Abstract
We describe the use of behavioral, neuroimaging, and genetic methods to examine individual differences in cognition and affect, guided by three criteria: (1) relevance to human performance in work and everyday settings; (2) interactions between working memory, decision-making, and affective processing; and (3) examination of individual differences. The results of behavioral, functional MRI (fMRI), event-related potential (ERP), and molecular genetic studies show that analyses at the group level often mask important findings associated with sub-groups of individuals. Dopaminergic/noradrenergic genes influencing prefrontal cortex activity contribute to inter-individual variation in working memory and decision behavior, including performance in complex simulations of military decision-making. The interactive influences of individual differences in anxiety, sensation seeking, and boredom susceptibility on evaluative decision-making can be systematically described using ERP and fMRI methods. We conclude that a multi-modal neuroergonomic approach to examining brain function (using both neuroimaging and molecular genetics) can be usefully applied to understanding individual differences in cognition and affect and has implications for human performance at work.
Introduction
Background
A patient undergoing a lengthy surgical procedure shows a sudden change in vital signs indicative of a critical, life-threatening condition. The attending surgeon must make a quick decision as to the appropriate course of action to take. Several possibilities must be juggled mentally while reaching for a diagnosis. The risks associated with different actions must also be evaluated. Individual physicians will differ in how effectively they carry out these mental operations. Can we account for why one physician may have sufficient working memory capacity to consider many possible diagnoses, while a colleague may be more prone to taking a risky course of action? A third one may experience anxiety and may not take action quickly, potentially endangering the patient. Yet another one may not cope well with the fatigue and monotony associated with the long operation and may reach a faulty decision.
In this paper we propose that evidence from neuroimaging and molecular genetics can provide insights into such inter-individual variation and their role in human decision-making and performance. We discuss interactions between individual differences in working memory, decision-making, and affective states. We also draw implications for applied issues such as selection, training, and the design of work settings so that the decision-making performance of individuals in naturalistic settings can be improved. Examination of such issues, whether raised in relation to physicians, military commanders, or laypersons engaged in everyday pursuits, falls within the realm of neuroergonomics. This emerging discipline represents the application of empirical findings and theories from neuroscience to the analysis of brain function and human behavior at work and other natural settings (Parasuraman, 2003, 2011; Parasuraman and Rizzo, 2008). As a case of translational neuroscience, neuroergonomics seeks to allow researchers and practitioners to develop new explanatory frameworks about humans at work in everyday settings and in relation to modern technology.
Neuroimaging techniques such as functional MRI (fMRI) and event-related potentials (ERPs) are among the major neuroscience methods that are being used in neuroergonomic research (Kramer and Parasuraman, 2007; Parasuraman, 2011; Parasuraman and Wilson, 2008). A more recent development is the use of molecular genetic methods, including candidate gene (Greene et al., 2008; Parasuraman, 2009; Posner et al., 2007) and genome wide association analyses (Butcher et al., 2008). We describe studies using these methods, guided by three criteria. First, whereas the neural bases of cognitive functions have been extensively studied, much of this work has been conducted using relatively simple laboratory tasks that do not capture the complexity and dynamics of behavior as it occurs naturally. Human factors researchers have examined such “cognition in the wild” (Hutchins, 1995), but typically using observational and not neurocognitive methods. Second, the role that different affective processes play in decision-making in complex tasks representative of everyday settings is relatively understudied (Bechara, 2007; de Visser and Parasuraman, 2010). Recent studies on the neural mechanisms of emotion can inform the study of naturalistic decision-making. Third, most neuroimaging studies of affective and cognitive processes report group results, neglecting individual differences (for an exception, see Miller et al., this issue). The contribution of molecular genetics to an understanding of such individual variability has been a focus of many recent studies. Accordingly, this review discusses all three of these aspects of cognition and affect. Clearly, many more topics within this general area could have been examined. Given space limitations, our review focuses on the cognitive processes of working memory and decision-making, affective processes and traits such as anxiety, sensation seeking, and boredom, the interactions between these processes, and their implications for improving our understanding of human performance in everyday settings.
Why individual differences matter
Why should one consider individual differences? There are many reasons. First, many theories of cognition thrive on their ability to provide good fits to the average performance of a group of participants. For example, acquisition of perceptual-motor and cognitive skills is well modeled by a power law function of practice (Newell and Rosenbloom, 1981). fMRI studies have also shown that posterior brain regions associated with learning a perceptual task show systematic reductions in activation over time that parallel such skill acquisition (e.g., Poldrack et al., 1998). But typically, the models apply less well to the skill acquisition functions of individuals (Parasuraman and Giambra, 1991). In some cases, adjusting model parameters can handle the problem. For example, slow and fast learners could be distinguished by altering the exponent in the same power law function of skill acquisition. But in other cases, the performance function for a group may not be characteristic of some or even many of the individuals making up the group. Furthermore, no function may adequately describe those rare, exceptional individuals who demonstrate extraordinarily high levels of cognitive competence—i.e. those who are literally “off the charts” (Parasuraman, 2009; Russell et al., 2009; Watson and Strayer, 2010).
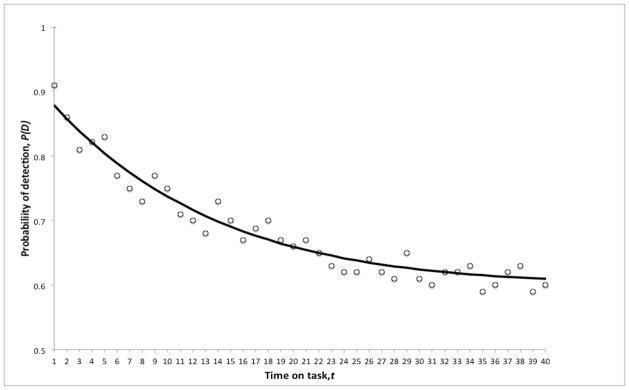
We provide three illustrative examples of these phenomena from our research. Consider first sustained attention, in which participants have to detect an infrequently and unpredictably occurring target among more frequent non-targets over a prolonged period of time—a task that typically confronts such workers as airport security screeners and air traffic controllers. When the performance of a group is considered, the decline in target detection sensitivity over time on task—the vigilance decrement (Parasuraman 1979)—can be well fit using an exponential function with two parameters (Giambra and Quilter 1987). Figure 1 shows such a function for a combined working memory/vigilance task (Caggiano and Parasuraman, 2004) for a group of 20 participants over a 40-minute period of performance (Parasuraman, 2009). The double exponential gave a good fit to the group decrement function: an initial rapid decline in the probability of detection, P(D) as a function of time t, followed by a slower drop. However, only 8 participants (40%) had individual performance trajectories that could be modeled, with varying degrees of fit, to the double-exponential function. The remainder either showed stable or variable detection rates over time, while one exceptional individual had 100% detection rate throughout the task (Parasuraman, 2009). Clearly functions other than the two-term exponential are required to model individual vigilance decrement, and other methods to explain the sources of individual differences in this basic cognitive function.
Figure 1.
Probability of detection, P(D), as a function of time on task, t, in a 40-minute visual vigilance task. Open circles show the mean detection rates for a group of 20 participants. Fitted line: P(D) = K (e−at + 1/(1 + e−bt)), where K is the asymptotic level of performance and a and b are free parameters.
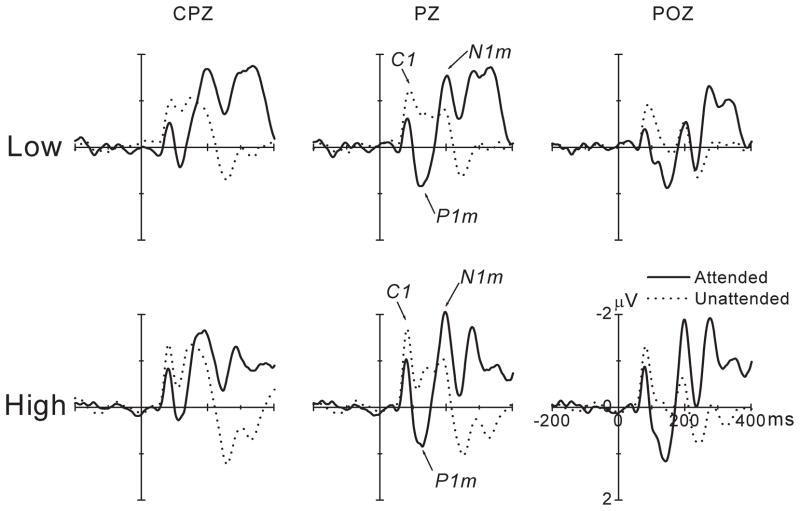
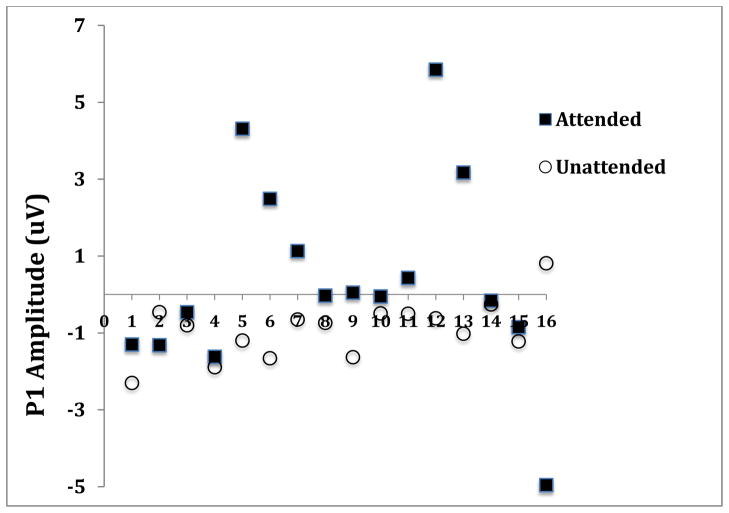
A second example involves attention not to a single, infrequently presented target at a fixed location, but to a target at a spatial location (left or right visual field) that is cued prior to target presentation. Voluntary covert attention to the cued location (while maintaining central fixation) enhances the early-latency P1 component of the ERP (Fu et al., 2008). Figure 2A shows group ERP results for the midline parietal electrode site (Pz) for attended and unattended stimuli. P1 amplitude was greater for attended than for unattended stimuli, an effect generally interpreted to reflect enhancement (“sensory gain”) by selective attention of early-stage cortical activity (Hillyard et al., 1998). However, the individual data for P1 amplitude, shown in Figure 2B for the Pz electrode, disclose a variable picture. First, there was considerable variability around the mean attended and unattended P1 amplitudes (shown in the solid and open symbols, respectively). Second, much of the P1 attention effect seemed to be driven by participants whose P1-attended values were much larger than for those of other participants, in whom the difference in P1 amplitude between attended and unattended was quite low. In 3 participants P1 amplitude was greater for unattended than for attended stimuli, and one of these (#16) showed a much greater P1 amplitude for unattended stimuli. Of course, to attribute differences in a physiological measure such as P1 primarily to stable individual differences assumes that random effects are not large and that the measure is reliable. The relatively high test-retest reliability of P1 and the consistency of the P1 enhancement effect in large-sample studies, where measurement variance is expected to be low (Hillyard et al., 1998), support this view. Thus, the standard model that attributes the neural effect of visuospatial attention to an enhancement of early cortical activity needs to explain why this effect is very large in some individuals but moderate in many, and why a few show a reversed effect.
