© 2008 Nature Publishing Group http://www.nature.com/natureneuroscience
B R I E F C O M M U N I C AT I O N S
The evolution of the arcuate
fasciculus revealed with
comparative DTI
James K Rilling1–4, Matthew F Glasser1, Todd M Preuss3,5,6,
Xiangyang Ma7, Tiejun Zhao7, Xiaoping Hu7 &
Timothy E J Behrens8,9
The arcuate fasciculus is a white-matter fiber tract that is
involved in human language. Here we compared cortical
connectivity in humans, chimpanzees and macaques
(Macaca mulatta) and found a prominent temporal lobe
projection of the human arcuate fasciculus that is much
smaller or absent in nonhuman primates. This human
specialization may be relevant to the evolution of language.
nonhuman species. Here, we use DTI to compare the organization of
the arcuate fasciculus in humans, chimpanzees and macaques.
We acquired DTI brain scans from ten live human subjects, three
postmortem chimpanzee brains and two postmortem macaque brains
(see Supplementary Table 1 and Supplementary Methods online). All
protocols were approved by the Emory University Institutional Animal
Care and Use Committee and Institutional Review Board, and written
informed consent was obtained from all human subjects. Postmortem
scanning makes it possible to scan longer and achieve higher spatial
resolution in the smaller nonhuman brains than would be possible with
in vivo scanning. In vivo scans obtained from one chimpanzee and one
macaque yielded results for the arcuate fasciculus that were very similar
to those obtained in the postmortem scans, confirming that any
interspecies differences were not a result of brain fixation (Supplementary Fig. 1 and Supplementary Methods online). Moreover, when
we compared the organization of the corticospinal tract and cingulum
bundle, two pathways for which we have no a priori grounds to expect
strong species differences, we found them to be very similar in the
in vivo human and postmortem nonhuman primate scans (Supplementary Fig. 2 and Supplementary Methods online).
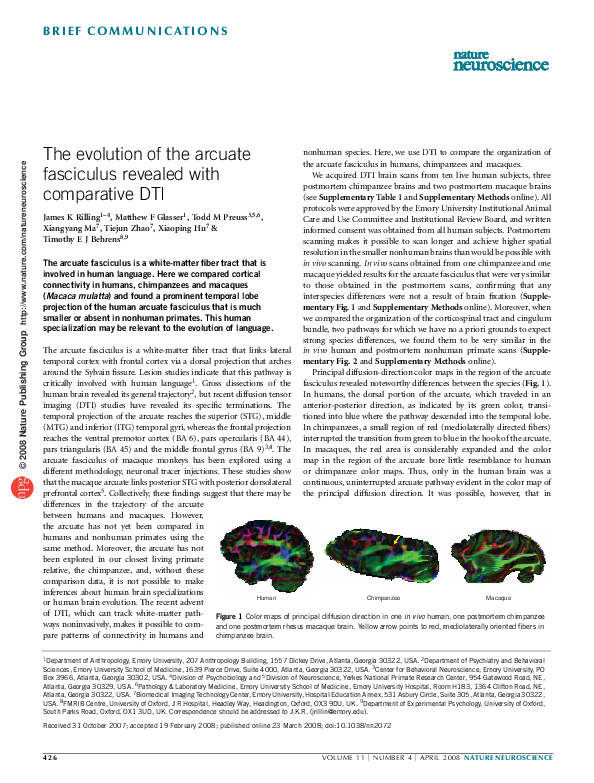
Principal diffusion-direction color maps in the region of the arcuate
fasciculus revealed noteworthy differences between the species (Fig. 1).
In humans, the dorsal portion of the arcuate, which traveled in an
anterior-posterior direction, as indicated by its green color, transitioned into blue where the pathway descended into the temporal lobe.
In chimpanzees, a small region of red (mediolaterally directed fibers)
interrupted the transition from green to blue in the hook of the arcuate.
