Abstract
Step index (STEPIX) is a recently developed compound muscle action potential (CMAP) scan method for evaluating motor unit loss and remodeling changes. This study investigates the influence of different stimulation parameters during CMAP scan on STEPIX and its examination of muscles affected by spinal cord injury (SCI). CMAP scan of the first dorsal interosseous (FDI) muscle was performed using different stimulus pulse widths (0.1 ms, 0.2 ms) and different numbers of stimuli (500, 1000) in 12 neurologically intact subjects. STEPIX was derived from each CMAP scan of all subjects. A significantly higher STEPIX was obtained using 1000 stimuli than 500 stimuli, while no significant difference in STEPIX was observed using 0.1 and 0.2 ms stimulus pulse widths. STEPIX was further applied to process CMAP scans of the FDI muscle from 13 tetraplegia and 13 healthy control subjects using the same stimulation parameter setting (0.1 ms, 500 stimuli), along with other methods including MScanFit motor unit number estimation (MUNE) and D50. STEPIX was significantly lower for the SCI subjects compared with the healthy control subjects. STEPIX was significantly correlated with MscanFit MUNE and D50, but had a smaller relative width of the overlapping zone (WOZ%) between tetraplegic and healthy control groups compared with MScanFit MUNE and D50. The findings of the study highlight the importance of maintaining a consistent stimulation parameter setting in CMAP scan studies and confirm the usefulness of STEPIX as a convenient CMAP scan parameter for examination of motor unit number changes.
Keywords: Compound muscle action potential (CMAP), CMAP scan, Step index (STEPIX), Amplitude index (AMPIX), Stimulation protocol, Spinal cord injury (SCI)
I. INTRODUCTION
With over half a century’s development, motor unit number estimation (MUNE) has become an established technique for the examination of neuromuscular diseases [1]. Traditional implementations of MUNE are based on estimating the average amplitude of a single motor unit action potential (MUAP) from a small number of near-threshold motor units. Subsequently, MUNE can then be estimated by dividing the maximal compound muscle action potential (CMAP) amplitude by the mean single MUAP amplitude. The mean single MUAP size can also be estimated by a range of other approaches, leading to various methods for calculating MUNE [2][3].
However, estimation of the size of a single MUAP from only a small number of near-threshold motor units can be inaccurate given the skewed size distribution of the motor unit pool [4]. This highlights the significance of the CMAP scan implementation of MUNE, which utilizes several hundred, gradually adjusted stimulation currents in a range between subthreshold and supramaximal intensity to obtain a sigmoidal curve describing the relation between stimulation and muscle response [5][6]. Two general approaches have been developed to process the data from a CMAP scan. One is to directly estimate the absolute number of motor units, such as via Bayesian MUNE [7][8] or MScanFit MUNE [9–11]. The other approach is to derive an index parameter [12–15] that can indirectly reflect the number of motor units and remodeling changes. Step index (STEPIX) is a representative example of the second CMAP scan processing approach [14]. Its calculation does not require any special program or software, and the results can be obtained immediately even using Microsoft Excel. This quick and convenient feature makes STEPIX desirable for clinical applications.
A CMAP scan can be administered using different stimulus pulse widths and different numbers of stimuli. The effect of these stimulation parameters on MScanFit MUNE and other CMAP scan characteristics has been examined previously [16–19]. However, it remains unknown how different stimulation parameters during a CMAP scan may eventually influence STEPIX and amplitude index (AMPIX), the latter being an accompanying parameter derived from the former. In this study, we set out to investigate the effect of different stimulus pulse widths and different numbers of stimuli involved in a CMAP scan on STEPIX and AMPIX. Furthermore, we present a novel application of STEPIX and AMPIX for the examination of muscles affected by spinal cord injury (SCI).
II. METHODS
A. Description of Data Sets
Two previously acquired data sets, Data Sets-Healthy Control (DS-HC) and Data Sets-Spinal Cord Injury (DS-SCI), were used in this study. Subject information and experimental details for both data sets have been reported in our previous studies [18][20], while a brief description is provided below. All experimental protocols generating the two data sets were approved by the Institutional Review Board of the University of Texas Health Science Center at Houston and TIRR Memorial Hermann Hospital (Houston, TX). All healthy control and SCI subjects provided written informed consent before any experimental procedures.
In both data sets, CMAP scans were performed on the first dorsal interosseous (FDI) muscle. Electrode placement followed that of routine nerve conduction studies (NCS) of the FDI muscle. A standard two-contact bar electrode was placed proximal to the wrist to stimulate the ulnar nerve. The diameter of each contact surface was 9 mm, and the distance between them was 20 mm. The optimal stimulating site was found by adjusting the electrode positions while stimulating at low intensities and observing for relatively large CMAPs. For EMG, disposable electrodes (10 mm diameter) were placed on the FDI muscle (active electrode) and on the thumb’s distal phalanx (reference electrode). A large self-adhesive ground electrode was placed on the dorsum of the hand. A clinical EMG/NCS system (UltraPro S100, Natus Inc, Middleton, USA) was used for this study. To record a CMAP scan, we first determined the lowest (subthreshold) and the highest (supramaximal) current intensity for the subject. After setting this stimulating intensity range, the CMAP scan was started at a stimulating frequency of 2Hz using the linear decline mode for the change in stimulus intensity. Information regarding the stimulating pulse width and the number of stimuli is described below for DS-HC and DS-SCI.
DS-HC contains CMAP scan data of the FDI muscle from 13 neurologically intact subjects (i.e., healthy controls). For 12 of the 13 subjects, CMAP scan recordings were performed using four sets of different numbers of stimuli and stimulus pulse widths: 500 stimuli @ pulse width 0.1 ms; 500 stimuli @ pulse width 0.2 ms; 1000 stimuli @ pulse width 0.1 ms; and 1000 stimuli @ pulse width 0.2 ms. The remaining one subject only participated in one CMAP scan recording (500 stimuli @ stimulus pulse width 0.1 ms). All recordings were performed on their dominant hand (11 right-handed and 2 left-handed).
