Article topic: Multiple Sclerosis
Name of the author: Najla O. Khalil
Keywords: Multiple sclerosis, MS, RRMS, SPMS, McDonald criteria, Demyelinating disorders.
Overview and Epidemiology
Multiple sclerosis (MS) is a chronic disabling inflammatory disease that leads to demyelination and neurodegeneration in the central nervous system (1). MS causes different signs and symptoms depending on the amount and type of nerves involved. Some people may experience symptom-free periods between attacks whilst others might lose their ability to walk altogether, as it can reach sensory, motor, visual, and autonomic systems (2). The course of multiple sclerosis is quite unpredictable, as physicians can never accurately predict who will be spared from the devastating and disabling outcomes of the disease or who will have relatively benign symptoms. The true cause of this altered connection between the brain and the rest of the body is still not fully understood, but it has been hypothesized that environmental factors and genetic susceptibility could be related to the pathogenesis of multiple sclerosis (3). MS is currently incurable but the ongoing search for a cure will always be underway.
MS mostly manifests in young adults; affecting 2.3 million people worldwide (as of 2013), with a female: male ratio of 2:1. It is most common in North America (140 cases per 100,000) followed by Europe (108 cases per 100,000), East Asia (2.2 cases per 100,000), and finally, sub-Saharan Africa (2.1 cases per 100,000). It is also the leading cause of non-traumatic disability in young adults (4). This high prevalence seen in North America and Europe could be related to the higher income, thus, the availability of neurologists and the ease of accessing imaging modalities leads to an easier and more accurate diagnosis.
Classification and Diagnostic Criteria
As of 2021, the 2017 McDonald criteria is being used for diagnosing multiple sclerosis with high accuracy. It is described in more detail below.
The 2017 revisions to the McDonald Criteria:
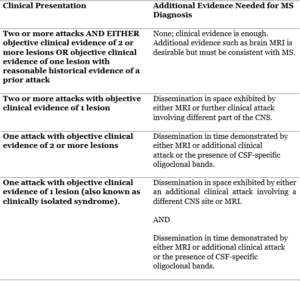
There is no specific test to diagnose MS so a complete neurological exam and medical history must be obtained from the patient, as it mainly relies on ruling out other diseases. The more unusual the symptoms are the more difficult it is to accurately diagnose the patient with multiple sclerosis. The most recent tool for the diagnosis of MS is the 2017 McDonald criteria and is described in (Table 1) and continues to be revised and updated. High-income countries use the 2017 McDonald criteria to diagnose MS, which relies on clinical presentations and MRI findings. It facilitates earlier diagnosis compared to other diagnostic criteria which mostly rely on clinical findings. This is especially helpful for those with the clinically isolated syndrome (CIS). In lower- and middle-income countries, diagnosis of MS relies more on clinical diagnostic criteria and less on MRI evidence, as it is not easily accessible. This makes it even more difficult to distinguish between MS and other inflammatory demyelinating diseases(1,4,5).
Table 1: The 2017 McDonald criteria for MS diagnosis (6)
- If the 2017 McDonald Criteria is fulfilled with no better explanation for the clinical presentation, MS will be diagnosed. However, if MS is suspected through CIS but the 2017 McDonald Criteria are not met, MS will be a possible diagnosis until a better explanatory diagnosis is met during the evaluation.
- Brain MRI should be done in all patients for whom the diagnosis of MS is being considered unless MRI isn’t possible. In addition, spinal cord MRI/ CSF examination must be performed in patients with inadequate previous findings supporting MS, patients with a presentation other than typical CIS, or presenting with atypical features. If tests are negative, alternative diagnoses should be considered before diagnosing MS.
- Clinical diagnosis based on objective clinical findings for two attacks is the best. In the absence of documented objective neurological findings, historical evidence for one past attack can include events with symptoms and evolution characteristics of a previous inflammatory demyelinating attack. However, at least one attack should be supported by objective findings. objective evidence is described in panel 1.
- The presence of CSF-specific oligoclonal bands is not exactly diagnostic for dissemination in time but can substitute for the requirement for demonstration of this measure.