Figure 2.
Figure 2A. Group-averaged ERPs (for 16 participants) at three midline electrode sites for attended and unattended stimuli. Reprinted from Figure 3 in Fu et al., NeuroImage, 39, 2008, 1349. Reprinted with permission from Elsevier Inc.
Figure 2B. Amplitudes of the P1 component (uV) at the Pz electrode site for attended and unattended stimuli for the 16 individual participants.
Assessment of individual differences in attention is important for understanding both decision-making and affective processing. For example, selective attention can bias decision-making if salient but irrelevant cues are attended to more than less salient, but decision-relevant cues (Payne et al., 1993). Individuals susceptible to attend to such salient, non-diagnostic cues will tend to make decision errors, particularly under time stress (Wallsten and Barton, 1982). Similarly, anxiety can bias attention to aversive or threatening stimuli (Bishop et al., 2004; Matthews and Mackintosh, 1998) and impair decision-making. A third example we provide therefore comes from an fMRI study on affective processing (Joseph et al., 2009).
Although the anterior cingulate cortex (ACC) is involved in emotion regulation and is often activated in affective processing tasks (Ridderinkhof et al., 2004), the degree of activation can vary considerably between individuals, so that the group pattern may not be representative. In this example, participants were exposed to affective images that were matched in valence but varied in the dimension of arousal (low or high). Individuals characterized as low in sensation seeking showed greater activation of the ACC to high-arousal stimuli. In contrast, high sensation seekers showed little activity in ACC but strong activation in brain regions associated with arousal and reinforcement, such as the insula. Clearly, an individual’s sensation seeking status modulates the pattern and intensity of brain reactivity to emotionally arousing stimuli. Miller et al. (this issue) similarly describe how fMRI patterns of brain activity in a memory retrieval task vary considerably between people, but that individual difference measures of cognitive style can account for such variability.
These examples from our previous work using behavioral, ERP, and fMRI measures show why individual differences matter and why group analyses have some limitations. The studies also show that some persons, including exceptional individuals, do not necessarily follow the group pattern. Methods are therefore needed for identifying and quantifying such individual differences. The dominant method has been the psychometric approach. Much has been learned using this method, and we also describe studies that have used psychometric tests to characterize individuals. However, molecular genetics provides an additional avenue for information on differences between people. We outline the general approach and describe findings that illustrate how molecular genetics can contribute to an understanding of the neural basis of normal person-to-person variation in affect and cognition.
Molecular Genetic Methods
Behavioral geneticists have traditionally examined the association between genetics and human behavior by comparing the performance of identical and fraternal twins to assess the heritability of a particular psychological function. For example, twin studies have established that general intelligence, or g, is strongly inherited, with heritability estimates ranging from 60 to 80% (Plomin and Crabbe, 2000). Twin studies have also demonstrated that components of cognition, e.g., working memory (Ando et al, 2001) and executive attention (Fan et al. 2001), and emotions such as anxiety and fear (Eley et al., 1999; Stevenson et al., 1992; Stein et al., 1999) have moderately high heritability values.
Genome wide analysis
Molecular genetic methods, including both candidate gene (allelic association) and genome wide association studies (GWAS) provide a different, complementary approach to the twin method. Early molecular genetic studies of cognition and affect typically examined only one or a few genes. However, the number of genes and single nucleotide polymorphisms (SNPs) that can now be examined for associations with neurocognitive phenotypes has risen rapidly in recent years. It is now possible to examine from 100,000 to more than one million SNPs, covering the entire human genome. Technical developments and advances in bioinformatics methods have made GWAS a current method of choice for many research groups, although its expense still precludes its widespread usage, a limitation that is likely to diminish in the future as mass genotyping costs come down.
The major advantage of the GWAS approach is that it allows for associations to be discovered in an unbiased or hypothesis-free manner between behavioral or neurocognitive phenotypes and all known genetic variations in the genome (including, in addition to SNPs, other changes such as insertions, deletions, repeats, copy number variation, etc.). In principle, GWAS can also provide information on multiple gene interactions and analysis of molecular pathways to cognition.
The limitations of GWAS are also widely recognized. Because of the number of statistical tests that must be conducted, the scope for Type 1 error is large, which is generally handled by setting a very stringent threshold (e.g, 10−8) for statistical significance. This raises the possibility of Type II error, so that potential genetic associations may be missed. The required sample sizes for GWAS can also be very large, making the use of other tools for the assessment of particular intermediate phenotypes, such as fMRI, difficult and further adding to the cost of such studies. Finally, the very fact that, unlike the candidate gene approach, GWAS is not hypothesis or theory driven can make interpretation of significant associations difficult in some cases.
GWAS research on neuropsychiatric diseases, such as autism (Weiss et al., 2009) and Alzheimer’s disease (Bertram and Tanzi, 2009) has increased in recent years but studies of normal variation in cognition are still rare. One example is a study of over 500,000 SNPs in a sample of 7,000 children (Butcher et al., 2008). Associations with g were found for six SNPs but none accounted for more than about 0.4% of the variance, consistent with a multiple genetic role in intelligence. Other genome wide studies of specific cognitive functions are currently in progress.
GWAS has also been combined with statistical modeling in a hybrid approach called genetic prediction. GWAS is first used to identify the SNPs most strongly associated with a trait in a sample. The effects of the top SNP “hits” are then combined in a regression model to predict the trait. Derringer et al. (2010) recently used this approach to show a putative link between dopaminergic genes and the sensation seeking personality trait. However, Powell and Zietsch (2011) cautioned that to be valid such statistical approaches must be confirmed in independent samples.
GWAS has been quite fruitful in finding SNPs related to rare human diseases. However, its use in the search for genetic factors linked to normal variation in cognition and affect has been less successful. To date the extant literature is still small and relatively weak effects have been found in the few non-disease studies. Hypothesis-driven candidate gene approaches offer another way to attack this problem.
Candidate gene studies
In the candidate gene approach, specific allelic variants are chosen on a theoretical basis, e.g., SNPs that are thought to influence neuromodulation in the brain. Several studies have used this method to examine individual differences in normal cognition (Greenwood et al. 2000; Fossella et al. 2002; Parasuraman et al. 2005; Posner et al. 2007; for reviews, see Green et al., 2008; Payton, 2009) and emotion (for reviews, see Xu et al., 2007; Feder et al., 2009). This method has also been used to examine associations and interactions between multiple specific genes and cognition (Espeseth et al. 2006, Greenwood et al. 2009).
Because the human genome has so many potential variations, candidate gene studies have followed various restricting steps. First, researchers have focused on SNPs that are likely to influence neurotransmitter or neurotrophic function. Second, neuroimaging studies on a cognitive function are reviewed to identify the brain networks mediating that function. Third, pharmacological and neurophysiological studies can identify the neurotransmitter innervation of these networks. These multiple levels of analysis can be combined into a joint bottom-up and top-down approach (Parasuraman and Greenwood, 2004). Bottom-up, one can use bioinformatics methods and a search of SNP databases to identify potential candidate genes. At the same time, a top-down approach can be used, beginning with the cognitive function in question. Often, genes have been identified that are associated with dysfunction in a cognitive process in a neurological or psychiatric disorder. The same genes may be involved in normal variation in that cognitive function. By identifying the neural networks known to mediate a particular cognitive function and the neurotransmitters that innervate those networks, the top-down and bottom-up approaches can converge on potential candidates for a genetic association study of cognition.
Imaging genomics
Although many early molecular genetic studies used behavioral phenotypes in forming gene-cognition links, recently there has been interest in using neuroimaging phenotypes, including ERPs (Espeseth et al., 2007; Parasuraman and Espeseth, 2007) and fMRI (Fan et al., 2003; de Geus et al., 2008; Egan et al., 2001), consistent with the view that such intermediate phenotypes, or “endophenotypes,” may provide for more direct genetic associations (Goldberg and Weinberger, 2004). The integration of neuroimaging and genetics, known as imaging genomics (Bigos and Weinberger, 2010) is thought to be beneficial in understanding the neurobiological pathways underlying abnormal brain functions and associated with various neuropsychiatric disorders. Additionally, imaging genomics allows researchers to bridge animal models with human studies by examining the intermediate links between behavior and genetic polymorphisms. The neuroimaging methods reviewed in this paper serve as effective endophenotypes related to affect and cognition. It is to be hoped that the new imaging genomics approach will provide new insights not only on the neurobiology of disease but also of normal individual variation in neurocognitive function in healthy individuals. We consider first such variation in working memory and decision-making.
