1. Introduction
Diabetes is a metabolic disease characterized by hyperglycemia, abnormal lipid, and protein metabolism, and in the long term, it affects the retina, kidney, liver, and the nervous system [1]. There are type 1 and type 2 diabetes, gestational diabetes, and some other uncommon forms. Also called non-insulin-dependent diabetes mellitus (NIDDM), T2D is characterized by insulin deficiency and peripheral insulin resistance resulting from the failure of pancreatic β-cell adaptation to increase in function and mass orchestrated by nutrient metabolism, hormones, and cytokines [2]. Reduction in insulin resistance and improvement of β-cell function and its mass are, therefore, important in the reduction of progression of T2D [2].
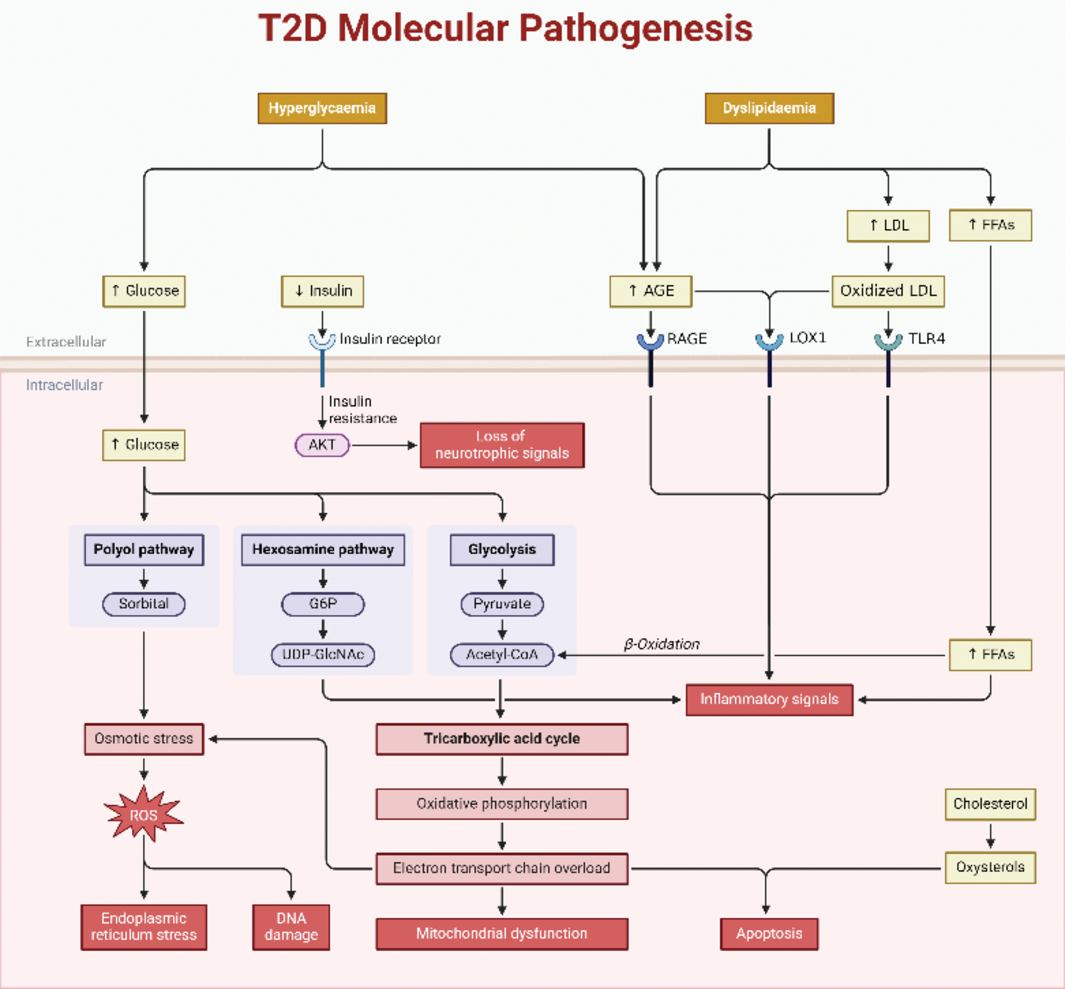
Advances in modern drug discovery have been able to uncover several antidiabetic strategies by evaluating the efficacy and safety of many bioactive scaffolds as modulators of experimentally confirmed molecular targets. These targets include enzymes involved in the sugar metabolism pathways such as glycolytic, hexosamine, advanced glycation end products, protein kinase C, polyol, and insulin signaling pathways [3] (Figure 1).
The mechanisms through which these compounds interact with these pathways are complex and involve multiple molecular targets [3]. In the glycolytic pathway, soy isoflavones, particularly genistein and daidzein, modulate enzymes such as adenosine monophosphate-activated protein kinase (AMPK), potentially enhancing glucose uptake and use by cells [4, 5]. In the hexosamine biosynthesis pathway (HBP), which is implicated in insulin resistance, soy bioactives, particularly isoflavones, may be modulated contributing to improved insulin sensitivity [6]. Specifically, it has been shown that genistein can inhibit the stimulation of the glutamine fructose-6-phosphate amidotransferase (GFAT) promoter. GFAT is a key enzyme in the HBP that catalyzes the conversion of fructose-6-phosphate to glucosamine-6-phosphate [7]. Soy compounds may have anti-glycation properties, reducing the formation of advanced glycation end products (AGEs) associated with complications of diabetes. Genistein can inhibit AGE-RAGE (receptor advanced glycation end products) binding, potentially reducing the downstream effects of RAGE activation [7]. Soy bioactives may influence the insulin signaling pathway in several ways. First, soy isoflavones, specifically genistein, have been reported to activate the insulin receptor and insulin receptor substrate (IRS) proteins. This activation enhances insulin signaling, leading to improved glucose uptake and metabolism [8]. Second, certain soy bioactives may inhibit tyrosine kinase, an enzyme that negatively regulates insulin signaling. The inhibition of tyrosine kinase can enhance insulin signaling [9]. Third, soy compounds may modulate the PI3K/Akt pathway which affects insulin signaling and can positively influence downstream signaling events involved in glucose homeostasis [10].
Figure 1
Molecular pathogenesis of T2D showing different signaling pathways. Created with BioRender.com.
Most T2D chemotherapeutic agents are replete with side effects, and many of these are contraindicated during pregnancy. This has resulted in the search for natural products that could treat the disease while keeping the safety of the patient [11]. Therefore, attention is being focused on edible plants that have therapeutic molecules to offer effective alternatives to the treatment of T2D with little or no side effects [11, 12]. Among the plants believed to have the potential to treat diabetes is the soybean. Soybean has been used globally for medicinal purposes, and several studies have proven its ability to lower hyperglycemia [13]. This plant has been an important protein source, and among Asians who consume the grain routinely, the incidence of T2D is said to be reduced [14]. The antidiabetic effects of soybeans and their phytochemicals are mediated through various mechanisms which include improving insulin secretion and sensitivity, regulating glucose metabolism, exerting antioxidant and anti-inflammatory effects, and modulating gut microbiota [15].
Soybean consists of isoflavones, protein, carbohydrate, and lipid fractions, and it has been reported to play a significant role in controlling metabolic disorders such as obesity and hyperglycemia [16, 17]. There are numerous methods of preparing soybeans, and the meals made from them can either be fermented or unfermented. Tofu, soymilk, edamame, soy nuts, and sprouts are examples of non-fermented foods. Fermented soy products include miso, tempeh, natto, and soy sauce [18].
Fermentation has been shown to improve the anti-oxidative and antidiabetic potentials of soybeans. Fermentation leads to the activation of certain enzymes which include proteases and peptidases, which results in the production of bioactive peptides [19]; β-glucosidase and α-galactosidase, which break down complex carbohydrates and oligosaccharides, making nutrients more bioavailable and potentially enhancing antidiabetic effects [20]; and lipase which influences the breakdown of lipids and the production of bioactive lipid compounds with potential health benefits [21]. Other enzymes that could be triggered by fermentation are polyphenol oxidase which is involved in the oxidation of polyphenols, affecting their levels of polyphenols and their antioxidant capacities in soybeans [22], and phytase which improves the mineral bioavailability and potentially enhances the nutritional quality of soybeans [23]. Fermentation can also ease the deglycosylation of certain compounds, making them more bioactive. For example, isoflavone glycosides in soybeans may be converted into their aglycone forms during fermentation, potentially enhancing their health-promoting effects [24].
When fermented, the soybean contents such as fermented soybean products, isoflavonoids, and soy peptides improve glucose metabolism and insulin secretion, attenuate insulin resistance, and increase β-cell mass [25]. Soybean, therefore, may prevent T2D as well as delay its progression [2]. This review article aims at highlighting the bioactive properties of soybeans with respect to the prevention, control, and management of diabetes given its importance as a disease of major public health concern.
2. Antidiabetic potential
The extracts of soybeans have been linked with the inhibition of key enzymes associated with T2D, hypertension, and other related bioactivities (see Table 1) such as anti-inflammation and antioxidation [26].
2.1. Anti-oxidative activity
Oxidation is a chemical reaction that results in the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS). In living things, free radicals are byproducts of metabolic processes in aerobic cells and exist in homeostasis with antioxidants. Accumulation of these highly unstable reactive species can result in oxidative stress, a phenomenon that occurs when there is an imbalance between the free radicals produced and the antioxidants that inhibit oxidation [27]. Oxidative stress has been remarkably connected to diabetes [28]. In diabetic patients, oxidative stress arises from an increase in the production of free radicals or a suppression of the activity of endogenous antioxidants, or both, and it induces dysfunction of β-cells as well as insulin resistance [29]. Hyperglycemia has been implicated in oxidative stress through the modulation of several molecular pathways involved in the metabolism of glucose and lipids such as glycolysis, hexosamine (HBP), advanced glycation end products (AGE), protein kinase C (PKC), polyol, and insulin signaling pathways [30] (Figure 1).
