Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser.
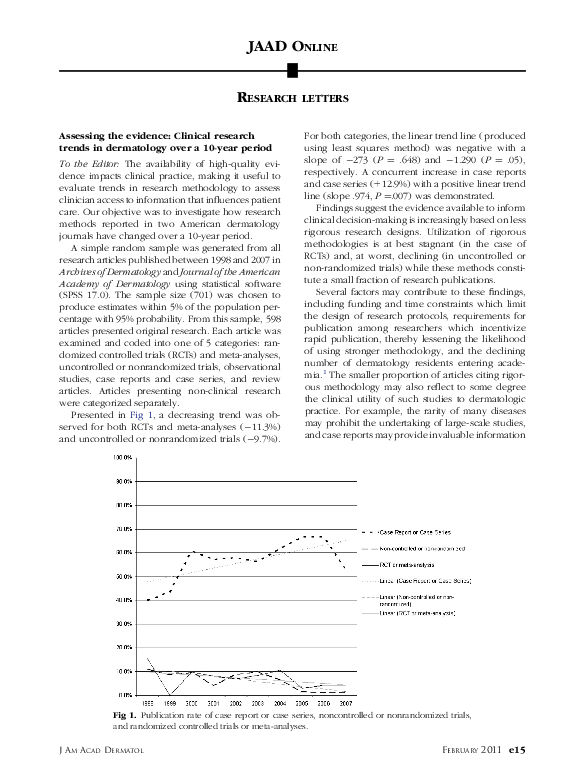
Assessing the evidence: Clinical research trends in dermatology over a 10-year period
Assessing the evidence: Clinical research trends in dermatology over a 10-year period
2011, Journal of the American Academy of Dermatology
Related Papers
Archives of Dermatology
Evidence-Based Medicine, the Research-Practice Gap, and Biases in Medical and Surgical Decision Making in Dermatology2010 •
Journal of Investigative Dermatology
The Growth of Clinical Trials and Systematic Reviews in Informing Dermatological Patient Care2012 •
Journal of Evidence Based Dental Practice
Evaluation of the Literature: Evidence Assessment Tools for Clinicians2013 •
A core principle of good public health practice is to base all policy decisions on the highest-quality scientific data, openly and objectively derived. 1 Determining whether data meet these conditions is difficult; uncertainty can lead to inaction by clinicians and public health decision makers. Although randomized, controlled trials (RCTs) have long been presumed to be the ideal source for data on the effects of treatment, other methods of obtaining evidence for decisive action are receiving increased interest, prompting new approaches to leverage the strengths and overcome the limitations of different data sources. 2-8 In this article, I describe the use of RCTs and alternative (and sometimes superior) data sources from the vantage point of public health, illustrate key limitations of RCTs, and suggest ways to improve the use of multiple data sources for health decision making. In large, well-designed trials, randomization evenly distributes known and unknown factors among control and intervention groups, reducing the potential for confounding. Despite their strengths, RCTs have substantial limitations. Although they can have strong internal validity, RCTs sometimes lack external validity ; generalizations of findings outside the study population may be invalid. 2,4,6 RCTs usually do not have sufficient study periods or population sizes to assess duration of treatment effect (e.g., waning immunity of vaccines) or to identify rare but serious adverse effects of treatment, which often become evident during post-marketing surveillance and long-term follow-up but could not be practically assessed in an RCT. The increasingly high costs and time constraints of RCTs can also lead to reliance on surrogate markers that may not correlate well with the outcome of interest. Selection of high-risk groups increases the likelihood of having adequate numbers of end points, but these groups may not be relevant to the broader target populations. These limitations and the fact that RCTs often take years to plan, implement, and analyze reduce the ability of RCTs to keep pace with clinical innovations; new products and standards of care are often developed before earlier models complete evaluation. These limitations also affect the use of RCTs for urgent health issues, such as infectious disease outbreaks, for which public health decisions must be made quickly on the basis of limited and often imperfect available data. RCTs are also limited in their ability to assess the individualized effect of treatment, as can result from differences in surgical techniques , and are generally impractical for rare diseases. Many other data sources can provide valid evidence for clinical and public health action. Observational studies, including assessments of results from the
British Journal of Dermatology
Publication of national dermatology guidelines as a Research Letter in the BJD : can less ever be enough?2020 •
Journal of Evaluation in Clinical Practice
Systematic reviews showed insufficient evidence for clinical practice in 2004: what about in 2011? The next appeal for the evidence-based medicine age2013 •
Journal of multidisciplinary healthcare
Does the medical literature remain inadequately described despite having reporting guidelines for 21 years? - A systematic review of reviews: an update2018 •
Reporting guidelines (eg, Consolidated Standards of Reporting Trials [CONSORT] statement) are intended to improve reporting standards and enhance the transparency and reproducibility of research findings. Despite accessibility of such guidelines, researchers are not required to adhere to them. Our goal was to determine the current status of reporting quality in the medical literature and examine whether adherence of reporting guidelines has improved since the inception of reporting guidelines. Eight reporting guidelines, such as CONSORT, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), STrengthening the Reporting of OBservational studies in Epidemiology (STROBE), Quality of Reporting of Meta-analysis (QUOROM), STAndards for Reporting of Diagnostic accuracy (STARD), Animal Research: Reporting In Vivo Experiments (ARRIVE), Consolidated Health Economic Evaluation Reporting Standards (CHEERS), and Meta-analysis of Observational Studies in Epidemiology (MOOSE)...
RELATED PAPERS
Academic Chronicles
From Awareness to Action: Enhancing Equity and Inclusion in Classrooms Through Culturally Responsive Teaching2023 •
2023 •
Advances in Interventional Cardiology
Coronary interventions via radial artery without pre procedural routine use of spasmolytic agents2020 •
Urban planning
Learning From Covid-19: Social Infrastructure in Disadvantaged Housing Areas in Denmark2022 •
2024 •
2018 •
Current Obesity Reports
Sarcopenic Obesity: Epidemiologic Evidence, Pathophysiology, and Therapeutic Perspectives2019 •
Life and Science
SARS-CoV-2 Leading Vaccine Candidates: Progress and Development2020 •
 Elizabeth Farley-Ripple
Elizabeth Farley-Ripple