Individual Differences in Working Memory and Decision Making
Neuroimaging studies
Working memory plays an important mediating role in many higher cognitive functions, including problem solving, reasoning, and decision-making (Baddeley, 1992). Working memory is also a key component of mental workload, which is assessed in studies of multitasking (Just et al., 2008) and more generally, in human performance in complex, work-relevant tasks (Parasuraman and Caggiano, 2002; Wickens and McCarley, 2007). Many human factors evaluations are concerned with designing interfaces or training methods that optimize operator workload at a moderate level, avoiding both underload and overload (Wickens and Hollands, 2000). Hence assessment of working memory and its role in decision-making is an important topic for neuroergonomic research. Also requiring further investigation is the development of neurocognitive tools to assess individual differences in working memory.
Functional MRI has revealed systematic individual differences in brain activity in relation to episodic (Van Horn et al., 2008) and working memory (Rypma and D’Esposito, 2000). Such differences in regional brain activation can also be linked to performance differences. We provide an example from a recent fMRI study conducted in our laboratory with an N-back task. Participants were asked to match a 3D object shape and rotation to the visual stimulus presented two items back. Figure 3 shows the brain responses of two individuals. The individual (Figure 3A) who performed well—with high accuracy and faster reaction times—activated typical working memory regions such as inferior frontal and dorsal lateral prefrontal cortex. The age-matched individual (Figure 3B) who was slower to respond and was less accurate, however, engaged additional regions associated with conflict control, default mode network, and emotion regulation (e.g., anterior and posterior cingulate, subcortical limbic areas).
Figure 3.
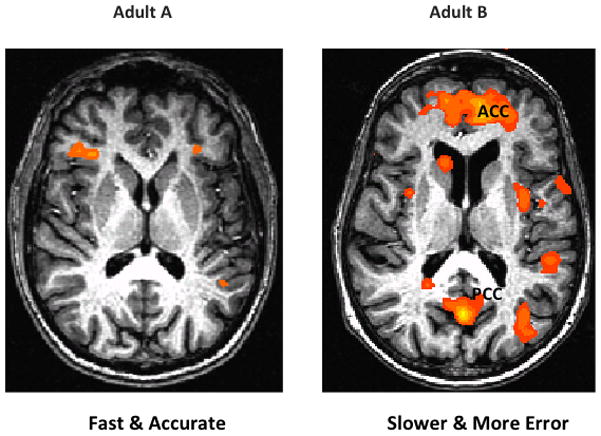
Individual differences in performance and fMRI responses during a 2-back working memory task. Participant A (left) showed activation in bilateral inferior frontal regions, typically related to working memory performance. An age- and gender-matched participant B (right image) was slower in reaction times and less accurate compared to A. Additional regions including anterior and posterior cingulate (part of the default mode network) were engaged in participant B during the 2-back working memory task.
While the N-back task has been the workhorse of human working memory studies, delayed match-to-sample tasks have been extensively used in working memory studies in behaving monkeys. Typically, a sample item is presented at the beginning of a trial and, after a delay, is followed by one or more test items that either match or do not match the sample. Enhanced prefrontal activity in the absence of stimuli during the delay period is the hallmark of maintenance in working memory (Miller and Desimone, 1994). This task is also useful in human studies because of the potential for linking the results from noninvasive human neuroimaging studies to those obtained with invasive techniques in animals. For example, Jiang et al. (2000) found enhanced neural responses for target matches compared to non-match responses, in a delayed face-matching task, primarily in the frontal cortex and less so in temporal cortex. Behaviorally, participants are also slower to respond to matching than to nonmatching items (Caggiano et al., 2006; Guo et al., 2008). As with the N-back task, individual differences in brain activation and performance are also readily apparent with the delayed match-to-sample task. Figure 4 shows individual differences in memory performance and fMRI responses from a recent study we conducted with a version of this task. The top eight good performers and bottom eight poor performers were selected from a sample of 30 participants based on their accuracy and reaction times. Compared to poor performers, good performers had reduced fMRI activity in the posterior precuneus (see Figure 4). The precuneus/posterior cingulate is part of the so-called “default network” that typically exhibits deactivation during cognitive tasks compared to resting state in healthy adults (Raichle et al., 2001; Fransson and Marelec, 2008).
Figure 4.
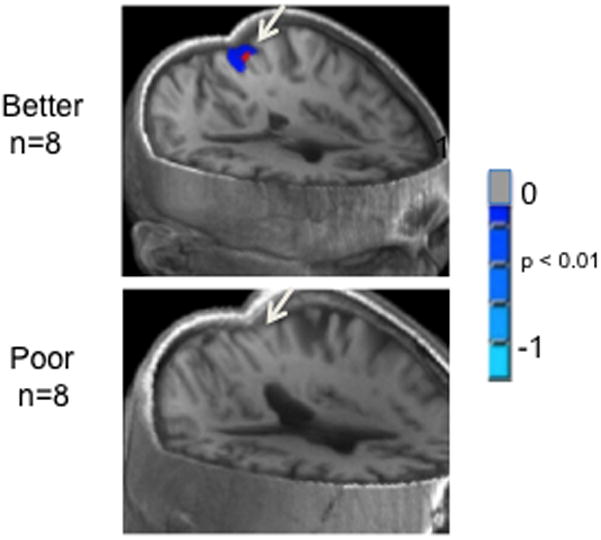
Top eight versus bottom eight performers in a delayed match-to-sample working memory task. Better performers on the working memory task showed deactivation of posterior precuneus/cingulate (top) during the working memory task, whereas poor performers failed to show the deactivation pattern (bottom).
These activation patterns and their link to individual differences in performance have implications for understanding both exceptional and impaired cognition. Similar to the present results seen in poor performers, patients with cognitive impairment do not deactivate the precuneus during memory tasks as healthy individuals do (see Buckner et al. 2008 for a review). At the other end of the spectrum, the current results for good performers in our working memory paradigm are consistent with a recent study on creativity. Individual creativity was significantly correlated with brain activity in the precuneus during a 2-back working memory task but not in a 0-back non-memory task (Takeuchi et al., 2011), thus pointing to the important role of working memory in higher cognition.
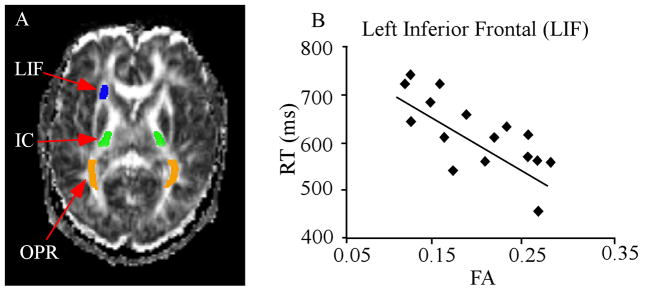
In addition to memory, the relationship between individual differences in language functions and brain structure has also been explored. For example, we examined the relationship between speed of visual word recognition and white matter fiber structure. Both gray (Draganski et al. 2004) and white matter (Scholz et al. 2009) change after months of intensive motor skills training. An example from our laboratory using diffusion tensor imaging (DTI) shows that such changes can be linked to performance (Gold et al., 2007). The speed of visual word recognition is known to vary widely between individuals. As Figure 5 shows, lexical decision time was correlated negatively with the regional fractional anisotropy (FA) value in white matter of inferior parietal and frontal regions, known to be associated with language processing. In contrast, no such association was found for white matter underlying visual or motor regions. The results suggest that individual differences in the speed with which lexical decisions are made are associated with the integrity of white matter linking frontal and parietal lobes. Additionally, individual differences in learning and task performance have also been linked to differences in plastic changes in brain structures associated with training or decision-making. The human brain retains its capacity to adapt to new skills and environmental alterations in adulthood and can change its structure in the process (Taubert et al. 2011).
Figure 5.
Speed of lexical decision correlates negatively with fractional anisotropy (FA) in left inferior frontal white matter. (A) Example of regions of interest (ROIs), shown on a representative participant’s FA images. ROIs included white matter of the left inferior frontal (LIF) region, the posterior limbs of the internal capsule (IC), and the optic radiations (OPR). (B) A significant negative correlation was observed between lexical decision reaction time and FA in white matter of the LIF region. Modified from Gold et al. (2007).
The results of these neuroimaging studies clearly show that patterns of brain activation and structure vary in systematic ways between individuals differing in working memory and other higher cognitive abilities. Both experience and genetic factors may contribute to such individual differences.
Molecular genetic studies
What is the genetic contribution to individual differences in working memory? Twin studies have shown that both verbal and spatial working memory (as measured by “span” tasks; Engle, 2002) have relatively high heritability values (Ando et al., 2001). Accordingly, studies have sought to identify the specific genes that contribute to normal variation in working memory.
Dopaminergic and noradrenergic receptor genes are good candidates for identifying genetic effects on working memory. Dopaminergic innervation of prefrontal cortical (PFC) neurons has been shown to be important for working memory in studies using dopamine agonists and antagonists in monkeys (Sawaguchi and Goldman-Rakic, 1991). Similarly, noradrenergic agonists such guanfacine, an α-2A adrenoreceptor agonist, improved spatial working memory performance in monkeys and increased blood flow in prefrontal but not temporal cortex in monkeys (Avery et al. 2000).
In human genetic work, several studies have examined the COMT and DBH genes, since they are known to be expressed in PFC. COMT modulates dopamine-signaling pathways and the availability of dopamine in the synaptic cleft (Goldberg and Weinberger, 2004).. A specific variation in the COMT gene (rs4680) has been associated with activation of PFC during working memory tasks (e.g., Egan et al., 2001). COMT has also been linked to other tasks that also activate PFC, such as the Wisconsin Card Sorting Task (Goldberg and Weinberger, 2004). This task taps many different cognitive functions subsumed under the rubric of executive control. Thus, a link between COMT and working memory has not been consistently established. A meta-analysis by Barnett et al. (2008) also found inconsistent association between the COMT gene and the N-back task (but see Goldman et al., 2009).
