SPATIAL STRUCTURE OF THE HEPTAPEPTIDE ANALOGUE OF NOCICEPTIN MOLECULE
Abstract
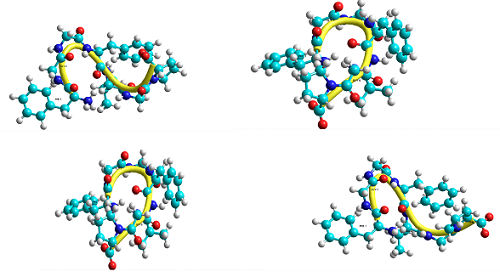
It is known that nociceptins are a new type of regulatory peptide. Knowledge of these peptide molecules' structural and functional properties is of great practical importance for medicine and pharmacology. Their mechanisms of action are considered anti-opioid. This scientific work is devoted to studying the spatial structure of the heptapeptide H-Phe1-Gly2-Gly3-Phe4-Val5-Gly6-Pro7-OH. It examines the conformational capabilities of this heptapeptide molecule. This neuropeptide molecule is a stable analog of the nociceptin. The biologically active conformation of the peptide molecule, which is realized upon interaction with the receptor, is included in the set of low-energy structures. Therefore, studying the spatial structure of peptide molecules is of great interest. Theoretical conformational analysis about nonvalent, electrostatic, and torsional interactions, the energy of the hydrogen bonds, and a special computer program carried out the calculations. The 10 low-energy conformations of this molecule and the values of the dihedral angles of the main chain and side chains are found, and the energy of the intra- and inter-residue interactions is estimated. It is revealed that low energy conformations of this molecule have the half-folded and folded type of backbone. The side chains of the Phe1 and Phe4 amino acids in low-energy conformations carry out effective interactions and are conformationally labile amino acids; they bring together the regions of the main chain and the side chains of the amino acids included in the heptapeptide. These folded forms bring parts of the backbone and the amino acids' side chains together, resulting in important interactions.
Full Text:
PDFReferences
J.M. Witkin, M.A. Statnick, L.M. Rorick-Khin, J.E. Pintar? The biology of Nociceptin/Orphanin FQ(N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence, Pharmacol. Ther., 2014, 141, 283-299.
E.A. Ivanova, N.Yu. Sarycheva, V.A. Dubynin, A.V. Malyshev, Behavioral effects of original tetrapeptide an analog of N-terminal nociceptin fragment, Bulletin of Experimental Biology and Medicine, 2012, 13, 851-857.
D. Reha, H. Valdes, J. Vondrasek, P. Hobza, Structure and IR spectrum of phenylalanyl-glycyl-glycine tripeptide in the gas-phase: IR/UV experiments ab initio quantum chemical calculations, and molecular dynamic simulations, Chemistry, A European Journal, 2005, 11,
-6817.
K.R. Wilson, M.J. Cruz, Ch. Nicolas, L. Belau, Thermal vaporization of biological nanoparticles: fragment-free vacuum ultraviolet photoionization mass spectra of tryptophan, phenylalanine-glycine-glycine, and beta-carotene, J. Phys. Chem., 2006, 110, 2106-2113.
I.S. Maksumov, L.I. Ismailova, N.M. Godjaev, A computer program for calculation of conformations of molecular systems, J. Struc. Chem., 1983, 24, 147-148.
J. Hermans, D. Ferro, Representation of a protein molecule as a tree and application to modular computer programs which calculate and modify atomic coordinates, Biopolymers, 1971, 10, 1121-1138.
V.G. Dashevskiy, Conformations of the organic molecules, Moskow, Chemistry, 1974, 432.
http//www.Hyper Chem.com
L.I. Ismailova, G.A. Akverdieva, S.D. Demukhamedova, N.A. Akhmedov, Molecular modelling of Pro-Gly gliproline and its complexes, Moscow University Physics Bulletin, 2023, 78, 668-680.