DS-SCI contains CMAP scan data of the FDI muscle from 13 SCI subjects with tetraplegia (post injury time: 1 to 24 years; ASIA Impairment Scale: A to D; GRASSP: 0 to 108/116; neurologic level: C1 to C7; grip force: 0 to 45.4 kg with a median of 0.9 kg; pinch force: 0 to 11.3 kg with a median of 1.4 kg). Each individual subject’s information can be found in [20]. More information on ASIA (American Spinal Injury Association) Impairment Scale and the GRASSP (graded redefined assessment of strength, sensibility and prehension) test can be found in [21][22]. The recording was performed on each subject’s right hand with 500 stimuli @ stimulus pulse width 0.1 ms.
B. CMAP Scan Analysis
STEPIX is calculated from steps, defined as the difference between adjacent amplitudes from sorted CMAP scan data points. The design of STEPIX assumes that there are relatively fewer large motor units and more small motor units in the motor unit pool, and therefore, the MUAP amplitude distribution can be modeled as a logarithmic distribution. Calculation of STEPIX includes the following procedures: (1) Steps are sorted from largest to smallest, and the smallest step whose amplitude is greater than 20 μV is defined as the R point (Figure 1B); (2) A logarithmic model is constructed using steps with amplitude greater than 50 μV, and the x-intercept of the regression curve is defined as the P point (Figure 1B); (3) The mean step amplitude is estimated by calculating the average amplitude value of the steps that are on the left side of the P point; (4) The step with x-value equal to the number of mean step amplitudes adding to 80% of the maximum CMAP amplitude is defined as the Q point. Graphically, this is shown in Figure 1C by finding the intersection of a line through the origin with a slope equal to the mean step amplitude and a horizontal line with y = 80% of the CMAP amplitude. This intersection is labeled as Q’, and the x-value of Q’ (Qx) is used to find the step of equal x-value (Q point). STEPIX is chosen between the smaller x-value (step number) of the Q point and the R point. Refer to [14], for further details of the STEPIX calculation. Lastly, the amplitude index (AMPIX) is calculated by dividing the maximum CMAP amplitude by the STEPIX.
Fig. 1.
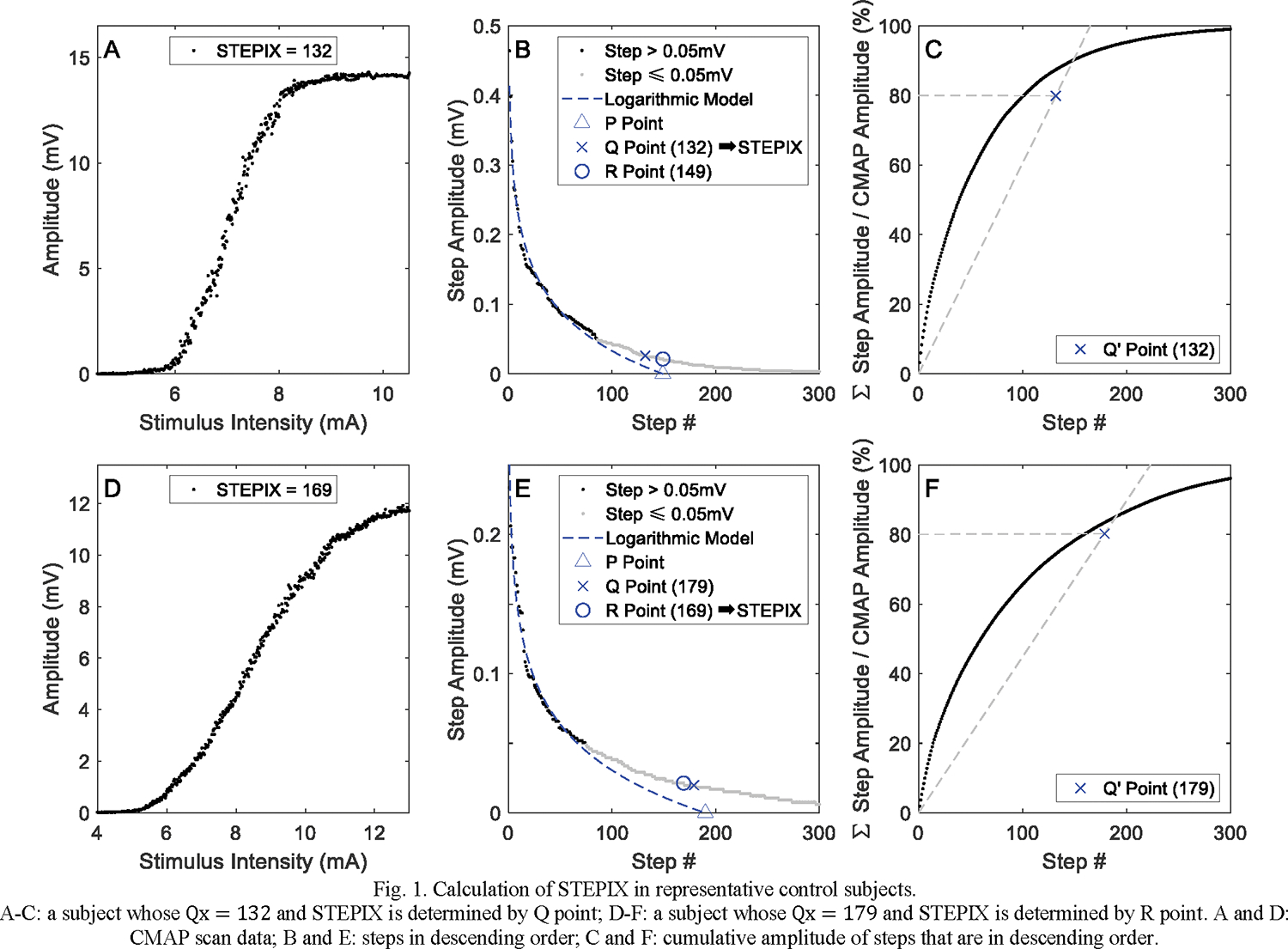
Calculation of STEPIX in representative control subjects.
A-C: a subject whose Qx = 132 and STEPIX is determined by Q point; D-F: a subject whose Qx = 179 and STEPIX is determined by R point. A and D: CMAP scan data; B and E: steps in descending order; C and F: cumulative amplitude of steps that are in descending order.