Given the role of dopamine and norepinephrine in PFC activation and working memory performance, the Dopamine Beta Hydroxylase (DBH) gene, which is known to modulate their differential availability in PFC neurons, provides another possible candidate for association with working memory. DBH SNPs have been associated with plasma and cerebrospinal fluid levels of the dopamine beta hydroxylase (DβH) enzyme (Bhaduri and Mukhopadhyay, 2008; Cubells and Zabetian, 2004). DβH controls the conversion of dopamine to norepinephrine in adrenergic vesicles. The DBH gene is about 23,000 base pairs (bp) long, with 12 coding regions (exons). Two of the major SNPs controlling DβH enzyme activity are 444G/A, involving a G to A substitution at location 444 in exon 2, and −1021 C/T, which involves a C/T change in the promoter region of the gene.
We have found that both DBH SNPs are associated with individual differences in spatial working memory using a delayed match-to-sample task (Parasuraman, 2009). Participants were shown target circles at one to three locations and asked to memorize them. The display was then blanked for a period of 3s, after which a single red test circle appeared alone, either at the same location as one of the target circles (match) or at a different location (non-match). Match accuracy decreased as the number of locations to be maintained in working memory increased from one to two to three, demonstrating the sensitivity of the task to variations in memory load. Working memory capacity (match accuracy) was significantly associated with variation in the −1021 C/T SNP of the DBH gene. Accuracy was higher for the C/T than for the C/C genotype, and higher still for the T/T genotype. The effect size of the −1021 C/T on working memory accuracy was greatest at the highest memory load. The genetic association is noteworthy because 444 G/A and −1021 C/T are so-called functional SNPs, meaning that they directly influence protein and amino acid production. The resulting product, the DβH enzyme, can be measured in blood samples. Cubells and colleagues (1998) first reported that the 444 G/A polymorphism of the DBH gene influences levels of the DβH enzyme in plasma. In a more recent study, however, Cubells and Zabetian (2004) found that the -1021 C/T SNP had a much greater effect on plasma levels of DβH. The CC allele combination of this SNP leads to a 10-fold increase in plasma DβH levels compared to only 2-fold for the 444 G/A SNP. Given an association between PFC dopamine levels and working memory, therefore, the CC genotype should be associated with poorer and the TT genotype with superior working memory capacity, respectively, as was found.
Time-stressed decision-making
Genetic associations between dopaminergic genes and working memory have typically been demonstrated for laboratory cognitive tasks, as in the DBH study described previously, or in studies of the COMT gene (Goldberg and Weinberger, 2004). The decisions that participants have to make in these laboratory tasks do not capture the complexities of performance in naturalistic settings. Can similar genetic associations be observed for more complex decision-making tasks? To examine this question, we used a simulated battlefield engagement task that we had previously developed for research examining decision-making under time stress (Rovira et al., 2007). Given the putative role of the dopaminergic system in decision-making, we focused on the same two SNPs of the DBH gene, 444 G/A and −1021 C/T.
The decision making task was a “sensor to shooter targeting system” showing a terrain view with enemy units, friendly artillery and battalion units, and a headquarters unit (see Figure 6). Participants were required to identify the most threatening enemy target in the terrain view and to select a corresponding friendly unit to engage the target within 10s, based on military rules for engagement. This task was carried out either manually or with automation support (a decision aid). Participants were free to follow the automation directive or to choose their own target to engage. The automation was not perfect, however, having an overall reliability of 80%. Rovira et al. (2007) showed that decision aiding speeded up engagement times, and hence reduced the overall sensor-to-shooter time. However, when the automation gave incorrect recommendations, decision-making accuracy was reduced, as participants tended to go along with the automated directives, a tendency that like other decision heuristics and biases (Tversky and Kahneman, 2004) has been called “automation bias” (Mosier et al., 1998).
Figure 6.
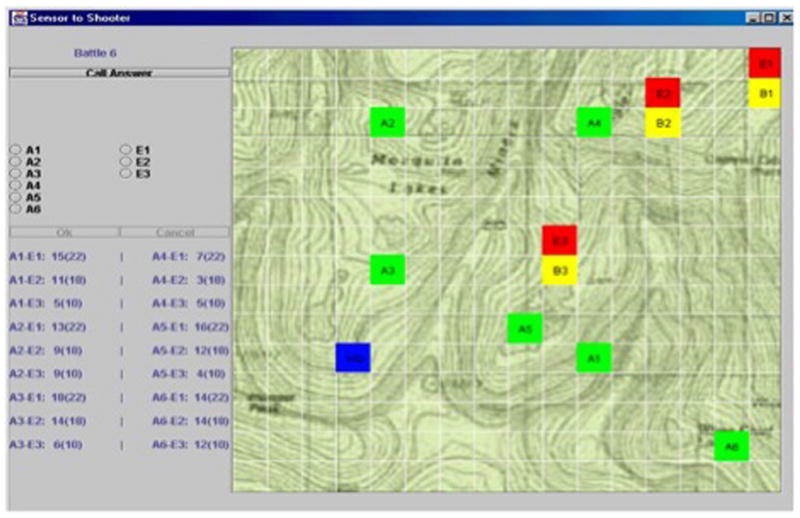
Simulated command and control task requiring decision-making under time pressure. Display shows a “sensor to shooter targeting system” with a terrain view containing enemy units, (red), friendly artillery and battalion units (green), and a headquarters unit (blue). Participants had 10 s to identify the most threatening enemy target and select a corresponding friendly unit to engage it, based on military rules of engagement.
The behavioral results reported by Rovira et al. (2007) were obtained in a task that was representative of military decision-making. In a further examination of the neurogenetic mechanisms underlying performance in this task, we investigated whether the DBH genotype would be associated with decision-making performance in this task both when the automation was reliable and when it was imperfect. Participants were genotyped for the 444 G/A and −1021 C/T SNPs of the DBH gene and divided into two groups. The low DβH enzyme activity group (genotype) included participants with the A/A and T/T combination of alleles of the two SNPs. The high enzyme activity group (genotype 2) included participants with the GA and C/C or G/G and C/C combinations of the two SNPs. We predicted that decision-making performance would be superior in the low (genotype 1) compared to the high enzyme activity group (genotype 2). This prediction was confirmed. Decision time was significantly shorter in the genotype 1 group than in the genotype 2 group, and was also faster with decision aiding than without (see Table 1). The effect of DBH genotype was observed both for unaided decision-making performance and under decision aiding. Overall, the low enzyme activity genotype group was about 24% faster in making target engagement decisions than the high enzyme activity group.
Table 1.
| Genotype Group | No Decision Aid | With Decision Aid |
|---|---|---|
| 1 - Low DβH activity | 7.31 (0.32) | 4.92 (0.42) |
| 2 - High DβH activity | 8.82 (0.45) | 7.34 (0.534) |
Mean times (s) for target engagement decisions. Standard errors are shown in parentheses.
DβH = dopamine beta hydroxylase.
There was no difference in overall decision accuracy as a function of DBH genotype, but performance on incorrect automation trials did differ with genotype. Consistent with Rovira et al. (2007), whereas decision-making accuracy was close to 100% when the decision aid was correct, performance was significantly poorer on the 20% of trials when the automated recommendation was incorrect. However, the genotype 1 group maintained significantly higher performance despite imperfect automated advice, at 96% correct in that condition vs. 82% for the genotype 2 group. Thus, possession of DBH genotypes associated with low activity of the DβH enzyme (higher dopamine/norepinephrine ratio) confirmed benefits not only for speeded decision making under time stress, but also for improved accuracy when given incorrect advice by an imperfect decision aid. Finally, emotional reactions to performing this complex decision-making task under time pressure were assessed using the Dundee Stress State Questionnaire (Matthews et al., 1999) and the NASA TLX subjective workload scale (Hart and Staveland, 1988). Whereas the genotype 1 group reported greater task engagement and low frustration after task completion, the genotype 2 group reported greater worry, distress, and frustration. These differential affective responses could also reflect trait differences in response to the task challenge, which would suggest an overlap between the effects of dopaminergic/noradrenergic genes on working memory, decision-making, and stress responses (Hess et al., 2009). We consider next such affective processes and their interaction with cognition.
Individual Differences in Affective Processing and Decision-Making
One major factor in individual variation in decision-making is differences in the ability of individuals to consider many alternative hypotheses in working memory. Thus, to the extent that genetic sources of variation in working memory capacity can be identified, we can also say something about individual differences in decision-making efficiency. However there is more to decision making than weighing options in working memory. Moreover, decision-making should not be considered solely from a “cold cognition” perspective, in which the accuracy or timeliness of decisions are linked to components of cognitive processing. A large body of research has clearly pointed to the important role that emotion plays in decision-making (Bechara, 2004; Damasio, 1994; Pfister and Bohm, 2008). Impairment in decision-making is often observed following damage to brain regions (e.g, ventromedial frontal cortex) subserving emotional experience, areas that are also generally involved in emotion regulation in healthy adults.
The process of decision-making can be divided into three major phrases: options assessment, behavioral output, and outcome evaluation (Ernst and Paulus, 2005; Paulus, 2005). The impact of affect is manifest in all three phases. Affect can influence the output of decision-making by providing heuristic information (Quartz, 2009; Slovic and Peters, 2006), by reinforcing and facilitating signal processing (Pessoa, 2009; Seymour and Dolan, 2008), by increasing the framing (i.e. wording) effect of decision choices (Fagley et al. 2010), or by directly interfering with the process of reflexes or reacting with the approach-avoidance response (Winkielman et al., 2005). Importantly, affective processes should not simply be considered as purely modulatory influences on these phases, but as an integral part of them (Pfister and Bohm, 2008).
Affective processing can influence decision making automatically, without conscious perception (Bechara, 2007). Thus, both automatic (unconscious) and conscious affective processing need to be taken into account in assessing performance in decision-making and other complex cognitive tasks. Accordingly, we first examine the phenomenon of affective priming, which has been extensively used to examine the automatic aspect of affective processing. We then briefly discuss three areas of individual differences in affective processing in relation to cognition and performance: anxiety, sensation seeking, and boredom susceptibility.
