Abstract
Aging of the nervous system underlies the behavioral and cognitive decline associated with senescence. Understanding the molecular and cellular basis of neuronal aging will therefore contribute to the development of effective treatments for aging and age-associated neurodegenerative disorders. Despite this pressing need, there are surprisingly few animal models that aim at recapitulating neuronal aging in a physiological context. We recently developed a C. elegans model of neuronal aging, and showed that age-dependent neuronal defects are regulated by insulin signaling. We identified electrical activity and epithelial attachment as two critical factors in the maintenance of structural integrity of C. elegans touch receptor neurons. These findings open a new avenue for elucidating the molecular mechanisms that maintain neuronal structures during the course of aging.
Keywords: C. elegans, aging, touch neuron, electrical activity, nerve attachment, axon degeneration
Our recent Caenorhabditis elegans model of neuronal aging presents two important features that distinguish it from previous vertebrate models of neurodegenerative diseases.1 First, through the first longitudinal imaging of single neurons across the entire adult lifespan, our model reveals unexpected dynamic features of neuronal aging that are not adequately appreciated in existing animal models of brain aging. Second, our model had identified factors that are specifically required for neuronal integrity without affecting organismal lifespan, indicating that lifespan and cellular aging could be mechanistically uncoupled.
Dynamic Features of Neuronal Aging
For most metazoan, including C. elegans, aging of the nervous system is not associated with significant neuronal loss.2,3 At the cellular level, the cardinal features of an aging human brain include dystrophic neurites, neurofibrillary tangles and insoluble protein aggregates.2,4,5 How these cellular defects evolve over time is still a mystery. Life-long tracking of individual aging neurons is difficult in lab mammals, however, due to their relatively long lifespan and the extraordinarily huge number of neurons in the mammalian brain. In contrast to previous reports that found no evidence for neuronal aging in C. elegans,3,6 we documented various types of age-dependent defects in C. elegans touch receptor and motor neurons, including misshapen neuronal soma, aberrant neurite formation, and beading or bubble-like lesions in the nerve processes.1 Similar to our findings, Tank et al. had recently reported age-dependent neurite sprouting in C. elegans.7 One striking feature revealed by our longitudinal imaging in touch neurons is the frequent growth and retraction of abnormal neurites. Age-dependent loss of dendritic branches had been found in mammalian neurons.2 By contrast, generation of new neurites in middle and late life of a neuron had never been documented. It is unclear whether these neurites form synaptic connections with other neurons, although they contain acetylated microtubules.1 The emergence of these neurites may represent a failure of the aged neuron to suppress unwanted growth rather than an enhanced ability to generate new structures, judging from their short length, random growth patterns and frequent retraction.1,7 Tank et al. had shown that activity of the Jun kinase pathway is required to suppress these age-dependent neuronal sprouting.7 Since rescued jnk-1 mutant animals overexpressing the C. elegans c-jun N-terminal kinase JNK-1 still show neuronal sprouting comparable to that in age-matched wild type, age-dependent loss of JNK-1 activity cannot be the sole explanation for senescent neuronal sprouting in C. elegans.7 It will be of interest to test whether aberrant neurite outgrowth is regulated by cytoskeletal machinery that also controls developmental axon extension, and what additional mechanisms normally keep these aberrant outgrowth in check.
Another prominent age-dependent defect of C. elegans touch neuron process is beading or bubble-like lesions (Fig. 1), and our longitudinal imaging suggests that some of the minor beading may represent bubble-like lesions in their early phase.1 Axon beading is common in neurons subjected to traumatic, hypoxic or inflammatory insults.8-11 In these conditions, beading may represent cytoskeletal defects or focal accumulation of axoplasmic cargos or organelles due to disrupted axonal transport.8-11 Identity of age-dependent axon beading remains obscure in both human and C. elegans. Interestingly, in some neurons at extremely old age, we observed axon splitting.1 Because multiple bubble-like lesions could be found to coexist on the same axon1 (Fig. 1), a wild speculation is that some of the bubble-like lesions are early focal splitting of the axon. Electron microscopy will be necessary to clarify the nature of these age-dependent axonal defects in C. elegans neurons.