STEPIX and AMPIX values of all CMAP scans from the two data sets were computed and compared with D50 and MScanFit MUNE methods. The D50 of a CMAP scan is defined as the number of the largest steps that are needed to build up to 50% of the maximum CMAP [13]. MScanFit MUNE was calculated using a free program provided by Bostock [9][10]. The program applies an initial mathematical model and sequentially adjusts model parameters to mimic the recorded CMAP scan. Pre-scan and post-scan segments were manually determined and default model parameter settings were used when implementing the MScanFit program. The percentage error for a valid MUNE was required to be less than 7%.
C. Statistical Analysis
Repeated measures analysis of variance was performed on the DS-HC data set using SPSS software (SPSS, Chicago, IL) in order to evaluate whether STEPIX and AMPIX were affected by different CMAP scan stimulation parameters, specifically, the number of stimuli and stimulus pulse width. Linear regression was applied to evaluate the agreement between MScanFit MUNE, D50, and STEPIX (or AMPIX).
In order to evaluate the sensitivity of STEPIX and AMPIX to paralyzed muscle changes after SCI, STEPIX and AMPIX values derived from DS-SCI and DS-HC (500 stimuli @ stimulus pulse width 0.1 ms only) data sets were analyzed using Wilcoxon rank sum test. The relative width of the overlapping zone (WOZ%) and the percentage of subjects whose data fall within the overlapping zone (POZ%) were calculated as well. The width of overlapping zone (WOZ) of is defined as
| (1) |
where represents the values of (STEPIX, AMPIX, D50, or the maximum CMAP) from all healthy subjects in DS-HC, and represents the values from all SCI subjects in DS-SCI.
And WOZ% of is calculated as:
| (2) |
Statistical significance was set as p < 0.05. All analyses were performed using MATLAB (MathWorks Inc., Natick, USA). Results are written in mean ± standard deviation.
III. RESULTS
A. Sensitivity to CMAP Scan Stimulation Protocols
The number of subjects whose STEPIX was calculated based on the R point instead of the Q point was 2 of 13 for 500 stimuli @ pulse width 0.1 ms, 1 of 12 for 500 stimuli @ pulse width 0.2 ms, 5 of 12 for 1000 stimuli @ pulse width 0.1 ms, and 5 of 12 for 1000 stimuli @ pulse width 0.2 ms. Figure 1 shows an example of STEPIX calculation in representative control subjects whose STEPIX was determined by the Q point and the R point. As shown in Figure 2, STEPIX derived from CMAP scans with 1000 stimuli (184.8 ± 31.5 for pulse width 0.1 ms, 186.7 ± 45.0 for pulse width 0.2 ms) was significantly greater than those derived from CMAP scans with 500 stimuli (139.8 ± 22.4 for pulse width 0.1 ms, 124.3 ± 23.7 for pulse width 0.2 ms) (p < 0.001). AMPIX derived from CMAP scans with 1000 stimuli (0.093 ± 0.034 mV for pulse width 0.1 ms, 0.092 ± 0.023 mV for pulse width 0.2 ms) was significantly smaller than those derived from CMAP scans with 500 stimuli (0.121 ± 0.029 mV for pulse width 0.1 ms, 0.137 ± 0.044 mV for pulse width 0.2 ms) (p < 0.001). Neither STEPIX nor AMPIX was sensitive to pulse width (p = 0.531 and p = 0.239, respectively).
Fig. 2.

Distribution of STEPIX and AMPIX derived from CMAP scans in DS-HC. Each box shows the maximum, minimum, median, and quartiles.
As shown in Figure 3, a strong correlation was observed between STEPIX and D50 for 500 stimuli @ pulse width 0.1 ms (correlation coefficient, r = 0.872, p < 0.001), 1000 stimuli @ pulse width 0.1 ms (r = 0.850, p < 0.001), 500 stimuli @ pulse width 0.2 ms (r = 0.935, p < 0.001), and 1000 stimuli @ pulse width 0.2 ms (r = 0.699, p = 0.011). A strong negative correlation was observed between AMPIX and D50 for 500 stimuli @ pulse width 0.1 ms (r = −0.750, p = 0.005), 1000 stimuli @ pulse width 0.1 ms (r = −0.801, p = 0.002), 500 stimuli @ pulse width 0.2 ms (r = −0.811, p = 0.001), and 1000 stimuli @ pulse width 0.2 ms (r = −0.822, p = 0.001).
Fig. 3.
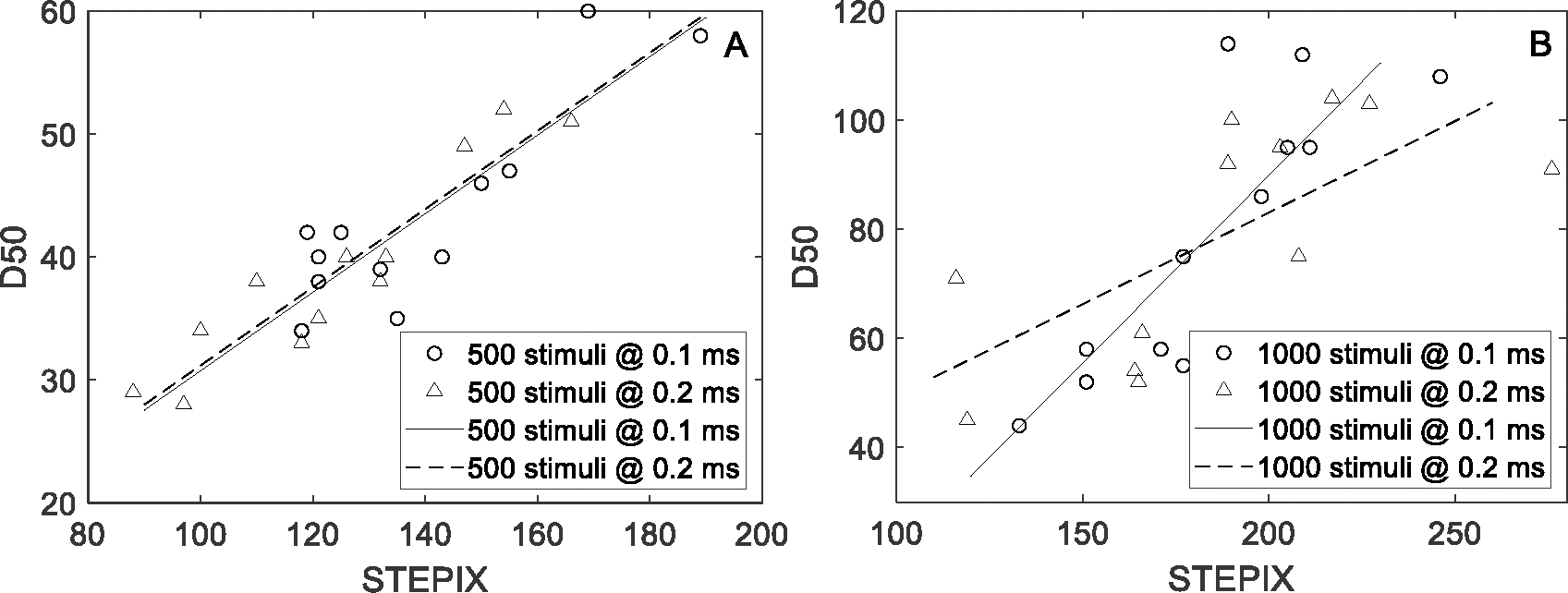
STEPIX and D50 derived from CMAP scans in DS-HC with regression lines. Each marker corresponds to one recording.
