Molecular docking for identification of novel potential COX inhibitors
of some isolated compounds from Clausena lansium for analgesic
treatment
Mohammad Shah Hafez Kabir1,4, Arkajyoti Paul2,4*, Md. Mominur Rahman1,4, Mir Muhammad Nasir
Uddin3, Abul Hasanat1,4, Mohammed Shoibe1,4, Mohuya Majumder2,4, Md. Golamur Rahman2, Joy
Chakraborty2,4
1 Department
of Pharmacy, International Islamic University Chittagong, Chittagong, Bangladesh, 2Department of Pharmacy, BGC Trust University
Bangladesh, Chittagong, Bangladesh, 3Department of Pharmacy, University of Chittagong, Chittagong-4331, Bangladesh, 4 GUSTO Research
Group , Chittagong, Bangladesh
Abstract
RESULTS
Developing a new agent in the analgesic field, plants secondary
metabolites can be a good source for the Non-Steroidal Antiinflammatory Drugs (NSAID) drug development. For this purpose we
subjected the active compounds Clausena lansium of to reveal its
potentiality by molecular docking analysis to find out its potent
compound against COX-1 and COX-2 which was done by Maestro v
10.1 (Schrodinger) docking analysis. Docking studies by Maestro v
10.1 (Schrodinger) showed that Murrayanine and Clausenaline E of
Clausena lansium had the lowest docking score respectively against
the COX-1 and COX2 which are -6.471 and -8.325 . Murrayanine and
Clausenaline E from Clausena lansium detected with significant
docking score which may be a potent analgesic compound because the
less docking score, the compound will be more potent.
Table 1: Docking score of different compounds with the receptors.
Compounds
claulamines E
clausemarin B
Clausenaline C
Clausenaline E
Murrayanine
vanillic acid
Xanthotoxol
Docking Score with
COX 1
-5.252
-5.958
-5.477
-6.412
-6.471
-4.702
-6.336
Docking Score with
COX 2
-6.93
-6.479
-8.325
-7.828
-6.592
-7.458
MATERIALS AND METHODS
Protein Preparation
Three dimensional crystal structure of COX 1 (PDB id:2OYE) and
COX 2 (PDB id:6COX) was downloaded in pdb format from the
protein data bank. After that, structure was prepared and refined using
the Protein Preparation Wizard of Schrödinger-Maestro v10.1.
Charges and bond orders were assigned, hydrogens were added to the
heavy atoms, selenomethionines were converted to methionines and
all waters were deleted. Using force field OPLS_2005, minimization
was carried out setting maximum heavy atom RMSD (root-meansquare-deviation) to 0.30 Å.
Ligand Preparation
Compounds were retrieved from Pubchem databases, i.e. claulamines
E, clausemarin B, Clausenaline C, Clausenaline E, Murrayanine,
vanillic acid and Xanthotoxol.
Glide Standard Precision (SP) ligand docking
SP flexible ligand docking was carried out in Glide of SchrödingerMaestro v10.1within which penalties were applied to non-cis/trans
amidebonds. Van der Waals scaling factor and partial charge cutoff
was selected to be 0.80 and 0.15, respectively for ligand atoms. Final
scoring was performed on energy-minimized poses and displayed as
Glide score. The best docked pose with lowest Glide score value was
recorded for each ligand.
Ligand based ADME/Toxicity prediction
This analysis is done by following server,
•http://www.scfbioiitd.res.in/software/drugdesign/lipinski.jsp#anchort
ag
•https://ilab.acdlabs.com/iLab2/index.php
•http://www.molinspiration.com/cgi-bin/properties
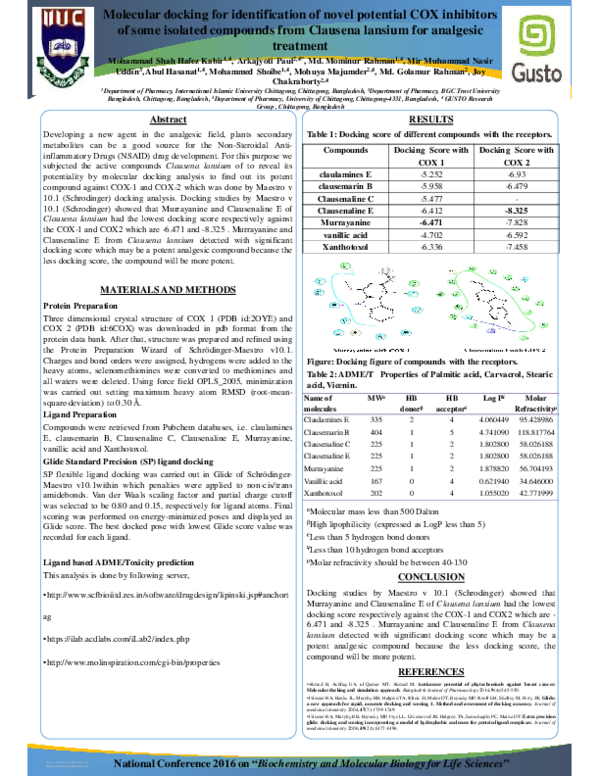
Figure: Docking figure of compounds with the receptors.
Table 2: ADME/T Properties of Palmitic acid, Carvacrol, Stearic
acid, Vicenin.
Name of
molecules
Claulamines E
MWα
Clausemarin B
335
HB
donorβ
2
HB
acceptor€
4
404
1
5
Log P¥
Molar
Refractivityµ
4.060449 95.428986
4.741090
118.817764
Clausenaline C
225
1
2
1.802800
58.026188
Clausenaline E
225
1
2
1.802800
58.026188
Murrayanine
225
1
2
1.878820
56.704193
Vanillic acid
167
0
4
0.621940
34.646000
Xanthotoxol
202
0
4
1.055020
42.771999
αMolecular
mass less than 500 Dalton
lipophilicity (expressed as LogP less than 5)
€Less than 5 hydrogen bond donors
¥Less than 10 hydrogen bond acceptors
µ Molar refractivity should be between 40-130
βHigh
CONCLUSION
Docking studies by Maestro v 10.1 (Schrodinger) showed that
Murrayanine and Clausenaline E of Clausena lansium had the lowest
docking score respectively against the COX-1 and COX2 which are 6.471 and -8.325 . Murrayanine and Clausenaline E from Clausena
lansium detected with significant docking score which may be a
potent analgesic compound because the less docking score, the
compound will be more potent.
REFERENCES
•Ahmed B, Ashfaq UA, ul Qamar MT, Ahmad M: Anticancer potential of phytochemicals against breast cancer:
Molecular docking and simulation approach. Bangladesh Journal of Pharmacology 2014, 9(4):545-550.
•Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK: Glide:
a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. Journal of
medicinal chemistry 2004, 47(7):1739-1749.
•Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT: Extra precision
glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. Journal of
medicinal chemistry 2006, 49(21):6177-6196.
RESEARCH POSTER PRESENTATION DESIGN © 2015
www.PosterPresentations.com
National Conference 2016 on “Biochemistry and Molecular Biology for Life Sciences”
�
 Arkajyoti Paul
Arkajyoti Paul