N.A. Akhmedov, Sh.N. Gadjiyeva, R.M. Abbasli, Structural organization of Asp-Pro-Lys-Gln-Asp-Phe-Met-Arg-Phe-NH2 molecule, Cur.Top. in Pept. & Prot.Res., 2009, 10, 57-62.
N.A. Akhmedov, L.I. Ismailova, L.N. Agayeva, N.M. Gocayev, The spatial structure of the cardio active peptides, Cur.Top. in Pept. & Prot. Res., 2010, 11, 87-93.
N.A. Akhmedov, L.I. Ismailova, R.M. Abbasli, L.N. Agayeva, S.R. Akhmedova, Spatial structure of Octarphin molecule, IOSR JAP., 2016, 8, 66-70.
N. Akhmedov, L. Agayeva, G. Akverdieva, R. Abbasli, L. Ismailova, Spatial structure of the ACTH-(6-9)-PGP molecule, J. Chem. Soc. Pak., 2021, 43, 500-504.
N. Akhmedov, L. Agayeva, S. Akhmedova, R. Abbasli, L. Ismailova, Spatial structure of the β-Casomorphin-7 Molecule, IOSR Journal of Applied Physics, 2021, 5, 62-67.
L.I. Ismailova, R.M. Abbasli, N.A. Akhmedov, Computer modeling of the spatial atructure of nonapeptide molecule, Control and Optimization with Industrial Applications, Proceedings of the 7-th International Conference, 2020, 218-220.
N.A. Akhmedov, L.N. Agayeva, R.M. Abbasli, L.I. Ismailova, Computer modeling of law-energy spatial structures of casoxin G molecule. Proceedings of the 8th International Conference on Control and Optimization with Industrial Applications (COIA-22), Azerbaijan, 2022, 1, 63-65.
L.I. Ismailova, R.M. Abbasli, N.A. Akhmedov, Structural organization of the Gly-Pro-Arg-Pro molecule, Actual questions of the biological physics and chemistry 2021, 6, 53-56.
L.N. Agaeva, A.A. Abdinova, S.R. Akhmedova, N.F. Akhmedov, N.A. Akhmedov, Spatial Structure of Soymorphin-6 molecule, Biophysics, 2023, 68, 929–933.
N.A. Akhmedov, L.N. Agaeva, R.M. Abbasli, L.I. Ismailova, Spatial Structure of the Casoxin C molecule, Biophysics, 2024, 69, 43–50.
L.N. Agayeva, N.A. Akhmedov, G.T. Imanova, Spatial Structure of the Casoxin D Molecule, Materials Research Innovations, 2024.
S. Tahirli, F. Aliyeva, H. Şenol, S. Demukhamedova, G. Akverdieva, I. Aliyeva, S. Veysova, N. Sadeghian, S. Günay, Y. Erden,
P. Taslimi, A. Sujayev,F.Chiragov, Novel complex compounds of nickel with 3-(1-phenyl-2,3-dimethyl-pyrazolone-5) azopentadione-2,4: synthesis, NBO analysis, reactivity descriptors and in silico and in vitro anti-cancer and bioactivity studies, Journal of Biomolecular Structure and Dynamics, 2024, 1-25.
S. Wang, Y. Li, H. Xu, Y. Sun, S. Xu, Design, structure of Amphiphiphilic peptide and its application from single molecule to nanoparticle, Results in Ingineering, 2022, 16, 100747-10055.
E.M. Popov, An Approach to calculations of the problem of structure functional organization of natural peptides, Mol. Biol., 1985, 19, 1107-1138.
A. Kotyk, Quantities, Units and Symbols in Physical Chemistry, Blackwell Scientific Publications, Oxford, 1999.
DOI: http://dx.doi.org/10.13171/mjc02407111782ismailova
Refbacks
- There are currently no refbacks.
Copyright (c) 2024 Mediterranean Journal of Chemistry