- MRI dissemination criteria in space and time are described in panel 1.
| Panel 1: Understanding dissemination in space, time and Objective clinical/paraclinical evidence. |
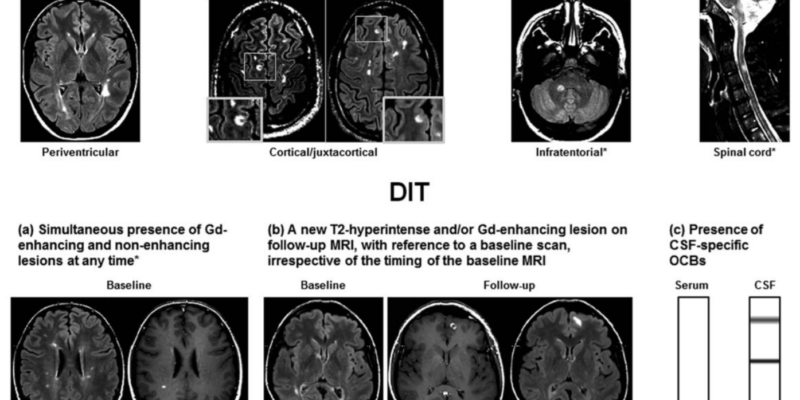
| 1. Dissemination in space (DIS) can be defined as the development of lesions in more than one distinct anatomical location within the central nervous system. It requires the detection of at least one T2-hyperintense lesion in at least two of these areas in the CNS; periventricular, juxtacortical, infratentorial, and the spinal cord. This can be proven if there is enough clinical evidence of these two lesions/areas affected, or if there is clinical evidence of one lesion and historical evidence of a second lesion as mentioned in the table above. |
| 2. Dissemination in time (DIT) reduces misdiagnosis of monophasic illness by requiring that the CNS lesions develop over time, this means comparing a patient’s baseline MRI with a follow-up MRI. What must be observed in DIT are gadolinium-enhancing and non-enhancing lesions, or, the appearance of a new T2-hyperintense or gadolinium-enhancing lesion on a follow-up MRI. The revised 2017 McDonald criteria added that a positive CSF for two or more CSF-specific oligoclonal bands can be a substitute for DIT in patients with no baseline MRI, this suggests that oligoclonal bands are an independent predictor of a second clinical attack(7). |
| 3. Objective clinical/paraclinical evidence is the association between the CNS lesion and the typical presenting symptom of the MS attack. It could be an abnormal neurological exam, MRI, OCT or neurophysiological exam that corresponds with the symptoms and anatomical location of the lesion. Examples include; optic disc pallor, p100 latency prolongation on visual evoked potentials, T2 hyperintensity of the optic nerve or a relative afferent pupillary defect in a patient presenting with optic neuritis symptoms, internuclear ophthalmoplegia in a patient presenting with diplopia, or hemisensory syndrome suggesting myelitis(6,7). |
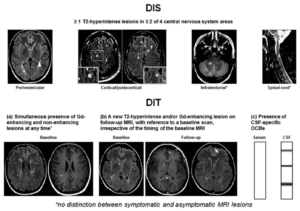
(Figure 1) Criteria for dissemination in space and dissemination in time in a patient with clinically isolated syndrome (a) a simultaneous presence of gadolinium-enhancing and non-enhancing lesions at any time, (b) a new T2-hyperintense and/or gadolinium-enhancing lesion on a follow-up MRI scan, with reference to a baseline scan, irrespective of timing of baseline of the MRI, or (c) Presence of cerebrospinal fluid-specific oligoclonal bands (lower row)(8)
Typical clinical presentation
MS is predominantly seen in early adulthood; this subsequently affects their quality of life which leads to patients seeking medical help and answers.
Multiple sclerosis can affect any part of the central nervous system making the signs, symptoms, and course of the disease extremely variable amongst patients. This adds to the difficulty of incorrect diagnosis because some MS symptoms may overlap with other demyelinating diseases. Imaging that is consistent with dissemination in space and time based on the 2017 McDonald criteria alongside typical symptoms are key components in accurately diagnosing MS. So, to accurately diagnose a patient with multiple sclerosis they must present with at least two attacks of typical MS syndromes; this includes optic neuritis, myelitis, brainstem and cerebellar syndromes(7).
- Optic neuritis
Optic neuritis is defined as an inflammation of the optic nerve. It has multiple different causes, one of those causes includes multiple sclerosis. It is more predominantly seen in white people. The diagnosis and treatment depend on the cause because of the many differential diagnoses for optic neuritis(9). The patient will present with subacute blurry vision or even loss of vision, which can be associated with pain and evolves over hours to days(10). Optic neuritis in multiple sclerosis is caused by acute demyelination of the optic nerve, it is the presenting symptom in about a fifth of MS patients(11). These patients typically present with an acute, unilateral, painful decrease in visual acuity that peaks within a few days and subsides within a few weeks(10).