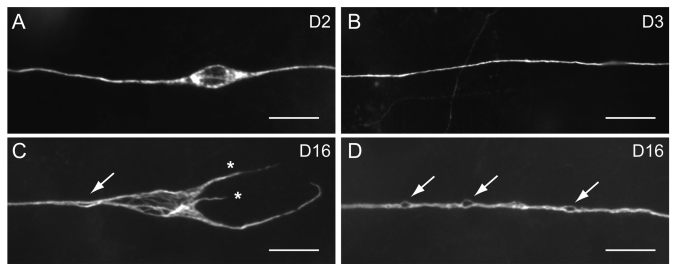
Figure 1.
Age-dependent defects of C. elegans touch receptor neurons, revealed by immunostaining of acetylated microtubules. Scale bar, 5 μm. (A) ALM in an animal 2 d of age in adulthood (D2) had a typical spindle-shape soma. (B) The PLM process in a D3 wild type animal. (C) ALM in a D16 animal showed misshapen soma with disorganized microtubule bundles, aberrant neurites (asterisks) and bubble-like lesion of the process (arrow). (D) Multiple bubble-like lesions (arrows) could be found in the PLM process of a D16 animal.
Neuron-Specific Anti-Aging Mechanisms
The stochastic feature of aging describes that in individual animals, the physiological age does not necessarily correlate with its chronological age. Thus, neurons from a mid-age C. elegans may have extensive defects, whereas neurons from an animal at advanced age may appear intact.1,3,6,7,12 On the other hand, individual tissues show great variations in the rate of aging.3,6 However, it is still believed that tissue aging worsens as the chronological age advances, and the regulation of lifespan and cellular aging is tightly linked.3,6,12 We and Tank et al. had identified electrical activities, nerve attachment, JNK and insulin signaling as potentially autonomous factors that contribute to neuronal integrity during aging.1,7 Surprisingly, in both studies, neuronal aging could be uncoupled from organismal aging. Mutations in mec-1 and mec-12, which encode an ECM protein and an α-tubulin, respectively, accelerate neuronal aging without affecting lifespan.1 Similarly, disrupting JNK signaling aggravates age-dependent neurite sprouting but does not decrease lifespan.7 These observations indicate that at the cellular level, aging is regulated in a tissue-specific manner, and is not necessarily linked to lifespan regulation.
Electrical activity had been shown to be essential for adult Drosophila olfactory neurons to survive and for newly generated hippocampal neurons to integrate into the adult neural circuits.13,14 Our study extends the roles of electrical activity in the maintenance of adult nervous system, and raises several issues that await exploration in the near future. First, does physiological membrane activity constantly activate signaling pathways that promote neuronal integrity, or does it act to suppress cellular machinery that dismantles the neurons? These two possibilities are not mutually exclusive and may cooperate to achieve optimal neuronal maintenance against aging.
Second, what is the signaling pathway that translates electrical activity on the plasma membrane into transcriptional and posttranslational mechanisms for neuronal maintenance? Calcium emerges as a promising target, in that its wide spectrum of intracellular dynamics makes it one of the most versatile second messengers in cellular physiology. Previous studies indicate that aged hippocampal neurons show more pronounced increase in intracellular calcium level under stimulation, compared with young neurons.15 Deranged regulation of intracellular calcium may underlie some of the functional decline seen in the aged neurons.16 In larval C. elegans, calcium had been shown to mediate necrotic neuronal death induced by excessive sodium channel activity.17 On the other hand, regeneration of touch neuron processes after laser axotomy requires calcium.18 Whether calcium promotes regenerative behaviors of injured neurons or causes the neuron to die may depend on its temporal dynamics. Rapid, massive calcium increase in the cytosol may activate proteolytic enzymes that dismantle the neuron, whereas persistent, low-grade calcium flow could activate cellular pathways that contribute to axon repair or neuronal integrity in the long run. One of the future challenges will be to monitor neuronal calcium dynamics in an extended temporal dimension that is relevant to aging.
