Adsorption of T.N.T. from Wastewater Using Ni-Oxide and Cu-Oxide Nanoparticles
Abstract
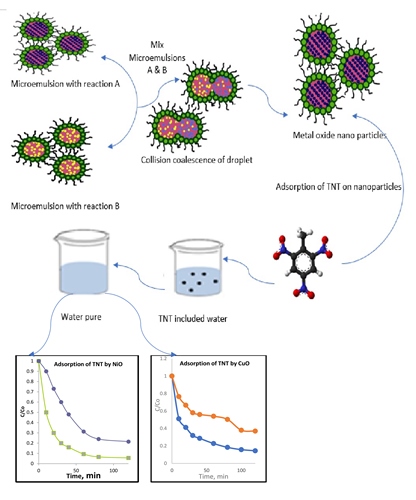
Nanocrystalline nickel oxide (NiO) and copper oxide (CuO) have been synthesized in a water-in-oil microemulsion. The as-synthesized samples were characterized by X-ray diffraction (XRD), Electron Spin Resonance (E.S.R.), transmission electron microscopy (T.E.M.), and Specific Surface Area (S.S.A.). The particle size of nickel oxide and copper oxide can be controlled from 10.0 to 21.5 and 12.5 to 25.0 nm, respectively, at a different time of calcination temperature with a fixed proportion of water, surfactant, and oil in the microemulsion. Also, the results showed that the specific surface area (89.96 m2 g-1) and pore diameter (8.11 nm) of the prepared nano NiO are higher than the specific surface area (71.96 m2 g-1) and pore diameter (3.71 nm) of the prepared nano CuO. An adsorption test was carried out to show the efficiency of these prepared NiO and CuO nanoparticles for the Adsorption of T.N.T. in an aqueous solution. The removal efficiencies of both nano NiO and CuO were achieved at 90.06% and 77.0%, respectively.
Additionally, NiO and CuO nanoparticles were regenerated for five cycles. The Kinetic models of Pseudo first-order and pseudo-second-order were described. The results demonstrated that T.N.T. adsorption on both nano adsorbents follows the pseudo-second-order model.
Full Text:
PDFReferences
N.A. Khan, S.U. Khan, S. Ahmed, I.H. Farooqi, A. Dhingra, A. Hussain and F. Changani, Applications of nanotechnology in water and wastewater treatment, Water Research, 2019, 47,3931–3946.
S. Pandey, A comprehensive review on recent developments in bentonite-based materials used as adsorbents for wastewater treatment, Journal of Molecular Liquids, 2017, 241, 1091–1113.
N.A. Khan, S.U. Khan, S. Ahmed, I.H. Farooqi, M. Yousefi, A.A. Mohammadi, F. Changani, Recent trends in disposal and treatment technologies of emerging-pollutants–A critical review, TrAC Trends Anal Chem., 2020, 122.
K.S. Ro, A. Venugopal, D.D. Adrian, D. Constant, K. Qaisi, K.T. Valsaraj, L.J. Thibodeaux, D. Roy, Solubility of 2,4,6-trinitrotoluene (TNT) in water, Chem. Eng. Data., 1996, 41, 758–761.
J.D. Nijs, M. Frank, Assessment of technologies for disposing explosive waste, J. Hazard. Mater., 2002, 90, 137–153.
J.C. Pennington, J.M. Brannon, Environmental fate of explosives, Thermochim. Acta., 2002, 384, 163–172.
J.L. Conder, T.W. La Point, J.A. Steevens, G.R. Lotufo, Recommendations for the assessment of T.N.T. toxicity in sediments, Environ. Toxicol. Chem., 2004, 23, 141–149.
M. Ikram, S. Ali, M. Aqeel, A. Ul-Hamid, M. Imrand, J. Haider, A.Haider, A. Shahbaz, S. Ali, Reduced graphene oxide nanosheets doped by Cu with highly efficient visible-light photocatalytic behavior, Journal of Alloys and Compounds, 2020, 837, 155588.