There is an association between the incidence of optic neuritis and multiple sclerosis risk variant HLA-DRB1*1501, suggesting the relation between risk factors for MS and the cause of optic neuritis in areas of the world where MS is commonly seen. There is also evidence that relapsing CNS demyelinating diseases that are characterized by optic nerve involvement have a relation with serum aquaporin-4 (AQP4) autoantibodies(12,13).
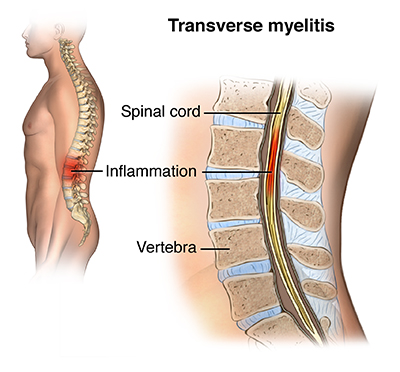
- Myelitis
Patients with MS may present with a band-like tightening sensation around their chest or abdomen (MS “hug”), this is a typical symptom of myelitis, suggesting the involvement of the posterior columns of the spinal cord. Myelitis in MS is usually partial and subacute. This corresponds with the typical imaging and neuropathological appearance of MS, which is the involvement of patchy asymmetric inflammatory-demyelinating lesions in the spinal cord. By contrast, complete transverse myelitis is the Inflammation and demyelination across both sides of one segment or level of the spinal cord resulting in symptoms of neurological disconnection and dysfunction below the level of the demyelinating area, such as impairment of motor, sensory, bowel, or bladder functions, it may also be accompanied with pain and absent reflexes(14–16).
- Brainstem and cerebellar syndromes
The brainstem is commonly involved in multiple sclerosis. Around 15% of patients with PPMS will present with a progressive cerebellar or brainstem syndrome, characterized most prominently by gradual worsening ataxia(17). The most common brainstem presentation in MS is diplopia (cranial nerves III, IV, VI involvement) due to internuclear ophthalmoplegia which may be bilateral, facial weakness or myokymia (cranial nerve VII involvement), vertigo (cranial nerve VIII or a lesion anywhere in the vestibular pathways), or bulbar (medullary) symptoms such as dysphagia, dysarthria, tongue weakness (cranial nerves IX, X, XII involvement) and ataxia (cerebellar lesion).
Facial weakness due to facial nerve involvement may accompany eye movement abnormalities. Usually due to multiple localizations, including the brainstem, cervical cord, and subcortical and cortical sensory pathways(18).
Diplopia may also suggest sixth cranial palsy and is not specific to MS. Third cranial nerve palsy is more suggestive of other causes. If the patient presents with persistent hiccups, nausea, or vomiting it’s more suggestive of neuromyelitis optica spectrum disorder (NMOSD) which is caused by a lesion in the area postrema. Usually, when a patient presents with hyperacute cerebellar symptoms this suggests a vascular cause rather than MS.
Patients may present with symptoms that are less specific to multiple sclerosis and may be seen in other demyelinating diseases. However, this doesn’t disapprove the need for further evaluation and testing for MS. Some atypical symptoms:
- Motor symptoms
Weakness due to the involvement of the corticospinal tract can be seen in about 89% of MS patients at some point in the course of their disease. Weakness is often accompanied by symptoms of an upper motor neuron lesion, such as hyperreflexia, positive response in Babinski and Chaddock reflexes, and spasticity(19). In the absence of weakness; stiffness, spams, cramping, and gait impairment may be present. When there are spinal cord or brainstem lesions, tonic seizures may occur which are usually confined to one side of the body(20).
- Cognitive impairment
Cognitive impairment is seen in all subtypes of multiple sclerosis, although, it is more common in progressive MS than in relapsing MS(1). Cognitive impairment accompanies other symptoms of MS so it cannot be used alone to diagnose MS, as other CNS lesions may also be accompanied by cognitive impairment.
3. Imbalance, Bowel and bladder dysfunction, Sensory impairment, Depression, and Fatigue are some other atypical symptoms of MS.
A patient with MS will usually present with attacks of neurological dysfunction including numbness, tingling, weakness, vision loss, gait abnormalities, incoordination, imbalance, and bladder dysfunction. This does not mean that every patient will present with the same symptoms as they vary considerably between patients, depending on where in the CNS is the neurological involvement. If the patient has RRMS then these symptoms would be completely gone and they’d be neurologically stable between attacks.