Affective decisions and visual processing style
Automatic processing of the affective valence of a stimulus can have a rapid influence on performance, such as speeding up reaction times to immediately following events. A good example comes from priming studies using the “evaluative decision” task (Fazio, 2001; Klauer and Musch, 2003; Frings and Wentura, 2008). In this task the time needed to evaluate a target item as either “happy” or “sad” is shorter when the preceding prime is affectively congruent with the subsequent target (e.g., “love-win”) than when prime-target pairs are affectively incongruent (e.g., “hate-win”).
ERPs can reveal the temporal characteristics of the neural mechanisms underlying affective priming in the evaluative decision task. Affectively incongruent word-word pairs elicit larger ERP responses for the N400 and late positive potential (LPP) components than do affectively congruent trials (Zhang, et al., 2006), which are associated with longer decision times in determining affective valence. The affective priming effect can also occur across domains (e.g., picture-word pairs). Zhang et al. (2010) conducted an ERP study with 480 such picture-word pairs presented 150 ms or 250 ms apart in time. Participants took significant longer to make valence decisions for incongruent than congruent pairs at either interval. The N400 effect (larger N400 for incongruent pairs) occurred at anterior scalp regions at the 150 ms interval. In contrast, the LPP, associated with attentional resource allocation (Johnson, 1986) occurred broadly across the scalp at the later interval of 250 ms. The ERP signatures at very short intervals provide neural evidence that affective pictures exert an automatic influence on the evaluation of affective target words.
At the group level, reaction times of evaluating cross-domain affective priming (visual pictures - verbal words) are longer than those of within-domain affective priming (verbal-verbal; Zhang et al., 2006). These affective priming results suggest that evaluative decisions might be linked to inter-individual variation in the way that people process images and verbal material. Psychometric evidence indicates that individuals differ in visual and verbal processing style: some are “visual “ and others “verbal” (Childers et al., 1985). In visual individuals, visual affective images are processed faster than words (Childers and Jiang, 2008). Thus depending on individual differences, there may be preferred brain pathways to process visual and cognitive information. This view is illustrated in Figure 7. Converging evidence supports the idea that the primary role of the amygdala in visual processing is to coordinate the function of cortical networks during evaluation of the biological significance of affective visual stimuli (Pessoa and Adolphs, 2010). The model links speedier transmission of visual information between the visual cortex and emotional processing in the amygdala for “visual” individuals, independent of whether participants make cognitive-evaluative judgments or not. For “verbal” people, the model proposes that preferred information processing associated with affective evaluation is faster via this frontal word processing-amygdala pathway.
Figure 7.
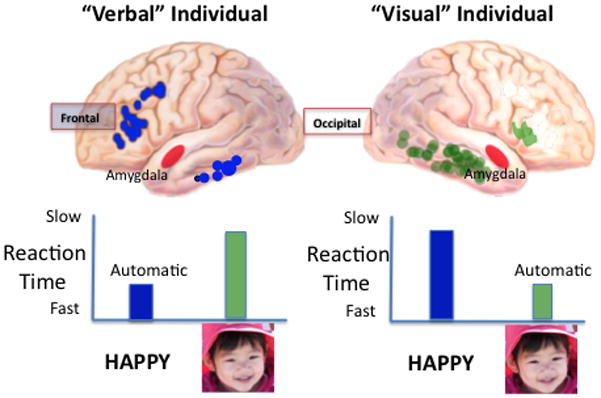
A model of individual differences in preferred processing style. A “verbal” individual prefers words and a more automatic information processing style. Hence when materials or emotional stimuli are presented in verbal form, reaction times are faster than when presented as images. The emotional center of the brain (amygdala in red) is proposed to be reached faster via the visual cortex in a “visual” individual. Modified from Childers and Jiang (2008).
Anxiety
Highly anxious people are known to be more likely to be risk-avoidant in economic decision-making, but the underlying mechanisms are not well understood (Eisenberg et al., 1998; Mitte, 2007). In a recent study, participants were given four kinds of possible feedback in a gambling task following their decision on a trial: positive, negative, neutral and ambiguous. Gu et al. (2010) reported that the amplitude of the feedback-related negativity (FRN), an ERP component associated with the outcome evaluation phase, was negatively correlated with levels of anxiety when the outcome was negative, but positively correlated with anxiety when the outcome was ambiguous. In contrast, the positive and neutral outcomes were not sensitive to anxiety. The FRN represents a binary categorization of the outcomes as either “good” or “not good” (Hajcak et al., 2007; Holroyd et al., 2006). Highly anxious individuals evaluate negative and ambiguous outcomes in different ways from low-anxious people (Gu et al., 2010).
Boredom susceptibility and sensation seeking
Individual differences in sensation seeking and its components, including boredom susceptibility and experience seeking, have been linked to differential brain functions related to behavioral or task performance. Using MRI, Martin et al. (2007) reported that individuals who scored high in experience seeking showed larger size of hippocampus. Boredom susceptibility (BS) is a subcomponent of sensation seeking (Zuckerman, 1994). High BS scores reflect a person’s tendency to be bored quickly. Repeated experience is not a natural tendency for such individuals. On the other hand, low sensation seeking individuals tend to enjoy repetitions of a familiar experience and tend to agree more with statements such as “I enjoy spending time in the familiar surroundings of home.”
Self-report scores on the BS subscale are good predictors of frontal ERP response latency. Individuals with high BS scores showed delayed frontal LPP responses during repeated visual exposure (Jiang et al., 2009). They were asked to make repeated judgments on a set of everyday pictures, deciding whether they were living (e.g. fish) or nonliving (e.g. umbrella). During visual stimulus adaptation, the ventral prefrontal cortex showed lack of frontal involvement among high sensation seekers, using the sLORETA (standardized low resolution brain electromagnetic tomography; Pascual-Marqui, 2002) EEG source localization method (see Figure 8).
Figure 8.
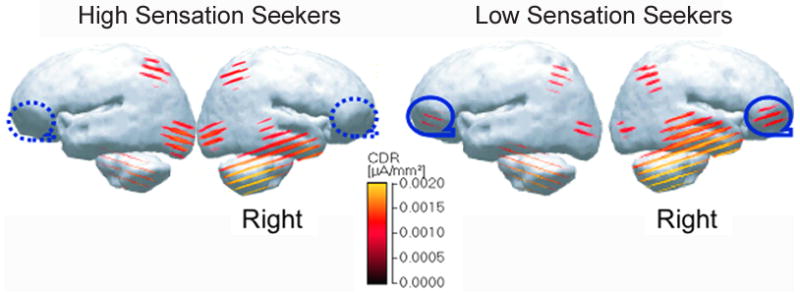
Inferior frontal activity of low sensation seekers, and lack of frontal responses in high sensation seekers during repeated visual experience (see circled area). Low-resolution current density reconstructions (CDRs) based on the sLORETA model using a color scale for CDRs as a source of designated time points from 444–500ms, which revealed the largest group differences in ERP responses. Modified from Jiang et al. (2009).
Discussion
This article began with a hypothetical example of physicians having to make a critical decision in a stressful situation. We asked if it is possible to explain variations in the effectiveness with which different individuals reach correct decisions in emotion-laden conditions in such work environments—whether in medicine, the military, or in other everyday situations. Studies using behavioral, fMRI, ERP, and molecular genetic methods—when used to examine the cognitive functions of attention, working memory, and decision-making and the affective processes of anxiety, sensation seeking, and boredom—can systematically describe individual differences in these functions, both in isolation and in interaction with each other. Importantly, inter-individual variability must be examined from multiple levels of analysis—gene, protein, neurotransmitter, brain networks, cognition, and performance, and cannot be reduced to any one of these levels (Parasuraman, 2003). Examples from each of these domains of cognition and emotion, and using all of these classes of methods, clearly show that analyses at the group level often mask important findings associated with sub-groups of individuals. Psychometric and molecular genetic methods can be used to identify such sub-groups. There is also growing interest in combining these two approaches. Studies using these diverse approaches show that group-level models of affect and cognition do not apply to all individuals, thus posing challenges for theory. A next step in theory development will involve the integration of the new psychometric and genetic information with neuroimaging data into more inclusive theories of cognition and affect.
We considered first individual differences in working memory and decision-making. Molecular genetic studies of individual variation in these functions point to variability in dopaminergic and noradrenergic genes. Given that working memory capacity is a key factor contributing to decision-making efficiency under time pressure, both may be mediated by overlapping molecular pathways. The experimental results indicate that a gene that regulates dopamine and norepinephrine availability, namely the DBH gene, plays a role in both functions. Specifically, variants of the DBH gene producing low levels of the DβH enzyme that converts dopamine to norpepinephrine (Cubells and Zabetian, 2004) were associated with both high working memory capacity and superior decision making in a simulated battlefield command and control task, as well as altered subjective stress response (Parasuraman, 2009). More importantly, the DBH gene also influenced the degree to which decision-making performance was adversely affected by imperfect decision aiding, with individuals with the low DβH enzyme variant showing less susceptibility. The modulation of the effects of a decision aid by genotype represents an example of a gene by task interaction. This represents a specific case of a gene-environment interaction. As Szalma (2009) pointed out, inter-individual variability in cognitive performance often reflects the interaction between person and task factors, rather than these factors in isolation. Future research on the cognitive effects of the DBH and other genes should systematically examine such interactive effects.
In terms of practical applications of these findings, since decision automation is increasingly used in many work domains (Parasuraman et al., 2000), these results carry implications for the design of these aids and for training individuals to use them effectively. Other implications for research and practice in human factors include providing practitioners with information on individual variation in decision-making so as to guide work design (Furham, 1992) or for adapting interfaces to individual workers (Szalma, 2009). Molecular genetic methods can also inform selection and training procedures aimed at developing teams of human operators who can make speedy, unbiased decisions in semi-automated systems (Parasuraman, 2009). However, additional work needs to be done using neuroergonomic methods in more complex tasks representative of naturalistic decision-making, such as decisions made by consumers, jury members and judges, physicians, pilots, and military commanders.
