Abstract
Conditional neuromodulation is a form of closed-loop bladder control where neurostimulation is applied in reaction to bladder pressure changes. Current methods based on external catheters have limited utility for chronic ambulatory therapy. We have developed a wireless pressure monitor to provide real-time, catheter-free detection of bladder contractions. The device is sized for chronic implantation in the bladder muscle. The pressure monitor consists of an ultra-low-power application specific integrated circuit (ASIC), micro-electro-mechanical (MEMS) pressure sensor, RF antennas, and rechargeable battery. Here we describe an overview of the system, including chronic in vivo test data of a non-hermetic polymer sensor package and chronic testing of the wireless sensor in large animal models. Test results show that the packaging method is viable for chronic encapsulation of implanted pressure sensors. Chronic testing of the pressure monitor revealed some obstacles relating to the chosen implant site within the bladder wall. However, chronic wireless device function was confirmed and data quality was sufficient to detect bladder compressions in large animals, with average correlation coefficient of 0.90.
I. Introduction
Conditional neuromodulation for bladder control relies on a feedback signal indicating bladder status [1]. For a chronic therapy, real-time feedback of bladder pressure is required, and existing catheters are not suitable for long-term use due to infection risk and their impractical and stigmatized nature. There is presently no option for ambulatory, catheter-free real-time bladder pressure feedback, other than relying on patient sensation (which may not be intact for all individuals).
Existing implantable bladder pressure sensors have mainly targeted acute diagnosis applications. Some wireless pressure sensors [2] [3] use primary cell batteries or transmit pressure data at millihertz rates to save energy; this data rate cannot provide real-time feedback for neuromodulation. A chronic bladder pressure sensor should include wireless energy transfer to maintain device operation over years of operation. Because the bladder is located in the pelvis and can be surrounded by more than 20 cm of tissue in obese patients, continuous RF-powering—which has been very successful in shallow-depth wireless sensors—is not feasible without reducing patient mobility.
We developed a wireless pressure sensor specifically for cystoscopic implantation within the bladder wall [4] (Fig. 1). The sensor uses a rechargeable battery which may be recharged while the user sleeps or sits atop a cushion with embedded RF induction coil. This scheme does not limit patient mobility and can provide continuous real-time bladder pressure telemetry.