Table 1
The soy constituents, class of molecules, and their antidiabetic properties
| Soy-constituent | Class of molecule | Anti-T2D effect |
|---|---|---|
| Genistein | Polyphenol | Anti-oxidative [31] |
| Daidzein | Polyphenol | Anti-oxidative [31] |
| Glycitein | Polyphenol | Anti-oxidative [31] |
| Equol | Polyphenol | Anti-oxidative [32] |
| Bowman-Birk inhibitor (BBI) | Protein | Anti-oxidative [33] |
| Lunasin | Peptide | Anti-oxidative [34] |
| GNPDIEHPE | Peptide | Anti-oxidative [35] |
| SVIKPPTDE | Peptide | Anti-oxidative [35] |
| VIKPPTDE | Peptide | Anti-oxidative [35] |
| GNPDIEHPET | Peptide | Anti-oxidative [35] |
| FEEPQQPQ | Peptide | Anti-oxidative [35] |
| D-10-R | Peptide | Anti-oxidative [36] |
| S-10-S | Peptide | Anti-oxidative [36] |
| V-11-R | Peptide | Anti-oxidative [36] |
| I-11-N | Peptide | Anti-oxidative [36] |
| ASP-3A | Polysaccharide | Anti-oxidative [37] |
| Raffinose | Polysaccharide | Anti-oxidative [38] |
| Phosphatidylcholine | Polyunsaturated fatty acid | Anti-oxidative [39] |
| Soybean germ phytosterols | Lipids | Anti-oxidative [40] |
| Cyanidin-3-O-glucoside | Polyphenol | Anti-inflammatory [41] |
| Daidzein | Polyphenol | Anti-inflammatory [42] |
| FLV | Peptide | Anti-inflammatory [43] |
| Val-Pro-Tyr | Peptide | Anti-inflammatory [44] |
| Lunasin | Peptide | Anti-inflammatory [45] |
| SSPS1 | Polysaccharide | Anti-inflammatory [46] |
| MET-SSPS-NPs | Polysaccharide | Anti-inflammatory [47] |
| O-3 PUFA | Polyunsaturated fatty acid | Anti-inflammatory [48] |
| Glucosylceramide | Sphingolipids | Anti-inflammatory [49] |
| Myricetin | Polyphenol | α-Amylase inhibition [50] |
| Glycitein | Polyphenol | α-Amylase inhibition [51] |
| Genistein | Polyphenol | α-Amylase inhibition [51] |
| Daidzein | Polyphenol | α-Amylase inhibition [51] |
| Peptide fractions | Peptides | α-Amylase inhibition [52] |
| SSPS | Polysaccharides | α-Amylase inhibition [53] |
| Insoluble dietary fibers | Polysaccharides | α-Amylase inhibition [54] |
| Soyasaponins | Saponins | α-Amylase inhibition [55] |
| Coumestrol | Polyphenol | α-Glucosidase inhibition [56] |
| Luteolin | Polyphenol | α-Glucosidase inhibition [57] |
| Glyceollin | Polyphenol | α-Glucosidase inhibition [57] |
| Daidzein | Polyphenol | α-Glucosidase inhibition [57] |
| Genistein | Polyphenol | α-Glucosidase inhibition [57] |
| 1-Deoxynojirimycin | Alkaloid | α-Glucosidase inhibition [58] |
| (ESPro1) | Protein | α-Glucosidase inhibition [59] |
| Gly-Ser-Arg | Peptide | α-Glucosidase inhibition [60] |
| Glu-Ala-Lys | Peptide | α-Glucosidase inhibition [60] |
| LLPLPVLK | Peptide | α-Glucosidase inhibition [61] |
| SWLRL | Peptide | α-Glucosidase inhibition [61] |
| WLRL | Peptide | α-Glucosidase inhibition [61] |
| Soyasaponin Ba | Saponins | α-Glucosidase inhibition [62] |
| Soyasaponin Bb | Saponins | α-Glucosidase inhibition [62] |
| SSPS | Polysaccharides | α-Glucosidase inhibition [63] |
| Morin | Polyphenol | Aldose reductase inhibition [64] |
| Quercitrin | Polyphenol | Aldose reductase inhibition [64] |
| Naringenin | Polyphenol | Aldose reductase inhibition [64] |
| Rutin | Polyphenol | Aldose reductase inhibition [64] |
| Genistein | Polyphenol | Aldose reductase inhibition [65] |
| Daidzein | Polyphenol | Aldose reductase inhibition [66] |
| 8-Hydroxydaid-zein | Polyphenol | Aldose reductase inhibition [67] |
| Glycitein | Polyphenol | Aldose reductase inhibition [68] |
| Stigmasterol | Lipid | DPP-4 inhibition [69] |
| VVNPDNNEN | Peptide | DPP-4 inhibition [69] |
| EEPQQPQQ | Peptide | DPP-4 inhibition [70] |
| QEPQESQQ | Peptide | DPP-4 inhibition [70] |
| SQRPQDRHQ | Peptide | DPP-4 inhibition [70] |
| PETMQQQQQQ | Peptide | DPP-4 inhibition [70] |
| SSPDIYNPQAGSVT | Peptide | DPP-4 inhibition [70] |
| APFL | Peptide | DPP-4 inhibition [70] |
| TPVGM | Peptide | DPP-4 inhibition [71] |
| EPAAV | Peptide | DPP-4 inhibition [71] |
| IAVPTGVA | Peptide | DPP-4 inhibition [72] |
| Daidzein | Polyphenol | Pancreatic lipase inhibition [73] |
| Glycitein | Polyphenol | Pancreatic lipase inhibition [73] |
| Genistein | Polyphenol | Pancreatic lipase inhibition [73] |
| PIL | Protein | Pancreatic lipase inhibition [74] |
| Lipoxygenase-1 | Protein | Pancreatic lipase inhibition [75] |
| 56.0 kDa α-Amylase | Protein | Pancreatic lipase inhibition [76] |
| FPFPRPPHQ, | Peptide | Pancreatic lipase inhibition [77] |
| QCCAFEM | Peptide | Pancreatic lipase inhibition [77] |
| FAPEFLK | Peptide | Pancreatic lipase inhibition [77] |
| SFFFPFELPRE | Peptide | Pancreatic lipase inhibition [77] |
| FMYL | Peptide | Pancreatic lipase inhibition [77] |
| PFLL | Peptide | Pancreatic lipase inhibition [77] |
| FPLL | Peptide | Pancreatic lipase inhibition [77] |
| LPHF | Peptide | Pancreatic lipase inhibition [77] |
| Galactomannan | Polysaccharide | Pancreatic lipase inhibition [78] |
| Chicoric acid | Polyphenol | ACE inhibition [79] |
| Beta-sitosterol | Lipid | ACE inhibition [80] |
| Nicotianamine | Metal chelator | ACE inhibition [81] |
| Val-Leu-Ile-Val-Pro | Peptide | ACE inhibition [82] |
| LPYP | Peptide | ACE inhibition [82] |
| IAVPTGVA | Peptide | ACE inhibition [82] |
| Leu-Val-Gln-Gly-Ser | Peptide | ACE inhibition [83] |
| Genistein | Polyphenol | PEPCK inhibition [84] |
| Daidzein | Polyphenol | PEPCK inhibition [85] |
| Genistein | Polyphenol | PPARγ activation [85] |
| Daidzein | Polyphenol | PPARγ activation [85] |
| Formononetin | Polyphenol | PPARγ activation [85] |
| Biochanin A | Polyphenol | PPARγ activation [85] |
Elevated levels of oxidized DNA, lipids, and proteins suggest that enhanced oxidative stress plays a major role in the pathogenesis of diabetes issues [86]. Several pathological mechanisms could trigger oxidative stress in diabetes mellitus. Excessive generation of superoxide anions happens when the mitochondria’s electron transport chain is disrupted by excessive glucose levels [87]. As advanced glycation end products (AGEs) form and accumulate, ROS are produced [88, 89]. As AGEs bind with their receptors (RAGE), ROS is further produced [90]. Moreover, the deactivation of antioxidant enzymes by glycation, as seen by the glycation of superoxide dismutase (SOD), compromises the antioxidant defense [91, 92]. An important additional avenue through which high glucose levels might cause oxidative damage is the polyol pathway [93]. Previous studies using aldose reductase (AR)–deficient mice models have demonstrated that the polyol pathway plays a critical role in diabetes-induced oxidative stress [94, 95].
The polyol pathway may cause oxidative stress by one of three mechanisms. Firstly, in a state of hyperglycemia, about 30% of the glucose is directed into the AR-dependent polyol pathway, which depletes NADPH and reduces glutathione (GSH) levels [96]. Secondly, when sorbitol dehydrogenase (SDH) converts sorbitol into fructose, oxidative stress is triggered. At this point, SDH converts the co-factor NAD + into NADH. Superoxide anions are produced by NADH oxidase using NADH as a substrate [97]. Thirdly, glucose is converted to fructose through the polyol pathway. Fructose can be further broken down to yield fructose-3-phosphate and 3-deoxyglucosone, which are two stronger non-enzymatic glycation agents than glucose [98, 99]. This implies that more AGEs would be produced as more glucose goes via the polyol route, which would lead to the generation of ROS [100].
Small molecule natural products
Soybean has polyphenolic antioxidants which exist in various forms determined by the color of the seed coat. Like other plant extracts, the quality and antioxidant potential of a soybean extract are dependent on both its source and method of extraction. Isoflavones are the polyphenols mostly found in soybean and are mainly made up of genistein, daidzein, and glycitein, and these are made more bioavailable by incorporating the hydroxyl group through fermentation by microorganisms [31, 101, 102]. In three different antioxidant assays (DPPH, FRAP, and ABTS), daidzein, glycitein, and genistein have shown the highest antioxidant capacity in human intestinal Caco-2 cells, out of the 12 major soybean isoflavones [9]. Equol, a metabolite formed from daidzein, has the highest estrogenic and antioxidant activities of all isoflavone-derived compounds. Equol has been shown to have even greater antioxidant power than 17-estradiol, alpha-tocopherol, and ascorbic acid [32].