In conclusion, the C. elegans model of neuronal aging reveals novel age-dependent defects that are highly dynamic, and had identified several factors specifically required for the maintenance of neuronal integrity during aging. It is important to verify these observations in other organisms such as Drosophila, mouse or human. A future goal will be to unravel the genetic control of age-dependent neuronal defects through forward and reverse genetics approaches.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/17138
References
- 1.Pan CL, Peng CY, Chen CH, McIntire SL. Genetic analysis of age-dependent defects of the Caenorhabditis elegans touch receptor neurons. Proc Natl Acad Sci USA. 2011;108:9274–9. doi: 10.1073/pnas.1011711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- 3.Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–14. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- 4.Wiśniewski HM, Ghetti B, Terry RD. Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys. J Neuropathol Exp Neurol. 1973;32:566–84. doi: 10.1097/00005072-197310000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Wiśniewski HM, Terry RD. Morphology of the aging brain, human and animal. Prog Brain Res. 1973;40:167–86. doi: 10.1016/S0079-6123(08)60686-X. [DOI] [PubMed] [Google Scholar]
- 6.Haithcock E, Dayani Y, Neufeld E, Zahand AJ, Feinstein N, Mattout A, et al. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2005;102:16690–5. doi: 10.1073/pnas.0506955102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tank EM, Rodgers KE, Kenyon C. Spontaneous Age-related neurite branching in Caenorhabditis elegans. J Neurosci. 2011;31:9279–88. doi: 10.1523/JNEUROSCI.6606-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JY, Shen S, Dietz K, He Y, Howell O, Reynolds R, et al. HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat Neurosci. 2010;13:180–9. doi: 10.1038/nn.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ochs S, Pourmand R, Jersild RA, Jr., Friedman RN. The origin and nature of beading: a reversible transformation of the shape of nerve fibers. Prog Neurobiol. 1997;52:391–426. doi: 10.1016/S0301-0082(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama Y, Aoki Y, Niitsu H. Studies on the mechanisms responsible for the formation of focal swellings on neuronal processes using a novel in vitro model of axonal injury. J Neurotrauma. 2001;18:545–54. doi: 10.1089/089771501300227341. [DOI] [PubMed] [Google Scholar]
- 11.Fayaz I, Tator CH. Modeling axonal injury in vitro: injury and regeneration following acute neuritic trauma. J Neurosci Methods. 2000;102:69–79. doi: 10.1016/S0165-0270(00)00282-X. [DOI] [PubMed] [Google Scholar]
- 12.Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–12. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang A, Priya R, Ramaswami M, Vijayraghavan K, Rodrigues V. Neuronal activity and Wnt signaling act through Gsk3-beta to regulate axonal integrity in mature Drosophila olfactory sensory neurons. Development. 2009;136:1273–82. doi: 10.1242/dev.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin CW, Sim S, Ainsworth A, Okada M, Kelsch W, Lois C. Genetically increased cell-intrinsic excitability enhances neuronal integration into adult brain circuits. Neuron. 2010;65:32–9. doi: 10.1016/j.neuron.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 2010;33:153–61. doi: 10.1016/j.tins.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toescu EC, Vreugdenhil M. Calcium and normal brain ageing. Cell Calcium. 2010;47:158–64. doi: 10.1016/j.ceca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Xu K, Tavernarakis N, Driscoll M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca2+ release from the endoplasmic reticulum. Neuron. 2001;31:957–71. doi: 10.1016/S0896-6273(01)00432-9. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci. 2010;30:3175–83. doi: 10.1523/JNEUROSCI.5464-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]