Fig. 1.
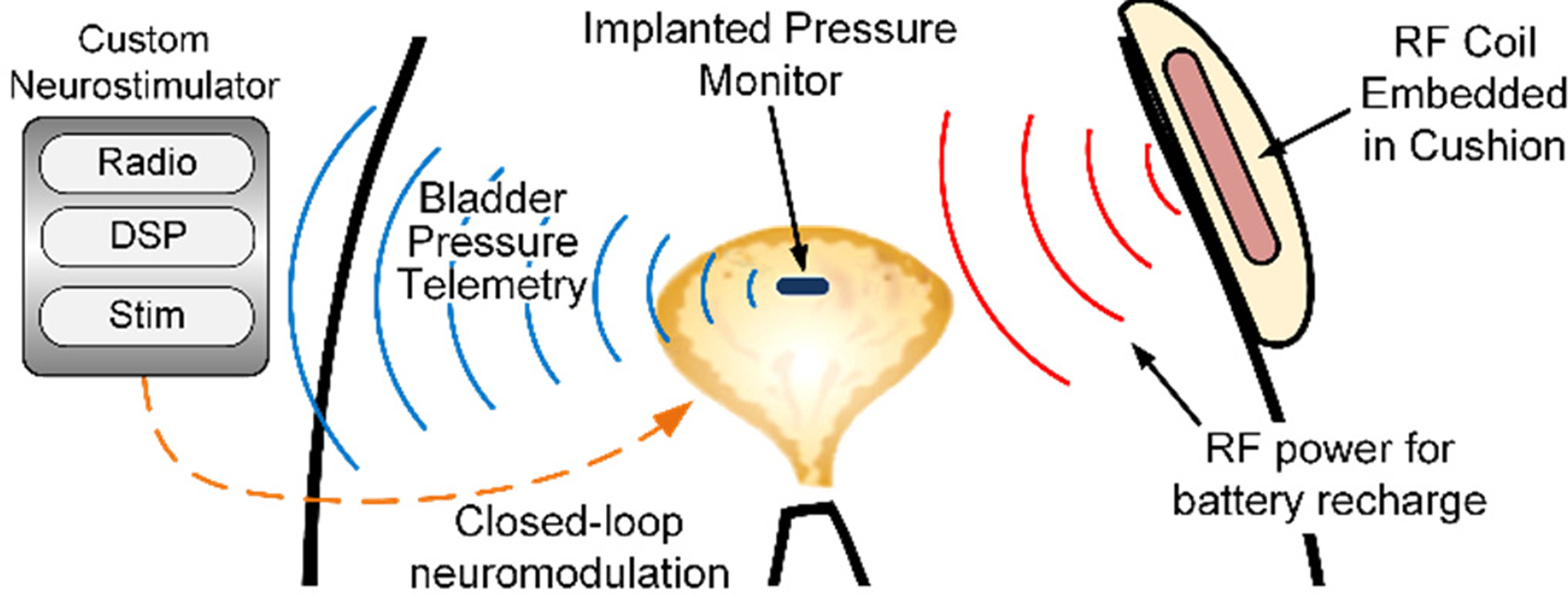
The pressure sensor is implanted within the bladder wall where pressure is transduced. Real-time wireless data transmission could enable conditional neurostimulation based on changing bladder pressure (after [6]).
Several details of this work have been previously published in various formats [4], [5]. Here, we present a cohesive overview of our approach to designing the wireless pressure monitor, specifically details on sensor encapsulation and chronic implant testing. We also are presenting new data gathered during large-animal implantation testing.
II. Wireless Pressure Monitor Microsystem
Achieving a small implant size in a chronic medical device requires ultra-low-power circuitry to reduce battery capacity. To maintain a small power budget while providing real-time transmission of pressure data, we created an ultra-low-power, fully-integrated pressure sensor ASIC. The ASIC was combined with a MEMS pressure sensor (Sensonor SW415 [7]), discrete capacitors, radio antennas, and a rechargeable battery to form a complete implantable microsystem.
Details on the ASIC and microsystem design have been previously discussed [6] and are summarized here (Fig. 2). The ASIC achieves ultra-low-power draw through switched-bias power control and adaptive transmission rate based on bladder pressure activity. Long-term pressure sensor drift is a known concern—especially in implanted sensors interfacing with tissue—so a novel DC offset removal feedback loop based on discrete-time computation was included [8].
Fig. 2.

The bladder pressure monitor (a,b) combined a MEMS pressure sensor with a highly-integrated ASIC with RF, power-management, and sensor interface circuits (c). External components of the monitoring system allowed for wireless battery charging, data reception, and closed-loop conditional neuromodulation.
III. Nonhermetic Pressure Sensor Encapsulation
We developed a nonhermetic polymer sensor package over a period of iteration using interdigitated electrodes soaked in hot saline to determine failure modes. Our key finding in these studies was that any inclusions of foreign particles provides water condensation points, leading to layer delamination and package failure. Our packaging process was performed in a Class-100 clean room to minimize contamination (Fig 3).
Fig. 3.

The nonhermetic package for chronic medical implants consisted of (a) ultrasonic cleaning, (b) sensor gel protection, (c) Parylene-C deposition, (d) PDMS negative mold creation, (e) mold loading, (f) vacuum injection molding of epoxy, and (g) final outer PDMS application (100-μm).
