Chemistry
Chemistry- Asymmetric Synthesis >>Chiral Building BlocksChiral Catalysts, Chiral Ligands and Chiral ReagentsChiral Resolution ReagentsBoronic acids/esters >>Aliphatic BoronsAromatic BoronsOther BoronsOxaborolesCatalysis Chemistry >>Achiral Crown LigandsC-H ActivationCarbon-Donor LigandsChiral Carbon LigandsChiral Crown LigandsChiral Olefin LigandsCross-CouplingCross-Coupling Using Transition Metal CatalystsDACH or Trost LigandsChemical Biology >>Conjugation Chemistry
- GlycoscienceLinkers and CrosslinkersNucleic Acid ChemistryPEGylationPeptide ChemistryPhotolabile Protecting GroupHeterocyclic Building Blocks >>AcridinesAliphatic HeterocyclesAromatic HeterocyclesAzetidinesBenzimidazolesBenzisoxazolesBenzodioxansBenzofuransBenzothiazolesInorganic reagents >>Inorganic ReagentOrganic Building Blocks >>Acid AnhydridesAcyl Chlorides
- AlcoholsAldehydesAliphatic Chain HydrocarbonsAliphatic Cyclic HydrocarbonsAlkenesAlkylsAlkynesOrganometallic Reagents >>Grignard ReagentsOrganoarsenicOrganobismuthOrganoboronOrganogermaniumOrganolithiumOrganomercuryOrganosiliconOrganotinSpecialty Synthesis >>Enzyme-Mediated SynthesisFluorous SynthesisIonic Liquids
- Solid Supported SynthesisStains and Dyes >>Acid DyesAzoic DyesBasic DyesDirect DyesDisperse DyesDye IntermediatesMordant DyesOil DyesOther Stains and DyesSynthetic Reagents >>Acids and BasesC-C Bond FormationC-X Bond Formation (Halogen)C-X Bond Formation (Non-Halogen)Chelation and Complexation CompoundsCondensation AgentsCouplingDehydrating ReagentsFluorination Reagent

 Pharmaceutical Intermediates
Pharmaceutical Intermediates- Analgesics >>BenzhydrocodoneBicifadineCapsaicinCelecoxibDebio-0827DexamethasoneElagolix SodiumEsketamine HydrochlorideIbuprofen SodiumAnesthetics >>CiprofolEsketamine HydrochlorideFospropofol Fospropofol DisodiumLevobupivacaine RemimazolamRemimazolam BesilateRemimazolam BesylateRopivacaineAnti-Addiction Agents >>Kakonein
- Lofexidine HydrochlorideVarenicline Anti-inflammatory Agents >>ApremilastAsunaprevirATB-346 RelatedBalsalazide BaricitinibBencycloquidium BromideBudesonideCefiderocol Sulfate TosylateCeftaroline Fosamil AcetateAntibacterials >>AFN-1252 RelatedAlatrofloxacin Bedaquiline FumarateBesifloxacin BrilacidinCaspofungin AcetateCefiderocol Sulfate TosylateCefilavancinCeftaroline Fosamil
- Anticonvulsants >>BrivaracetamCannabidiolEscitalopram OxalateEslicarbazepine Acetate RelatedFosphenytoinGabapentin EnacarbilJNJ-10234094LacosamideLevetiracetam RelatedAntidementia Agents >>Azeliragon RelatedDavunetideDonepezil HydrochlorideDonepezil RelatedEnsaculinFlorbetaben Flortaucipir F 18Flutemetamol IdalopirdineAntidepressants >>4-Chlorokynurenine
- AgomelatineAgomelatine RelatedAllopregnanolone RelatedAmibegronAmitifadineAripiprazole RelatedBasimglurantBefloxatoneAntiemetics >>AprepitantAprepitant RelatedDolasetron RelatedDolasetron Mesylate HydrateFosaprepitant DimeglumineFosaprepitant RelatedOlanzapinePalonosetron RelatedPalonosetron HydrochlorideAntifungals >>Anidulafungin RelatedCabotegravirMore >>

 Inhibitors/Agonists
Inhibitors/Agonists- ADC >>ADC AntibodyADC LinkerADC Linker with PayloadADC ToxinAntibody-Drug Conjugates (ADCs)Drug-Linker Conjugates for ADCAngiogenesis >>ALKBcr-AblBTKEGFRFAKFGFRFLT3HER2HIFAnti-infection >>3CLproAntibacterialAntibioticAntifungal
- AntiparasiticAntiprotozoalAntiviralArenavirusBVDVApoptosis >>Apoptosis InducerBcl-2Bcl-6BRKc-Mycc-RETC/EBPCARDCaspaseAutophagy >>Atg4ATG4BATTECsAUTACsAutophagyBeclin1
- FKBPLC3LRRK2Biochemical Reagent >>Cell Cycle >>AntifolateAPCAurora KinaseBUBCasein KinaseCdc25CDKChkCRISPR/Cas9Cytoskeleton >>ActinArp2/3 ComplexCYTHCytoplasmic DyneinDynaminFAKMore >>

 Material Science
Material Science- Aggregation-Induced Emission >>Other AIE BlocksTetraphenylethylene SeriesCOFs Linkers >>Aldehyde COFs LinkersAlkynyl COFs LinkersAmine COFs LinkersBoric COFs LinkersCyano COFs LinkersMultifunctional COFs linkersOther COFs linkersTriptycene SeriesElectronic Materials >>Battery MaterialsDye-Sensitized Solar Cell (DSSC) MaterialsElectronic MaterialLiquid Crystal (LC) MaterialsMolecular ConductorsOrganic Light-Emitting Diode (OLED) MaterialsOrganic Solar Cell (OPV) MaterialsOrganic Transistor (OFET) MaterialsPerovskite Solar Cell (PSC) MaterialsMagnetic Materials >>Magnetic Ionic LiquidsMagnetic MaterialMagnetic Metal Complexes
- Organic Radicals Material Chemical Compounds >>Ligands for Functional Metal ComplexesLiquid Crystal (LC) Building BlocksPhthalocyanine Building BlocksPolymer and Macromolecule Semiconductor Building BlocksSmall Molecule Semiconductor Building BlocksSolubility Enhancing Reagents Supramolecular Host Materials MOF Ligands >>Carboxylic Acid MOF LigandsCarboxylic Acid Nitrogen-Containing Mixed MOF LigandsNitrogen-Containing MOF LigandsOther MOF LigandsNanomaterials >>Carbon NanomaterialsCeramic MembranesDendrimersGold NanoparticlesIron Oxide NanoparticlesMaterials by ApplicationMesoporous MaterialsMetals and Metal AlloysNano Minerals: NanoclaysOLED Materials >>Dopant
- HostHTMOLED IntermediatesOther OLED related materialsOptical Materials >>Coumarin DyesCyanine and Squarylium DyesDCM DyesDipyrromethene DyesFluorescence MaterialsFluorescent ProbesHeat and Pressure Sensitive DyesNear-Infrared (NIR) DyesOrganic Non-Linear Optical (NLO) MaterialsOrganic Pigments >>Organic PigmentOther Materials >>Material OtherPolymer Science >>MonomersPolymer AdditivesPolymerization ReagentsPolymersResins
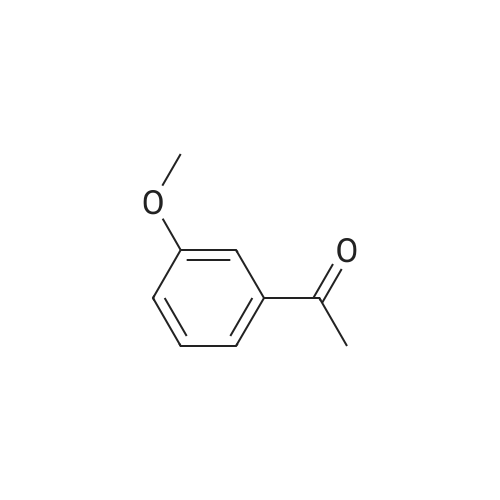
[ CAS No. 579-74-8 ] {[proInfo.proName]}
,{[proInfo.pro_purity]}



| Purity | Size | Price | VIP Price | USA Stock *0-1 Day | Global Stock *5-7 Days | Quantity | |||||
| {[ item.p_purity ]} | {[ item.pr_size ]} |
{[ getRatePrice(item.pr_usd, 1,1) ]} {[ getRatePrice(item.pr_usd,item.pr_rate,item.mem_rate) ]} |
{[ getRatePrice(item.pr_usd, 1,1) ]} | Inquiry {[ getRatePrice(item.pr_usd,item.pr_rate,item.mem_rate) ]} {[ getRatePrice(item.pr_usd,1,item.mem_rate) ]} | {[ item.pr_usastock ]} | Inquiry - | {[ item.pr_chinastock ]} | Inquiry - |
Product Details of [ 579-74-8 ]
| CAS No. : | 579-74-8 | MDL No. : | MFCD00008725 |
| Formula : | C9H10O2 | Boiling Point : | - |
| Linear Structure Formula : | - | InChI Key : | DWPLEOPKBWNPQV-UHFFFAOYSA-N |
| M.W : | 150.17 | Pubchem ID : | 68481 |
| Synonyms : |
|
Calculated chemistry of [ 579-74-8 ] Expand+
Physicochemical Properties
| Num. heavy atoms : | 11 |
| Num. arom. heavy atoms : | 6 |
| Fraction Csp3 : | 0.22 |
| Num. rotatable bonds : | 2 |
| Num. H-bond acceptors : | 2.0 |
| Num. H-bond donors : | 0.0 |
| Molar Refractivity : | 43.13 |
| TPSA : | 26.3 Ų |
Pharmacokinetics
| GI absorption : | High |
| BBB permeant : | Yes |
| P-gp substrate : | No |
| CYP1A2 inhibitor : | Yes |
| CYP2C19 inhibitor : | No |
| CYP2C9 inhibitor : | No |
| CYP2D6 inhibitor : | No |
| CYP3A4 inhibitor : | No |
| Log Kp (skin permeation) : | -5.92 cm/s |
Lipophilicity
| Log Po/w (iLOGP) : | 1.71 |
| Log Po/w (XLOGP3) : | 1.82 |
| Log Po/w (WLOGP) : | 1.9 |
| Log Po/w (MLOGP) : | 1.44 |
| Log Po/w (SILICOS-IT) : | 2.16 |
| Consensus Log Po/w : | 1.81 |
Druglikeness
| Lipinski : | 0.0 |
| Ghose : | None |
| Veber : | 0.0 |
| Egan : | 0.0 |
| Muegge : | 1.0 |
| Bioavailability Score : | 0.55 |
Water Solubility
| Log S (ESOL) : | -2.19 |
| Solubility : | 0.971 mg/ml ; 0.00647 mol/l |
| Class : | Soluble |
| Log S (Ali) : | -1.99 |
| Solubility : | 1.53 mg/ml ; 0.0102 mol/l |
| Class : | Very soluble |
| Log S (SILICOS-IT) : | -2.84 |
| Solubility : | 0.216 mg/ml ; 0.00144 mol/l |
| Class : | Soluble |
Medicinal Chemistry
| PAINS : | 0.0 alert |
| Brenk : | 0.0 alert |
| Leadlikeness : | 1.0 |
| Synthetic accessibility : | 1.0 |
Safety of [ 579-74-8 ]
| Signal Word: | Warning | Class: | N/A |
| Precautionary Statements: | P305+P351+P338 | UN#: | N/A |
| Hazard Statements: | H302-H319 | Packing Group: | N/A |
| GHS Pictogram: |

|
||
Application In Synthesis of [ 579-74-8 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Upstream synthesis route of [ 579-74-8 ]
- Downstream synthetic route of [ 579-74-8 ]
[ 579-74-8 ] Synthesis Path-Upstream 1~9
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 96% | With copper(ll) bromide In chloroform; ethyl acetate at 70℃; for 8 h; Inert atmosphere; Reflux | Copper (II) bromide (2.97 g, 6.66 mmol) was placed in a twonecked round-bottomed flask fitted with a reflux condenser. EtOAc (10.0 mL) was added to it and the mixture was stirred at 70C for 5 min under nitrogen atmosphere. 1-(2-Methoxyphenyl)ethanone (6) (0.50 g, 3.33 mmol) in CHCl3 (10.0 mL) was slowly added to it and then the mixture was refluxed for 8 h. After completion of the reaction, it was cooled to room temperature, filtered through Celite® pad, and washed with EtOAc (20 mL). The filtrate was concentrated under reduced pressure. The crude was puri- fied by column chromatography (EtOAc:hexane = 1:4) to afford the pure product 7a (0.73 g, 96percent) as brown liquid. Rf = 0.43 (EtOAc/hexane = 1/4); 1 H NMR (300 MHz, CDCl3): δ 7.81 (1H, dd, J = 7.8, 1.8 Hz), 7.52 (1H, td, J = 7.8, 1.8 Hz), 7.05–6.96 (2H, m), 4.61 (2H, s), 3.94 (3H, s); 13C NMR (75 MHz, CDCl3): δ 192.3, 158.8, 134.9, 131.6, 124.9, 121.2, 111.7, 56.0, 38.0. |
| 83% | Stage #1: at 60℃; Stage #2: at 60℃; for 0.25 h; |
General procedure: The round-bottom flask containing acetophenone derivative 1, 10 (4 mmol) and PTSA (0.076 g,0.4 mmol) was heated to 60 °C to turn the reaction mixture into a paste and NBS (0.854 g, 4.8 mmol) thenadded slowly. After 15 min, the reaction mixture was cooled to room temperature and water (20 mL)added. The crude product was extracted with dichloromethane (2 x 20 mL), dried over anhydrousNa2SO4 and purified by crystallization from n-hexane–dichloromethane to give pure product. 2-Methoxyphenacyl bromide (2): Yellow solid, soluble in acetone, dichloromethane, chloroform, insolublein water; yield 83percent; m.p.: 43–45 °C; 1H-NMR (500 MHz, CDCl3) (δ, ppm): 7.78 (dd, 1H, J = 1.5,7.5 Hz, Ar–H), 7.51–7.47 (m, 1H, Ar–H), 7.02–6.96 (m, 2H, Ar–H), 4.57 (s, 2H, CH2), 3.93 (s, 3H, OCH3).This result showed consistency with the data in literature [26]. |
| 330 g | With bromine In acetonitrile at 20 - 25℃; for 4 h; | 1 -(2-methoxyphenyl)ethan-1 -one 2-bromo-1 - MW: 150.17 (2-methoxyphenyl)ethan-1-one MW: 229.07 [00242] l-(2-methoxyphenyl)ethanone, 1.1, (300 g, 1.0 eq) was added to a reactor containing acetonitrile (1.2 L, 4.0 V). Br2 (319.62 g, 1.0 eq) was added by cooling the reaction to below 25°C. The reaction mixture was stirred for 4 h at 20-25 °C and sampled for IPC until the content of l-(2-methoxyphenyl)ethanone was 6.5percent. NaHSCb (600 ml, 2V) was added to quench the reaction and then stirred for an additional 0.5h at 20-25°C. Product was extracted with methyl tert-butyl ether (600 ml, 2V), three times, to yield a black oil (418 g, crude), which was purified using a column to yield 330 g of 2-bromo-l-(2-methoxyphenyl)ethan-l-one, 1.2, as an off-white solid (98percent purity). |
[2] Tetrahedron Letters, 2006, vol. 47, # 27, p. 4707 - 4710
[3] Tetrahedron Letters, 2009, vol. 50, # 6, p. 700 - 703
[4] Chemistry - A European Journal, 2011, vol. 17, # 28, p. 7953 - 7959
[5] Molecules, 2017, vol. 22, # 5,
[6] Dalton Transactions, 2008, # 6, p. 822 - 831
[7] Journal of Medicinal Chemistry, 1987, vol. 30, # 8, p. 1497 - 1502
[8] Chemical and Pharmaceutical Bulletin, 1992, vol. 40, # 5, p. 1170 - 1176
[9] Australian Journal of Chemistry, 1994, vol. 47, # 11, p. 2001 - 2012
[10] Bioorganic and Medicinal Chemistry Letters, 2002, vol. 12, # 4, p. 719 - 722
[11] Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 5, p. 1291 - 1295
[12] Bioorganic and Medicinal Chemistry Letters, 2008, vol. 18, # 4, p. 1297 - 1303
[13] Tetrahedron, 2008, vol. 64, # 22, p. 5191 - 5199
[14] Chemistry - An Asian Journal, 2010, vol. 5, # 10, p. 2258 - 2265
[15] European Journal of Medicinal Chemistry, 2012, vol. 54, p. 403 - 412
[16] Angewandte Chemie - International Edition, 2013, vol. 52, # 44, p. 11632 - 11636[17] Angew. Chem., 2013, vol. 125, # 44, p. 11846 - 11850,5
[18] Advanced Synthesis and Catalysis, 2013, vol. 355, # 18, p. 3570 - 3574
[19] Chemical Communications, 2014, vol. 50, # 51, p. 6726 - 6728
[20] Journal of Medicinal Chemistry, 2014, vol. 57, # 15, p. 6572 - 6582
[21] Journal of Materials Chemistry A, 2015, vol. 3, # 12, p. 6258 - 6264
[22] MedChemComm, 2015, vol. 6, # 6, p. 1036 - 1042
[23] European Journal of Medicinal Chemistry, 2015, vol. 104, p. 1 - 10
[24] Phosphorus, Sulfur and Silicon and the Related Elements, 2016, vol. 191, # 8, p. 1166 - 1173
[25] Bioorganic and Medicinal Chemistry Letters, 2016, vol. 26, # 15, p. 3669 - 3674
[26] Angewandte Chemie - International Edition, 2016, vol. 55, # 29, p. 8444 - 8447[27] Angew. Chem., 2016, vol. 128, # 29, p. 8584 - 8587,4
[28] Patent: KR101642378, 2016, B1, . Location in patent: Paragraph 0062-0066
[29] ChemMedChem, 2018, vol. 13, # 1, p. 37 - 47
[30] Patent: US2018/44459, 2018, A1, . Location in patent: Paragraph 0367
[31] Bioorganic and Medicinal Chemistry, 2018, vol. 26, # 8, p. 1986 - 1995
[32] Patent: US2018/44284, 2018, A1, . Location in patent: Paragraph 0175; 0176
[33] Organic Letters, 2018, vol. 20, # 8, p. 2257 - 2260
[34] Patent: WO2018/161008, 2018, A1, . Location in patent: Paragraph 00241-00242
[35] Chemical Communications (Cambridge, United Kingdom), 2018, vol. 54, # 86, p. 12182 - 12185
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 99% | With N-Bromosuccinimide; iodine In acetonitrile for 12 h; Darkness | General procedure: To a reaction tube charged with NBS (1.5 equiv, 0.3 mmol), catalyst (10 molpercent, 0.02 mmol) and CH3CN (1.0 mL),was added para-chloroanisole 1a (0.2 mmol). After being stirred at room temperature for 12 h in dark, the reaction was quenched by saturated aq. solution of Na2S2O3 (2 mL). The resulting mixture was extracted by ethyl acetate (3 5 mL). The combined organic extracts were washed by brine (10 mL), dried over Na2SO4 and filtered through a pad of Celite. The filtrate was concentrated under reduced pressure and the residuewas purified by flash chromatography on a silica gel column with petroleum ether/dichloromethane (5:1) as the eluent to give 4.3.1. 2-Bromo-4-chloroanisole (2a) |
| 95% | With hydrogenchloride; N-Bromosuccinimide In water; acetone at 20℃; for 3 h; | Compound 41-2 (0492) To a solution of 41-1 (2.0 g, 13.32 mmol) in acetone (25 mL) was added NBS (2.37 g, 13.32 mmol) and 1M HCl aq. (0.13 mL, 0.13 mmol). The reaction mixture was stirred at room temperature for 3 h, and then concentrated to dryness under reduced pressure. The residue was dissolved with PE (40 mL) the resultant precipitate was filtered and dried in vacuum to afford 41-2 as a white solid (2.90 g, yield: 95percent). |
[2] Tetrahedron Letters, 2006, vol. 47, # 27, p. 4707 - 4710
[3] Patent: US9138427, 2015, B2, . Location in patent: Page/Page column 312-313
[4] Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1987, p. 1423 - 1428
[5] Synthesis (Germany), 2013, vol. 45, # 11, p. 1497 - 1504
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 72% | With ammonium hydroxide; ammonium acetate; sodium cyanoborohydride; zinc In ethanol; water at 80℃; for 36 h; | General procedure: To a saturated solution of NH4OAc in EtOH (40 mL) were added activated Zn (5 equiv), acetophenone (1 equiv), NaBCNH3(3 equiv) and 30percent aq NH3 (10 mL) respectively. The mixture wasstirred at 80 C for 36 h. The reaction mixture was cooled to rt and concentrated under reduced pressure. The residue was dissolved in CH2Cl2 and made basic using 1 M NaOH (50 mL). Theorganic phase was separated and aqua phase was extracted with (2x25 mL) CH2Cl2. The organic phases were combined and acidified with HCl (pH: 2.0). The organic layer was separated andH2O layer was extracted with CH2Cl2 (2 25 mL). The H2O layer was made basic with NaOH (pH: 10.0), The organic layer was extracted with CH2Cl2 (3 25 mL). Combined organic layers weredried over Na2SO4 and evaporation of the solvent afforded thedesired amines. |
[2] Chemistry - A European Journal, 2014, vol. 20, # 1, p. 245 - 252
[3] Farmaco, 1991, vol. 46, # 7-8, p. 861 - 872
[4] Bioorganic and Medicinal Chemistry, 2015, vol. 23, # 13, p. 3592 - 3602
[5] Chirality, 2018, vol. 30, # 7, p. 900 - 906
[6] Journal of the Chemical Society. Perkin Transactions 2, 2000, # 7, p. 1339 - 1347
[7] Advanced Synthesis and Catalysis, 2011, vol. 353, # 11-12, p. 2085 - 2092
[8] Molecules, 2014, vol. 19, # 12, p. 21386 - 21397
[9] Bioorganic and Medicinal Chemistry, 2016, vol. 24, # 4, p. 554 - 569
[10] Green Chemistry, 2017, vol. 19, # 2, p. 474 - 480
[ 579-74-8 ] Synthesis Path-Downstream 1~103
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 100% | With potassium-t-butoxide; 1-hydrosilatrane In tetrahydrofuran; N,N-dimethyl-formamide for 48h; | 2 General Procedure for the Reduction of Ketones General procedure: To a 25 ml round bottom flask containing 5 ml N,N-dimethylformamide, was added 1-hydrosilatrane (0.263 g, 2.0 mmol), and ketone (1.0 mmol). The resulting solution was stirred for 1 minute, after which 1 M t-BuOK in THF (1.0 mmol, 1.0 ml) was added. Reaction mixture was allowed to stir for 30 min. Reaction was quenched with 25 ml 3 M HCl, and extracted with 30 mL ethyl acetate. Organic layer was washed with brine (50 ml×3), and dried with anhydrous sodium sulphate. After filtration, the solvent was removed under vacuum to yield product. |
| 99% | Stage #1: 1-(2-methoxyphenyl)ethan-1-one With (dppe)2Fe(H)2*(C7H8)2; Na-tetrakis(ethoxy)borate In toluene at 100℃; for 2h; visible light irradiation; Inert atmosphere; Stage #2: With lithium hydroxide monohydrate; sodium hydroxide In methanol; toluene at 20℃; for 16h; | |
| 99% | With dichloro-[1-(2,3,4,5,6-pentamethylbenzyl)-3-(2-methoxyethyl)benzimidazol-2-ylidene]ruthenium (II); isopropanol; potassium hydroxide at 80℃; for 1h; Inert atmosphere; |
| 99% | With C37H28Cl2N5PRu; isopropanol; sodium hydroxide at 82℃; for 0.0166667h; Inert atmosphere; | |
| 99% | With C15H18BF3; hydrogen; phosphazene base P1-t-bu-tris(tetramethylene) In tetrahydrofuran at 75℃; for 40h; | |
| 99% | With hydrogen; anhydrous silver perchlorate; 1,1,1,3,3,3-hexamethyldisilazane potassium In toluene at 25℃; for 17h; Glovebox; | |
| 98% | With potassium-t-butoxide In isopropanol at 80℃; for 12h; | |
| 98.9% | With C55H54Cl4N2O2PRu2; sodium hydroxide In isopropanol at 82℃; for 0.333333h; | |
| 98% | Stage #1: 1-(2-methoxyphenyl)ethan-1-one With C44H44CuN2P2(1+)*F6P(1-) In toluene at 25℃; for 1h; Inert atmosphere; Stage #2: With sodium hydroxide In methanol; lithium hydroxide monohydrate Inert atmosphere; | 3. Typical procedure for catalytic hydrosilylation of ketones General procedure: Under nitrogen atmosphere the copper based catalyst 1 (8 7 mmg0.01 mmol), tBuOK ( 5 6 mmg0.05 mmol) and toluene (3 mL) were placed in a tube equipped with a Teflon coated magnetic stirring bar. T he mixture was stirred at 25 °C for 15 min and then polymethylhydrosiloxane (PMHS, 0.0 9 m L, 1.5 mmol mmol) was injected. After 15mins, ketone (0.5 mmol) was introduced and the mixture was stirred at 25 °C for therequired reaction time. The mixture was quenched with MeOH (1 mL) and 10%NaOH solution (3 mL), and the mixture was stirred for 4 h. T he mixture was extractedwith ethyl acetate (5 mL × 3) and the combined organic layer was washed with waterand saturated sodium chloride solution, dried over anhydrous Na2SO4. the solvent was removed under vacuum and the residue was purified by flash chromatography (silica gel) to afford the desired product. All the product alcohols were analyzed by 1H NMR, 13C NMR, or GC analysis. |
| 97% | With methyldiethoxysilane; ferrous acetate In tetrahydrofuran at 65℃; for 24h; | |
| 97% | With manganese(I) pentacarbonyl bromide; potassium-t-butoxide; Ethane-1,2-diamine In isopropanol at 80℃; for 3h; | 2.1. Representative procedure for transfer hydrogenation reaction ofacetophenone General procedure: To a solution of acetophenone (58 μL, 0.5 mmol) in 2-propanol (0.5 mL) was added a stock solution of manganese pentacarbonyl bromide (0.5 mL, 0.005 mol·L-1; 2.7 mg, 0.010 mmol, in 2 mL 2-propanol)followed, in this order, by a stock solution of ethylenediamine (0.5 mL,0.005 mol·L-1; 1.0 μL, 0.0125 mmol, in 2.5 mL 2-propanol) and tBuOK (0.5 mL, 0.010 mol·L-1; 2.4 mg, 0.020 mmol, in 2 mL 2-propanol). The reaction mixture was stirred for 3 h at 80 °C in an oil bath. The solution was then filtered through a small pad of silica (2 cm in a Pasteur pip-ette). The silica was washed with ethyl acetate. The filtrate was evaporated and the conversion was determined by 1H NMR. The crude residue was then puried by column chromatography (SiO 2 , mixture of petroleum ether/ethyl acetate or dietyl ether as eluent. Enantiomeric excesses were determined by GC analyses performedon GC-2014 (Shimadzu) 2010 apparatus equipped with Supelco beta-DEX 120 column (30 m × 0.25 mm). The determination of the absoluteconguration was done by comparison with (S)-alcohol obtained bykinetic resolution of racemic alcohols with Novozym 435 (CandidaAntarctica Lipase B) and by comparison of the retention times with the literature [32-34]. |
| 97% | With formic acid; C18H24ClIrN3 In lithium hydroxide monohydrate at 80℃; for 4h; Schlenk technique; Inert atmosphere; chemoselective reaction; | |
| 95% | Stage #1: 1-(2-methoxyphenyl)ethan-1-one With Trimethyl borate; dimethylsulfide borane complex; (-)-3-amino-6,6-dimethyl-2-hydroxy-bicyclo[3.1.1]heptane In tetrahydrofuran at 20℃; Stage #2: With methanol In tetrahydrofuran | |
| 95% | With C53H46ClN3P2Ru; potassium isopropoxide; isopropanol at 82℃; for 1h; | |
| 94% | With N,N,N,N,-tetramethylethylenediamine; methyldiethoxysilane In tetrahydrofuran at 65℃; for 24h; | |
| 93% | With C28H36ClF3N2O3RuS; potassium-t-butoxide; isopropanol at 82℃; for 12h; Inert atmosphere; | |
| 93% | With 1-hydrosilatrane; potassium-t-butoxide In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 0.5h; | |
| 93% | With isopropanol; sodium hydroxide for 3h; Inert atmosphere; Schlenk technique; Reflux; | 2.3 General procedure for transfer hydrogenation of ketones catalyzed by 4 General procedure: Under nitrogen atmosphere, a mixture of ketone (2 mmol), catalyst 4 (0.008 mmol), and 2-propanol (17.6 mL) was stirred at 82°C. After 5 min, 2.4 mL of 0.1 M NaOH (0.24 mmol) solution in 2-propanol was introduced to initiate the reaction, and the reaction mixture was stirred at refluxing temperature. At the specified time, 0.1 mL of the reaction mixture was filtered through a short pad of celite to remove the complex catalyst, and immediately diluted with 0.2 mL of 2-propanol. The filtrate was used for GC analysis. After the reaction was finished, the mixture was condensed under reduced pressure and subject to flash silica gel column chromatography to afford the alcohol product (detected under 254 nm UV light or by alkaline potassium permanganate solution; eluent: petroleum ether (60-90 °C)/ethyl acetate = 10:1 or petroleum ether (30-60 °C)/dichloromethane= 1:1, v/v). The alcohol products were identified by comparison with the authentic sample through NMR and GC analyses. |
| 92% | With potassium-t-butoxide; isopropanol at 80℃; for 12h; Inert atmosphere; | |
| 92% | Stage #1: 1-(2-methoxyphenyl)ethan-1-one With ferrous acetate; tricyclohexylphosphine In tetrahydrofuran at 65℃; Inert atmosphere; Stage #2: In tetrahydrofuran at 65℃; Inert atmosphere; Stage #3: With lithium hydroxide monohydrate; sodium hydroxide In tetrahydrofuran; methanol at 0 - 20℃; Inert atmosphere; | |
| 92% | With hydrogen In lithium hydroxide monohydrate at 20℃; for 72h; Autoclave; | 2.4. General procedure for hydrogenation under hydrogen pressure General procedure: The stainless steel autoclave was charged with the previously prepared aqueous suspension of Col-Ni-CMC Nps (8 mL, from 40 mg of NiCl2·6H2O and 10 mL, from 50 mg of NiCl2·6H2O) for ketone hydrogenation. The appropriate nitro-aromatic ([substrate]/[metal] = 100) in 2 mL of MeOH, was added into the autoclave and dihydrogen was admitted to the system at constant pressure (10 to 40 bars). The mixture was stirred until the reaction was finished. Samples for gas chromatographic analysis were removed from time to time. The residue was extracted with diethyl ether (3 × 25 mL). The organic layer was dried over Na2SO4 and the solvent was removed under reduced pressure Pure products were obtained by column chromatography over silica gel using hexane/ethyl acetate as eluent. |
| 92% | With [Re(NH{CH2CH2P(iPr2)}2)(CO)3]Br; potassium-t-butoxide; hydrogen In toluene at 110℃; for 2h; Inert atmosphere; Glovebox; Autoclave; | |
| 90% | With trimethylamine-N-oxide; (N,N,N-trimethyl-2-(5-oxo-4,6-bis(trimethylsilyl)cyclopenta[c]pyrrol-2-(1H,3H,5H)-yl)ethanaminium) iron tricarbonyl; hydrogen In lithium hydroxide monohydrate at 85℃; for 14h; Autoclave; Inert atmosphere; | |
| 89% | Stage #1: 1-(2-methoxyphenyl)ethan-1-one With Triethoxysilane; diethylzinc(II); N,N-dimethyl-N'-(4-tert-butylphenyl)formamidine In tetrahydrofuran; hexane at 20 - 60℃; Inert atmosphere; Stage #2: With sodium hydroxide In tetrahydrofuran; hexane; lithium hydroxide monohydrate at 0℃; for 1h; | |
| 88% | With C56H55ClN3P2Ru(1+)*F6P(1-); potassium 2-methyl-2-butoxide In isopropanol at 20 - 80℃; for 1.5h; Schlenk technique; Inert atmosphere; | |
| 85% | With sodium hydroxide; ytterbium(III) tris(trifluoromethanesulfonate) In isopropanol for 0.5h; Heating; | |
| 85% | With [Ru(CH3CN)3(κ2-o-DPPBS)Cl] In methanol at 70℃; for 15h; Autoclave; | |
| 85% | With sodium tetrahydridoborate; lithium hydroxide monohydrate In tetrahydrofuran at 20℃; for 6h; Schlenk technique; Inert atmosphere; | |
| 81% | With potassium-t-butoxide; 1-hydrosilatrane In tetrahydrofuran; N,N-dimethyl-formamide for 0.5h; Inert atmosphere; | 5. General procedure for the racemic reduction of ketones General procedure: To a 25 mL round-bottomed flask containing 5 mL N,N-dimethylformamide, were added 1-hydrosilatrane(0.263 g, 2.0 mmol), and ketone (1.0 mmol). The resulting solution was stirred for 1 minute, after which 1M tBuOK in THF (1.0 mmol, 1.0 mL) was added. Reaction mixture was allowed to stir for 30 min. Reaction was quenched with 25 mL 3M HCl, and extracted with 30 mL ethyl acetate. Organic layer was washed with brine (50 mL x 3), and dried with anhydrous sodium sulphate. After filtration, the solvent was removed under vacuum to yield product. No further steps were taken for purification. |
| 80% | Stage #1: 1-(2-methoxyphenyl)ethan-1-one With 2.6-dimethylphenol; Triethoxysilane; diethylzinc(II) In tetrahydrofuran; hexane at 60℃; for 1h; Inert atmosphere; Stage #2: With sodium hydroxide In tetrahydrofuran; methanol; hexane; lithium hydroxide monohydrate at 0℃; for 1h; | |
| 80% | With C21H26ClNORu; hydrogen In methanol; lithium hydroxide monohydrate Autoclave; Heating; | |
| 80% | With C70H68Cl4N7O2P3Ru2; potassium hydroxide In isopropanol at 82℃; for 2h; | 2.4. Catalytic experiments General procedure: Complex 21 (the same for complexes 22-26) (1 mmol) were dissolved in 15 mL 2-propanol and acetophenone (or acetophenone derivatives) (100 mmol) and KOH (5 mmol) were added then reac- tion was refluxed at 82 °C for 2 h. The solvent was removed by ro- tary evaporator under the vacuum and washed with diethyl ether (2 ×20 ml). Oily mixture was purified with flash chromatography silica gel (petroleum ether/ethyl acetate (5:1)). The obtained oily mixture was analyzed from HPLC. |
| 79% | With formic acid; [(dpa)(p-cymene)RuCl]Cl; anhydrous sodium formate In lithium hydroxide monohydrate at 65℃; for 24h; Inert atmosphere; | |
| 77% | Stage #1: 1-(2-methoxyphenyl)ethan-1-one With C40H32Cl2N3PRu; isopropanol at 82℃; for 0.166667h; Schlenk technique; Inert atmosphere; Stage #2: With sodium hydroxide for 3h; Schlenk technique; Inert atmosphere; | |
| 74% | Stage #1: 1-(2-methoxyphenyl)ethan-1-one With sodium tetrahydridoborate In methanol at 0 - 20℃; Inert atmosphere; Stage #2: With lithium hydroxide monohydrate | |
| 74% | With sodium tetrahydridoborate In methanol at 0 - 20℃; Inert atmosphere; | |
| 69% | With diisobutylaluminum borohydride In tetrahydrofuran at 25℃; for 1h; Inert atmosphere; | |
| 61% | With bis[chlorido(η2,η2-cycloocta-1,5-diene)rhodium(I)]; anhydrous potassium trifluoroacetate; diphenyl(methyl)phosphine; 4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl-4',4',5',5'-tetramethyl-1,3,2-dioxaborolane; 4,4,5,5-tetramethyl-1,3,2-dioxaborolane In tetrahydrofuran; cyclohexane at 20℃; for 48h; regioselective reaction; | |
| 59% | With potassium-t-butoxide In 1,4-dioxane at 80℃; for 16h; Inert atmosphere; Sealed tube; | |
| 53% | With C18H37BrNO4P2Re; potassium-t-butoxide; hydrogen In toluene at 120℃; for 20h; Glovebox; Autoclave; | |
| 46% | With sodium tetrahydridoborate In methanol at 20℃; | |
| With tris isopropylate aluminium | ||
| 18 % Spectr. | With sodium tetrahydridoborate; glacial acetic acid at 16 - 21℃; for 0.166667h; | |
| With sodium tetrahydridoborate In methanol for 0.75h; | ||
| With sodium tetrahydridoborate | ||
| With sodium tetrahydridoborate | ||
| With sodium tetrahydridoborate In ethanol for 1.5h; Heating; | ||
| 100 % Chromat. | With dodecacarbonyltri-iron; sodium isopropanolate; 5,10,15,20-tetra(pyridin-4-yl)-21H,23H-porphyrin In isopropanol at 100℃; for 7h; | |
| 97 % Chromat. | With 1H-imidazol-3-ium; sodium isopropanolate In isopropanol at 100℃; for 24h; | |
| With sodium tetrahydridoborate | ||
| With methanol; sodium tetrahydridoborate | ||
| With samarium diiodide In tetrahydrofuran | ||
| With potassium hydroxide; di-μ-chlorobis-[(η6-p-cymene)chlororuthenium(II)]; (1R,2R)-3-(diphenylmethoxy)-1-methylamino-1-phenylpropan-2-ol In isopropanol at 25℃; for 4h; | ||
| 99 % Chromat. | With potassium-t-butoxide; hydrogen In isopropanol at 25℃; for 8h; | |
| With sodium tetrahydridoborate | ||
| With lithium isopropoxide In isopropanol at 180℃; for 0.333333h; microwave irradiation; | ||
| 84 % ee | With potassium-t-butoxide; hydrogen In isopropanol at 20 - 25℃; for 24h; | |
| With sodium hydroxide In tetrahydrofuran; lithium hydroxide monohydrate | 4.1 1-(2-Methoxyphenyl)ethanol Step 1 1-(2-Methoxyphenyl)ethanol Lithium aluminum hydride (1.52 g) was suspended in tetrahydrofuran (100 ml), 2'-methoxyacetophenone (2.76 ml) was added under ice-cooling, and the mixture was stirred for 30 min. To the reaction mixture were successively added water (1.5 ml), 15% aqueous sodium hydroxide solution (1.5 ml) and water (4.5 ml). The organic layer was dried over magnesium sulfate and concentrated under reduced pressure to give the title compound (3.05 g). | |
| With sodium isopropanolate; isopropanol at 100℃; | ||
| With sodium tetrahydridoborate | ||
| With C42H39ClN2OsP2; sodium isopropanolate In isopropanol at 82℃; for 0.0333333h; Inert atmosphere; | ||
| 93 %Spectr. | With RuCl2(NC6H4NHCC5H3NN2C3H(CH3)2)(P(C6H5)3); potassium isopropoxide; isopropanol at 28℃; for 0.0166667h; Air; | |
| With [Ir(cod)(κ2-o-tBu2P-C6H4-NMe2)](+)PF6(-); isopropanol; sodium tertiary butoxide at 82℃; for 0.0833333h; Inert atmosphere; | ||
| With sodium hydroxide; isopropanol for 0.333333h; Heating / reflux; | 5 Examole 5: catalytic reduction of linear and cyclic dialkvl ketones, alkylaryl ketonesand diaryl ketones in presence of complex 8; Complex 8 (3.0 mg, 0.005 mmol) is suspended in 3 ml of 2-propanol in a 10mltailed tubes (Schlenk) and 2 ml of a 0.1M solution of NaOH in 2-propanol added.The complex is completely solubilised in a few minutes by stirring.Separately, in a 50ml tailed tubes (Schlenk), the necessary amount ofacetophenone (240 ul, 2 mmol) is dissolved in 19 ml of 2-propanol, the system isrefluxed and 1 ml of the previously prepared catalyst solution added (the addition of the complex is considered to be the starting time of the reaction). The molarratios between acetophenone/catalyst/NaOH are 2000/1/40; the reaction ismonitored at 1, 2, 5 minutes by collecting 0.5 ml of the solution and adding 4.5 mlof diethyl ether. This solution is passed through a silica column in order toeliminate the catalyst and sodium hydroxide, and finally analysed by gaschromatography. The gas chromatograph analysis data are reported in table 2. | |
| With [Ru(chloro)(p-cymene)(N,N'-bis[(1S)-1-benzyl-2-O-(diphenylphosphinite)ethyl]ethanediamide)] chloride; isopropanol; sodium hydroxide at 82℃; for 1h; Inert atmosphere; | ||
| With sodium tetrahydridoborate In methanol at 20℃; | ||
| 98 %Spectr. | With C51H53ClN3O2P2Ru(1+)*Cl(1-); Cs2CO3; isopropanol at 83℃; for 6h; Inert atmosphere; | |
| With [Ru((Ph2P)2N-C6H4-2-CH(CH3)2)(η6-p-cymene)Cl]Cl; isopropanol; sodium hydroxide at 82℃; for 0.25h; Inert atmosphere; | ||
| With [Ru{chloro(p-cymene)(N,N'-bis[(1R)-1-ethyl-2-O-(diphenylphosphinite)ethyl]ethanediamide)}]chloride; isopropanol; sodium hydroxide at 82℃; for 1h; | ||
| With lithium aluminium hydride In tetrahydrofuran at 0℃; for 0.5h; | 4.1 Example 4; (2R)-1-[1,1-Dimethyl-2-(naphthalen-2-yl)ethylamino]-3-[1-(2-methoxyphenyl)ethoxy]propan-2-ol; Step 1; 1-(2-Methoxyphenyl)ethanol Lithium aluminum hydride (1.52 g) was suspended in tetrahydrofuran (100 ml), 2'-methoxyacetophenone (2.76 ml) was added under ice-cooling, and the mixture was stirred for 30 min. To the reaction mixture were successively added water (1.5 ml), 15% aqueous sodium hydroxide solution (1.5 ml) and water (4.5 ml). The organic layer was dried over magnesium sulfate and concentrated under reduced pressure to give the title compound (3.05 g). | |
| 96 %Chromat. | With Ru(η6-cymene)(PPh2NH-C6H4-2-CH(CH3)2)Cl2; isopropanol; sodium hydroxide at 82℃; for 0.5h; Inert atmosphere; | |
| With C58H50Cl2N2P4RuS2; isopropanol; sodium hydroxide at 82℃; for 1h; Inert atmosphere; | ||
| With sodium tetrahydridoborate In methanol at 0 - 20℃; for 2h; Inert atmosphere; | ||
| With [Ru((PPh2)2NCH2-C4H3S)(η6-p-cymene)Cl]Cl; isopropanol; sodium hydroxide at 82℃; for 0.166667h; Inert atmosphere; | ||
| With Candida tenuis AKR2B5 xylose reductase [E.(1)C11175]; lithium hydroxide monohydrate; NADH In ethanol at 25℃; aq. phosphate buffer; Enzymatic reaction; | ||
| 97 %Chromat. | With anhydrous sodium carbonate; isopropanol at 82℃; for 8h; Inert atmosphere; | |
| 94 %Chromat. | With C71H56N5P2Ru(1+)*Cl(1-); potassium isopropoxide; isopropanol; potassium hydroxide at 82℃; for 4h; Inert atmosphere; | |
| 98 %Chromat. | Stage #1: 1-(2-methoxyphenyl)ethan-1-one With [RuCl2(η6-benzene)tris(4-methoxyphenyl)phosphane] In isopropanol at 82℃; for 0.166667h; Inert atmosphere; Stage #2: With potassium isopropoxide In isopropanol at 82℃; for 0.5h; Inert atmosphere; | |
| With [RuCl(bis(2-(dimethylamino)phenyl)amide)(PPh3)]; isopropanol; potassium hydroxide at 83℃; for 2h; Inert atmosphere; | ||
| > 99 %Chromat. | Stage #1: 1-(2-methoxyphenyl)ethan-1-one With n-butyllithium; ferrous acetate; 1,3-bis(2,6-diisopropylphenyl)imidazolinium chloride In tetrahydrofuran; hexane at 65℃; Inert atmosphere; Stage #2: With sodium hydroxide In tetrahydrofuran; methanol; hexane; lithium hydroxide monohydrate at 20℃; for 3h; Inert atmosphere; | |
| With bis[dichlorido(η5-1,2,3,4,5-pentamethyl-cyclopentadienyl)rhodium (III)]; lithium formate; (S)-prolinehydroxamic acid In lithium hydroxide monohydrate at 24℃; for 14h; enantioselective reaction; | ||
| With [(η6-p-cymene)RuCl(6,6’-dihydroxy-2,2’-bipyridine)]Cl; isopropanol; potassium hydroxide at 85℃; for 24h; | ||
| With Ru(Ph2PNHCH2-C4H3O)(η-6-benzene)Cl2; isopropanol; potassium hydroxide at 82℃; for 0.333333h; | ||
| With sodium tetrahydridoborate In ethanol Inert atmosphere; | ||
| With [C10H6N2{NHPPh2Ru(η6-benzene)Cl2}2]; sodium hydroxide In isopropanol for 0.333333h; Reflux; Inert atmosphere; | 4.3.4 General procedure for the transfer hydrogenation of ketones General procedure: Typical procedure for the catalytic hydrogen transfer reaction: a solution of complexes [C10H6N2{NHPPh2Ru(η6-benzene)Cl2}2], 1, [C10H6N2{PPh2NHRh(cod)Cl}2], 2 and [C10H6N2{NHPPh2Ir(η5-C5Me5)Cl2}2], 3 (0.005 mmol), NaOH (0.025 mmol) and the corresponding ketone (0.5 mmol) in degassed iso-PrOH (5 mL) were refluxed for 10 min for 1, 1 h for 2 and 3 h for 3. After this period a sample of the reaction mixture was taken off, diluted with acetone and analyzed immediately by GC. Conversions obtained are related to the residual unreacted ketone. | |
| With formic acid; [Cp*Rh(2,2'-dipyridylamine-κ2N1,N1')Cl]Cl; anhydrous sodium formate In lithium hydroxide monohydrate at 60℃; for 18h; | ||
| With [(bis(5-methyl-3-phenyl-1,2,4-triazolyl)(3-methyl-5-phenyl-1,2,4-triazolyl)borate)Ru(p-cymene)Cl]; potassium hydroxide In isopropanol for 24h; Inert atmosphere; Reflux; | ||
| > 99 %Chromat. | With Triethoxysilane; C27H56FeN2P4Si In tetrahydrofuran at 100℃; for 24h; Inert atmosphere; Schlenk technique; | |
| With sodium tetrahydridoborate In ethanol | ||
| With sodium tetrahydridoborate In methanol at 20℃; Schlenk technique; | Racemic: General procedure: The ketone (1mmol) was dissolved in MeOH (5cm3) in a schlenk tube, then NaBH4 (3mmol) was added slowly and the mixture was stirred at r.t. for o/n. The solvent was removed and the mixture was dissolved in DCM (10 cm3), washed with water (10cm3), filtered and solvent removed. a small amount of the residue was dilluted inEtOAc and then injected on the GC to determine the conversion. | |
| Stage #1: 1-(2-methoxyphenyl)ethan-1-one With C33H58FeN3PSi2; phenylsilane In toluene at 20℃; for 4h; Inert atmosphere; Glovebox; Green chemistry; Stage #2: With sodium hydroxide In toluene for 1h; Green chemistry; | ||
| 92 %Chromat. | Stage #1: 1-(2-methoxyphenyl)ethan-1-one With (η6-C6H6)RuCl2[μ-(PPh2CH2N)2CH2(C10H6)]RuCl2(η6-C6H6); isopropanol for 0.166667h; Inert atmosphere; Schlenk technique; Stage #2: With potassium isopropoxide In isopropanol at 82℃; for 0.5h; Inert atmosphere; Schlenk technique; | |
| With [Rh((Ph2P)2NCH2-C4H3O)(Cp*)Cl]Cl; sodium hydroxide In isopropanol at 82℃; for 10h; Schlenk technique; Inert atmosphere; | ||
| With OsCl[PPh2(CH2)4PPh2](CNN-H); sodium isopropanolate In isopropanol for 0.0333333h; Inert atmosphere; Reflux; | 19 Example 19: catalytic reduction of linear and cyclic dialkyl ketones, alkylaryl ketones and diarylketones in the presence of the osmium complex (7a) The catalyst solution was prepared in a 10 ml Schlenk by adding 5 ml of 2-propanol to the complex (7a) (1.8 mg, 0.0021 mmol). By stirring, the complex dissolves completely over a period of a few minutes. Separately, in a second Schlenk (50 ml), 200 µl of the previously prepared solution containing the catalyst and 0 4 ml of a 0.1 M NaOiPr solution in 2-propanol were added to a solution of ketone (2 mmol) in 19 ml of 2-propanol under reflux. The start of the reaction was considered to be when the complex was added. The molar ratios of ketone/catalyst/NaOiPr were 20000/1 /400. The GC analysis data are given in table 2. | |
| With [Ru(Cy2PNHCH2-C4H3S)(η6-p-cymene)Cl2]; isopropanol; sodium hydroxide at 82℃; for 2h; Inert atmosphere; Schlenk technique; | ||
| With [Rh(Cy2PNHCH2-C4H3O)(cod)Cl]; isopropanol; sodium hydroxide at 82℃; for 0.416667h; Inert atmosphere; Schlenk technique; | 4.2. General procedure for the transfer hydrogenation of ketones General procedure: Typical procedure for the catalytic hydrogen transfer reaction: a solution of complexes [Rh(Cy2PNHCH2-C4H3O)(cod)Cl], (1),[Rh(Cy2PNHCH2-C4H3S)(cod)Cl], (2), [Ir(Cy2PNHCH2-C4H3O)(η5-C5Me5)Cl2], (3) and [Ir(Cy2PNHCH2-C4H3S)(η5-C5Me5)Cl2], (4)(0.005 mmol), NaOH (0.025 mmol) and the corresponding ketone(0.5 mmol) in degassed iso-PrOH (5 mL) were refluxed until the reactions were completed. After this period a sample of the reactionmixture was taken off, diluted with acetone and analyzed immediately by GC. Conversions obtained are related to the residual unreacted ketone. | |
| With [Ru((Ph2PO)-C7H14N2Cl)(η6-p-cymene)Cl2]Cl; potassium hydroxide In isopropanol at 82℃; Inert atmosphere; Schlenk technique; | 3.2 Transfer hydrogenation of ketones General procedure: Typical procedure for the catalytic hydrogen transfer reaction: a solution of the complexes [Ru((Ph2PO)-C7H14N2Cl)(η6-arene)Cl2]Cl and [Ru((Ph2PO)-C7H14N2Cl)(η6-arene)Cl2]Cl {arene: benzene 4, 5; p-cymene 6, 7} (0.005 mmol), KOH (0.025 mmol) and the corresponding ketone (0.5 mmol) in degassed 2-propanol (5 mL) was refluxed until the reactions were completed. Then, a sample of the reaction mixture was taken off, diluted with acetone and analyzed immediately by GC. The conversions are related to the residual unreacted ketone. GC analyses were performed a Shimadzu 2010 Plus Gas Chromatograph equipped with a capillary column (5 % biphenyl, 95 % dimethylsiloxane) (30 m×0.32 mm×0.25 μm). The GC parameters for transfer hydrogenation of the ketones were as follows: initial temperature, 50° C; initial time, hold min 1 min; solvent delay, 4.48 min; temperature ramp 15° C/min; final temperature, 270° C, hold min 5 min; final time, 20.67 min; injector port temperature, 200° C; detector temperature, 200° C, injection volume, 2.0 μL | |
| 98 %Spectr. | With C36H103AlO4Si14; isopropanol In neat (no solvent) at 80℃; for 24h; Glovebox; Schlenk technique; | |
| With sodium tetrahydridoborate In ethanol at 0 - 20℃; |