M. Ikram, A. Raza, M. Imran, A. Ul-Hamid, A. Shahbaz and S. Ali, Hydrothermal Synthesis of Silver Decorated Reduced Graphene Oxide (rGO) Nanoflakes with Effective Photocatalytic Activity for Wastewater Treatment, Nano scale Research Letters, 2020, 15, 95.
J. Hassan, M. Ikram, A. Ul-Hamid, M. Imran, M. Aqeel and S. Ali, Application of Chemically Exfoliated Boron Nitride Nanosheets Doped with Co to Remove Organic Pollutants Rapidly from Textile Water, Nanoscale Research Letters, 2020, 15, 75.
A. Raza, M. Ikram, M. Aqeel, M. Imran, A. Ul Hamid, K.N. Riaz, S. Ali, Enhanced industrial dye degradation using Co-doped in chemically exfoliated MoS2 nanosheets, Applied Nanoscience, 2020, 10, 1535-1544.
M. Ikram, E. Umar, A. Raza, A. Haider, S. Naz, A. Ul-Hamid, J. Haider, I. Shahzadi, J. Hassan and S. Alic, Dye degradation performance, bactericidal behavior and molecular docking analysis of Cu-doped TiO2 nanoparticles, R.S.C. Adv., 2020, 10, 24215.
M. krama, S. Ali, M. Aqeel, A. Ul-Hamid, M. Imran, J. Haider, A. Haider, A. Shahbaz, S. Ali, Reduced graphene oxide nanosheets doped by Cu with highly efficient visible-light photocatalytic behavior, Journal of Alloys and Compounds, 2020, 837, 155588.
S. Pandey, J. Yeon Do, J. Kim, M. Kang, Fast and highly efficient catalytic degradation of dyes using κ-carrageenan stabilized silver nanoparticles nanocatalyst, Carbohydrate polymers, 2020, 230, 11559.
S. Pandey, J. Yeon Do, J. Kim, M. Kang, Fast and highly efficient removal of dye from aqueous solution using natural locust bean gum-based hydrogels adsorbent, International Journal of Biological Macromolecules, 2020, 143, 60-75.
S.G. Mohammad, S.M. Ahmed, A.E. Amr and A.H. Kamel, Porous Activated Carbon from Lignocellulosic Agricultural Waste for the Removal of Acetampirid Pesticide from Aqueous Solutions, Molecules, 2020, 25, 2339
Z.G. Gök, M.İna, M. Yiğitoğlu, Hacettepe Effective Degradation of 2,4,6-Trinitrotoluene (T.N.T.) with a Bacterial Consortium Developed from High TNT-degrading Bacteria Isolated from TNT-contaminated Soil, J. Biol. & Chem., 2018, 46, 445-455.
S.M. Solyman, S. Shaban, S. Morsy, A.Y. El-Naggar, A.M. Badawi, S.M. Ahmed, Alkaline Hydrolysis of T.N.T. in Micellar System, Journal of Dispersion Science and Technology, 2011, 32, 731–736.
A.M. Badaw, S.A. Shaban, S.M. Ahmed, S.M. Morsy and A.Y. El-Naggar, Kinetics of T.N.T. Degradation in The Presence of Zero Valent Iron Nanocatalyst, Egypt. J. Chem., 2012, 55, 339–353.
X. Zhang, Y. Lin, X. Shan, Z. Chen, Degradation of 2,4,6-trinitrotoluene (T.N.T.) from explosive wastewater using nanoscale zero-valent iron, Chemical Engineering Journal, 2010, 158,
–570.
S.A. Shaban, A.M. Badawi, S.L. Sheltawy, M.M. ELShahid, Degradation of T.N.T. from aqueous solution using catalytic liquid, Int. J. Chem. Sci., 2014, 12, 495-507.
A.M. Badawi, S.M, Ahmed, S.A. Shaban, S.M. Morsy, Nanotechnology: the next revolution for wastewater treatment (T.N.T. contaminate), Desalination and Water Treatment, 2012, 40, 1–6.
S.M. Ahmed, S.A. Shaban, D.S. El-Desouki, N.K. Aboul-Gheit, S.M. Abdel-Azim, Photocatalytic degradation of T.N.T. in wastewater using Fe-doped TiO2 nanoparticles, Desalination and Water Treatment, 2018, 104, 241–249.