Full details of the packaging process were published [9], and are only briefly summarized here. Devices were cleaned and the MEMS pressure sensor protected by a convex drop of PDMS gel (Sylgard 527, Dow Corning). Two 5-μm layers of Parylene-C (Specialty Coating Systems) were deposited (PDS 2010 reactor) as vapor barriers and to create a flexible pressure-sensitive diaphragm (Fig. 4c,d). PDMS molds were cast from 3D-printed parts; the pressure diaphragm was fixed to the inner mold wall by PDMS (MDX-4210, Dow Corning) to prevent ingress of molding epoxy. Epoxy (EB-107LP, Epoxyset, Inc) was injected into the mold under vacuum and cured. A final 100-μm layer of PDMS was applied by a roller to the exterior of the device to enhance biocompatibility.
Fig. 4.

Eighty wireless sensors (a) were packaged (b) for chronic in vivo testing. One unique component of the polymer package is convex, gel-filled pressure diaphragms created by Parylene-C coating (c,d).
A. Chronic In Vivo Sensor Packaging Test
The nonhermetic pressure sensor packaging process was validated in vivo using a rodent model in which devices were implanted subcutaneously for 1–6 months. We created a miniature, wireless pressure-sensing test module measuring 14 × 25 × 3 mm to test the nonhermetic package (Fig. 4a,b). 80 test platforms were constructed, encapsulated, and sterilized.
24 devices were implanted subcutaneously into 3 cohorts of rats. Device function was checked weekly by gently pressing on the rat skin near the implanted device. Pressure data were transmitted and recorded in real-time. On average, implanted sensors registered pressures of 1.5 cm H2O from finger presses.
Some implanted devices failed after 4 months due to battery damage during soldering. Moisture ingress was ruled out as a failure mode after inspecting the devices for condensate. Nonfunctional devices regained function after battery replacement which suggested failures were not due to package defects.