B. Sensitivity to Motor Unit Loss
Figure 4 shows an example of STEPIX calculation in representative SCI subjects whose STEPIX was determined by the Q point and the R point, respectively. The selected subject with Q point accepted had a much larger grip force (15.4 kg) but a smaller maximum CMAP amplitude than the other R point accepted subject (whose grip force was 2.7 kg). STEPIX, AMPIX, D50, and the maximum CMAP amplitude of each CMAP scan in DS-SCI and DS-HC (500 stimuli @ pulse width 0.1 ms only) are shown in Figure 5. STEPIX of four SCI subjects were based on the R point instead of the Q point. There were significant differences in STEPIX, MUNE and the maximum CMAP amplitude (p < 0.001) between the healthy control and the SCI groups, but not in D50 or AMPIX (Table 1). The lowest WOZ% value (10.9%) was achieved using STEPIX.
Fig. 4.
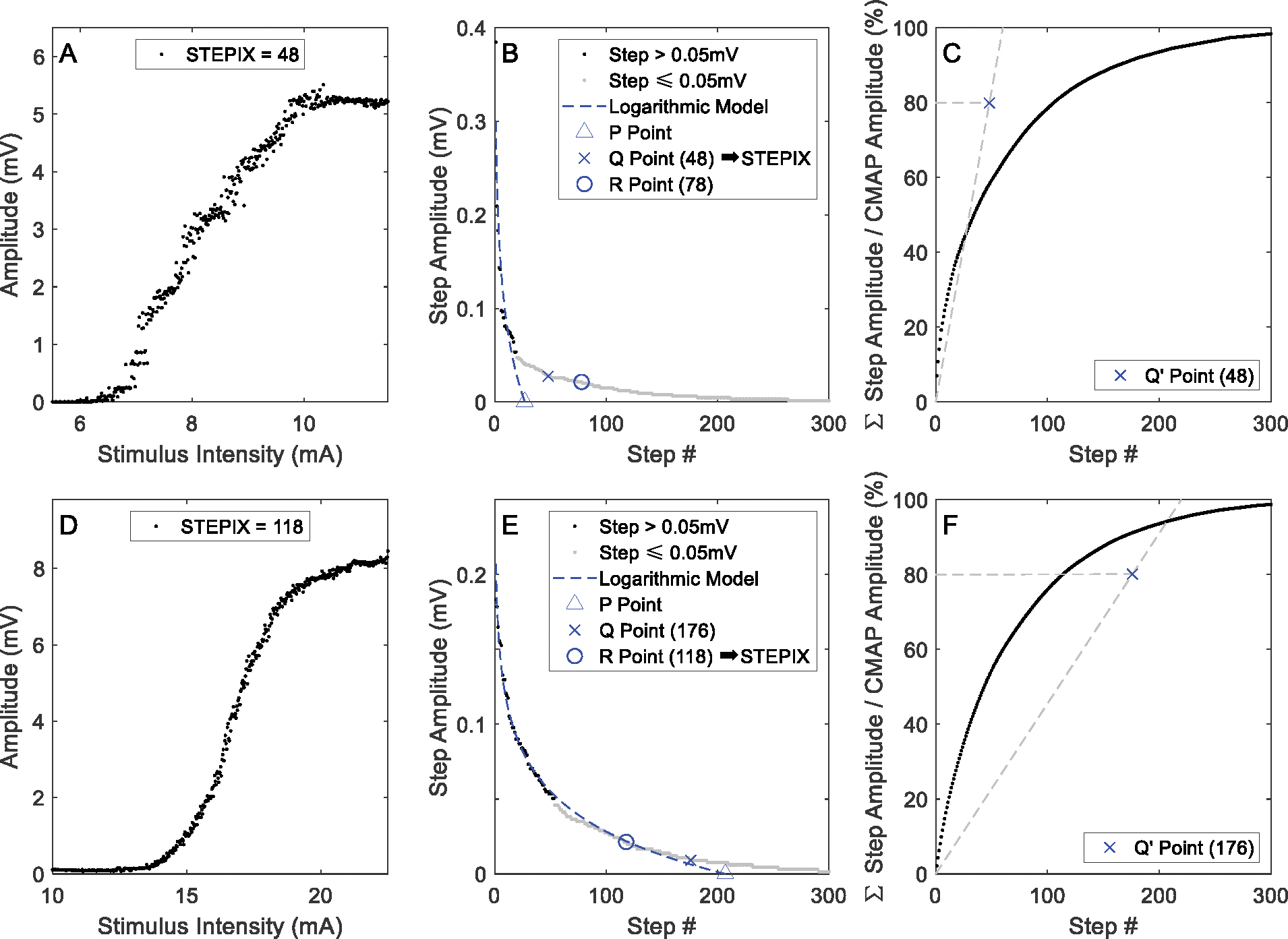
Calculation of STEPIX of representative SCI subjects。 A-C: a subject whose STEPIX is determined by Q point; D-F: a subject whose STEPIX is determined by R point. A and D: CMAP scan data; B and E: steps in descending order; C and F: cumulative amplitude of steps that are in descending order.