The antioxidant and anti-hyperglycemic activities of black soybean (Glycine max (L) Merr) seed coat treated with either ethyl acetate or hydro-ethanol both in vitro and in vivo using diabetes-induced Wistar rats have been investigated in a recent study. The results showed that in vitro, both ethyl acetate and hydroethanolic extracts of black soybean scavenged both 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical and hydrogen peroxide in a dose-dependent manner, and significantly reduced blood glucose level. In addition, the in vivo study reveals that the levels of GSH and activity of SOD in the pancreas and brain were elevated [13]. Interestingly, the wild soybean, Glycine soja, which has been a source of traditional food for the Chinese has isoflavones at levels significantly higher than its cultivated counterpart G. max [103]. In a recent study, the phenolic and flavonoid composition as well as the antioxidant activity of G. max and its wild relative G. soja was examined. The results of DPPH and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays showed that the scavenging activity of soybean extracts obtained using 70% methanol was notably more elevated in wild soybean than the cultivated one [104].
Compared to non-fermented soyfoods, fermented soyfoods—especially those that are long-fermented—have a greater potential for antioxidants. The foods with the greatest H-ORAC values are tempeh, natto, and soy sauce, and then Soybean-koji miso. Given the high antioxidant content of fermented soy products, this may be related to the powerful radical scavenging properties of aglycone isoflavones [105]. Certain bacteria are involved in the fermentation process of soybeans, which helps change the substances found in soybeans, such as isoflavones. These microbes are essential to the fermentation process because they break down complicated molecules and produce beneficial metabolites. They include Bacillus spp. [106], Streptococcus spp. [107], Aspergillus spp. [108], Lactobacillus spp. [109], Enterococcus spp. [110], and Rhizopus spp. [111].
The microbes involved in soybeans fermentation produce the β-glucosidase enzyme, which transforms the glycosides and polyphenols in these products into their aglycone form. These changes result in increased therapeutic benefits and anti-cancer, anti-obesity, neuroprotective, and antidiabetic activities by increasing the bioavailability of these phenolic compounds in the human gut [31]. In a 12-week, randomized, controlled, crossover clinical trial (NCT03429920) involving 27 participants, it was discovered that a non-probiotic fermented soy product reduced total and LDL cholesterol levels [112]. Also, in another 12-week, randomized, multi-center, placebo-controlled, double-blind clinical trial, it was proven that Lactobacillus plantarum C29–fermented soybean (DW2009) has an ameliorative effect on mild cognitive impairment (MCI). After DW2009 was administered, there was an increase in serum brain-derived neurotrophic factor (BDNF) levels. This finding implies the involvement of the gut–brain axis in improving cognitive deficits in MCI and may offer some preliminary insight into the underlying effects of cognitive improvement. The clinical trial’s findings show that DW2009 can be given to MCI patients in a way that is safe and effective at improving their cognitive function [113].
Proteins/peptides
Soybean has bioactive peptides with antioxidant and antidiabetic activities. The Bowman-Birk inhibitor (BBI) is an 8 kDa natural soybean-derived protein that has been shown to have antioxidant properties. BBI inhibits superoxide anion radical generation and reduces oxidative stress, which can be beneficial for overall health [33]. Nuclear factor erythroid 2-related factor 2 (Nrf2) is typically sequestered in the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1). Under conditions of oxidative stress or exposure to specific compounds, Nrf2 is released from Keap1, translocates to the nucleus, and induces the expression of genes coding antioxidant enzymes such as SOD or catalase, heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase 1 (NQO1), and glutathione S-transferase (GST). These enzymes play a crucial role in cellular defense against oxidative stress [114]. The peptides of BBI exhibit strong binding with Keap1 at the binding site of Nrf2. This makes Nrf2 more available to combat oxidative stress [115].
Also, Lunasin, a 5 KDa soy peptide, inhibits the oxidation of linoleic acid and acts as an ABTS radical scavenger [34, 116]. According to published reports, lunasin possesses several antioxidant qualities, including the ability to block signaling pathways triggered by oxidative stress, activate the Nrf2 pathway, and alter the activity of antioxidant enzymes including SOD and catalase. By neutralizing ROS, these enzymes are essential for the body’s defense against oxidative stress [117]. Lunasin also has metal-chelating properties. By chelating metals, lunasin may help prevent oxidative damage. Metal chelation is relevant to oxidative stress, as transition metals can catalyze the production of ROS [115].
To test the anti-oxidative potential of soybean peptides, Zhang et al. [35] hydrolyzed soybean using alcalase, and the resultant protein hydrolysate was digested with pepsin and pancreatin under simulated gastrointestinal conditions. A significant free-radical scavenging activity was seen in DPPH and ABTS antioxidant assays as well as in Caco-2cells with H2O2-induced oxidative stress, and was enhanced by digestion. Moreover, the peptides GNPDIEHPE, SVIKPPTDE, VIKPPTDE, GNPDIEHPET, and FEEPQQPQ remained intact after LC-MS absorption and could show antioxidant properties after being absorbed by the intestinal epithelia [35]. In another study, fermented soy products obtained using Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus and sequentially digested with pepsin and pancreatin were investigated for their antioxidant activity. The peptides produced D-10-R, S-10-S, V-11-R, and I-11-N had weak antioxidant activity in vitro (ABTS test), but in Caco-2 cells, they strongly inhibited the binding of Keap1 protein to nuclear factor erythroid 2-related factor 2 (Nrf2). Thus, the levels of Nrf2 were elevated suggesting that they offered protection against oxidative stress induced by tert-butyl hydroperoxide [36].
Polysaccharides
Dietary polysaccharides also have an antioxidant role in combating diabetes. Their antioxidant potential has been attributed to their ability to enhance the activity of antioxidant enzymes, as well as the possible conjugation of small moieties of peptides, flavones, polyphenols, and proteins on the polysaccharides [118]. Soy hulls are primarily made up of cellulose, hemicellulose, and pectin, and these polysaccharides have been suggested to play a role in regulating blood glucose and scavenging free radicals based on their varying physicochemical properties [119]. A study by Song et al. [37] using superoxide radical, hydroxy radical, ABTS, and DPPH scavenging tests revealed that ASP-3A, a pectic polysaccharide, displayed dose-dependent antioxidant activities, activated the NRF2/ARE pathway, and thus improved the antioxidant activity of hepatocytes in mice with CCl4-induced oxidative stress. In another study, polysaccharides from small black soybean were found to change the abundance of Oscillospira, Ruminococcus, Mucispirillum, and Dorea bacteria in the gut of STZ-induced diabetic rats, and control the metabolism of linoleic acid in addition to improving the symptoms of both hyperglycemia and lipidemia. Also, the soybean bran conferred stronger cytoprotection to hepatocytes [120]. Soybeans have raffinose, and this polysaccharide has been shown to improve the oxidative status of the in Nile Tilapia (Oreochromis niloticus). Feeding raffinose increased SOD activity at all levels, while glutathione peroxidase (GPX) and SOD improved by 1 to 1.5 g/kg above the control. Raffinose, on the other hand, reduced the level of malondialdehyde (MDA), and the lowest concentrations were found at 1 or 1.5 g/kg levels [38, 121].
Lipids
It has been proven that the polyunsaturated fatty acid phosphatidylcholine (PC), which is present in soybeans, has antioxidant properties. PC may be a useful functional material for hepatic and renal injuries involving oxidative stress generated by AGE during diabetes conditions since cells treated with PC showed a considerable reduction in cytotoxicity and oxidative stress [39]. Soybeans are rich in saponins [122], and in T2D mouse models, these compounds have demonstrated the potential to restore body weight, suppress hyperglycemia and hyperlipidemia, restore insulin sensitivity, improve adipocyte thermogenesis, reduce the underlying hepatic damage caused by steatosis, and increase the ratio of hepatic phosphorylated adenosine monophosphate-activated protein kinase (p-AMPK) to the total protein [123]. A study was conducted utilizing DPPH˙ and OH˙ radical scavenging assays, a beta-carotene protection assay, and a heating oil system to examine the antioxidant ability of soybean germ and its oil from Northeast (NE-SG) and Shandong Province (SD-SG) of China. According to the findings, soybean germ phytosterols were more effective antioxidants in oil systems than in non-oil systems. Since it was more effective at lower temperatures (60°C) with longer times as opposed to higher temperatures (120°C and 180°C) with shorter times, these phytosterols’ antioxidant capacity was temperature- and time-dependent. These findings prove that soybean germ phytosterols can act as antioxidants to stop lipid oxidation in foods that have been kept at low temperatures for an extended period [40].
2.2. Anti-inflammatory activity
The critical role of inflammation in the pathophysiology of T2D has been proven. Chronic inflammation is often seen in individuals with T2D, and it may lead to insulin resistance which is a hallmark of the disease [124]. High sugar levels can lead to oxidative stress and inflammation which can contribute to the complications associated with T2D such as cardiovascular disease and nerve damage [125]. The function of tumor necrosis factor-alpha (TNF-α) in obesity, notably in insulin resistance and diabetes, is revealed by empirical research. Epidemiologic links between inflammation and obesity and T2D have been set up because of elevated levels of circulating pro-inflammatory markers and mediators which include fibrinogen, C-reactive protein, interleukin (IL)-6, plasminogen activator inhibitor-1, sialic acid, and white blood cells, in these conditions [126].