Cohorts carried the implants for 1–6 months (Table 1). At each endpoint animals were euthanized and tissue harvested. Histology revealed no granulation tissue and a fibrous capsule present around each device. These tests confirmed that the sensor package is chronically viable.
Table 1.
Summary of Chronic In Vivo Packaging Test
| n | Implant Duration | Failures | Histological Findings |
|---|---|---|---|
| 8 | 1 month | 0 | None |
| 8 | 3 month | 0 | None |
| 8 | 6 month | 3* | None |
| 8 | 6 month saline | 2* | N/A |
failures due to battery lead wire attachment, not moisture ingress
B. Chronic Bladder Pressure Monitor Assembly
The ASIC and MEMS sensor were directly wirebonded to a gold-plated circuit board and protected with epoxy. After attaching the battery, transmission antenna, and wireless charging coil, the microsystem was encapsulated with the nonhermetic package as previously described (Fig. 5a). The completed device measured 3.5 × 7 × 17 mm and weighed 0.7 g (Fig. 5b,c).
Fig. 5.
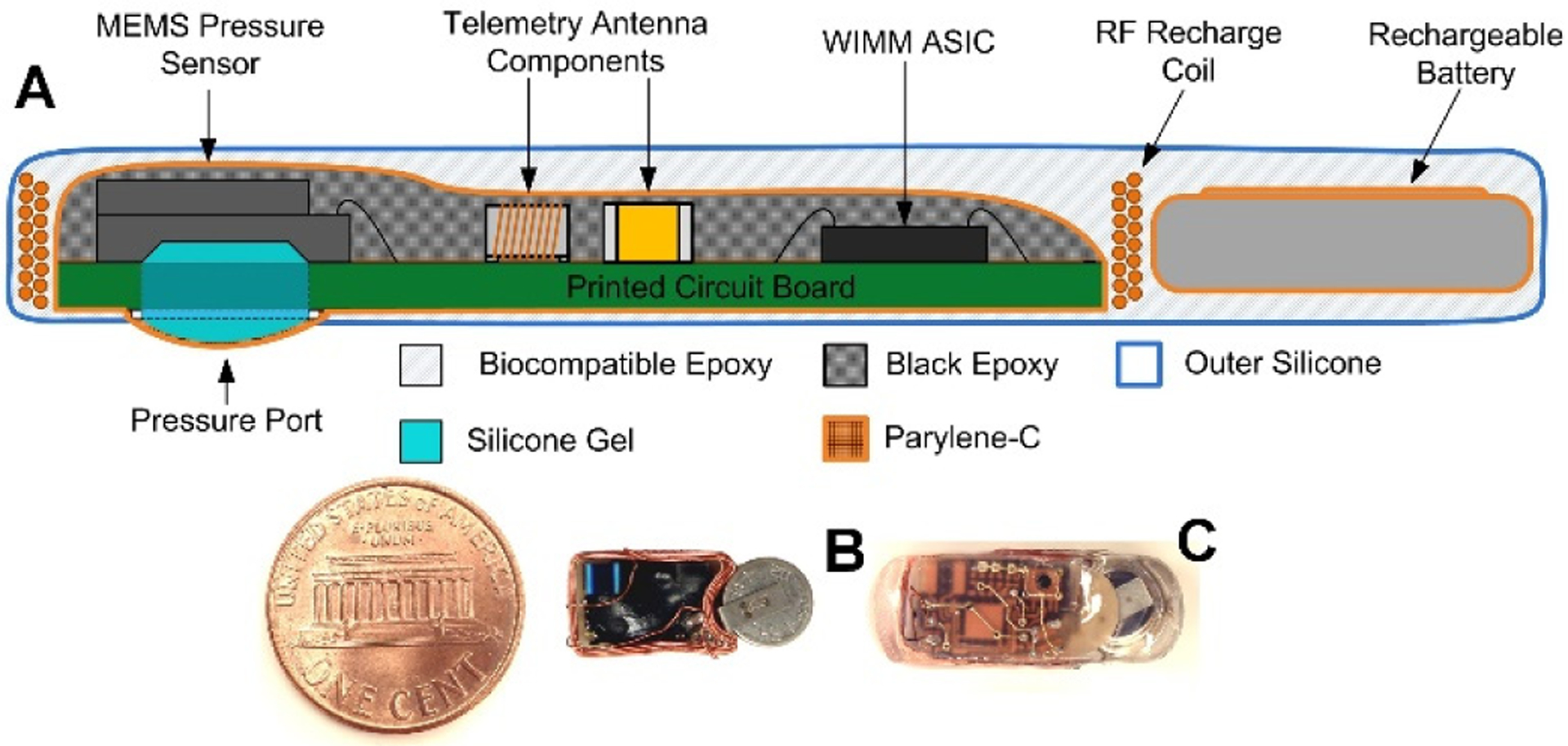
Cross-section of the packaged pressure monitor (a). Packaging increased the footprint of pressure monitors (b) by approximately 10% (c).
IV. In Vivo Wireless Bladder Pressure Sensing
Sterilized wireless pressure monitors (n=6) were implanted with conventional urologic instruments in large animal models (female Jersey calves) weighing 60–75 kg (n=5). Device position was confirmed by X-ray, and the wireless recharge antenna alignment was determined (Fig. 6). Animals carried the implanted wireless sensors for 2–4 weeks. We recorded wireless data and performed conscious wireless battery charging every other day. Animals were anesthetized weekly to assess the bladder healing response, bladder capacity, and device position.
Fig. 6.