Several affective processes play integral roles in decision-making. We discussed three areas of individual differences in affective processing in relation to decision-making and cognitive performance: affective evaluation, anxiety, and boredom susceptibility. Firstly, the influence of affective processes on decision-making can occur both consciously and automatically (Bechara, 2007). The automatic aspect of affective processing is apparent in the phenomenon of affective priming. Results from affective priming studies indicate that evaluative decisions about words are influenced by automatically processed affective information presented between 150–250 ms before the decision stimulus, as reflected in both the LPP and N400 components of the ERP (Zhang et al., 2006; 2010). Affective priming can also occur across stimulus domains (pictures and words), but the impact on evaluative decision-making differs as a function of processing style between individuals. A psychometrically validated scale (Childers et al., 1985) indicates that some individuals are “visual” while others are “verbal”. In visual individuals, visual affective images are processed faster than words (Childers and Jiang, 2008). Such differences in processing style could be used to assess everyday decision-making, as in consumers making choices of items to purchase. In a recent study, ERPs were measured as consumers made buying decisions for items presented as “on-sale” (e.g., a 15% reduction) or simply at regular price. Individuals with low-math anxiety had larger P300 responses than high-anxiety persons when making their non-buy purchasing decisions (Jones et al, 2011). P300 had a more fronto-central distribution for high-math anxiety individuals whereas it was more centro-posterior for low-math anxietypersons. Thus, it is possible to identify neural correlates of individual differences in affect and cognition that influence performance in tasks that capture some of the characteristics of everyday decision-making.
Anxiety clearly impairs performance on many other cognitive tasks, although the effects tend to be most robust for tasks with working memory or multitasking components (Eysenck, 1992). Trait anxiety also impairs economic decision-making, and some of the underlying mechanisms can be revealed using ERPs. For example, the FRN component of the ERP, which reflects outcome evaluation in the Iowa Gambling task, differentiates between individuals low and high in trait anxiety (Gu et al., 2010). This finding suggests that anxiety involves a conscious strategy of evaluation of decisions, and is particularly debilitating when outcomes are ambiguous. Of course, this does not rule out the view that anxiety also impairs decision-making automatically, as studies using masked stimuli in the affective priming (Li et al., 2008) and “emotional Stroop” paradigms (Gotlib et al., 1984) have shown.
Individual differences in the interactive effects between anxiety and the cognitive processes of working memory and decision-making may be influenced by genetic factors in dopaminergic molecular pathways. A “worrier vs. warrior” model has been proposed to account for such individual differences between cognition and affect (Goldman et al., 2005). Whereas “worriers” with the COMT (Met158) variation may have high working memory (Goldberg and Weinberger, 2004), they also tend to exhibit high trait anxiety. In contrast, those with the COMT (Val158) variation have the opposite pattern, not high in working memory performance but with little or no anxiety. This “warrior” genotype has better stress resiliency but also modest diminution of executive cognitive performance.
Thirdly, individual differences in sensation seeking and its sub-component boredom susceptibility also play a role in cognitive performance. Brain activation patterns during a matching task differentiate between individuals low and high sensation seeking (Jiang et al., 2009). Boredom susceptibility is also associated with poor performance on repetitive tasks. Not surprisingly, participants with trait boredom do poorly on long-duration vigilance tasks (Davies et al., 1983; Thackray et al., 1973). Such tasks typically induce stress and fatigue (Szalma et al., 2004), which is exacerbated in individuals with high boredom susceptibility. Given that many modern work environments, such as airport security screening (McCarley et al., 2004; Wolfe et al., 2005) and military sonar monitoring (Arabito et al., 2007) involve vigilance, boredom susceptibility can negatively impact performance in many industrial tasks (Smith, 1981). Trait boredom is also associated with job dissatisfaction and absenteeism in manufacturing (Kass et al., 2001) and healthcare (Watt and Hargis, 2009).
Finally, some limitations of the neuroergonomic approach we have outlined must be acknowledged. Candidate gene studies have been criticized because of failure to replicate reported associations, although this has been primarily in the context of neuropsychiatric disorders (Iannonidis et al., 2001). The purported solution—studies examining variation across the entire genome—is expensive due to the need for very large samples, and the results to date have not offered significant improvement. A compromise in which both GWAS and candidate gene studies with multiple phenotypes are conducted in a theory-driven manner appears to be a measured approach (Reinvang et al., 2010). Another limitation of the present review is that much—but not all—of the evidence discussed has not involved examination of brain function and performance in ecologically valid simulations of work or everyday environments, which is one goal of neuroergonomics (Parasuraman and Rizzo, 2008). But some data from such studies were discussed, and the methods described here could be extended to more complex situations representative of real-world performance.
Conclusions
To return to the opening example: is there a basis for systematic description of variation in the decision-making performance of different physicians? The studies reviewed in this paper suggest that it may be possible to do so, although much additional validation research would need to be conducted. Individual differences in working memory can be assessed using fMRI and by variations in noradrenergic/dopaminergic genes such as DBH, which has also been linked to decision-making. ERPs and psychometric correlates of anxiety, sensation seeking, and boredom, coupled with their genetic correlates, can be identified. Such evidence could be used for selection and training of physicians so that the variability noted in the opening example can be reduced, resulting in better outcomes for the patient.
In summary, the neuroergonomic approach to examining brain function and human performance in work and everyday environments can be usefully applied to understanding individual differences in cognition, affect, and performance. The results of studies using behavioral, neuroimaging (including fMRI and ERPs), and molecular genetic methods, have provided insights into the sources of variability in working memory, decision-making and the affective processes that interact with these cognitive functions. Findings from complex simulations of military command and control as well as everyday decision-making indicate that the approach we have described can be applied to complex, naturalistic behavior. In addition, as the other papers in this special issue attest, more such studies are being conducted. As an interdisciplinary endeavor, neuroergonomics will continue to benefit from and grow alongside developments in neuroscience, psychology, engineering, and other fields. The basic enterprise of human factors—how humans design, interact with, and use technology—can be considerably enriched if we also consider the human brain that makes such activities possible. There have already been some achievements in basic research and application in neuroergonomics. The future should yield more such advances.
Acknowledgments
Supported by AFOSR/AFRL grant FA9550-10-1-0385 and the Center of Excellence in Neuroergonomics, Technology, and Cognition (CENTEC) to RP and NIH grant P50 DA 05312 to the Center for Drug Abuse Research Translation (CDART) at the University of Kentucky. The authors thank CENTEC colleagues for comments and discussion and Ruolei Gu and Sarah Wing for assistance in the manuscript preparation.
References
- Ando J, Ono Y, Wright MJ. Genetic structure of spatial and verbal working memory. Behav Gen. 2001;31:615–624. doi: 10.1023/a:1013353613591. [DOI] [PubMed] [Google Scholar]
- Arrabito GR, Abel SM, Lam K. Methods for mitigating the vigilance decrement in an auditory sonar monitoring task: A research synthesis. Canad Acoust. 2007;35:15–24. [Google Scholar]
- Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AF. The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology. 2000;23:240–249. doi: 10.1016/S0893-133X(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafò MR. Meta-analysis of the cognitive effects of the catechol-O- methyltransferase gene Val158/108Met polymorphism. Biol Psychiat. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A. The neurology of emotions and feelings, and their role in behavioral decisions. In: Parasuraman R, Rizzo M, editors. Neuroergonomics: The Brain at Work. Oxford University Press; New York, NY: 2007. pp. 178–192. [Google Scholar]
- Bechara A, Damásio AR, Damásio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. Genome-wide association studies in Alzheimer’s disease. Hum Mol Genet. 2009;18(R2):137–145. doi: 10.1093/hmg/ddp406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaduri N, Mukhopadhyay K. Correlation of plasma dopamine beta-hydroxylase activity with polymorphisms in DBH Gene: A study on eastern Indian population. Cell Mol Neurobiol. 2008;28:343–350. doi: 10.1007/s10571-007-9256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigos K, Weinberger D. Imaging genetics: Days of future past. NeuroImage. 2010;53:804–809. doi: 10.1016/j.neuroimage.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: Controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Butcher LM, Davis OS, Craig IW, Plomin R. Genome-wide quantitative trait locus association scan of general cognitive ability using pooled DNA and 500K single nucleotide polymorphism microarrays. Genes Brain Behav. 2008;7:435–446. doi: 10.1111/j.1601-183X.2007.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiano D, Jiang Y, Parasuraman R. Aging and repetition priming for targets and distracters in a working memory task. Aging Neuropsychol Cogn. 2006;13:552–573. doi: 10.1080/138255890969555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiano D, Parasuraman R. The role of memory representation in the vigilance decrement. Psychon Bull Rev. 2004;11:932–937. doi: 10.3758/bf03196724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers TL, Houston MJ, Heckler SE. Measurement of individual differences in visual and verbal information processing. J Consum Res. 1985;12:125–134. [Google Scholar]