Small molecule natural products
The anti-inflammatory properties of soybeans also have antidiabetic effects. In a study conducted by Matsukawa et al. [41], black soybean seed coat extract (BSSCE), together with its active ingredient cyanidin-3-O-glucoside (Cy3G), has been shown to have antidiabetic benefits on db/db mice via enhancing adipocyte development. Additionally, it was found that 3T3-Ll cells treated with BSSCE and Cy3G differentiated into smaller adipocytes, which was associated with upregulated PPARγ and C/EBP gene expressions, upregulated adiponectin secretion, downregulated TNF production, activated insulin signaling, and upregulated glucose uptake. PGC-1, SIRT1, and UCP-3 gene expressions were all noticeably elevated in C2C12 myotubes cultivated in conditioned media made from 3T3-L1 adipocyte cultures treated with Cy3G.
Beneficial bioactive substances found in soybeans that have been proven to be effective against chronic inflammatory diseases such as T2D include isoflavones, peptides, fibers, polysaccharides, and lipids [127]. The isoflavone, daidzein, obtained from fermented soybean can prevent inflammation associated with T2D [42]. A study investigated the inhibitory effect of daidzein on the expression of pro-inflammatory cytokines, CCL2 and IL-6, in a co-culture of 3T3L1 adipocytes and RAW264 macrophages. The results revealed that treatment with daidzein significantly suppressed mRNA expression of CCL2 and IL6 in both the adipocytes and macrophages. In addition, daidzein inhibited the transcription of pro-inflammatory genes and reduced the mRNA levels of CXCL2 in TNFα-treated cells [42].
Peptides
Enzymatic hydrolysis, fermentation, food processing, and gastrointestinal digestion of soy protein give rise to soybean peptides (SPs), which aid in the absorption of amino acids in the body among other physiological activities [127]. Earlier studies have reported that administering synthesized soy peptide FLV caused a decrease in the production and effect of inflammatory molecules and increased the sensitivity of adipocytes to insulin [43]. An in vitro study using a Caco-2 cell line reported the anti-inflammatory effect of soy tripeptide Val-Pro-Tyr which decreased the expression of TNF-α, IL-6, IL-1, and IL-17 in those cells [44]. Another soy peptide, lunasin, also showed anti-inflammatory properties by decreasing the expression of COX-2 in mice colon induced by dextran sodium sulfate (DSS) [45].
Polysaccharides
Through underlying molecular pathways connected to inflammatory factors, numerous studies have proven a link between dietary polysaccharides and the alleviation of diabetes. The use of these polysaccharides has been shown to have hypoglycemic, hypolipidemic, antioxidant, and anti-inflammatory properties, which increase pancreatic β-cell mass and reduce β-cell dysfunction in in vivo and in vitro studies. Dietary polysaccharides activate the PI3K/Akt pathway, enhance insulin signaling pathways via insulin receptors, and eventually modulate the ERK/JNK/MAPK pathway [128]. Specifically, soluble soybean polysaccharide (SSPS1) with a molecular weight of 2,737 kDa obtained from soy whey enhanced the growth rate and phagocytic activity of RAW 264.7 macrophages and modulate the secretion of IL-1β, IL-6, INF-β, TNF-α, and nitric oxide [46]. The addition of soluble soybean polysaccharides to dairy products as a source of dietary fiber has been shown to reduce blood cholesterol, improve laxation, and reduce the risk of diabetes [129]. Also, He et al. [47] discovered that in a high-fat-diet animal model, metformin-soluble soybean polysaccharide nanoparticles (MET-SSPS-NPs) had a lipid-lowering effect. MET-SSPS-NPs can significantly downregulate PCSK9 and fatty acid-binding proteins (FABP5 and FABP7), and upregulate peroxisome proliferator-activated receptor gamma (PPARγ) expressions, consequently increasing the level of adipokines.
Lipids
Soybeans have omega-3 polyunsaturated fatty acids (O-3 PUFAs). Clinical and empirical data associates these compounds with decreased pro-inflammatory mediators in chronic inflammatory diseases like diabetes [130]. Bioactive metabolites produced by fatty acid oxygenases mediate the biological activities of O-3 PUFAs [48]. These compounds can alter the diversity and abundance of the gut microbiota, which in turn modulates T2DM [48, 131]. Specifically, O-3 PUFA reduces inflammation by inhibiting the production of pro-inflammatory cytokines, such as IL-17, in monocytes that have been exposed to harmful lipopolysaccharides (LPS) and stimulating the production of anti-inflammatory factors such as IL-10 [48]. In addition, the role of dietary sphingolipids in the prevention of metabolic syndrome has been studied. Dietary glucosylceramide (a sphingolipid) has anti-inflammatory properties which include suppressing the mRNA expression of pro-inflammatory cytokines such as IL-1β and IL-6, downregulating the activation of TNF-α, and suppressing tissue edema and leukocyte infiltration to the inflammatory site [49].
2.3. Alpha-amylase inhibition
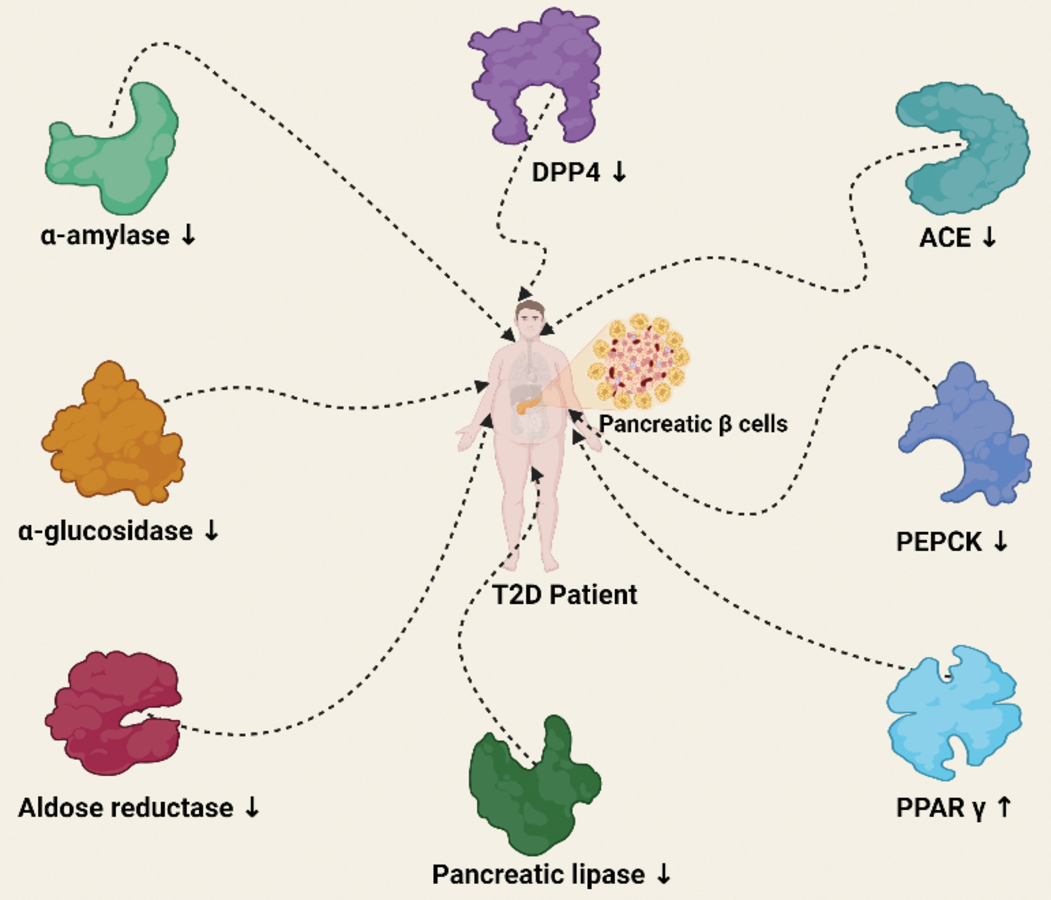
Many enzymes are involved in the T2D pathogenesis (Figure 2). α-Amylase is a calcium metalloenzyme that plays a critical role in the breakdown of complex polysaccharides into smaller units such as glucose and maltose to ease their absorption into the body [132]. The enzyme also elevates blood sugar levels and produces postprandial hyperglycemia, and one of the strategies for regulating glucose levels in diabetes is the use of drugs or substances that can block the hydrolysis of carbohydrates by α-amylase [70, 133].
Figure 2
Enzyme targets for management of T2D. ↑ means activation. ↓ means inhibition. Created with BioRender.com
Small molecule natural products
Isoflavones obtained from the ethanolic extract of Tempe (made from un-germinated soybeans) and Tempe flour (made from germinated soybeans) have been proven to be α-amylase inhibitors [134]. This study showed that the ability of the soybeans to inhibit α-amylase activity increased with germination, and this is evidenced by the IC50 values which were significantly lower in un-germinated soy Tempe. The results suggest that germination of soybeans helps increase the bioactive compound contents in soybeans, and this is due to various biochemical and enzymatic reactions such as the activation of protease enzymes, changes in pH, the breakdown of storage compounds, and changes in gene expression patterns. These changes occur in the seeds during germination, thereby improving their nutritional value and digestibility unlike un-germinated seeds [134].
In a study by Tan and Chang [50], high-performance liquid chromatography (HPLC) analysis of fractions of phenolic extract of black soybean was performed. Results showed that black soybean had phenolic acids such as syringic, gallic, p-hydroxybenzoic, vanillic, ferulic, protocatechuic, caffeic, sinapic, chlorogenic, and salicylic acids. Other flavonoids contained in black soybeans include catechin, quercetin-3-O-glucoside, myricetin, epicatechin, quercetin-3-O-glucoside, kaempferol-3-O-rutinoside, and kaempferol-3-O-glucoside. All the pure phenolic acids and flavonoids had anti-α-amylase inhibitory activity with myricetin as the strongest enzyme inhibitor [50].