Fig. 5.
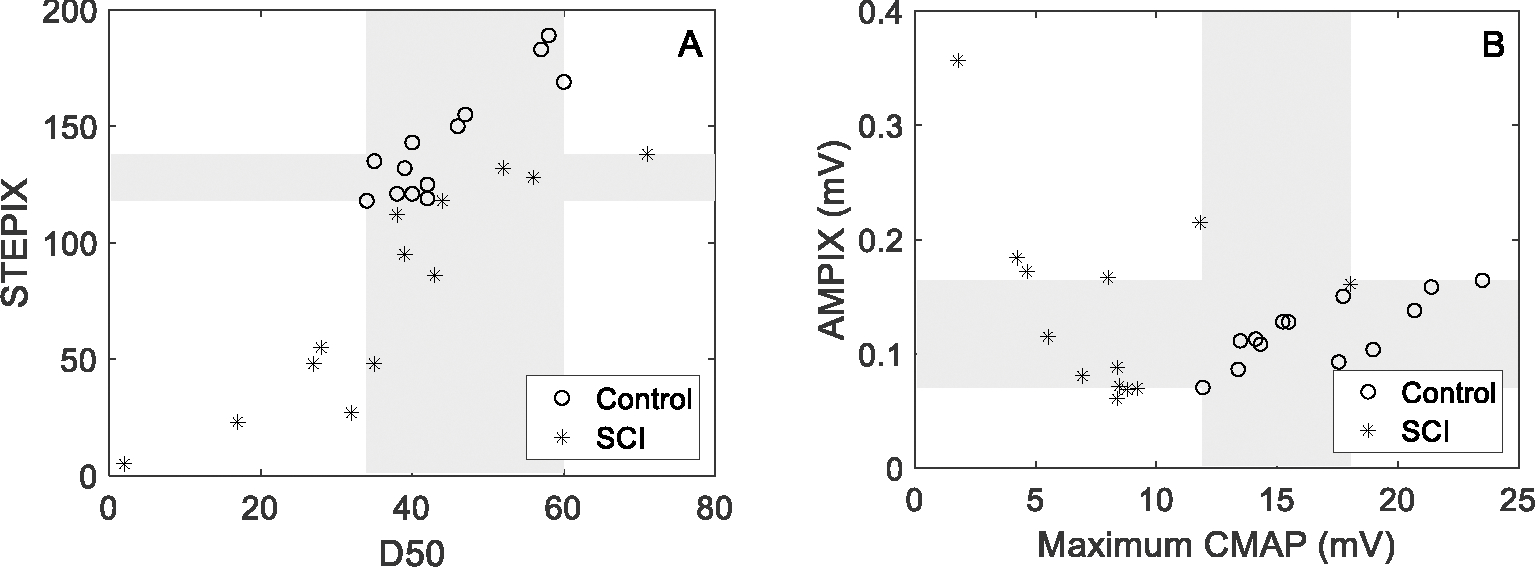
STEPIX, D50, AMPIX and the maximum CMAP amplitude of each CMAP scan data in DS-SCI and DS-HC (500 stimuli @ pulse width 0.1 ms). Each marker represents one subject. Grey area refers to the overlapping zone between the healthy control group and the SCI group.
Table 1.
Different CMAP scan parameters for examination of SCI and healthy control subjects
| Control | SCI | Group Difference | WOZ% | POZ% | |
|---|---|---|---|---|---|
| D50 | 44.5 ± 8.7 | 37.2 ± 17.5 | p = 0.158 | 37.7% | 76.9% |
| MUNE | 108.2 ± 21.4 | 59.4 ± 36.8 | p = 6.4 × 10−4 | 44.2% | 57.7% |
| STEPIX | 143.1 ± 24.6 | 78.1 ± 46.0 | p = 7.2 × 10−4 | 10.9% | 42.3% |
| AMPIX (mV) | 0.119 ± 0.028 | 0.139 ± 0.084 | p = 0.878 | 31.7% | 69.2% |
| CMAP (mV) | 16.7 ± 3.6 | 8.0 ± 4.0 | p = 1.2 × 10−4 | 28.2% | 38.5% |
D50 was correlated with MScanFit MUNE (r = 0.660, p < 0.001) across SCI and the healthy control subjects. A stronger correlation was observed between STEPIX and MScanFit MUNE (r = 0.821, p < 0.001, Figure 6A) and a moderate negative correlation was observed between AMPIX and MScanFit MUNE (r = −0.569, p = 0.002, Figure 6B).
Fig. 6.