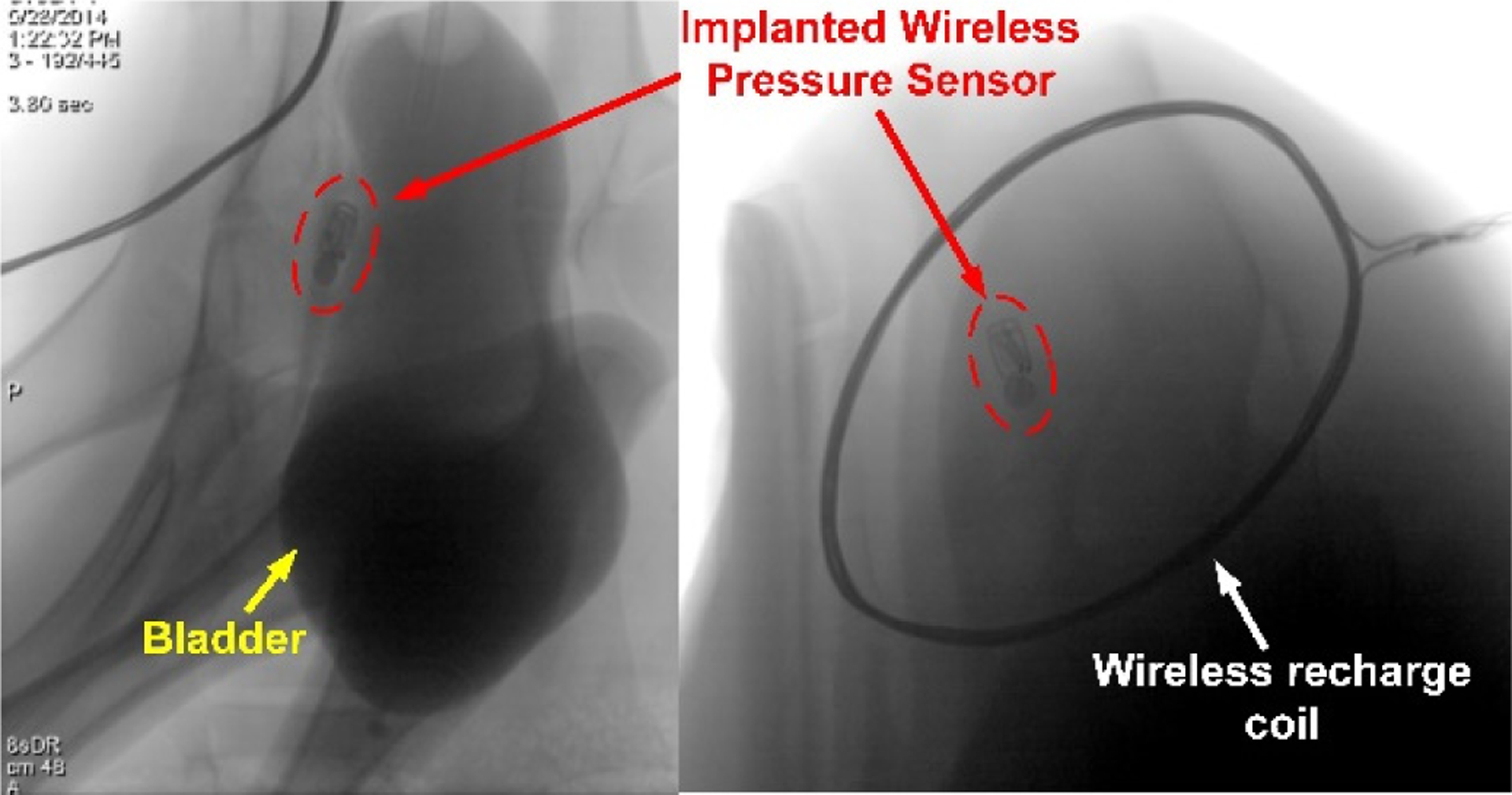
X-ray images of the implanted pressure monitor confirmed location within the bladder wall. Optimal placement of the external wireless recharge coil was determined by observing the device orientation when implanted.
During anesthetized checkups, data were collected from the pressure monitor and a reference catheter. A loss of device sensitivity was apparent after implantation. During manual bladder compressions, for example, the wireless sensor detected attenuated pressure changes (Fig. 7a,b). Correlation coefficients (Table 2) during compressions were high (mean ) and correlation during 300-s recordings was moderate (r=0.68–0.77).
Fig. 7.

Simultaneous catheter and wireless pressure monitor pressure recordings showed a nonlinear damping (a). Pressure readings from the implant were attenuated but correlated with manual compressions (analysis windows shown by black bar) (b). After implantation, sensor response matched the reference linearly (c), suggesting the damping may be attributable the implant location.
Table 2.