Tiss et al. [51] proved that unfermented soy milk (USM) and kefir fermented soymilk (FSM) inhibit α-amylases with IC50 values of 72.60 and 52.71 μg/mL, respectively, with the strongest α-amylase inhibition activity recorded at 16 hours of fermentation by kefir. The strong inhibition of α-amylase might be due to increased amounts of isoflavone aglycones, including genistein, daidzein, and glycitein in the soy after fermentation by kefir. It was also observed that feeding high-fat fructose diet (HFFD) to rats with FSM led to a significant decrease in intestinal and pancreatic α-amylase activities by 26% and 31%, respectively. This, in turn, led to reduced hydrolysis of starch and a decrease in blood glucose level by 26%. This shows that FSM is more beneficial than USM in controlling glucose levels due to the increased bioavailability of soy isoflavones after fermentation [51].
Peptides
The benefits of soy peptides in the management of diabetes and insulin resistance have also been described by earlier studies [135]. Germinated SPs have been shown in vitro to control hyperglycemia through the inhibition of α-amylase in the saliva [135]. Bioactive peptides in soybeans are released through the activity of proteolytic enzymes which include proteases and peptidases produced by microorganisms during a fermentation process. These peptides are abundant in fermented soymilk, and when soymilk is fermented by kefir, it inhibits α-amylase. Studies in both animals and humans have revealed that fermented products of soybeans have better antidiabetic properties, unlike unfermented products. In a study of streptozotocin (STZ)-induced diabetic rats fed with fermented soybean, α-amylase was found to be inhibited [135]. Also, González-Montoya et al. [52] saw that six days germinated soybean protein digest (6GSPD) can inhibit salivary α-amylase with a lower IC50 of 1.70 compared to the positive control Acarbose with an IC50 of 0.16. Different peptide fractions were obtained from 6GSPD by ultrafiltration and evaluated. They showed varying levels of α-amylase inhibitory activity. Peptides that show anti-α-amylase activity have been found to contain high-molecular-weight amino acids and aromatic residues like phenylalanine, tryptophan, tyrosine, and arginine [52]. Apart from amino acid composition, peptides’ structures may modulate the inhibition of α-amylase through steric hindrance obstructing the active site of the enzyme; charge interactions affecting the binding affinity; secondary structures influencing the overall shape and flexibility of the peptide; hydrophobic interactions influencing the stability of the enzyme–peptide complex; and inducing conformational changes after binding with the enzyme [136].
Polysaccharides
Water-soluble soybean polysaccharide (SSPS) that has been derived from the leftover residue of soy processing is an acidic polysaccharide made up of arabinan and galactan as side chains and the primary backbone of galacturonan and rhamnogalacturonan. SSPS has a relatively low viscosity and great stability in an aqueous solution, and is primarily composed of the dietary fiber found in soybean cotyledons [137, 138]. Lin et al. [53] proved that SSPS has an α-amylase-inhibiting activity which is significantly increased by fermentation and microwave treatment. When compared to unfermented SSPS, the inhibitory activity of α-amylase in fermented SSPS and microwaved SSPS was 2.69 and 2.41 times greater at 11.69% and 10.47%, respectively. Also, insoluble dietary fibers from soybean hull demonstrated an α-amylase inhibition activity and a significant effect on the fecal microbiota increasing the abundance of Lactobacillales and Bifidobacteriales. In this regard, the citric acid extract of the insoluble dietary fiber from soy hull performed better than the oxalic acid extract [54].
The intricate interplay of molecular forces and structural properties characterizes the physical and chemical interactions between α-amylase and soy polysaccharides. By occupying the active site of α-amylase, soy polysaccharides could interact with it through non-specific binding and competitive inhibition [139]. Soy polysaccharides can also alter α-amylase’s kinetic properties, such as substrate binding affinity and catalytic rate, which can impact how the enzyme interacts with polysaccharides. This modification could lead to variations in the enzyme’s starch hydrolysis effectiveness [140]. α-Amylase can also undergo structural modifications because of binding with soy polysaccharides. These modifications could have an impact on the geometry or general structure of the enzyme’s active site, which would affect how well it interacts with starch molecules [141]. Moreover, there may be a pH-dependent interaction between α-amylase and soy polysaccharides. The charge distribution on the surfaces of the polysaccharides and the enzyme can change due to pH changes, which can affect how the two interact [142].
Lipids
Quan et al. [55] investigated the hypoglycemic effects of soyasaponins (SS) in GK/Jcl T2D rats. Through colorimetric methods, it was discovered that soybean hypocotyl extract fed to the diabetic rats showed low α-amylase inhibitory activity at the concentration of 1 g/L.
The distinctive structure of soyasaponins is derived from a triterpene or steroidal aglycone that is linked to one or more sugar moieties. The potential interactions and inhibitory effects of soyasaponins on α-amylase may be limited in comparison to other compounds found in soybeans [143]. This limitation might result from soyasaponins’ overall hydrophilic nature due to the presence of sugar moieties. This could cause steric hindrance when interacting with the hydrophobic active site of α-amylase. Furthermore, the sugar moieties could increase molecular weight, which may affect binding kinetics [144].
2.4. Alpha-glucosidase inhibition
The mammalian α-glucosidase is synthesized in the mucosal brush border of the small intestine, and its major physiological function is to catalyze the digestion of disaccharides and starch which make up a huge percentage of the human diet [145]. The enzyme selectively catalyzes the endonucleolytic or exonucleolytic hydrolysis of various sugars producing monosaccharides, oligosaccharides, or glycosaminoglycans, which increases postprandial blood glucose [146–148]. This enzyme is considered a crucial clinical target for the treatment of T2D [149].
Small molecule natural products
Evidence has shown the availability of α-glucosidase inhibitors in soybeans. Both the wild soybean (G. soja; WS) and the cultivated soybean (G. max; CS) extracts displayed dose-dependent α-glucosidase inhibitory activities. The α-glucosidase inhibitory activity of the extracts from WS (84.82 0.34%) was higher than that of the extracts from CS (56.06 0.24%). This could be because 42 of the 79 flavonoid compounds that were found to be present in these plants after metabolomic analysis were elevated in WS. The most distinct compounds in WS were protocatechuic aldehyde, garbanzol, 2′-hydroxygenistein, ligustilide, and resveratrol [104].
The most prevalent polyphenol in soy leaves, coumestrol, proves strong α-glucosidase inhibitory activity, with inhibition levels rising as the plant develops [56]. Other polyphenols in soybeans – which include luteolin, glyceollin, daidzein, and genistein – have been shown to exert α-glucosidase inhibitory activity [57]. Daidzein is converted into glyceollin in soybeans during fermentation due to the activity of Aspergillus sojae. Glyceollin has been revealed to be a competitive inhibitor of α-glucosidase, while genistein is noncompetitive – luteolin showed a mixed mode of activity. Glyceollin and luteolin present a synergistic effect on α-glucosidase inhibition [57]. Also, through a molecular docking study, it was discovered that 6-gingerdiol and stigmasterol have a potent inhibitory effect on α-glucosidase activity [57]. Gao et al. [58] reported that the Japanese traditional fermented soybean dish, Okara, has a remarkable α-glucosidase inhibitory capacity due to the activity of a newly isolated strain of Bacillus amyloliquefaciens SY07. 1-Deoxynojirimycin (DNJ) was shown to be one of the bioactive constituents responsible for the anti-α-glucosidase activity of the fermented broth. The good α-glucosidase inhibitory activity of the Okara broth could make soybean a novel potential microbial source of antidiabetic products.
Protein
A soybean protein (ESPro1) with a significant α-glucosidase inhibitory activity was discovered in a recent study when it was extruded at 160°C and 30 rpm. The fraction (ESPro1 F3) with the highest inhibitory activity was isolated using digestion, ultrafiltration, and gel filtration chromatography. The ESPro1 F3 fraction was then used to screen and produce six peptides with α-glucosidase inhibitory activity, with LLRPPK displaying the highest inhibitory activity (46.98 0.63%) [59]. In another study, soybean protein hydrolysate was produced by hydrolyzing soybean protein with trypsin after being pre-treated with ultrasound. Ultrafiltration, ion exchange chromatography, gel filtration chromatography, and reversed-phase HPLC were used to separate the soybean protein hydrolysate. The fasting blood glucose levels of experimental mice were significantly lowered by an ultrafiltration-derived, highly active part with a molecular weight of less than 5 kDa. The reversed-phase HPLC fraction C-III-2a had the highest α-glucosidase-inhibitory activity, and the half-inhibitory concentration (IC50) was 0.049 mg/mL. By using ion trap tandem mass spectrometry, the amino acid sequences of two tripeptides from fraction C-III-2a were determined to be Gly-Ser-Arg and Glu-Ala-Lys [60]. Also, three peptides (LLPLPVLK, SWLRL, and WLRL) isolated from soy protein with alkaline proteinase digestion, purified by size-exclusion and anion-exchange chromatography, and found using tandem MS have been shown to possess high α-glucosidase inhibitory [61].
Lipids
Ha et al. [62] investigated the α-glucosidase inhibitory activity of soyasaponin derivatives. Through simple reversible slow-binding inhibition, two derivatives, soyasaponin Ba and soyasaponin Bb, significantly inhibited the activity with the IC50 of ca. 25 and 32 μM. In a different study, soyasaponins extracted from soybean hypocotyls using ODS column chromatography and HPLC showed noncompetitive inhibition of α-glucosidase with IC50 values ranging from 10 to 40 μmol/L. The findings imply that soyasaponins, which have α-glucosidase-inhibiting properties, may be physiologically helpful in reducing postprandial hyperglycemia in diabetic patients [150].
Polysaccharides
Acidic polysaccharides, known as soluble soybean polysaccharides (SSPS), are isolated from soybean cotyledons and have a high degree of stability and low viscosity in an aqueous solution. SSPS have a structure like pectin. The major backbone of SSPS was shown to be formed of galacturonan and rhamnogalacturonan [63]. Soluble polysaccharides have been proven to inhibit α-glucosidase in several plant species [151–153].