[2]Castro, Luis C. Misal; Bezier, David; Sortais, Jean-Baptiste; Darcel, Christophe [Advanced Synthesis and Catalysis, 2011, vol. 353, # 8, p. 1279 - 1284]
[3]Location in patent: experimental part Guerbue, Nevin; Yasar, Sedat; Ozean, Emine Ozge; Ozdemir, Ismail; Cetinkaya, Bekir [European Journal of Inorganic Chemistry, 2010, # 19, p. 3051 - 3056]
[4]Li, Ke; Niu, Jun-Long; Yang, Ming-Ze; Li, Zhen; Wu, Li-Yuan; Hao, Xin-Qi; Song, Mao-Ping [Organometallics, 2015, vol. 34, # 7, p. 1170 - 1176]
[5]Mummadi, Suresh; Brar, Amandeep; Wang, Guoqiang; Kenefake, Dustin; Diaz, Rony; Unruh, Daniel K.; Li, Shuhua; Krempner, Clemens [Chemistry - A European Journal, 2018, vol. 24, # 62, p. 16526 - 16531]
[6]Wang, Shengdong; Huang, Haiyun; Tsareva, Svetlana; Bruneau, Christian; Fischmeister, Cédric [Advanced Synthesis and Catalysis, 2019, vol. 361, # 4, p. 786 - 790]
[7]Oezdemir, Ismail; Sahin, Neslihan; Cetinkaya, Bekir [Monatshefte fur Chemie, 2007, vol. 138, # 3, p. 205 - 209]
[8]Location in patent: experimental part Aydemir, Murat; Baysal, Akin; Meric, Nermin; Gümgüm, Bahattin [Journal of Organometallic Chemistry, 2009, vol. 694, # 16, p. 2488 - 2492]
[9]Li, Zhi-Wen; An, Dong-Li; Wei, Zan-Bin; Li, Yan-Yun; Gao, Jing-Xing [Tetrahedron Letters, 2022, vol. 97]
[10]Furuta, Akihiro; Nishiyama, Hisao [Tetrahedron Letters, 2008, vol. 49, # 1, p. 110 - 113]
[11]Wang, Ding; Bruneau-Voisine, Antoine; Sortais, Jean-Baptiste [Catalysis Communications, 2018, vol. 105, p. 31 - 36]
[12]Liu, Ji-Tian; Yang, Shiyi; Tang, Weiping; Yang, Zhanhui; Xu, Jiaxi [Green Chemistry, 2018, vol. 20, # 9, p. 2118 - 2124]
[13]Krzemiński, Marek P.; Wojtczak, Andrzej [Tetrahedron Letters, 2005, vol. 46, # 48, p. 8299 - 8302]
[14]Du, Wangming; Wang, Liandi; Wu, Ping; Yu, Zhengkun [Chemistry - A European Journal, 2012, vol. 18, # 37, p. 11550 - 11554]
[15]Nishiyama, Hisao; Furuta, Akihiro [Chemical Communications, 2007, # 7, p. 760 - 762]
[16]Location in patent: experimental part Yigcit, Beyhan; Yigcit, Murat; Oezdemir, Ismail; Cetinkaya, Engin [Transition Metal Chemistry, 2012, vol. 37, # 3, p. 297 - 302]
[17]Varjosaari, Sami E.; Skrypai, Vladislav; Suating, Paolo; Hurley, Joseph J. M.; Gilbert, Thomas M.; Adler, Marc J. [European Journal of Organic Chemistry, 2017, vol. 2017, # 2, p. 229 - 232]
[18]Wang, Liandi; Liu, Tingting [Tetrahedron Letters, 2019, vol. 60, # 36]
[19]Location in patent: experimental part Keles, Mustafa; Keles, Tugba; Serindag, Osman; Yasar, Sedat; Oezdemir, Ismail [Phosphorus, Sulfur and Silicon and the Related Elements, 2010, vol. 185, # 1, p. 165 - 170]
[20]Location in patent: experimental part Addis, Daniele; Shaikh, Nadim; Zhou, Shaolin; Das, Shoubhik; Junge, Kathrin; Beller, Matthius [Chemistry - An Asian Journal, 2010, vol. 5, # 7, p. 1687 - 1691]
[21]Harrad, Mohamed Anouar; Boualy, Brahim; El Firdoussi, Larbi; Mehdi, Ahmad; Santi, Claudio; Giovagnoli, Stefano; Nocchetti, Morena; Ait Ali, Mustapha [Catalysis Communications, 2013, vol. 32, p. 92 - 100]
[22]Wei, Duo; Roisnel, Thierry; Darcel, Christophe; Clot, Eric; Sortais, Jean-Baptiste [ChemCatChem, 2017, vol. 9, # 1, p. 80 - 83]
[23]Merel, Delphine S.; Elie, Margaux; Lohier, Jean-Francois; Gaillard, Sylvain; Renaud, Jean-Luc [ChemCatChem, 2013, vol. 5, # 10, p. 2939 - 2945]
[24]Location in patent: experimental part Enthaler, Stephan; Schroeder, Kristin; Inoue, Shigeyoshi; Eckhardt, Bjoern; Junge, Kathrin; Beller, Matthias; Driess, Matthias [European Journal of Organic Chemistry, 2010, # 25, p. 4893 - 4901]
[25]Hsu, Shih-Fan; Plietker, Bernd [Chemistry - A European Journal, 2014, vol. 20, # 15, p. 4242 - 4245]
[26]Matsunaga, Hirofumi; Yoshioka, Naoko; Kunieda, Takehisa [Tetrahedron Letters, 2001, vol. 42, # 50, p. 8857 - 8859]
[27]Jiang, Fan; Yuan, Kedong; Achard, Mathieu; Bruneau, Christian [Chemistry - A European Journal, 2013, vol. 19, # 31, p. 10343 - 10352]
[28]Beller, Matthias; Jackstell, Ralf; Maes, Bert U. W.; Schneider, Carolin [European Journal of Organic Chemistry, 2020]
[29]Varjosaari, Sami E.; Skrypai, Vladislav; Herlugson, Sharon M.; Gilbert, Thomas M.; Adler, Marc J. [Tetrahedron Letters, 2018, vol. 59, # 29, p. 2839 - 2843]
[30]Location in patent: experimental part Enthaler, Stephan; Eckhardt, Bjoern; Inoue, Shigeyoshi; Irran, Elisabeth; Driess, Matthias [Chemistry - An Asian Journal, 2010, vol. 5, # 9, p. 2027 - 2035]
[31]Yao, Zi-Jian; Zhu, Jing-Wei; Lin, Nan; Qiao, Xin-Chao; Deng, Wei [Dalton Transactions, 2019, vol. 48, # 21, p. 7158 - 7166]
[32]Balkaner, Orçun; Sarıoğulları, Derya Işıl; Uslu, Aylin [Journal of Molecular Structure, 2022, vol. 1261]
[33]Location in patent: experimental part Romain, Charles; Gaillard, Sylvain; Elmkaddem, Mohammed K.; Toupet, Loic; Fischmeister, Cedric; Thomas, Christophe M.; Renaud, Jean-Luc [Organometallics, 2010, vol. 29, # 8, p. 1992 - 1995]
[34]Maity, Apurba; Sil, Amit; Patra, Sanjib K. [European Journal of Inorganic Chemistry, 2018, vol. 2018, # 36, p. 4063 - 4073]
[35]Location in patent: experimental part Barros-Filho, Bartholomeu A.; de Oliveira, Maria da Conceicao F.; Lemos, Telma L.G.; de Mattos, Marcos C.; Gonzalo, Gonzalo de; Gotor-Fernandez, Vicente; Gotor, Vicente [Tetrahedron Asymmetry, 2009, vol. 20, # 9, p. 1057 - 1061]
[36]Location in patent: experimental part Vieira, Gizelle A. B.; De Freitas Araujo, Daniel M.; Lemos, Telma L. G.; De Mattos, Marcos Carlos; Da Conceição F. De Oliveira, Maria; Melo, Vânia M. M.; De Gonzalo, Gonzalo; Gotor-Fernández, Vicente; Gotor, Vicente [Journal of the Brazilian Chemical Society, 2010, vol. 21, # 8, p. 1509 - 1516] Location in patent: experimental part Barros-Filho, Bartholomeu A.; Nunes, Fatima M.; de Oliveira, Maria da Conceicao F.; Lemos, Telma L.G.; de Mattos, Marcos C.; de Gonzalo, Gonzalo; Gotor-Fernandez, Vicente; Gotor, Vicente [Journal of Molecular Catalysis B: Enzymatic, 2010, vol. 65, # 1-4, p. 37 - 40]
[37]Amberchan, Gabriella; Snelling, Rachel A.; Moya, Enrique; Landi, Madison; Lutz, Kyle; Gatihi, Roxanne; Singaram, Bakthan [Journal of Organic Chemistry, 2021, vol. 86, # 9, p. 6207 - 6227]
[38]Zhang, Bing; Xu, Xin; Tao, Lei; Lin, Zhenyang; Zhao, Wanxiang [ACS Catalysis, 2021, vol. 11, # 15, p. 9495 - 9505]
[39]Wang, Xueqiang; Li, Chenchen; Wang, Xia; Wang, Qingli; Dong, Xiu-Qin; Duan, Abing; Zhao, Wanxiang [Organic Letters, 2018, vol. 20, # 14, p. 4267 - 4272]
[40]Li, Haoran; Wei, Duo; Bruneau-Voisine, Antoine; Ducamp, Maxime; Henrion, Mickaël; Roisnel, Thierry; Dorcet, Vincent; Darcel, Christophe; Carpentier, Jean-François; Soulé, Jean-François; Sortais, Jean-Baptiste [Organometallics, 2018, vol. 37, # 8, p. 1271 - 1279]
[41]Ciszek, Benjamin; Fleischer, Ivana [Chemistry - A European Journal, 2018, vol. 24, # 47, p. 12259 - 12263]
[42]Adkins et al. [Journal of the American Chemical Society, 1949, vol. 71, p. 3622,3623]
[43]Nieminen, Tuula, E. A.; Hase, Tapio A. [Tetrahedron Letters, 1987, vol. 28, # 40, p. 4725 - 4728]
[44]Knauer, Birgit; Krohn, Karsten [Liebigs Annalen, 1995, # 4, p. 677 - 684]
[45]Athawale, Vilas; Manjrekar, Narendra [Synlett, 2000, # 2, p. 225 - 226]
[46]Wu, Yikang; Shen, Xin; Huang, Jia-Hui; Tang, Chao-Jun; Liu, He-Hua; Hu, Qi [Tetrahedron Letters, 2002, vol. 43, # 36, p. 6443 - 6445]
[47]Fujio; Keeffe; More O'Ferrall; O'Donoghue [Journal of the American Chemical Society, 2004, vol. 126, # 32, p. 9982 - 9992]
[48]Enthaler, Stephan; Erre, Giulia; Tse, Man Kin; Junge, Kathrin; Beller, Matthias [Tetrahedron Letters, 2006, vol. 47, # 46, p. 8095 - 8099]
[49]Enthaler, Stephan; Jackstell, Ralf; Hagemann, Bernhard; Junge, Kathrin; Erre, Giulia; Beller, Matthias [Journal of Organometallic Chemistry, 2006, vol. 691, # 22, p. 4652 - 4659]
[50]Morris, David J.; Hayes, Aidan M.; Wills, Martin [Journal of Organic Chemistry, 2006, vol. 71, # 18, p. 7035 - 7044]
[51]Pinder; Smith [Journal of the Chemical Society, 1954, p. 113,117]
[52]Prasad, Edamana; Flowers II, Robert A. [Journal of the American Chemical Society, 2002, vol. 124, # 22, p. 6357 - 6361]
[53]Pasto, Mireia; Riera, Antoni; Pericas, Miquel A. [European Journal of Organic Chemistry, 2002, # 14, p. 2337 - 2341]
[54]Yu; Zeng; Sun; Deng; Dong; Chen; Wang; Pei [Journal of Organometallic Chemistry, 2007, vol. 692, # 11, p. 2306 - 2313]
[55]Shailaja; Kaanumalle, Lakshmi S.; Sivasubramanian, Karthikeyan; Natarajan, Arunkumar; Ponchot, Keith J.; Pradhan, Ajit; Ramamurthy [Organic and Biomolecular Chemistry, 2006, vol. 4, # 8, p. 1561 - 1571]
[56]Kodama, Koichi; Kobayashi, Yuka; Saigo, Kazuhiko [Chemistry - A European Journal, 2007, vol. 13, # 7, p. 2144 - 2152]
[57]Ekstroem, Jesper; Wettergren, Jenny; Adolfsson, Hans [Advanced Synthesis and Catalysis, 2007, vol. 349, # 10, p. 1609 - 1613]
[58]Current Patent Assignee: JOHNSON MATTHEY PLC - WO2005/7662, 2005, A2 Location in patent: Page/Page column 12
[59]Current Patent Assignee: JAPAN TOBACCO INC - EP1308436, 2003, A1
[60]Enthaler, Stephan; Spilker, Björn; Erre, Giulia; Junge, Kathrin; Tse, Man Kin; Beller, Matthias [Tetrahedron, 2008, vol. 64, # 17, p. 3867 - 3876]
[61]Chen, Gang; Wang, Zheng; Wu, Jiang; Ding, Kuiling [Organic Letters, 2008, vol. 10, # 20, p. 4573 - 4576]
[62]Location in patent: experimental part Baratta, Walter; Ballico, Maurizio; Baldino, Salvatore; Chelucci, Giorgio; Herdtweck, Eberhardt; Siega, Katia; Magnolia, Santo; Rigo, Pierluigi [Chemistry - A European Journal, 2008, vol. 14, # 30, p. 9148 - 9160]
[63]Location in patent: scheme or table Zhao, Miao; Yu, Zhengkun; Yan, Shenggang; Li, Yang [Tetrahedron Letters, 2009, vol. 50, # 32, p. 4624 - 4628]
[64]Location in patent: experimental part Lundgren, Rylan J.; Stradiotto, Mark [Chemistry - A European Journal, 2008, vol. 14, # 33, p. 10388 - 10395]
[65]Current Patent Assignee: UNIVERSITY OF UDINE - WO2005/51965, 2005, A2 Location in patent: Page/Page column 12-14
[66]Location in patent: experimental part Aydemir, Murat; Meriç, Nermin; Durap, Feyyaz; Baysal, Akin; Toǧrul, Mahmut [Journal of Organometallic Chemistry, 2010, vol. 695, # 9, p. 1392 - 1398]
[67]Location in patent: experimental part Bizerra, Ayla M.C.; Gonzalo, Gonzalo de; Lavandera, Ivan; Gotor-Fernandez, Vicente; de Mattos, Marcos Carlos; de Oliveira, Maria da Conceicao F.; Lemos, Telma L.G.; Gotor, Vicente [Tetrahedron Asymmetry, 2010, vol. 21, # 5, p. 566 - 570]
[68]Location in patent: experimental part Zhang, Yao; Li, Xingwei; Hong, Soon Hyeok [Advanced Synthesis and Catalysis, 2010, vol. 352, # 10, p. 1779 - 1783]
[69]Location in patent: experimental part Aydemir, Murat; Baysal, Akn [Journal of Organometallic Chemistry, 2010, vol. 695, # 23, p. 2506 - 2511]
[70]Location in patent: scheme or table Aydemir, Murat; Meric, Nermin; Baysal, Akin; Kayan, Cezmi; Togrul, Mahmut; Guemgum, Bahattin [Applied Organometallic Chemistry, 2010, vol. 24, # 3, p. 215 - 221]
[71]Current Patent Assignee: JAPAN TOBACCO INC - US2004/6130, 2004, A1 Location in patent: Page 23
[72]Location in patent: scheme or table Aydemir, Murat; Baysal, Akin [Polyhedron, 2010, vol. 29, # 4, p. 1219 - 1224]
[73]Location in patent: experimental part Aydemir, Murat; Baysal, Akin; Özkar, Saim; Yildirim, Leyla Tatar [Inorganica Chimica Acta, 2011, vol. 367, # 1, p. 166 - 172]
[74]Crittall, Matthew R.; Rzepa, Henry S.; Carbery, David R. [Organic Letters, 2011, vol. 13, # 5, p. 1250 - 1253]
[75]Location in patent: experimental part Aydemir, Murat; Baysal, Akin; Özkar, Saim; Yildirim, Leyla Tatar [Polyhedron, 2011, vol. 30, # 5, p. 796 - 804]
[76]Location in patent: experimental part Vogl, Michael; Kratzer, Regina; Nidetzky, Bernd; Brecker, Lothar [Organic and Biomolecular Chemistry, 2011, vol. 9, # 16, p. 5863 - 5870]
[77]Location in patent: scheme or table Bogar, Krisztian; Krumlinde, Patrik; Bacsik, Zoltan; Hedin, Niklas; Baeckvall, Jan-E. [European Journal of Organic Chemistry, 2011, # 23, p. 4409 - 4414]
[78]Location in patent: scheme or table Wang, Lei; Pan, Hai-Ran; Yang, Qin; Fu, Hai-Yan; Chen, Hua; Li, Rui-Xiang [Inorganic Chemistry Communications, 2011, vol. 14, # 9, p. 1422 - 1427]
[79]Location in patent: experimental part Wang, Lei; Yang, Qin; Fu, Hai-Yan; Chen, Hua; Yuan, Mao-Lin; Li, Rui-Xiang [Applied Organometallic Chemistry, 2011, vol. 25, # 8, p. 626 - 631]
[80]Location in patent: experimental part Ren, Peng; Vechorkin, Oleg; Csok, Zsolt; Salihu, Isuf; Scopelliti, Rosario; Hu, Xile [Dalton Transactions, 2011, vol. 40, # 35, p. 8906 - 8911]
[81]Location in patent: experimental part Buitrago, Elina; Zani, Lorenzo; Adolfsson, Hans [Applied Organometallic Chemistry, 2011, vol. 25, # 10, p. 748 - 752]
[82]Location in patent: experimental part Ahlford, Katrin; Adolfsson, Hans [Catalysis Communications, 2011, vol. 12, # 12, p. 1118 - 1121]
[83]Location in patent: scheme or table Nieto, Ismael; Livings, Michelle S.; Sacci, John B.; Reuther, Lauren E.; Zeller, Matthias; Papish, Elizabeth T. [Organometallics, 2011, vol. 30, # 23, p. 6339 - 6342]
[84]Location in patent: experimental part Aydemir, Murat; Baysal, Akin; Guemguem, Bahattin [Applied Organometallic Chemistry, 2012, vol. 26, # 1, p. 1 - 8]
[85]Zhang, Juanni; Yang, Xiangren; Zhou, Han; Li, Yanyun; Dong, Zhenrong; Gao, Jingxing [Green Chemistry, 2012, vol. 14, # 5, p. 1289 - 1292]
[86]Aydemir, Murat; Meric, Nermin; Baysal, Akn [Journal of Organometallic Chemistry, 2012, vol. 720, p. 38 - 45,8]
[87]Madern, Nathalie; Talbi, Barisa; Salmain, Michele [Applied Organometallic Chemistry, 2013, vol. 27, # 1, p. 6 - 12]
[88]Kumar, Mukesh; Depasquale, Joseph; White, Nicholas J.; Zeller, Matthias; Papish, Elizabeth T. [Organometallics, 2013, vol. 32, # 7, p. 2135 - 2144]
[89]Blom, Burgert; Enthaler, Stephan; Inoue, Shigeyoshi; Irran, Elisabeth; Driess, Matthias [Journal of the American Chemical Society, 2013, vol. 135, # 17, p. 6703 - 6713]
[90]Vitale, Paola; D'Introno, Cinzia; Perna, Filippo Maria; Perrone, Maria Grazia; Scilimati, Antonio [Tetrahedron Asymmetry, 2013, vol. 24, # 7, p. 389 - 394]
[91]Darwish, Moftah O.; Wallace, Alistair; Clarkson, Guy J.; Wills, Martin [Tetrahedron Letters, 2013, vol. 54, # 32, p. 4250 - 4253]
[92]Ruddy, Adam J.; Kelly, Colin M.; Crawford, Sarah M.; Wheaton, Craig A.; Sydora, Orson L.; Small, Brooke L.; Stradiotto, Mark; Turculet, Laura [Organometallics, 2013, vol. 32, # 19, p. 5581 - 5588]
[93]Fu, Qi; Zhang, Lei; Yi, Tao; Zou, Mingjun; Wang, Xiaoyan; Fu, Haiyan; Li, Ruixiang; Chen, Hua [Inorganic Chemistry Communications, 2013, vol. 38, p. 28 - 32]
[94]Ok, Fatih; Aydemir, Murat; Durap, Feyyaz [Applied Organometallic Chemistry, 2014, vol. 28, # 1, p. 38 - 43]
[95]Current Patent Assignee: UNIVERSITY OF UDINE - EP2178843, 2013, B1 Location in patent: Paragraph 0090
[96]Kayan, Cezmi; Meric, Nermin; Aydemir, Murat; Ocak, Yusuf Selim; Temel, Hamdi [Applied Organometallic Chemistry, 2014, vol. 28, # 2, p. 127 - 133]
[97]Rafikova, Khadichakhan; Kystaubayeva, Nurzhamal; Aydemir, Murat; Kayan, Cezmi; Ocak, Yusuf Selim; Temel, Hamdi; Zazybin, Alexey; Gürbüz, Nevin; Özdemir, Ismail [Journal of Organometallic Chemistry, 2014, vol. 758, p. 1 - 8]
[98]Aydemir, Murat; Rafikova, Khadichakhan; Kystaubayeva, Nurzhamal; Paşa, Salih; Meriç, Nermin; Ocak, Yusuf Selim; Zazybin, Alexey; Temel, Hamdi; Gürbüz, Nevin; Özdemir, Ismail [Polyhedron, 2014, vol. 81, p. 245 - 255]
[99]McNerney, Brian; Whittlesey, Bruce; Cordes, David B.; Krempner, Clemens [Chemistry - A European Journal, 2014, vol. 20, # 46, p. 14959 - 14964]
[100]Bigler, Raphael; Mezzetti, Antonio [Organic Letters, 2014, vol. 16, # 24, p. 6460 - 6463]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 99% | With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; hydroxylamine; oxygen In water monomer; 1,2-dichloro-ethane at 80℃; for 6h; Autoclave; | |
| 99% | With C53H46ClN3P2Ru; potassium-t-butoxide; acetone at 56℃; for 0.0833333h; | |
| 98% | With methyl 3,5-bis((1H-1,2,4-triazol-1-yl)methyl)benzoate; oxygen; anhydrous Sodium acetate; nickel(II) bromide at 120℃; for 72h; |
| 97% | With hydrogenchloride; 2,2,6,6-tetramethyl-1-piperidinyloxy free radical; Amberlite IRA 900 chlorite In dichloromethane at 20℃; for 4.5h; | |
| 97% | With 1,10-Phenanthroline; tris(2,4-pentanedionato)iron(III); potassium carbonate In toluene for 48h; Reflux; Green chemistry; | |
| 96% | With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; polymer-bound {NMe3(1+)*Br(OAc)2(1-)} In dichloromethane at 40℃; for 24h; | |
| 96% | With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; bisacetoxybromate(I) resin In dichloromethane at 20℃; for 24h; | |
| 95% | With [(2-(benzoimidazol-2-yl)-6-(3,5-dimethylpyrazol-1-yl)pyridine)RuCl2(PPh3)]; potassium-t-butoxide; acetone In methanol at 56℃; for 0.333333h; Inert atmosphere; | A typical procedure for the catalytic oxidation of alcohols General procedure: The catalyst solutionwas prepared by dissolving complex 3(36.1 mg,0.05mmol) in methanol (5.0 mL).Under a nitrogen atmosphere, the mixture of an alcohol substrate (2.0 mmol) and1.0 mL of the catalyst solution (0.01mmol) in 20mL acetone was stirred at 56 Cfor 10 minutes. tBuOK(22.4mg, 0.2 mmol)was then added to initiate the reaction.At the stated time, 0.1 mL of the reaction mixture was sampled and immediately diluted with 0.5 mL acetone pre-cooled-to-0 C for GC or NMR analysis. After the reaction was complete, the reaction mixture was condensed under reduced pressure and subject to purification by flash silica gel column chromatography to afford the corresponding ketone product, which was identified by comparison with the authentic sample through NMR and GC analysis. |
| 92% | With C6H4MoNO7(1-)*C19H42N(1+); oxygen In water monomer at 100℃; for 24h; Green chemistry; chemoselective reaction; | 2.3. General procedure for the catalytic oxidation of alcohols toaldehydes General procedure: A mixture of alcohol (0.75 mmol), and catalyst Mo1 (13 mg,3.0 mol%) taken in 0.5 mL of water was stirred at 100 ° C under oxygenatmosphere (O2 bladder) and the stirring was continued for16-24 h as per requirement. The progress of reaction was monitoredby TLC. After completion of the reaction, ethyl acetate was added to the mixture. The aqueous phase was extracted with ethyl acetate 2-3 times. Then the combined organic extracts were driedover anhydrous sodium sulfate and the solvent was removed under reduced pressure. The crude product so obtained was purified by column chromatography using hexane-ethyl acetate as eluent. While the known products were characterized by spectroscopic techniques and compared with reported data and the new products 22b and 36b were characterized completely. The characterization detail is provided in supporting information section. |
| 91% | With iron (ΙΙΙ) nitrate nonahydrate; oxygen; 2,3-dicyano-5,6-dichloro-p-benzoquinone In 1,2-dichloro-ethane at 60℃; for 3h; Schlenk technique; Green chemistry; | |
| 90% | With tert.-butylhydroperoxide In hexane; water monomer at 50℃; for 23.5h; | |
| 89% | With Cs2CO3 In toluene at 110℃; for 18h; | |
| 88% | With C14H14N6O2; oxygen; anhydrous Sodium acetate; palladium diacetate at 120℃; for 96h; | |
| 88% | With copper(II) selenite dihydrate; potassium hydroxide In toluene for 28h; Reflux; | |
| 87% | With oxygen; potassium carbonate In toluene at 110℃; for 24h; Green chemistry; chemoselective reaction; | |
| 86% | With tert.-butylhydroperoxide; Eosin In decane; acetonitrile at 25℃; for 28h; Inert atmosphere; Irradiation; Molecular sieve; Green chemistry; chemoselective reaction; | General procedure for oxidation of alcohols: General procedure: Oven dried round bottom flask was charged with Eosin Y (5 mmol) alcohol (1 mmol) and 3 equiv. of TBHP (5.5 M in decane) in dry ACN. The resulting mixture was degassed for 15 mins, followed by back filling N2, and then irradiated under Blue LED light (12W, 455 nm) at room temperature (25 oC). After reaction completion monitored through TLC, the mixture was diluted with 15 ml of 10% NaHCO3 solution, and extracted with EtOAc (3 × 20 ml). The combined organic extracts were washed with brine (20 ml), dried over Na2SO4, and concentrated on vacuo. Purification of the crude product on silica gel using EtOAc:Hexane as solvent system afforded the desired product. |
| 85% | With calcium hypochlorite; water monomer for 0.0166667h; microwave irradiation; | |
| 85% | With phosphotungstate-Fe3+ dual-metal-site modulated graphitic carbon nitride In acetonitrile at 25 - 35℃; for 20h; Sealed tube; Irradiation; | |
| 80% | With Montmorillonite K10; iron nitrate (III) In hexane at 60℃; for 2h; | |
| 78% | With [RuCl2(PPh3)2(2-PyCH21,3,5-triaza-7-phosphadamantane)].Br; potassium hydroxide In water monomer for 48h; Schlenk technique; Reflux; Inert atmosphere; | General procedure for dehydrogenation of alcohols General procedure: Ruthenium complex 2 (5 mol%), KOH (15 mol%), alcohol (5 mmol) and H2O (1.0 mL) wereplaced in Schlenk tube. The reaction mixture was stirred under reflux for 48 h. After completion of thereaction, the product was extracted with dichloromethane. Then all DCM were evaporated under vacuo,the product ketones and aldehydes were isolated from crude mixture by column chromatography usinghexane/EtOAc as eluent. The formation of products was confirmed by comparing the 1H-NMR datawith literature reports. |
| 73% | With dihydrogen peroxide; acetic acid; sodium bromide In water monomer at 60℃; for 2h; Inert atmosphere; | General Procedure for NaBr-Catalyzed Oxidation General procedure: Under nitrogen atmosphere, to a solution of substrate alcohol (0.5 mmol) in aceticacid (1.0 mL) was added a stock-solution of aqueous NaBr solution (1.94 M, 25 μL)and 30% aqueous H2O2 (50 μL, 0.5 mmol). After stirring the mixture for one hour at60 °C, additional 30% aqueous H2O2 (50 μL, 0.5 mmol) was added, and stirring wascontinued for another one hour. After cooling, the mixture was poured into a saturatedaqueous NaHCO3 solution (ca. 30 mL) with the aid of CH2Cl2, and resulting mixturewas extracted with CH2Cl2. The combined organic layers were dried over anhydrousMgSO4, filtered and concentrated in vacuo. The residue was chromatographed onsilica gel (flash column or preparative TLC) to afford the corresponding ketone. |
| 61% | In acetonitrile at 25℃; for 15h; Schlenk technique; Inert atmosphere; Sealed tube; Irradiation; | |
| 60.7% | With tert.-butylhydroperoxide at 70℃; | |
| 58% | With ruthenium(III) trichloride hydrate; C13H19N4(1+)*Br(1-) In toluene at 115℃; for 24h; Schlenk technique; Inert atmosphere; | 4.3. General procedure for dehydrogenation of alcohol General procedure: RuCl3nH2O (0.5 mol %), HMTA-Bz (1 mol %), alcohol (0.25 ml)and dry toluene (1.0 ml) were placed in a Schlenk tube. The reactionmixture was stirred under open condition to nitrogen andrefluxed for 24 h. After completion of the reaction all toluene wereevaporated under vacuo, the oxidized products were isolated fromcrude mixture with the help of column chromatography using hexane/EtOAc as eluent. The formation of products was confirmed bycomparing the 1H NMR data with literature reports. |
| 55% | With barium permanganate Irradiation; | |
| 45% | With oxygen; triethylamine In tetrahydrofuran; toluene at 45℃; for 20h; | |
| 45% | With iron (ΙΙΙ) nitrate nonahydrate; N-hydroxyphthalimide; oxygen In acetonitrile at 25℃; | 3 2.2. General procedure of oxidation of secondary alcohols General procedure: Substrate (1 mmol) and the desired amounts of Fe(NO3)3·9H2Oand NHPI were added to 1.5 mL of acetonitrile in a 15 mL test tube.The solution was maintained for 20 h under an atmospheric pres-sure of O2and at 25C. After the reaction was quenched by Na2S2O3solution, 60 mg of nitrobenzene, serving as an internal standard,was added to the reaction system. The solution was centrifugedand the supernatant was diluted with diethyl ether and dried withanhydrous Na2SO4for 30 min. The products were analyzed by GC,and further confirmed by GC-MS. The isolated yield was obtainedthrough column chromatography generally performed on silica gel(200-300 mesh). |
| 24% | With sodium tetrafluoroborate; potassium hydroxide In acetone at 100℃; for 12h; Inert atmosphere; Schlenk technique; | 3.8. General procedure for catalytic Oppenauer-type oxidation General procedure: To a mixture of ruthenium complex (0.04 mmol), KOH (0.4 mmol) and NaBF 4 (0.16 mmol) were added acetone (2 mL) and a substrate (0.8 mmol), the resulting mixture was heated at reflux in argon for 12 h or 100 °C for 12 h with some inactive substrates ( Table 3 , 2o-2r ). After the reaction completed, the prod- uct was isolated by column chromatography on silica gel with dichloromethane-petroleum ether as eluents. |
| With chloroform; dinitrogen tertoxide | ||
| With potassium dichromate||potassium bichromate||K2Cr2O7||Cr2O7K2; sulfuric acid | ||
| 8 % Chromat. | With oxygen In benzene for 10h; Ambient temperature; Irradiation; | |
| With manganese(IV) oxide; molecular sieve In hexane for 12h; Heating; Yield given; | ||
| 3 % Chromat. | With oxygen In benzene for 16h; Ambient temperature; Irradiation; other time, other solvent,; | |
| With potassium peroxodisulfate; <i>tert</i>-butyl alcohol In water monomer at 25℃; Radiolysis; | ||
| With C30H26N3OP; potassium carbonate; copper chloride (I) at 110℃; for 14h; | ||
| With palladium diacetate; oxygen; triethylamine In tetrahydrofuran; toluene at 20℃; for 12h; | ||
| 97 %Spectr. | With Br(1-)*C20H27BrN5O2Pd(1+); oxygen; anhydrous Sodium acetate at 20 - 120℃; for 96h; | |
| 86 %Spectr. | With [Cp*Ir(6,6'-dihydroxy-2,2'-bipyridine)(H2O)](OTf)2 In water monomer for 20h; Inert atmosphere; Reflux; | |
| With C22H28BF2NOS; isopropanol; potassium hydroxide for 9h; Reflux; | ||
| 92 %Spectr. | With Cp*Ir(6,6'-dionato-2,2'-bipyridine)(H2O) In n-Pentane at 90℃; for 5h; | |
| 88 %Chromat. | With styrene; 2C13H8NS(1-)*C12H27P*Ir(3+)*CF3O3S(1-); potassium-t-butoxide In toluene at 110℃; for 12h; Schlenk technique; Inert atmosphere; | |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; 6,8-di(tert-butyl)-3-[2-(1H-imidazol-4-yl)ethyl]-3,4-dihydro-2H-1,3-benzoxazine; oxygen; copper trifluoromethanesulfonate In dichloromethane at 40℃; for 28.5h; | Typical procedure for the oxidation of secondary alcohols. General procedure: A 5-mL two-necked, round-bottom flask equipped with a magnetic stirrer and an oxygen balloon was charged in succession with 0.0106 g(0.05 mmol) of Cu(OTf), 0.0078 g (0.05 mmol) of TEMPO, 0.0171 g (0.05 mmol) of benzoxazine ligand L, and 2 mL of methylene chloride. The corresponding alcohol, 1 mmol, was then added at 40°C under stirring, and oxygen from the balloon was introduced through a three-way valve. The progress of the reaction was monitored by GLC using a suitable column. | |
| With sodium chlorine monoxide; N-Bromosuccinimide; [(R,R)-(N,N-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminato)]manganese(III) chloride In dichloromethane; water monomer at 25℃; for 4.5h; chemoselective reaction; | Catalytic oxidation General procedure: In a typical experiment an alcohol (1 mmol), salen-Mn(III) complex (0.02 mmol), NBS (0.13 mmol), and CH2Cl2 (2 mL) was loaded into a 5 mL flask at room temperature. NaOCl (2.3 mmol) was added dropwise within 10 min and progress of the reaction was monitored by GC. Upon completion of the process the reaction mixture was treated twice with10 mL of 10% NaHSO3 solution and the organic phase was dried over anhydrous sodium sulfate and filtered off. The solvent was removed by distillation. The residue was distilled under low pressure. | |
| With Pd/TiO2(at)MIL-101 at 90℃; for 24h; Inert atmosphere; | ||
| With nickel (II) chloride In water monomer at 20℃; for 6h; Inert atmosphere; Irradiation; | ||
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; Trametes versicolor laccase In tert-butyl methyl ether at 30℃; for 16h; Enzymatic reaction; | ||
| With sodium chlorine monoxide; Sodium hydrogenocarbonate; sodium bromide In water monomer at 0℃; for 0.0833333h; | ||
| With chromium(VI) oxide | ||
| 86 %Spectr. | With 2-bromoanthracene-9,10-dione; nitrobenzene In acetonitrile at 30℃; for 20h; Sealed tube; Irradiation; Inert atmosphere; | |
| 88 %Chromat. | With C25H16N2O10Ru3; anhydrous sodium carbonate; acetone at 60℃; for 8h; Inert atmosphere; Schlenk technique; |

[2]Du, Wangming; Wang, Liandi; Wu, Ping; Yu, Zhengkun [Chemistry - A European Journal, 2012, vol. 18, # 37, p. 11550 - 11554]
[3]Urgoitia; Sanmartin; Herrero; Domínguez [Chemical Communications, 2015, vol. 51, # 23, p. 4799 - 4802]
[4]Kloth, Katrin; Bruenjes, Marco; Kunst, Eike; Joege, Thomas; Gallier, Florian; Adibekian, Alexander; Kirschning, Andreas [Advanced Synthesis and Catalysis, 2005, vol. 347, # 10, p. 1423 - 1434]
[5]Song, Hansoo; Kang, Byungjoon; Hong, Soon Hyeok [ACS Catalysis, 2014, vol. 4, # 9, p. 2889 - 2895]
[6]Sourkouni-Argirusi, Georgia; Kirschning, Andreas [Organic Letters, 2000, vol. 2, # 24, p. 3781 - 3784]
[7]Bruenjes, Marco; Sourkouni-Argirusi, Georgia; Kirschning, Andreas [Advanced Synthesis and Catalysis, 2003, vol. 345, # 5, p. 635 - 642]
[8]Wang, Qingfu; Du, Wangming; Liu, Tingting; Chai, Huining; Yu, Zhengkun [Tetrahedron Letters, 2014, vol. 55, # 9, p. 1585 - 1588]
[9]Thiruvengetam, Prabaharan; Chakravarthy, Rajan Deepan; Chand, Dillip Kumar [Journal of Catalysis, 2019, vol. 376, p. 123 - 133]
[10]Hu, Yongke; Chen, Lei; Li, Bindong [Catalysis Communications, 2018, vol. 103, p. 42 - 46]
[11]Zhang, Yuecheng; Huo, Wenge; Zhang, Hong-Yu; Zhao, Jiquan [RSC Advances, 2017, vol. 7, # 75, p. 47261 - 47270]
[12]Hu, Xinyu; Zhu, Haiyan; Sang, Xinxin; Wang, Dawei [Advanced Synthesis and Catalysis, 2018, vol. 360, # 22, p. 4293 - 4300]
[13]Urgoitia, Garazi; Maiztegi, Ainhoa; Sanmartin, Raul; Herrero, María Teresa; Domínguez, Esther [RSC Advances, 2015, vol. 5, # 125, p. 103210 - 103217]
[14]Choudhury, Prabhupada; Behera, Pradyota Kumar; Bisoyi, Tanmayee; Sahu, Santosh Kumar; Sahu, Rashmi Ranjan; Prusty, Smruti Ranjita; Stitgen, Abigail; Scanlon, Joseph; Kar, Manoranjan; Rout, Laxmidhar [New Journal of Chemistry, 2021, vol. 45, # 13, p. 5775 - 5779]
[15]Kumaravel, Sangeetha; Thiruvengetam, Prabaharan; Ede, Sivasankara Rao; Karthick; Anantharaj; Sam Sankar, Selvasundarasekar; Kundu, Subrata [Dalton Transactions, 2019, vol. 48, # 45, p. 17117 - 17131]
[16]Devari, Shekaraiah; Rizvi, Masood Ahmad; Shah, Bhahwal Ali [Tetrahedron Letters, 2016, vol. 57, # 30, p. 3294 - 3297]
[17]Mojtahedi, Mohammad M.; Saidi, Mohammad R.; Bolourtchian, Mohammad; Shirzi, Jafar S. [Monatshefte fur Chemie, 2001, vol. 132, # 5, p. 655 - 658]
[18]Duan, Limei; Li, Peihe; Li, Wanfei; Liu, Jinghai; Liu, Ying; Liu, Zhifei; Lu, Ye; Sarina, Sarina; Wang, Jinghui; Wang, Yin; Wang, Yingying; Zhu, Huaiyong [Catalysis science and technology, 2021, vol. 11, # 13, p. 4429 - 4438]
[19]Hirano, Masao; Komiya, Kan; Morimito, Takashi [Organic Preparations and Procedures International, 1995, vol. 27, # 6, p. 703 - 706]
[20]Bhatia, Anita; Muthaiah, Senthilkumar [Synlett, 2018, vol. 29, # 12, p. 1644 - 1648]
[21]Komagawa, Hiromi; Maejima, Yukako; Nagano, Takashi [Synlett, 2016, vol. 27, # 5, p. 789 - 793]
[22]Sun, Danhui; Li, Peihe; Wang, Xia; Wang, Yingying; Wang, Jinghui; Wang, Yin; Lu, Ye; Duan, Limei; Sarina, Sarina; Zhu, Huaiyong; Liu, Jinghai [Chemical Communications, 2020, vol. 56, # 79, p. 11847 - 11850]
[23]Baskaran, Thangaraj; Kumaravel, Raju; Christopher, Jayaraj; Sakthivel, Ayyamperumal [RSC Advances, 2014, vol. 4, # 22, p. 11188 - 11196]
[24]Barteja, Parul; Devi, Preeti; Kannan, Muthukumar; Muthaiah, Senthilkumar [Journal of Catalysis, 2020, vol. 386, p. 1 - 11]
[25]Mojtahedi, Mohammad M.; Sharifi, Ali; Kaamyabi, Sharif; Saidi, Mohammad R. [Journal of Chemical Research - Part S, 2002, # 6, p. 286 - 287]
[26]Batt, Frédéric; Bourcet, Emmanuel; Kassab, Youssef; Fache, Fabienne [Synlett, 2007, # 12, p. 1869 - 1872]
[27]Zhao, Hanqing; Sun, Wei; Miao, Chengxia; Zhao, Quanyi [Journal of Molecular Catalysis A: Chemical, 2014, vol. 393, p. 62 - 67]
[28]Liu, Hongming; Luo, Shubiao; Xue, Peng; Zhou, Jun [Journal of Organometallic Chemistry, 2022, vol. 965-966]
[29]Grundy [Journal of the Chemical Society, 1957, p. 5087]
[30]Klages [Chemische Berichte, 1903, vol. 36, p. 3589]
[31]Suzuki; Yamazaki; Takabe; Morioka; Mizuno; Matsushima [Bulletin of the Chemical Society of Japan, 1984, vol. 57, # 7, p. 1870 - 1875]
[32]Hirano, Masao; Yakabe, Sigetaka; Chikamori, Hideki; Clark, James H.; Morimoto, Takashi [Journal of Chemical Research - Part S, 1998, # 6, p. 308 - 309]
[33]Suzuki; Yamazaki; Takabe; Morioka; Mizuno; Matsushima [Bulletin of the Chemical Society of Japan, 1984, vol. 57, # 7, p. 1870 - 1875]
[34]Baciocchi, Enrico; Bietti, Massimo; Ercolani, Gianfranco; Steenken, Steen [Tetrahedron, 2003, vol. 59, # 5, p. 613 - 618]
[35]Tsai, Weiwen; Liu, Yi-Hung; Peng, Shie-Ming; Liu, Shiuh-Tzung [Journal of Organometallic Chemistry, 2005, vol. 690, # 2, p. 415 - 421]
[36]Schultz, Mitchell J.; Hamilton, Steven S.; Jensen, David R.; Sigman, Matthew S. [Journal of Organic Chemistry, 2005, vol. 70, # 9, p. 3343 - 3352]
[37]Location in patent: experimental part Urgoitia, Garazi; Sanmartin, Raul; Herrero, Maria Teresa; Dominguez, Esther [Green Chemistry, 2011, vol. 13, # 8, p. 2161 - 2166]
[38]Kawahara, Ryoko; Fujita, Ken-Ichi; Yamaguchi, Ryohei [Journal of the American Chemical Society, 2012, vol. 134, # 8, p. 3643 - 3646]
[39]Location in patent: experimental part Kilic, Ahmet; Kayan, Cezmi; Aydemir, Murat; Durap, Feyyaz; Durgun, Mustaf; Baysal, Akin; Tas, Esref; Guemguem, Bahattin [Applied Organometallic Chemistry, 2011, vol. 25, # 5, p. 390 - 394]
[40]Kawahara, Ryoko; Fujita, Ken-Ichi; Yamaguchi, Ryohei [Angewandte Chemie - International Edition, 2012, vol. 51, # 51, p. 12790 - 12794][Angew. Chem., 2012, p. 12962 - 12966]
[41]Wang, Dawei; Zhao, Keyan; Yang, Shuyan; Ding, Yuqiang [Russian Journal of General Chemistry, 2014, vol. 84, # 10, p. 2016 - 2020][Zh. Obshch. Khim., 2014, vol. 84, # 10, p. 2016 - 2020,5]
[42]Zhang; Huang; Lü; Cao; Zhao [Russian Journal of General Chemistry, 2015, vol. 85, # 8, p. 1965 - 1972][Zh. Obshch. Khim.]
[43]Zhang; Lü; Cui; Zhao [Russian Journal of General Chemistry, 2014, vol. 84, # 10, p. 2021 - 2026][Zh. Obshch. Khim.]
[44]Tilgner, Dominic; Friedrich, Martin; Hermannsdörfer, Justus; Kempe, Rhett [ChemCatChem, 2015, vol. 7, # 23, p. 3916 - 3922]
[45]Zhao, Lei-Min; Meng, Qing-Yuan; Fan, Xiang-Bing; Ye, Chen; Li, Xu-Bing; Chen, Bin; Ramamurthy, Vaidhyanathan; Tung, Chen-Ho; Wu, Li-Zhu [Angewandte Chemie - International Edition, 2017, vol. 56, # 11, p. 3020 - 3024][Angew. Chem., 2017, vol. 129, # 11, p. 3066 - 3070]
[46]Martínez-Montero, Lía; Gotor, Vicente; Gotor-Fernández, Vicente; Lavandera, Iván [Green Chemistry, 2017, vol. 19, # 2, p. 474 - 480]
[47]Chen, Tao; Xu, Zhenkai; Zhou, Lei; Qiu, Jiaqi; Wang, Maolin; Wang, Jiping [Molecular catalysis, 2019, vol. 474]
[48]Dhami,K.S.; Stothers,J.B. [Canadian Journal of Chemistry, 1965, vol. 43, p. 479]
[49]Chen, Guanghui; Liao, Shengfu; Liu, Jianguo; Liu, Qiying; Ma, Longlong; Yan, Long [RSC Advances, 2020, vol. 10, # 61, p. 37014 - 37022]
[50]Dong, Qing; Ma, Zongwen; Hao, Zhiqiang; Han, Zhangang; Lin, Jin; Lu, Guo-Liang [Applied Organometallic Chemistry, 2021, vol. 35, # 9]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 86% | Stage #1: o-hydroxyacetophenone With lithium hydroxide monohydrate In tetrahydrofuran at 20℃; for 1h; Stage #2: dimethyl sulfate In tetrahydrofuran for 60h; | |
| 86% | Stage #1: o-hydroxyacetophenone With lithium hydroxide monohydrate In tetrahydrofuran at 20℃; for 1h; Stage #2: dimethyl sulfate In tetrahydrofuran for 60h; | 4.1.5. 2-Methoxyphenyl methyl ketone 14 A solution of 1-(2-hydroxy-phenyl)-ethanone 13 (35.2 mL, 300 mmol) and LiOH·H2O (24.8 g, 590 mmol) in THF (400 mL) was stirred at room temperature for 1 h, and then dimethyl sulfate (42 mL, 293 mmol) was added. After completion of the reaction (TLC, 60 h), the solvent was removed under reduced pressure. The residue was dissolved in 2 M NaOH, and the resulting solution was extracted with diethyl ether. The combined extracts were dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure to afford compound 14 (38.5 g, 256.0 mmol). Yield: 86%. 1H NMR (400 MHz, CDCl3): δ 7.71-7.69 (dd, J = 7.7, 1.8 Hz, 1H), 7.44-7.40 (m, 1H), 6.97-6.92 (m, 2H), 3.86 (s, 3H), 2.58 (s, 3H). 13C NMR (100.6 MHz, CDCl3): δ 199.8, 158.9, 133.7, 130.3, 128.2, 120.5, 111.6, 55.5, 31.8. IR (KBr): 3074, 3003, 2944, 2840, 1674, 1598, 1578, 1486, 1437, 1358, 1292, 1247, 1163, 1126, 1023, 967, 805, 758, 595, 534 cm-1. |
| 81% | With tetrabutylammomium bromide; sodium hydroxide In diethoxymethane; water at 50 - 55℃; for 4h; |
| 75% | With lithium hydroxide In tetrahydrofuran at 70℃; for 1.5h; | |
| With potassium hydroxide |