Measured Correlation Coefficients Between Device and Reference Intravesical Catheter.
| Cystometric Tracings (300 s each) | 0.75 | 0.68 | 0.77 |
| Compression 1 | 0.85 | Compression 4 | 0.91 |
| Compression 2 | 0.86 | Compression 5 | 0.86 |
| Compression 3 | 0.96 | Compression 6 | 0.94 |
| Average Compression Correlation | 0.90 | ||
After the device was explanted, the static pressure sensor response was linear (Fig. 7c). Therefore, we hypothesize that a biomechanical effect may have caused the sensitivity loss when the sensor was implanted within the bladder muscle.
The outcomes from in vivo tests of the pressure monitor showed that the pressure sensor circuits functioned in large mammals. Pressure accuracy was affected by implant location, but correlation with compressions was noted, which is still useful for conditional bladder neuromodulation. No device failures due to moisture ingress were observed, confirming efficacy of the nonhermetic package. Device retention within the bladder was poor, with erosion into the bladder occurring at 2–4 weeks. Improvements to device anchoring may mitigate this effect.
V. Conclusion
We designed and tested a wireless pressure sensor intended for chronic implantation in the bladder wall. A nonhermetic polymer encapsulation package was tested to be chronically viable over 6 months of implantation in rodents. Pressure monitors were implanted in large animals using conventional urologic instruments and minimally-invasive methods. Wireless pressure measurements and battery recharge were performed in freely moving animals. Simultaneous pressure recordings from the pressure monitor and a reference catheter showed a damping effect caused by sensor measurement from within the bladder muscle—wireless data were attenuated but correlated with bladder compressions. Ultimately, the implant location was found to be unsuitable due to the damping effect and poor device retention rates at one month. By addressing these effects, a chronic pressure monitor capable of providing real-time pressure feedback for conditional neuromodulation may be realized.
Acknowledgments
This work was sponsored by the US Department of Veterans Affairs, Case Western Reserve University, and the Cleveland Clinic
References
- [1].Kirkham A, Shah N, Knight S, Shah P and Craggs M, “The acute effects of continuous and conditional neuormodulation on the bladder in spinal cord injury,” Spinal Cord, vol. 39, pp. 420–428, 2001. [DOI] [PubMed] [Google Scholar]
- [2].Axisa F et al. “Design and fabrication of a low cost impantable bladder pressure monitor,” Conf. IEEE Eng in Med and Biol Society (EMBC), 2009. [DOI] [PubMed] [Google Scholar]
- [3].Wang C et al. “A mini-invasive long-term bladder urine pressure measurement ASIC and system,” IEEE Transactions on Biomedical Circuits and Systems, vol. 2, no. 1, pp. 44–49, 2008. [DOI] [PubMed] [Google Scholar]
- [4].Majerus S, Fletter P, Damaser M and Garverick S, “Low-power wireless micromanometer system for acute and chronic bladder-pressure monitoring,” IEEE Trans on Biomedical Engineering, vol. 58, no. 3, pp. 763–768, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Makovey I et al. “Wireless implantable rechargeable bladder pressure sensor: cystoscopic implantation and ambulatory data collection,” The Journal of Urology, vol. 193, no. 4, p. e489, 2015. [Google Scholar]
- [6].Majerus S et al. , “Wireless implantable pressure monitor for conditional bladder neuromodulation,” IEEE Bio Circuits and Sys Conf, Atlanta, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sensonor AS, “SW415 Pressure Sensor Die,” August 2012. [Online]. Available: http://www.sensonor.com/media/8274/2012-08-13-product-brief-sw415-a4-web.pdf. [Accessed 20 January 2014].
- [8].Majerus S and Damaser M, “Automatic drift cancellation of implanted bladder pressure sensor,” in IEEE Bio Circuits and Sys Conf, Atlanta, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang P et al. “Long-Term Evaluation of Non-hermetic Micropackage Technology for Pressure Sensor in Medical Microsystem,” in Conf. Solid-State Sensors, Actuators, and Microsystems, Anchorage, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