T. Guerra, I.B. Jr, Adsorption of Trinitrotoluene on a MgO Surface Including Surface Relaxation Effects, Journal of Chemistry, 2013, 1-8.
I. Bharti, A.K. Khurana, A. Shaw, J.M. Saxena, P. Khurana, K. Rai, Removal of Trinitrotoluene with Nano Zerovalent Iron Impregnated Graphene Oxide, Water Air Soil Pollut., 2018, 229, 17.
P. Hu, Y. Zhang, K. Tong, F. Wei, Q. An, X. Wang, P.K. Chu, F. Lv, Removal of organic pollutants from red water by magnetic-activated coke, Desalination and Water Treatment, 2015, 54, 2710-2722.
M.T. Raad, M. Kassir, W. El Khatib, M. Issa, A. Hijazi, H. Bazzi, A comparative adsorption study of trinitrotoluene onto graphene oxide, reduced graphene oxide and reduced graphene oxide coated silica nanoparticles through equilibrium, kinetic and thermodynamic modeling, Graphene Technol, 2017, 2, 63-73.
J. Hassan, M. Ikram, A. Ul-Hamid, M. Imran, M. Aqeel, S. Ali, Application of Chemically Exfoliated Boron Nitride Nanosheets Doped with Co to Remove Organic Pollutants Rapidly from Textile, Water Nanoscale Research Letters, 2020, 15, 75.
M. Ikram, M.I. Khan, A. Raza, M. Imran, A. Ul-Hamid, S. Ali, Outstanding performance of silver-decorated MoS2 nanopetals used as nanocatalyst for synthetic dye degradation, Physica E: Low-dimensional Systems and Nanostructures, 2020, 124, 114246.
J. Suárez-Cerda, H. Espinoza-Gómez, G. Alonso-Núñez, I.A. Rivero, Y. Gochi-Ponce, L.Z. Flores-López, A green synthesis of copper nanoparticles using native cyclodextrins as stabilizing agents, Journal of Saudi Chemical Society, 2017, 21, 341–348.
N. Sebeia, M. Jabli, A. Ghith, T.A. Saleh, Eco-friendly synthesis of Cynomorium coccineum extract for controlled production of copper nanoparticles for sorption of methylene blue dye, Arabian Journal of Chemistry, 2020, 13, 4263–4274.
M. Rashad, H. A. Al-Aoh, Promising adsorption studies of bromophenol blue using copper oxide nanoparticles, Desalination and Water Treatment, 2019, 139, 360–368.
K.H. Hassan, A.A. Jarullah, S.K. Saadi, synthesis of copper oxide nanoparticle as an adsorbent for removal of Cd (II) and Ni (II) ions from binary system, International Journal of Applied Environmental Sciences, 2017, 12, 1841–1861.
R. Hosseini, M.H. Sayadi, H. Shekari, Adsorption of nickel and chromium from aqueous solutions using copper oxide nanoparticles: Adsorption isotherms, kinetic modeling, and thermodynamic studies, Avicenna Journal of Environmental Health Engineering, 2019, 6, 1-9.
A. Phasuk, S. Srisantitham, T. Tuntulani, W. Anutrasakda, Facile synthesis of magnetic hydroxyapatite-supported nickel oxide nanocomposite and its dye adsorption characteristics, adsorption, 2017, 24, 157–167.
G. Bai, H. Dai, J. Deng, Y. Liu, W. Qiu, Z. Zhao, X. Li, H. Yang, The microemulsion preparation and high catalytic performance of mesoporous NiO nanorods and nanocubes for toluene combustion, Chemical Engineering Journal, 2013, 219, 200-208.
Y. Dehmani, S. Abouarnadasse, Study of the adsorbent properties of nickel oxide for phenol depollution, Arabian Journal of Chemistry, 2020, 5312-5325.
Y. Zheng, B. Zhu, H. Chen, W. You, C. Jiang, J. Yu, Hierarchical flower-like nickel (II) oxide microspheres with a high adsorption capacity of Congo red in water, J. Colloid Interface Sci., 2017, 504, 688–696.