[2]Location in patent: experimental part Dhondge, Attrimuni P.; Shaikh, Ajam C.; Chen, Chinpiao [Tetrahedron Asymmetry, 2012, vol. 23, # 10, p. 723 - 733]
[3]Location in patent: experimental part Coleman, M. Todd; Leblanc, Gabriel [Organic Process Research and Development, 2010, vol. 14, # 3, p. 732 - 736]
[4]Basak, Anindita; Nayak, Mrinal K.; Chakraborti, Asit K. [Tetrahedron Letters, 1998, vol. 39, # 27, p. 4883 - 4886]
[5]v. Auwers; Brink [Justus Liebigs Annalen der Chemie, 1932, vol. 493, p. 218,231]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 96% | Stage #1: 2-Methoxyacetophenone With potassium hydroxide In ethanol; water at 0℃; for 0.5h; Stage #2: benzaldehyde In ethanol; water for 48h; | 1.1 [Step 1] Preparation of ((E)-1-(2-methoxyphenyl)-3-phenylprop-2-en-1-one 2’-Methoxyacetophenone (1.09 g, 6.60 mmol) and 10% potassium hydroxide aqueous solution (80 mL, 142mmol) were dissolved in ethanol (15mL), and stirred at 0°C for 30 min. before adding benzaldehyde (0.800 mL, 7.92mmol) dropwise, and the mixture was stirred at room temperature for about 2 days. Ethanol was removed by evaporationusing an evaporator, and the remaining mixture was extracted 4 times with ethyl acetate (30mL), after which the extractwas dried with magnesium sulfate, and the filtered solution was concentrated. The residue was purified using silica gelcolumn chromatography (silica gel 60, eluant: ethyl acetate/hexane=1:7 (v/v)) to obtain the subject compound (1.52 mg,6.36 mmol, yield: 96%) as a yellow, oil-like substance. 1H NMR (400 MHz, CDCl3): δ: 7.64 (d, J = 4.8 Hz, 1H), 7.61-7.57 (m, 3H), 7.48 (ddd, J = 15.2, 15.2, 2.0 Hz,1H), 7.41-7.35 (m, 4H), 7.05 (dd, J = 6.4, 6.4 Hz, 1H), 7.01 (d, J = 8.5 Hz, 1H), 3.91 (s, 3H). |
| 88% | With sodium hydroxide In methanol; water for 12h; Inert atmosphere; | |
| 87% | With sodium hydroxide In water at 0 - 20℃; diastereoselective reaction; |
| 79.6% | With sodium hydroxide In ethanol; water Heating; | 4.1.2. Synthesis and spectroscopic characterization of chalcones General procedure: All syntheses were carried out in the same fashion. Each reaction was monitored by TLC for 24 h to determine when the starting materials had been consumed. All TLC analyses were run on Selecto Scientific flexible silica gel-coated plates containing a fluorescent indicator and were developed using a hexanes-ethyl acetate (4:1) solution as the eluent. The following procedure is representative of the synthesis of all chalcones (see [Chart 1] and [Chart 2] for structures): A 25-mL round-bottomed flask was charged with the appropriate derivatives of both acetophenone (3 mmol) and benzaldehyde (3 mmol), and mixed with 10 mL of 95% EtOH. The mixture was then stirred magnetically while being gently heated (in a 30 °C water bath) until both starting materials dissolved. In a separate flask, NaOH (3.5 mmol) was added to 10-mL of an ethanol-water (1:1) and the mixture was stirred magnetically until the solid dissolved. The NaOH solution was then added dropwise (using a Pasteur pipet) to the ethanolic solution of acetophenone and benzaldehyde described above. In most cases, the reaction mixture turned yellow and solidified within a few minutes. Ice water (2 mL) was added to the flask and the mixture was stirred vigorously. The solid was collected on a Hirsch funnel, washed with cold water, and air-dried overnight. The purity of the crude product was determined at this point using a combination of TLC analysis, melting point measurement, and 1H NMR spectroscopy. In case of an oily product, the reaction mixture was extracted with two 10-mL portions of CH2Cl2 and the organic phase was collected, dried over Na2SO4, and removed using a rotary evaporator. The purity of the oily product was then determined as described above. All impure products (solid or oil) were purified by column chromatography using Merck Silica gel (grade 60, 230-400 mesh) and 4:1 hexanes-ethyl acetate as eluent. In case of a solid, chromatographic separation was followed by recrystallization from either methanol or ethanol-water mixture. In all cases, the purity of the final product was checked again as described above; the spectral characteristics were found to be in good general agreement with those found in the literature.4 The organic chalcones prepared for this study were either pale-yellow solids or oils of the same color (as specified); the ferrocenyl analogs were reddish-orange solids or oils. For each of the reported compounds below, the 1H NMR data is presented as δ (multiplicity, integral ratio), and the IR data as νCO, νCC. The following % yield and physical data are for new chalcones prepared for this study. Data for other chalcones (not given below) have been reported elsewhere[4], [5] and [6] and are also available online as Supplementary data. |
| 78% | With sodium hydroxide In ethanol; water at 20℃; for 4h; | |
| 78% | With sodium hydroxide In water at 20℃; Cooling with ice; | Synthesis of (E)-chalcones 1a-j General procedure: Into a 25 mL flask was added ethanol (1 mL), acetophenone (1 mmol) and a solution of NaOH (507 mg) in H2O (450 μL). The reaction mixture was placed in an ice bath, and benzaldehyde was slowly added (102 μL, 1 mmol). The mixture was stirred for 2 to 6 h at room temperature and then neutralized with aqueous solution of NH4Cl (5 mL), extracted with AcOEt (3 x 30 mL). The organic phase was dried over Na2SO4 and then concentrated under vacuum. |
| 72.8% | With sodium hydroxide In methanol at 28℃; for 12h; | |
| 71% | Stage #1: 2-Methoxyacetophenone With lithium hydroxide monohydrate In ethanol at 20℃; for 0.166667h; Stage #2: benzaldehyde In ethanol at 20℃; for 2h; | |
| 71% | With lithium hydroxide monohydrate In ethanol at 20℃; for 2h; | C.1.b In a 14-mL vial, the substituted acetophenone (1.25 mmol) and lithium hydroxide monohydrate (0.251 mmol) were dissolved in ethanol (5 mL) and the mixture was stirred at room temperature (RT) for 10 min followed by addition of substituted benzaldehyde (1.272 mmol). The reaction mixture was then stirred at RT and monitored by TLC using 25% ethyl acetate/hexanes as the solvent system. The reaction was quenched after 2 hrs by pouring into 50 mL of stirring ice cold water. If the product precipitated out after quenching with cold water, it was filtered off and crystallized with hot ethanol. In some examples, a sticky mass was observed in the aqueous solution after quenching. In those cases, the product was extracted by ethyl acetate (3 x 50 mL), dried over sodium sulfate, and concentrated under vacuum. The crude product was purified by flash chromatography using ethyl acetate/hexanes as the solvent system in increasing order of polarity. (E)-l-(2-methoxyphenyl)-3-phenylprop-2-en-l-one (lb): It was obtained as yellow oil in 71% yield. *H NMR (400 MHz, DMS) δ =7.76 -7.67 (m, 2H), 7.56 - 7.46 (m, 3H), 7.44 -7.37 (m, 4H), 7.18 (d, 7 =7.9 Hz, 1H), 7.05 (td, 7 =7.5, 0.9 Hz, 1H), 3.85 (s, 3H). 13C NMR (101 MHz, DMSO) δ 192.60, 158.17, 142.97, 135.01, 133.53, 130.94, 129.98, 129.45, 129.23, 128.97, 127.41, 121.01, 112.79, 56.27. LC- MS (ESI-TOF): m/z 239.1072 ([Ci6H1402 + H]+ calcd. 239.1067). Purity 98.02% (rt 7.21 min). |
| 70% | With carbon tetrabromide In neat (no solvent) at 60℃; for 27h; | |
| 68% | With sodium hydroxide In ethanol; water at 20℃; | |
| 64% | With iodine; triphenylphosphine In 1,4-dioxane for 11h; Reflux; | General procedure for the synthesis of compounds 3 General procedure: A mixture of PPh3 (524 mg, 2.0 mmol, 1.0 equiv), I2 (507 mg, 2.0 mmol, 1.0 equiv) and 1,4-dioxane (4 mL) was stirred at rt for 30 min. Then, compounds 1 (2.0 mmol) and 2 (2.4 mmol) were added to the mixture, and stirred for appropriate time (Tabel 2) under reflux temperuture. After completion of the reaction (TLC), DCM (20 mL) was added and the solution was washed with NaS2O3 (20 mL, 10%). Then, organic layer was washed with water (2 × 20 mL) and brine (2 × 20 mL) and was dried over anhyd. MgSO4. The organic layer was concentrated under reduced pressure and the crude product was purified by column chromatography to afford the corresponding product 3. |
| 60% | With potassium hydroxide In ethanol; water for 12h; Heating; | |
| 52% | With lithium hydroxide monohydrate In ethanol at 20℃; for 2.25h; | Preparation of (E)-1-(2-methoxyphenyl)-3-phenylprop-2-en-1-one (8a) General procedure: To an ethanol solution of the substituted acetophenones(1.0 equiv) was added LiOHH2O (1.0 equiv). The reaction mixture was stirred for 15 min at room temperature and then treated withthe desired benzaldehyde (1.2 equiv). The mixture was stirred for2 h at room temperature. The reaction mixture was quenched withH2O (100 mL) and then diluted with EtOAc (200 mL) and washedwith H2O (2 200 mL) and brine (200 mL). The organic layer wasdried with anhydrous Na2SO4 and concentrated in vacuo. The residuewas purified by column chromatography on SiO2 |
| 33% | With sodium hydroxide at 20℃; for 48h; | 2.1. Chemistry General procedure: Synthesis of methoxychalcones 5-21 was achieved byClaisen-Schmidt reaction, using protocols reported by ourgroup with minor modifications [16,17]. Appropriateacetophenone derivatives (5 mmol) and respectivebenzaldehyde derivatives (5 mmol) were solubilized inethanol (10 mL), and ethanolic solution of NaOH (1.0 mol/L,5 mL) was added dropwise to the reaction medium, whichwas submitted to magnetic stirring at room temperature.Progress of reaction was checked by thin-layerchromatography analyses. After 48 h, the reaction mediumwas poured onto crushed ice from deionized water.Precipitates were removed by filtration, washed with colddeionized water and dried at room temperature. All crudeproducts were purified over successive silica gel columns,using mixtures of hexane and ethyl acetate as mobile phase. |
| 16% | With sodium hydroxide In ethanol for 24h; Ambient temperature; | |
| With sodium hydroxide; ethanol | ||
| With sodium hydroxide In 1,4-dioxane; water | ||
| With ethanol; sodium hydroxide at 20℃; for 3h; | ||
| With sodium hydroxide In ethanol; water at 20℃; | General procedure for the preparation of α,β-unsaturated ketones (1b-1k). General procedure: A 50 mL round-bottomed flask was charged with the appropriate ketone (3.0 mmol), aldehyde (3.0 mmol) and 10 ml of 95% EtOH, to which a NaOH solution [3.5 mmol of NaOH in 10 mL of ethanol-water (1:1, v/v)] was added dropwise. The mixture was stirred at room temperature. In most cases, a precipitate produced after stirring for several hours. The solid was collected by filtration, washed with water and recrystallized from EtOH to get the products. In the cases that oily products were produced, the solvent of reaction mixture was removed by rotary evaporator, the residue was treated with water (20 mL) and extracted with CH2Cl2 (3×20 mL). The organic phase was dried over anhydrous Na2SO4, and solvent was removed by a rotary evaporator. The residue was purified by column chromatography with petroleum ether/ethyl acetate (15:1-5:1) as an eluent to give products. | |
| 89 %Spectr. | With A26 basic resin In ethanol; toluene at 40℃; Flow reactor; chemoselective reaction; | |
| With potassium hydroxide In methanol; water at 0 - 20℃; | ||
| With sodium hydroxide In methanol | ||
| With sodium hydroxide In ethanol; water at 20℃; for 12h; | Chalcones 2a-2d were prepared following the known proceduce:[7] benzaldehyde and NaOH were added to a solution of acetophenone in EtOH at room temperature. The mixture was stirred for 12 h, neutralized with diluted HCl, and extracted with EA. The organic layer was dried over Na2SO4 and the solvents evaporated. The residue was purified by flash column chromatography to give corresponding chalcones. | |
| With sodium hydroxide In methanol; water at 0 - 20℃; |

[2]Bhunia, Anup; Patra, Atanu; Puranik, Vedavati G.; Biju, Akkattu T. [Organic Letters, 2013, vol. 15, # 7, p. 1756 - 1759]
[3]Shaykhutdinova, Polina; Oestreich, Martin [Organometallics, 2016, vol. 35, # 16, p. 2768 - 2771]
[4]Attar, Saeed; O'Brien, Zachary; Alhaddad, Hasan; Golden, Melissa L.; Calderón-Urrea, Alejandro [Bioorganic and Medicinal Chemistry, 2011, vol. 19, # 6, p. 2055 - 2073]
[5]Vieira, Lucas C. C.; Matsuo, Bianca T.; Martelli, Lorena S. R.; Gall, Mayara; Paixão, Marcio W.; Corrêa, Arlene G. [Organic and Biomolecular Chemistry, 2017, vol. 15, # 29, p. 6098 - 6103]
[6]Martelli, Lorena S.R.; Vieira, Lucas C.C.; Paixão, Márcio W.; Zukerman-Schpector, Julio; de Souza, Juliana O.; Aguiar, Anna Caroline C.; Oliva, Glaucius; Guido, Rafael V.C.; Corrêa, Arlene G. [Tetrahedron, 2019, vol. 75, # 25, p. 3530 - 3542]
[7]Liu, Xiaoling; Go, Mei-Lin [Bioorganic and Medicinal Chemistry, 2006, vol. 14, # 1, p. 153 - 163]
[8]Location in patent: experimental part Kumar, Vineet; Kumar, Sarvesh; Hassan, Mohammad; Wu, Hailong; Thimmulappa, Rajesh K.; Kumar, Amit; Sharma, Sunil K.; Parmar, Virinder S.; Biswal, Shyam; Malhotra, Sanjay V. [Journal of Medicinal Chemistry, 2011, vol. 54, # 12, p. 4147 - 4159]
[9]Current Patent Assignee: THE JOHNS HOPKINS UNIVERSITY - WO2012/116362, 2012, A2 Location in patent: Page/Page column 48-49
[10]Kazi, Imran; Guha, Somraj; Sekar, Govindasamy [Organic Letters, 2017, vol. 19, # 5, p. 1244 - 1247]
[11]Pal, Krisztina; Kallay, Mihaly; Kubinyi, Miklos; Bako, Peter; Mako, Attila [Tetrahedron Asymmetry, 2007, vol. 18, # 13, p. 1521 - 1528]
[12]Xue, Kangsheng; Sun, Guoxiang; Zhang, Yanzhi; Chen, Xubing; Zhou, Yang; Hou, Jinjun; Long, Huali; Zhang, Zijia; Lei, Min; Wu, Wanying [Synthetic Communications, 2021, vol. 51, # 4, p. 625 - 634]
[13]Loudet, Aurore; Bandichhor, Rakeshwar; Wu, Liangxing; Burgess, Kevin [Tetrahedron, 2008, vol. 64, # 17, p. 3642 - 3654]
[14]Choi, Ji Won; Jang, Bo Ko; Cho, Nam-Chul; Park, Jong-Hyun; Yeon, Seul Ki; Ju, Eun Ji; Lee, Yong Sup; Han, Gyoonhee; Pae, Ae Nim; Kim, Dong Jin; Park, Ki Duk [Bioorganic and Medicinal Chemistry, 2015, vol. 23, # 19, p. 6486 - 6496]
[15]Marques, Beatriz C.; Santos, Mariana B.; Anselmo, Daiane B.; Monteiro, Diego A.; Gomes, Eleni; Saiki, Marilia F. C.; Rahal, Paula; Rosalen, Pedro L.; Sardi, Janaina C. O.; Regasini, Luis O. [Medicinal Chemistry, 2020, vol. 16, # 7, p. 881 - 891]
[16]Batt; Goodman; Jones; Kerr; Mantegna; McAllister; Newton; Nurnberg; Welch; Covington [Journal of Medicinal Chemistry, 1993, vol. 36, # 10, p. 1434 - 1442]
[17]v. Auwers; Brink [Justus Liebigs Annalen der Chemie, 1932, vol. 493, p. 218,231]
[18]Yasui, Shinro; Fujii, Masayuki; Ohno, Atsuyoshi [Bulletin of the Chemical Society of Japan, 1987, vol. 60, # 11, p. 4019 - 4026]
[19]Corbett, Jennifer L.; Weavers, Rex T. [Synthetic Communications, 2008, vol. 38, # 4, p. 489 - 498]
[20]Location in patent: experimental part Karaman, Isa; Gezegen, Hayreddin; Guerdere, M. Burcu; Dingil, Alparslan; Ceylan, Mustafa [Chemistry and biodiversity, 2010, vol. 7, # 2, p. 400 - 408]
[21]Tang, Fang; Tang, Li; Guan, Zhi; He, Yan-Hong [Tetrahedron, 2018, vol. 74, # 46, p. 6694 - 6703]
[22]Laroche, Benjamin; Saito, Yuki; Ishitani, Haruro; Kobayashi, Shū [Organic Process Research and Development, 2019, vol. 23, # 5, p. 961 - 967]
[23]Corsini, Emanuela; Facchetti, Giorgio; Esposito, Sara; Maddalon, Ambra; Rimoldi, Isabella; Christodoulou, Michael S. [Archiv der Pharmazie, 2020, vol. 353, # 7]
[24]Singh; Gousuddin, Mohammad; Khan, Huma [European Journal of Molecular and Clinical Medicine, 2021, vol. 8, # 2, p. 182 - 200]
[25]Chen, De-Yin; Song, Shuai; Chen, Ling-Yan; Ren, Xinfeng; Li, Ya [Tetrahedron Letters, 2021, vol. 68]
[26]Iacovino, Luca G.; Pinzi, Luca; Facchetti, Giorgio; Bortolini, Beatrice; Christodoulou, Michael S.; Binda, Claudia; Rastelli, Giulio; Rimoldi, Isabella; Passarella, Daniele; Di Paolo, Maria Luisa; Dalla Via, Lisa [ACS Medicinal Chemistry Letters, 2021, vol. 12, # 7, p. 1151 - 1158]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 96% | With copper(ll) bromide In chloroform; ethyl acetate at 70℃; for 8h; Inert atmosphere; Reflux; | 2-Bromo-1-(2-Methoxyphenyl)Ethanone (7a). Copper (II) bromide (2.97 g, 6.66 mmol) was placed in a twonecked round-bottomed flask fitted with a reflux condenser. EtOAc (10.0 mL) was added to it and the mixture was stirred at 70C for 5 min under nitrogen atmosphere. 1-(2-Methoxyphenyl)ethanone (6) (0.50 g, 3.33 mmol) in CHCl3 (10.0 mL) was slowly added to it and then the mixture was refluxed for 8 h. After completion of the reaction, it was cooled to room temperature, filtered through Celite pad, and washed with EtOAc (20 mL). The filtrate was concentrated under reduced pressure. The crude was puri- fied by column chromatography (EtOAc:hexane = 1:4) to afford the pure product 7a (0.73 g, 96%) as brown liquid. Rf = 0.43 (EtOAc/hexane = 1/4); 1 H NMR (300 MHz, CDCl3): δ 7.81 (1H, dd, J = 7.8, 1.8 Hz), 7.52 (1H, td, J = 7.8, 1.8 Hz), 7.05-6.96 (2H, m), 4.61 (2H, s), 3.94 (3H, s); 13C NMR (75 MHz, CDCl3): δ 192.3, 158.8, 134.9, 131.6, 124.9, 121.2, 111.7, 56.0, 38.0. |
| 96% | With copper(ll) bromide In chloroform; ethyl acetate for 8h; Reflux; Inert atmosphere; | {2-Bromo-1- (2-methoxyphenyl) ethanone} (Compound 7a): A two-necked round bottom flask equipped with a reflux condenser was charged with copper (II)Bromide (2.97 g, 6.66 mmol) was added and EtOAc (10.0 mL)And the mixture was stirred at 70 ° C for 5 minutes. To a solution of 1- (2-methoxyphenyl) ethanone (Compound 6) in CHCl3 (10.0 mL)(0.50 g, 3.33 mmol) was slowly added and the mixture was refluxed for 8 hours.After the reaction was completed, the reaction mixture was cooled to room temperature, filtered through a pad of Celite (R), and washed with EtOAc (20 mL). The filtrate was concentrated under reduced pressure.The crude product was purified by column chromatography (EtOAc: hexane = 1: 4)To give pure brown compound 7a (0.73 g, 96%) as a brown liquid phase. |
| 96% | With copper(ll) bromide In chloroform; ethyl acetate for 8h; Inert atmosphere; Reflux; | 2-bromo-1-(2-methoxyphenyl)ethanone(2-bromo-1-(2-methoxyphenyl)ethanone}(compound 7a) Equipped with a reflux condenserCopper (II) bromide (2.97 g, 6.66 mmol) was added to a two-neck round bottom flask,Was added, EtOAc (10.0 mL) was added, and the mixture was stirred at 70 ° C for 5 minutes.1- (2-methoxyphenyl) ethanone (Compound 6) (0.50 g, 3.33 mmol) was slowly added to CHCl 3 (10.0 mL) under nitrogen atmosphere,The mixture was refluxed for 8 hours.After completion of the reaction,After cooling to room temperature,Filtration through a Celite pad,And washed with EtOAc (20 mL).The filtrate was concentrated under reduced pressure.The crude product was purified by column chromatography (EtOAc: hexane = 1: 4) to give pure compound 7a (0.73 g, 96%) as a brown liquid phase. |
| 91% | With N-Bromosuccinimide at 20℃; for 2h; | |
| 91% | With N-Bromosuccinimide; toluene-4-sulfonic acid at 20℃; for 2h; | |
| 91% | With copper(ll) bromide In ethyl acetate for 3h; Reflux; | |
| 90% | With N-Bromosuccinimide; toluene-4-sulfonic acid In dichloromethane at 40℃; for 0.25h; Microwave irradiation; Green chemistry; | |
| 84% | With N-Bromosuccinimide; toluene-4-sulfonic acid | |
| 83% | Stage #1: 2-Methoxyacetophenone With toluene-4-sulfonic acid at 60℃; Stage #2: With N-Bromosuccinimide at 60℃; for 0.25h; | 3.2.1. General Procedure for the Synthesis of Phenacyl Bromides 2, 11 General procedure: The round-bottom flask containing acetophenone derivative 1, 10 (4 mmol) and PTSA (0.076 g,0.4 mmol) was heated to 60 °C to turn the reaction mixture into a paste and NBS (0.854 g, 4.8 mmol) thenadded slowly. After 15 min, the reaction mixture was cooled to room temperature and water (20 mL)added. The crude product was extracted with dichloromethane (2 x 20 mL), dried over anhydrousNa2SO4 and purified by crystallization from n-hexane-dichloromethane to give pure product. 2-Methoxyphenacyl bromide (2): Yellow solid, soluble in acetone, dichloromethane, chloroform, insolublein water; yield 83%; m.p.: 43-45 °C; 1H-NMR (500 MHz, CDCl3) (δ, ppm): 7.78 (dd, 1H, J = 1.5,7.5 Hz, Ar-H), 7.51-7.47 (m, 1H, Ar-H), 7.02-6.96 (m, 2H, Ar-H), 4.57 (s, 2H, CH2), 3.93 (s, 3H, OCH3).This result showed consistency with the data in literature [26]. |
| 83% | With N-Bromosuccinimide; toluene-4-sulfonic acid In neat (no solvent) at 60℃; for 0.25h; regioselective reaction; | General procedure for the synthesis of phenacyl bromides (3a-i) General procedure: The round-bottom flask containing acetophenone derivative 2a-i (4 mmol) and PTSA (0.076 g, 0.4 mmol) was heated to 60 oC to turn the reaction mixture into a paste and then NBS (0.854 g, 4.8 mmol) was added slowly. After 15 min., the reaction mixture was cooled to room temperature and water (20 ml) was added. The crude product was extracted with dichloromethane (2 x 20 mL), dried over anhydrous Na2SO4 and puried by crystallization from hexane-dichloromethane to give pure product. 2-Methoxyphenacyl bromide (3a):[20,29] Yellow solid, soluble in acetone, dichloromethane, chloroform, insoluble in water; yield 83%; mp: 43-45 oC; 1H NMR (500 MHz, CDCl3) (δ, ppm): 7.78 (d, 1H, J = 7.5 Hz, Ar-H), 7.51-7.47 (m, 1H, Ar-H), 7.02-6.96 (m, 2H, Ar-H), 4.57 (s, 2H, CH2), 3.93 (s, 3H, OCH3). |
| 77% | With bis(1,3-dimethyl-2-imidazolidinone) hydrotribromide In dichloromethane at 20℃; for 1h; | 4.3 Typical procedure for the α-bromination of carbonyl compounds with 3a General procedure: To a solution of propiophenone (9a, 285mg, 2.15mmol) in CH2Cl2 (4mL) was added 3a (1.23g, 2.62mmol), and the mixture was stirred at 20°C for 1h. The reaction mixture was quenched with saturated NaHCO3 aqueous solution and then extracted with diethyl ether (15mL×3). The combined organic layers were washed with water (20mL×2), dried over Na2SO4 and concentrated in vacuo. The resulting crude residue was purified using silica gel column chromatography (eluent: hexane/chloroform=1:2) to afford bromide 10a (433mg, 94%) [24] as a white crystalline solid |
| 60% | With bromine In tetrachloromethane for 0.833333h; | |
| With bromine In chloroform for 0.25h; Ambient temperature; | ||
| With bromine In 1,4-dioxane; diethyl ether for 1h; | ||
| With aluminium trichloride; bromine In diethyl ether | ||
| With hydrogenchloride; bromine In acetic acid at 20℃; | ||
| With bromine In diethyl ether at 20℃; | ||
| With bromine In chloroform Heating; | ||
| With N-Bromosuccinimide; toluene-4-sulfonic acid at 20℃; for 2h; | ||
| With copper(ll) bromide In chloroform; ethyl acetate for 0.333333h; Reflux; Inert atmosphere; | ||
| With toluene-4-sulfonic acid; N,N'-dibromohydantoin In methanol at 30℃; for 8h; | 5.2. General procedure for the preparation of compounds 2 General procedure: To a solution of the appropriate acetophenone 1 and p-toluenesulfonic acid (15 mmol) in dry methanol (20 mL), dibromohydantoin (15 mmol), diluted in methanol (20 mL) was added dropwise and the mixture was stirred for 8 h at 30 °C. After the completion of the reaction, excess solvent was removed under reduced pressure. Ice water was added to the residue. The precipitated solid was filtered, and washed with ethanol. The resulting crude solid was directly used in the next step without purification. | |
| With bromine In diethyl ether Inert atmosphere; | ||
| With copper(ll) bromide In chloroform; ethyl acetate for 8h; Reflux; | ||
| With copper(ll) bromide In chloroform; ethyl acetate for 8h; Reflux; | ||
| With copper(ll) bromide In chloroform; ethyl acetate at 85℃; for 3h; | ||
| With copper(ll) bromide In tetrahydrofuran | ||
| With tetrabutylammomium bromide In acetonitrile at 20℃; | ||
| With copper(ll) bromide In chloroform; ethyl acetate Reflux; | Substituted 2-bromo-1-arylethanone 7a-o General procedure: Substituted 2-bromo-1-arylethanone 7a-o A solution of the appropriate ketone 3a-o (25 mmol) in hot chloroform (25 mL) was added to a preheated, stirred suspension of copper (II) bromide (50 mmol) in ethyl acetate (25 mL). The resulting reaction mixture was refluxed with vigorous stirring till completion of the reaction which detected by TLC monitoring. The reaction mixture was filtered while hot to remove copper (I) bromide then concentrated under reduced pressure. After cooling, the separated solid was filtered, washed with petroleum ether and dried to give compounds 7a-o [49] . | |
| With hydrogen bromide; bromine; acetic acid at 0 - 5℃; | General procedure to obtainα-bromoacetophenonederivatives General procedure: General procedure to obtainα-bromoacetophenonederivatives Acetophenone derivative (10 mmol) was solved in acetic acid(50 mL) and hydrobromic acid (0.5 mL) mixture. Bromine(10 mmol, 0.52 mL) was added dropwise to this mixture at 0-5°Ctemperatureandthemixturewasstirredfor6-7h.Afterthisperiod, the mixture was poured into ice-water, collapsed portionwas filtrated and after dryness it was crystallized from ethanol. | |
| With tetra-N-butylammonium tribromide In acetonitrile at 20℃; for 12h; | Step (i) General procedure: (i) The commercial available 8a-8l (4 mmol, 1.0 equiv.)was respectively dissolved in acetonitrile (50 mL), adding tetrabutylammoniumtribromide(4 mmol, 1.0 equiv.) later. The mixture was stirred overnight under roomtemperature until the solution turned light yellow or colorless. The solventwas removed in vacuo, the residue wasextracted with dichloromethane and washed with water. The organic layers werecombined and concentrated under vacuum to provide the crude products 9a-9l, which using for next step withoutany purification. | |
| With bromine In diethyl ether | ||
| With bromine In toluene at 5 - 10℃; for 3h; | 1 (2-phenyl-4 - (2-methoxy) phenyl imidazole for manufacturing) O- [...] (300g) is toluene (500 ml) to a solution dissolving the bromine (320g) and is adapted to hold a 5-10 °C while agitating 3 was dripped over time. Furthermore, 20 °C hereinafter water (500 ml) adding an, potassium carbonate (300g) of 1 was including neutralization with an and introduced over time. Furthermore, ethyl acetic acid (500 ml) and water (500 ml) by the addition of extracted organic layer. Saline saturated organic layer (500 ml) to 2 times cleaning and, generated α-bromo (2-methoxy) acetophenone with a biocatalyst, without isolating the ground terminal of to next reactor being. [...] benzamide with an organic layer (320g), potassium carbonate (800g) and water (600 ml) in 60 °C by mixing he stirring time 6. A reaction solution are the first using of potassium hydroxide after including neutralization with an, acetic acid ethyl (1 ℓ) extracted organic layer by the addition of. Saline saturated organic layer (1 ℓ) to 3 times cleaning and, in evaporator 150 ml was concentrating up. Hexane (1 ℓ) adding an, at room temperature to filter out generated members 150g of 2-phenyl-4 - (2-methoxy) phenyl imidazole is obtained. | |
| With copper(ll) bromide In dichloromethane; ethyl acetate at 60℃; Microwave irradiation; Sealed tube; | ||
| With N-Bromosuccinimide; toluene-4-sulfonic acid In acetonitrile at 80℃; for 3.5h; Inert atmosphere; | 5.A Step A. A 200 mL flask was charged with 2′-methoxyacetophenone (7.51 g, 50 mmol), N-bromosuccinimide (9.79 g, 55 mmol), p-toluenesulfonic acid (10.79 g, 55 mmol) and MeCN (100 mL) under nitrogen stream and the mixture was stirred for 3.5 hours at 80° C. The mixture was allowed to cool to room temperature and then MeCN was removed by an evaporator. CHCl3 (150 mL) and H2O (50 mL) were added to a residue and the lower layer was separated and then washed with saturated aqueous NaHCO3 (50 mL) twice and brine (50 mL). The solution was dried over anhydrous MgSO4 and filtrated. Solvents were then removed in an evaporator and the crude product as orange-brown oil (11.74 g) in >99.9% yield was obtained. This product was identified as 2-Bromo-2′-methoxyacetophenone contaminated with some dibromo compound. 1H-NMR (CDCl3), delta (ppm): 3.93 (s, 3H), 4.59 (s, 2H), 6.97 (d, J=8.4 Hz, 1H), 7.00-7.06 (m, 1H), 7.50 (ddd, J=1.8, 7.3, 8.4 Hz, 1H), 7.81 (dd, J=1.8, 7.7 Hz, 1H). | |
| With copper(ll) bromide In ethyl acetate for 12h; Reflux; | General procedure: In the synthesis process, different 1-(aryl/heteroaryl)ethanone(50 mmol) derivatives were brominated in the presence of copper(II) bromide (100 mmol) followed by a reaction with equimolarpyrimidine-2-carbothioamide (50 mmol). In the course of the reaction,the components were mixed in ethanol acetate for approximately1 h in room temperature and then refluxed at 77-78 Cfor 15-20 min. After cooling, the precipitate was filtered, neutralizedwith sodium acetate, and recovered from the HBr salt. Allthe synthesized compounds were purified twice by crystallizationfrom ethanol. Pyrimidine-2-carbonitrile and phosphorus pentasulfidewere mixed in dioxane for 48 h to obtain pyrimidine-2-carbothioamideas the starting material. This is the first time in theliterature that synthesis of pyrimidine thioamides has been undertakenwith this method | |
| With N-Bromosuccinimide; toluene-4-sulfonic acid In water; acetonitrile at 80℃; for 3.5h; Inert atmosphere; | 7.A Step A. A 200 mL flask was charged with 2′-methoxyacetophenone (7.51 g, 50 mmol), N-bromosuccinimide (9.79 g, 55 mmol), p-toluenesulfonic acid (10.79 g, 55 mmol) and MeCN (100 mL) under nitrogen stream and the mixture was stirred for 3.5 hours at 80° C. The mixture was allowed to cool to room temperature and then MeCN was removed by an evaporator. CHCl3 (150 mL) and H2O (50 mL) were added to a residue and the lower layer was separated and then washed with saturated aqueous NaHCO3 (50 mL) twice and brine (50 mL). The solution was dried over anhydrous MgSO4 and filtrated. Solvents were then removed in an evaporator and the crude product as orange-brown oil (11.74 g) in >99.9% yield was obtained. This product was identified as 2-Bromo-2′-methoxyacetophenone contaminated with some dibromo compound. 1H-NMR (CDCl3), delta (ppm): 3.93 (s, 3H), 4.59 (s, 2H), 6.97 (d, J=8.4 Hz, 1H), 7.00-7.06 (m, 1H), 7.50 (ddd, J=1.8, 7.3, 8.4 Hz, 1H), 7.81 (dd, J=1.8, 7.7 Hz, 1H). | |
| With bromine In dichloromethane at 20℃; for 3h; | ||
| 330 g | With bromine In acetonitrile at 20 - 25℃; for 4h; | 5.1 [00241] Example 5.1: Preparation of 2-bromo-l-(2-methoxyphenyl)ethan-l-one (compound 1.2) 1 -(2-methoxyphenyl)ethan-1 -one 2-bromo-1 - MW: 150.17 (2-methoxyphenyl)ethan-1-one MW: 229.07 [00242] l-(2-methoxyphenyl)ethanone, 1.1, (300 g, 1.0 eq) was added to a reactor containing acetonitrile (1.2 L, 4.0 V). Br2 (319.62 g, 1.0 eq) was added by cooling the reaction to below 25°C. The reaction mixture was stirred for 4 h at 20-25 °C and sampled for IPC until the content of l-(2-methoxyphenyl)ethanone was 6.5%. NaHSCb (600 ml, 2V) was added to quench the reaction and then stirred for an additional 0.5h at 20-25°C. Product was extracted with methyl tert-butyl ether (600 ml, 2V), three times, to yield a black oil (418 g, crude), which was purified using a column to yield 330 g of 2-bromo-l-(2-methoxyphenyl)ethan-l-one, 1.2, as an off-white solid (98% purity). |
| With bromine In diethyl ether at 0 - 20℃; | ||
| With copper(ll) bromide In chloroform; ethyl acetate for 2h; Reflux; | ||
| With copper(ll) bromide In 2-methyltetrahydrofuran at 20℃; for 24h; | General procedure for synthesis of α-haloketones(7-12) General procedure: To a solution of acetophenones 1-6 (16.65 mmol) in 2-Metetrahydrofuran(20 mL), copper(II)bromide (19.98 mmol)was added (Raghunath et al. 2015). The reaction mixturewas allowed to stir at room temperature for 24 h. Aftercompletion of the reaction (monitored by TLC), reactionmixture was filtered off. The filtrate containing the 2-bromo-1-phenylethanones 7-12 was taken to the next stepwithout isolation. However, to check the purity of theformed phenacyl bromides, two representative compounds(8 and 10) were isolated in standard procedure by evaporatingthe solvent under vacuum and confirmed by spectralanalysis (1H NMR, Mass, and HPLC). As all the derivativeswere pure by TLC, they were preceded to next step withoutpurification/isolation. | |
| With N-Bromosuccinimide; toluene-4-sulfonic acid In acetonitrile at 60℃; for 4h; | ||
| With N-Bromosuccinimide; toluene-4-sulfonic acid In chloroform at 20℃; for 24h; | 3.1.4 General procedure for the synthesis of b1-b5 General procedure: To a solution of acetophenone (3mmol) in CHCl3 were added p-TsOH·H2O (100mg, 0.53mmol) and N-bromosuccinimide (NBS, 214mg, 3.6mmol). The mixture was stirred at room temperature for 24h. The aqueous mixture was extracted with ether (50mL). The organic layer was dried (Na2SO4), after filtration, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (Petroleum ether and CH2Cl2, 2:1) to obtain white solid. To a solution of the white solid in DMF (50mL), NaN3 (143mg, 2.2mmol) was added. After stirred at r.t for 3h, the solid was disappeared completely. Added ascorbic acid (528mg, 3mmol) and anhydrous K2CO3 (414mg, 3mmol) into the reaction solution, stirred at r.t for 30min. Then added the intermediate 2 (680mg, 2mmol) and CuSO4·5H2O (625mg, 2.5mmol) into the reaction solution, stirred at r.t for 3h. Method as described in 4.1.2.2 (acidified to pH 5-6 and extracted with EtOAc, washed with brine solution, dried over anhydrous Na2SO4, filtered, and concentrated). The residues of b1-b5, were purified by flash column chromatography (CHCl3/Petroleum ether/mehanol=1/1, v/v) [30] (white crystal or powder, yield 36-80% for this step reaction, Scheme 1). | |
| With bromine In methanol | ||
| With N-Bromosuccinimide; toluene-4-sulfonic acid In acetonitrile at 50℃; for 24h; Inert atmosphere; | ||
| Stage #1: 2-Methoxyacetophenone With copper(I) bromide In ethyl acetate Inert atmosphere; Stage #2: | ||
| With copper(ll) bromide In ethyl acetate for 5h; Schlenk technique; Inert atmosphere; Reflux; | ||
| With N-Bromosuccinimide; toluene-4-sulfonic acid In water; acetonitrile Reflux; | ||
| With copper(ll) bromide In dichloromethane; ethyl acetate at 60℃; for 0.25h; Microwave irradiation; |