Phenotypic subtypes of multiple sclerosis
There are three phenotypic subtypes of multiple sclerosis: relapsing-remitting MS (RRMS), progressive MS (primary or secondary), and clinically isolated syndrome (CIS)(21).
A clinically isolated syndrome (CIS) is when a patient presents with MS after a single event or attack. These patients present with an acute demyelinating attack, but the MRI conducted does not fully meet the criteria for RRMS. In this case, DIS can be satisfied with clinical evidence of only one lesion incorporating the patient’s MRI, or if there is any evidence of another lesion during neurological examination.
A CIS diagnosis does not exclude the possibility of CIS progressing into RRMS, or any other classification of MS. A brain MRI at the presentation of the first demyelinating attack can predict the development of MS, as well as the type and extent of disability in the long run (1,22,23).
85% of patients diagnosed with MS showed RRMS features characterized by recurring symptoms followed by their total or partial disappearance(4). MS relapses/attacks occur due to demyelination evolving over 24 hours which persists from days to weeks, patients then relatively improve(2). Relapsing-remitting MS is defined by the International Panel on the Diagnosis of multiple sclerosis as ‘patient-reported symptoms or objectively observed signs typical of an acute inflammatory demyelinating event in the CNS, current or historical, with a duration of at least 24 h, in the absence of fever or infection’(8).
This pattern of RRMS can become increasingly progressive after an initial relapse in 50% of untreated patients 10-15 years into the course of the disease, this stage is defined as secondary-progressive MS (24). It is usually difficult to define the point of transition. SPMS is characterized by a gradual decline in neurological functions, usually involving the areas of the CNS that were previously involved during the relapse stage. At first, patients with SPMS experience will have relapsing and remitting symptoms, but over time it turns into an insidious progression of the disease without any remission.
Some research is shedding light on better characterizing the point of transition from RRMS to SPMS or even predicting it by imaging and laboratory biomarkers as a way of distinguishing between SPMS and RRMS. These biomarkers are IL-17 and INF-gamma, which are pro-inflammatory cytokines increased in RRMS. TGF-beta1 is another biomarker, it is decreased in RRMS and has immunosuppressant activity. This could potentially prove that different cytokines are responsible for RRMS compared to SPMS, but is still under research and needs further validation(25).
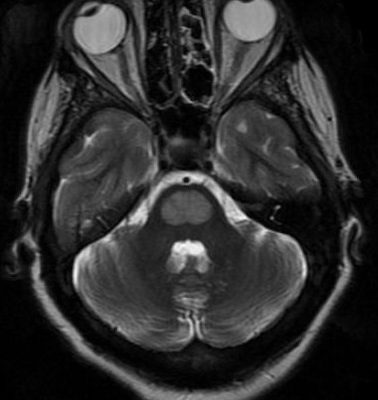
(Figure 2) extensive brainstem and cerebellar involvement, case courtesy of Dr Bita(26)
Primary Progressive multiple sclerosis (PPMS) represents 10-15% of MS cases and is characterized at the time of disease onset by a slow decline in neurological functions and has no relapses or remissions. In contrast, SPMS usually occurs after relapse and not from the beginning of the disease.(27)
Table 2. 2017 McDonald criteria for the diagnosis of MS in patients with a disease course characterized by having being progressed from onset (primary progressive multiple sclerosis) (28)
| Primary progressive multiple sclerosis can be diagnosed based on cases with
· ≥1 year of disability progression which can be determined either prospectively or retrospectively. |
| With two of the following:
· ≥1 T2-hyperintense lesion characteristic of multiple sclerosis in one or more of the following regions: periventricular, cortical or juxtacortical, or infratentorial · ≥2 T2-hyperintense lesions in the spinal cord · Presence of CSF-specific oligoclonal bands |
Pathogenesis and Etiology
The exact pathogenesis of multiple sclerosis is still not fully understood but there has been recent evidence proving the relationship between genetics (e.g., HLA DRB1*15:01) and MS. other factors that contribute to the development of MS include environmental factors (e.g., vitamin D deficiency), immunizations, emotional and physical stressors, diet and lifestyle (e.g., Cigarette smoking). Environmental factors have a bigger role in susceptibility to MS contrary to what most people think. Both the innate and adaptive immune systems are known to contribute to the pathogenesis of MS. New treatment targets are now being developed due to the discovery that B cells have a major role in the disease(29).