2.5. Aldose reductase inhibition
AR is an enzyme in the polyol pathway involved in glucose metabolism by catalyzing the conversion of glucose into sorbitol (which is after oxidized to fructose). Sorbitol can accumulate in various tissues, leading to oxidative stress and tissue damage. The activation of AR has been implicated in the development of diabetic complications, such as neuropathy, nephropathy, and retinopathy. Inhibiting this enzyme in T2D can delay or prevent the pathogenic process [154]. Even in normoglycemic states, AR plays a critical role in promoting inflammatory and cytotoxic conditions. Its activation under hyperglycemic settings leads to the development of chronic diabetes complications. As a result, it is an established antidiabetic drug target [155].
Small molecule natural products
AR inhibitors are substances that can block the activity of this enzyme, potentially reducing the accumulation of sorbitol and preventing related tissue damage [155]. Some studies have shown that certain compounds present in soybeans, such as isoflavones, may possess AR inhibitory activity. These findings suggest that consuming soybeans or soy-derived products could potentially help regulate blood sugar levels and reduce the risk of diabetic complications [64]. Using NMR, UV, and IV spectroscopy, the flavonoids genistein, kaempherol, naringenin, quercetin, morin, and rutin were identified and extracted from the UFV-5′ cultivar of G. max (soya). At a concentration of 10−4 M, quercitrin and morin showed the greatest inhibitory activity. But at a concentration of 10−5 M, quercetrin, rutin, and naringenin had the strongest effects [64].
Found in Korean fermented soybean pastes (Doen-jang), genistein was shown to have an AR inhibiting activity. With an IC50 value of 20 μM, genistein inhibited the pig lens’ AR [156]. In another study, genistein has been shown to inhibit AR activity in human lens epithelial cells (HLE-B3). HLE-B3 cells cultivated in high-glucose conditions show downregulated expression of TGF-β2, αB-crystallin, and fibronectin mRNAs when exposed to purified genistein derived from Pueraria lobate roots. Furthermore, there was a decrease in thiobarbituratic acid-reactive compounds and glutathione (GSH) levels. These findings prove that genistein protects HLE-B3 cells against lens opacity and prevents high glucose–mediated harmful effects. Since genistein is probably able to inhibit AR and cellular oxidation to achieve these effects, it could be a promising therapeutic agent for the treatment and prevention of problems related to T2D, including diabetic cataracts [65].
Daidzein is another isoflavone found in soybeans that has been shown to have AR inhibitory activity. In experimental animals, it has been proven to ameliorate diabetic retinopathy protecting against retinal damage in hyperglycemic conditions. In this study, it was discovered that AR and sorbitol dehydrogenase levels were markedly lowered (p < 0.001 and p < 0.01, respectively) upon treatment with 100 mg/kg of daidzein. The results of the 28-day daidzein treatment showed a considerable reduction in oxidative stress as well. Also, retinal thickness decreased with daidzein administration, according to histopathological studies [66]. An analog of daidzein, 8-hydroxydaid-zein, has also been proven to be an AR inhibitor. 8-Hydroxydaid-zein was isolated and found from a methanolic extract of okara (soybean pulp) fermented with the fungal strain Aspergillus sp. HK-388 and showed noncompetitive inhibition of human recombinant AR with respect to DL-glyceraldehyde, its Ki value being evaluated as 7.0 μM [67]. Daidzein can be transformed into ortho-dihydroxy isoflavones and glycitein by the bacteria Bacillus subtilis Roh-1. Glycitein, which is also known to be an inhibitor of AR, can be produced from natural-fermented soybean paste Doenjang [68].
2.6. DPP-4 inhibition
The DPP4 is a transmembrane exopeptidase which is involved in the selective degradation of Incretins, and increased levels and activity of this enzyme have been implicated in T2D [157, 158]. Secreted from the intestines, Incretins such as glucagon-like peptide-1 and -2 (GLP-1 and GLP-2) and glucose-dependent insulinotropic peptide (GIP) are metabolic hormones that induce the production of insulin by the pancreatic β cells to reverse elevated glycaemia [159]. The “incretin effect” is caused by GLP-1 and GIP binding to their respective receptors, GLP-1R and GIPR. This initiates an intracellular signaling cascade that results in changes to ion channel function, increased levels of cytosolic calcium that help fuse insulin granules to the plasma membrane, and improved exocytosis of granules that carry insulin. This culminates in an increase in insulin secretion in a glucose-dependent way. Other insulinotropic activities include increased β-cell proliferation and improved resistance to apoptosis [160]. Consequently, targeting DPP4 has become the focal point of many recent studies seeking diverse approaches in the management of T2D and other metabolic disorders [159].
Small molecule natural products
In a recent study, the antidiabetic potential of soybean and ginger (Zingiber officinale) was investigated in silico and from the ethanolic extracts from both plants. The results revealed that stigmasterol from soybeans and 12-shogaol from ginger were strongly potent against DPP4. Comparatively, the soybean extract was found to be a more potent inhibitor of DPP4 than ginger [69].
Hydrolysates and peptides
Other studies have explored the inhibition of DPP4 using natural alternatives such as hydrolysates and bioactive peptides from foods rich in protein. The bioactivity of hydrolysates and biopeptides is dependent on the composition, length, and sequence of amino acids. DPP4 inhibitory peptides in soybean can be released by enzymatic hydrolysis in the gut, germination, and fermentation [52], and can be found using approaches such as liquid chromatography-mass spectrometry (LC-MS) and fractionation driven by bioactivity [161]. To investigate the potential of germinated soybean hydrolysate and biopeptides to inhibit DPP4, pepsin and pancreatin were used to hydrolyze soybean germinate for six days [70]. Results obtained show that the hydrolysate mostly impaired the activity of DPP4 at an inhibitory concentration (IC50) value of 1.49 mg/mL, and peptides ranging from 5 to 10 and >10 kDa had an IC50 value of 0.91 and 1.18 mg/mL, respectively. Their research also showed that peptides with proline found between the first and fourth positions at the N-terminal (VVNPDNNEN, EEPQQPQQ, QEPQESQQ, SQRPQDRHQ, PETMQQQQQQ, and SSPDIYNPQAGSVT) displayed higher inhibitory properties [70].
Based on their evaluation, Rivero-Pino et al. [162] reported that treating soybean with subtilisin, trypsin, and flavorzyme yielded soybean hydrolysate as the highest DPP4 inhibitor at a minimal concentration as compared to chickpea, lentil, and pea with an IC50 value of 2.68 mg/mL. Furthermore, APFL, TPVGM, and EPAAV were identified as biopeptides from soybean which had DPP4 inhibitory activity, with EPAAV as the most potent. This differential attribute of soybean was suggested to be due to the higher proportion of proline and alanine in its amino acid composition [71]. The DPP4 inhibitory activity of Soy1 (IAVPTGVA) peptide was studied in situ using a Caco-2 cell line from the human intestine and ex vivo using human serum from venous blood. Lammi et al. [72] report that Soy1 inhibited the activity of DPP4 both in situ (IC50 value = 223.2 μM) and ex vivo (27.7% and 35.0% at 100.0 and 300.0 μM concentrations) in a dose-dependent manner.
Besides, results from assays conducted by Lammi et al. [163] using pepsin and trypsin showed that both enzymes yielded protein hydrolysates and biopeptides from soybean which inhibited the activity of DPP4 both in vitro and in situ. For the in vitro tests, the human recombinant enzyme (H-Gly-Pro-AMC) was used as substrate, while the in situ assays were performed on human Caco-2 cells. In vitro and at concentrations of 1.0 and 2.5 mg/mL, pepsin reduced the activity of DPP4 by 16.3% and 31.4%, respectively, and trypsin by 15.3% and 11.0%, respectively. Using Caco-2 cells, the activity of DPP4 was also impaired in a dose-dependent trend (pepsin: 15.4%, 19.6 %, and 37.0%; trypsin: 17.0%, 25.2%, and 43.3%; both enzymes at concentrations of 1.0, 2.5, and 5.0 mg/mL, respectively).
2.7. Pancreatic lipase inhibition
Pancreatic lipase (PL) enzyme mainly hydrolyzes triacylglycerols into monoacylglycerols and free fatty acids, its inhibition being therefore considered as a potential treatment for obesity and T2D [77]. A lipase inhibitor may work through at least three different primary ways. The first is by directly attaching to lipase, the second is by acting as a substrate analog that either permanently or reversibly affects the active site, and the third method is by acting on the substrate to reduce the substrate’s availability. [164]. The crude extracts of legumes could inhibit PL, and they can be used to produce nutritional supplements to fight obesity. These beans could also be used to create nutritionally dense meal replacements and substitute foods that could reduce the rate at which lipids are absorbed [165]. In an extensive comparative study carried out to examine the PL inhibitory effects of 13 commonly consumed dietary legumes grown in China, it was discovered that yellow soybean saponin extract demonstrated a 34.1% inhibitory activity, and it could be a target for more research on the anti-obesity and anti-diabetes properties using cell and animal models [166].
Small molecule natural products
Natural compounds such as saponins, alkaloids, polyphenols, and other substances have been found as effective anti-obesity agents [166]. It is well recognized that the presence of isoflavone aglycones, such as daidzein, glycitein, and genistein, is what gives soybeans their health benefits because they are absorbed more quickly by humans than isoflavone glucosides. Furthermore, according to numerous research, the bioconversion of isoflavone aglycones by bacteria in fermented soybean meals such soy milk, soy yogurt, Cheonggukjang, and Doenjang results in larger amounts of these compounds than isoflavone glucosides [167].