[2]Current Patent Assignee: HALLYM UNIVERSITY - KR101916106, 2018, B1 Location in patent: Paragraph 0060; 0066-0068
[3]Current Patent Assignee: HALLYM UNIVERSITY - KR101926612, 2018, B1 Location in patent: Paragraph 0023; 0029; 0030; 0031
[4]Pravst, Igor; Zupan, Marko; Stavber, Stojan [Tetrahedron Letters, 2006, vol. 47, # 27, p. 4707 - 4710]
[5]Location in patent: scheme or table Tatar, Jovana; Baranac-Stojanović, Marija; Stojanović, Milovan; Marković, Rade [Tetrahedron Letters, 2009, vol. 50, # 6, p. 700 - 703]
[6]Dai, Weiyang; Samanta, Soma; Xue, Ding; Petrunak, Elyse M.; Stuckey, Jeanne A.; Han, Yanyan; Sun, Duxin; Wu, Yong; Neamati, Nouri [Journal of Medicinal Chemistry, 2019, vol. 62, # 6, p. 3068 - 3087]
[7]Rodríguez, Juan C.; Maldonado, Rony A.; Ramírez-García, Gonzalo; Díaz Cervantes, Erik; de la Cruz, Fabiola N. [Journal of Heterocyclic Chemistry, 2020, vol. 57, # 5, p. 2279 - 2287]
[8]Location in patent: experimental part Kaerkaes, Markus D.; Johnston, Eric V.; Karlsson, Erik A.; Lee, Bao-Lin; Akermark, Torbjoern; Shariatgorji, Mohammadreza; Ilag, Leopold; Hansson, Oerjan; Baeckvall, Jan-E.; Akermark, Bjoern [Chemistry - A European Journal, 2011, vol. 17, # 28, p. 7953 - 7959]
[9]Phan, Phuong-Thuy T.; Nguyen, Thu-Trang T.; Nguyen, Hong-Nhung T.; Le, Bao-Khanh N.; Vu, Thao T.; Tran, Dong C.; Pham, Tuan-Anh N. [Molecules, 2017, vol. 22, # 5]
[10]Phan, Phuong-Thuy T.; Nguyen, Hong-Nhung T.; Kim, Son N.; Pham, Tuan-Anh N. [Synthetic Communications, 2021, vol. 51, # 5, p. 786 - 796]
[11]Nishio, Yuya; Yubata, Kotaro; Wakai, Yutaro; Notsu, Kotaro; Yamamoto, Katsumi; Fujiwara, Hideki; Matsubara, Hiroshi [Tetrahedron, 2019, vol. 75, # 10, p. 1398 - 1405]
[12]Kermagoret, Anthony; Braunstein, Pierre [Dalton Transactions, 2008, # 6, p. 822 - 831]
[13]Ogata, Masaru; Matsumoto, Hiroshi; Kida, Shiro; Shimizu, Sumio; Tawara, Katsuya; Kawamura, Yoshimi [Journal of Medicinal Chemistry, 1987, vol. 30, # 8, p. 1497 - 1502]
[14]Rival; Grassy; Michel [Chemical and Pharmaceutical Bulletin, 1992, vol. 40, # 5, p. 1170 - 1176]
[15]Barlin, Gordon B.; Davies, Les P.; Davis, Rohan A.; Harrison, Peter W. [Australian Journal of Chemistry, 1994, vol. 47, # 11, p. 2001 - 2012]
[16]Kim, Yong; Nam, Nguyen-Hai; You, Young-Jae; Ahn, Byung-Zun [Bioorganic and Medicinal Chemistry Letters, 2002, vol. 12, # 4, p. 719 - 722]
[17]Lee, Sunkyung; Kim, Taemi; Lee, Byung Ho; Yoo, Sung-eun; Lee, Kyunghee; Yi, Kyu Yang [Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 5, p. 1291 - 1295]
[18]Skoumbourdis, Amanda P.; Huang, Ruili; Southall, Noel; Leister, William; Guo, Vicky; Cho, Ming-Hsuang; Inglese, James; Nirenberg, Marshall; Austin, Christopher P.; Xia, Menghang; Thomas, Craig J. [Bioorganic and Medicinal Chemistry Letters, 2008, vol. 18, # 4, p. 1297 - 1303]
[19]Pravst, Igor; Zupan, Marko; Stavber, Stojan [Tetrahedron, 2008, vol. 64, # 22, p. 5191 - 5199]
[20]An, Xiao-Lei; Chen, Jia-Rong; Li, Chang-Feng; Zhang, Fu-Gen; Zou, You-Quan; Guo, Ying-Cen; Xiao, Wen-Jing [Chemistry - An Asian Journal, 2010, vol. 5, # 10, p. 2258 - 2265]
[21]Location in patent: experimental part Song, Ming-Xia; Zheng, Chang-Ji; Deng, Xian-Qing; Wang, Qing; Hou, Shao-Pu; Liu, Ting-Ting; Xing, Xiao-Lan; Piao, Hu-Ri [European Journal of Medicinal Chemistry, 2012, vol. 54, p. 403 - 412]
[22]Chen, Jianzhong; Liu, Delong; Butt, Nicholas; Li, Chao; Fan, Dongyang; Liu, Yangang; Zhang, Wanbin [Angewandte Chemie - International Edition, 2013, vol. 52, # 44, p. 11632 - 11636][Angew. Chem., 2013, vol. 125, # 44, p. 11846 - 11850,5]
[23]Boominathan, Siva Senthil Kumar; Hu, Wan-Ping; Senadi, Gopal Chandru; Wang, Jeh-Jeng [Advanced Synthesis and Catalysis, 2013, vol. 355, # 18, p. 3570 - 3574]
[24]Boominathan, Siva Senthil Kumar; Hu, Wan-Ping; Senadi, Gopal Chandru; Vandavasi, Jaya Kishore; Wang, Jeh-Jeng [Chemical Communications, 2014, vol. 50, # 51, p. 6726 - 6728]
[25]Bellale, Eknath; Naik, Maruti; Vb, Varun; Ambady, Anisha; Narayan, Ashwini; Ravishankar, Sudha; Ramachandran, Vasanthi; Kaur, Parvinder; McLaughlin, Robert; Whiteaker, James; Morayya, Sapna; Guptha, Supreeth; Sharma, Sreevalli; Raichurkar, Anandkumar; Awasthy, Disha; Achar, Vijayshree; Vachaspati, Prakash; Bandodkar, Balachandra; Panda, Manoranjan; Chatterji, Monalisa [Journal of Medicinal Chemistry, 2014, vol. 57, # 15, p. 6572 - 6582]
[26]Huo, Jingpei; Zeng, Heping [Journal of Materials Chemistry A, 2015, vol. 3, # 12, p. 6258 - 6264]
[27]Luo, Yong; Zhu, Yongxia; Ran, Kai; Liu, Zhihao; Wang, Ningyu; Feng, Qiang; Zeng, Jun; Zhang, Lidan; He, Bing; Ye, Tinghong; Zhu, Shirui; Qiu, Xiaolong; Yu, Luoting [MedChemComm, 2015, vol. 6, # 6, p. 1036 - 1042]
[28]Abdel-Aziz, Hatem A.; Ghabbour, Hazem A.; Eldehna, Wagdy M.; Al-Rashood, Sara T.A.; Al-Rashood, Khalid A.; Fun, Hoong-Kun; Al-Tahhan, Mays; Al-Dhfyan, Abdullah [European Journal of Medicinal Chemistry, 2015, vol. 104, p. 1 - 10]
[29]Yurttaş, Leyla; Özkay, Yusuf; Duran, Murat; Turan-Zitouni, Gülhan; Özdemir, Ahmet; Cantürk, Zerrin; Küçükoğlu, Kaan; Kaplancıklı, Zafer Asım [Phosphorus, Sulfur and Silicon and the Related Elements, 2016, vol. 191, # 8, p. 1166 - 1173]
[30]Ran, Kai; Gao, Chao; Deng, Hongxia; Lei, Qian; You, Xinyu; Wang, Ningyu; Shi, Yaojie; Liu, Zhihao; Wei, Wei; Peng, Cuiting; Xiong, Lu; Xiao, Kunjie; Yu, Luoting [Bioorganic and Medicinal Chemistry Letters, 2016, vol. 26, # 15, p. 3669 - 3674]
[31]Chen, Jianzhong; Zhang, Zhenfeng; Liu, Delong; Zhang, Wanbin [Angewandte Chemie - International Edition, 2016, vol. 55, # 29, p. 8444 - 8447][Angew. Chem., 2016, vol. 128, # 29, p. 8584 - 8587]
[32]Current Patent Assignee: TAMURA CORPORATION - KR101642378, 2016, B1 Location in patent: Paragraph 0062-0066
[33]Tung, Truong-Thanh; Dao, Trong T.; Junyent, Marta G.; Palmgren, Michael; Günther-Pomorski, Thomas; Fuglsang, Anja T.; Christensen, Søren B.; Nielsen, John [ChemMedChem, 2018, vol. 13, # 1, p. 37 - 47]
[34]Current Patent Assignee: CENTRAL GLASS CO LTD; INTERNATIONAL BUSINESS MACHINES CORP - US2018/44459, 2018, A1 Location in patent: Paragraph 0367
[35]Sahin, Zafer; Ertas, Merve; Berk, Barkın; Biltekin, Sevde Nur; Yurttas, Leyla; Demirayak, Seref [Bioorganic and Medicinal Chemistry, 2018, vol. 26, # 8, p. 1986 - 1995]
[36]Current Patent Assignee: CENTRAL GLASS CO LTD; INTERNATIONAL BUSINESS MACHINES CORP - US2018/44284, 2018, A1 Location in patent: Paragraph 0175; 0176
[37]Teske, Johannes; Plietker, Bernd [Organic Letters, 2018, vol. 20, # 8, p. 2257 - 2260]
[38]Current Patent Assignee: GILEAD SCIENCES INC; Monsanto (in: Bayer); BAYER AG - WO2018/161008, 2018, A1 Location in patent: Paragraph 00241-00242
[39]Yang, Yuwen; Yang, Weibo [Chemical Communications, 2018, vol. 54, # 86, p. 12182 - 12185] Deng, Lei; Kleij, Arjan W.; Yang, Weibo [Chemistry - A European Journal, 2018, vol. 24, # 72, p. 19156 - 19161]
[40]Chen, Zhili; Hu, Fangli; Huang, Shengli; Zhao, Zhengxing; Mao, Hui; Qin, Wenling [Journal of Organic Chemistry, 2019, vol. 84, # 12, p. 8100 - 8111]
[41]Sanivarapu, Sumalatha; Vaddiraju, Namratha; Velide, Lakshmi [Medicinal Chemistry Research, 2019, vol. 28, # 9, p. 1461 - 1470]
[42]Li, Ying; Li, Wendian; Tian, Jiangyan; Huang, Guozheng; Lv, Hui [Organic Letters, 2020, vol. 22, # 14, p. 5353 - 5357]
[43]Zheng, Yan; Zhang, Yi-long; Li, Zeng; Shi, Wen; Ji, Ya-ru; Guo, Ya-Hui; Huang, Cheng; Sun, Guo-ping; Li, Jun [European Journal of Medicinal Chemistry, 2021, vol. 213]
[44]Mishra, Ashish A.; Bhanage, Bhalchandra M. [New Journal of Chemistry, 2021, vol. 45, # 12, p. 5357 - 5362]
[45]Gaykar, Rahul N.; George, Malini; Guin, Avishek; Bhattacharjee, Subrata; Biju, Akkattu T. [Organic Letters, 2021, vol. 23, # 9, p. 3447 - 3452]
[46]Bantzi, Marina; Augsburger, Fiona; Loup, Jérémie; Berset, Yan; Vasilakaki, Sofia; Myrianthopoulos, Vassilios; Mikros, Emmanuel; Szabo, Csaba; Bochet, Christian G. [Journal of Medicinal Chemistry, 2021, vol. 64, # 9, p. 6221 - 6240]
[47]Lv, Hao-Peng; Yang, Xiao-Peng; Wang, Bai-Lin; Yang, Hao-Di; Wang, Xing-Wang; Wang, Zheng [Organic Letters, 2021, vol. 23, # 12, p. 4715 - 4720]
[48]Zermeño-Macías, María de los Ángeles; González-Chávez, Marco Martín; Méndez, Francisco; Richaud, Arlette; González-Chávez, Rodolfo; Ojeda-Fuentes, Luis Enrique; Niño-Moreno, Perla Del Carmen; Martínez, Roberto [Molecules, 2021, vol. 26, # 16]
[49]Quoc, Thang Nguyen; Thanh, Tung Truong; Xuan, Huy Luong [RSC Advances, 2021, vol. 11, # 46, p. 28797 - 28808]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 99% | With N-Bromosuccinimide; iodine; In acetonitrile; for 12h;Darkness; | General procedure: To a reaction tube charged with NBS (1.5 equiv, 0.3 mmol), catalyst (10 mol%, 0.02 mmol) and CH3CN (1.0 mL),was added para-chloroanisole 1a (0.2 mmol). After being stirred at room temperature for 12 h in dark, the reaction was quenched by saturated aq. solution of Na2S2O3 (2 mL). The resulting mixture was extracted by ethyl acetate (3 5 mL). The combined organic extracts were washed by brine (10 mL), dried over Na2SO4 and filtered through a pad of Celite. The filtrate was concentrated under reduced pressure and the residuewas purified by flash chromatography on a silica gel column with petroleum ether/dichloromethane (5:1) as the eluent to give 4.3.1. 2-Bromo-4-chloroanisole (2a) |
| 95% | With hydrogenchloride; N-Bromosuccinimide; In water; acetone; at 20℃; for 3h; | Compound 41-2 (0492) To a solution of 41-1 (2.0 g, 13.32 mmol) in acetone (25 mL) was added NBS (2.37 g, 13.32 mmol) and 1M HCl aq. (0.13 mL, 0.13 mmol). The reaction mixture was stirred at room temperature for 3 h, and then concentrated to dryness under reduced pressure. The residue was dissolved with PE (40 mL) the resultant precipitate was filtered and dried in vacuum to afford 41-2 as a white solid (2.90 g, yield: 95%). |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 92% | With ammonia; hydrogen In water at 130℃; for 24h; Autoclave; | |
| 91% | With formic acid; C26H34ClIrN4O; ammonium formate In methanol at 80℃; for 4h; Inert atmosphere; Schlenk technique; | |
| 88% | With ammonia; hydrogen In tetrahydrofuran at 120℃; for 15h; |
| 84% | With 4-methoxy-N-(1-(naphthalen-2-yl)ethylidene)aniline; ammonium formate In methanol at 80℃; for 12h; Inert atmosphere; chemoselective reaction; | |
| 82% | With ammonium acetate; sodium cyanoborohydride In methanol for 96h; Ambient temperature; | |
| 72% | With ammonium hydroxide; ammonium acetate; sodium cyanoborohydride; zinc In ethanol; water at 80℃; for 36h; | 4.2.1. General procedure for the synthesis of amines via reductive amination of acetophenones General procedure: To a saturated solution of NH4OAc in EtOH (40 mL) were added activated Zn (5 equiv), acetophenone (1 equiv), NaBCNH3(3 equiv) and 30% aq NH3 (10 mL) respectively. The mixture wasstirred at 80 C for 36 h. The reaction mixture was cooled to rt and concentrated under reduced pressure. The residue was dissolved in CH2Cl2 and made basic using 1 M NaOH (50 mL). Theorganic phase was separated and aqua phase was extracted with (2x25 mL) CH2Cl2. The organic phases were combined and acidified with HCl (pH: 2.0). The organic layer was separated andH2O layer was extracted with CH2Cl2 (2 25 mL). The H2O layer was made basic with NaOH (pH: 10.0), The organic layer was extracted with CH2Cl2 (3 25 mL). Combined organic layers weredried over Na2SO4 and evaporation of the solvent afforded thedesired amines. |
| 57% | Stage #1: 2-Methoxyacetophenone With titanium(IV) isopropylate; ammonia In isopropyl alcohol at 20℃; Stage #2: With sodium tetrahydroborate In isopropyl alcohol at 20℃; for 2h; | |
| With ammonium acetate; sodium cyanoborohydride In methanol at 20℃; | ||
| Multi-step reaction with 3 steps 1: titanium(IV) isopropylate / Isopropyl acetate / 0.5 h 2: 5%-palladium/activated carbon; hydrogen / Isopropyl acetate / 24 h / 24 °C / 11251.1 Torr 3: 5%-palladium/activated carbon; hydrogen / Isopropyl acetate / 24 h / 55 °C | ||
| With ammonium acetate; sodium cyanoborohydride In methanol at 20℃; for 12h; | General procedure: 1-(2-methylphenyl)ethanone (0.50 mL, 3.73 mmol) and ammonium acetate (2.88 g, 37.3 mmol) were dissolved in methanol (20 mL). To the stirred solution was added slowly a solution of NaCNBH3 (0.79 g, 11.2 mmol) dissolved in methanol (15 mL) through a dropping funnel. The whole mixture was stirred at room temperature. After stirring for 12 h, solvent was removed by using rotary evaporator. The residue was dissolved in ethyl acetate and then the solution was treated with 6 N HCl solution to makethe solution to be acidic (pH = 12-13). The two layers were separated and then 6 N NaOH solutionwas added to the separated aqueous solution to make the solution to be basic (pH = 11-12). The basic aqueous solution was extracted with ethyl acetate twice and then combined organic solution was dried over anhydrous Na2SO4. Solvent was removed by using rotary evaporator to afford an intermediateamine B, 1-(2-methylphenyl)ethylamine (Ar = 2-methylphenyl, R1 = CH3, compound B in Figure 5) (0.21 g, 46% yield). | |
| Multi-step reaction with 2 steps 1: formic acid; formamide / 6 h / 190 °C 2: hydrogenchloride / water / 12 h / Reflux | ||
| With ammonium acetate; sodium cyanoborohydride In methanol at 20℃; for 24h; | ||
| With water; formamide In neat (no solvent) at 160℃; |

[2]Tanaka, Kouichi; Miki, Takashi; Murata, Kunihiko; Yamaguchi, Ayumi; Kayaki, Yoshihito; Kuwata, Shigeki; Ikariya, Takao; Watanabe, Masahito [Journal of Organic Chemistry, 2019, vol. 84, # 17, p. 10962 - 10977]
[3]Jagadeesh, Rajenahally V.; Murugesan, Kathiravan; Alshammari, Ahmad S.; Neumann, Helfried; Pohl, Marga-Martina; Radnik, Jörg; Beller, Matthias [Science, 2017, vol. 358, # 6361, p. 326 - 332]
[4]Talwar, Dinesh; Salguero, Noemi Poyatos; Robertson, Craig M.; Xiao, Jianliang [Chemistry - A European Journal, 2014, vol. 20, # 1, p. 245 - 252]
[5]Giardina; Marrazzo; Marucci; Grazia Piloni; Quaglia [Il Farmaco, 1991, vol. 46, # 7-8, p. 861 - 872]
[6]Akincioʇlu, Akin; Akincioʇlu, Hülya; Gülçin, Ilhami; Durdagi, Serdar; Supuran, Claudiu T.; Göksu, Süleyman [Bioorganic and Medicinal Chemistry, 2015, vol. 23, # 13, p. 3592 - 3602]
[7]Issaraseriruk, Natthapol; Sritana-anant, Yongsak; Shitangkoon, Aroonsiri [Chirality, 2018, vol. 30, # 7, p. 900 - 906]
[8]Kinbara, Kazushi; Harada, Yoshiko; Saigo, Kazuhiko [Journal of the Chemical Society. Perkin Transactions 2 (2001), 2000, # 7, p. 1339 - 1347]
[9]Nugent, Thomas C.; Negru, Daniela E.; El-Shazly, Mohamed; Hu, Dan; Sadiq, Abdul; Bibi, Ahtaram; Umar, M. Naveed [Advanced Synthesis and Catalysis, 2011, vol. 353, # 11-12, p. 2085 - 2092]
[10]Lee, Ga Ram; Hyun, Myung Ho [Molecules, 2014, vol. 19, # 12, p. 21386 - 21397]
[11]Kiefer, Lionel; Beaumard, Floriane; Gorojankina, Tatiana; Faure, Hélène; Ruat, Martial; Dodd, Robert H. [Bioorganic and Medicinal Chemistry, 2016, vol. 24, # 4, p. 554 - 569]
[12]Martínez-Montero, Lía; Gotor, Vicente; Gotor-Fernández, Vicente; Lavandera, Iván [Green Chemistry, 2017, vol. 19, # 2, p. 474 - 480]
[13]Dong, Wei-Li; Du, Xiu-Jiang; Liu, Xing-Hai; Peng, Xing-Jie; Zhao, Rui-Qi; Zhao, Wei-Guang [Journal of Enzyme Inhibition and Medicinal Chemistry, 2020, vol. 35, # 1, p. 682 - 691]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 99% | With lithium hydroxide; hydrogen; triphenylphosphine; iridium; (8R,9R)-9-amino(9-deoxy)epicinchonin In methanol at 40℃; for 20h; optical yield given as %ee; enantioselective reaction; | |
| 99% | With hydrogen; potassium hydroxide; 9-amino-9-deoxyepicinchonine In isopropyl alcohol at 40℃; for 5h; Autoclave; optical yield given as %ee; enantioselective reaction; | |
| 99% | With bis(1,5-cyclooctadiene)diiridium(I) dichloride; (R)-N-(3-methylpyridine-2-methyl)-7-bis-(3,5-di-tert-butylphenyl)phosphino-7′-amino-1,1′-spirodihydroindane; potassium <i>tert</i>-butylate; hydrogen In ethanol at 25 - 30℃; for 0.666667h; Autoclave; optical yield given as %ee; enantioselective reaction; |
| 99% | With bis(1,5-cyclooctadiene)diiridium(I) dichloride; (R)-7′-bis-(3,5-di-tert-butylphenyl)phosphino-7′-amino-1,1′-spiroindene; potassium <i>tert</i>-butylate; hydrogen In propan-1-ol at 25 - 30℃; for 4h; Autoclave; optical yield given as %ee; enantioselective reaction; | |
| 99% | With bis(1,5-cyclooctadiene)diiridium(I) dichloride; hydrogen; C41H44N3O3P; barium(II) hydroxide In ethanol at 40℃; for 1h; Autoclave; enantioselective reaction; | |
| 96% | With formic acid; triethylamine In water at 50℃; for 6h; enantioselective reaction; | |
| 96% | With bis(1,5-cyclooctadiene)diiridium(I) dichloride; C37H35FeN2P; hydrogen; potassium carbonate In methanol at 20℃; for 12h; Glovebox; Autoclave; enantioselective reaction; | General procedure for asymmetric hydrogenation of ketones General procedure: In a nitrogen-filled glovebox, a stainless steel autoclave was charged with [Ir(COD)Cl]2(3.4 mg, 0.005 mmol) andL2(6.6 mg, 0.11 mmol) in 1.0 mL of dry MeOH. After stirring for 1h at room temperature, a solution of the substrates1(1.0 mmol) andK2CO3(6.9 mg, 0.05 mmol) in 2.0 mL of MeOH was added to the reaction mixture, and then the hydrogenation was performed at room temperature under an H2pressure of 20 bar for 12 h. The solvent was then evaporated and the residue was purified by flash column chromatography to give the corresponding hydrogenation product which was analyzed by chiral HPLC to determine the enantiomeric excesses. |
| 94% | With (S)-bis[[N-2-diphenylphosphinite propyl]-1,1'-ferrocenylmethyldiamine(chloro ɳ4-1,5-cyclooctadiene rhodium(I))]; isopropyl alcohol; sodium hydroxide for 0.5h; Reflux; Schlenk technique; Inert atmosphere; enantioselective reaction; | 2.2. General procedure for the transfer hydrogenation of ketones General procedure: Typical procedure for the catalytic hydrogen-transfer reaction:a solution of the Rh(I)-complexes 17-24, (0.01 mmol), NaOH(0.05 mmol) and the corresponding ketone (1 mmol) in degassedisoPrOH (10 mL) was refluxed until the reaction completed. Then,a sample of the reaction mixture is taken off, diluted with acetoneand analyzed immediately by GC, conversions obtained are relatedto the residual unreacted ketone. |
| 94% | With C36H40Cl2N2P2Ru; potassium <i>tert</i>-butylate In dichloromethane; isopropyl alcohol at 23℃; for 2h; enantioselective reaction; | |
| 93% | With RuBr2[(S,S)-2,4-bis(diphenylphosphino)pentane](2-aminomethyl-3,5-dimethylpyridine); potassium <i>tert</i>-butylate; hydrogen In isopropyl alcohol at 40℃; for 19h; Inert atmosphere; Autoclave; | 6 General procedure: In an autoclave, 1.32 mg of RuBr2[(S,S)-xylskewphos] (3,5-Me2pica) (1.29×10-3 mmol, S/C=10000) and 5.79 mg of potassium tert-butoxide (5.16×10-2 mmol) are placed, and replaced with argon gas. Under argon gas flow, 1.5 mL of acetophenone (12.9 mmol) and 2.9 mL of ethanol was added while measuring by a syringe, pressurized with hydrogen to 10 atm, stirred at 40° C. for 19 hours, then the reduction of the hydrogen pressure was confirmed and phenylethanol was obtained at 100% yield. The optical purity was 88.0% ee as measured by GC (CP-Chirasil-DEX CB (0.25 mml. D×25 m, DF=0.25 μm, from VARIAN), constant at 110° C., pressure: 102.0 kPa, column flow: 1.18 mL/min, vaporizing chamber temperature: 250° C., detector temperature: 275° C., the retention time of each enantiomer was: (R): 11.7 min, (S): 12.4 min), and (S) isomer has predominantly been generated. |
| 92% | Stage #1: 2-Methoxyacetophenone With pyridine; C56H63N2O4Yb In 1,2-dimethoxyethane at -10℃; for 0.5h; Inert atmosphere; Stage #2: With 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane In 1,2-dimethoxyethane at -10℃; for 8h; Inert atmosphere; enantioselective reaction; | |
| 89% | Stage #1: 2-Methoxyacetophenone With C32H41CrN3O2Si; C7H14O4Si In toluene at -40 - 20℃; for 2h; Stage #2: With potassium carbonate In methanol for 1h; enantioselective reaction; | |
| 85% | With 2-(N-morpholino)ethanesulfonic acidbuffer; NAD; isopropyl alcohol at 30℃; acetone powder of Geotrichum candidum; | |
| 85% | Stage #1: 2-Methoxyacetophenone With (1R,2R)-N,N’-bis(3,5-di-tert-butylbenzyl)-1,2-diphenylethane-1,2-diamine; diethoxymethylane; zinc diacetate In tetrahydrofuran at 25℃; for 24h; Inert atmosphere; Stage #2: With potassium carbonate In tetrahydrofuran; methanol for 0.5h; enantioselective reaction; | |
| 80% | With sodium hydroxide; isopropyl alcohol for 0.0333333 - 0.5h; Heating / reflux; | 13; 15 The chiral catalyst (12) (3.6 mg, 0.005 mmol) is suspended in 3 ml of 2-propanol in a 10 ml Schlenk, and 2 ml of a 0.1 M NaOH solution in 2-propanol are added, with consequent dissolution of the product.Separately, in a 50 ml Schlenk the ketone (2 mmol) is dissolved in 19 ml of de-aerated 2-propanol. The system is heated under reflux and 1 ml of the solution containing the previously prepared catalyst is added.The molar ratios of acetophenone/catalyst/NaOH are 2000/11/40. The results obtained from the gas chromatographic analysis are given in table 5.; EXAMPLE 15; Synthesis of (S)-2'-methoxy-1-phenylethanol; Using the same procedure as was used for (S) 2'-chloro-1-phenylethanol, synthesis of (S)-2'-methoxy-1-phenylethanol was undertaken starting from 2-methoxyacetophenone. The molar ratios used for ketone/(12)/base are equal to 5000/1/100. Starting from 1.4 ml (10 mmol) of 2-methoxyacetophenone, 1.24 g of alcohol (80% yield) of S configuration (94% ee) were obtained. |
| 80% | With sodium hydroxide; isopropyl alcohol for 1h; Heating / reflux; | 15 Using the same procedure as was used for (S) 2'-chloro-1-phenylethanol, synthesis of (S)-2'-methoxy-1-phenylethanol was undertaken starting from 2- methoxyacetophenone. The molar ratios used for ketone/(12)/base are equal to 5000/1/100. Starting from 1.4 ml (10 mmol) of 2-methoxyacetophenone, 1.24 g of alcohol (80% yield) of S configuration (94% ee) were obtained. |
| 69% | With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; N-(tert-butoxycarbonyl)-L-valine-(6-amido-1-O-benzyl-6-deoxy-2,3-O-isopropylidene-α-D-mannofuranose); potassium <i>tert</i>-butylate; lithium chloride In tetrahydrofuran; isopropyl alcohol at 20℃; for 3h; enantioselective reaction; | |
| 68% | With formic acid; C20H23ClIrNO3; isopropylamine In dichloromethane at 20℃; enantioselective reaction; | |
| 67% | With Lactobacillus paracasei BD101 In aq. buffer at 30℃; for 48h; Microbiological reaction; enantioselective reaction; | |
| 50% | With NAD; isopropyl alcohol at 30℃; for 5h; Enzymatic reaction; optical yield given as %ee; enantiospecific reaction; | |
| 50% | With Kluyveromyces marxianus CBS 6556 growing cells In ethanol at 30℃; for 96h; enantioselective reaction; | 4.4. Bioreduction of 1a-n by using Kluyveromyces marxianus growing cells: general procedure General procedure: Kluyveromyces marxianus CBS 6556 was stored on agar slants at 4 °C. For the inoculum preparation, a 250-mL conical Erlenmeyer flask containing 100 mL of yeast maintenance medium (YMM) (previously autoclaved at 121 °C, 1 atm, for 15 min), was inoculated with a single loopful of the microorganisms from the agar slants. The flask was then incubated aerobically at 30 °C in a rotary shaker at 200 rpm for 24 h. Next, the growing cultures were inoculated (5% v/v) into a 250 mL conical Erlenmeyer flask containing 100 mL of YMM and incubated for an additional 24 h under the same conditions. These cultures were then used as the final inoculum (1% v/v) into a 1000 mL flask containing 400 mL of YMM. After 24 h incubation at 30 °C in the shaker (200 rpm), 100 mg of the ketone 1a-n dissolved in 1 mL of absolute ethanol was added. The reactions were monitored by GC, by collecting suspension of aliquots of 1 mL after 1, 3, 4, and 5 days of reaction from each flask: after extraction with ethyl acetate (2 mL), the organic phase was analyzed by GC. After appropriate conversion, the suspension was centrifuged (3000 rpm, 6 min, 4 °C), and the aqueous phase was extracted with ethyl acetate (4 × 150 ml). The yellow organic phase was dried over Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified by silica gel column chromatography using petroleum ether and ethyl acetate (90:10 or 80:20) as eluents to yield the desired alcohols. The absolute configurations of alcohols 2a-n obtained from the bioprocess were determined by comparison of their specific rotations with those previously reported in the literature, from commercially available compounds, or by comparison of retention times with previously published data. |
| 45% | Stage #1: 2-Methoxyacetophenone With [RhCl2(p-cymene)]2; isopropyl alcohol; lithium chloride; Boc-L-alanine(2S)-hydroxypropylamide In tetrahydrofuran at 30℃; for 0.25h; Inert atmosphere; Stage #2: With sodium isopropylate In tetrahydrofuran at 30℃; for 1h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | |
| 10% | With Synechococcus sp. PCC 7942 at 20℃; for 72h; Irradiation; | |
| With (-)-diisopinocamphenylborane chloride In tetrahydrofuran at -25℃; for 8h; | ||
| 100 % Chromat. | With diisopinocamphenylchloroborane In tetrahydrofuran at -25℃; for 1h; | |
| With D-glucose; D-glucose dehydrogense; Pyrococcus furiosus alcohol dehydrogenase In dimethyl sulfoxide at 37℃; | ||
| With 1,4-dihydronicotinamide adenine dinucleotide; (S)-alcohol dehydrogenase from Rhodococcus erythropolis In phosphate buffer at 30℃; | ||
| Multi-step reaction with 2 steps 1: (1S,2R)-2-amino-1,2-diphenylethanol; benzoic acid / acetonitrile; H2O / 20 °C | ||
| With sodium isopropylate; isopropyl alcohol at 60℃; for 0.166667h; | ||
| With C49H57ClFeN2OsP2; sodium isopropylate In isopropyl alcohol at 60℃; for 0.5h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| With (R)-1-[(S)-2-(diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine ethanol; dichlorotris(triphenylphosphine)osmium(II); sodium isopropylate; 2,2-dimethyl-1-(pyridin-2-yl)propan-1-amine; isopropyl alcohol at 60℃; for 1h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| With C55H69ClFeN2O2P2Ru; potassium <i>tert</i>-butylate; hydrogen In methanol; ethanol at 40℃; for 0.5h; | ||
| With tris(triphenylphosphine)ruthenium(II) chloride; 1-(benzo[h]quinolin-2-yl)-2,2-dimethylpropanamine; (S,R)-Josiphos*; sodium isopropylate In isopropyl alcohol at 60℃; for 0.5h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| With Lentinus strigellus In water at 28℃; for 144h; optical yield given as %ee; stereoselective reaction; | ||
| 94 % ee | With sodium hydroxide; isopropyl alcohol for 0.0333333h; Heating / reflux; | 13 chiral catalyst (12) (3.6 mg, 0.005 mmol) is suspended in 3 ml of 2-propanol in a 10 ml Schlenk, and 2 ml of a 0.1 M NaOH solution in 2-propanol are added, with consequent dissolution of the product. Separately, in a 50 ml Schlenk the ketone (2 mmol) is dissolved in 19 ml of de- aerated 2-propanol. The system is heated under reflux and 1 ml of the solution containing the previously prepared catalyst is added. The molar ratios of acetophenone/catalyst/NaOH are 2000/1/40. The results obtained from the gas chromatographic analysis are given in table 5. |
| With growing cells of Lasiodiplodia theobromae (strain #009) at 28℃; for 24h; potato dextrose culture medium; Microbiological reaction; optical yield given as %ee; enantioselective reaction; | ||
| With potassium <i>tert</i>-butylate; hydrogen In isopropyl alcohol at 20℃; for 24h; Autoclave; optical yield given as %ee; enantioselective reaction; | ||
| 97 % ee | With potassium <i>tert</i>-butylate; hydrogen; triphenylphosphine In toluene; <i>tert</i>-butyl alcohol at 25 - 30℃; for 5h; | 24 Accurately weighed amounts of (SS,S)-9 (0.9 mg, 1 μmol), solid KO-t-C4H9 (6 mg, 0.05 mmol) and sometimes derivatives [e.g. PPh3 (0.3 mg, 1 μmol)] were placed in a pre-oven-dried (120° C.) 350-mL autoclave containing a magnetic stirring bar, and placed under high vacuum for at least 20 min before purging with argon. Freshly distilled solvent (toluene, 2.7 mL; t-BuOH, 0.3 mL) and purified ketones (1 mmol, S/C=1,000) were placed into a pre-dried Schlenk and degassed by 3 cycles of freeze-and-thaw and then added to the autoclave under an Ar atmosphere. H2 was introduced under 20 atm pressure with several quick release-fill cycles before being set to 8 atm. The solution was vigorously stirred at 25° C. and H2 consumption monitored. The H2 was carefully released after a period of time, the solution passed through a short pad of silica gel and solvent removed under reduced pressure. The crude product mixture was analyzed by 1H NMR to determine conversion and chiral GC or HPLC to determine ee of the chiral alcohol products. The hydrogenation results are given in Table 5. TABLE 5 Screening of aromatic substrates using complex (SS,S)-9a Entry subPPh3b t/h Conv. % Ee % (S) 1 acetophenone 1 100 96 2 acetophenone 3eq 1 62 98 3 acetophenone 1eq 1 100 98 4 2-Me-acetophenone 13.5 87 90 5 2-Me-acetophenone 1eq 8.5 74 97 6 4-MeO-acetophenone 5 99 97 7 4-MeO-acetophenone 1eq 3 99 99.6 8 4-Br-acetophenone 15 100 93 9 4-Br-acetophenone 1eq 6 99 98 10 4-F-acetophenone 1eq 9.5 100 98 11 4-Me-acetophenone 1eq 12 99 98 12 3-Br-acetophenone 1eq 1.5 99 98 133,5-CF3-acetophenone 1eq 3 100 92 14 1eq 5 100 99 15 1eq 2 99 99 16 1eq 10 90 98 17 1eq 3 97 91 18c 1eq 8 99 99 aConditions: Substrate/Catalyst/Base = 1000/1/50; VT = 3 mL, 25-30° C.bCompared to catalyst;cThe H2 pressure was 20 atm and the configuration of the product was R. |
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; C27H42N2O8; sodium isopropylate; isopropyl alcohol; lithium chloride In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| With C10H22N2*C44H32Cl2P2Ru; hydrogen; potassium hydroxide In isopropyl alcohol at 20℃; for 12h; enantioselective reaction; | ||
| 90 % ee | With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; (2S)-2-{benzyl[2-(benzyl{(2S)-1-[(diphenylphosphanyl)oxy]-3-phenylpropan-2-yl}amino)ethyl]amino}-3-phenylpropyl diphenylphosphinite; potassium hydroxide In isopropyl alcohol at 82℃; for 5h; Inert atmosphere; Schlenk technique; enantioselective reaction; | |
| 94 % ee | With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; C38H50N2O2P2; isopropyl alcohol; potassium hydroxide at 82℃; for 0.75h; Schlenk technique; Inert atmosphere; enantioselective reaction; | General procedure for the transfer hydrogenation of ketones General procedure: A typical experimental procedure is described. A flame dried Schlenk flask was charged with [Ru(η6-p-cymene)(μ-Cl)Cl]2 (0.005mmol), bis(phosphinite) ligand (0.02mmol) and a stir bar. To these components was added iso-PrOH (5mL, dried and degassed), and the resultant solution was heated at 82°C until the reaction was completed, followed by cooling to ambient temperature. A solution of ketone (0.5mmol) in iso-PrOH (5mL) was then added to catalyst mixture and the solution was heated to the desired temperature (generally, 25°C, 50°C or 82°C). The reaction was initiated with the addition of a solution of KOH (2.5mL, 0.1M iso-PrOH). As soon as the reaction completed, an aliquot of the catalytic solution (1mL) was removed via syringe and evaporated under reduced pressure. The resultant oil was subjected to flash chromatography (silica gel-60, Et2O) and subsequent evaporation under reduced pressure to yield clear liquids in each case. After this time a sample of the reaction mixture was taken off, diluted with acetone and analyzed immediately by GC, conversions obtained are related to the residual unreacted ketone. |
| 97 % ee | With [(2S)-2-(ferrocenylmethylamino)-2-phenylethyldiphenylphosphinito(dichloro(η6-benzene)ruthenium(II))]; sodium hydroxide In isopropyl alcohol at 82℃; for 0.5h; Inert atmosphere; Schlenk technique; enantioselective reaction; | General procedure for the transfer hydrogenation of ketones General procedure: 2.2;General procedures for synthesis of ferrocene based Ru(II)-phosphinites complexes (17-24) ;[Ru(η6-benzene)(μ-Cl)Cl]2 (0.15 mmol) and ferrocene based phosphinite (0.30 mmol) were dissolved in 20 mL of toluene and stirred for 1 h at room temperature.The volume was concentrated to ca.1-2 mL under reduced pressure and addition of petroleum ether (20 mL) gave the desired complex a tile red solid which was collected by filtration and dried in vacuum. |
| 91 % ee | With (2S)-2-(ferrocenylmethylamino)-2-phenylethyl diphenylphosphinito(dichloro(η6-p-cymene)ruthenium(II)); isopropyl alcohol; potassium hydroxide for 0.5h; Reflux; Inert atmosphere; Schlenk technique; enantioselective reaction; | 4.2 General procedure for the transfer hydrogenation of ketones General procedure: Typical procedure for the catalytic hydrogen-transfer reaction: a solution of ruthenium complexes 9-12 (0.005mmol), KOH (0.025mmol), and the corresponding ketone (0.5mmol) in degassed iso-PrOH (5mL) was refluxed until the reaction was completed. Next a sample of the reaction mixture was taken off, diluted with acetone, and analyzed immediately by GC, conversions obtained are related to the unreacted ketone. |
| > 99.9 % ee | With hydrogen; lithium hydroxide; (8R,9R)-9-amino(9-deoxy)epicinchonin In methanol at 25℃; for 48h; Autoclave; enantioselective reaction; | 2.4 General procedure for the asymmetric hydrogenation reaction General procedure: Asymmetric hydrogenation of aromatic ketones was performed in a 60mL stainless steel autoclave with a magnetic stirred bar at room temperature, by using 9-amino(9-deoxy)epicinchonine as modifier, which is derived from cinchonine. In a typical run, the catalyst, chiral diamine, solvent, base and acetophenone were placed in the autoclave, followed by five purges hydrogen. The hydrogen pressure was thereafter increased to desired level. The mixture was stirred at room temperature for the appropriate duration. |
| >= 99 % ee | With seeds of Linum usitatissimum In aq. phosphate buffer at 25℃; for 72h; Microbiological reaction; enantioselective reaction; | General procedure: Studies were previously performed to determine the optimal reaction conditions among the parameters: amount ofbiocatalyst (2 g, 5 g, 10 g, and 20 g), time of reaction (24 h, 48, 72, and 96 h), use of co-solvent isopropyl alcohol in theproportions (v/v) 2, 5, and 10% in distilled water and a buffer solution previously prepared from Na2HPO4-KH2PO4, withpH 6.0, 7.0, and 8.0, using 50 mg of acetophenone as pattern substrate. Therefore, the reactions were carried out in the bestreaction conditions among the parameters tested, using 50 mg of substrate and 20 g of biocatalyst in a buffer solution (Na2HPO4-KH2PO4), pH 6.0, over a period of 72 h at 25C without a co-solvent. In these reaction conditions, 70.4% ofbioconversion and an ee of 93.7% were obtained for pattern acetophenone. All biotransformation reactions were performedusing a modified methodology proposed by Machado et al. [11]. Whole seeds of Linum usitatissimum L. were washed with 5%sodium hypochlorite and rinsed with sterile distilled water. Each individual carbonyl substrate, 1-14 (50 mg), was added to asuspension of 20 g of L. usitatissimum seeds in 40 mL of a buffered solution (Na2HPO4-KH2PO4), pH 6.0, and incubated at25C in an orbital shaker (175 rpm) for 72 h. Controls were similarly processed, except that no substrates were added. Allreactions were performed in triplicate. The course of all reactions was monitored by TLC (Merck, silica gel 60 F254) and thesubstances revealed by spraying with vanillin solution. After completion of the reaction, each suspension was filtered andwashed with water, and the aqueous solutions were extracted with CH2Cl2 (3 50 mL). The organic phases were dried withNa2SO4 and removed in a rotator evaporator. The reaction products were purified by column chromatography on silica gel60 VETEC with a binary mixture of hexane-ethyl acetate (8:2, v/v) as eluent to afford the (S)-alcohols (Scheme 1). The opticalrotations were measured on a PerkinElmer 241 digital polarimeter. |
| 97 % ee | With (R)-bis[[N-(2-diphenylphosphinite-2-phenyl)ethyl]-1,1'-ferrocenylmethyldiamine(dichloro η6-p-cymene ruthenium(II))]; sodium hydroxide In isopropyl alcohol at 82℃; for 4h; Inert atmosphere; Schlenk technique; enantioselective reaction; | |
| 92 % ee | With (S)-bis[[N-2-diphenylphosphinite propyl]-1,1'-ferrocenylmethyldiamine(dichloro η5-pentamethylcyclopentadienyl iridium(III))]; isopropyl alcohol; sodium hydroxide at 82℃; for 3h; Inert atmosphere; Schlenk technique; enantioselective reaction; | 4.2. General procedure for the transfer hydrogenation ofketones General procedure: Typical procedure for the catalytic hydrogen-transfer reaction:a solution of the Ir(III)-complexes 1-8, (0.01 mmol), NaOH(0.05 mmol) and the corresponding ketone (1 mmol) in degassed2-propanol (10 mL) was refluxed until the reaction wascompleted. A sample of the reaction mixture was then takenoff, diluted with acetone and analyzed immediately by GC.The conversions obtained are related to the residual unreactedketone. |
| 99 % ee | With (2S)-2-(ferrocenylmethylamino)-2-phenylethyldiphenylphosphinito(dichloro(ɳ5-pentamethylcyclopentadienyl)iridium(III)); isopropyl alcohol; potassium hydroxide at 82℃; for 0.5h; Inert atmosphere; Schlenk technique; enantioselective reaction; | General procedure for the transfer hydrogenation of ketones General procedure: Typical procedure for the catalytic hydrogen transfer reaction: asolution of iridium complexes 17-24 (0.005 mmol), KOH (0.025 mmol) and the corresponding ketone (0.5 mmol) in degassed 2-propanol (5 mL) was refluxed until the reaction completed. Then, a sample of the reaction mixture is taken off, diluted with acetone and analyzed immediately by GC, conversions obtained are related to the residual unreacted ketone. |
| > 99 % ee | With D-glucose In aq. phosphate buffer; ethanol at 30℃; for 24h; enantioselective reaction; | 4.3 Resting Cell Collection and Biocatalytic Asymmetric Reductions of Prochiral Ketones General procedure: 2 g strain cells were collected by centrifuging at 4 C, 6000 r/min for 10 min. Then, the collected strain cells were repeatedly purged for three times by 0.1 mol/L phosphate buffer (pH 6.6) and suspended with 10 mL 0.1 mol/L phosphate buffer (pH 6.6) with 60 mmol/L final substrate concentration and 2 % (w/v) glucose. The conditions of biocatalytic asymmetric reductions were 30°C, pH 6.6, 150 r/min, 24 h. At the end of reactions, the product and the residual substrate were extracted by ethyl acetate (1:1, v/v) and dried by MgSO4. The substrate conversion and product e.e. value were determined by GC analysis. |
| Multi-step reaction with 2 steps 1: (1R,2R)-N,N’-bis(3,5-di-tert-butylbenzyl)-1,2-diphenylethane-1,2-diamine; zinc diacetate / neat (no solvent) / 6 h / 25 °C / Inert atmosphere; Sealed tube 2: tetrabutyl ammonium fluoride / tetrahydrofuran / 0.08 h / 0 °C / Inert atmosphere | ||
| Multi-step reaction with 2 steps 1: <SUP>Ph</SUP>boxmi-MnCH<SUB>2</SUB>SiMe<SUB>3</SUB> / toluene / 2 h / -40 - 20 °C / Schlenk technique; Inert atmosphere; Glovebox 2: silica gel | ||
| > 99 % ee | With yeast strain Candida zeylanoides P1 In aq. buffer at 30℃; for 48h; Enzymatic reaction; enantioselective reaction; | |
| 73 %Chromat. | With C50H47Cl2N6O5PRuS2; potassium isopropoxide; isopropyl alcohol at 28℃; for 0.333333h; Inert atmosphere; enantioselective reaction; | |
| 67 %Spectr. | With formic acid; RuCl[(S,S)-FsDPEN](p-cymene); triethylamine at 40℃; for 24h; Inert atmosphere; Schlenk technique; enantioselective reaction; | |
| > 99 % ee | With formic acid; triethylamine at 50℃; for 5h; Schlenk technique; enantioselective reaction; | |
| >99 %Spectr. | With phenylsilane In aq. buffer at 20℃; for 3h; Enzymatic reaction; enantioselective reaction; | |
| 90.5 % ee | With Grubbs catalyst first generation; tris(o-methoxyphenyl)phosphine; hydrogen; lithium hydroxide; (8R,9R)-9-amino(9-deoxy)epicinchonin In propan-1-ol at 30℃; for 2h; Autoclave; enantioselective reaction; | 2.2. Typical procedure for asymmetric hydrogenation of ketones General procedure: Ruthenium precursor, ligands, solvent, base and ketones were added to a 50 mL stainless autoclave with a magnetic stirrer. The autoclave is then charged H2. After stirring for 2 h, the conversions and enantioselectivity values were analyzed by GC. The ee values of chiral alcohols were calculated from the equations enantioselectivity values (ee, %) = |R - S| / |R + S|. |

