Editorial Board Members' Collection Series: Diagnostic Approaches to Gastrointestinal and Pancreatic Diseases
Share This Topical Collection
Editors
 Dr. Paolo Aseni
Dr. Paolo Aseni
 Dr. Paolo Aseni
Dr. Paolo Aseni
E-Mail
Website
Collection Editor
1. Department of Emergency, ASST Grande Ospedale Metropolitano Niguarda, Piazza Ospedale Maggiore 3, 20162 Milan, Italy
2. Morfologia Umana Macroscopica, Dipartimento Di Scienze Biomediche E Cliniche “L. Sacco”, Università Degli Studi Di Milano, Milan, Italy
Interests: pancreatitis; emergencies in organ transplantation; acute liver failure; acute postoperative complications; post-operative hemodynamic monitoring; acute gastrointestinal bleeding; complications in portal hypertension; training and simulation in the emergency department
Topical Collection Information
Dear Colleagues,
Gastrointestinal and pancreatic diseases are a significant global health concern, necessitating precise and timely diagnoses for effective patient care. We invite research papers focusing on innovative diagnostic methods to further our understanding of these conditions.
We encourage submissions that explore recent advancements in diagnostic technologies, such as cutting-edge imaging modalities and biomarker discoveries. It is our aim to share insights on how these approaches can enhance diagnostic accuracy and expedite patient treatment, spanning common gastroenterological and pancreatic disorders to rare and complex ailments.
Additionally, we welcome research on genetics and molecular diagnostics in identifying hereditary gastrointestinal and pancreatic conditions. Emphasizing the role of artificial intelligence and machine learning in data analysis for gastroenterology and pancreatic disorders is also encouraged.
Your contributions may significantly impact patient well-being and advance gastroenterology diagnostics. We await your valuable insights.
Dr. Paolo Aseni
Dr. Ervin Toth
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Diagnostics is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- gastrointestinal diseases
- diagnostics
- biomarkers
- imaging techniques
- precision medicine
- EUS
- pancreatic diseases
- inflammatory bowel diseases
Published Papers (2 papers)
2024
Open AccessArticle
Circulating IgG Fragments for Gastric Cancer and Esophageal Cancer
by
Eugene I. Goufman, Nataliia B. Tikhonova, Andrey P. Aleksankin, Karina B. Gershkovich, Alexander A. Stepanov, Irina I. Stepanova, Liudmila M. Mikhaleva, Natalia V. Nizyaeva, Olga V. Kovaleva, Alexander A. Alferov, Yury B. Kuzmin and Nikolay E. Kushlinskii
Viewed by 825
Abstract
Blood serum of patients with gastric (
n = 68) and esophageal (
n = 43) cancer was assessed for proteolytic fragments of IgG. Serum samples of 20 healthy donors were used as a control. We analyzed indicators of hemostasis (prothrombin time, fibrinogen,
[...] Read more.
Blood serum of patients with gastric (
n = 68) and esophageal (
n = 43) cancer was assessed for proteolytic fragments of IgG. Serum samples of 20 healthy donors were used as a control. We analyzed indicators of hemostasis (prothrombin time, fibrinogen, plasminogen activity, a2-antiplasmin activity, protein C activity) in blood plasma and the level of total IgG in the blood serum. The median IgG-LysK of healthy donors was lower than in esophageal cancer and in patients with gastric cancer. ROC-analysis showed high sensitivity (91%) and specificity (85%) in the group with esophageal cancer but 68% and 85%, respectively, in patients with gastric cancer. Analysis of false negatives IgG-LysK in cancer patients showed that most patients had an advanced stage of cancer accompanied by metastases. Total IgG in the plasma of patients with false-negative IgG-LysK values was 30% lower than in samples with positive values, while the level of a2-antiplasmin was increased and the prothrombin time was shorter. These changes in blood homeostasis may be the reason for an increase in the proportion of false-negative values of the IgG-LysK coefficient. Circulatory IgG-LysK levels increase in the early stages of such cancers as gastric and esophageal cancers. Thus, when used in a panel with other more specific markers for these pathologies, this indicator can significantly increase the early detection of cancer.
Full article
►▼
Show Figures
Open AccessArticle
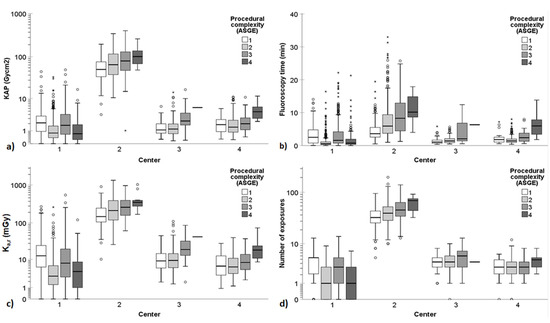
Assessing Patient Radiation Exposure in Endoscopic Retrograde Cholangiopancreatography: A Multicenter Retrospective Analysis of Procedural Complexity and Clinical Factors
by
Touko Kaasalainen, Ekaterina Saukko, Outi Lindström, Marianne Udd, Sara Regnér, Arto Saarela, Ervin Toth, Gabriele Wurm Johansson, Anna-Leena Manninen, Juha Grönroos and Leena Kylänpää
Viewed by 1539
Abstract
Background and aims: Endoscopic retrograde cholangiopancreatography (ERCP) procedures can result in significant patient radiation exposure. This retrospective multicenter study aimed to assess the influence of procedural complexity and other clinical factors on radiation exposure in ERCP. Methods: Data on kerma-area product (KAP), air-kerma
[...] Read more.
Background and aims: Endoscopic retrograde cholangiopancreatography (ERCP) procedures can result in significant patient radiation exposure. This retrospective multicenter study aimed to assess the influence of procedural complexity and other clinical factors on radiation exposure in ERCP. Methods: Data on kerma-area product (KAP), air-kerma at the reference point (K
a,r), fluoroscopy time, and the number of exposures, and relevant patient, procedure, and operator factors were collected from 2641 ERCP procedures performed at four university hospitals. The influence of procedural complexity, assessed using the American Society for Gastrointestinal Endoscopy (ASGE) and HOUSE complexity grading scales, on radiation exposure quantities was analyzed within each center. The procedures were categorized into two groups based on ERCP indications: primary sclerosing cholangitis (PSC) and other ERCPs. Results: Both the ASGE and HOUSE complexity grading scales had a significant impact on radiation exposure quantities. Remarkably, there was up to a 50-fold difference in dose quantities observed across the participating centers. For non-PSC ERCP procedures, the median KAP ranged from 0.9 to 64.4 Gy·cm
2 among the centers. The individual endoscopist also had a substantial influence on radiation dose. Conclusions: Procedural complexity grading in ERCP significantly affects radiation exposure. Higher procedural complexity is typically associated with increased patient radiation dose. The ASGE complexity grading scale demonstrated greater sensitivity to changes in radiation exposure compared to the HOUSE grading scale. Additionally, significant variations in dose indices, fluoroscopy times, and number of exposures were observed across the participating centers.
Full article
►▼
Show Figures