- Childers TL, Jiang Y. Neurobiological perspectives on the nature of visual and verbal processes. J Consum Psychol. 2008;18:264–269. [Google Scholar]
- Cubells JF, van Kammen DP, Kelley ME, Anderson GM, O’Connor DT, Price LH, Gelernter J. Dopamine beta-hydroxylase: Two polymorphisms in linkage disequilibrium at the structural gene DBH associate with biochemical phenotypic variation. Hum Gen. 1998;102:533–540. doi: 10.1007/s004390050736. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Zabetian CP. Human genetics of plasma dopamine β hydroxylase activity: Applications to research in psychiatry and neurology. Psychopharmacology. 2004;174:463–476. doi: 10.1007/s00213-004-1840-8. [DOI] [PubMed] [Google Scholar]
- Damasio A. Descarte’s Error: Emotion, Reason, and the Human Brain. Putman; New York, NY: 1994. [Google Scholar]
- Davies DR, Shackelton VJ, Parasuraman R. Monotony and boredom. In: Hockey GRJ, editor. Stress and Fatigue in Human Performance. Wiley; London: 1983. pp. 1–32. [Google Scholar]
- De Geus R, Goldberg T, Boomsma DI, Posthuma D. Imaging the genetics of brain structure and function. Biol Psychol. 2008;79:1–8. doi: 10.1016/j.biopsycho.2008.04.002. [DOI] [PubMed] [Google Scholar]
- de Visser E, Parasuraman R. The social brain: Behavioral, computational, and neuroergonomic perspectives. In: Hayes C, Miller C, editors. Human-Computer Etiquette: Understanding the Impact of Human Culture and Expectations on the Use and Effectiveness of Computers and Technology. Taylor & Francis; New York, NY: 2010. pp. 263–288. [Google Scholar]
- Derringer J, Krueger RF, Dick DM, Saccone S, Grucza RA, Agrawal A, et al. Predicting sensation seeking from dopamine genes: A candidate-system approach. Psychol Sci. 2010;21:1282–1290. doi: 10.1177/0956797610380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg AE, Baron J, Seligman MEP. Individual differences in risk aversion and anxiety. Psychol Bull. 1998;87:245–251. [Google Scholar]
- Eley TC, Bolton D, O’Connor TG, Perrin S, Smith P, Plomin R. A twin study of anxiety-related behaviours in pre-school children. J Child Psychol Psychiat. 2003;44:945–960. doi: 10.1111/1469-7610.00179. [DOI] [PubMed] [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Curr Dir Psychol Sci. 2002;11:19–23. [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: A selective review from a neurocognitive and clinical perspective. Biol Psychiat. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Espeseth T, Endestad T, Rootwelt H, Reinvang I. Nicotine receptor gene CHRNA4 modulates early event-related potentials in auditory and visual oddball target detection tasks. Neuroscience. 2007;147:974–985. doi: 10.1016/j.neuroscience.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Espeseth T, Greenwood PM, Reinvang I, Fjell AM, Walhovd KB, Westlye LT, Parasuraman R. Interactive effects of APOE and CHRNA4 on attention and white matter volume in healthy middle-aged and older adults. Cogn Affect Behav Neurosci. 2006;6:31–43. doi: 10.3758/cabn.6.1.31. [DOI] [PubMed] [Google Scholar]
- Eysenck MW. Anxiety: The Cognitive Perspective. Hove, UK: Erlbaum Associates; 1992. [Google Scholar]
- Fagley N, Coleman JG, Simon AF. Effects of framing, perspective taking, and perspective (affective focus) on choice. Pers Indiv Diff. 2010;48:264–269. [Google Scholar]
- Fan J, Fossella JA, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of attention onto brain activity. Proc Natl Acad Sci USA. 2003;100:7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio RH. On the automatic activation of associated evaluations: an overview. Cogn Emot. 2001;15:115–141. [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nature Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, Posner MI. Assessing the molecular genetics of attention networks. BMC Neurosci. 2002;3:14–19. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage. 2008;42:1178–84. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Frings C, Wentura D. Trial-by-trial effects in the affective priming paradigm. Acta Psychol. 2008;128:318–323. doi: 10.1016/j.actpsy.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Fu S, Zinni M, Squire PN, Kumar R, Caggiano DM, Parasuraman R. When and where perceptual load interacts with voluntary visuospatial attention: An event-related potential and dipole modeling study. NeuroImage. 2008;39:1345–1355. doi: 10.1016/j.neuroimage.2007.09.068. [DOI] [PubMed] [Google Scholar]
- Furham A. Personality at Work: The Role of Individual Differences in the Workplace. Routledge; New York, NY: 1992. [Google Scholar]
- Giambra LM, Quilter RE. A two-term exponential functional description of the time course of sustained attention. Hum Factors. 1987;29:635–643. doi: 10.1177/001872088702900603. [DOI] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia. 2007;45:2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci. 2004;8:325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci Fm. The genetics of addictions: uncovering the genes, Nat. Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Goldman D, Weinberger DR, Malhotra AK, Goldberg TE. The role of COMT Val158Met in cognition. Biol Psychiatry. 2009;65:1–2. doi: 10.1016/j.biopsych.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, McCann CD. Construct accessibility and depression: An examination of cognitive and affective factors. J Pers Soc Psychol. 1984;47:427–439. doi: 10.1037//0022-3514.47.2.427. [DOI] [PubMed] [Google Scholar]
- Green AE, Munafò M, DeYoung C, Fossella JA, Fan J, Gray JR. Using genetic data in cognitive neuroscience: from growing pains to genuine insights. Nat Rev Neurosci. 2008;9:710–720. doi: 10.1038/nrn2461. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lin MK, Sundararajan R, Fryxell KJ, Parasuraman R. Synergistic effects of genetic variation in nicotinic and muscarinic receptors on visual attention but not working memory. Proc Natl Acad Sci USA. 2009;106:3633–3638. doi: 10.1073/pnas.0807891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Sunderland T, Friz JL, Parasuraman R. Genetics and visual attention: Selective deficits in healthy adult carriers of the e4 allele of the apolipoprotein E gene. Proc Natl Acad Sci USA. 2000;97:11661–11666. doi: 10.1073/pnas.97.21.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R, Ge Y, Jiang Y, Luo YJ. Anxiety and outcome evaluation: the good, the bad and the ambiguous. Biol Psychol. 2010;85:200–206. doi: 10.1016/j.biopsycho.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R, Huang YX, Luo YJ. Anxiety and feedback negativity. Psychophysiol. 2010;47:961–967. doi: 10.1111/j.1469-8986.2010.00997.x. [DOI] [PubMed] [Google Scholar]
- Guo CY, Lawson A, Zhang Q, Jiang Y. Brain potentials of new and studied objects during working memory. Hum Brain Mapp. 2008;29:441–452. doi: 10.1002/hbm.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. It’s worse than you thought: The feedback negativity and violations of reward prediction in gambling tasks. Psychophysiol. 2007;44:905–912. doi: 10.1111/j.1469-8986.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Hess C, Reif A, Strobel A, Boreatti-Hummer A, Heine M, Lesch K-P, Jacob CP. A functional dopamine-b-hydroxylase gene promoter polymorphism is associated with impulsive personality styles, but not with affective disorders. J Neural Transm. 2009;116:121–130. doi: 10.1007/s00702-008-0138-0. [DOI] [PubMed] [Google Scholar]
- Hart SG, Staveland LE. Development of the NASA-TLX (Task Load Index): Results of empirical and theoretical research. In: Hancock PA, Meshkati N, editors. Human Mental Workload. Elsevier; Amsterdam: 1988. pp. 156–170. [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Phil Trans Royal Soc London-Series B: Biol Sci. 1998;353:1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Hajcak G, Larsen JT. The good, the bad and the neutral: Electrophysiological responses to feedback stimuli. Brain Res. 2006;1105:93–101. doi: 10.1016/j.brainres.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Hutchins E. Cognition in the Wild. The MIT Press; Cambridge, MA: 1996. [Google Scholar]
- Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genetics. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Haxby JV, Martin A, Ungerleider LG, Parasuraman R. Complementary neural mechanisms for tracking familiar items in human working memory. Science. 2000;287:643–646. doi: 10.1126/science.287.5453.643. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lianekhammy J, Lawson A, Guo C, Lynam D, Joseph J, Gold BT, Kelly TH. Brain responses to repeated visual experience among low and high sensation seekers: role of boredom susceptibility. Psychiatry Res: NeuroImaging. 2009;173:100–106. doi: 10.1016/j.pscychresns.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Jr A triarchic model of P300 amplitude. Psychophysiol. 1986;23:367–384. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Jones WJ, Childers TL, Jiang Y. The Shopping Brain: Neural Correlates of Buying under Different Promotional Formats. Paper presented at the Society for Consumer Psychology; Atlanta, GA. 2011. [Google Scholar]
- Joseph J, Liu X, Jiang Y, Lynam DL, Kelly T. Neural correlates of emotional reactivity in sensation seeking. Psychol Sci. 2009;20:215–223. doi: 10.1111/j.1467-9280.2009.02283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just M, Keller TA, Cynkar J. A decrease in brain activation associated with driving when listening to someone speak. Brain Res. 2008;1205:70–80. doi: 10.1016/j.brainres.2007.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass SJ, Vodanovich SJ, Callender A. State-trait boredom: Relationship to absenteeism, tenure, and job satisfaction. J Bus Psychol. 2001;16:317–327. [Google Scholar]
- Klauer KC, Musch J. Affective priming: Findings and theories. In: Musch K, Klauer KC, editors. The Psychology of Evaluation: Affective Processes in Cognition and Emotion. Lawrence Erlbaum; Mahwah, NJ: 2003. pp. 7–49. [Google Scholar]
- Kramer A, Parasuraman R. Neuroergonomics—application of neuroscience to human factors. In: Caccioppo J, Tassinary L, Berntson G, editors. Handbook of psychophysiology. 2. Cambridge University Press; New York, NY: 2007. pp. 704–722. [Google Scholar]
- Lang KL, Livesley WJ, Vemon PA. Heritability of the big five personality dimensions and their facets: A twin study. J Personality. 1996;64:577–592. doi: 10.1111/j.1467-6494.1996.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Li W, Zinbarg RE, Boehm SG, Paller KA. Neural and behavioral evidence for affective priming from unconsciously perceived emotional facial expressions and the influence of trait anxiety. J Cogn Neurosci. 2008;20:95–107. doi: 10.1162/jocn.2008.20006. [DOI] [PubMed] [Google Scholar]