[2]Location in patent: experimental part Jiang, He-yan; Chen, Hua; Li, Rui-xiang [Catalysis Communications, 2010, vol. 11, # 7, p. 584 - 587]
[3]Xie, Jian-Hua; Liu, Xiao-Yan; Xie, Jian-Bo; Wang, Li-Xin; Zhou, Qi-Lin [Angewandte Chemie - International Edition, 2011, vol. 50, # 32, p. 7329 - 7332]
[4]Xie, Jian-Bo; Xie, Jian-Hua; Liu, Xiao-Yan; Zhang, Qian-Qian; Zhou, Qi-Lin [Chemistry - An Asian Journal, 2011, vol. 6, # 3, p. 899 - 908]
[5]Zhang, Lin; Zhang, Ling; Chen, Qian; Li, Linlin; Jiang, Jian; Sun, Hao; Zhao, Chong; Yang, Yuanyong; Li, Chun [Organic Letters, 2022, vol. 24, # 1, p. 415 - 419]
[6]Xu, Xiao; Wang, Rui; Wan, Jingwei; Ma, Xuebing; Peng, Jingdong [RSC Advances, 2013, vol. 3, # 19, p. 6747 - 6751]
[7]Qin, Chao; Hou, Chuan-Jin; Liu, Hongzhu; Liu, Yan-Jun; Huang, De-Zhi; Hu, Xiang-Ping [Tetrahedron Letters, 2018, vol. 59, # 8, p. 719 - 722]
[8]Ak, Bünyamin; Aydemir, Murat; Durap, Feyyaz; Meriç, Nermin; Baysal, Akin [Inorganica Chimica Acta, 2015, vol. 438, p. 42 - 51]
[9]Chen, Fumin; He, Dongxu; Chen, Li; Chang, Xiaoyong; Wang, David Zhigang; Xu, Chen; Xing, Xiangyou [ACS Catalysis, 2019, vol. 9, # 6, p. 5562 - 5566]
[10]Current Patent Assignee: KANTO KAGAKU HOLDINGS K.K.; HOKKAIDO UNIVERSITY - US2015/31920, 2015, A1 Location in patent: Page/Page column 0159; 0160; 0162
[11]Song, Peng; Lu, Chengrong; Fei, Zenghui; Zhao, Bei; Yao, Yingming [Journal of Organic Chemistry, 2018, vol. 83, # 11, p. 6093 - 6100]
[12]Schiwek, Christian H.; Vasilenko, Vladislav; Wadepohl, Hubert; Gade, Lutz H. [Chemical Communications, 2018, vol. 54, # 66, p. 9139 - 9142]
[13]Nakamura; Matsuda [Journal of Organic Chemistry, 1998, vol. 63, # 24, p. 8957 - 8964]
[14]Szewczyk, Marcin; Stanek, Filip; Bezłada, Agata; Mlynarski, Jacek [Advanced Synthesis and Catalysis, 2015, vol. 357, # 16-17, p. 3727 - 3731]
[15]Current Patent Assignee: UNIVERSITY OF UDINE - US2008/249308, 2008, A1 Location in patent: Page/Page column 8; 9
[16]Current Patent Assignee: UNIVERSITY OF UDINE - WO2005/105819, 2005, A1 Location in patent: Page/Page column 23-24
[17]Margalef, Jèssica; Slagbrand, Tove; Tinnis, Fredrik; Adolfsson, Hans; Diéguez, Montserrat; Pàmies, Oscar [Advanced Synthesis and Catalysis, 2016, vol. 358, # 24, p. 4006 - 4018]
[18]Zhou, Gang; Aboo, Ahmed H.; Robertson, Craig M.; Liu, Ruixia; Li, Zhenhua; Luzyanin, Konstantin; Berry, Neil G.; Chen, Weiping; Xiao, Jianliang [ACS Catalysis, 2018, vol. 8, # 9, p. 8020 - 8026]
[19]Yılmaz, Durmuşhan; Şahin, Engin; Dertli, Enes [Chemistry and biodiversity, 2017, vol. 14, # 11]
[20]Location in patent: experimental part Matsuda, Tomoko; Marukado, Ryo; Mukouyama, Masaharu; Harada, Tadao; Nakamura, Kaoru [Tetrahedron Asymmetry, 2008, vol. 19, # 19, p. 2272 - 2275]
[21]Vitale, Paola; D'Introno, Cinzia; Perna, Filippo Maria; Perrone, Maria Grazia; Scilimati, Antonio [Tetrahedron Asymmetry, 2013, vol. 24, # 7, p. 389 - 394]
[22]Location in patent: experimental part Wettergren, Jenny; Buitrago, Elina; Ryberg, Per; Adolfsson, Hans [Chemistry - A European Journal, 2009, vol. 15, # 23, p. 5709 - 5718]
[23]Nakamura, Kaoru; Yamanaka, Rio; Tohi, Keiko; Hamada, Hiroki [Tetrahedron Letters, 2000, vol. 41, # 35, p. 6799 - 6802]
[24]Ramachandran, P. Veeraraghavan; Gong, Baoqing; Brown, Herbert C. [Tetrahedron Letters, 1994, vol. 35, # 14, p. 2141 - 2144]
[25]Zhao, Mangzhu; King, Anthony O.; Larsen, Robert D.; Verhoeven, Thomas R.; Reider, Paul J. [Tetrahedron Letters, 1997, vol. 38, # 15, p. 2641 - 2644]
[26]Zhu, Dunming; Malik, Hiba T.; Hua, Ling [Tetrahedron Asymmetry, 2006, vol. 17, # 21, p. 3010 - 3014]
[27]Gröger, Harald; Hummel, Werner; Rollmann, Claudia; Chamouleau, Francoise; Hüsken, Hendrik; Werner, Helge; Wunderlich, Christine; Abokitse, Kofi; Drauz, Karlheinz; Buchholz, Stefan [Tetrahedron, 2004, vol. 60, # 3, p. 633 - 640]
[28]Kodama, Koichi; Kobayashi, Yuka; Saigo, Kazuhiko [Chemistry - A European Journal, 2007, vol. 13, # 7, p. 2144 - 2152]
[29]Baratta, Walter; Chelucci, Giorgio; Herdtweck, Eberhardt; Magnolia, Santo; Siega, Katia; Rigo, Pierluigi [Angewandte Chemie - International Edition, 2007, vol. 46, # 40, p. 7651 - 7654]
[30]Baratta, Walter; Ballico, Maurizio; Chelucci, Giorgio; Siega, Katia; Rigo, Pierluigi [Angewandte Chemie - International Edition, 2008, vol. 47, # 23, p. 4362 - 4365]
[31]Location in patent: experimental part Baratta, Walter; Ballico, Maurizio; Del Zotto, Alessandro; Siega, Katia; Magnolia, Santo; Rigo, Pierluigi [Chemistry - A European Journal, 2008, vol. 14, # 8, p. 2557 - 2563]
[32]Location in patent: experimental part Baratta, Walter; Chelucci, Giorgio; Magnolia, Santo; Siega, Katia; Rigo, Pierluigi [Chemistry - A European Journal, 2009, vol. 15, # 3, p. 726 - 732]
[33]Location in patent: experimental part Baratta, Walter; Ballico, Maurizio; Baldino, Salvatore; Chelucci, Giorgio; Herdtweck, Eberhardt; Siega, Katia; Magnolia, Santo; Rigo, Pierluigi [Chemistry - A European Journal, 2008, vol. 14, # 30, p. 9148 - 9160]
[34]Location in patent: experimental part Barros-Filho, Bartholomeu A.; de Oliveira, Maria da Conceicao F.; Lemos, Telma L.G.; de Mattos, Marcos C.; Gonzalo, Gonzalo de; Gotor-Fernandez, Vicente; Gotor, Vicente [Tetrahedron Asymmetry, 2009, vol. 20, # 9, p. 1057 - 1061]
[35]Current Patent Assignee: UNIVERSITY OF UDINE - WO2005/105819, 2005, A1 Location in patent: Page/Page column 22-23
[36]Location in patent: experimental part Barros-Filho, Bartholomeu A.; Nunes, Fatima M.; de Oliveira, Maria da Conceicao F.; Lemos, Telma L.G.; de Mattos, Marcos C.; de Gonzalo, Gonzalo; Gotor-Fernandez, Vicente; Gotor, Vicente [Journal of Molecular Catalysis B: Enzymatic, 2010, vol. 65, # 1-4, p. 37 - 40]
[37]Stegink, Bart; Van Boxtel, Lonneke; Lefort, Laurent; Minnaard, Adriaan J.; Feringa, Ben L.; De Vries, Johannes G. [Advanced Synthesis and Catalysis, 2010, vol. 352, # 14-15, p. 2621 - 2628]
[38]Current Patent Assignee: CHINESE ACADEMY OF SCIENCES; ENANTIOTECH - US2011/92712, 2011, A1 Location in patent: Page/Page column 9; 10
[39]Coll, Mercedes; Pamies, Oscar; Adolfsson, Hans; Dieguez, Montserrat [Chemical Communications, 2011, vol. 47, # 44, p. 12188 - 12190]
[40]Location in patent: scheme or table Ma, Meng Lin; Ren, Chuan Hong; Lv, Ya Jing; Chen, Hua; Li, Xian Jun [Chinese Chemical Letters, 2011, vol. 22, # 2, p. 155 - 158]
[41]Aydemir, Murat; Durap, Feyyaz; Kayan, Cezmi; Baysal, Akin; Turgut, Ylmaz [Synlett, 2012, vol. 23, # 19, p. 2777 - 2784]
[42]Elma, Duygu; Durap, Feyyaz; Aydemir, Murat; Baysal, Akin; Meric, Nermin; Ak, Bünyamin; Turgut, Ylmaz; Gümgüm, Bahattin [Journal of Organometallic Chemistry, 2013, vol. 729, p. 46 - 52]
[43]Işik, Uǧur; Aydemir, Murat; Meriç, Nermin; Durap, Feyyaz; Kayan, Cezmi; Temel, Hamdi; Baysal, Akin [Journal of Molecular Catalysis A: Chemical, 2013, vol. 379, p. 225 - 233]
[44]Ak, Buenyamin; Elma, Duygu; Meric, Nermin; Kayan, Cezmi; Isik, Ugur; Aydemir, Murat; Durap, Feyyaz; Baysal, Akin [Tetrahedron Asymmetry, 2013, vol. 24, # 20, p. 1257 - 1264]
[45]Li, Chun; Zhang, Lin; Liu, Hailong; Zheng, Xueli; Fu, Haiyan; Chen, Hua; Li, Ruixiang [Catalysis Communications, 2014, vol. 54, p. 27 - 30]
[46]Tavares, Leonardo C.; Arriaga, Angela M. C.; De Lemos, Telma L. G.; Souza, Juliana M. O.; Teixeira, Maria V. S.; Santiago, Gilvandete M. P. [Chemistry of Natural Compounds, 2015, vol. 51, # 4, p. 752 - 755][Khim. Prir. Soedin., 2015, # 4, p. 645 - 647,3]
[47]Ak; Durap; Aydemir; Baysal [Applied Organometallic Chemistry, 2015, vol. 29, # 11, p. 764 - 770]
[48]Ak, Bünyamin; Aydemir, Murat; Durap, Feyyaz; Meriç, Nermin; Elma, Duygu; Baysal, Akin [Tetrahedron Asymmetry, 2015, vol. 26, # 23, p. 1307 - 1313]
[49]Meriç, Nermin; Aydemir, Murat [Journal of Organometallic Chemistry, 2016, vol. 819, p. 120 - 128]
[50]Wang, Dan; Yang, Zhirong; Zhang, Jinhua; Han, Yunlei; Hao, Junli; He, Lang [Catalysis Letters, 2016, vol. 146, # 6, p. 1079 - 1086]
[51]Szewczyk, Marcin; Bezłada, Agata; Mlynarski, Jacek [ChemCatChem, 2016, vol. 8, # 23, p. 3575 - 3579]
[52]Vasilenko, Vladislav; Blasius, Clemens K.; Wadepohl, Hubert; Gade, Lutz H. [Angewandte Chemie - International Edition, 2017, vol. 56, # 29, p. 8393 - 8397][Angew. Chem., 2017, vol. 129, p. 8513 - 8517,5]
[53]Şahin, Engin; Dertli, Enes [Chemistry and biodiversity, 2017, vol. 14, # 9]
[54]Chai, Huining; Liu, Tingting; Yu, Zhengkun [Organometallics, 2017, vol. 36, # 21, p. 4136 - 4144]
[55]Matsunami, Asuka; Ikeda, Marika; Nakamura, Hitomi; Yoshida, Minori; Kuwata, Shigeki; Kayaki, Yoshihito [Organic Letters, 2018, vol. 20, # 17, p. 5213 - 5218]
[56]Doherty, Simon; Knight, Julian G.; Alshaikh, Hind; Wilson, James; Waddell, Paul G.; Wills, Corinne; Dixon, Casey M. [European Journal of Inorganic Chemistry, 2021, vol. 2021, # 3, p. 226 - 235]
[57]Ji, Pengfei; Park, Jeeyoung; Gu, Yang; Clark, Douglas S.; Hartwig, John F. [Nature Chemistry, 2021, vol. 13, # 4, p. 312 - 318]
[58]Wang, Mengna; Zhang, Ling; Sun, Hao; Chen, Qian; Jiang, Jian; Li, Linlin; Zhang, Lin; Li, Li; Li, Chun [Catalysis Communications, 2021, vol. 154]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 96% | With aluminum (III) chloride In dichloromethane at -5 - 25℃; | |
| 89% | With hydrogen iodide at 25℃; for 24h; Inert atmosphere; | 5 Synthesis of o-hydroxyacetophenone A 2-way cock with a septum at the tip was attached to a recovery flask containing o-methoxyacetophenone (159 mg, 1.05 mmol), dried by allowing to stand overnight under reduced pressure in the presence of diphosphorus pentoxide, At atmospheric pressure. After reducing the pressure with a diaphragm pump again, hydrogen iodide gas (257 mg, 2.01 mmol) was introduced from the septum using a 50 mL syringe, and after returning to atmospheric pressure by filling with nitrogen, it was allowed to stand at 25 ° C. for 24 hours I put it. After the reaction, the mixture was reduced in pressure by a diaphragm pump to obtain a mixture of o-hydroxyacetophenone and o-methoxyacetophenone (128.6 mg). The yield was determined to be 89%: 1% of o-hydroxyacetophenone: o-methoxyacetophenone from the integral ratio of 1 H-NMR using phenanthrene as an internal standard. |
| 85% | With 1-n-butyl-3-methylimidazolim bromide at 220℃; for 0.666667h; Inert atmosphere; Microwave irradiation; |
| 85% | With magnesium iodide at 50℃; for 4h; Ionic liquid; | |
| 80% | With dimethyl diselenide; sodium In N,N,N,N,N,N-hexamethylphosphoric triamide for 8h; Heating; | |
| 77% | With cerium(III) chloride; sodium iodide In acetonitrile for 8h; Heating; |

[2]Current Patent Assignee: CHIBA UNIVERSITY; GODO SHIGEN CO LTD - JP5725506, 2015, B2 Location in patent: Paragraph 0072-0075
[3]Location in patent: experimental part Park, Jiyeon; Chae, Junghyun [Synlett, 2010, # 11, p. 1651 - 1656]
[4]Location in patent: experimental part Lee, Kwan Soo; Kim, Kee D. [Bulletin of the Korean Chemical Society, 2010, vol. 31, # 12, p. 3842 - 3843]
[5]Evers, Michel; Christiaens, Leon [Tetrahedron Letters, 1983, vol. 24, # 4, p. 377 - 380]
[6]Yadav; Subba Reddy; Madan; Riaz Hashim [Chemistry Letters, 2000, # 7, p. 738 - 739]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 92% | With silver trifluoromethanesulfonate; In water; acetic acid; at 110℃; for 6h;Schlenk technique; | General procedure: To a 25mL Schlenk tube, AuSBA-15 (6wt%, 20mg), AgOTf (0.05mmol) was added to a solution of phenylacetylene (1.0mmol) in HOAc/H2O (3.0mL, 15:1) under ambient air, the resulting mixture was stirred for 6hat 110C. It was monitored by TLC. After the reaction was completed, the solvent was removed under reduced pressure and purified of the crude product by column chromatography on silica-gel afforded the desired compound. |
| 90% | With Perfluorooctanesulfonic acid; C8AgF17O3S*H2O; In water; at 100℃; for 8h;Darkness; | General procedure: To the mixture of phenylacetylene (1 mmol), water (3.0 mL),silver perfluorooctanesulfonate (5 mol%) and perfluorooctane sulfonateacid (2 mol%) was added. The mixture was stirred at 100 Cfor 8 h. The solution was extracted with n-hexane (diethyl ether)(3 5 mL), the combined extract was dried with anhydrous MgSO4. The rest of the solution was used for the next cycle of reaction. Theextraction solvent was removed and the crude product was separatedby column chromatography to give the pure sample. |
| 88% | With silver tetrafluoroborate; water; In acetic acid; at 110℃; for 10h; | General procedure: To the mixture of terminal alkyne (1mmol), water (2.0 equiv), and acetic acid (2 mL), silver tetrafluoroborate (5 mol%) was added. The mixture was stirred at 110C for 10 h. Water (10 mL) was added and the solution was extracted with ethyl acetate (3×8 mL), the combined extract was dried with anhydrous MgSO4. The solvent was removed and the crude product was separated by column chromatography to give the pure sample. 4.2.9. 1-(2-Methoxyphenyl)ethanone (2i). 1H NMR (400 MHz, CDCl3): delta=7.69-7.72 (m, 1H), 7.41 (m, 1H), 6.93-6.98 (m, 2H), 3.88 (s, 3H), 2.59 (s, 3H). 13C NMR (100 MHz, CDCl3): delta=199.8, 158.9, 133.6, 130.3, 128.2, 120.5, 111.6, 55.4, 31.8. MS (EI) m/z: 150, 135, 105, 92, 77, 63. |
| 85% | With indium(III) triflate; water; toluene-4-sulfonic acid; In 1,2-dichloro-ethane; for 0.5h;Sealed tube; Reflux; | General procedure: The reaction mixture of In(OTf)3 (11.2 mg, 2 mol %), PTSA (57.1 mg, 30 mol %), DCE (2.0 mL), alkynes 1a-1n or 1p-1t (1.0 mmol) and water (0.2 mL) in a 10 mL flask or in a 10 mL sealed tube was stirred at reflux and monitored periodically by TLC. Upon completion, DCE was removed under reduced pressure using an aspirator, and then the residue was purified by flash chromatography (PE/EA) on silica gel to afford corresponding carbonyl compounds 2a-2n or 2p-2t. |
| 84% | With water; In methanol; at 150℃; under 8250.83 Torr; for 14h;Autoclave; Inert atmosphere; Green chemistry; | General procedure: In a 100 mL capacity of autoclave vessel a 60 mL solution of methanol and water (1:2) was added, further 1 mmol alkynes were added to this solution. The autoclave was three times purged withthe gas and then nally pressurized up to the 11 bar pressure. The reaction mixture was vigorously stirred at 150 C for continuous 14 h. After the completion of the reaction, the reactor was cooled to room temperature, and then the argon pressure was carefully released to the atmospheric pressure. Methanol from the reaction mixture is removed using rotatory evaporator. After that, the reaction mixture was transferred in a separating funnel, and it wasworked up with ethyl acetate. The organic layer was separatedand dried over Na2SO4. Afterwards, it was filtered and concentrated under reduced pressure. The resulted crude mixture waspuried by silica gel column chromatography using ethyl acetate/n-hexane as eluent, and pure keto product was isolated. |
| 84% | With methanol; In water; at -5℃; for 0.5h;Irradiation; Green chemistry; | General procedure: To a 100 mL capacity borosilicate immersion well of UV reactor, 80 mL aqueous methanol MeOH:H2O (1:2), alkyne (1 mmol), Rh catalyst (1.5 mol%) was added, and the reaction mixture was cooled to-5 C. It was irradiated using a Hg vapor UV lamp, 125 W, 289 nm for30 min with continuous stirring. After completion of the reaction, the reaction mixture was left to warm to room temperature and then concentrated in vacuo. The residue was extracted with dichloromethaneand water, the organic phase was collected, dried with anhydrous Na2SO4, concentrated at reduced pressure, and puried by flash columnchromatography using hexane/ethyl acetate as eluent to obtain the corresponding product.The catalyst was synthesized as per the procedure reported in reference 15. |
| 76% | With iron(III) sulfate hydrate; acetic acid; at 95℃; for 7h;Schlenk technique; | General procedure: Ferric sulfate hydrate (I, 8 mol%), glacial acetic acid (5 mL) and the alkyne (1 - 2 mmol) were introducedinto a 50 mL Schlenk tube, equipped with an air condenser, and the mixture kept under stirring at 95 C or120 C, until consumption of the substrate or no further conversion, as evidenced by TLC or GC. Uponcooling, the supernatant solution was poored into water and the residue washed twice with diethyl ether.After extraction with diethyl ether ( 2), the combined organic layers were washed with a saturated aqueoussolution of sodium bicarbonate and then water until neutrality. Alternatively, the crude from the reactions ofsubstrates featuring hydroxyl or carbonyl groups, as for 12, 15, 20 and 22, was obtained by removing aceticacid under vacuum, in order to reduce loss of material during biphasic extraction. The products were purified |
| 99%Chromat. | With methanol; water; gold(I) chloride; at 65℃; for 3h;Green chemistry; | General procedure: In a 4 mL reaction vial equipped with a magnetic stirring bar,AuCl (5.8 mg, 0.025 mmol, 5 mol%) was added to MeOH (1 mL)under argon atmosphere. The reaction mixture was stirred for 5min, and then starting material (0.5 mmol, 1.0 equiv) and internalstandard dodecane (1.0 equiv) were added, followed bydefined amount of distilled H2O (4.0 equiv). The resulting reactionmixture was heated for 3 h, or for 24 h when needed, at65 C. After completion of the reaction, the reaction mixturewas diluted and filtered using CH2Cl2 and injected in GC foranalysis. |

[2]Green Chemistry,2015,vol. 17,p. 532 - 537
[3]RSC Advances,2016,vol. 6,p. 69822 - 69827
[4]Inorganic Chemistry Communications,2019,vol. 109
[5]Angewandte Chemie - International Edition,2002,vol. 41,p. 4563 - 4565
[6]Chemical Communications,2017,vol. 53,p. 6926 - 6929
[7]Journal of Organometallic Chemistry,2017,vol. 851,p. 46 - 51
[8]Journal of Organometallic Chemistry,2015,vol. 799-800,p. 122 - 127
[9]Tetrahedron,2013,vol. 69,p. 6116 - 6120
[10]Tetrahedron,2013,vol. 69,p. 3775 - 3781
[11]Tetrahedron Letters,2018,vol. 59,p. 2075 - 2078
[12]Catalysis Communications,2018,vol. 115,p. 78 - 81
[13]Tetrahedron Letters,2014,vol. 55,p. 1608 - 1612
[14]Tetrahedron Letters,2013,vol. 54,p. 4414 - 4417
[15]Organic Letters,2014,vol. 16,p. 948 - 951
[16]Journal of the American Chemical Society,1984,vol. 106,p. 2728 - 2730
[17]Synlett,2015,vol. 26,p. 2517 - 2520
[18]Organic Syntheses,2006,vol. 83,p. 55 - 60
[19]European Journal of Organic Chemistry,2018,vol. 2018,p. 3974 - 3981
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 82% | With dipotassium peroxodisulfate; copper(I) sulfate In water; acetonitrile at 65 - 70℃; for 3h; | |
| 77% | With pyridine; N-hydroxyphthalimide; tetrabutylammonium tetrafluoroborate; oxygen In 2,2,2-trifluoroethanol; acetonitrile at 25 - 30℃; Electrolysis; | 2.2.1 Procedure for mono-oxidation General procedure: An undivided cell was equipped with a magnet stirrer, platinum plate electrode (1.0×1.0×0.3 cm3), as the working electrode and counter electrode. Substrate (0.5 mmol),nBu4NBF4 (0.5 mmol, 164.6 mg), N-hydroxyphthalimide (NHPI, 0.1 mmol, 16.3 mg), and pyridine (1.0 mmol, 82 μL)were added to MeCN/2,2,2-trifluoroethan-1-ol (TFE) (5:1,3 mL). The electrolysis was conducted in an undivided cell equipped with O2 balloon at a constant current of 5 mA at room temperature (25-30 °C). When the reaction was completed, the solvent was removed under reduced pressure and the remaining crude product was purified by column chromatography over silica gel (petroleum ether/ethyl acetate(PE/EA)=30:1-10:1) to afford the corresponding aromatic ketone product. |
| 72% | With cerium(III) chloride In water at 25℃; for 24h; Irradiation; Green chemistry; |
| 56% | With 2,6-dimethylpyridine; oxygen; C34H36NO6(1+)*ClO4(1-) In water; acetonitrile at 20℃; for 18h; Irradiation; Green chemistry; | |
| 50% | With oxygen; 2,6-dimethylpyridine perchlorate In acetonitrile at 20℃; for 12h; Electrolysis; | |
| 40 %Chromat. | With tert.-butylhydroperoxide; C66H60Fe2N12O4(4+)*4BF4(1-)*4H2O In water; acetonitrile at 20℃; for 6h; | Catalytic tests A Schlenk tube was charged with the substrate (0.3 mmol) andthe catalyst (4.5 lmol) dissolved in ACN (0.5 mL). The oxidanttBuOOH (0.32 mL, 70 wt% in H2O, 2.4 mmol) was slowly addedwithin 30 min, and then solution was shaken for 6 h at room temperature.0.2 mL of the solution were dissolved in 1 mL of DCM andthe yield was determined by GC |
| With ketoreductase-P3-B03; oxygen; NADPH; 9‑mesityl-10-methylacridinium perchlorate In water; acetonitrile at 23℃; for 24h; Irradiation; Enzymatic reaction; |

[2]Li, Zhibin; Zhang, Yan; Li, Kuiliang; Zhou, Zhenghong; Zha, Zhenggen; Wang, Zhiyong [Science China Chemistry, 2021, vol. 64, # 12, p. 2134 - 2141]
[3]Xie, Pan; Xue, Cheng; Shi, Sanshan; Du, Dongdong [ChemSusChem, 2021, vol. 14, # 13, p. 2689 - 2693]
[4]García Mancheño, Olga; Kuhlmann, Jan H.; Pérez-Aguilar, María Carmen; Piekarski, Dariusz G.; Uygur, Mustafa [Green Chemistry, 2021, vol. 23, # 9, p. 3392 - 3399]
[5]Li, Xue; Bai, Fang; Liu, Chaogan; Ma, Xiaowei; Gu, Chengzhi; Dai, Bin [Organic Letters, 2021, vol. 23, # 19, p. 7445 - 7449]
[6]Martínez-Ferraté, Oriol; Britovsek, George J.P.; Claver, Carmen; Van Leeuwen, Piet W.N.M. [Inorganica Chimica Acta, 2015, vol. 431, p. 156 - 160]
[7]Betori, Rick C.; May, Catherine M.; Scheidt, Karl A. [Angewandte Chemie - International Edition, 2019, vol. 58, # 46, p. 16490 - 16494][Angew. Chem., 2019, vol. 131, p. 16642 - 16646,5]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 75% | With potassium hydroxide In ethanol at 20℃; | Chalcones 3a-3d (general procedure) General procedure: Benzaldehyde1a-1c (12 mmol) and acetophenone 2a or 2b(12 mmol) were dissolved in ethanol (10 mL), and40% potassium hydroxide solution was added dropwisewhile stirring at room temperature over a period of30 min. The mixture was then stirred at room temperatureuntil the reaction was complete (4-6 h, TLCmonitoring), the solvent was removed on a rotaryevaporator under reduced pressure, the residue waspoured into ice water, and the product was filtered offand recrystallized from methanol. |
| 60% | Stage #1: 2-Methoxyacetophenone With potassium hydroxide In ethanol at 0℃; for 0.25h; Stage #2: benzaldehyde In ethanol at 25℃; for 4h; | |
| 46% | With potassium hydroxide; water monomer In methanol for 24h; |
| With ethanol; sodium hydroxide at 25℃; for 3h; Combinatorial reaction / High throughput screening (HTS); | ||
| With sodium hydroxide In ethanol at 25℃; | ||
| With potassium hydroxide In ethanol at 20℃; for 48h; | ||
| With sodium hydroxide In ethanol; water monomer at 20℃; for 5h; | General procedure for the preparation of α,β-unsaturated ketones General procedure: In a 250 mL flask, corresponding aldehyde (20 mmol),corresponding ketone (20 mmol) and ethanol (50 mL) were placed, and thesolution was stirred at room temperature. To the solution, NaOH aqueoussolution (1.5 M, 20 mL) was slowly added. The mixture was stirred for about 5 h.Crude-unsaturated ketone was obtained after filtration. Then, the crude productwas recrystallized from ethanolIn a 250 mL flask, corresponding aldehyde (20 mmol),corresponding ketone (20 mmol) and ethanol (50 mL) were placed, and thesolution was stirred at room temperature. To the solution, NaOH aqueoussolution (1.5 M, 20 mL) was slowly added. The mixture was stirred for about 5 h.Crude-unsaturated ketone was obtained after filtration. Then, the crude productwas recrystallized from ethanol. | |
| In ethanol; water monomer at 20℃; | General procedure for the synthesis of chalcone dienophiles 2a-d General procedure: A mixture of the corresponding acetophenone (1 equiv.) and benzaldehyde (1 equiv.) in EtOH (5 mL/1 mmol of acetophenone) was stirred at rt for 30 min. Then a solution of 50% w/w aqueous KOH (1 mL/1 mmol) was added. The reaction mixture was stirred at rt until all benzaldehyde was consumed by monitoring on TLC. Afterwards the mixture was poured into ice-water acidified with 3N HCl. In cases in which the chalcones precipitated, they were filtered, purified using column chromatography and crystallised from ethanol (yields: 85-90%). | |
| With sodium hydroxide In methanol at 20℃; | ||
| With sodium hydroxide In ethanol at 0 - 20℃; for 12h; | 3.2.1. General method for the synthesis of substituted 1,3-diaryl-2-propene-1-one (1-19) General procedure: 1,3-Diaryl-2-propene-1-one derivatives (1{19) were prepared according to the procedure described elsewhere inthe literature. 53 61 Equimolar quantities of substituted acetophenone (10 mmol) and substituted benzaldehyde(10 mmol) in ethanol (10 mL) were stirred at 0{5C for 30 min. Sodium hydroxide (40%, 4 mL) was thenadded dropwise. Stirring was continued for 12 h at room temperature and the reactions were stopped after TLCmonitoring. The reaction mixture was poured into crushed ice and acidied if necessary with 6 N HCl. Theprecipitated solid mass was ltered, washed with water, and crystallized from ethanol to give white, orange, oryellow products puried by column chromatography if necessary. | |
| With sodium hydroxide at 20℃; for 1.5h; Sealed tube; Cooling with ice; | ||
| Stage #1: 2-Methoxyacetophenone; benzaldehyde In ethanol at 20℃; for 0.5h; Stage #2: In ethanol; water monomer at 20℃; Alkaline conditions; | ||
| Stage #1: 2-Methoxyacetophenone With sodium hydroxide In methanol; water monomer at 0 - 20℃; for 1h; Stage #2: benzaldehyde In methanol; water monomer at 20℃; | ||
| With sodium hydroxide In ethanol at 20℃; for 0.0833333h; |

[2]Shadakshari, Uma; Nayak, Sandip K. [Tetrahedron, 2001, vol. 57, # 38, p. 8185 - 8188]
[3]Stahl, Ingfried; Schomburg, Sabine; Kalinowski, Hans Otto [Chemische Berichte, 1984, vol. 117, # 6, p. 2247 - 2260]
[4]Cox, Christopher D.; Breslin, Michael J.; Mariano, Brenda J.; Coleman, Paul J.; Buser, Carolyn A.; Walsh, Eileen S.; Hamilton, Kelly; Huber, Hans E.; Kohl, Nancy E.; Torrent, Maricel; Yan, Youwei; Kuo, Laurence C.; Hartman, George D. [Bioorganic and Medicinal Chemistry Letters, 2005, vol. 15, # 8, p. 2041 - 2045]
[5]Location in patent: experimental part Gezegen, Hayreddin; Dingil, Alparslan; Ceylan, Mustafa [Journal of Heterocyclic Chemistry, 2010, vol. 47, # 5, p. 1017 - 1024]
[6]Location in patent: experimental part Karaman, Isa; Gezegen, Hayreddin; Ceylan, Mustafa; Dilma, Merve [Phosphorus, Sulfur and Silicon and the Related Elements, 2012, vol. 187, # 5, p. 580 - 586]
[7]Beniyama, Yoko; Matsuno, Kenji; Miyachi, Hiroyuki [Bioorganic and Medicinal Chemistry Letters, 2013, vol. 23, # 6, p. 1662 - 1666]
[8]Huang, Qiang; Wu, Ji-Wei; Xu, Hua-Jian [Tetrahedron Letters, 2013, vol. 54, # 29, p. 3877 - 3881]
[9]Tee, Jia Ti; Chee, Chin Fei; Buckle, Michael J.C.; Lee, Vannajan Sanghiran; Chong, Wei Lim; Khaledi, Hamid; Rahman, Noorsaadah Abd [Tetrahedron Letters, 2015, vol. 56, # 36, p. 5082 - 5085]
[10]Gandolfi, Raffaella; Facchetti, Giorgio; Christodoulou, Michael S.; Fusè, Marco; Meneghetti, Fiorella; Rimoldi, Isabella [ChemistryOpen, 2018, vol. 7, # 5, p. 393 - 400]
[11]Fandakli, Seda; Kahriman, Nuran; Yücel, Tayyibe Beyza; Alpay Karaoglu, Şengül; Yayli, Nurettin [Turkish Journal of Chemistry, 2018, vol. 42, # 2, p. 520 - 535]
[12]Du, Ding; Feng, Jie; Ma, Rui; Zhang, Beichen; Zhang, Kuili [Organic and Biomolecular Chemistry, 2020, vol. 18, # 38, p. 7549 - 7553]
[13]Chang, Weixing; Kong, Jingyang; Li, Jing; Liu, Lingyan; Wang, Hongkai; Zeng, Tianlong [Advanced Synthesis and Catalysis, 2021, vol. 363, # 16, p. 4024 - 4032]
[14]Biswal, Priyabrata; Samser, Shaikh; Meher, Sushanta Kumar; Chandrasekhar, Vadapalli; Venkatasubbaiah, Krishnan [Advanced Synthesis and Catalysis, 2022, vol. 364, # 2, p. 413 - 419]
[15]Wang, Hui; Yang, Ren-Yin; Xu, Bo [Chemistry - A European Journal, 2022, vol. 28, # 23]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 88% | With sodium hydroxide In ethanol; water at 60℃; for 2h; | |
| 83% | With sodium hydroxide In ethanol; water at 60℃; for 2h; | Typical experimental procedure: General procedure: To a solution of acetophenone (40 mmol) and salicylaldehyde (40 mmol) in EtOH (50 mL) was added 40% NaOH (10 mL) aqueous solution dropwise and the reaction was refluxed at 60 °C for 2 h. The solution/suspension was poured onto cold H2O and the mixture neutralized with 2 M HCl until the solution was acidic. The resulting yellow precipitate was collected, washed with H2O and recrystallized from EtOH to yield a yellow solid (4.2 g, 85% yield of 3). |
| 60% | With sodium hydroxide In ethanol at 20℃; |
| 22 g | With sodium hydroxide In methanol; water for 96h; | |
| With potassium hydroxide In methanol at 20℃; | ||
| With potassium hydroxide In ethanol at 20℃; for 24h; | ||
| With sodium hydroxide In methanol; water at 0 - 20℃; | ||
| With sodium hydroxide In methanol Reflux; | ||
| With potassium hydroxide In ethanol; water at 0 - 25℃; for 48h; |

[2]Location in patent: experimental part Mazimba, Ofentse; Masesane, Ishmael B.; Majinda, Runner R. [Tetrahedron Letters, 2011, vol. 52, # 50, p. 6716 - 6718]
[3]Jiang, Shanshan; Hu, Bin; Yu, Xingxin; Deng, Weiping [Chinese Journal of Chemistry, 2014, vol. 32, # 8, p. 694 - 698]
[4]Bird, T. Geoffrey C.; Brown, Ben R.; Stuart, Ian A.; Tyrrell, William R. [Journal of the Chemical Society. Perkin transactions I, 1983, # 8, p. 1831 - 1846]
[5]Jia, Zhen-Xin; Luo, Yong-Chun; Cheng, Xi-Na; Xu, Peng-Fei; Gu, Yu-Cheng [Journal of Organic Chemistry, 2013, vol. 78, # 13, p. 6488 - 6494]
[6]Rouh, Hossein; Liu, Yangxue; Katakam, Nandakumar; Pham, Lilian; Zhu, Yi-Long; Li, Guigen [Journal of Organic Chemistry, 2018, vol. 83, # 24, p. 15372 - 15379]
[7]Harish, Battu; Subbireddy, Manyam; Obulesu, Owk; Suresh, Surisetti [Organic Letters, 2019]
[8]Bhoi, Manoj N.; Borad, Mayuri A.; Jethava, Divya J.; Pandya, Himanshu A.; Patel, Chirag N.; Patel, Hitesh D. [Journal of Molecular Structure, 2020, vol. 1222]
[9]Cao, Jiangying; Dong, Hang; Xu, Qifu; Zhang, Yingjie; Zhao, Chunlong [RSC Advances, 2021, vol. 11, # 35, p. 21426 - 21432]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 72.4% | Stage #1: 2-Methoxyacetophenone; Glyoxilic acid at 105 - 108℃; for 3h; Stage #2: With hydrazine hydrate In water for 2h; Reflux; | General procedure for the synthesis of 6-substitutedpyridazin-3(2H)-one derivatives (1a, 1b, 1c) General procedure: The mixture of glyoxylic acid monohydrate (5.52g, 0.06mol) and appropriate acetophenone derivative (27.04g, 0.18mol) was heated at 105-108°C for 3h. At the end of the time, the reaction medium was cooled to room temperature and to added 24mL of water and 4.8mL of conc. ammonium hydroxide. Then, the mixture was extracted with dichloromethane (2×50mL), and 2.9mL (0.06mol) hydrazine hydrate was added onto the separated aqueous phase. The mixture was heated under reflux for 2h. At the end of the period, the reaction medium was cooled, the precipitate was filtered and crystallized from the appropriate solvent. |
| With ammonium hydroxide; hydrazine hydrate 1) heating, 2) water, reflux; Yield given. Multistep reaction; | ||
| With hydrazine hydrate |
[2]Coates; McKillop [Synthesis, 1993, # 3, p. 334 - 342]
[3]George, Riham F.; Saleh, Dalia O. [European Journal of Medicinal Chemistry, 2016, vol. 108, p. 663 - 673]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 88% | Sodium hydride (480mg, 20.0 mmmol) was taken in dry THF (18mL) in a 100mL round bottom flask under N2 and cooled it down to 0C. To it was added a solution of ethyl 3-(2-methoxyphenyl)-3-oxopropanoate (1.0g, 6.7mmol) in THF (2mL). The reaction mixture was stirred at rt for 30min followed by the addition of diethyl carbonate (3.2mL, 26.8mmol). The reaction mixture was then stirred at rt for 14h. Ice-cooled water was added dropwise to quench the reaction. It was extracted with EtOAc (3×75mL). The combined organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude was further purified by flash chromatography on silica gel (60-120 mesh) using 10% hexane-EtOAc as eluent to afford 2c as a colourless liquid (1.3g, 88%). LC-MS (ESI) m/z 223.32 [M+H+]; 97% (purity). | |
| 86% | With sodium hydride; In toluene; mineral oil; | General procedure: The substrate b-ketoesters 10 a-n were either purchased or synthesized following published procedures. Some benzoylacetates were commercially available. Ethyl 3-oxo-3-phenyl propanoate (10a) was purchased. The reaction of benzoylacetates 10 b-n was prepared as described in previous reports. 25-27 A solution of a substituted acetophenone 8 a-n (0.05 mol) dissolved in toluene (50 mL) was added dropwise to a solution containing diethyl carbonate (9) (0.10 mol) and sodium hydride (0.15 mol 60% dispersion in mineral oil). The mixture was stirred at room temperature, and then refluxed for 30 min. The mixture was poured into ice water,acidified with glacial acetic acid, and extracted with EtOAc (3x100 mL). The EtOAc extract was then dried over anhydrous MgSO4. After removal of the solvent in vacuo, the crude products were purified by silica gel column chromatography eluting with dichloromethane to afford benzoylacetates 10 b-n. All synthetic compounds were in agreement with 1H NMR, 13C NMR, IR and mass spectroscopic data. |
| In tetrahydrofuran; | A. Ethyl O-methoxybenzoylacetate To a suspension of 7.7 g. (0.32 mole of oil-free sodium hydride in 200 ml. of cold (0 C.) tetrahydrofuran containing 72.6 g. (0.61 mole) of diethyl carbonate was added dropwise under a nitrogen atmosphere 44 g. (0.29 mole) of O-methoxyacetophenone in 200 ml. of tetrahydrofuran. When the addition was complete, the reaction was allowed to stir at 0 C. for 2 hours. The reaction was cooled, poured into a 10% hydrochloric acid solution and extracted with ether. The combined extracts were washed with brine and water, dried over magnesium sulfate and concentrated in vacuo, 36 g. |
[2]Bioorganic and Medicinal Chemistry,2013,vol. 21,p. 5064 - 5075
[3]Chemical and pharmaceutical bulletin,1987,vol. 35,p. 3909 - 3913
[4]Journal of Medicinal Chemistry,1997,vol. 40,p. 2266 - 2275
[5]Patent: US4575507,1986,A
[6]European Journal of Organic Chemistry,2015,vol. 2015,p. 3656 - 3660
[7]Journal of Medicinal Chemistry,2018,vol. 61,p. 791 - 803
[8]Organic and Biomolecular Chemistry,2018,vol. 16,p. 4683 - 4687
- 24
-
 [ 579-74-8 ]
[ 579-74-8 ] 
- (1S)-1-(2-methoxyphenyl)ethan-1-ol [ No CAS ]