The inhibitory activities of unfermented soy-powder milk (UFSPM) and soy-powder milk fermented using L. plantarum P1201 (FSPM) on PL have been set up. PL showed a dose-dependent inhibitory activity in response to UFSPM and FSPM. In comparison to the control group, FSPM, but not UFSPM, inhibited adipogenesis in 3T3-L1 cells and decreased their triglyceride content by 23.1% after treatment with 1,000 μg/mL of FSPM. The anti-obesity effect of FSPM can be attributed to conjugated linoleic acid (CLA) and isoflavone aglycones, which downregulated lipoprotein lipase (LPL), adiponectin, adipocyte fatty acid-binding protein (aP2), fatty acid synthase (FAS), and acetyl CoA carboxylase (ACC) mRNA. Additionally, FSPM increased the anti-obesity activity and pancreatic enzymes’ inhibitory function [168]. These findings suggest that the fermented hydrolysate milks may have helped to increase the value of the soybean [168]. Hwang et al. [169] also revealed that in soy-powder yogurts (SPYs) made using Lactobacillus brevis and L. plantarum, the total average isoflavones decreased while there was an increase in aglycones. Also, the highest antioxidant potential and enzyme-inhibiting activities were seen in SPY employing mixed strains.
In another study, isoflavone-enriched soybean leaves (IESLs) were solid-lactic acid fermented using starters Lactiplantibacillus plantarum P1201 and Levilactobacillus brevis BMK184. This solid-lactic acid fermentation resulted in alterations in the isoflavone levels and the inhibition of digestive enzymes. The amount of isoflavones in total declined throughout fermentation, while the amount of isoflavone aglycones increased. This fermentation process using P1201 + BMK184 made the IESL attain a PL inhibition activity value of 37.3% [170]. Suh et al. [73] used ultra-performance liquid chromatography with quadrupole-time-of-flight mass spectrometry (UPLC + QTFMS) to examine the changes in metabolites during the fermentation of soybeans. The results showed that the soybean’s isoflavone glycosides and soyasaponins were reduced, but their aglycones, like daidzein, glycitein, and genistein, were elevated. These aglycones also proved PL inhibition activities. Specifically, genistein has been shown to lower hyperlipidemia by preventing the production of hepatic lipids. Because genistein prevents PL activity, which promotes increased dietary fat excretion without absorption and reduces adipocyte development, it can be used as an effective treatment for obesity. Large amounts of proteins are discharged through the urine because of glomerulonephritis, which causes hypoalbuminemia and secondary hyperlipidemia that are brought on by the liver producing more lipid and lipoproteins. By preventing the production of hepatic lipids, genistein can lower hyperlipidemia [171].
Proteins
A protein molecule obtained from the crude aqueous extract of soybean meal, called the protein inhibitor of lipase (PIL), has been shown to inhibit the activity of PL on tributyrin or triolein. The activity of PIL, which has a molecular weight of approximately 70kDa, is remarkably similar to those of hydrophobic proteins such as serum albumin and lactoglobulin which inhibit PL in the absence of colipase and bile salt [74]. The ability of PIL to interact with lipids (by penetrating the monomolecular films of different glycerides and phospholipids) and alter the nature of the substrate–water interface is a factor that enhances its ability to inhibit PL [74]. The binding of PIL to substrate micelles stops lipase from acting normally on the interfacial region between the micelle surface and the aqueous medium [172]. PIL, which also inhibits the lipase activity of powdered porcine pancreas, has been proved to be a protein by the fact that it’s rendered inactive by heat and pronase treatment [173, 174].
Another lipase-inhibiting protein in the soybean seed is lipoxygenase-1 (LOX-1). A study revealed that the amount of LOX-1 needed to achieve a 50% reduction in lipase activity was 3.2 × 10−7 M, and it reduced the release of fatty acids from a soybean oil emulsion in a dose-dependent manner. The soybean LOX-1 gene was transfected into Escherichia coli and the recombinant LOX-1 product also inhibited the activity of the lipase [75, 174]. From soybean seeds lacking in lipoxygenase (LOX), another 56.0 kDa lipase-inhibiting protein has been discovered. The estimated masses of the hypothetically expected lysyl endopeptidase–treated peptides of soybean seed protein were nearly identical to the masses of the lysyl endopeptidase–digested peptides of the 56.0 kDa inhibitory protein. The protein identified as an α-amylase prevented PL from hydrolyzing soybean oil though to a lesser extent than by LOX-1 [76].
The pancreatic lipase-inhibiting activity of mature (MSPHs) and young (YSPHs) soybean enzymatic protein hydrolysates was investigated in a recent study. Using LC-MS QTOF and molecular binding studies, PHs with strong anti-lipidemic effects were then chosen for sequencing. It was predicted that the peptides FPFPRPPHQ, QCCAFEM, and FAPEFLK from MSPHs and SFFFPFELPRE, FMYL, PFLL, FPLL, and LPHF from YSPHs would have strong inhibitory effects on PL. The findings suggest that MSPHs and YSPHs beans could be identified as possible components in the treatment of hypercholesterolemia [77]. In another study, in vitro and in silico analyses have also identified PL-inhibiting low-molecular-mass (LMMH) and high-molecular-mass (HMMH) peptides obtained from a protein hydrolysate from soybean protein concentrate produced by papain [175].
Polysaccharides
Dietary fibers, which include a range of gums, mucilages, and algal polysaccharides, are mostly indigestible complex carbohydrates found in plant cell walls (cellulose, hemicellulose, and pectin). Plant cell walls include lignin, a non-carbohydrate part of dietary fiber [176]. Soluble dietary fibers (SDFs) have proven their ability to control blood cholesterol and sugar levels due to their structural and functional properties [177]. Soybean hull galactomannan has also been shown to inhibit PL activity. Following pre-incubation of galactomannan with the enzyme, the impact of galactomannan on PL was investigated at various concentrations (50, 100, 150, and 200 ppm). Data showed that pre-incubating lipase with galactomannan resulted in a concentration-dependent suppression of enzyme activity, with the inhibition percentages ranging between 5.06% and 19.68% for the lowest concentration (50 ppm) and the highest concentration (200 ppm), respectively [78]. The inhibitory activity of galactomannan may result from one or more of the following mechanisms: the direct effect of galactomannan on enzyme, the reduction of enzyme–substrate binding due to a relatively high viscosity of the reaction medium (galactomannan), changing the conformation of the enzyme, interaction between galactomannan and enzyme (as protein), and interaction between galactomannan and enzyme substrate. These findings suggest that galactomannan can be used to inhibit lipase activity [78].
2.8. Angiotensin-converting enzyme inhibition
Angiotensin-converting enzyme (ACE) has been extensively investigated in relation to glucose metabolism and renal injury in diabetes [178]. Studies show that most diabetic complications such as nephropathy and hypertension are of vascular origin with the involvement of the renin–angiotensin–aldosterone system (RAAS). ACE is an important enzyme that plays a crucial role in the RAAS, which is involved in regulating blood pressure, fluid balance, and electrolyte homeostasis in the body. ACE is responsible for converting angiotensin I to angiotensin II, a potent vasoconstrictor that also stimulates the release of aldosterone [179]. ACE inhibitor therapy reduces both microvascular and macrovascular complications in diabetes and appears to improve insulin sensitivity and glucose metabolism [180].
In the context of diabetes, ACE and the RAAS have been implicated in the development and progression of diabetic complications [178]. Some of these include diabetic nephropathy ACE contributes to the pathogenesis of diabetic nephropathy through renal vasoconstriction, inflammation, oxidative stress, and the deposition of extracellular matrix proteins, ultimately causing kidney damage [181] and diabetic retinopathy where ACE promotes retinal vascular permeability and inflammation, contributing to the development of retinopathy [182]. Cardiovascular complication is another condition where ACE is associated with an increased risk of cardiovascular diseases such as hypertension, coronary artery disease, and heart failure [183].
ACE inhibitors are drugs commonly used for the treatment of hypertension and heart failure. They work by inhibiting the activity of ACE, an enzyme involved in the production of angiotensin II, which causes blood vessel constriction and increases blood pressure [183]. ACE inhibitors have also shown potential benefits in managing diabetes by enhancing insulin sensitivity and improving glucose metabolism [184]. By reducing angiotensin II levels, ACE inhibitors may trigger insulin activity and promote glucose uptake in tissues [185].
Small molecule natural products
Soy consumption has been associated with improved endothelial function and reduced markers of inflammation, which may have beneficial effects on diabetes and cardiovascular health [186]. This may be because soybeans contain phenolic-rich extracts that can inhibit ACE linked with T2D and hypertension [13]. A compound found in soybeans, chicoric acid, a polyphenol, has also been implicated in enhancing insulin secretion and increasing glucose uptake, suggesting its potential in diabetes management [79]. When ACE-inhibitory compounds from soybeans were analyzed in silico, beta-sitosterol showed the most potential for inhibiting ACE I when compared to 33 other soy phytomolecules [80]. Nicotianamine, found in soybeans, is a new inhibitor of ACE 2 [81].
Protein and peptides
Glycinin is the major storage protein of soybean. Different enzymatic hydrolysates of glycinin have shown ACE-inhibitory properties. The protease P hydrolysate, having a pentapeptide with the sequence Val-Leu-Ile-Val-Pro, demonstrated the greatest ACE-inhibitory activity (1.69 ± 0.17 μM). This peptide, which is produced from glycinin, has antihypertensive properties that may be useful in the creation of functional foods for medicinal purposes [82]. ACE-inhibitory fractions, namely, fractions 2, 3, and 4, were obtained from the hydrolysis of soybean protein isolate using pepsin and pancreatin. The values of the hydrolysate fraction that inhibited 50% of ACE activity were 1.09, 0.42, and 0.25 mg/mL, respectively, for fractions 2, 3, and 4. As revealed by kinetic studies, fractions 2, 3, and 4 are noncompetitive, competitive, and mixed ACE inhibitors, respectively. The ACE-inhibitory activity is due to the scavenging properties of cationic peptides in the fractions. The strongest antioxidant was fraction 3, whereas fraction 4 hardly showed any scavenging activity. Because fraction 3 has significant ACE-inhibitory and free-radical scavenging qualities, we determined that it had the greatest potential for application as a bioactive component [187]. Another soy storage protein, β-conglycinin, has also been implicated in regulating insulin sensitivity and ACE activity in rats fed with a high-salt diet, potentially slowing the progression of diabetic nephropathy [188].