In macaques, the red area is considerably expanded and the color
map in the region of the arcuate bore little resemblance to human
or chimpanzee color maps. Thus, only in the human brain was a
continuous, uninterrupted arcuate pathway evident in the color map of
the principal diffusion direction. It was possible, however, that in
The arcuate fasciculus is a white-matter fiber tract that links lateral
temporal cortex with frontal cortex via a dorsal projection that arches
around the Sylvain fissure. Lesion studies indicate that this pathway is
critically involved with human language1. Gross dissections of the
human brain revealed its general trajectory2, but recent diffusion tensor
imaging (DTI) studies have revealed its specific terminations. The
temporal projection of the arcuate reaches the superior (STG), middle
(MTG) and inferior (ITG) temporal gyri, whereas the frontal projection
reaches the ventral premotor cortex (BA 6), pars opercularis (BA 44),
pars triangularis (BA 45) and the middle frontal gyrus (BA 9)3,4. The
arcuate fasciculus of macaque monkeys has been explored using a
different methodology, neuronal tracer injections. These studies show
that the macaque arcuate links posterior STG with posterior dorsolateral
prefrontal cortex5. Collectively, these findings suggest that there may be
differences in the trajectory of the arcuate
between humans and macaques. However,
the arcuate has not yet been compared in
humans and nonhuman primates using the
same method. Moreover, the arcuate has not
been explored in our closest living primate
relative, the chimpanzee, and, without these
comparison data, it is not possible to make
inferences about human brain specializations
Human
Chimpanzee
Macaque
or human brain evolution. The recent advent
of DTI, which can track white-matter path- Figure 1 Color maps of principal diffusion direction in one in vivo human, one postmortem chimpanzee
ways noninvasively, makes it possible to com- and one postmortem rhesus macaque brain. Yellow arrow points to red, mediolaterally oriented fibers in
pare patterns of connectivity in humans and chimpanzee brain.
1Department of Anthropology, Emory University, 207 Anthropology Building, 1557 Dickey Drive, Atlanta, Georgia 30322, USA. 2Department of Psychiatry and Behavioral
Sciences, Emory University School of Medicine, 1639 Pierce Drive, Suite 4000, Atlanta, Georgia 30322, USA. 3Center for Behavioral Neuroscience, Emory University, PO
Box 3966, Atlanta, Georgia 30302, USA. 4Division of Psychobiology and 5Division of Neuroscience, Yerkes National Primate Research Center, 954 Gatewood Road, NE,
Atlanta, Georgia 30329, USA. 6Pathology & Laboratory Medicine, Emory University School of Medicine, Emory University Hospital, Room H183, 1364 Clifton Road, NE,
Atlanta, Georgia 30322, USA. 7Biomedical Imaging Technology Center, Emory University, Hospital Education Annex, 531 Asbury Circle, Suite 305, Atlanta, Georgia 30322,
USA. 8FMRIB Centre, University of Oxford, J R Hospital, Headley Way, Headington, Oxford, OX3 9DU, UK. 9Department of Experimental Psychology, University of Oxford,
South Parks Road, Oxford, OX1 3UD, UK. Correspondence should be addressed to J.K.R. (jrillin@emory.edu).
Received 31 October 2007; accepted 19 February 2008; published online 23 March 2008; doi:10.1038/nn2072
426
VOLUME 11
[
NUMBER 4
[
APRIL 2008 NATURE NEUROSCIENCE
�B R I E F C O M M U N I C AT I O N S
Figure 2 Three-dimensional tractography results.
(a) Average tractography results for humans,
chimpanzees and macaques, showing left and
right hemisphere results. (b) Schematic summary
of results shown in a. Center of gravity of human
MTG projections at x ¼ ± 48 are at Montreal
Neurological Institute coordinates, x ¼ –48,
y ¼ –42, z ¼ –3 and x ¼ 48, y ¼ –36, z ¼ –7.
abSF, ascending branch of the Sylvian fissure;
AOS, anterior occipital sulcus; AS, arcuate sulcus;
CS, central sulcus; FOS, fronto-orbital sulcus;
hbSF, horizontal branch of the Sylvian fissure;
IFS, inferior frontal sulcus; IPS, intraparietal
sulcus; ITS, inferior temporal sulcus; LCaS, lateral
calcarine sulcus; LuS, lunate sulcus; PoCS,
postcentral sulcus; PrCS, precentral sulcus; PS,
principal sulcus; SF, Sylvian fissure; SFS, superior
frontal sulcus; STS, superior temporal sulcus.
© 2008 Nature Publishing Group http://www.nature.com/natureneuroscience
a
Human
Chimpanzee
Macaque
b
CS IPS
46
10
IFS
9
CS
39
8 PrCS
IFS 46
44
45
40
PrCS
22
45 44
6
47
37
STS
6
47
IPS
PS
39
40
22
37
45
STS
21
Chimpanzee
Human
chimpanzees, at least, the arcuate actually did pass into the temporal
lobe, but that standard tractography algorithms, which consider only the
principal diffusion direction, cannot follow it through a region where it
intermingles with a different, mediolaterally oriented pathway. For this
reason, we used a newly developed algorithm designed to track through
crossing fibers by also considering the secondary diffusion direction6.