- (R)-1-(2-methoxyphenyl)ethanol [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 99% | With (mer-[(S,S)-1,5-dimethyl-2,4-bis(4-phenyl-1,3-oxazolin-2-yl)benzene(1-)]Ru(CO)Cl)2(ZnCl2); hydrogen; sodium methylate; (-)-(S)-1-Anthracen-9-ylethanol In isopropyl alcohol at 40℃; for 42h; Inert atmosphere; Autoclave; optical yield given as %ee; enantioselective reaction; | |
| 97% | With dichloro(benzene)ruthenium(II) dimer; potassium <i>tert</i>-butylate; hydrogen; (R)-N,N'-((1R,2R)-cyclohexane-1,2-diyl)bis(2-((R)-tert-butyl(methyl)phosphino)benzamide) In isopropyl alcohol at 20℃; for 8h; Schlenk technique; Overall yield = 97 percent; | 3.General procedu re for asymmetric hydrogenation General procedure: The [Ru(C6H6)Cl2]2(1.5 mg, 3 10-3 mmol, 1.0 mol%), (Rc,Rc,Rp,Rp)-L1 (3.5 mg, 6.610-3 mmol, 1.1 mol%) and isopropanol (1.5 mL) were added to a schlenk tube under an atmosphere of argon. After stirring at room temperature for 1.0 h, the orange-yellow solution was transferred to a stainless-steel reactor via syringe in a glovebox. Next, tBuOK (6.7 mg, 0.060 mmol, 10.0 mol%), and acetophenone (72.0 mg, 0.60 mmol, 1.0 eq) were added into the solution, and the resulting mixture was stirred under a hydrogen pressure (3.0 MPa) at room temperature for 8.0 h. The pure products were obtained by flash column chromatographic purification using petroleum and ethyl acetate as the eluent. The pure products were obtained by flash column chromatography using petroleum and ethyl acetate as the eluent. The enantiomeric excesses were measured by chiral HPLC using the Daicel chiral OD-H (4.6 mm × 250 mm, 5 μm) and REGIS (R,R)-WHELK-O1 (2.1 mm × 150 mm, 5 μm) columns and 2-propanol/hexane as the eluent. The absolute configurations of the corresponding products were determined by comparison of the results with literature reports. |
| 1: 95.5% 2: 4.5% | With 4 A molecular sieve; (1R,2R,4S,6S)-2-(2-methoxyphenyl)-bicyclo[2.2.2]octane-2,6-diol; benzo[1,3,2]dioxaborole In tetrahydrofuran at -20℃; for 24h; Title compound not separated from byproducts.; |
| 91% | With trans-[OsCl2(py){2,6-bis[4’-(S)-isopropyloxazolin-2-yl]pyridine}]; caesium carbonate In isopropyl alcohol at 82℃; for 10h; Inert atmosphere; Schlenk technique; | 4.7. General procedure for hydrogen transfer reactions General procedure: The catalyst [0.4 mol % (complexes 12-22) or 0.2 mol % (dinuclearcomplex 24)] and the ketone (2.5 mmol) were placed in a three-bottomSchlenk flask under dry argon atmosphere and 2-propanol (45 mL) wasadded. After stirring the mixture for 15 min at 82 °C, 5 ml of a 0.06Msolution of base (Cs2CO3) in 2-propanol (0.3 mmol) were added. Thereaction was monitored by gas chromatography using an HP-6890equipment. The corresponding alcohol and ketone were the only productsdetected in all cases. The conversion and e.e.values were determinedby GC with a Supelco β-DEX 120 chiral capillary column. |
| 50% | With D-glucose In aq. phosphate buffer at 37℃; for 24h; Microbiological reaction; Inert atmosphere; Green chemistry; enantioselective reaction; | Bioreduction of 1a-o by using Lactobacillus reuteri restingcells General procedure: L. reuteri strains were grown in MRS medium. This pre-culture was inoculated in 500 mL of MRS and incubated for 24 h (37° C). Cells were harvested by centrifugation (2800 × g, 10 min)and washed twice with phosphate buffered saline pH 7.4 (PBS,Sigma-Aldrich). Then the cells were suspended in the same bufferand adjusted for cell density. To this cell suspension, 1% glucose and the desired concentration of arylketone were added (total volume50 mL). To ensure the anaerobic conditions, flasks were degassed with a N2flux for 3 min. The reaction mixture was incubated at 37° C, 200 rpm. Sugars and organic acids were analyzed by HPLCbefore the extraction of the bioconversion products. The reactions progress was monitored by collecting suspension aliquots in a timecourse: after extraction with diethyl ether, the organic phase wasanalyzed by NMR or HPLC. After the appropriate conversion time(see Table 3), the suspension was centrifuged (2800 × g, 10 min,4C), and the aqueous phase was extracted with diethyl ether(3 × 15 mL). The organic phase was dried over Na2SO4, filtered, andevaporated under reduced pressure. The product was isolated bysilica gel column chromatography using hexane and ethyl acetate(90:10 or 80:20) as eluents to yield the desired alcohols (2a-o,Table 2). Absolute configurations of alcohols 2a-o obtained from the bioprocess were determined by comparison of their optical spe-cific rotation signs and/or retention times with known data (See Supporting informations). |
| With benzo[1,3,2]dioxaborole In hexane at -30℃; for 1h; different titanium alkoxide catalysts, other solvents and reaction time; also reduction of further ketones; | ||
| With potassium hydroxide; chloro(1,5-cyclooctadiene)rhodium(I) dimer; (R,R)-trans-1,2-bis<γ-(triethoxysilyl)propyl>cyclohexane In isopropyl alcohol Ambient temperature; other acetophenones, var. rhodium complexes; | ||
| With n-butyllithium; polymethylhydrosiloxane; chiral titanocene 1,1'-binaphth-2,2'-diolate; tetrabutyl ammonium fluoride 1.) benzene, hexane, 0.8 d, 2.) benzene, hexane, THF; Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; | ||
| With benzo[1,3,2]dioxaborole In hexane at -30℃; for 1h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | ||
| With lithium aluminium tetrahydride; (1R,2S,3S,5R)-(-)-2-anilinomethyl-6,6-dimethyl-3-ethoxybicyclo<3.1.1>heptan-2-ol In tetrahydrofuran; diethyl ether at -78℃; for 1h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | ||
| With dimethylsulfide borane complex; (S)-tetrahydro-1-butyl-3,3-diphenyl-1H,3H-pyrrolo{2,1-c}{1,3,2}oxazaborole In tetrahydrofuran for 0.166667h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | ||
| With potassium <i>tert</i>-butylate; hydrogen In isopropyl alcohol at 24 - 30℃; for 10h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | ||
| With hydrogenchloride; Rh-(R,R)-t-Bu-MiniPHOS; 1-naphthylphenylsilane 1.) THF, -15 deg C, 2.) H2O; Yield given; Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; | ||
| With lithium aluminium tetrahydride; (1R,2S,3S,5R)-2-aminomethyl-6,6-dimethyl-3-ethoxybicyclo<3.1.1>heptan-2-ol In tetrahydrofuran; diethyl ether at -78℃; for 1h; Yield given; Yields of byproduct given. Title compound not separated from byproducts; | ||
| With dimethylsulfide borane complex; (4R,5R)-2-methyl-4-phenyl-5-methoxymethyl-1,3,2-oxazaborolidine In tetrahydrofuran; toluene at 10℃; for 0.166667h; Title compound not separated from byproducts; | ||
| Stage #1: 2-Methoxyacetophenone With diphenylsilane In 1,2-dimethoxyethane at -40℃; for 6h; Stage #2: With methanol; potassium carbonate In 1,2-dimethoxyethane at 20℃; for 4h; Title compound not separated from byproducts; | ||
| With potassium hydroxide; hydrogen; (R)-3,3'-dimethyl-[1,1'-binaphthalene]-2,2'-diamine In isopropyl alcohol at 20℃; Title compound not separated from byproducts; | ||
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; (1R,2R)-1-NH2-2-(p-MeOC6H4NHCONH)-cyclohexane In isopropyl alcohol at 20℃; for 4h; Title compound not separated from byproducts; | ||
| With formic acid; triethylamine; L-prolyl-(o-fluoroanilide) In dichloromethane at 30℃; for 6h; | ||
| Stage #1: 2-Methoxyacetophenone With (((1S,2S)-2-t-BuS-c-hexyloxy)PPh2)-(2,5-norbornadiene)RhOTf; 1-naphthylphenylsilane In tetrahydrofuran at -20℃; for 2h; Stage #2: With methanol In tetrahydrofuran at 20℃; for 0.5h; Title compound not separated from byproducts; | ||
| With dimethylsulfide borane complex In tetrahydrofuran Heating; Title compound not separated from byproducts; | ||
| With phosphate buffer; Daucus carota L In acetone for 48h; Enzymatic reaction; Title compound not separated from byproducts; | ||
| With sodium hydroxide; isopropyl alcohol at 82℃; for 0.0833333h; Title compound not separated from byproducts; | ||
| With N-(p-toluenesulfonyl)-(1R,2R)-diphenylethylenediamine; sodium formate at 40℃; for 2h; Title compound not separated from byproducts; | ||
| With C49H47O2N2(1+)*BF4(1-); diphenylsilane; silver trifluoromethanesulfonate In tetrahydrofuran at 0℃; for 15h; Title compound not separated from byproducts; | ||
| With potassium <i>tert</i>-butylate; hydrogen In propan-1-ol at 25℃; for 10h; Title compound not separated from byproducts; | ||
| With potassium <i>tert</i>-butylate; hydrogen In isopropyl alcohol at 25 - 30℃; for 24h; Title compound not separated from byproducts; | ||
| With potassium <i>tert</i>-butylate; hydrogen In isopropyl alcohol at 25 - 30℃; for 24h; Title compound not separated from byproducts; | ||
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; triethylamine; (R,R)-N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine In dichloromethane at 28℃; for 48h; Title compound not separated from byproducts; | ||
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; (S,S)-N-(1-naphthylSO2)-1,2-ethylenediamine dendritic ligand; triethylamine In dichloromethane at 28℃; for 48h; Title compound not separated from byproducts; | ||
| With sodium formate; cetyltrimethylammonim bromide In water at 28℃; for 10h; Title compound not separated from byproducts; | ||
| With 1,2-O-isopropylidene-3-O-Ph2P-5-deoxy-5-tBuS-D-xylofuranose; chloro(1,5-cyclooctadiene)rhodium(I) dimer; diphenylsilane In toluene at -15℃; Title compound not separated from byproducts; | ||
| With potassium <i>tert</i>-butylate; hydrogen; (1S,2S)-1,2-bis(4-methoxyphenyl)ethane-1,2-diamine In isopropyl alcohol; toluene at 28℃; for 48h; | ||
| With sodium tetrahydroborate; 2-(3-nitrophenyl)-1,3,2-dioxaborolane-(4R,5R)-dicarboxylic acid In tetrahydrofuran for 0.5h; | ||
| With formic acid; triethylamine at 25℃; for 48h; Title compound not separated from byproducts; | ||
| With dimethylsulfide borane complex; tris[(S)-2-(diphenyl(hydroxy)methyl)pyrrolidino]P=O In tetrahydrofuran at 70℃; Title compound not separated from byproducts; | ||
| With dimethylsulfide borane complex; (S)-chiral pyrrolidine-based ionic liquid In toluene Heating; Title compound not separated from byproducts; | ||
| With sodium hydroxide; isopropyl alcohol; Boc-L-alanine(2S)-hydroxypropylamide at 20℃; Title compound not separated from byproducts; | ||
| With potassium <i>tert</i>-butylate; hydrogen In isopropyl alcohol; <i>tert</i>-butyl alcohol at 25℃; Title compound not separated from byproducts; | ||
| With (S)-(+)-2-(5-phenyl-4,5-dihydro-1,3-oxazol-2-yl)pyridine; trichlorosilane In chloroform at -20℃; for 24h; Title compound not separated from byproducts; | ||
| With sodium hydroxide; (R)-[2-(6-(tBuNHCH2)pyridin-2-yl)-5-CH3phenyl](dppb)Ru(II)Cl; isopropyl alcohol In toluene Heating; Title compound not separated from byproducts; | ||
| With air; sodium formate at 40℃; for 1h; Title compound not separated from byproducts; | ||
| With formic acid; triethylamine at 40℃; for 1.25h; Title compound not separated from byproducts; | ||
| With dimethylsulfide borane complex; chiral C3-symmetric tris(β-hydroxyamide) In tetrahydrofuran at 50℃; Title compound not separated from byproducts; | ||
| With sodium formate at 40℃; for 21h; Title compound not separated from byproducts; | ||
| With potassium <i>tert</i>-butylate; hydrogen In isopropyl alcohol; <i>tert</i>-butyl alcohol at 18 - 20℃; for 2.5h; Title compound not separated from byproducts.; | ||
| With sodium formate; (2S)-N-phenylpyrrolidine-2-carboxamide In water at 30℃; for 4h; | ||
| With -methyl-2-oxoethyl)carbamate; tert-butyl N-((1S)-2-[(1R)-2-hydroxy-1-phenylethyl]amino}-1; isopropyl alcohol at 20℃; for 10h; Title compound not separated from byproducts.; | ||
| With sodium hydroxide; hydrogen; l-1,2-bis-(4-bromophenyl)-N,N'-dimethylethane-1,2-diamine In methanol at 50℃; for 21h; | ||
| With sodium hydroxide; Boc-L-alanine(2S)-hydroxypropylamide In isopropyl alcohol at 20℃; for 3.5h; Title compound not separated from byproducts.; | ||
| With potassium isopropoxide; (1S,3R,4R)-2-azabicyclo[2.2.1]heptane-3-(R)-methylmethanol In isopropyl alcohol at 20℃; for 2h; Title compound not separated from byproducts.; | ||
| With chiral dipyridylphosphine Ru; potassium <i>tert</i>-butylate; hydrogen In isopropyl alcohol; <i>tert</i>-butyl alcohol at 20℃; for 30h; Title compound not separated from byproducts.; | ||
| With (-)-diisopinocamphenylborane chloride In diethyl ether at -25℃; Title compound not separated from byproducts.; | ||
| With tris(3,5-dimethylphenyl)phosphine; hydrogen; sodium t-butanolate In isopropyl alcohol at 30℃; for 16h; | ||
| With tetraethylammonium bromide; sodium formate In water at 39.84℃; | ||
| With sodium isopropylate In isopropyl alcohol at 20℃; for 2h; Title compound not separated from byproducts.; | ||
| With sodium formate; triethylamine In water at 40℃; for 1.5h; Title compound not separated from byproducts.; | ||
| With triethylamine In formic acid at 25℃; for 22h; Title compound not separated from byproducts.; | ||
| With sodium isopropylate; lithium chloride at 20℃; for 2h; | ||
| With potassium hydroxide; hydrogen In isopropyl alcohol at 27℃; for 4h; | ||
| With dimethylsulfide borane complex In tetrahydrofuran for 1h; Heating; Title compound not separated from byproducts.; | ||
| With sodium hydroxide; isopropyl alcohol at 82℃; for 4h; | ||
| With hydrogen; sodium methylate In isopropyl alcohol at 40℃; for 24h; Title compound not separated from byproducts.; | ||
| With diethoxymethylane; iron(II) acetate In tetrahydrofuran at 20℃; for 32h; Title compound not separated from byproducts.; | ||
| With potassium hydroxide; [RuCl2(p-cymene)]2; (2S,3aS,7aS)-(octahydro-indol-2-yl)-methanol at 20℃; for 16h; optical yield given as %ee; | ||
| With HCOONa; [Cp*RhCl2]2; (R,R)-N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine In water at 40℃; for 24h; optical yield given as %ee; enantioselective reaction; | ||
| Stage #1: 2-Methoxyacetophenone With polymethylhydrosiloxane; (S)-(1,1'-binaphthalene)-2,2'-diylbis(diphenylphosphine) In toluene at 20℃; for 18h; Inert atmosphere; Stage #2: With tetrabutyl ammonium fluoride; water In tetrahydrofuran; diethyl ether for 0.5h; optical yield given as %ee; enantioselective reaction; | ||
| With lithium hydroxide; (S)-quinolin-4-yl((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methanamine; hydrogen; triphenylphosphine; iridium In methanol at 30℃; for 3h; optical yield given as %ee; enantioselective reaction; | ||
| Stage #1: 2-Methoxyacetophenone With aluminum(III) nitrate nonahydrate; sodium hydroxide; polymethylhydrosiloxane; copper(II) nitrate trihydrate; sodium carbonate; (S)-(1,1'-binaphthalene)-2,2'-diylbis(diphenylphosphine) In toluene at 20℃; Inert atmosphere; Stage #2: With tetrabutyl ammonium fluoride In tetrahydrofuran; diethyl ether for 0.5h; | ||
| Stage #1: 2-Methoxyacetophenone With polymethylhydrosiloxane; copper(II) ferrite; (S)-(1,1'-binaphthalene)-2,2'-diylbis(diphenylphosphine) In toluene at 20℃; for 30h; Stage #2: With tetrabutyl ammonium fluoride; water In tetrahydrofuran; diethyl ether; toluene for 0.5h; optical yield given as %ee; enantioselective reaction; | ||
| With iridium(III) chloride; (S,S)-naphthalene-2-sulfonic acid (1,2-diphenyl-2-propylaminoethyl)-amide; hydrogen; sodium hydroxide In methanol at 40℃; for 24h; optical yield given as %ee; enantioselective reaction; | ||
| With [RhCl2(p-cymene)]2; potassium hydroxide In isopropyl alcohol at 20℃; Inert atmosphere; enantioselective reaction; | ||
| With [RhCl2(p-cymene)]2; [(1R,3S)-6,7-dimethoxy-1-phenyl-1,2,3,4-tetrahydroisoquinolin-3-yl]methanol; potassium <i>tert</i>-butylate; isopropyl alcohol at 20℃; for 1h; Inert atmosphere; optical yield given as %ee; | ||
| With sodium tetrahydroborate; 2Br(1-)*C18H32N2(2+) In methanol at 20℃; for 0.416667h; optical yield given as %ee; | ||
| With [Ru{chloro(p-cymene)(N,N'-bis[(1S)-1-sec-butyl-2-O-(diphenylphosphinyl)ethyl]ethanediamide)}] chloride; isopropyl alcohol; potassium hydroxide at 82℃; for 1h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| Stage #1: 2-Methoxyacetophenone With (BOPA-dpm)H; iron(II) acetate In tetrahydrofuran at 65℃; Inert atmosphere; Stage #2: With diethoxymethylane In tetrahydrofuran at 65℃; optical yield given as %ee; | ||
| With C40H58Cl2FeN4O6P2; potassium <i>tert</i>-butylate; isopropyl alcohol at 22 - 24℃; for 3h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| With potassium formate In water at 50℃; for 24h; Inert atmosphere; | A.6 The reaction was carried out with the same conditions as in Comparative example A-1, except that 2.10 mg (3.33 μmol) of RuCl[(S,S)-(C2H5)2CHCH2SO2dpen](p-cymene) was used as the catalyst. GC analysis of the reaction product confirmed that 1-(2'-methoxyphenyl)ethanol with 88.1% ee optical purity was produced at 99% yield. Comparison with Comparative examples A-1 and A-2 indicated the superiority of this complex. | |
| With Candida tropicalis CE017 at 28℃; for 216h; Microbiological reaction; Potato-dextrose medium; optical yield given as %ee; enantioselective reaction; | ||
| With C68H78Cl2N2O6P2Ru; hydrogen; potassium hydroxide In isopropyl alcohol at 20℃; for 12h; Autoclave; optical yield given as %ee; enantioselective reaction; | ||
| With iridium(III) chloride hydrate; C24H28N2O2S; hydrogen; sodium hydroxide In methanol at 40℃; for 24h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| Stage #1: 2-Methoxyacetophenone With (S,S)-(BOPA-dpm)FeCl2; zinc In tetrahydrofuran at 65℃; for 1h; Inert atmosphere; Stage #2: With Triethoxysilane In tetrahydrofuran at 65℃; for 48h; Inert atmosphere; Stage #3: With potassium fluoride; tetrabutyl ammonium fluoride In tetrahydrofuran; methanol Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| With [RhCl2(p-cymene)]2; 5-[(2S,4R)-4-(tert-butyldiphenylsilyloxy)pyrrolidin-2-yl]-1H-1,2,3-triazole; potassium hydroxide In isopropyl alcohol at 20℃; for 4h; Inert atmosphere; optical yield given as %ee; | ||
| With μ-(2R)-2-[benzyl{(2-((dicyclohexylphosphanyl)oxy)ethyl)}amino]butyldicyclohexylphosphinito-bis[dichloro(η6-p-isopropyltoluene)ruthenium(II)]; sodium hydroxide In isopropyl alcohol at 82℃; for 9h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 3.2. General procedure for the transfer hydrogenation of ketones General procedure: a solution of the ruthenium complexesμ-(2R)-2-[benzyl{(2-(diphenylphosphanyl)oxy)ethyl)}amino]butyldiphenylphosphinito-bis[dichloro(η6-p-isopropyltoluene)ruthenium(II)], 3 and μ-(2R)-2-[benzyl{(2-((dicyclohexylphosphanyl)oxy)ethyl)}amino]butyldicyclohexylphosphinito-bis[dichloro(η6-p-isopropyltoluene)ruthenium(II)], 4 (0.005 mmol), KOH (0.025 mmol) and the corresponding ketone (0.5 mmol) in degassed iso-PrOH (5 mL) was refluxed up to the reaction completed. After this time a sample of the reaction mixture is taken off, diluted with acetone and analyzed immediately by GC, conversions obtained are related to the residual unreacted ketone. | |
| With formic acid; C24H28ClN2O2PolRuS; triethylamine In dichloromethane at 40℃; for 24h; Inert atmosphere; enantioselective reaction; | ||
| With formic acid; [RhCl2(p-cymene)]2; C18H24N2O2S; triethylamine at 40℃; for 96h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| Stage #1: 2-Methoxyacetophenone With C54H50Cl2N2O2P2Ru; potassium <i>tert</i>-butylate In butan-1-ol for 0.333333h; Stage #2: With hydrogen In butan-1-ol at 20℃; for 48h; Autoclave; optical yield given as %ee; enantioselective reaction; | ||
| With dmap; (R)-3,3'-bis(9-anthracenyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate; benzo[1,3,2]dioxaborole In toluene at -20℃; for 24h; Molecular sieve; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; (S)-N-[(1R,2R)-2-(4-methylphenylsulfonamido)-1,2-diphenylethyl]pyrrolidine-2-carboxamide; sodium formate In water at 60℃; for 24h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; lithium formate; L-prolinamide In water at 35℃; for 18h; optical yield given as %ee; enantioselective reaction; | ||
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; 2-((3R,3aS,6R,6aR)-3-(benzyloxy)hexahydrofuro[3,2-b]furan-6-ylamino)ethanol; potassium <i>tert</i>-butylate; isopropyl alcohol at 25℃; for 2h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; C25H36N2O7S; sodium isopropylate; lithium chloride In tetrahydrofuran; isopropyl alcohol at 20℃; for 24h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| With bis(triphenylphosphine)carbonyliridium(I) chloride; (R)-N,N'-bis[2-(piperidin-1-yl)benzylidene]propane-1,2-diamine; potassium hydroxide In isopropyl alcohol at 75℃; for 5h; optical yield given as %ee; enantioselective reaction; | ||
| With (S)-(+)-2-(5-phenyl-4,5-dihydro-1,3-oxazol-2-yl)pyridine; trichlorosilane In chloroform at -20℃; for 16h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| With dichloro(benzene)ruthenium(II) dimer; (1<i>Ξ</i>,4<i>R</i>)-1-benzylamino-<i>p</i>-menth-8-en-2-one oxime; potassium hydroxide In isopropyl alcohol at 80℃; for 5h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | ||
| With (S,S)-DPENDS; C36H24Cl2O18P2RuS6(6-)*6Na(1+); hydrogen; potassium hydroxide In water at 30℃; for 3h; Autoclave; optical yield given as %ee; enantioselective reaction; | 4.2. Typical procedure for asymmetric hydrogenation of aromatic ketones General procedure: To a 60 mL stainless autoclave with a glass liner and magnetic stirrer were added PEG-400, H2O, RuCl2(TPPTS)2, (S,S)-DPENDS, KOH, and reactant. Hydrogen was introduced to the desired pressure after the reaction mixture had been purged with H2 five times. The products were extracted by n-hexane and analyzed by GC-960 with a FID detector and β-DEX120 capillary column (30 m × 0.25 mm, 0.25 μm film) at 115 °C. The enantiomeric excess (ee value) was calculated from the equation: ee (%) = 100 × (R - S)/(R + S). | |
| 85 % ee | With dodecacarbonyl-triangulo-triruthenium; (R,R)-N-(1-benzyl-1,2,3-triazole-4-ylmethyl)-N’-4-toluenesulphonyl-1,2-diphenylethylenediamine; isopropyl alcohol at 80℃; for 16h; Inert atmosphere; | |
| 80 % ee | With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; (2S)-2-[benzyl(2-{benzyl[(2S)-1-[bis(propan-2-yl)phosphanyl]oxy}-3-phenylpropan-2-yl]amino}ethyl)amino]-3-phenylpropyl bis(propan-2-yl)phosphinite; potassium hydroxide In isopropyl alcohol at 82℃; for 7h; Inert atmosphere; Schlenk technique; enantioselective reaction; | |
| 80 % ee | With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; (2R)-2-[benzyl(2-{benzyl[(2R)-1-[bis(propan-2-yl)phosphanyl]oxy}-3-phenylpropan-2-yl]amino}ethyl)amino]-3-phenylpropyl bis(propan-2-yl)phosphinite; potassium hydroxide In isopropyl alcohol at 82℃; for 8h; Inert atmosphere; Schlenk technique; enantioselective reaction; | |
| 74 % ee | With trans-[OsCl2(P(OMe)3){2,6-bis[4′-(S)-isopropyloxazolin-2′-yl]pyridine}]; potassium <i>tert</i>-butylate; isopropyl alcohol at 82℃; for 1h; Inert atmosphere; Schlenk technique; enantioselective reaction; |

[2]Du, Peng; Liu, Yan-Lan; Lu, Xiao-Bing [Tetrahedron Letters, 2020, vol. 61, # 42]
[3]Sarvary, Ian; Almqvist, Fredrik; Frejd, Torbjoern [Chemistry - A European Journal, 2001, vol. 7, # 10, p. 2158 - 2166]
[4]de Julián, Eire; Fernández, Nuria; Díez, Josefina; Lastra, Elena; Gamasa, M. Pilar [Molecular catalysis, 2018, vol. 456, p. 75 - 86]
[5]Perna; Ricci; Scilimati; Mena; Pisano; Palmieri; Agrimi; Vitale [Journal of Molecular Catalysis B: Enzymatic, 2016, vol. 124, p. 29 - 37]
[6]Giffels, Guido; Dreisbach, Claus; Kragl, Udo; Weigerding, Michael; Waldmann, Herbert; Wandrey, Christian [Angewandte Chemie, 1995, vol. 107, # 18, p. 2165 - 2166]
[7]Adima, Augustin; Moreau, Joel J. E.; Wong Chi Man, Michel [Journal of Materials Chemistry, 1997, vol. 7, # 12, p. 2331 - 2333]
[8]Carter, Mary Beth; Schiøtt, Birgit; Gutiérrez, Alberto; Buchwald, Stephen L. [Journal of the American Chemical Society, 1994, vol. 116, # 26, p. 11667 - 11670]
[9]Giffels, Guido; Dreisbach, Claus; Kragl, Udo; Weigerding, Michael; Waldmann, Herbert; Wandrey, Christian [Angewandte Chemie, 1995, vol. 107, # 18, p. 2165 - 2166]
[10]Cherng, Yie-Jia; Fang, Jim-Min; Lu, Ta-Jung [Tetrahedron Asymmetry, 1995, vol. 6, # 1, p. 89 - 92]
[11]Meier, Chris; Laux, Wolfgang H. G. [Tetrahedron, 1996, vol. 52, # 2, p. 589 - 598]
[12]Doucet, Henri; Ohkuma, Takeshi; Murata, Kunihiko; Yokozawa, Tohru; Kozawa, Masami; Katayama, Eiji; England, Anthony F.; Ikariya, Takao; Noyori, Ryoji [Angewandte Chemie - International Edition, 1998, vol. 37, # 12, p. 1703 - 1707]
[13]Yamanoi, Yoshinori; Imamoto, Tsuneo [Journal of Organic Chemistry, 1999, vol. 64, # 9, p. 2988 - 2989]
[14]Cherng, Yie-Jia; Fang, Jim-Min; Lu, Ta-Jung [Journal of Organic Chemistry, 1999, vol. 64, # 9, p. 3207 - 3212]
[15]Puigjaner, Cristina; Vidal-Ferran, Anton; Moyano, Albert; Pericas, Miquel A.; Riera, Antoni [Journal of Organic Chemistry, 1999, vol. 64, # 21, p. 7902 - 7911]
[16]Kuwano, Ryoichi; Sawamura, Masaya; Shirai, Junya; Takahashi, Masatoshi; Ito, Yoshihiko [Bulletin of the Chemical Society of Japan, 2000, vol. 73, # 2, p. 485 - 496]
[17]Mikami, Koichi; Korenaga, Toshinobu; Ohkuma, Takeshi; Noyori, Ryoji [Angewandte Chemie - International Edition, 2000, vol. 39, # 20, p. 3707 - 3710]
[18]Bied, Catherine; Moreau, Joel J.E.; Wong Chi Man, Michel [Tetrahedron Asymmetry, 2001, vol. 12, # 2, p. 329 - 336]
[19]Rhyoo, Hae Yoon; Yoon, Young-Ae; Park, Hee-Jung; Chung, Young Keun [Tetrahedron Letters, 2001, vol. 42, # 30, p. 5045 - 5048]
[20]Evans, David A.; Michael, Forrest E.; Tedrow, Jason S.; Campos, Kevin R. [Journal of the American Chemical Society, 2003, vol. 125, # 12, p. 3534 - 3543]
[21]Zhang, Yong-Xin; Du, Da-Ming; Chen, Xiao; Lue, Shao-Feng; Hua, Wen-Ting [Tetrahedron Asymmetry, 2004, vol. 15, # 1, p. 177 - 182]
[22]Maczka, Wanda K.; Mironowicz, Agnieszka [Tetrahedron Asymmetry, 2004, vol. 15, # 13, p. 1965 - 1967]
[23]Cuervo, Dario; Gamasa, M. Pilar; Gimeno, Jose [Chemistry - A European Journal, 2004, vol. 10, # 2, p. 425 - 432]
[24]Wu, Xiaofeng; Li, Xiaoguang; Hems, William; King, Frank; Xiao, Jianliang [Organic and Biomolecular Chemistry, 2004, vol. 2, # 13, p. 1818 - 1821]
[25]Song, Chun; Ma, Changqin; Ma, Yudao; Feng, Wenhua; Ma, Shutao; Chai, Qiang; Andrus, Merritt B. [Tetrahedron Letters, 2005, vol. 46, # 18, p. 3241 - 3244]
[26]Jing, Qing; Zhang, Xue; Sun, Jie; Ding, Kuiling [Advanced Synthesis and Catalysis, 2005, vol. 347, # 9, p. 1193 - 1197]
[27]Grasa, Gabriela A.; Zanotti-Gerosa, Antonio; Medlock, Jonathan A.; Hems, William P. [Organic Letters, 2005, vol. 7, # 8, p. 1449 - 1451]
[28]Grasa, Gabriela A.; Zanotti-Gerosa, Antonio; Medlock, Jonathan A.; Hems, William P. [Organic Letters, 2005, vol. 7, # 8, p. 1449 - 1451]
[29]Liu, Weiguo; Cui, Xin; Cun, Linfeng; Zhu, Jin; Deng, Jingen [Tetrahedron Asymmetry, 2005, vol. 16, # 15, p. 2525 - 2530]
[30]Liu, Weiguo; Cui, Xin; Cun, Linfeng; Zhu, Jin; Deng, Jingen [Tetrahedron Asymmetry, 2005, vol. 16, # 15, p. 2525 - 2530]
[31]Wang, Fei; Liu, Hui; Cun, Linfeng; Zhu, Jin; Deng, Jingen; Jiang, Yaozhong [Journal of Organic Chemistry, 2005, vol. 70, # 23, p. 9424 - 9429]
[32]Dieguez, Montserrat; Pamies, Oscar; Claver, Carmen [Tetrahedron Asymmetry, 2005, vol. 16, # 23, p. 3877 - 3880]
[33]Liu, Weiguo; Cui, Xin; Cun, Linfeng; Wu, Jun; Zhu, Jin; Deng, Jingen; Fan, Qinghua [Synlett, 2005, # 10, p. 1591 - 1595]
[34]Cordes, David B.; Nguyen, Thao M.; Kwong, Tracey J.; Suri, Jeff T.; Luibrand, Richard T.; Singaram, Bakthan [European Journal of Organic Chemistry, 2005, # 24, p. 5289 - 5295]
[35]Matharu, Daljit S.; Morris, David J.; Kawamoto, Aparecida M.; Clarkson, Guy J.; Wills, Martin [Organic Letters, 2005, vol. 7, # 24, p. 5489 - 5491]
[36]Du, Da-Ming; Fang, Tao; Xu, Jiaxi; Zhang, Shi-Wei [Organic Letters, 2006, vol. 8, # 7, p. 1327 - 1330]
[37]Yang, Shang-Dong; Shi, Yun; Sun, Zhen-Hua; Zhao, Ya-Bin; Liang, Yong-Min [Tetrahedron Asymmetry, 2006, vol. 17, # 12, p. 1895 - 1900]
[38]Wettergren, Jenny; Bogevig, Anders; Portier, Maud; Adolfsson, Hans [Advanced Synthesis and Catalysis, 2006, vol. 348, # 10-11, p. 1277 - 1282]
[39]Grasa, Gabriela A.; Zanotti-Gerosa, Antonio; Hems, William P. [Journal of Organometallic Chemistry, 2006, vol. 691, # 10, p. 2332 - 2334]
[40]Malkov, Andrei V.; Stewart Liddon, Angus J. P.; Ramirez-Lopez, Pedro; Bendova, Lada; Haigh, David; Kocovsky, Pavel [Angewandte Chemie - International Edition, 2006, vol. 45, # 9, p. 1432 - 1435]
[41]Baratta, Walter; Chelucci, Giorgio; Gladiali, Serafino; Siega, Katia; Toniutti, Micaela; Zanette, Matteo; Zangrando, Ennio; Rigo, Pierluigi [Angewandte Chemie - International Edition, 2005, vol. 44, # 38, p. 6214 - 6219]
[42]Wu, Xiaofeng; Vinci, Daniele; Ikariya, Takao; Xiao, Jiangliang [Chemical Communications, 2005, # 35, p. 4447 - 4449]
[43]Morris, David J.; Hayes, Aidan M.; Wills, Martin [Journal of Organic Chemistry, 2006, vol. 71, # 18, p. 7035 - 7044]
[44]Fang, Tao; Xu, Jiaxi; Du, Da-Ming [Synlett, 2006, # 10, p. 1559 - 1563]
[45]Li, Xiaohong; Blacker, John; Houson, Ian; Wu, Xiaofeng; Xiao, Jianliang [Synlett, 2006, # 8, p. 1155 - 1160]
[46]Burk, Mark J.; Hems, William; Herzberg, Daniela; Malan, Christophe; Zanotti-Gerosa, Antonio [Organic Letters, 2000, vol. 2, # 26, p. 4173 - 4175]
[47]Rhyoo; Park; Chung [Chemical Communications, 2001, # 20, p. 2064 - 2065]
[48]Pastor, Isidro M.; Vaestilae, Patrik; Adolfsson, Hans [Chemistry - A European Journal, 2003, vol. 9, # 17, p. 4031 - 4045]
[49]Maillet, Celine; Praveen, Thoniyot; Janvier, Pascal; Minguet, Sebastien; Evain, Michel; Saluzzo, Christine; Tommasino, M. Lorraine; Bujoli, Bruno [Journal of Organic Chemistry, 2002, vol. 67, # 23, p. 8191 - 8196]
[50]Bogevig, Anders; Pastor, Isidro M.; Adolfsson, Hans [Chemistry - A European Journal, 2004, vol. 10, # 1, p. 294 - 302]
[51]Alonso, Diego A.; Nordin, Sofia J. M.; Roth, Peter; Tarnai, Tibor; Andersson, Pher G.; Thommen, Marc; Pittelkow, Ulrich [Journal of Organic Chemistry, 2000, vol. 65, # 10, p. 3116 - 3122]
[52]Wu, Jing; Chen, Hua; Kwok, Waihim; Guo, Rongwei; Zhou, Zhongyuan; Yeung, Chihung; Chan, Albert S. C. [Journal of Organic Chemistry, 2002, vol. 67, # 22, p. 7908 - 7910]
[53]Ramachandran, P. Veeraraghavan; Gong, Baoqing; Brown, Herbert C.; Francisco, Joseph S. [Tetrahedron Letters, 2004, vol. 45, # 12, p. 2603 - 2605]
[54]Shimizu, Hideo; Igarashi, Daisuke; Kuriyama, Wataru; Yusa, Yukinori; Sayo, Noboru; Saito, Takao [Organic Letters, 2007, vol. 9, # 9, p. 1655 - 1657]
[55]Yang, Hengquan; Li, Jun; Yang, Jie; Liu, Zhimin; Yang, Qihua; Li, Can [Chemical Communications, 2007, # 10, p. 1086 - 1088]
[56]Ahlford, Katrin; Zaitsev, Alexey B.; Ekström, Jesper; Adolfsson, Hans [Synlett, 2007, # 16, p. 2541 - 2544]
[57]Jiang, Lin; Wu, Tong-Fei; Chen, Ying-Chun; Zhu, Jin; Deng, Jin-Gen [Organic and Biomolecular Chemistry, 2006, vol. 4, # 17, p. 3319 - 3324]
[58]Matharu, Daljit S.; Morris, David J.; Clarkson, Guy J.; Wills, Martin [Chemical Communications, 2006, # 30, p. 3232 - 3234]
[59]Vaestilae, Patrik; Zaitsev, Alexey B.; Wettergren, Jenny; Privalov, Timofei; Adolfsson, Hans [Chemistry - A European Journal, 2006, vol. 12, # 12, p. 3218 - 3225]
[60]Korenaga, Toshinobu; Nomura, Kenji; Minami, Shinichi; Sasaki, Hitomi; Sakai, Takashi [Tetrahedron Asymmetry, 2008, vol. 19, # 6, p. 695 - 700]
[61]Niu, Yan-Ning; Yan, Ze-Yi; Li, Gao-Qiang; Wei, Hai-Long; Gao, Guo-Lin; Wu, Lu-Yong; Liang, Yong-Min [Tetrahedron Asymmetry, 2008, vol. 19, # 8, p. 912 - 920]
[62]Madrigal, Cesar A.; García-Fernández, Almudena; Gimeno, José; Lastra, Elena [Journal of Organometallic Chemistry, 2008, vol. 693, # 15, p. 2535 - 2540]
[63]Ito, Jun-Ichi; Ujiie, Satoshi; Nishiyama, Hisao [Chemical Communications, 2008, # 16, p. 1923 - 1925]
[64]Shaikh, Nadim S.; Enthaler, Stephan; Junge, Kathrin; Beller, Matthias [Angewandte Chemie - International Edition, 2008, vol. 47, # 13, p. 2497 - 2501]
[65]Location in patent: scheme or table Liegault, Benoit; Tang, Xiaoping; Bruneau, Christian; Renaud, Jean-Luc [European Journal of Organic Chemistry, 2008, # 5, p. 934 - 940]
[66]Location in patent: scheme or table Wu, Xiaofeng; Li, Xiaohong; Zanotti-Gerosa, Antonio; Pettman, Allan; Liu, Jianke; Mills, Allan James; Xiao, Jianliang [Chemistry - A European Journal, 2008, vol. 14, # 7, p. 2209 - 2222]
[67]Kantam, M. Lakshmi; Laha, Soumi; Yadav, Jagjit; Likhar, Pravin R.; Sreedhar, Bojja; Jha, Shailendra; Bhargava, Suresh; Udayakiran; Jagadeesh [Organic Letters, 2008, vol. 10, # 14, p. 2979 - 2982]
[68]Jiang, He-Yan; Yang, Chao-Fen; Li, Chun; Fu, Hai-Yan; Chen, Hua; Li, Rui-Xiang; Li, Xian-Jun [Angewandte Chemie - International Edition, 2008, vol. 47, # 48, p. 9240 - 9244]
[69]Kantam, M. Lakshmi; Laha, Soumi; Yadav, Jagjit; Likhar, Pravin R.; Sreedhar; Jha, Shailendra; Bhargava, Suresh; Udayakiran; Jagadeesh [Organic Letters, 2008, vol. 10, # 19, p. 4391 - 4391]
[70]Kantam, M. Lakshmi; Yadav, Jagjit; Laha, Soumi; Srinivas, Pottabathula; Sreedhar, Bojja; Figueras [Journal of Organic Chemistry, 2009, vol. 74, # 12, p. 4608 - 4611]
[71]Location in patent: scheme or table Martins, José E.D.; Wills, Martin [Tetrahedron, 2009, vol. 65, # 29-30, p. 5782 - 5786]
[72]Location in patent: experimental part Michalek, Florian; Lagunas, Anna; Jimeno, Ciril; Pericas, Miquel A. [Journal of Materials Chemistry, 2008, vol. 18, # 39, p. 4692 - 4697]
[73]Location in patent: experimental part Chakka, Sai Kumar; Andersson, Pher G.; Maguire, Glenn E. M.; Kruger, Hendrik G.; Govender, Thavendran [European Journal of Organic Chemistry, 2010, # 5, p. 972 - 980]
[74]Location in patent: experimental part Hajipour, Abdol Reza; Karimi, Fatemeh [Synthetic Communications, 2010, vol. 40, # 12, p. 1784 - 1793]
[75]Location in patent: experimental part Aydemir, Murat; Meric, Nermin; Baysal, Akin; Guemguem, Bahattin; Togrul, Mahmut; Turgut, Yilmaz [Tetrahedron Asymmetry, 2010, vol. 21, # 6, p. 703 - 710]
[76]Inagaki, Tomohiko; Phong, Le Thanh; Furuta, Akihiro; Ito, Jun-Ichi; Nishiyama, Hisao [Chemistry - A European Journal, 2010, vol. 16, # 10, p. 3090 - 3096]
[77]Naik, Anu; Maji, Tapan; Reiser, Oliver [Chemical Communications, 2010, vol. 46, # 25, p. 4475 - 4477]
[78]Current Patent Assignee: KANTO KAGAKU HOLDINGS K.K. - US2010/261924, 2010, A1 Location in patent: Page/Page column 88-89
[79]Location in patent: experimental part Vieira, Gizelle A. B.; De Freitas Araujo, Daniel M.; Lemos, Telma L. G.; De Mattos, Marcos Carlos; Da Conceição F. De Oliveira, Maria; Melo, Vânia M. M.; De Gonzalo, Gonzalo; Gotor-Fernández, Vicente; Gotor, Vicente [Journal of the Brazilian Chemical Society, 2010, vol. 21, # 8, p. 1509 - 1516]
[80]Location in patent: scheme or table Ma, Meng Lin; Peng, Zong Hai; Guo, Yu; Chen, Li; Chen, Hua; Li, Xian Jun [Chinese Chemical Letters, 2010, vol. 21, # 5, p. 576 - 579]
[81]Martins, José E.D.; Morris, David J.; Wills, Martin [Tetrahedron Letters, 2009, vol. 50, # 6, p. 688 - 692]
[82]Inagaki, Tomohiko; Ito, Akihiro; Ito, Jun-Ichi; Nishiyama, Hisao [Angewandte Chemie - International Edition, 2010, vol. 49, # 49, p. 9384 - 9387]
[83]Location in patent: experimental part Cambeiro, Xacobe C.; Pericas, Miquel A. [Advanced Synthesis and Catalysis, 2011, vol. 353, # 1, p. 113 - 124]
[84]Aydemir, Murat; Meric, Nermin; Baysal, Akin; Turgut, Yilmaz; Kayan, Cezmi; Şeker, Sevil; Toǧrul, Mahmut; Gümgüm, Bahattin [Journal of Organometallic Chemistry, 2011, vol. 696, # 8, p. 1541 - 1546]
[85]Location in patent: experimental part Marcos, Rocio; Jimeno, Ciril; Pericas, Miquel A. [Advanced Synthesis and Catalysis, 2011, vol. 353, # 8, p. 1345 - 1352]
[86]Location in patent: scheme or table Zhang, Bo; Wang, Hui; Lin, Guo-Qiang; Xu, Ming-Hua [European Journal of Organic Chemistry, 2011, # 22, p. 4205 - 4211]
[87]Location in patent: experimental part Cui, Yu Ming; Wang, Lai Lai; Kwong, Fuk Yee; Sun, Wei [Chinese Chemical Letters, 2010, vol. 21, # 12, p. 1403 - 1406]
[88]Zhang, Zuhui; Jain, Pankaj; Antilla, Jon C. [Angewandte Chemie - International Edition, 2011, vol. 50, # 46, p. 10961 - 10964]
[89]Location in patent: experimental part Manville, Charles V.; Docherty, Gordon; Padda, Ranbir; Wills, Martin [European Journal of Organic Chemistry, 2011, # 34, p. 6893 - 6901]
[90]Location in patent: experimental part Ahlford, Katrin; Adolfsson, Hans [Catalysis Communications, 2011, vol. 12, # 12, p. 1118 - 1121]
[91]Location in patent: experimental part Huynh, Khanh-Duy; Ibrahim, Houssein; Kolodziej, Emile; Toffano, Martial; Vo-Thanh, Giang [New Journal of Chemistry, 2011, vol. 35, # 11, p. 2622 - 2631]
[92]Location in patent: experimental part Coll, Mercedes; Ahlford, Katrin; Pamies, Oscar; Adolfsson, Hans; Dieguez, Montserrat [Advanced Synthesis and Catalysis, 2012, vol. 354, # 2-3, p. 415 - 427]
[93]Location in patent: scheme or table Yu, Shen Luan; Li, Yan Yun; Dong, Zhen Rong; Gao, Jing Xing [Chinese Chemical Letters, 2012, vol. 23, # 4, p. 395 - 398]
[94]Location in patent: experimental part Malkov, Andrei V.; Stewart-Liddon, Angus J. P.; McGeoch, Grant D.; Ramirez-Lopez, Pedro; Kocovsky, Pavel [Organic and Biomolecular Chemistry, 2012, vol. 10, # 25, p. 4864 - 4877]
[95]Location in patent: experimental part Ibn El Alami, Mohammed Samir; El Amrani, Mohamed Amin; Dahdouh, Abdelaziz; Roussel, Pascal; Suisse, Isabelle; Mortreux, Andre [Chirality, 2012, vol. 24, # 8, p. 675 - 682]
[96]Location in patent: experimental part Qin, Ruixiang; Wang, Jinbo; Xiong, Wei; Chen, Hua; Feng, Jian; Liu, Derong [Tetrahedron Asymmetry, 2012, vol. 23, # 11-12, p. 834 - 837]
[97]Johnson, Tarn C.; Totty, William G.; Wills, Martin [Organic Letters, 2012, vol. 14, # 20, p. 5230 - 5233,4] Johnson, Tarn C.; Totty, William G.; Wills, Martin [Organic Letters, 2012, vol. 14, # 20, p. 5230 - 5233]
[98]Aydemir, Murat; Durap, Feyyaz; Kayan, Cezmi; Baysal, Akin; Turgut, Ylmaz [Synlett, 2012, vol. 23, # 19, p. 2777 - 2784]
[99]Aydemir, Murat; Durap, Feyyaz; Kayan, Cezmi; Baysal, Akin; Turgut, Ylmaz [Synlett, 2012, vol. 23, # 19, p. 2777 - 2784]
[100]Vega, Esmeralda; Lastra; Gamasa, M. Pilar [Inorganic Chemistry, 2013, vol. 52, # 10, p. 6193 - 6198]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 95% | Stage #1: ethyl trifluoroacetate, With sodium hydride In 1,2-dimethoxyethane; mineral oil Stage #2: 2-Methoxyacetophenone In 1,2-dimethoxyethane; mineral oil at 160℃; for 0.25h; Microwave irradiation; Stage #3: With hydrogenchloride In 1,2-dimethoxyethane; water; mineral oil | 5.1 Step 1. Synthesis of 4,4,4-trifluoro-1-(2-methoxyphenyl)butane-1,3-dione (C9) To a suspension of sodium hydride (60% in mineral oil, 202 mg, 8.0 mmol) in 1,2-dimethoxyethane (4 mL) was added drop-wise ethyl trifluoroacetate (954 μL, 8.0 mmol) followed by 1-(2-methoxyphenyl)ethanone (552 μL, 4.0 mmol) and the reaction was heated to 160° C. in a microwave for 15 minutes. Aqueous hydrochloric acid (1 N, 20 mL) was added and the mixture was extracted with tert-butyl methyl ether (3*10 mL). The combined organic layers were dried over sodium sulfate and concentrated in vacuo. Purification via silica gel chromatography (Gradient: 0% to 10% tert-butyl methyl ether in heptanes) afforded the title compound as a red solid. Yield: 933 mg, 3.79 mmol, 95%. GCMS m/z 246 (M+). 1H NMR (400 MHz, CDCl3) δ 3.96 (s, 3H), 6.99 (s, 1H), 7.02 (br d, J=8.5 Hz, 1H), 7.08 (ddd, J=8, 7, 1.0 Hz, 1H), 7.55 (ddd, J=8.4, 7.4, 1.8 Hz, 1H), 7.99 (dd, J=7.9, 1.9 Hz, 1H). |
| 85% | Stage #1: 2-Methoxyacetophenone With sodium hydride In tetrahydrofuran at -5 - 0℃; for 0.5h; Inert atmosphere; Stage #2: ethyl trifluoroacetate, In tetrahydrofuran at 20℃; for 6h; | 12.1 (1) in 100 ml round bottom flask is added 2 - methoxy hypnone (compound 3k) 14.9mmol, dry THF 30 ml, lowering the temperature to - 5 - 0 °C, under the protection of nitrogen addition of NaH 0.715g (29.8mmol), in this particularity stirring 30min, adding trifluoro ethyl acetate 3.175g (22.4mmol), stirring at the room temperature reaction 6h, evaporating the solvent under reduced pressure, adding ice water dilution solution, for 1 mol/L HCl solution of adjusting PH value to 6, extracted with ethyl acetate three times (10 ml × 3), recovery, combined with the organic layer, the organic layer using 5 ml water washing, and the anhydrous magnesium sulfate drying, desolvation, the concentration of the product after the drying, to obtain compound 4k (4, 4, 4 - trifluoro -1 - (2 - methoxyphenyl) - 1, 3 - Butanedione), yield 85%; |
| With sodium methylate In methanol for 24h; Heating; |
| Stage #1: ethyl trifluoroacetate, With sodium methylate In diethyl ether for 0.0833333h; Stage #2: 2-Methoxyacetophenone In diethyl ether at 20℃; | ||
| Stage #1: 2-Methoxyacetophenone With sodium hydride In N,N-dimethyl-formamide at -5 - 0℃; for 0.5h; Stage #2: ethyl trifluoroacetate, In N,N-dimethyl-formamide at 20℃; for 5h; | ||
| Stage #1: ethyl trifluoroacetate, With sodium methylate In diethyl ether for 0.0833333h; Stage #2: 2-Methoxyacetophenone In diethyl ether at 20℃; Further stages.; | ||
| Stage #1: 2-Methoxyacetophenone With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #2: ethyl trifluoroacetate, In N,N-dimethyl-formamide at 0℃; for 4.5h; | Compound 77: 5-(2-methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazole 2-Methoxyacetophenone (1 mL, 7.27 mmol) was stirred in DMF (10 mL) at 0° C. Sodium hydride (240 mg, 9.45 mmol) was added. After 30 minutes, ethyl trifluoroacetate (1.13 mL, 9.45 mmol) was added and the cold bath was removed. After 4.5 hours, the mixture was poured into ice-cold 1M NaHSO4. The organics were extracted with ether and the ether layer was subsequently washed once with brine, dried and concentrated. This crude material containing Compound 76 was used without further purification in the next reaction. The crude material containing Compound 76 (1.69 g, 6.87 mmol) was stirred in ethanol (10 mL). Anhydrous hydrazine (5 mL) was added. The mixture was heated at 60° C. overnight. After cooling, the reaction mixture was partitioned between ethyl acetate and brine. The organics were dried and concentrated. Chromatography (SiO2, 10 to 30% ethyl acetate:hexanes) gave Compound 77. 1H NMR (400 MHz, CDCl3) δ: 7.66 (dd, J=1.6, 7.7 Hz, 1H), 7.37 (m, 1H), 7.07 (m, 2H), 6.87 (s, 1H), 4.03 (s, 3H). [M+H] calc'd for C11H10F3N2O, 243; found 243. | |
| Stage #1: 2-Methoxyacetophenone With sodium hydride In tetrahydrofuran at -5 - 0℃; for 0.5h; Inert atmosphere; Stage #2: ethyl trifluoroacetate, In tetrahydrofuran at -5 - 20℃; for 6h; Inert atmosphere; Stage #3: With hydrogenchloride In water Cooling with ice; | 4.2. General procedure for preparation of 4,4,4-trifluoro-1-(substituted phenyl)butane-1,3-dione (2) General procedure: Substituted acetophenone 1 (149 mmol) was dissolved in dry THF (300 mL) under nitrogen atmosphere and NaH (7.15 g, 298 mmol) was added in portions maintaining the temperature between -5 and 0 °C. After stirring at this temperature for 30 min, ethyl trifluoroacetate (31.75 g, 224 mmol) was added and the reaction mixture was allowed to stir at room temperature for 6 h. The reaction mixture was evaporated, added with ice-water, acidified with HCl (1 N) to pH 6, and extracted with EtOAc (3 × 100 mL). The combined organic layer was washed with water (50 mL), dried over MgSO4, and concentrated. The solid residue was washed with hexane and dried under high vacuum to provide a solid mass, which was re-dissolved in CH2Cl2, and dried under high vacuum to give a white solid diketone 2 in 92 +/- 2% (n = 3) yield, Rf = 0.22-0.25 (25% EtOAc/Hexanes). | |
| With sodium methylate In tetrahydrofuran; methanol at 0 - 20℃; | Step a General procedure: To a freshly prepared sodium methylate solution in methanol and THF ethyl trifluoroacetate (1.2 equiv) was added under stirring at 0° followed by addition of ketone 2 (1.0 equiv). The reaction mixture was allowed to stir for additional 3-24 h until the starting materials were consumed, as determined by thin-layer chromatography (TLC). Then the solvent was removed under reduced pressure and the residue was acidified with hydrochloric acid (1 N), followed by extracted with acetic ether. The combined organic layers were dried (MgSO4), Fitered and the filtrate was concentrated under reduced pressure. The crude product was puried by column chromatography. Yield: 40-90%. For some cases, the crude products can be straight used for step c without the column chromatography procedure. | |
| With sodium hydride In tetrahydrofuran Reflux; | ||
| With sodium methylate In methanol for 2h; Reflux; | General Synthetic Procedure for 4,4,4-Trifluoro-1-phenylbutane-1,3-dione Compounds II General procedure: Referring to Scheme 1, to the appropriate acetophenone derivative (0.05 mol) and ethyltrifluoroacetate (0.075 mol) in methanol (20 mL), sodium methoxide solution (0.1 mol of Na + 15 mL ofCH3OH) was added dropwise at room temperature, and the mixture was refluxed for 2 h. After themethanol was evaporated under vacuum, the residue was dissolved in ethyl acetate (50 mL), washedwith 5% HCl (25 mL) and water (25 mL), and dried over sodium sulfate. After the solvent wasevaporated under vacuum, the corresponding compound II was obtained. | |
| Stage #1: 2-Methoxyacetophenone With sodium hydride In tetrahydrofuran Stage #2: ethyl trifluoroacetate, In tetrahydrofuran at 0 - 20℃; for 24h; |