Two SPs, LPYP and IAVPTGVA (Soy1), have been shown to have hypoglycemic and hypocholesterolemic properties in vitro. Examining the effects of Soy1 and LPYP in vitro, the results show that the intestinal and renal ACE activities were reduced, with IC50 values of 14.7 ± 0.28 and 5.0 ± 0.28 μM (Caco-2 cells) and 6.0 ± 0.35 and 6.8 ± 0.20 μM (HK-2 cells), respectively [189]. ACE-inhibitory peptides have also been found in fermented soybean extract (FSE). After 3 days of aging, FSE exhibited ACE-inhibitory activity with an IC50 value of 1.46 mg/mL. Ultrafiltration and consecutive chromatographic methods revealed that FSE has a novel ACE-inhibitory peptide with the sequence Leu-Val-Gln-Gly-Ser. The peptide had an IC50 value of 22 μg/mL (43.7 μM) which is a 66-fold increase in ACE-inhibitory activity compared to that of FSE [83].
2.9. Phosphoenolpyruvate carboxykinase inhibition
The phosphoenolpyruvate carboxykinase (PEPCK) is an enzyme involved in gluconeogenesis, a metabolic pathway that generates glucose from non-carbohydrate sources such as amino acids, lactate, and glycerol. In T2D, patients produce excess glucose through gluconeogenesis with the help of PEPCK [190].
In HepG2 cells and in mice with alloxan-induced diabetes, the impact of genistein on PEPCK and glucose synthesis has been investigated. The expression of cytosolic PEPCK (PEPCK-C) and the signaling pathways were examined in HepG2 cells after exposure to various concentrations of genistein in the presence or absence of modulators. Genistein boosted AMPK, MEK½, ERK½, and CRTC2 phosphorylation states while decreasing PEPCK-C expression and glucose production in HepG2 cells [84]. Genistein-induced MEK½ and ERK½ activities were increased after treatment with the AMPK inhibitor (compound C), suggesting that the two signaling pathways may interact. In mice with alloxan-induced diabetes, genistein treatment also decreased fasting glucose levels, along with decreased PEPCK-C expression and enhanced AMPK and ERK12 phosphorylation states in the liver. By lowering glucose production and inhibiting PEPCK-C expression in HepG2 cells as well as in mice that have been given alloxan to cause diabetes, genistein satisfies the requirements for an effective antidiabetic drug. These findings suggest that genistein is a strong candidate for preventing T2DM by altering the AMPK-CRTC2 and MEK/ERK signaling pathways, which may open the door to a novel strategy for changing dysfunction in hepatic gluconeogenesis in T2DM [191].
Small molecule natural products
It has been discovered that the soy isoflavone daidzein has a remarkable therapeutic potential for treating the pathophysiology of T2D. The main source of daidzein is fermented soybean, but it can also be made by consuming the glycosylated moiety of daidzein and then having an intestine bacterial enzyme hydrolyze it. Daidzein has been shown in numerous studies to have a preventative effect on the reduction of hyperglycemia, insulin resistance, dyslipidemia, obesity, inflammation, and other T2D complications [42].
In a study by Choi et al. [191], genistein and daidzein supplements enhanced the insulin/glucagon ratio and C-peptide level in non-obese diabetic (NOD) mice while keeping the insulin-staining cell of the pancreas. When compared to the control group, genistein and daidzein supplements decreased the activities of the lipogenic enzymes glucose-6-phosphatase (G6Pase) and PEPCK, and increased the activities of malic enzyme and glucose-6-phosphate dehydrogenase (G6PD). Significantly, genistein and daidzein supplementation decreased the activities of carnitine palmitoyltransferase (CPT) and fatty acid oxidation in these animals. In comparison to the control group, genistein and daidzein enhanced plasma triglyceride and free fatty acid (FFA) concentrations. These findings imply that genistein and daidzein regulate glucose homeostasis by decreasing G6Pase, PEPCK, fatty acid oxidation, and CPT activities, while increasing malic enzyme and G6PD activities in the liver and keeping pancreatic beta-cells [191].
Another strategic target for T2D control is the peroxisome proliferator-activated receptors (PPAR) which regulate insulin sensitivity and blood glucose homeostasis. Isoflavones such as genistein, daidzein, formononetin, and biochanin A bind and activate PPARγ and PPARδ. This isoflavone mechanism of action is of special importance in the context of diabetes [85].
3. Conclusion
Empirical data reveal that the consumption of soybeans reduced the risk of T2D by 17% [192]. Small molecules, polysaccharides, lipids, and peptides derived from soybeans have demonstrated modulatory properties on key enzymes that affect T2D pathogenesis. Interestingly, isoflavones such as genistein and daidzein, and glycitein affect multiple targets. Overall, soybeans decrease in fasting blood glucose levels, improve insulin sensitivity, control lipid profile, and offer promising health benefits, particularly in relation to T2D. While soybeans have been shown to have good impacts on diabetes management, individual differences may exist in responses. However, more research is needed to determine specific dose–response effects and any interactions with other drugs or diets.
Abbreviations
ABTS:
2,2’-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)
ACC:
Acetyl CoA carboxylase
ACE:
Angiotensin-converting enzyme
AGE:
Advanced glycation end products
Akt:
RAC (Rho family)-alpha serine/threonine-protein kinase
AMPK:
Adenosine Monophosphate-Activated Protein Kinase
aP2:
Adiponectin, adipocyte fatty acid-binding protein
ARE:
Antioxidant responsive element
BBI:
Bowman-Birk Inhibitor
BDNF:
Brain-derived neurotrophic factor
BSSCE:
Black soybean seed coat extract.
C/EBP:
CCAAT-enhancer-binding proteins
CCL2:
Chemokine (C-C motif) ligand 2
CLA:
Conjugated linoleic acid
CPT:
Carnitine palmitoyltransferase
CRTC2:
CREB-regulated transcription coactivator 2
CXCL2:
C-X-C motif chemokine ligand 2
Cy3G:
Cyanidin-3-O-glucoside
DNA:
Deoxyribonucleic acid
DPPH:
2,2-Diphenyl-1-picrylhydrazyl
ERK:
Extracellular signal-regulated kinase
FABP:
Fatty acid-binding protein
FAS:
Fatty acid synthase
FFA:
Free fatty acid
FRAP:
Ferric-reducing ability of plasma
FSE:
Fermented soybean extract.
FSM:
Fermented soymilk
G6Pase:
Glucose-6-phosphatase
G6PD:
Glucose-6-phosphate dehydrogenase
GFAT:
Glutamine fructose-6-phosphate amidotransferase
GIP:
Glucose-dependent insulinotropic peptide
GLP:
Glucagon-like peptide
GPX:
Glutathione peroxidase
GSH:
Glutathione
GST:
Glutathione S-transferase
HBP:
Hexosamine biosynthesis pathway
HFFD:
High-fat fructose diet
HO-1:
Heme oxygenase-1
HORAC:
Hydrophilic oxygen radical absorbance capacity
HPLC:
High-performance liquid chromatography
IL:
Interleukin
IRS:
Insulin receptor substrate
JNK:
c-Jun N-terminal kinases
Keap 1:
Kelch-like ECH-associated protein 1
LC-MS QTOF:
Liquid chromatograph-mass spectrometry. quadrupole time-of-flight
LDL:
Low-density lipoprotein
LOX-1:
Lipoxygenase-1
LPL:
Lipoprotein lipase
MAPK:
Mitogen-activated protein kinases
MCI:
Mild cognitive impairment
MDA:
Malondialdehyde
MEK:
Mitogen-activated protein kinase kinase
mRNA:
Messenger RNA
NAD+:
Nicotinamide adenine dinucleotide
NADH:
Nicotinamide adenine dinucleotide hydrogen
NADPH:
Nicotinamide adenine dinucleotide phosphate
NIDDM:
Non-insulin-dependent diabetes mellitus
NOD:
Non-obese Diabetic
NQO1:
NAD(P)H quinone oxidoreductase 1
Nrf2:
Nuclear factor erythroid 2-related factor 2
O-3 PUFA:
Omega-3 polyunsaturated fatty acids
p-AMPK:
Phosphorylated adenosine monophosphate-activated protein kinase
PC:
Phosphatidylcholine
PEPCK:
Phosphoenolpyruvate carboxykinase
PGC-1:
Peroxisome proliferator-activated receptor gamma coactivator
PI3K:
Phosphoinositide 3-kinase
PIL:
Protein Inhibitor of Lipase
PKC:
Protein kinase C
PL:
Pancreatic lipase
PPAR:
Peroxisome proliferator-activated receptors
RAAS:
Renin–angiotensin–aldosterone system
RAGE:
Receptor for advanced glycation end products
RNS:
Reactive nitrogen species
ROS:
Reactive oxygen species
SDF:
Soluble dietary fibers
SDH:
Sorbitol dehydrogenase
SIRT1;
Sirtuin 1
SOD:
Superoxide dismutase
SSPS:
Soybean soluble polysaccharides
STZ:
Streptozocin
T2D:
Type 2 diabetes
T2DM:
Type 2 diabetes mellitus
TGF-β2:
Transforming growth factor beta-2
TNF-α:
Tumor necrosis factor-alpha
UCP-3:
Mitochondrial uncoupling protein 3
UPLC + QTFMS:
Ultra-performance liquid chromatography with quadrupole-time-of-flight mass spectrometry
USM:
Unfermented soy milk