- Martin SB, Covell DJ, Joseph JE, Chebrolu H, Smith CD, Kelly TH, Jiang Y, Gold BT. Human experience seeking correlates with hippocampus volume: Convergent evidence from manual tracing and voxel-based morphometry. Neuropsychologia. 2007;45:2874–2881. doi: 10.1016/j.neuropsychologia.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cogn Ther Res. 1998;22:539–560. [Google Scholar]
- Matthews G, Joyner L, Gilliland K, Campbell SE, Huggins J, Falconer S. Validation of a comprehensive stress state questionnaire: Towards a state “Big Three”? In: Mervielde I, Deary IJ, De Fruyt F, Ostendorf F, editors. Personality Psychology in Europe. Vol. 7. Tilburg: Tilburg University Press; 1999. pp. 236–251. [Google Scholar]
- McCarley JS, Kramer AF, Wickens CD, Vidoni E, Boot WR. Visual skills in airport security screening. Psychol Sci. 2004;15:302–306. doi: 10.1111/j.0956-7976.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263:520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- Miller M, Donovan CL, Bennett CM, Elissa M, Aminoff EM, Mayer RE. Individual differences cognitive style and strategy predict similarities in the patterns of brain activity between individuals. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.05.060. this issue. [DOI] [PubMed] [Google Scholar]
- Mitte K. Anxiety and risk decision-making: The role of subjective probability and subjective cost of negative events. Pers Indiv Diff. 2007;43:243–253. [Google Scholar]
- Mosier KL, Skitka LJ, Heers S, Burdick M. Automation bias: Decision-making and performance in high-tech cockpits. Int J Aviat Psychol. 1998;8:47–63. doi: 10.1207/s15327108ijap0801_3. [DOI] [PubMed] [Google Scholar]
- Newell A, Rosenbloom PS. Mechanisms of skill acquisition and the law of practice. In: Anderson JR, editor. Cognitive Skills and their Acquisition. Erlbaum; Hillsdale, NJ: 1981. pp. 1–55. [Google Scholar]
- Parasuraman R. Memory load and event rate control sensitivity decrements in sustained attention. Science. 1979;205:924–927. doi: 10.1126/science.472714. [DOI] [PubMed] [Google Scholar]
- Parasuraman R. Neuroergonomics: Research and practice. Theor Iss Ergon Sci. 2003;4:5–20. [Google Scholar]
- Parasuraman R. Assaying individual differences in cognition with molecular genetics: theory: theory and application. Theor Iss Ergon Sci. 2009;10:399–416. [Google Scholar]
- Parasuraman R. Neuroergonomics: Brain, cognition, and performance at work. Curr Dir Psychol Sci. 2011 in press. [Google Scholar]
- Parasuraman R, Caggiano D. Mental workload. In: Ramachandran VS, editor. Encyclopedia of the Human Brain. Vol. 3. Academic Press; San Diego, CA: 2002. pp. 17–27. [Google Scholar]
- Parasuraman R, Espeseth T. Genetic and neuroimaging studies of cholinergic and neurotrophic modulation of visual attention. Prog Nat Sci. 2007;17:7–18. [Google Scholar]
- Parasuraman R, Giambra L. Skill development in vigilance: Effects of event rate and age. Psychol Aging. 1991;6:155–169. doi: 10.1037//0882-7974.6.2.155. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM. Molecular genetics of visuospatial attention and working memory. In: Posner MI, editor. Cognitive Neuroscience of Attention. Guilford; New York, NY: 2004. pp. 245–259. [Google Scholar]
- Parasuraman R, Greenwood PM, Kumar R, Fossella J. Beyond heritability: Neurotransmitter genes differentially modulate visuospatial attention and working memory. Psychol Sci. 2005;16:200–207. doi: 10.1111/j.0956-7976.2005.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman R, Rizzo M. Neuroergonomics: The Brain at Work. Oxford University Press; New York, NY: 2008. [Google Scholar]
- Parasuraman R, Sheridan TB, Wickens CD. A model for types and levels of human interaction with automation. IEEE Trans Sys Man Cyber Part A. 2000;30:286–297. doi: 10.1109/3468.844354. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Wilson GF. Putting the brain to work: Neuroergonomics past, present and future. Hum Factors. 2008;50:468–474. doi: 10.1518/001872008X288349. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;24D:5–12. [PubMed] [Google Scholar]
- Paulus MP. Neurobiology of decision-making: Quo vadis? Cogn Brain Res. 2005;23:2–10. doi: 10.1016/j.cogbrainres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Payne JW, Bettman JR, Walrath LC. The Adaptive Decision Maker. Cambridge University Press; Cambridge, UK: 1993. [Google Scholar]
- Payton A. The impact of genetic research on our understanding of normal cognitive ageing: 1995 to 2009. Neuropsychol Rev. 2009;19:451–477. doi: 10.1007/s11065-009-9116-z. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister HR, Bohm G. The multiplicity of emotions: A framework of emotional functions in decision making. Judgment Dec Mak J. 2008;3:5–17. [Google Scholar]
- Plomin R, Crabbe J. DNA. Psychol Bull. 2000;26:806–828. doi: 10.1037/0033-2909.126.6.806. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Desmond JE, Glover GH, Gabrieli JD. The neural basis of visual skill learning: an fMRI study of mirror reading. Cereb Cortex. 1998;8:1–10. doi: 10.1093/cercor/8.1.1. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE. Attention genes. Develop Sci. 2007;10:24–29. doi: 10.1111/j.1467-7687.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- Powell JE, Zietsch BP. Predicting sensation seeking from dopamine genes: Use and misuse of genetic prediction. Psychol Sci. 2011 Jan 26;2011 doi: 10.1177/0956797610397669. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Quartz SR. Reason, emotion and decision-making: risk and reward computation with feeling. Trends Cogn Sci. 2009;13:209–215. doi: 10.1016/j.tics.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinvang I, Deary IJ, Fjell AM, Steen VM, Espeseth T, Parasuraman R. Neurogenetic effects on cognition in aging brains: a window of opportunity for intervention? Frontiers Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rovira E, McGarry K, Parasuraman R. Effects of imperfect automation on decision making in a simulated command and control task. Hum Factors. 2007;49:76–87. doi: 10.1518/001872007779598082. [DOI] [PubMed] [Google Scholar]
- Russell R, Duchaine B, Nakayama K. Super-recognizers: People with extraordinary face recognition ability. Psychon Bull Rev. 2009;16:252–257. doi: 10.3758/PBR.16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251(4996):947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, Dolan R. Emotion, decision making, and the amygdala. Neuron. 2008;58:662–671. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Slovic P, Peters E. Risk perception and affect. Curr Dir Psychol Sci. 2006;15:322–325. [Google Scholar]
- Smith RP. Boredom: A review. Hum Factors. 1981;23:329–340. doi: 10.1177/001872088102300308. [DOI] [PubMed] [Google Scholar]
- Stein MB, Jang KL, Livesley WJ. Heritability of anxiety sensitivity: A twin study. Am J Psychiat. 1999;156:246–251. doi: 10.1176/ajp.156.2.246. [DOI] [PubMed] [Google Scholar]
- Stevenson J, Batten N, Cherner M. Fears and fearfulness in children and adolescents: a genetic analysis of twin data. J Child Psychol Psychiat. 1992;33:977–85. doi: 10.1111/j.1469-7610.1992.tb00919.x. [DOI] [PubMed] [Google Scholar]
- Sundararajan R, Fryxell KJ, Lin M, Greenwood PM, Parasuraman R. Comparison of the effects of two SNPs in the DBH gene on working memory. Soc Neurosci Abst 2006 [Google Scholar]
- Szalma JF. Individual differences in human technology interaction: incorporating variation in human characteristics into human factors and ergonomics research and design Theor. Iss Ergon Sci. 2009;10:381–397. [Google Scholar]
- Szalma JL, Warm JS, Matthews G, Dember WN, Weiler E, Meier A, Eggemeier FT. Effects of sensory modality and task duration on performance, workload, and stress in sustained attention. Hum Factors. 2004;46:219–233. doi: 10.1518/hfes.46.2.219.37334. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R. Failing to deactivate: The association between brain activity during a working memory task and creativity. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2010.11.052. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Taubert M, Draganski B, Anwander A, Müller K, Horstmann A, Villringer A, Ragert P. Dynamic properties of human brain structure: Learning-related changes in cortical areas and associated fiber connections. J Neurosci. 2010;30:11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray RI, Jones KN, Touchstone RM. Self-estimates of distractibility as related to performance decrement on a task requiring sustained attention. Ergonomics. 1973;16:141–52. doi: 10.1080/00140137308924490. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Judgement under uncertainty: Heuristics and biases. Science. 1974;185:1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- Van Horn JD, Grafton ST, Miller MB. Individual variability in brain activity: A nuisance or an opportunity? Brain Imag Behav. 2008;2:327–334. doi: 10.1007/s11682-008-9049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsten TS, Barton C. Processing multidimensional information for decisions. J Exp Psychol: Learn Mem Cogn. 1982;8:361–384. [Google Scholar]
- Watson JM, Strayer D. Supertaskers: Profiles in extraordinary multitasking ability. Psychon Bull Rev. 2010;17:479–485. doi: 10.3758/PBR.17.4.479. [DOI] [PubMed] [Google Scholar]
- Watt JD, Hargis MB. Boredom proneness: Its relationship with subjective underemployment, perceived organizational support, and job performance. J Bus Psychol. 2009;25:163–174. [Google Scholar]
- Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, et al. Autism Consortium. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- Wickens CD, Hollands JG. Engineering psychology and human performance. 3. Longman; New York, NY: 2000. [Google Scholar]
- Wickens CD, McCarley JS. Applied attention theory. CRC Press, Taylor and Francis; New York, NY: 2007. [Google Scholar]
- Winkielman P, Berridge KC, Wilbarger JL. Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Pers Soc Psychol Bull. 2005;31:121–135. doi: 10.1177/0146167204271309. [DOI] [PubMed] [Google Scholar]
- Wolfe JM, Horowitz TS, Kenner NM. Rare items often missed in visual searches. Nature. 2005;435:439–440. doi: 10.1038/435439a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Lawson A, Guo CY, Jiang Y. Electrophysiological correlates of visual affective priming. Brain Res Bull. 2006;71:316–323. doi: 10.1016/j.brainresbull.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Li X, Gold BT, Jiang Y. Neural correlates of cross-domain affective priming. Brain Res. 2010;1329:142–151. doi: 10.1016/j.brainres.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral Expressions and Biosocial Bases of Sensation Seeking. Cambridge University Press; Cambridge, MA: 1994. [Google Scholar]