We used this technique to track the arcuate fasciculus, along with
two additional pathways that convey fibers between frontal and
parietal-temporal cortex, the superior longitudinal fasciculus and the
extreme capsule. These pathways can be clearly identified in a coronal
section through the color map at the level of the precentral sulcus
(Supplementary Fig. 3 online). In all three species, we tracked between
a coronal region of interest (ROI) that encompassed these three
pathways and an ROI in the white matter underlying the STG, MTG
and ITG, as well as the inferior parietal lobule (Supplementary Fig. 3
and Supplementary Methods). Note that the surface anatomy of the
macaque temporal lobe differs from those of humans and chimpanzees:
the less-convoluted macaques lack an inferior temporal sulcus, so that
there is no distinction between MTG and ITG.
Tracking results in macaques revealed that the pathway of
highest probability ran in the vicinity of the extreme capsule deep to
the insula, with weaker pathways running both dorsal and lateral to the
insula. Posteriorly, cortical terminations were observed in posterior
STG (area 22) and anterior inferior parietal cortex (area 7b). Anteriorly,
terminations were found in the frontal operculum, insular cortex and
NATURE NEUROSCIENCE VOLUME 11
[
NUMBER 4
[
APRIL 2008
the inferolateral margin of the frontal lobe
(area 6), including the extreme ventral aspects
of areas 44 and 45 in the arcuate sulcus, with
the strongest terminations being in area 45
(Fig. 2a,b and Supplementary Fig. 1). These
results are consistent with prior tracer5 and
DTI7 studies in macaques (Fig. 3a).
Tracking in chimpanzees revealed that,
unlike macaques, the pathway running dorsal
to the insula was stronger than that of the
extreme capsule (Fig. 3a). Cortical terminaAS CS
IPS
7a
tions were more widespread than in maca7b
22
6
ques, involving prominently the inferior
44
parietal lobule, including both the supramarSTS
ginal gyrus (area 40) and the angular gyrus
(area 39), as well as dorsolateral prefrontal and
Macaque
dorsal premotor cortex (Fig. 2a,b and Supplementary Fig. 1). In chimpanzees, this
dorsal pathway was dominated by connections with the inferior parietal
lobe (supramarginal gyrus and angular gyrus).
Tracking in humans, as in chimpanzees, revealed that the dorsal
pathway was dominant to the extreme capsule pathway (Fig. 3a). The
cortical terminations of humans also differed from chimpanzees and
macaques, with humans having much stronger terminations posteriorly, in the MTG and ITG, as well as anteriorly, in pars opercularis
(BA 44), pars triangularis (BA 45), pars orbitalis (BA 47) and
surrounding regions (Fig. 2a,b and Supplementary Fig. 1). Terminations in the MTG and ITG were found in 10 of 10 human brains, 1 of 4
chimpanzee brains and 0 of 3 macaque brains (w2 ¼ 56.5, degrees of
freedom ¼ 2, P o 0.0001), and human terminations were more
extensive than those of the lone chimpanzee subject who also had
them (Supplementary Fig. 1). Connectivity with the MTG was more
widespread and of higher probability in the left than in the right
hemisphere, consistent with functional imaging evidence that lexicalsemantic (word-meaning) processing is lateralized to left MTG and
angular gyrus8, and with studies reporting leftward asymmetries in the
human arcuate fasciculus3,4. In humans, tracts extending into the
temporal lobe via the arcuate fasciculus made a much greater contribution to the dorsal pathway (Fig. 3b) than they did in chimpanzees.
Substantial evidence indicates that the MTG is involved in lexicalsemantic processing8 and that pars triangularis and pars orbitalis are
involved in syntactic processes of sentence comprehension9. To explore
whether these two regions were specifically connected with one
427
�B R I E F C O M M U N I C AT I O N S
a
b
SLFII, SLFIII
and arcuate
Extreme capsule
STS
Extreme
capsule
Human
Arcuate to
MTG
Extreme capsule
© 2008 Nature Publishing Group http://www.nature.com/natureneuroscience
SLFII, SLFIII
and arcuate
STS
Extreme
capsule
Arcuate to MTG
Chimpanzee
displacement of extrastriate visual areas in humans compared with
macaques10,11 and is consistent with evidence that both frontal-12,13
and temporal-lobe white-matter volume increased disproportionately
in human evolution10. Our results also suggest that the evolution of
language entailed modifications of cortical areas and pathways that
mediate specific linguistic functions and was not an incidental byproduct of selection for general brain-size enlargement14. This does not
preclude the possibility that the modified pathways mediate functions
in addition to language, such as tool use15, although the correspondence between the structures modified in human evolution identified
in this study and structures known to be involved in language function
is notable.