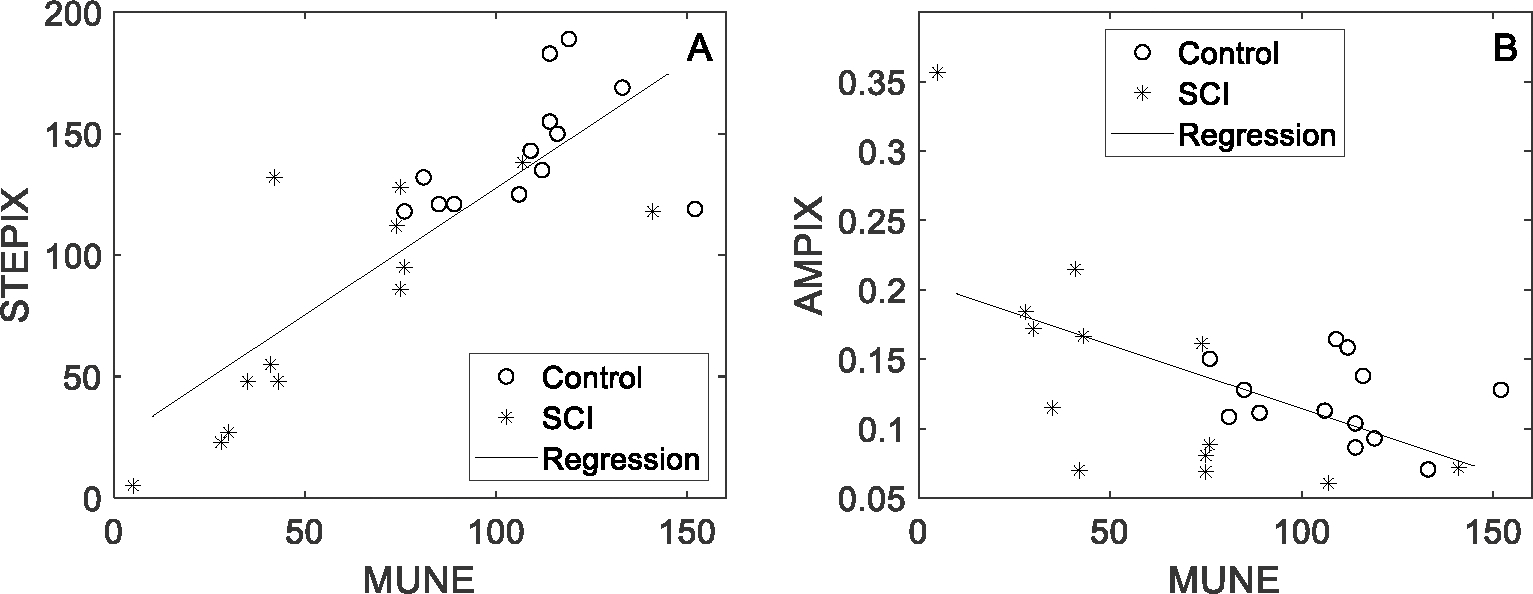
STEPIX, AMPIX, and MUNE values of each CMAP scan data in DS-SCI and DS-HC (500 stimuli @ pulse width 0.1 ms). Each marker represents one subject.
IV. DISCUSSION
The influence of different CMAP scan stimulation parameters on STEPIX (and AMPIX) was explored in this study. For the stimulus pulse width, it was found that there was no significant difference in STEPIX values derived from the two different (0.1 and 0.2 ms) stimulus pulse widths. This is different from MScanFit MUNE, which was previously shown to be significantly lower when using 0.2 ms stimulus pulse width than 0.1 ms [18]. To reach the same excitation level (total charge delivered), a shorter stimulus pulse duration would require higher stimulus intensity. As a result, the range of the x-axis (stimulus intensity) is wider at 0.1 ms stimulus pulse width while the y-axis (CMAP amplitude) remains almost the same, compared with the width of 0.2 ms. Therefore, the CMAP scan using 0.1 ms pulse width is expected to demonstrate a more flattened pattern (i.e., less steep slope for the middle region) compared with 0.2 ms pulse width. The significant increase in MScanFit MUNE from 0.2 to 0.1 ms can be attributed to such a CMAP scan pattern alteration, which plays a key role in the MScanFit model [9]. However, for STEPIX, its calculation primarily depends on CMAP amplitude differences (y-axis) and is not sensitive to the rate of CMAP amplitude change per step (x-axis). This justifies the lack of influence of stimulus pulse width on STEPIX.
For the number of stimuli, a previous study has revealed that it was not a major factor affecting MScanFit MUNE. In contrast, the current study found that STEPIX was significantly affected by the number of stimuli. Given a fixed stimulus pulse width and the amplitude range of stimuli for a CMAP scan, increasing the number of stimuli will make the CMAP scan denser, with more points within the range of stimuli, but will not change its overall pattern. Therefore, it is not surprising that no significant difference was observed in MScanFit MUNE using two different numbers of stimuli. However, denser points within the same range of stimuli tends to reduce the step amplitude, thus affecting the logarithmic regression. As a result, the mean step amplitude is reduced, which consequently increases STEPIX. This is consistent to our observation that STEPIX at 1000 stimuli was significantly higher than at 500 stimuli. In fact, the reduced step amplitude with higher stimuli count is expected to increase the chance of rejecting the Q point results in the STEPIX calculation. As demonstrated in this study, there was a higher rejection rate of Q point trials at 1000 stimuli than at 500 stimuli. Therefore, for the FDI muscle, we recommend 500 stimuli over 1000 for STEPIX analysis, for a more meaningful estimate. A relatively lower number of stimuli is also more bearable to patients and saves recording time.
A combination of two stimulus pulse widths and two numbers of stimuli was tested in this study and in a previous study [18] to examine their influence on CMAP scan processing. The demonstrated effect of stimulus pulse width on MScanFit MUNE and the number of stimuli on STEPIX can facilitate the selection of more reasonable CMAP scan stimulation parameters and appropriate interpretation of CMAP scan processing results. Nonetheless, it remains difficult to determine the most optimal stimulation parameter settings, which can vary in different muscles or situations. For example, stimulus pulse width longer than 0.2 ms might be required for peroneus longus and tibialis anterior muscles [23–25], and for those patients who have increased axonal thresholds [26] [27], due to a stimulator’s output limit in current intensity. Although not practical to recommend a universal stimulation parameter setting for different muscles or situations, it is important to have a consistent stimulation parameter setting when investigating motor unit property changes using CMAP scan, so the derived parameters (STEPIX, AMPIX, MScanFit MUNE) will be least affected by non-physiological factors.
This study further demonstrates a novel application of STEPIX and AMPIX for the examination of (partially) paralyzed muscles using the same stimulation parameter setting for both SCI and healthy control groups. Previous MUNE and other electrodiagnostic investigations have revealed motor unit loss and remodeling post SCI [28–32]. All SCI subjects in DS-SCI had some motor function deficit, and their STEPIX demonstrated the expected pattern where the STEPIX of the SCI subjects was significantly lower than that of the healthy control subjects. Among the examined parameters it was found that STEPIX, maximum CMAP, and MScanFit MUNE were able to separate the two groups. Notably, STEPIX achieved a smaller WOZ% compared with MScanFit MUNE or maximum CMAP amplitude, implying its effectiveness or sensitivity in detecting neuromuscular alterations of the SCI subjects. Although a strong correlation was observed between STEPIX and D50, there was no significant difference in D50 between the SCI and healthy control subjects. Given that both D50 and STEPIX are derived from sorted step amplitudes, and STEPIX appears to be more sensitive to motor unit loss than D50 (as demonstrated in this study), STEPIX can be viewed as an improved version of D50.
