plasma Aβ levels and low plasma levels of IL-2 and IFN-γ exhibited the best cognitive performance. The increase in plasma Aβ seen with Th2 recipients occurred without a reduction in dense-core parenchyma Aβ deposits or ELISA-measured brain Aβ. Nonetheless, for Tg and Tg/Th2 mice collectively, higher soluble Aβ1–40 in the hippocampus correlated with poorer RAWM performance (not shown), consistent with prior studies indicating that soluble Aβ oligomers contribute to cognitive impairment (Arendash et al., 2004; 2006). Compared to Tg controls, Tg mice infused with Th2 cells also had significantly fewer CAA-laden vessels within the hippocampal formation. When APP+PS1 mice were grouped in terms of high versus low CAA, mice with low hippocampal CAA had decreased levels of soluble Aβ1–42 in the hippocampus and exhibited better cognitive function. Higher plasma levels of Aβ in Th2 cell recipients are most likely due to greater Aβ efflux from the brain, although decreased peripheral degradation of Aβ cannot be ruled out. These findings suggest that the reduction of soluble Aβ in the brains of Tg/Th2 mice was mediated by greater efflux – as opposed to degradation within the brain.
The lack of significant and consistent T cell infiltration into the brains of Aβ-specific Th2 cell recipients suggests a mechanism(s) that may involve systemic or cerebrovascular components, as opposed to direct interactions between Aβ-responsive T cells and dense-core deposits or cells deep in the brain parenchyma. This premise is supported by the lack of effect Th2 infusions had on hippocampal Aβ.
Strong correlations between behavioral improvements and lower serum levels of GM-CSF in this study may indicate a critical role for this cytokine in immune responses to Aβ. In addition to its effects on granulocytes and macrophages, GM-CSF also promotes the survival of microglia and may prime them for dendritic cell function (Esen and Kielen, 2007), which is notable as Th2 infusions resulted in both lower serum GM-CSF and a ~30% reduction in plaque-associated microglia. GM-CSF also coordinates anti-tumor activities to self-antigens by dendritic cells and macrophages (Dranoff et al., 1993), with tonic GM-CSF expression by tumor cells antagonizing anti-tumor responses (Sotomayor et al., 1991; Serafini et al., 2004); more recently, GM-CSF has been shown to play an important role in stimulating the phagocytosis of apoptotic cells by antigen presenting cells (APCs), and subsequent APC stimulation of regulatory T cells (Tregs) that suppress autoimmune responses (Jinushi et al., 2007). High systemic levels of GM-CSF in APP+PS1 mice may contribute to an expansion of Tregs that suppress effector T cell responses to Aβ, which could be countered by the adoptive transfer of Aβ-specific effector T cells that overwhelm those Tregs. However, correlation does not imply causality so it is also possible that higher levels of GM-CSF are unrelated to the beneficial effects that resulted from Th2 cell infusions.
Studies from our labs, and others, have demonstrated the therapeutic benefits of bolstering immune responses to Aβ, yet the role of the immune system in preventing or delaying Alzheimer’s pathophysiology remains unresolved. Several advocates for the therapeutic use of anti-Aβ monoclonal antibodies have suggested that similar antibodies play a critical role in delaying disease onset (Brettschneider et al., 2005; Henkel et al., 2007). However, given the amount of Aβ that is constitutively produced throughout life, the immunological resources required to maintain such a response over 60 or more years would be inordinate. In contrast, our findings suggest that the influence of Aβ-specific Th2 cells extend beyond antibody responses to provide significant and long lasting cognitive benefits in APP+PS1 mice through another mechanism. The CAA that often occurs in AD hinders perivascular macrophages or pericytes that move along the interstitial space of blood vessels, causing them to recruit effector cells from the bloodstream. While a massive infiltration of mononuclear cells results in encephalitis, as occurs in multiple sclerosis and the rodent model experimental autoimmune encephalomyelitis, small episodic responses provided by Th2 cells may be sufficient to eliminate these Aβ aggregations in an ongoing process to maintain the flow of fluid and cells through the cerebrovascular/parenchyma interface. Aβ-specific T cells would be well suited to provide such immune responses over the course of many years until they are diminished by the expansion of Tregs or age-related immune deficiencies. This type of episodic response may be more in line with normal immune function than the continuous presence of large quantities of Aβ-specific antibodies inherent to many active and passive Aβ immunotherapeutic approaches to AD.