SLFII and arcuate
SLFIII
Extreme capsule
STS
Extreme capsule
Macaque
Figure 3 Two-dimensional tractography results. (a,b) Coronal (a) and axial (b)
sections from an individual human, chimpanzee and macaque, illustrating
the relative strength of the dorsal and ventral pathways. SLFII and SLFIII,
superior longitudinal fasciculus II and III.
another, we quantified the probability of connectivity between the
region of the MTG cortex where terminations were found and each of
two anatomically defined ROIs: one spanning pars opercularis (BA 44)
and the other including both pars triangularis (BA 45) and pars
orbitalis (BA 47). In both hemispheres, MTG had a higher probability
of connectivity with pars triangularis and pars orbitalis than with pars
opercularis (Supplementary Fig. 4 online). This raises the possibility
that the expanded pathway in humans supports the transmission of
word-meaning information stored in the MTG and angular gyrus to
pars triangularis and orbitalis for both sentence comprehension and
sentence construction during spontaneous speech.
In conclusion, our results indicate that the organization and cortical
terminations of the arcuate fasciculus were strongly modified in human
evolution. Notably, in humans, but not chimps or macaques, frontal
cortex of the left hemisphere was strongly connected via the arcuate
fasciculus with the left MTG and ITG, ventral and anterior to the cortex
usually included in Wernicke’s area (Fig. 2b). In macaques, this region
consists mainly of extrastriate visual cortex, whereas in humans it
represents word meaning10. We suggest that the MTG and ITG cortex
enlarged disproportionately in the human lineage, following the
divergence of the human and chimpanzee lineages, possibly with the
addition of new cortical fields, and that in humans, new connections
were established between this region and Broca’s area, linking regions
that are involved in lexical-semantic and syntactic processing in
modern humans. This would account for the apparent posterior
428
Note: Supplementary information is available on the Nature Neuroscience website.
ACKNOWLEDGMENTS
We thank Q. Shen and F. Zhao for technical assistance, and M.F.S. Rushworth
and M.J. Konner for many helpful comments. We also thank P. Croxson for
collecting the human scans and M.-M. Carrasco for assistance with tracking
control pathways. This work was supported by the Center for Behavioral
Neuroscience Science and Technology Center Program of the National Science
Foundation under agreement no. IBN-9876754, Emory University Research
Committee, James S. McDonnell Foundation grant 21002093 to T.M.P.,
RO1EB002009 to X.H., the Yerkes Base Grant (NIH RR-00165) and the UK
Medical Research Council to T.E.J.B.
AUTHOR CONTRIBUTIONS
J.K.R. designed the study, acquired the nonhuman data, supervised analyses and
wrote the paper. M.F.G. analyzed the data. T.M.P. acquired the nonhuman brains,
assisted with data analysis and presentation, and wrote the paper. X.M., T.Z. and
X.H. assisted with nonhuman primate protocol development, and T.E.J.B. and
Oxford colleagues acquired the human data. T.E.J.B. oversaw the data-analysis
strategy with the exception of the in vivo chimpanzee and macaque data
presented in the supplementary information.
Published online at http://www.nature.com/natureneuroscience
Reprints and permissions information is available online at http://npg.nature.com/
reprintsandpermissions
1. Geschwind, N. Science 170, 940–944 (1970).
2. Dejerine, J. Anatomie des Centres Nerveux (Rueff et Cie, Paris, 1895).
3. Glasser, M.F. & Rilling, J.K. Cereb. Cortex published online, doi:10.1093/cercor/
bhn011 (14 February 2008).
4. Powell, H.W. et al. Neuroimage 32, 388–399 (2006).
5. Petrides, M. & Pandya, D.N. in Principles of Frontal Lobe Function (eds. Stuss, D.T.
& Knight, R.T.) 31–50 (Oxford University Press, New York, 2002).
6. Behrens, T.E., Berg, H.J., Jbabdi, S., Rushworth, M.F. & Woolrich, M.W. Neuroimage 34,
144–155 (2007).
7. Croxson, P.L. et al. J. Neurosci. 25, 8854–8866 (2005).
8. Price, C.J. J. Anat. 197, 335–359 (2000).
9. Sakai, K.L. Science 310, 815–819 (2005).
10. Rilling, J.K. & Seligman, R.A. J. Hum. Evol. 42, 505–533 (2002).
11. Preuss, T.M. in The Cognitive Neurosciences 3rd edn. (ed. Gazzaniga, M.S.) 5–22 (MIT
Press, Cambridge, Massachusetts, 2004).
12. Deacon, T. The Symbolic Species (W.W. Norton, New York, 1997).
13. Schoenemann, P.T., Sheehan, M.J. & Glotzer, L.D. Nat. Neurosci. 8, 242–252 (2005).
14. Gould, S.J. J. Soc. Issues 47, 43–65 (1991).
15. Johnson-Frey, S.H., Newman-Norlund, R. & Grafton, S.T. Cereb. Cortex 15, 681–695
(2005).
VOLUME 11
[
NUMBER 4
[
APRIL 2008 NATURE NEUROSCIENCE
�
 Todd M Preuss
Todd M Preuss