H. A. Al-Aoh, Adsorption performances of nickel oxide nanoparticles (NiO N.P.s) towards bromophenol blue dye (B.B.), Desalination and Water Treatment, 2018, 110, 229–238.
W.T. Yao, S.H. Yu, Y. Zhou, Formation of uniform CuO nanorods by spontaneous aggregation: selective synthesis of CuO, Cu2O, and Cu nanoparticles solid-liquid phase arc discharge process, J. Phys. Chem. B, 2005, 109, 14011–14016.
R. Ahmadi, A. Razzaghian, Z. Eivazi, K. Shahidi, Synthesis of Cu-CuO and Cu-Cu2O Nanoparticles via Electro-Explosion of Wire Method, Int. J. Nanosci. Nanotechnol., 2018, 4, 93-99.
S.J. Brunauer, L. S.Deming, W. Deming, E.Teller, On a Theory of the van der Waals Adsorption of Gases, Journal of American Chemical Society, 1940, 62, 1723-1732.
J.H. de Boer, E.P. Everette, F.S. Stone, Eds, The structure and properties of porous materials. Butterworths, (London), 1985, 68.
A.M. Mahmoud, F.A. Ibrahim, S.A. Shaban, N.A. Youssef, adsorption of heavy metal ion from aqueous solution by nickel oxide nanocatalyst prepared by different methods, Egyptian Journal of Petroleum, 2015, 24, 27–35.
F.Y.A. El-Kady, M.G. Abd El Wahed, S. Shaban, A.O. Abo El Naga, Hydrotreating of heavy gas oil using CoMo/γ-Al2O3 catalyst prepared by equilibrium deposition filtration, J. Fuel, 2010, 89, 3193–3206.
M. Rubinstein, R.H. Kodama, S.A. Makhlouf, Electron spin resonance study of NiO antiferromagnetic nanoparticles, Journal of Magnetism and Magnetic Materials, 2001, 234, 289-293
F. Amano, T. Tanaka, T. Funabiki, Auto-reduction of Cu(II) species supported on Al2O3 to Cu(I) by Thermo vacuum treatment, J. Mol. Catal. A, 2004, 221, 89-95.
F. Amano, S. Suzuki, T. Yamamoto, T. Tanaka, One-electron reducibility of isolated copper oxide on alumina for selective NO–C.O. reaction, applied catalysis B: Environmental, 2006, 64, 282-289.
Y.S. Ho, G. McKay, Pseudo-second order model for sorption processes, Process Biochemistry, 1999, 34, 451-465.
Y.S. Ho, G. McKay, Sorption of dye from aqueous solution by peat, Chemical Engineering Journal, 1998, 70, 115-124.
R.A.-Cuevas-Villanueva, A.R. Hidalgo-Vázquez, C. de Jesús, Cortés Penagos, R. Cortés-Martínez, Thermodynamic, kinetic, and equilibrium.
parameters for the removal of lead and cadmium from aqueous solutions with calcium alginate beads, Scientific. World J., 2014, 1–9
R.O. Abo El Naga, S.A. Shaban, F.Y. El Kady, Metal-organicframework-derived nitrogen-doped nanoporous carbon as an efficient adsorbent for methyl orange removal from aqueous solution, Journal of the Taiwan Institute of Chemical Engineers, 2018, 93, 363–373.
N.F Nejad, E. Shams, M.K. Amini, J.C. Bennett, Synthesis of magnetic mesoporous carbon and its application for adsorption of dibenzothiophene, Fuel Process. Technol., 2013, 106, 376.
B.H. Hameed, A.A. Rahman, Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material, J. Hazard. Mater., 2008, 160, 576.
N.A. Youssef, S.A. Shaban, F.A. Ibrahim, A.M. Mahmoud, Preparation of nano-Fe2O3 particles and evaluation of their catalytic activity in Adsorption of Pb (II), Mn (II) and Zn(II) from aqueous solution, Desalination and Water Treatment, 2017, 69, 284–293.
DOI: http://dx.doi.org/10.13171/mjc02101051534aat
Refbacks
- There are currently no refbacks.
Copyright (c) 2021 Mediterranean Journal of Chemistry