The limitations of the study should be acknowledged. First, the different CMAP scan settings were tested only in healthy control subjects. Although we expect the findings can be generalized to patient data, a confirmation analysis using CMAP scans under different stimulation protocols from other patients is necessary, such as patients with amyotrophic lateral sclerosis, the primary target population of CMAP scan examinations. Second, this study lacks EMG testing of the SCI subjects to provide confirmable or supplementary information about motor unit changes after SCI. Altered STEPIX and AMPIX can reflect motor unit number and size changes associated with spinal motor neuron degeneration and muscle fiber reinnervation. Additionally, muscle fiber atrophy is a confounding factor that may affect STEPIX and AMPIX. A simultaneous EMG test, i.e., fiber density analysis with single fiber EMG or quantitative MUAP analysis with concentric needle EMG, can help confirm CMAP scan findings and obtain more definite information about motor unit changes. These two limitations will be addressed in our future work.
In summary, this study investigated the effect of different stimulation parameters on CMAP scan’s STEPIX analysis. Contrary to MScanFit MUNE (which is significantly affected by stimulus pulse width, not by the number of stimuli), STEPIX and AMPIX are significantly affected by the number of stimuli, not by stimulus pulse width. The findings of this study can help determine reasonable CMAP scan stimulation parameters and facilitate appropriate interpretation of STEPIX and related results. The findings highlight the importance of applying the same CMAP scan stimulation parameters for STEPIX comparison. Application of the STEPIX analysis to SCI subjects supports that it provides an effective parameter in detecting motor unit loss after SCI. The data processing of STEPIX is automatic and fast, making it a promising CMAP scan method suitable for clinical applications.
Acknowledgments
This paragraph of the first footnote will contain the date on which you submitted your paper for review. This study was supported by National Natural Science Foundation of China under grant number 82102179, and Shandong Provincial Natural Science Foundation under grant numbers ZR2021QH267, ZR2021QH053, and ZR2020KF012. XL was supported by the National Institutes of Health under grant number 7 R21 NS113716–02 and the National Institute on Disability and Rehabilitation Research under grant number 90REMM0001–01-00.
Contributor Information
Zhiyuan Lu, School of Rehabilitation Science and Engineering, University of Health and Rehabilitation Sciences, Qingdao, Shandong, 266072, China.
Maoqi Chen, School of Rehabilitation Science and Engineering, University of Health and Rehabilitation Sciences, Qingdao, Shandong, 266072, China.
Ya Zong, School of Rehabilitation Science and Engineering, University of Health and Rehabilitation Sciences, Qingdao, Shandong, 266072, China; Department of Rehabilitation Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
Chengjun Huang, Department of Neuroscience, Baylor College of Medicine, Houston, TX 77030 USA.
Xiaoyan Li, Department of Neurology, Medical College of Wisconsin, Milwaukee, WI, USA, and also with Fischell Department of Bioengineering, University of Maryland at College Park, College Park, MD, USA.
Ping Zhou, School of Rehabilitation Science and Engineering, University of Health and Rehabilitation Sciences, Qingdao, Shandong, 266072, China.
REFERENCES
- [1].Wright RD, Sivak A, Abrahão A, Jones KE. Fifty Years of Motor Unit Number Estimation. Can J Neurol Sci. 2021. Dec 1:1–3. doi: 10.1017/cjn.2021.500. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- [2].Gooch CL, Doherty TJ, Chan KM, Bromberg MB, Lewis RA, Stashuk DW, Berger MJ, Andary MT, Daube JR. Motor unit number estimation: a technology and literature review. Muscle Nerve. 2014. Dec;50(6):884–93 [DOI] [PubMed] [Google Scholar]
- [3].de Carvalho M, Barkhaus PE, Nandedkar SD, Swash M. Motor unit number estimation (MUNE): Where are we now? Clin Neurophysiol. 2018. Aug; 129(8):1507–16. [DOI] [PubMed] [Google Scholar]
- [4].Fuglevand AJ, Winter DA, Patla AE. Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol. 1993. Dec;70(6):2470–88. [DOI] [PubMed] [Google Scholar]
- [5].Blok JH, Ruitenberg A, Maathuis EM, and Visser GH, “The electrophysiological muscle scan,” Muscle Nerve, vol. 36, no. 4, pp. 436–46, Oct, 2007. [DOI] [PubMed] [Google Scholar]
- [6].Visser GH, Blok JH. The CMAP scan. Suppl Clin Neurophysiol. 2009; 60:65–77. [DOI] [PubMed] [Google Scholar]
- [7].Ridall PG, Pettitt AN, Henderson RD, and McCombe PA, “Motor unit number estimation--a Bayesian approach,” Biometrics, vol. 62, no. 4, pp. 1235–50, Dec, 2006. [DOI] [PubMed] [Google Scholar]
- [8].Ridall PG, Pettitt AN, Friel N, McCombe PA, and Henderson R, “Motor unit number estimation using reversible jump Markov chain Monte Carlo methods,” Journal of the Royal Statistical Society: Series C (Applied Statistics), vol. 56, no. 3, pp. 235–269, 2007. [Google Scholar]
- [9].Bostock H, “Estimating motor unit numbers from a CMAP scan,” Muscle Nerve, vol. 53, no. 6, pp. 889–896, Jun. 2016, doi: 10.1002/mus.24945. [DOI] [PubMed] [Google Scholar]
- [10].Jacobsen AB, Bostock H, and Tankisi H, “CMAP scan MUNE (MScan) - a novel motor unit number estimation (MUNE) method,” J. Vis. Exp, vol. 2018, no. 136, p. 56805, Jun. 2018, doi: 10.3791/56805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tankisi H MScanFit Motor Unit Number Estimation: A Novel Method for Clinics and Research. Neurol Sci Neurophysiol 2021;38:1–5. [Google Scholar]