Environmental risk factors
Vitamin D deficiency, infectious agents, and cigarette smoking have been shown to contribute to the pathogenesis of multiple sclerosis. Some physicians say that obesity might also be a risk factor but that could be due to vitamin D deficiency. Therefore, correcting vitamin D levels is important in reducing the risk of MS. On the other hand, Vitamin D deficiency in neonates hasn’t shown any correlation with the incidence of MS. The incidence of the disease increases with the increase of smoking duration, this is seen more in men than in women(30–34).
Exposure to infectious agents; usually Epstein-Barr virus infection as a young adult increases the risk of developing the disease. Others may say that the hygiene hypothesis is related. The more pathogens and infectious agents a growing child is exposed to the less likely they are to develop allergies and autoimmune diseases when they are older. Because of that, some may say that people who were raised in conditions where they were not exposed to many pathogens have a higher risk of developing MS later on in life(35,36).
Genetic factors
It has been observed that the incidence of Multiple sclerosis increases within families. This suggests that genetics have a significant role in the development of MS. Carriers of DRB1*15:01 are three times more likely to develop MS than non-carriers of this allele, it is known as the strongest association with MS susceptibility. Recent population-based studies have estimated a sibling of a patient with MS has a sevenfold increase in the risk of developing the disease. Environmental factors have also been shown to interact with genetic risk (e.g., HLA), thus, increasing the overall risk of developing MS (37). More specifically, the HLA region on chromosome 6 has been noted to have significant associations with most autoimmune diseases in humans. And ever since the 1970s, the connection between serotype DR2 (HLA-DR15 and HLA-DR16 serotype) and multiple sclerosis has been established(38,39).
In addition to clear MS genetic variants, a genome-wide association study (GWAS) has identified the first two non-HLA genetic variants with minor effects, including genes in IL2RA and IL7RA(40).
Work up and Diagnosis
Diagnosis of MS requires a combination of findings during clinical history, neurological exam, MRI, and the exclusion of other possible inflammatory demyelinating diseases. CSF, urodynamic studies of the bladder, and OCT are usually supportive for the diagnosis. Some patients may come between attacks so all the symptoms might not be present, some may present with persisting fatigue or heat sensitivity between attacks so that is something physicians must take into account(41).
MRI
The most sensitive tool to diagnose MS is MRI, it detects brain and spinal cord lesions. It is also necessary for the exclusion of other diseases (42). The presence or absence of red flags in the MRI will help guide the differential diagnosis, which includes metabolic diseases, toxicity, malignancy, and infiltrative processes(1). This is important in preventing the misdiagnosis of multiple sclerosis as any other demyelinating disease. The specific guidelines for the clinical implementation of the brain and spinal cord MRI were mentioned above (McDonald criteria 2017).
Lumbar puncture
CSF may show the presence of IgG oligoclonal bands in CSF that are absent in serum, with an elevated IgG index in multiple sclerosis patients(43), The presence of CSF-specific oligoclonal bands doesn’t demonstrate dissemination in time but can substitute the requirement for demonstration of this measure.
Treatment and management
Treatment of multiple sclerosis (MS) is divided into 3 groups: treatment of the exacerbations, slowing the progression of the disease with disease-modifying therapies (DMTs), and symptomatic relief therapies.
- Relapse management
A real relapse occurs when a patient experiences a new symptom not experienced before. A pseudo-relapse is when an acute attack of physical or emotional stress exacerbates their already existing symptoms. Treatment of acute relapses aims to speed recovery from neurologic deficits, alleviate the severity of the attack and lessen or eliminate potentially persistent deficits.
Corticosteroids are the first-line treatment modality for MS relapses. The use of corticosteroids over time shortens the time to recover from an acute attack, reduces symptoms, and improves motor function(44). If patients don’t respond to corticosteroids and are still experiencing severe relapses, plasmapheresis should be used(45). Although relapses are uncommon during pregnancy, if it occurs, intravenous immunoglobulin can be used safely during pregnancy. Methylprednisolone can also be given to pregnant women as it is the preferred drug because it is metabolized before crossing the placenta(46,47).
Treatment with low doses of carbamazepine or related agents often provides rapid and substantial relief for motor symptoms of MS(48).
- Disease-Modifying Therapies
There is no cure for multiple sclerosis, so the aim is to reduce the progression of the disease. There are several disease-modifying therapies (DMTs) available, an example of DMTs (are Interferon Beta, Glatiramer Acetate, Natalizumab, Fingolimod, Dimethyl Fumarate).
Prognosis
MS on its own is rarely fatal, but the secondary complications of MS-like difficulties swallowing, respiratory infections, and bladder infections may decrease the patient’s life expectancy by 10-15 years. However, with the continuous development of newer therapies; these complications may be a thing of the past.