Clinical trials with direct Aβ vaccination were largely abandoned due to Th1 driven encephalitogenic side-effects in some patients, which subsequently led to the avoidance of strategies that stimulate T cell responses. However, that unfortunate complication in an early clinical trial may have led many investigators to throw out the baby with the bathwater. Our findings indicate that a non-inflammatory Aβ-specific Th2 response is beneficial and is not necessarily accompanied by extensive mononuclear cell infiltration of the CNS. Indeed, anti-inflammatory effects may contribute to the cognitive benefits we observed as lower plasma cytokine levels consistently correlated with better working memory in APP+PS1 mice. These results further suggest that a general dysfunction of immune responses in APP+PS1 mice can be ameliorated by the infusion of a small number of Aβ-specific Th2 cells.
Supplementary Material
Acknowledgments
Supported by grants within the NIA-designated Florida Alzheimer’s Disease Research Center (AG025711), and the Byrd Alzheimer’s Center & Research Institute to GA. This research was also supported by UCR funds to DE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ansel KM, Djuretic I, Tanasa B, Rao A. Differentiation and IL4 locus accessibility. Ann Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Schleif W, Rezai-Zadeh K, Jackson E, Zacharia LC, Cracchiolo JR, Shippy D, Tan J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain β-amyloid production. Neuroscience. 2006;142:941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, et al. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64(1):94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- Brettschneider S, Morgethaler NG, Teipel SJ, Fischer-Schulz C, Buerger K, Dodel R, et al. Decreased serum antibody beta(1–42) autoantibody levels in Alzheimer’s disease, determined by newly developed immuno-precipitation assay with a radio-labeled amyloid beta(1–42) peptide. Biol Psychiatry. 2005;57(7):813–816. doi: 10.1016/j.biopsych.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esen N, Kielian T. Effects of low dose GM-CSF on microglial inflammatory profiles to diverse pathogen-associated molecular patterns (PAMPs) J Neuroinflammation. 2007;20:4–10. doi: 10.1186/1742-2094-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethell DW, Shippy D, Cao C, Cracchiolo JR, Runfeldt M, Blake B, Arendash GW. Abeta-specific T-cells reverse cognitive decline and synaptic loss in Alzheimer’s mice. Neurobiol Dis. 2006;23(2):351–361. doi: 10.1016/j.nbd.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Nakazaki Y, Dougan M, Carrasco DR, Mihm M, Dranoff G. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and anti-inflammatory activities of GM-CSF. J Clin Invest. 2007;117(7):1902–1913. doi: 10.1172/JCI30966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel AW, Dittrich PS, Groemer TW, Lemke EA, Klingauf J, Klafki HW, et al. Immune complexes of auto-antibodies against A-beta 1–42 peptides patrol cerebrospinal fluid of non-Alzheimer’s patients. Mol Psychiatry. 2007;12(6):601–610. doi: 10.1038/sj.mp.4001947. [DOI] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408(6815):982–985. doi: 10.1038/35050116. Erratum in: Nature 2001; 412(6847):660. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, Dartiques JF, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- Sotomayor EM, Fu YX, Lopez-Cepero M, Herbert L, Jimenez JJ, Albarracin C, Lopez DM. Role of tumor-derived cytokines on the immune system of mice bearing a mammary adenocarcinoma. II. Downregulation of macrophage-mediated cytotoxicity by tumor-derived granulocyte-macrophage colony-stimulating factor. J Immunol. 1991;147:2816–2823. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.