[2]Current Patent Assignee: HEBEI UNIVERSITY - CN104974135, 2017, B Location in patent: Paragraph 0211
[3]Penning, Thomas D.; Talley, John J.; Bertenshaw, Stephen R.; Carter, Jeffery S.; Collins, Paul W.; Docter, Stephen; Graneto, Matthew J.; Lee, Len F.; Malecha, James W.; Miyashiro, Julie M.; Rogers, Roland S.; Rogier; Yu, Stella S.; Anderson, Gary D.; Burton, Earl G.; Cogburn, J. Nita; Gregory, Susan A.; Koboldt, Carol M.; Perkins, William E.; Seibert, Karen; Veenhuizen, Amy W.; Zhang, Yan Y.; Isakson, Peter C. [Journal of Medicinal Chemistry, 1997, vol. 40, # 9, p. 1347 - 1365]
[4]Sloop, Joseph C.; Bumgardner, Carl L.; Loehle, W. David [Journal of Fluorine Chemistry, 2002, vol. 118, # 1-2, p. 135 - 147]
[5]Singh, Sunil K.; Reddy, P. Ganapati; Rao, K. Srinivasa; Lohray, Braj B.; Misra; Rajjak, Shaikh A.; Rao, Yeleswarapu K.; Venkateswarlu [Bioorganic and Medicinal Chemistry Letters, 2004, vol. 14, # 2, p. 499 - 504]
[6]Sloop, Joseph C.; Bumgardner, Carl L.; Washington, Gary; Loehle, W. David; Sankar, Sabapathy S.; Lewis, Adam B. [Journal of Fluorine Chemistry, 2006, vol. 127, # 6, p. 780 - 786]
[7]Current Patent Assignee: TAKEDA PHARMACEUTICAL COMPANY LIMITED - US2007/197532, 2007, A1 Location in patent: Page/Page column 59
[8]Location in patent: experimental part Gao, Mingzhang; Wang, Min; Miller, Kathy D.; Zheng, Qi-Huang [European Journal of Medicinal Chemistry, 2011, vol. 46, # 9, p. 4760 - 4767]
[9]Wang, Ning-Yu; Zuo, Wei-Qiong; Xu, Ying; Gao, Chao; Zeng, Xiu-Xiu; Zhang, Li-Dan; You, Xin-Yu; Peng, Cui-Ting; Shen, Yang; Yang, Sheng-Yong; Wei, Yu-Quan; Yu, Luo-Ting [Bioorganic and Medicinal Chemistry Letters, 2014, vol. 24, # 6, p. 1581 - 1588]
[10]Xing, Li-Juan; Lu, Tao; Fu, Wei-Li; Lou, Mei-Mei; Chen, Bo; Wang, Zhi-Shen; Jin, Yang; Li, Dan; Wang, Bin [Advanced Synthesis and Catalysis, 2015, vol. 357, # 14-15, p. 3076 - 3080]
[11]Liu, Chunhui; Cui, Zining; Yan, Xiaojing; Qi, Zhiqiu; Ji, Mingshan; Li, Xinghai [Molecules, 2016, vol. 21, # 7]
[12]Stevenson, Ralph J.; Azimi, Iman; Flanagan, Jack U.; Inserra, Marco; Vetter, Irina; Monteith, Gregory R.; Denny, William A. [Bioorganic and Medicinal Chemistry, 2018, vol. 26, # 12, p. 3406 - 3413]
- 26
-
 [ 579-74-8 ]
[ 579-74-8 ] 
-
 [ 120-57-0 ]
[ 120-57-0 ] 
- (E)-1-(2-methoxyphenyl)-3-(3,4-methylenedioxyphenyl)prop-2-en-1-one [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 85% | With sodium hydroxide In methanol; water at 20℃; | 2.2. General Procedure for the Preparation of Chalcones General procedure: Sodium hydroxide solution (50 % w/v, 5.3 ml, 66.7mmol) was added to a stirred solution of the acetophenone(6.67 mmol) and aldehyde (6.67 mmol) in methanol (30 ml). The resulting mixture was stirred at room temperature and sequentially monitored by TLC until the reaction was complete. The reaction was quenched with water (30 ml) and extracted with ethyl acetate (3x30 ml). The combined organic extracts were washed with brine (50 ml), dried with anhydrous magnesium sulfate and the solvent removed invacuo. The crude product was recrystalised from ethanol |
| With sodium hydroxide In ethanol for 1.5h; Ambient temperature; |
[2]Patt, William C.; Edmunds, Jeremy J.; Repine, Joseph T.; Berryman, Kent A.; Reisdorph, Billy R.; Lee, Chet; Plummer, Mark S.; Shahripour, Aurash; Haleen, Stephen J.; Keiser, Joan A.; Flynn, Mike A.; Welch, Kathleen M.; Reynolds, Elwood E.; Rubin, Ron; Tobias, Brian; Hallak, Hussein; Doherty, Annette M. [Journal of Medicinal Chemistry, 1997, vol. 40, # 7, p. 1063 - 1074]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With potassium hydroxide In ethanol at 80℃; | ||
| With potassium hydroxide In ethanol; water Heating; |
[2]Li, Jian; Chen, Jing; Gui, Chunshan; Zhang, Li; Qin, Yu; Xu, Qiang; Zhang, Jian; Liu, Hong; Shen, Xu; Jiang, Hualiang [Bioorganic and Medicinal Chemistry, 2006, vol. 14, # 7, p. 2209 - 2224]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 1: 36% 2: 35% | With samarium diiodide In tetrahydrofuran at 0 - 20℃; for 3h; |
- 29
-
 [ 579-74-8 ]
[ 579-74-8 ] 
-
 [ 15062-88-1 ]
[ 15062-88-1 ] 
- N-Benzooxazol-2-yl-N'-[1-(2-methoxy-phenyl)-eth-(Z)-ylidene]-hydrazine [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 81% | With acetic acid In ethanol for 5h; Heating; |
- 33
-
 [ 13513-82-1 ]
[ 13513-82-1 ] 
-
 [ 579-74-8 ]
[ 579-74-8 ] 
- (1S)-1-(2-methoxyphenyl)ethan-1-ol [ No CAS ]

- (R)-1-(2-methoxyphenyl)ethanol [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With 3 A molecular sieve; oxygen; caesium carbonate In chloroform at 23℃; for 164h; | ||
| With dichloro bis(acetonitrile) palladium(II); oxygen; (-)-sparteine In 1,2-dichloro-ethane at 70℃; Title compound not separated from byproducts.; | ||
| With oxygen; caesium carbonate; (-)-sparteine In chloroform at 23℃; for 164h; Molecular sieve; optical yield given as %ee; |
| With Arthrobacter atrocyaneus In N,N-dimethyl-formamide at 32℃; for 48h; Microbiological reaction; | ||
| 56 % ee | Stage #1: 1-(2-methoxyphenyl)ethanol With C28H36ClMnN2O2; potassium acetate In dichloromethane; water for 0.0833333h; Stage #2: With N-Bromosuccinimide In dichloromethane; water at 20℃; for 4h; enantioselective reaction; |

[2]Jensen; Pugsley; Sigman [Journal of the American Chemical Society, 2001, vol. 123, # 30, p. 7475 - 7476]
[3]Ebner, David C.; Bagdanoff, Jeffrey T.; Ferreira, Eric M.; McFadden, Ryan M.; Caspi, Daniel D.; Trend, Raissa M.; Stoltz, Brian M. [Chemistry - A European Journal, 2009, vol. 15, # 47, p. 12978 - 12992]
[4]Silva, Camila R.; Souza, Juliana C.; Araujo, Lidiane S.; Kagohara, Edna; Garcia, Thais P.; Pelizzari, Vivian H.; Andrade, Leandro H. [Journal of Molecular Catalysis B: Enzymatic, 2012, vol. 83, p. 23 - 28]
[5]Xu, Daqian; Wang, Shoufeng; Shen, Zhiqiang; Xia, Chungu; Sun, Wei [Organic and Biomolecular Chemistry, 2012, vol. 10, # 14, p. 2730 - 2732]
- 34
-
 [ 579-74-8 ]
[ 579-74-8 ] 
-
 [ 7677-24-9 ]
[ 7677-24-9 ] 
- 2-(trimethylsilyloxy)-2-(2'-methyloxyphenyl) propanenitrile [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 94.5% | With sodium tetrahydroborate at 50℃; for 8.5h; Inert atmosphere; | 17 Example 17 In a 250 ml three-necked flask, sodium borohydride (12.5 mmol) and 2-methoxyacetophenone (1.25 mol) were added. Under the protection, slowly warm up to 50 °C, stirring while dropping cyanotrimethylsilane (1.5 mol) through the dropping funnel for 0.5 hour, keeping the reaction temperature, continuing to stir the reaction for 8 hours, standing, cooling to room temperature, vacuum distillation The corresponding fractions were collected and analyzed by GC. The yield of the addition product, 2-(2-methoxyphenyl)-2-trimethylsilyloxypropionitrile, was 94.5 %. |
| 90% | at 20℃; for 0.166667h; | |
| 59% | Stage #1: 2-Methoxyacetophenone With (Ra)-4-hydroxy-2,6-diphenyldinaphtho[1,3,2]dioxaphosphepine 4-oxide; n-butyllithium In hexane; toluene at -78℃; for 0.5h; Inert atmosphere; Stage #2: trimethylsilyl cyanide In hexane; toluene at 0℃; for 24h; Inert atmosphere; |
| 0.25 g | With [CH3(Ph)Si(9H-fluoren-9-yl)2]LaCl In chloroform at 25℃; for 15h; | |
| Stage #1: trimethylsilyl cyanide With N,N-dimethylaniline N-oxide In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: 2-Methoxyacetophenone With 2,2,2-trifluoroethanol; (R,R)-N,N'-bis(3,5-di-tert-butyl-salicylidene)-1,2-cyclohexanediamino methylaluminium In tetrahydrofuran at -20℃; for 14h; Inert atmosphere; enantioselective reaction; | ||
| With C43H61N3Th In benzene-d6 at 80℃; for 24h; Sealed tube; |

[2]Khan, Noor-ul H.; Agrawal, Santosh; Kureshy, Rukhsana I.; Abdi, Sayed H.R.; Singh, Surendra; Jasra, Raksh V. [Journal of Organometallic Chemistry, 2007, vol. 692, # 20, p. 4361 - 4366]
[3]Hatano, Manabu; Ikeno, Takumi; Matsumura, Tokihiko; Torii, Shinobu; Ishihara, Kazuaki [Advanced Synthesis and Catalysis, 2008, vol. 350, # 11-12, p. 1776 - 1780]
[4]Mei, Luo; Zhu, Ma Huai [Synthetic Communications, 2005, vol. 35, # 20, p. 2615 - 2623]
[5]Location in patent: experimental part Alaaeddine, Ali; Roisnel, Thierry; Thomas, Christophe M.; Carpentiera, Jean-Francois [Advanced Synthesis and Catalysis, 2008, vol. 350, # 5, p. 731 - 740]
[6]Deka, Hemanta; Fridman, Natalia; Eisen, Moris S. [Inorganic Chemistry, 2022, vol. 61, # 8, p. 3598 - 3606]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 92% | With potassium hydroxide In methanol; water at 20℃; Cooling with ice; | 7 3.2 General procedure for enone synthesis General procedure: To a an ice-cooled solution of the appropriate ketone (10 mmol) in MeOH (50 mL) 10% KOH aq solution (30 mL) was added, followed by gradual addition of the corresponding aryl aldehyde (10 mmol). The mixture was left to attain room temperature and stirred overnight. The solid product was filtered and washed three times with MeOH/H2O mixture (5:3) and left to dry. In case of oily products the reaction mixture was extracted by CH2Cl2 (4 × 20 mL) and the combined organic layers were washed with water, filtered over anhydrous MgSO4, evaporated under reduced pressure and used without further purification. |
| 41.7% | With sodium hydroxide In methanol at 28℃; for 12h; |
[2]Liu, Xiaoling; Go, Mei-Lin [Bioorganic and Medicinal Chemistry, 2006, vol. 14, # 1, p. 153 - 163]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 100% | Stage #1: 2-Methoxyacetophenone With sodium hydroxide In ethanol; water at 0℃; for 0.5h; Stage #2: 2-chloro-benzaldehyde In ethanol; water at 20℃; for 48h; | 1.1 [Step 1] Preparation of (E)-3-(2-chlorophenyl)-1-(2-methoxyphenyl)prop-2-en-1-one 2’-Methoxyacetophenone (1.0 g, 6.7 mmol, 1.0 eq) and 2.5 mol/L sodium hydroxide aqueous solution (30 ml)were dissolved in ethanol (50 ml), and the solution was stirred at 0°C for 30 min. before adding 2-chlorobenzaldehyde(1.123 g, 8.0 mmol, 1.2 eq) dropwise, then the mixture was stirred for two days at room temperature. Ethanol wasremoved by evaporation, the remaining mixture was extracted with ethyl acetate, and the organic phase was dried withanhydrous magnesium sulfate and concentrated to obtain the subject compound as a solid (2.178 g, yield 100%). Rf value: 0.21 (n-hexane/ethyl acetate = 7:1 v/v).1H-NMR (300 MHz, CDCl3) δ: 8.01 (d, J = 16.0 Hz, 1H), 7.71-7.62 (m, 2H), 7.50-7.39 (m, 2H), 7.35 (d, J = 16.0Hz, 1H), 7.30-7.26 (m, 2H), 7.06-6.98 (m, 2H), 3.89 (s, 3H). |
| 84% | With potassium hydroxide In methanol; water at 20℃; Cooling with ice; | 9 3.2 General procedure for enone synthesis General procedure: To a an ice-cooled solution of the appropriate ketone (10 mmol) in MeOH (50 mL) 10% KOH aq solution (30 mL) was added, followed by gradual addition of the corresponding aryl aldehyde (10 mmol). The mixture was left to attain room temperature and stirred overnight. The solid product was filtered and washed three times with MeOH/H2O mixture (5:3) and left to dry. In case of oily products the reaction mixture was extracted by CH2Cl2 (4 × 20 mL) and the combined organic layers were washed with water, filtered over anhydrous MgSO4, evaporated under reduced pressure and used without further purification. |
| 27.8% | With sodium hydroxide In methanol at 28℃; for 12h; |
[2]Abdel-Halim, Mohammad; Keeton, Adam B.; Gurpinar, Evrim; Gary, Bernard D.; Vogel, Simon M.; Engel, Matthias; Piazza, Gary A.; Boeckler, Frank M.; Hartmann, Rolf W.; Abadi, Ashraf H. [Bioorganic and Medicinal Chemistry, 2013, vol. 21, # 23, p. 7343 - 7356]
[3]Liu, Xiaoling; Go, Mei-Lin [Bioorganic and Medicinal Chemistry, 2006, vol. 14, # 1, p. 153 - 163]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 100 % Chromat. | With sodium hydroxide at 82℃; for 8h; | |
| 99 %Chromat. | With C36H35Cl2N4PRu; potassium isopropoxide at 82℃; for 0.5h; Inert atmosphere; | |
| With (N3,N3'-di-2-(diphenylphosphinite)benzylidene-[2,2']bipyridinyl-3,3'-diamine)bis((η6-benzene)dichlororuthenium(II)); potassium hydroxide for 0.25h; Reflux; Inert atmosphere; |
| With (thiophene-2-(N-diphenylphosphino)methylamine)(η6-benzene)dichlororuthenium(II); potassium hydroxide for 0.5h; Inert atmosphere; Reflux; | ||
| With [rhodium(I)((Ph2P)2N-C6H4-2-CH(CH3)2)(1,5-cyclooctadiene)](tetrafluoroborate); sodium hydroxide at 82℃; for 0.416667h; Reflux; Inert atmosphere; Schlenk technique; | General procedure for the transfer hydrogenation of ketones General procedure: Typical procedure for the catalytic hydrogen transfer reaction: asolution of ruthenium complexes the [Rh((Ph2P)2NCH2-C4H3S)2]-BF4, 5, [Rh((Ph2P)2NCH2-C4H3O)2]BF4, 6, Rh((Ph2P)2N-C6H4-2-CH(CH3)2)(cod)]BF4, 7, [Rh((Ph2P)2N-C6H4-4-CH(CH3)2)(cod)]BF4,8, [Rh((Ph2P)2N-C6H4-2-CH(CH3)2)2]BF4, 9 or [Rh((Ph2P)2N-C6H4-4-CH(CH3)2)2]BF4, 10, NaOH (0.025 mmol) and the corresponding ketone (0.5 mmol) in degassed iso-PrOH (5 mL) were refluxed until the reactions were completed. After this period a sample of the reaction mixture was taken off, diluted with acetone and analyzed immediately by GC. Conversions obtained are related to the residual unreacted ketone. | |
| With [Rh(cod)(PPh2NH-C6H4-4-CH(CH3)2)Cl]; sodium hydroxide at 82℃; for 0.5h; Inert atmosphere; Schlenk technique; |

[2]Location in patent: experimental part Zhao, Miao; Yu, Zhengkun; Yan, Shenggang; Li, Yang [Journal of Organometallic Chemistry, 2009, vol. 694, # 19, p. 3068 - 3075]
[3]Location in patent: experimental part Aydemir, Murat; Durap, Feyyaz; Baysal, Akin; Meric, Nermin; Buldaǧ, Ayşegül; Gümgüm, Bahattin; Özkar, Saim; Yildirim, Leyla Tatar [Journal of Molecular Catalysis A: Chemical, 2010, vol. 326, # 1-2, p. 75 - 81]
[4]Location in patent: experimental part Aydemir, Murat; Baysal, Akin; Turgut, Yilmaz [Applied Organometallic Chemistry, 2011, vol. 25, # 4, p. 270 - 275]
[5]Aydemir, Murat; Meric, Nermin; Kayan, Cezmi; Ok, Fatih; Baysal, Akin [Inorganica Chimica Acta, 2013, vol. 398, p. 1 - 10]
[6]Aydemir, Murat; Ocak, Yusuf Selim; Rafikova, Khadichakhan; Kystaubayeva, Nurzhamal; Kayan, Cezmi; Zazybin, Alexey; Ok, Fatih; Baysal, Akin; Temel, Hamdi [Applied Organometallic Chemistry, 2014, vol. 28, # 6, p. 396 - 404]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| Multi-step reaction with 2 steps 1: Br2 / diethyl ether / 20 °C 2: ethanol; H2O / 20 °C |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 84% | With boron trifluoride diethyl etherate; hydrazine In tetrahydrofuran at 0℃; for 0.5h; Inert atmosphere; | General method for hydrazones synthesis 2-22 General procedure: To a solution of acetophenone or isatin (5 mmol) in THF (10 mL), BF3·OEt2 (7.5 mmol) was added at 0°C under the atmosphere of N2 and the mixture was stirred for 10 minutes. To the cooled mixture, anhydrous hydrazine (7.5 mmol) was added in one portion and the reaction mixture was stirred at 0°C under the atmosphere of N2 for 30 min. The reaction was quenched by the addition of a saturated solution of NH4Cl (4 mL), extracted by ethyl acetate (3 × 15 mL) and purified by column chromatography (ethyl acetate-CH2Cl2; 3 : 7) to afford hydrazones 2-22. |
| Multi-step reaction with 2 steps 1: ethanol / Heating 2: N2H4 / ethanol / Heating |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 93% | Sodium cyanoborohydride (2.46 g, 39.1 mmol) was added to a mixture of 1-(2-methoxyphenyl)ethanone (5.87 g, 39.1 mmol), ammonium acetate (30 g, 391 mmol) and 3 molecular sieves (20 g) in methanol (200 mL) and the mixture was stirred at room temperature for 3 days. The mixture was filtered and the solvent was evaporated under reduced pressure. The residue was dissolved in aqueous sodium hydroxide (1M) and the mixture was extracted with ethyl acetate. The combined organic fractions were washed with water, dried (MgSO4), and the solvent was evaporated under reduced pressure. The residue was dissolved in diethyl ether and ethereal hydrogen chloride (1M, 50 mL) was added. The solid was collected, washing with diethyl ether, and dried in vacuo to give the title compound as a colorless solid (6.82 g, 93%). m/z (ES+) 135 (M+1-NH3). |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 95% | With water In methanol at 70℃; for 1h; | 28 Example 28 Example 28 To a solution in which 0.001 g of methyl(triphenylphosphine)gold (0.002 mmol) was dissolved in 1 ml of methanol, 0.13 g of o-anisylacetylene (1 mmol) and an aqueous solution in which 0.05 g of concentrated sulfuric acid (0.5 mmol) was dissolved in 0.5 ml of water were added. After stirring at 70° C. for 1 hour, the yield of 2'-methoxyacetophenone was 95% (catalyst turnover number: 475). |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 86% | With tris(2,2′-bipyridyl)ruthenium(II) chloride; 1,4-dioxane dibromide; sodium ascorbate powder In acetonitrile at 20℃; for 8h; Irradiation; Green chemistry; | General procedure for the visible light mediated oxidation of acetoarylones General procedure: To an oven-dried round bottom flask equipped with a magnetic stir bar was charged with dioxane dibromide (1.1 equiv.), tris(2,2′-bipyridyl)ruthenium(II) chloride (2 mol%), acetoarylone (AA, 1.0 equiv.), sodium ascorbate (3.0 equiv.) and dry CH3CN. The mixture was irradiated under a 5W Blue LED bulb at a distance of 5 cm under open-air atmosphere. After stirring at room temperature for 8-10 h, the solvent was removed under reduced pressure and the residue was purified by either recrystallization or filtration thru short pad silica gel column chromatography using hexane-ethyl acetate mixtures. The purity of the compound was confirmed by IR, 1H and 13CNMR measurements, vide infra. |
| With SeO2 In ethanol | 40 (2-Methoxyphenyl)glyoxal PREPARATION 40 (2-Methoxyphenyl)glyoxal SeO2 (12.4 g., 0.112 mol) was dissolved in 75 ml. of 95% ethanol by warming to 55°. o-Methoxyacetophenone (15.3 g., 0.102 mol) was added in one portion and the mixture refluxed 21 hours. The reaction mixture was treated with activated carbon, filtered over diatomaceous earth, and the filtrate stripped to yield to an oil, 23.2 g. The latter was distilled to yield purified title product, 8.4 g., bp 94°-96°/0.5 mm, which solidified on cooling. | |
| With selenium(IV) dioxide; water monomer In 1,4-dioxane for 4h; Reflux; |
| With selenium(IV) dioxide In 1,4-dioxane; water monomer | ||
| With selenium(IV) oxide In 1,4-dioxane; water monomer | General procedure: Commercial unavailable (oxo)acetaldehydes were synthesized via the reaction where the corresponding substituted 1 -phenylethanone was oxygenated with Se02 in water/dioxane solution. The reactions were carried out according to available literature | |
| With iodine; dimethyl sulfoxide at 90℃; | ||
| With selenium(IV) dioxide; water monomer In 1,4-dioxane at 100 - 103℃; for 6h; | General Procedure for the Synthesis of Compounds 1-17 General procedure: An oven dried round-bottom flask was charged with thecorresponding acetophenone (0.01mol) in 1,4-dioxane/watermixture (10:0.5, v/v). Selenium dioxide (0.01 mol), was thenadded in one portion to the stirred solution. The resultingmixture was then heated at reflux for 3 h until the startingmaterial was consumed as judged by TLC analysis. The reactionmixture was allowed to cool and then the mixture wasfiltered. The filtrate was then evaporated under vacuum on arotary evaporator. Water (10 mL) was added to the residueand the resulting mixture was heated at 101-103 °C for further3 h. The solution of 1, 2-diaminobenzene (0.012 mol) indioxane (20 mL) was added in one portion to the reactionmixture and stirred for 30 min at room temperature. The reactionmixture was diluted with water (100 mL) and the resultingprecipitates were collected by filtration. The crudemixture was purified on silica gel column (EtOAc / Hexane,1/9 3/7) to afford the desired quinoxalines in good to excellentyields (64-97%) [24]. The structures of all quinoxalinederivatives 1-17 were confirmed with different spectroscopictechniques. | |
| With iodine In dimethyl sulfoxide at 100℃; for 0.333333h; Microwave irradiation; | ||
| With selenium(IV) dioxide In 1,4-dioxane; water monomer at 115℃; for 12h; Inert atmosphere; | ||
| With iodine; dimethyl sulfoxide at 130℃; for 1h; Sealed tube; |

[2]Current Patent Assignee: PFIZER INC - US4675186, 1987, A
[3]Location in patent: experimental part Pellegatti, Laurent; Vedrenne, Emeline; Leger, Jean-Michel; Jarry, Christian; Routier, Sylvain [Tetrahedron, 2010, vol. 66, # 24, p. 4383 - 4389]
[4]Mupparapu, Nagaraju; Khan, Shahnawaz; Battula, Satyanarayana; Kushwaha, Manoj; Gupta, Ajai Prakash; Ahmed, Qazi Naveed; Vishwakarma, Ram A. [Organic Letters, 2014, vol. 16, # 4, p. 1152 - 1155]
[5]Current Patent Assignee: VICHEM CHEMIE KUTATO KFT - WO2014/106763, 2014, A1 Location in patent: Page/Page column 13
[6]Battini, Narsaiah; Padala, Anil K.; Mupparapu, Nagaraju; Vishwakarma, Ram A.; Ahmed, Qazi Naveed [RSC Advances, 2014, vol. 4, # 50, p. 26258 - 26263]
[7]Zeb, Aurang; Hameed, Abdul; Khan, Latifullah; Khan, Imran; Dalvandi, Kourosh; Choudhary, M. Iqbal; Basha, Fatima Z. [Medicinal Chemistry, 2014, vol. 10, # 7, p. 724 - 729]
[8]Balaguez, Renata A.; Betin, Eduardo S.; Barcellos, Thiago; Lenardão, Eder J.; Alves, Diego; Schumacher, Ricardo F. [New Journal of Chemistry, 2017, vol. 41, # 4, p. 1483 - 1487]
[9]Ghosh, Prithwish; Kwon, Na Yeon; Kim, Saegun; Han, Sangil; Lee, Suk Hun; An, Won; Mishra, Neeraj Kumar; Han, Soo Bong; Kim, In Su [Angewandte Chemie - International Edition, 2021, vol. 60, # 1, p. 191 - 196][Angew. Chem., 2021, vol. 133, # 1, p. 193 - 198,6]
[10]Zhuang, Shi-Yi; Tang, Yong-Xing; Liu, Jin-Yi; Chen, Xiang-Long; Ma, Jin-Tian; Wu, Yan-Dong; Zheng, Kai-Lu; Wu, An-Xin [Organic Letters, 2022, vol. 24, # 15, p. 2858 - 2862]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With sodium methylate In carbonic acid dimethyl ester | 5 EXAMPLE 5 EXAMPLE 5 The procedure is as described in Example 1, except that 95 g of sodium methylate in 800 ml dimethylcarbonate are used and 250 g of o-methoxyacetophenone are added dropwise over a period of 5 hours at 90°C, the alcohol formed being simultaneously distilled off at normal pressure. Working up in accordance with Example 1 gives 100 g of unreacted starting material and 162 g of o-methoxybenzoylacetic acid methyl ester boiling at 130° to 133°C/0.07 torr (= 78% of the theoretical amount based on the reacted o-methoxyacetophenone). |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 1: 66% 2: 25% | In tetrahydrofuran at 20℃; for 3h; |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With diethylamine In ethanol at 20℃; | General two-step procedure for aryl-OVK General Procedure: An isatin (1 eq.) with the desired substitution pattern or N-alkylation (see general procedure above) was condensed with the desired aryl methyl ketone (1 eq.) using diethylamine (1 eq.) in anhydrous ethanol for 2-48 hours (most reactions were complete after 2-3 h). The condensation product could be precipitated in quantitative yield using pentane and isolated by filtration. Without further purification, it was subjected to a 4:1 mixture of glacial acetic acid with concentrated hydrochloric acid and carefully heated to boiling with a heatgun for 20-30 min. The acidic mixture was diluted tenfold with water and neutralized with sodium hydroxide and sodium bicarbonate, whereby the highly colored OVK products precipitated. After filtration and drying, the crude products were dissolved in a minimal amount of acetone and DCM. The solution was gradually triturated with pentane and any precipitating dark impurities filtered off. The final product either crystallized from this solution or could be obtained by removing the volatiles. In most cases, the product was sufficiently pure without any further effort. If necessary, a small quantity of product could be purified by preparative TLC on 1 mm glass-backed silica gel plates, typically using 3:2 ethyl acetate - pentane as developing solvent and 100% ethyl acetate as eluent. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 98% | With sodium hydride In toluene; mineral oil for 0.5h; Reflux; | General Procedure A: Synthesis of the phenyliodonium ylides of β-ketoesters General procedure: In a dry two neck flask equipped with a reflux condenser and rubber septum and a stir bar, NaH (60% wt in mineral oil, 4.0 g, 100 mmol) was suspended in toluene (8 mL) and dimethylcarbonate (9.1 g, 100 mmol, 2.5 eq) was added. The mixture was heated to reflux and acetophenone (4.8 g, 40 mmol, 1.0 eq) in toluene (20 mL) was added dropwise. The reaction mixture was stir for 0.5 h when hydrogen evolution had ceased.The suspension was cooled to 0 °C, diluted with EtOAc (150 mL) and carefully quenched with MeOH (10 mL), followed by H2O, and then acidified to pH < 5 using 1N HCl. The organic layer is separated and then washed with H2O (200 mL) then brine (100 mL), and dried over MgSO4, and concentrated in vacuo. The resulting biphasic liquid was partitioned between MeCN (20 mL) and hexanes (10 mL) and then washed with hexanes (25 mL x 2) and concentrated in vacuo to give the β-ketoesters. Dicarbonyl compound (6mmol) was dissolved in MeOH (6 mL) and then cooled in a brine-ice bath. Then, 30% KOH in MeOH (6 mL) solution was added dropwise an the mixture was allowed to stir for 2 minutes after which a solution of iodobenzene diacetate (6 mmol) was added dropwise and the resulting mixture was allowed to stir for 1 hr and then poured onto icewater (100 mL). The suspension is then extracted with CH2Cl2 (50 mL x 3). The combined extracts were dried over MgSO4, filtered through Buchner funnel, then concentrated in vacuo to approximately one third of the original volume. The ylide was slowly precipitated using Et2O and/or hexanes, and cooled to 0 °C and isolated by filtration. |
| 87% | With sodium hydride at 20 - 50℃; for 3.5h; | 14 Methyl 3-(2-methoxyphenyl)-3-oxopropanoate Methyl 3-(2-methoxyphenyl)-3-oxopropanoate Sodium hydride (1.9 g, 55.42 mmol, 3.00 equiv, 70%) was added portionwise to a solution of 1-(2-methoxyphenyl)ethanone (4 g, 26.67 mmol, 1.00 equiv) in dimethyl carbonate (40 mL) at 0° C. The reaction was stirred for 1 h at room temperature, then for an additional 2.5 h at 50° C. Then the reaction was quenched by the addition of 50 mL of ice/water. The pH of the aqueous solution was adjusted to 2 with HCl (1N). The resulting solution was extracted with 2*20 mL of ethyl acetate. The organic layers were combined and concentrated in vacuo. The residue was purified by silica gel column chromatography with ethyl acetate/petroleum ether (1:2). This resulted in 4.8 g (87%) of methyl 3-(2-methoxyphenyl)-3-oxopropanoate as yellow green oil. |
| 21% | With sodium hydride In tetrahydrofuran; toluene at 70℃; for 4h; |
| Stage #1: carbonic acid dimethyl ester With sodium hydride In tetrahydrofuran for 0.5h; Reflux; Stage #2: 2-Methoxyacetophenone In tetrahydrofuran Reflux; | ||
| With sodium hydride In toluene; mineral oil for 5h; Reflux; | 1.2.2 General procedure B: preparation of β-ketoesters 1b. General procedure: To a solution of ketone (20 mmol) in toluene (80 mL) was added dimethyl carbonate (60 mmol) and NaH (40 mmol, 60%). The reaction mixture was refluxed for 5 h until TLC indicated the total consumption of the ketone. After cooling, the reaction mixture was poured into ice-water (100 mL), acidified with 3 M HCl to pH 2-3 and extracted with EtOAc (100 mL x3). The combined organic layer was dried over Na2SO4 and evaporated under reduced pressure. The residue was purified by flash column chromatography (petroleum ether /EtOAc = 10/1) to afford the desired compound. | |
| With sodium hydride In tetrahydrofuran at 0℃; Reflux; |

[2]Current Patent Assignee: SYNERGENICS LCC - US2012/277224, 2012, A1 Location in patent: Page/Page column 20
[3]Píša, Ondřej; Rádl, Stanislav [European Journal of Organic Chemistry, 2016, vol. 2016, # 13, p. 2336 - 2350]
[4]Location in patent: experimental part Li, Miao; Liu, Chang-Ling; Yang, J.I.-Chun; Zhang, Jin-B.O.; Li, Zhi-Nian; Zhang, Hong; Li, Zheng-Ming [Journal of Agricultural and Food Chemistry, 2010, vol. 58, # 5, p. 2664 - 2667]
[5]Chong, Siying; Su, Yingpeng; Wu, Lili; Zhang, Weigang; Ma, Junyan; Chen, Xiaowei; Huang, Danfeng; Wang, Ke-Hu; Hu, Yulai [Synthesis, 2016, vol. 48, # 9, p. 1359 - 1370]
[6]Sun, Desong; Zhao, Xiaoyuan; Zhang, Bobo; Cong, Ying; Wan, Xintong; Bao, Mingmai; Zhao, Xue; Li, Bing; Zhang-Negrerie, Daisy; Du, Yunfei [Advanced Synthesis and Catalysis, 2018, vol. 360, # 8, p. 1634 - 1638]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| With tert.-butylhydroperoxide In chlorobenzene at 99.84℃; for 10h; Overall yield = 87 %; |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 79% | With sodium ethanolate In ethanol at 50℃; for 3h; Reflux; | 4.1.1. 2-Hydroxy-4-oxo-4-phenyl-2-but-2-enoic acid ethyl ester25 (1a) General procedure: Ethanol (200 mL) was converted to sodium ethoxide by portion wise addition of sodium (6.26 g; 0.27 mol) before a solution of diethyl oxalate (37 mL; 0.27 mol) and acetophenone (16.36 g; 0.14 mol) in ethanol (100 mL) was added dropwise at 50 C. The mixture was heated at reflux for 3 h. After cooling, the precipitate formed was filtered and then washed with absolute ethanol. The residue was taken up in water (20 mL) and acidified with 1 N HCl solution (5 mL). The precipitate formed was filtered and washed with petroleum ether to give 1a as yellow crystal (yield: 62%), mp 72-74 C. |
| 74% | With sodium ethanolate In ethanol at 50℃; for 2h; Reflux; | 2 4.1.2. General procedure for the preparation of ethyl 2-hydroxy-4-oxo-4-aryl-2-butenoates (11-21) To a stirred solution of sodium ethanolate, freshly prepared by reacting Na (66 mmol, 2 equiv) with 50 mL absolute EtOH, the corresponding aryl ketone (33 mmol, 1 equiv) and diethyl oxalate (66 mmol, 2 equiv) diluted in 30 mL of absolute EtOH were added dropwise at 50°C. The mixture was refluxed for 2 h. The solvent was evaporated under reduce pressure and the residue was dissolved in 1 N aqueous HCl (20 mL) and stirred for additional 1 h. Then, the solution was extracted with EtOAc (220 mL) and washed with distilled water (20 mL). The organic layers were dried over MgSO4 and evaporated under reduce pressure. Finally, the residue was triturated in cyclohexane to give compounds 11-21. |
| With sodium acetate In methanol for 4h; Reflux; |
| With sodium ethanolate In diethyl ether; ethanol at 20℃; |

[2]Tourteau, Aurélien; Andrzejak, Virginie; Body-Malapel, Mathilde; Lemaire, Lucas; Lemoine, Amélie; Mansouri, Roxane; Djouina, Madjid; Renault, Nicolas; El Bakali, Jamal; Desreumaux, Pierre; Muccioli, Giulio G.; Lambert, Didier M.; Chavatte, Philippe; Rigo, Benoît; Leleu-Chavain, Natascha; Millet, Régis [Bioorganic and Medicinal Chemistry, 2013, vol. 21, # 17, p. 5383 - 5394]
[3]Xi, Mei-Yang; Jia, Jian-Min; Sun, Hao-Peng; Sun, Zhong-Ying; Jiang, Jie-Wei; Wang, Ya-Jing; Zhang, Min-Ye; Zhu, Jun-Feng; Xu, Li-Li; Jiang, Zheng-Yu; Xue, Xin; Ye, Ming; Yang, Xi; Gao, Yuan; Tao, Lei; Guo, Xiao-Ke; Xu, Xiao-Li; Guo, Qing-Long; Zhang, Xiao-Jin; Hu, Rong; You, Qi-Dong [Journal of Medicinal Chemistry, 2013, vol. 56, # 20, p. 7925 - 7938]
[4]Johansson, Niklas G.; Turku, Ainoleena; Vidilaseris, Keni; Dreano, Loïc; Khattab, Ayman; Ayuso Pérez, Daniel; Wilkinson, Aaron; Zhang, Yuezhou; Tamminen, Matti; Grazhdankin, Evgeni; Kiriazis, Alexandros; Fishwick, Colin W. G.; Meri, Seppo; Yli-Kauhaluoma, Jari; Goldman, Adrian; Boije Af Gennäs, Gustav; Xhaard, Henri [ACS Medicinal Chemistry Letters, 2020, vol. 11, # 4, p. 605 - 610]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 88% | With 10% Ru/C In ethanol at 78℃; for 24h; | 10 Example 10: Preparation of methylene malononitrile compounds (III-10)The reaction formula is as follows: Add 7.509g (50mmol) o-methoxyacetophenone (I-10), 1.0g (Ru molar amount is (I-10) 10 ‰) to the reaction flaskRu / C catalyst, 3.303 g (50 mmol) of malononitrile II and 120 mL of ethanol were stirred at 78 ° C. for 24 h. The following operations were the same as in Example 8.Finally, 8.772g of solid was obtained, the yield was 88.0%, and the purity of GC-MS was 98.0%. |
| 51% | With ammonium acetate; acetic acid In toluene Reflux; Dean-Stark; | |
| With sodium acetate; acetic acid In toluene Reflux; |
[2]Longstreet, Ashley R.; Campbell, Brian S.; Gupton, B. Frank; McQuade, D. Tyler [Organic Letters, 2013, vol. 15, # 20, p. 5298 - 5301]
[3]Althagafi, Ismail; Elagamy, Amr; Kumar, Abhinav; Pratap, Ramendra; Shally; Shaw, Ranjay [Organic and biomolecular chemistry, 2020, vol. 18, # 32, p. 6407 - 6417]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 85% | With potassium hydroxide; In methanol; water; at 40℃; for 4h;Inert atmosphere; | Fill the round bottom flask with raw material 4106 (0.69mol),Raw material 4206 (0.63mol),Add 1.2L of methanol and stir to dissolve,Aqueous potassium hydroxide solution (80 mL, 3.15 mol) was slowly added dropwise to the mixture.After the dropwise addition was completed, the reaction mixture was heated to 40 C under a nitrogen atmosphere and stirred for 4 hours.After the reaction mixture was cooled to room temperature, 4M HCl solution was added to adjust the pH of the mixture to neutral,And placed at -20 to crystallize.After dissolving the filtered solid with an organic solvent, after evaporating the solvent,The solid product obtained was beaten with methanol at -20 C.Suction filtration and drying to obtain a white solid with a yield of 85% and a purity of 99.8%. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 91% | With indium trifluoromethanesulfonate; copper (I) iodide; oxygen In 1-methyl-pyrrolidin-2-one at 100℃; for 30h; Schlenk technique; | |
| 82% | With N-iodo-succinimide In water monomer at 80℃; for 6h; Green chemistry; | 4.3 Typical procedure for the synthesis of 2-arylimidazo [1,2-a]pyridines General procedure: A mixture of 2-aminopyridine 1a (94.11mg, 1mmol) and acetophenone 2a (120.15mg, 1mmol, 1 equiv.) in aqueous medium was stirred and heated at 80°C for 6h, after addition of NIS (224.98mg, 1mmol, 1 equiv.). After completion of the reaction (TLC), the mixture was cooled to room temperature and diluted with Et2O (10mL) and transferred into a separatory funnel. The organic layer was collected and further extracted with Et2O (2×10mL). The combined organic extract were dried (anh Na2SO4), filtered, then filtrate concentrated under rotary vacuum evaporation, and the residue was charged on to chromatography (100-200 mesh silica gel) column and eluted with EtOAc-hexane to afford pure 3a (192.36mg, 90%). All the remaining reactions were performed following this general procedure. |
| 82% | With N-iodo-succinimide In water monomer at 80℃; for 6h; Green chemistry; | 4.3 Typical procedure for the synthesis of 2-arylimidazo [1,2-a]pyridines General procedure: A mixture of 2-aminopyridine 1a (94.11mg, 1mmol) and acetophenone 2a (120.15mg, 1mmol, 1 equiv.) in aqueous medium was stirred and heated at 80°C for 6h, after addition of NIS (224.98mg, 1mmol, 1 equiv.). After completion of the reaction (TLC), the mixture was cooled to room temperature and diluted with Et2O (10mL) and transferred into a separatory funnel. The organic layer was collected and further extracted with Et2O (2×10mL). The combined organic extract were dried (anh Na2SO4), filtered, then filtrate concentrated under rotary vacuum evaporation, and the residue was charged on to chromatography (100-200 mesh silica gel) column and eluted with EtOAc-hexane to afford pure 3a (192.36mg, 90%). All the remaining reactions were performed following this general procedure. |
| 69% | In ethanol at 70℃; for 24h; |