- [12].Zhou P Appropriate index parameters may serve a useful purpose in motor unit number estimation. Clin Neurophysiol. 2022. Jul;139:117–118. [DOI] [PubMed] [Google Scholar]
- [13].Sleutjes BTHM et al. , “CMAP scan discontinuities: automated detection and relation to motor unit loss,” Clin. Neurophysiol, vol. 125, no. 2, pp. 388–395, Feb. 2014, doi: 10.1016/J.CLINPH.2013.07.016. [DOI] [PubMed] [Google Scholar]
- [14].Nandedkar SD, Barkhaus PE, and Stålberg EV, “Analysis of the compound muscle action potential scan: Step index (STEPIX) and amplitude index (AMPIX),” Clin. Neurophysiol., Apr. 2022, doi: 10.1016/j.clinph.2022.04.011. [DOI] [PubMed] [Google Scholar]
- [15].Lu Z, Chen M, Zong Y, Li X, Zhou P. A Novel Analysis of CMAP Scans from Perspective of Information Theory: CMAP Distribution Index (CDIX). IEEE Trans on Biomed Eng; 2022. Oct 5; doi: 10.1109/TBME.2022.3212312. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maathuis EM, Henderson RD, Drenthen J, Hutchinson NM, Daube JR, Blok JH, Visser GH (2012) Optimal stimulation settings for CMAP scan registrations. J Brachial Plex Peripher Nerve Inj. 7(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sleutjes BTHM, Ruisch J, Nassi TE, Buitenweg JR, van Schelven LJ, van den Berg LH, Franssen H, Stephan Goedee H. Impact of stimulus duration on motor unit thresholds and alternation in compound muscle action potential scans. Clin Neurophysiol. 2021. Feb;132(2):323–331. [DOI] [PubMed] [Google Scholar]
- [18].Zong Y, Lu Z, Zhang L, Li X, and Zhou P, “Motor unit number of the first dorsal interosseous muscle estimated from CMAP scan with different pulse widths and steps,” J. Neural Eng, vol. 17, no. 1, p. 014001, Feb. 2020, doi: 10.1088/1741-2552/ab57cc. [DOI] [PubMed] [Google Scholar]
- [19].Zong Y, Lu Z, Xu P, Chen M, Deng L, Li S, Zhang Y, Xie Q and Zhou P (2022) MScanFit motor unit number estimation of abductor pollicis brevis: Findings from different experimental parameters. Front. Aging Neurosci. 14:953173. doi: 10.3389/fnagi.2022.953173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zong Y et al. , “CMAP scan examination of the first dorsal interosseous muscle after spinal cord injury,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 29, pp. 1199–1205, Jun. 2021, doi: 10.1109/TNSRE.2021.3088061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Burns S, Biering-Sørensen F, Donovan W, Graves D, Jha A, Johansen M, Jones L, Krassioukov A, Kirshblum, Mulcahey MJ, Schmidt Read M, Waring W. International Standards for Neurological Classification of Spinal Cord Injury, Revised 2011. Top Spinal Cord Inj Rehabil 2012;18(1):85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kalsi-Ryan S, Beaton D, Curt A, et al. (2012) The Graded Redefined Assessment of Strength Sensibility and Prehension (GRASSP) Reliability and Validity. J Neurotrauma 29(5):905–14. [DOI] [PubMed] [Google Scholar]
- [23].Sørensen DM, Bostock H, Ballegaard M, Fuglsang-Frederiksen A, Graffe CC, Grötting A, et al. Assessing inter-rater reproducibility in MScanFit MUNE in a 6-subject, 12-rater “Round Robin” setup, Neurophysiol Clin. 2022. Apr;52(2):157–169 [DOI] [PubMed] [Google Scholar]
- [24].Tankisi DA, Alaydin HC, Boran E, Cengiz B. Feasibility and reliability of MScanFit motor unit number estimation in peroneus longus muscle, Muscle Nerve 2022. Jun 28. doi: 10.1002/mus.27667. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- [25].Kristensen AG, Khan KS, Bostock H, Khan BS, Gylfadottir S, Andersen H, Finnerup NB, Jensen TS, Tankisi H MScanFit motor unit number estimation and muscle velocity recovery cycle recordings in diabetic polyneuropathy. Clin Neurophysiol. 2020. Nov;131(11):2591–2599. doi: 10.1016/j.clinph.2020.07.017. [DOI] [PubMed] [Google Scholar]
- [26].Nodera H et al. 2004. Nerve excitability properties in charcotmarie-tooth disease type 1A Brain 127 203–11 [DOI] [PubMed] [Google Scholar]
- [27].Henderson RD, Ridall GR, PettittA N, McCombe PA and Daube JR 2006. The stimulus-response curve and motor unit variability in normal subjects and subjects with amyotrophic lateral sclerosis Muscle Nerve 34 34–43 [DOI] [PubMed] [Google Scholar]
- [28].Korupolu R, Stampas A, Singh M, Zhou P, Francisco G. Electrophysiological Outcome Measures in Spinal Cord Injury Clinical Trials: A Systematic Review. Top Spinal Cord Inj Rehabil. 2019. Fall;25(4):340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang JF, Stein RB, Jhamandas J, Gordon T. Motor unit numbers and contractile properties after spinal cord injury. Ann Neurol. 1990; 28: 496–502. [DOI] [PubMed] [Google Scholar]
- [30].Xiong GX, Zhang JW, Hong Y, Guan Y, Guan H. Motor unit number estimation of the tibialis anterior muscle in spinal cord injury. Spinal Cord 2008;46: 696–702. [DOI] [PubMed] [Google Scholar]
- [31].Li X, Jahanmiri-Nezhad F, Rymer WZ, Zhou P. An examination of the motor unit number index (MUNIX) in muscles paralyzed by spinal cord injury. IEEE Trans Inf Technol Biomed. 2012;16(6):1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Li L, Li X, Liu J, Zhou P. Alterations in multidimensional motor unit number index of hand muscles after incomplete cervical spinal cord injury. Front Hum Neurosci. 2015;9:238. [DOI] [PMC free article] [PubMed] [Google Scholar]