[2]Jahan, Kousar; Sofi, Firdoos Ahmad; Salim, Sumi Aisha; Bharatam, Prasad V. [Tetrahedron, 2022, vol. 112]
[3]Jahan, Kousar; Sofi, Firdoos Ahmad; Salim, Sumi Aisha; Bharatam, Prasad V. [Tetrahedron, 2022, vol. 112]
[4]Meng, Xu; Wang, Yanmin; Yu, Chaoying; Zhao, Peiqing [RSC Advances, 2014, vol. 4, # 52, p. 27301 - 27307]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 62% | With ammonium peroxydisulfate; dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer In water at 110℃; for 3h; Inert atmosphere; Sealed tube; |
- 60
-
 [ 579-74-8 ]
[ 579-74-8 ] 
-
 [ 118994-84-6 ]
[ 118994-84-6 ] 
- 3-hydroxy-1-(2-methoxyphenyl)-3-(oxazol-4-yl)propan-1-one [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 68% | To THF (20 ml) at -84 C was added LDA (2.5 ml, 5.5 mmol). The resultant solution of LDA was added dropwise to a solution of o-methoxyacetophenone (0.6 ml, 4.5 mmol). The reaction mixture was stirred at -84 C for 30 min before a solution of <strong>[118994-84-6]oxazole-4-carboxaldehyde</strong> (0.5 g, 5.5 mmol) in a minimal amount of THF was added dropwise at -84 C. The reaction mixture was stirred for 30 min at -84 C, quenched with saturated aqueous NH4Cl (20 ml) and allowed to warm to room temperature. The layers were allowed to separate, and the aqueous layer was extracted with EtOAc (2 × 20 ml). The combined organic layers were dried (MgSO4), filtered and concentrated. The product was recrystallized from CH2Cl2/hexanes yielding 5 as pale yellow crystals (0.751 g, 68%), Mpt: 104-107 C, HRMS: found: 270.0735, calculated for C12H12NO4Na [M + Na]+: 270.0737, 1H NMR (400.13 MHz, DMSO-d6) delta ppm 3.87 (s, 2-OCH3), 5.36 (d, J = 5.4 Hz, OH), 3.26 (dd J = 8.2, 16.2 Hz, Ha-2?), 3.37 (dd J = 4.6, 16.2 Hz, Hb-2?), 5.06 (m, H-3?), 7.89 (s, H-5?), 8.26 (s, H-6?), 7.16 (d, J = 8.6 Hz, H-3), 7.54 (t, J = 7.5 Hz, H-4), 7.03 (t, J = 7.5 Hz, H-5), 7.52 (d, J = 8.6 Hz, H-6), 13C NMR (100.62 MHz, DMSO-d6 delta ppm 199.6 (C-1?), 50.4 (C-2?), 62.6 (C-3?), 143.3 (C-4?), 135.1 (C-5?), 151.5 (C-6?), 128.1 (C-1), 158.0 (C-2), 112.3 (C-3), 133.6 (C-4), 120.9 (C-5), 129.4 (C-6). |
- 61
-
 [ 579-74-8 ]
[ 579-74-8 ] 
-
 [ 256417-11-5 ]
[ 256417-11-5 ] 
- (E)-3-(2,3-difluoro-4-methoxyphenyl)-1-(2-methoxyphenyl)prop-2-en-1-one [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 96% | With potassium hydroxide In methanol; water at 20℃; |
- 62
-
 [ 579-74-8 ]
[ 579-74-8 ] 
-
 [ 3392-97-0 ]
[ 3392-97-0 ] 
- (E)-1-(2-methoxyphenyl)-3-(2,6-dimethoxyphenyl)prop-2-en-1-one [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 84% | General procedure: A 50 mL flask was charged with substituted acetophenone (5 mmol) and a solution of sodium hydroxide (10 mmol) in a 4:1 (v/v) mixture of ethanol/H2O (25 mL), and the resulting mixture was stirred at room temperature for 5 min. A substituted benzaldehyde (5 mmol) was then added to the reaction, and the resulting mixture was stirred at room temperature. The reaction was then monitored byTLC using ethyl acetate/petroleum ether (1:4 or 1:2 v/v) as the solvent system. Upon completion of the reaction, the crude product was filtered off and recrystallized from a mixture of dichloromethane and ethanol or purified by column chromatography over silica gel eluting with a mixture of petroleum ether and ethyl acetate to give the pure product. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 1: 39 %Chromat. 2: 61 %Chromat. | With (x)C4H8O*(x)C18H15P*Ru; hydrogen In tetrahydrofuran at 30℃; for 2.5h; Autoclave; Glovebox; |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 1: 11 %Chromat. 2: 73 %Chromat. 3: 16 %Chromat. | With (x)C4H8O*(x)C28H28P2*Rh; hydrogen In tetrahydrofuran at 30℃; for 5h; Autoclave; Glovebox; |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 1: 20 %Chromat. 2: 50 %Chromat. 3: 26 %Chromat. | With Rh*C4H8O*C18H15P; hydrogen In tetrahydrofuran at 30℃; for 5h; Autoclave; Glovebox; |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 89% | With toluene-4-sulfonic acid In water at 80℃; for 2h; Sealed tube; Green chemistry; | |
| 85% | With ytterbium(III) triflate In acetonitrile at 20℃; for 24h; | 4.2. General procedure for the preparation of compounds 1a-e and 2a-e General procedure: To a solution of naphthalene-1,8-diamine 3 (200.0 mg, 1.3 mmol) in MeCN (6 mL) was added acetophenones (1.3 mmol) or benzaldehydes (1.3 mmol) and a catalytic amount of Yb(OTf)3 (0.1 equiv) at room temperature. The resulting mixture was stirred room temperature for 24 h. The solution was then concentrated in vacuo, and the product was extracted with CH2Cl2/water. The combined organic layer was dried over MgSO4, concentrated in vacuo, and the product was purified by column chromatography to obtain the desired compound. |
| 81% | With squaric acid In water at 80℃; for 2h; Green chemistry; | 2.2 General procedure for the synthesis of 2,3-dihydro-1H-perimidine derivatives (3a-o) General procedure: The mixture of 1, 8-diamino naphthalene 1 (1 mmol), ketones2a-o (1 mmol) and squaric acid (10 mol %) was heated in water (5 mL) at 80 °C for the appropriate time shown inTable 2. The reaction mixture was then cooled to room temperatureand pure products were afforded by filtration of thereaction mixture with multiple washing of water. Most of thesynthesized compounds (3a-o) being well reported were primarilyidentified by melting points.13-17 The synthesizedcompounds were further confirmed by 1H and 13C NMR. |
[2]Wu, Chun-Ku; Liou, Teau-Jiuan; Wei, Hao-Yi; Tsai, Pei-Shan; Yang, Ding-Yah [Tetrahedron, 2014, vol. 70, # 44, p. 8219 - 8225]
[3]Khopkar, Sushil; Shankarling, Ganapati [Journal of Chemical Sciences, 2020, vol. 132, # 1]
- 68
-
 [ 579-74-8 ]
[ 579-74-8 ] 
-
 [ 116308-35-1 ]
[ 116308-35-1 ] 
- (E)-1-(2-methoxyphenyl)-3-(2-(trifluoromethyl)pyridine-3-yl)-prop-2-en-1-one [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 35% | A solution of lithium hydroxide (0.8 mg, 0.03 mmol) and 2'-methoxyacetophenone (26 mg, 0.157 mmol) in absolute methanol (1.5 mL) was stirred at room temperature for 15 min. To the resulting mixture was added a solution of <strong>[116308-35-1]2-(trifluoromethyl)-3-pyridinecarboxaldehyde</strong> (6a, 28 mg, 0.16 mmol) in absolute methanol (15 mL). The reaction was stirred overnight at room temperature (approx. 18 h). The reaction was then concentrated on a rotary evaporator and the resulting oily residue purified by chromatography on silica gel using a gradient of 0-100% ethyl acetate in hexane to provide the desired product (17 mg, 35%) as a light yellow waxy solid. 1H NMR (CDCl3): delta 8.71 (d, J = 4.9 Hz, 1H), 8.12 (d, J = 8.1 Hz, 1H), 7.93 (dd, J = 15.8 Hz, 2.0 Hz, 1H), 7.68 (dd, J = 7.6, 1.8 Hz, 1H), 7.56 (dd, J = 8.0, 4.6 Hz, 1H), 7.55 (d, J = 7.4 Hz, 1H), 7.54 (dt, J = 7.4, 1.9 Hz, 1H), 7.36 (d, J = 15,8 Hz, 1H), 7.09 (t, J = 7.6, 1H), 7.03 (d, J = 8.2 Hz, 1H), 3.93 (s, 3H). 13C NMR (CDCl3) delta 190.8, 157.3, 148.2, 147.1, 145.2, 136.5, 135.1, 134.4, 132.6, 131.6, 129.7, 129.6, 127.2, 125.5, 120.0, 110.6, 54.7. HRMS (FAB): calcd C16H12F3NO2 + H = 308.0898, found 308.0889. |
- 69
-
 [ 579-74-8 ]
[ 579-74-8 ] 
-
 [ 1083197-78-7 ]
[ 1083197-78-7 ] 
- (E)-1-(2-methoxyphenyl)-3-(4-(trifluoromethyl)-pyridin-3-yl)prop-2-en-1-one [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 54% | General procedure: A solution of lithium hydroxide (0.8 mg, 0.03 mmol) and 2'-methoxyacetophenone (26 mg, 0.157 mmol) in absolute methanol (1.5 mL) was stirred at room temperature for 15 min. To the resulting mixture was added a solution of 2-(trifluoromethyl)-3-pyridinecarboxaldehyde (6a, 28 mg, 0.16 mmol) in absolute methanol (15 mL). The reaction was stirred overnight at room temperature (approx. 18 h). The reaction was then concentrated on a rotary evaporator and the resulting oily residue purified by chromatography on silica gel using a gradient of 0-100% ethyl acetate in hexane to provide the desired product (17 mg, 35%) as a light yellow waxy solid. Prepared using the general method from lithium hydroxide (3 mg, 0.125 mmol)), 2'-methoxyacetophenone (94 mg, 0.625 mmol)) and <strong>[1083197-78-7]4-trifluoromethyl-3-pyridinecarboxaldehyde</strong> (6c, 111 mg, 0.635 mmol) in absolute methanol (final reaction volume = 4 mL). The reaction mixture was purified by chromatography on silica gel (gradient of 0-100% ethyl acetate in hexane) to give the desired product as a yellow oil (104 mg, 54%). 1H NMR (CDCl3) delta 8.99 (s, 1H), 8.70 (d, J = 5.5 Hz, 1H), 7.81 (dd, J = 15.8, 2.0 Hz, 1H), 7.63 (dd, J = 7.6, 1.8 Hz, 1H), 7.51 (d, J = 5.5 Hz, 1H), 7.46 (dt, J = 8.4, 1.8 Hz, 1H), 7.42 (d, J = 15.8 Hz, 1H), 7.00 (t, J = 7.6 Hz, 1H), 6.94 (d, J = 8.4 Hz, 1H), 3.94 (s, 3H). 13C NMR (CDCl3) delta 191.2, 158.5, 151.4, 150.7, 149.4, 136.1, 134.2, 133.9, 132.4, 130.9, 129.1, 128.2, 121.7, 119.5, 111.6, 55.6. HRMS (FAB): calcd C16H12F3NO2 + H = 308.0898, found 308.0900. |
- 70
-
 [ 579-74-8 ]
[ 579-74-8 ] 
-
 [ 131747-62-1 ]
[ 131747-62-1 ] 
- (E)-1-(2-methoxyphenyl)-3-(3-(trifluoromethyl)-pyridin-2-yl)prop-2-en-1-one [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 54% | General procedure: A solution of lithium hydroxide (0.8 mg, 0.03 mmol) and 2'-methoxyacetophenone (26 mg, 0.157 mmol) in absolute methanol (1.5 mL) was stirred at room temperature for 15 min. To the resulting mixture was added a solution of 2-(trifluoromethyl)-3-pyridinecarboxaldehyde (6a, 28 mg, 0.16 mmol) in absolute methanol (15 mL). The reaction was stirred overnight at room temperature (approx. 18 h). The reaction was then concentrated on a rotary evaporator and the resulting oily residue purified by chromatography on silica gel using a gradient of 0-100% ethyl acetate in hexane to provide the desired product (17 mg, 35%) as a light yellow waxy solid. Prepared using the general method from lithium hydroxide (1.2 mg, 0.01 mmol), 2'-methoxyacetophenone (72 mg, 0.48 mmol) and <strong>[131747-62-1]3-trifluoromethyl-2-pyridinecarboxaldehyde</strong> (6d, 85 mg, 0.49 mmol) in absolute methanol (final reaction volume = 4 mL). The reaction mixture was purified by chromatography on silica gel (gradient of 0-100% ethyl acetate in hexane) to give the desired product as a yellow oil that hardened upon standing (77 mg, 54%). 1H NMR (CDCl3) delta 8.82 (d, J = 4.3 Hz 1H), 8.12 (d, J = 15.0 Hz, 1H), 8.01 (dd, J = 8.0, 1.6 Hz, 1H), 7.93 (dd, J = 15.0, 1.9 Hz, 1H), 7.70 (dd, J = 7.6, 1.8 Hz, 1H), 7.51 (t, J = 7.4 Hz, 1H), 7.41 (dd, J = 7.5, 4.6 Hz, 1H), 7.07 (t, J = 7.5 Hz, 1H), 7.02 (d, J = 8.1 Hz, 1H), 3.93 (s, 3H). 13C NMR (CDCl3) delta 192.4, 158.6, 152.3, 151.6, 135.4, 134.1, 133.9, 133.5, 130.6, 128.8, 125.8, 125.1, 123.2, 120.8, 111.6, 55.7. HRMS (FAB): calcd C16H12F3NO2 + H = 308.0898, found 308.0897. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 71% | With sodium hydroxide In ethanol at 0 - 20℃; for 48h; | General method for preparation of chalcone and analogues (1a-21a, 1b-2b, 1c-3c, 1d-4d) General procedure: The corresponding aldehyde (1.0 equiv) and ketone (1.0 equiv) were dissolved in ethanol at 0 °C and 10% NaOH (1.0 equiv.) solution was slowly added. After that, the mixture was allowed to warm up to room temperature and stirred for 48 h. The resulting mixture was evaporated to dryness and the residue was purified by chromatography on silica gel eluted with petroleum ether-ethyl acetate to afford the final compound. The characterization data of the synthesized compounds are in the Supporting information. |
| With sodium hydroxide In ethanol; water at 60℃; for 16h; | ||
| With sodium hydroxide In ethanol |
[2]Zhang, Hongyu; Cheng, Xiao; Wang, Kai; Huang, Shuo; Zhang, Houyu; Wang, Yue [Angewandte Chemie - International Edition, 2015, vol. 54, # 29, p. 8369 - 8373][Angew. Chem., 2015, vol. 127, # 29, p. 8489 - 8493,5]
[3]Zhou, Bo; Jiang, Peixin; Lu, Junxuan; Xing, Chengguo [Archiv der Pharmazie, 2016, p. 539 - 552]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 67% | With iodine; 4-aminobenzene sulfonic acid In dimethyl sulfoxide at 100℃; for 5h; | Typical Procedure for the Preparation of 2,5-Diphenyloxazole General procedure: A test tube was charged with 1a (0.32 mmol), 2a (0.38 mmol), I2 (2.0 equiv.) and PABS (0.5equiv.). Then 2 mL DMSO was added to the reaction system. The reaction was stirred at 100 oCfor 5 h. After cooling to room temperature, the solvent diluted with 10 mL ethyl acetate andwashed with 5 mL brine and dried over anhydrous Na2SO4. After the solvent was evaporated invacuo, the residues were purified by column chromatography, eluting with petroleum ether/EtOAc to afford pure 3aa. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 94% | With hantzsch ester In 2,2,2-trifluoroethanol at 20℃; for 4h; UV-irradiation; Sealed tube; Green chemistry; | |
| 88% | With diethyl ether In lithium hydroxide monohydrate at 20℃; for 1.5h; Schlenk technique; Irradiation; | 3. General procedure for the reaction of reductive dehalogenation General procedure: To a 10 mL schlenk tube, 1 (0.2 mmol), H2O (1.6 mmol) and Et2O (4 mL) were added in sequence. The reaction mixture was stirred and irradiated by 40 W Kessil purple LED (390 nm) at room temperature . After the reaction was completed, the reaction mixture was purified by flash column chromatography using PE/EtOAc as the eluent to give the desired product. |
| 87% | With hantzsch ester; sulforhodamine 101; N-ethyl-N,N-diisopropylamine; Eosin In lithium hydroxide monohydrate at 20℃; for 8h; Irradiation; Inert atmosphere; |
| 86% | With lithium hydroxide monohydrate; triphenylantimony at 120℃; for 0.166667h; Microwave irradiation; | |
| 81% | With hantzsch ester; N-ethyl-N,N-diisopropylamine In lithium hydroxide monohydrate at 20℃; for 8h; Irradiation; Inert atmosphere; | |
| Multi-step reaction with 2 steps 1: N,N,N-tributyl-1-butanaminium iodide; potassium carbonate / dichloromethane; lithium hydroxide monohydrate / 20 °C 2: bis[(2,2,2-trifluoroacetyl)oxy]palladium; 5,5'-bis[di(3,5-di-t-butyl-4-methoxyphenyl)phosphino]-4,4'-bi-1,3-benzodioxole; hydrogen / propan-2-one / 24 h / 20 °C / 22502.3 Torr / Glovebox; Autoclave | ||
| Multi-step reaction with 2 steps 1: potassium carbonate / propan-2-one / Reflux 2: 4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl-4',4',5',5'-tetramethyl-1,3,2-dioxaborolane; sodium tertiary butoxide; 2,6-bis[1-(2,6-dimethylphenylimino)ethyl]pyridine cobalt(II)dichloride / tetrahydrofuran; methanol / 3 h / 65 °C / Schlenk technique; Inert atmosphere |

[2]Chen, Jiajia; Chen, Jianhui; Luo, Yanshu; Xia, Yuanzhi; Zhang, Jinrong [Tetrahedron Letters, 2022]
[3]Jiang, Man; Li, Xing-Long; Liu, Hui; Wang, Rong-Zhou; Wang, Ying; Xing, Ling-Bao; Yu, Shengsheng; Zhang, Ming-Hui [Dyes and Pigments, 2022, vol. 197]
[4]Murata, Yuki; Sugawara, Yoshiyuki; Matsumura, Mio; Kakusawa, Naoki; Yasuike, Shuji [Chemical and Pharmaceutical Bulletin, 2017, vol. 65, # 11, p. 1081 - 1084]
[5]Li, Xinglong; Wang, Ying; Song, Ao; Zhang, Minghui; Chen, Mengning; Jiang, Man; Yu, Shengsheng; Wang, Rongzhou; Xing, Lingbao [Chinese Journal of Chemistry, 2021, vol. 39, # 10, p. 2725 - 2730]
[6]Chen, Jianzhong; Zhang, Zhenfeng; Liu, Delong; Zhang, Wanbin [Angewandte Chemie - International Edition, 2016, vol. 55, # 29, p. 8444 - 8447][Angew. Chem., 2016, vol. 128, # 29, p. 8584 - 8587]
[7]Gao, Kecheng; Xu, Man; Cai, Cheng; Ding, Yanghao; Chen, Jianhui; Liu, Bosheng; Xia, Yuanzhi [Organic Letters, 2020, vol. 22, # 15, p. 6055 - 6060]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 59.1% | With sodium hydroxide In ethanol; water at 0 - 20℃; for 12h; | 1. Synthesis of chalcones using NaOH: General Procedure General procedure: A mixture of theacetophenone (1mmol) and the aldehyde (1 mmol) was dissolved in EtOH (10 mL). Anaqueous solution of NaOH (40%, 1 mL) was added to this solution at 0-5oC.The reaction mixture was allowed to attain room temperature and then stirringwas continued for 12h. TLC examination (30% ethyl acetate in hexane) indicatedthe completion of the reaction. The reaction mixture was then poured overcrushed ice and acidified to pH ~2 with 1N. HCl. The light yellow solid precipitatedwas filtered, washed with water, and dried. The product obtained was either purifiedon column chromatography (Si gel with 10% ethyl acetate in hexane) orrecrystallized from EtOH. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 57% | With ferrocene In benzene-d6 at 25℃; Glovebox; Inert atmosphere; Schlenk technique; Sealed tube; |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 89% | With sodium hydroxide In ethanol; water at 20℃; for 6h; | 2.3. Synthesis of (E)-1-(2-methoxyphenyl)-3-(pyren-1-yl)prop-2-en-1-one(PC-OCH3) The corresponding pyrene chalcones were synthesized by Claisen-Schmidt condensation reaction from the respective aldehydes and ketone[33-38]. 2-Methoxyacetophenone (1 mol) and pyrenealdehyde(1 mol) were dissolved in absolute ethanol and stirred for about 10 minto get homogeneous solution. NaOH (1 mol) was dissolved in aminimum amount of H2O and added slowly to the solution for over20 min. The reaction mixture was slowly turned to yellow color precipitate,then stirred for 6 h at RT. The entire reaction was monitored byTLC technique. At the end of the reaction, the resultant mixture wasquenched with ice water, and set apart for 4 h to form a yellow coloredprecipitate which was filtered, dried for 12 h and subjected to columnchromatography to get the product. PC-OCH3, Yield: 89%: FT-IR (KBr,cm-1):3041 (vAr-C-H),2971 (vAli-C-H), 1599 (vC]O), 1589(v-C]C-). GC-MS calculated for [C26H18O2] 362.4397; found362.1308 M+. |
| With sodium hydroxide In ethanol at 60℃; for 16h; | 4.2 General synthesis of chalcone General procedure: In a 100 mL round bottom flask, acetophenone or acetonaphthone derivatives (1 equiv) and the 1-pyrenecarboxaldehyde (1 equiv) were solubilized in ethanol. Subsequently, sodium hydroxide (2.5 equiv) in water was added to the solution. The solution was stirred for 16 h at 60°C. After cooling down, the precipitate was filtered on a glass filter. The solid obtained was the recrystallized in a mixture of dichloromethane and ethanol two times to yield the pure ligands. |
- 78
-
 [ 579-74-8 ]
[ 579-74-8 ] 
-
 [ 4460-86-0 ]
[ 4460-86-0 ] 
- (E)-1-(2-methoxyphenyl)-3-(2,4,5-trimethoxyphenyl)prop-2-en-1-one [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 85% | With sodium hydroxide In ethanol at 20℃; for 3h; | Synthesis of chalcones (3a-g): General procedure: A mixture of 2,4,5-trimethoxybenzaldehyde (1, 5 mmol), substituted acetophenone (2a-g, 5 mmol) and sodium hydroxide (5 mmol) in 95 % ethyl alcohol (25 mL) was stirred at room temperature for 3 h. The progress of the reaction was monitored by TLC. After the completion of the reaction, the mixture was poured in to ice cold water and kept in the refrigerator for overnight. The solid formed was filtered and washed with cold hydrochloric acid (5 %). Crude products obtained were crystallized from methyl alcohol to obtain pure chalcones (3a-g). |
- 81
-
 [ 579-74-8 ]
[ 579-74-8 ] 
-
 [ 132838-25-6 ]
[ 132838-25-6 ] 
- (E)-3-(3-(2-fluoroethoxy)phenyl)-1-(2-methoxyphenyl)prop-2-en-1-one [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 86% | With potassium hydroxide In ethanol; water at 20℃; for 0.5h; | 3.5. General Procedure for the Preparation of Fluoroethoxychalcones General procedure: An aqueous solution of KOH (1.2 eq.) was added at room temperature to a solution of the appropriate acetophenone (1.0 eq.), and benzaldehyde (1.2 eq.) in ethanol (10 mL). The reaction mixture was then stirred for 30 min. After completion of reaction, the reaction was judged by TLC. The reaction mixture was filtered through a Buckner funnel under vacuum. The solid was washed several times with a 1:1 ethanol-water mixture. The solid was finally dried under vacuum. The crude product was further purified by column chromatography or recrystallization. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 71% | With carbon tetrabromide; caesium carbonate; In acetonitrile; at 80℃; for 6h;Inert atmosphere; | In an oven dried 100 mL round bottomed flask 1-nitrosonaphthalene-2-ol 4a or 2-nitrosophenol 4b (1.0 mmol), acetophenones 5a-k (1.0 mmol), CBr4 (0.5 mmol, 165 mg),Cs2CO3 (2.1 mmol, 682 mg) and 10 mL CH3CN were added successively and the reactionmixture was heated for 6h at 80 C under nitrogen atmosphere. After completion of thereaction (progress was monitored by TLC; SiO2, Hexane/EtOAc = 9:1), the reaction mixturewas allowed to cool at room temperature and then solvent was removed under vacuum. Thecrude product was purified by using column chromatography over silica gel using hexane /ethyl acetate = 9:1 as an eluent to obtain the desired products (3a-f, 3h-3i, 3k, 3m, 3w, 3x) inhigh yields.. |
- 83
-
 [ 5834-16-2 ]
[ 5834-16-2 ] 
-
 [ 579-74-8 ]
[ 579-74-8 ] 
- 1-(2-methoxyphenyl)-3-(3-methylthiophen-2-yl)prop-2-en-1-one [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 86% | With potassium hydroxide; In methanol; at 20℃; | General procedure: To a solution mixture of 3-methylthiophene-2-carbaldehyde, 1 (5 mmol) and acetophenones, 2(a-h) (5 mmol) in methyl alcohol, potassium hydroxide solution (40%, 2 mL) was added. Then the solution mixture was stirred at room temperature for 2-3 h. The progress of the reaction was monitored by TLC. After the completion, the reaction mixture was cooled to room temperature and poured into ice cold water. The solids separated were filtered, washed successively with cold hydrochloric acid (5%) and cold water. The crude solids were recrystallized from methyl alcohol to obtain the compounds 3(a-h). |
| With potassium hydroxide; In methanol; at 20℃; | General procedure: To a solution mixture of 3-methylthiophene-2-carbaldehyde, 1 (10mmole) and acetophenones, 2(a-h) (10 mmole) in methyl alcohol,potassium hydroxide solution (40%, 2 mL) was added. Then the solutionmixture was stirred at room temperature for 2-3 h. The progress ofthe reaction was monitored by TLC. After the completion, the mixturewas cooled to room temperature and poured into ice cold water. Thesolids separated were filtered, washed successively with cold hydrochloricacid (5%) and cold water. The crude solids were recrystallizedfrom methyl alcohol to obtain compounds 3(a-h) [26]. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 78% | With sulfonic chloride acid at 0 - 20℃; | 1.2 2) Synthesis of Compound 3 ClSO3H (30 mL, 0.46 mol, 24.6 eq) was added to a vial, cooled to 0 ° C, and then compound 2 (2.8 g, 18.7 mmol, 1.0 eq) was added.Stir for twenty minutes, remove the ice water bath, and react at room temperature for 16 hours.The TLC dot plate shows complete reaction and new points are generated. The reaction solution was poured into ice water with stirring and quenched, and extracted with dichloromethane.Dry over anhydrous sodium sulfate and spin dry the organic phase.After passing through a column (PE/EA = 20: 1-10: 1), Compound 3 (3.6 g, yield: 78%) was obtained as white solid. |
| 78% | With sulfonic chloride acid at 0 - 25℃; for 16.3333h; | |
| 78% | With sulfonic chloride acid at 0 - 25℃; for 16.3333h; | 4.1.1.3. 3-Acetyl-4-methoxybenzenesulfonyl chloride (3). Compound 2 (2.8g, 18.7mmol) was added to sulfonyl chloride (30mL, 0.46mmol) at 0°C, stirred at 0°C for 20min. Then the solution was warmed up to 25°C and stirred for 16h. Ice water was poured to the mixture, extracted the mixture with 330mL DCM. The combined organic layers were separated. The organic layer was dried by Na2SO4, and the dried solution was filtered. The filtrate was concentrated and the residue obtained was purified by flash-column chromatography (eluting with 5-10% ethyl acetate-hexanes) to afford 3 as white solid (3.6g, yield: 78%). ESI-MS: m/z=248.1 [M+ H]+. |
| 78% | With sulfonic chloride acid at 0 - 25℃; for 16.3333h; | 4.1.1.3. 3-Acetyl-4-methoxybenzenesulfonyl chloride (3). Compound 2 (2.8g, 18.7mmol) was added to sulfonyl chloride (30mL, 0.46mmol) at 0°C, stirred at 0°C for 20min. Then the solution was warmed up to 25°C and stirred for 16h. Ice water was poured to the mixture, extracted the mixture with 330mL DCM. The combined organic layers were separated. The organic layer was dried by Na2SO4, and the dried solution was filtered. The filtrate was concentrated and the residue obtained was purified by flash-column chromatography (eluting with 5-10% ethyl acetate-hexanes) to afford 3 as white solid (3.6g, yield: 78%). ESI-MS: m/z=248.1 [M+ H]+. |
| 200 mg | With sulfonic chloride acid In dichloromethane at 0 - 20℃; for 12h; | 24 3-acetyl-4-methoxybenzenesulfonyl chloride Add 3mL of chlorosulfonic acid into a 50mL reaction flask, cool to 0 in an ice bath, slowly add 1mL of 1.87mmol 2'-methoxyacetophenone in methylene chloride solution, stir for 20 minutes, remove the ice bath and return to room temperature. , Reaction for 12h. Under ice bath conditions, the reaction was quenched with 10 mL ice water, a large amount of hydrochloric acid gas was generated, and a white solid gradually precipitated. After standing for crystallization, filtration with suction, and drying, 200 mg of white solid was obtained. Directly cast to the next step without purification. |

[2]Chen, Lei; Su, Mei; Jin, Qiu; Wang, Wei; Wang, Chun-Gu; Assani, Israa; Wang, Mu-Xuan; Zhao, Shi-Feng; Lv, Shen-Min; Wang, Jia-Wei; Sun, Bo; Li, Yan; Liao, Zhi-Xin [Journal of Medicinal Chemistry, 2021, vol. 64, # 21, p. 16106 - 16131]
[3]Assani, Israa; Chen, Lei; Huang, Ri-Zhen; Jin, Qiu; Kang, Yang-yang; Li, Yan; Liao, Zhi-Xin; Lv, Shen-Min; Su, Mei; Sun, Bo; Wang, Chun-Gu; Wang, De-Zhong; Wang, Jia-Wei; Wang, Mu-Xuan; Wu, Xian-Zhi; Zhao, Shi-Feng [European Journal of Medicinal Chemistry, 2022, vol. 229]
[4]Assani, Israa; Chen, Lei; Huang, Ri-Zhen; Jin, Qiu; Kang, Yang-yang; Li, Yan; Liao, Zhi-Xin; Lv, Shen-Min; Su, Mei; Sun, Bo; Wang, Chun-Gu; Wang, De-Zhong; Wang, Jia-Wei; Wang, Mu-Xuan; Wu, Xian-Zhi; Zhao, Shi-Feng [European Journal of Medicinal Chemistry, 2022, vol. 229]
[5]Current Patent Assignee: CHINA PHARMACEUTICAL UNIVERSITY - CN112876482, 2021, A Location in patent: Paragraph 0183; 0193-0194
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 84% | With tetrakis(triphenylphosphine) palladium(0); potassium acetate; silver fluoride; triphenylphosphine In hexane at 140℃; for 20h; Sealed tube; Inert atmosphere; | 4.1-4.3 In this embodiment, a preparation method of a benzoyl para-difluoroalkylated derivative is shown as follows: 2-methoxyacetophenone is used as a raw material, and the reaction formula is as follows: (1) 2-methoxyacetophenone 0.0300 g (0.2 mmol) and tetrakistriphenylphosphine palladium 0.0116 g were added to the reaction tube.(0.01 mmol), potassium acetate 0.0786 g (0.8 mmol), triphenylphosphine 0.0157 g (0.06 mmol), silver fluoride 0.0077 g (0.06 mmol), ethyl bromodifluoroacetate 0.0203 g (1.00 mmol) and 0.25 mL Hexane, protected by argon, and reacted at 140 ° C for 20 hours;(2) TLC tracks the reaction until it is completely over;(3) The crude product obtained after the completion of the reaction was separated by column chromatography ( petroleum ether: ethyl acetate = 15:1) to give the objective product (yield 84%). |
| 84% | With tetrakis(triphenylphosphine) palladium(0); potassium acetate; silver fluoride; triphenylphosphine In hexane at 140℃; for 20h; Inert atmosphere; Sealed tube; | |
| 55% | With (bis(tricyclohexyl)phosphine)palladium(II) dichloride; iron(II) acetate; potassium carbonate In 1,2-dichloro-ethane at 110℃; for 48h; Schlenk technique; Inert atmosphere; Sealed tube; |
[2]Tu, Guangliang; Yuan, Chunchen; Li, Yuting; Zhang, Jingyu; Zhao, Yingsheng [Angewandte Chemie - International Edition, 2018, vol. 57, # 47, p. 15597 - 15601][Angew. Chem., 2018, vol. 130, # 47, p. 15823 - 15827,5]
[3]Mao, Yang-Jie; Wang, Bing-Xin; Wu, Qiu-Zi; Zhou, Kun; Lou, Shao-Jie; Xu, Dan-Qian [Chemical Communications, 2019, vol. 55, # 14, p. 2019 - 2022]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 81% | With sodium hydroxide In ethanol for 4h; Reflux; Inert atmosphere; | 3.1. Synthesis of ligands General procedure: A suspension of 2-aminonicotinaldehyde (500 mg; 4.09 mmol), substituted acetophenone (4.09 mmol), and NaOH (200 mg; 5 mmol) in EtOH (40 mL) was refluxed under argon for 4 h. The solvent was removed under vacuum. The residue was extracted from a mixture of CH2Cl2/water and the organic layer was dried over magnesium sulfate; the product was purified by column chromatography on silica gel eluting with CH2Cl2:EtOAc (6:1). After the solvent was removed, the desired product was obtained as a white solid. (more information about ligands characterization see supplementary material). |
| 81% | With sodium hydroxide In ethanol for 4h; Reflux; Inert atmosphere; | 2.1.1. Synthesis of the ligands. General procedure: A suspension of 2-aminonicotinaldehyde (500 mg, 4.09 mmol), 2- or 4-methoxy-substituted acetophenone (4.09 mmol) and NaOH (200 mg,5 mmol) in EtOH (40 ml) was refluxed under argon for 4 h, as depicted in Scheme 3. After this period, the solvent was removed under reduced pressure. The residue was extracted with CH2Cl2 from an aqueous mixture and the organic layer was dried over magnesium sulfate. The product was purifiedby column chromatography on silica gel, eluting with CH2Cl2-EtOAc (6:1 v/v). After the solvent had been removed, the desired product was obtained as a white solid. |
[2]Guajardo, Juana; Ibañez, AndrÃs; Guerchais, Veronique; Vega, AndrÃs; Moya, Sergio; Aguirre, Pedro [Acta Crystallographica Section C: Structural Chemistry, 2018, vol. 74, # 11, p. 1547 - 1552]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 2.49 g (74%) | With stannous chloride; triethylamine; N-ethyl-N,N-diisopropylamine;TiCl4; In N,N-dimethyl-formamide; | EXAMPLE 146 2-Methoxy-1-[2-methyl-4-piperidylpyrrolo[4,5-d]pyrimidin-6-yl]benzene Hydrochloride Monohydrate. Using the method described in Example 30 by employing 2-methoxy-1-(1-pyrrolidinylvinyl)furan (freshly prepared before use from 2'-methoxy acetophenone (Aldrich Chemical Company), pyrrolidine and TiCl4 (2.14 g, 10.5 mmol), <strong>[13162-43-1]2-methyl-4,6-dichloro-5-nitropyrimidine</strong> (Example 76(b)) (2.25 g, 10.5 mmol), N,N-diisopropylethylamine (1.8 mL, 10.5 mmol), piperidine (1.7 mL, 16.8 mmol), NEt3 (1.8 mL) and SnCl2 (32 mL of a 2 M soln in DMF). In this example the SnCl2 solution was added to the reaction mixture at 140 C. The mixture was stirred at 140 C. for an additional 16 h then the heating was discontinued and the mixture was allowed to cool to room temperature. The residue was purified by flash chromatography on silica gel with 95:5 CHCl3/MeOH as eluant to give 2.49 g (74%) of 2-methoxy-1-[2-methyl-4-piperidylpyrrolo[4,5-d]pyrimidin-6-yl]benzene as a beige colored foam. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 31.8% | With sodium hydride; In tetrahydrofuran; mineral oil;Reflux; | General procedure: To a suspension of sodium hydride (60% in mineral oil, 5 eq) in dryTHF was added solution of substituted methyl benzoate (2 eq) in dryTHF. After mixture refluxed, substituted acetophenone (1 eq) in dryTHF was added dropwise. The mixture was refluxed overnight, then quenched with 1 N HCl. The mixture was extracted with ethyl acetatetwice, and the combined organic phase was washed with brine solution,dried with anhydrous Na2SO4, filtrated, and concentrated under reduced pressure. The residue was purified on a silica gel column. |
- 96
-
 [ 579-74-8 ]
[ 579-74-8 ] 
-
 [ 2150-37-0 ]
[ 2150-37-0 ] 
- 1-(2-methoxyphenyl)-3-(3,5-dimethoxyphenyl)propane-1,3-dione [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 80% | With sodium hydride In tetrahydrofuran; mineral oil at 70℃; for 8h; Inert atmosphere; |
- 102
-
 [ 579-74-8 ]
[ 579-74-8 ] 
-
 [ 5147-80-8 ]
[ 5147-80-8 ] 
- (2Z,4E)‑2‑cyano‑3,5‑bis(methylsulfanyl)‑5‑(2‑methoxyphenyl)penta‑2,4‑dienoic acid amide [ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 34% | With sodium hydroxide; In dimethyl sulfoxide; at 20℃; | General procedure: To a solution of 3,3-bis(methylsulfanyl)methylenemalononitrile 1 (1.70 g, 10 mmol) in 20 mL of DMSO, keton 2a - j (10 mmol) and powdered sodium hydroxide (0.8 20 mmol) were added, and the mixture was magnetically stirred for 4 - 5 h at room temperature. After addition of 300 mL of water to the mixture, the solution was stirred for 12 h at room temperature. The formed precipitate was collected by filtra- tion and washed several times with water. After drying under air, the formed product was recrystallized using methanol or ethanol to obtain the pure products. |
| Yield | Reaction Conditions | Operation in experiment |
|---|---|---|
| 74% | With silver(I) acetate; palladium diacetate; C20H17F6N3O4; N-acetylglycine at 80℃; for 24h; |
Tags: 579-74-8 synthesis path| 579-74-8 SDS| 579-74-8 COA| 579-74-8 purity| 579-74-8 application| 579-74-8 NMR| 579-74-8 COA| 579-74-8 structure
Related Functional Groups of
[ 579-74-8 ]
Aryls
-
[ 829-20-9 ]
1-(2,4-Dimethoxyphenyl)ethanone
Similarity: 0.98
-
[ 43113-94-6 ]
1-(3-Methoxy-5-methylphenyl)ethanone
Similarity: 0.95
-
[ 13185-18-7 ]
3,4-Dihydro-8-methoxynaphthalen-1(2H)-one
Similarity: 0.93

-
[ 131-57-7 ]
(2-Hydroxy-4-methoxyphenyl)(phenyl)methanone
Similarity: 0.93
Ethers

-
[ 829-20-9 ]
1-(2,4-Dimethoxyphenyl)ethanone
Similarity: 0.98

-
[ 43113-94-6 ]
1-(3-Methoxy-5-methylphenyl)ethanone
Similarity: 0.95

-
[ 13185-18-7 ]
3,4-Dihydro-8-methoxynaphthalen-1(2H)-one
Similarity: 0.93

-
[ 131-57-7 ]
(2-Hydroxy-4-methoxyphenyl)(phenyl)methanone
Similarity: 0.93
Ketones

-
[ 829-20-9 ]
1-(2,4-Dimethoxyphenyl)ethanone
Similarity: 0.98

-
[ 43113-94-6 ]
1-(3-Methoxy-5-methylphenyl)ethanone
Similarity: 0.95

-
[ 13185-18-7 ]
3,4-Dihydro-8-methoxynaphthalen-1(2H)-one
Similarity: 0.93

-
[ 131-57-7 ]
(2-Hydroxy-4-methoxyphenyl)(phenyl)methanone
Similarity: 0.93
Precautionary Statements-General | |
| Code | Phrase |
| P101 | If medical advice is needed,have product container or label at hand. |
| P102 | Keep out of reach of children. |
| P103 | Read label before use |
Prevention | |
| Code | Phrase |
| P201 | Obtain special instructions before use. |
| P202 | Do not handle until all safety precautions have been read and understood. |
| P210 | Keep away from heat/sparks/open flames/hot surfaces. - No smoking. |
| P211 | Do not spray on an open flame or other ignition source. |
| P220 | Keep/Store away from clothing/combustible materials. |
| P221 | Take any precaution to avoid mixing with combustibles |
| P222 | Do not allow contact with air. |
| P223 | Keep away from any possible contact with water, because of violent reaction and possible flash fire. |
| P230 | Keep wetted |
| P231 | Handle under inert gas. |
| P232 | Protect from moisture. |
| P233 | Keep container tightly closed. |
| P234 | Keep only in original container. |
| P235 | Keep cool |
| P240 | Ground/bond container and receiving equipment. |
| P241 | Use explosion-proof electrical/ventilating/lighting/equipment. |
| P242 | Use only non-sparking tools. |
| P243 | Take precautionary measures against static discharge. |
| P244 | Keep reduction valves free from grease and oil. |
| P250 | Do not subject to grinding/shock/friction. |
| P251 | Pressurized container: Do not pierce or burn, even after use. |
| P260 | Do not breathe dust/fume/gas/mist/vapours/spray. |
| P261 | Avoid breathing dust/fume/gas/mist/vapours/spray. |
| P262 | Do not get in eyes, on skin, or on clothing. |
| P263 | Avoid contact during pregnancy/while nursing. |
| P264 | Wash hands thoroughly after handling. |
| P265 | Wash skin thouroughly after handling. |
| P270 | Do not eat, drink or smoke when using this product. |
| P271 | Use only outdoors or in a well-ventilated area. |
| P272 | Contaminated work clothing should not be allowed out of the workplace. |
| P273 | Avoid release to the environment. |
| P280 | Wear protective gloves/protective clothing/eye protection/face protection. |
| P281 | Use personal protective equipment as required. |
| P282 | Wear cold insulating gloves/face shield/eye protection. |
| P283 | Wear fire/flame resistant/retardant clothing. |
| P284 | Wear respiratory protection. |
| P285 | In case of inadequate ventilation wear respiratory protection. |
| P231 + P232 | Handle under inert gas. Protect from moisture. |
| P235 + P410 | Keep cool. Protect from sunlight. |
Response | |
| Code | Phrase |
| P301 | IF SWALLOWED: |
| P304 | IF INHALED: |
| P305 | IF IN EYES: |
| P306 | IF ON CLOTHING: |
| P307 | IF exposed: |
| P308 | IF exposed or concerned: |
| P309 | IF exposed or if you feel unwell: |
| P310 | Immediately call a POISON CENTER or doctor/physician. |
| P311 | Call a POISON CENTER or doctor/physician. |
| P312 | Call a POISON CENTER or doctor/physician if you feel unwell. |
| P313 | Get medical advice/attention. |
| P314 | Get medical advice/attention if you feel unwell. |
| P315 | Get immediate medical advice/attention. |
| P320 | |
| P302 + P352 | IF ON SKIN: wash with plenty of soap and water. |
| P321 | |
| P322 | |
| P330 | Rinse mouth. |
| P331 | Do NOT induce vomiting. |
| P332 | IF SKIN irritation occurs: |
| P333 | If skin irritation or rash occurs: |
| P334 | Immerse in cool water/wrap n wet bandages. |
| P335 | Brush off loose particles from skin. |
| P336 | Thaw frosted parts with lukewarm water. Do not rub affected area. |
| P337 | If eye irritation persists: |
| P338 | Remove contact lenses, if present and easy to do. Continue rinsing. |
| P340 | Remove victim to fresh air and keep at rest in a position comfortable for breathing. |
| P341 | If breathing is difficult, remove victim to fresh air and keep at rest in a position comfortable for breathing. |
| P342 | If experiencing respiratory symptoms: |
| P350 | Gently wash with plenty of soap and water. |
| P351 | Rinse cautiously with water for several minutes. |
| P352 | Wash with plenty of soap and water. |
| P353 | Rinse skin with water/shower. |
| P360 | Rinse immediately contaminated clothing and skin with plenty of water before removing clothes. |
| P361 | Remove/Take off immediately all contaminated clothing. |
| P362 | Take off contaminated clothing and wash before reuse. |
| P363 | Wash contaminated clothing before reuse. |
| P370 | In case of fire: |
| P371 | In case of major fire and large quantities: |
| P372 | Explosion risk in case of fire. |
| P373 | DO NOT fight fire when fire reaches explosives. |
| P374 | Fight fire with normal precautions from a reasonable distance. |
| P376 | Stop leak if safe to do so. Oxidising gases (section 2.4) 1 |
| P377 | Leaking gas fire: Do not extinguish, unless leak can be stopped safely. |
| P378 | |
| P380 | Evacuate area. |
| P381 | Eliminate all ignition sources if safe to do so. |
| P390 | Absorb spillage to prevent material damage. |
| P391 | Collect spillage. Hazardous to the aquatic environment |
| P301 + P310 | IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
| P301 + P312 | IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
| P301 + P330 + P331 | IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. |
| P302 + P334 | IF ON SKIN: Immerse in cool water/wrap in wet bandages. |
| P302 + P350 | IF ON SKIN: Gently wash with plenty of soap and water. |
| P303 + P361 + P353 | IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
| P304 + P312 | IF INHALED: Call a POISON CENTER or doctor/physician if you feel unwell. |
| P304 + P340 | IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
| P304 + P341 | IF INHALED: If breathing is difficult, remove victim to fresh air and keep at rest in a position comfortable for breathing. |
| P305 + P351 + P338 | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. |
| P306 + P360 | IF ON CLOTHING: Rinse Immediately contaminated CLOTHING and SKIN with plenty of water before removing clothes. |
| P307 + P311 | IF exposed: call a POISON CENTER or doctor/physician. |
| P308 + P313 | IF exposed or concerned: Get medical advice/attention. |
| P309 + P311 | IF exposed or if you feel unwell: call a POISON CENTER or doctor/physician. |
| P332 + P313 | IF SKIN irritation occurs: Get medical advice/attention. |
| P333 + P313 | IF SKIN irritation or rash occurs: Get medical advice/attention. |
| P335 + P334 | Brush off loose particles from skin. Immerse in cool water/wrap in wet bandages. |
| P337 + P313 | IF eye irritation persists: Get medical advice/attention. |
| P342 + P311 | IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. |
| P370 + P376 | In case of fire: Stop leak if safe to Do so. |
| P370 + P378 | In case of fire: |
| P370 + P380 | In case of fire: Evacuate area. |
| P370 + P380 + P375 | In case of fire: Evacuate area. Fight fire remotely due to the risk of explosion. |
| P371 + P380 + P375 | In case of major fire and large quantities: Evacuate area. Fight fire remotely due to the risk of explosion. |
Storage | |
| Code | Phrase |
| P401 | |
| P402 | Store in a dry place. |
| P403 | Store in a well-ventilated place. |
| P404 | Store in a closed container. |
| P405 | Store locked up. |
| P406 | Store in corrosive resistant/ container with a resistant inner liner. |
| P407 | Maintain air gap between stacks/pallets. |
| P410 | Protect from sunlight. |
| P411 | |
| P412 | Do not expose to temperatures exceeding 50 oC/ 122 oF. |
| P413 | |
| P420 | Store away from other materials. |
| P422 | |
| P402 + P404 | Store in a dry place. Store in a closed container. |
| P403 + P233 | Store in a well-ventilated place. Keep container tightly closed. |
| P403 + P235 | Store in a well-ventilated place. Keep cool. |
| P410 + P403 | Protect from sunlight. Store in a well-ventilated place. |
| P410 + P412 | Protect from sunlight. Do not expose to temperatures exceeding 50 oC/122oF. |
| P411 + P235 | Keep cool. |
Disposal | |
| Code | Phrase |
| P501 | Dispose of contents/container to ... |
| P502 | Refer to manufacturer/supplier for information on recovery/recycling |
Physical hazards | |
| Code | Phrase |
| H200 | Unstable explosive |
| H201 | Explosive; mass explosion hazard |
| H202 | Explosive; severe projection hazard |
| H203 | Explosive; fire, blast or projection hazard |
| H204 | Fire or projection hazard |
| H205 | May mass explode in fire |
| H220 | Extremely flammable gas |
| H221 | Flammable gas |
| H222 | Extremely flammable aerosol |
| H223 | Flammable aerosol |
| H224 | Extremely flammable liquid and vapour |
| H225 | Highly flammable liquid and vapour |
| H226 | Flammable liquid and vapour |
| H227 | Combustible liquid |
| H228 | Flammable solid |
| H229 | Pressurized container: may burst if heated |
| H230 | May react explosively even in the absence of air |
| H231 | May react explosively even in the absence of air at elevated pressure and/or temperature |
| H240 | Heating may cause an explosion |
| H241 | Heating may cause a fire or explosion |
| H242 | Heating may cause a fire |
| H250 | Catches fire spontaneously if exposed to air |
| H251 | Self-heating; may catch fire |
| H252 | Self-heating in large quantities; may catch fire |
| H260 | In contact with water releases flammable gases which may ignite spontaneously |
| H261 | In contact with water releases flammable gas |
| H270 | May cause or intensify fire; oxidizer |
| H271 | May cause fire or explosion; strong oxidizer |
| H272 | May intensify fire; oxidizer |
| H280 | Contains gas under pressure; may explode if heated |
| H281 | Contains refrigerated gas; may cause cryogenic burns or injury |
| H290 | May be corrosive to metals |
Health hazards | |
| Code | Phrase |
| H300 | Fatal if swallowed |
| H301 | Toxic if swallowed |
| H302 | Harmful if swallowed |
| H303 | May be harmful if swallowed |
| H304 | May be fatal if swallowed and enters airways |
| H305 | May be harmful if swallowed and enters airways |
| H310 | Fatal in contact with skin |
| H311 | Toxic in contact with skin |
| H312 | Harmful in contact with skin |
| H313 | May be harmful in contact with skin |
| H314 | Causes severe skin burns and eye damage |
| H315 | Causes skin irritation |
| H316 | Causes mild skin irritation |
| H317 | May cause an allergic skin reaction |
| H318 | Causes serious eye damage |
| H319 | Causes serious eye irritation |
| H320 | Causes eye irritation |
| H330 | Fatal if inhaled |
| H331 | Toxic if inhaled |
| H332 | Harmful if inhaled |
| H333 | May be harmful if inhaled |
| H334 | May cause allergy or asthma symptoms or breathing difficulties if inhaled |
| H335 | May cause respiratory irritation |
| H336 | May cause drowsiness or dizziness |
| H340 | May cause genetic defects |
| H341 | Suspected of causing genetic defects |
| H350 | May cause cancer |
| H351 | Suspected of causing cancer |
| H360 | May damage fertility or the unborn child |
| H361 | Suspected of damaging fertility or the unborn child |
| H361d | Suspected of damaging the unborn child |
| H362 | May cause harm to breast-fed children |
| H370 | Causes damage to organs |
| H371 | May cause damage to organs |
| H372 | Causes damage to organs through prolonged or repeated exposure |
| H373 | May cause damage to organs through prolonged or repeated exposure |
Environmental hazards | |
| Code | Phrase |
| H400 | Very toxic to aquatic life |
| H401 | Toxic to aquatic life |
| H402 | Harmful to aquatic life |
| H410 | Very toxic to aquatic life with long-lasting effects |
| H411 | Toxic to aquatic life with long-lasting effects |
| H412 | Harmful to aquatic life with long-lasting effects |
| H413 | May cause long-lasting harmful effects to aquatic life |
| H420 | Harms public health and the environment by destroying ozone in the upper atmosphere |
Sorry,this product has been discontinued.
HomeInquiry
* Country/Region
* Quantity Required :
* Cat. No.:
* CAS No :
* Product Name :
* Additional Information :














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping