Prospects of soft biopotential interfaces for wearable human-machine interactive devices and applications
Abstract
Human interaction with machines can be made easy, comfortable, and accessible by introducing user-friendly interfaces. In the case of wearable devices, their sensors and other interfacing elements are very well within the proximity of users. Since biopotential signals can be accessed from the surface of the human skin, users can have seamless interaction with wearable human-computer interactive devices. Rigid interfaces can hinder the user experience, and therefore, the need for soft biopotential interfaces is important. Imperceptible and unobtrusive soft biopotential interfaces will drastically enhance many aspects of human-computer interaction. This paper reviews the use of soft, flexible, and stretchable biopotential interfaces in wearable human-machine interactive devices. Additionally, attention is brought to the scope of other possible applications of soft biopotential interfaces in wearable devices.
Keywords
INTRODUCTION
Human-machine interface (HMI), in layman’s terms, is an interactive system designed to allow communication between humans and machines[1]. Since the dawn of human-computer interaction (HCI) technologies, it was evident that HMI technology would have to adapt and evolve to improve its usability[2]. Eventually, wearable devices with HCI capabilities were introduced to the world, and considering human aspects such as physical, cognitive, and emotional characteristics of these devices is essential during their development[3]. Refining designs, configurations, and ergonomics of rigid wearable devices for HCI technologies would not be enough to enhance the user experience. Therefore, using soft and advanced engineering materials is crucial for making HMIs more competent and up to par to pass as a mode of communication in wearable HCI devices[4].
With the constant progress in health and lifestyle technologies, there has been an uprising in wearable devices and the features they can offer[5-9]. Wearable devices are essential tools for HCI technologies that can record individual biological and neural activities through close contact with human skin[10]. All kinds of physical and mental activities in the human body generate electrical potentials, called biopotentials, that provide colossal amounts of information, which can be used for various applications, such as health monitoring and controlling electronic devices[11-16]. For instance, neural activities in the brain can be recorded, analyzed, and used for various purposes. Motor actions that are intention-based, such as walking or moving arms, can be monitored in the form of neural activities in the brain. Similarly, when sensory inputs are perceived in the brain, such as touch or taste, the associated neural activities can be observed in the brain. The most common technique for non-invasive brain recording is electroencephalography (EEG)[17]. Although EEG allows the recording of neuronal activity non-invasively, it is difficult to isolate and record specific areas of the brain[18]. Neural signals from the brain travel in the human body and branch out to different areas through the nervous system. Similarly, the sensory inputs from the body are sent to the brain via these nervous systems. Apart from EEG, electromyogram (EMG), electrocardiograph (ECG), and electrooculogram (EOG) signals are the major biopotential signals that can be acquired using non-invasive interfaces[19]. Other than acquiring data from these signals, non-invasive interfaces can also perform stimulation-based applications for specific rehabilitation purposes[20-22].
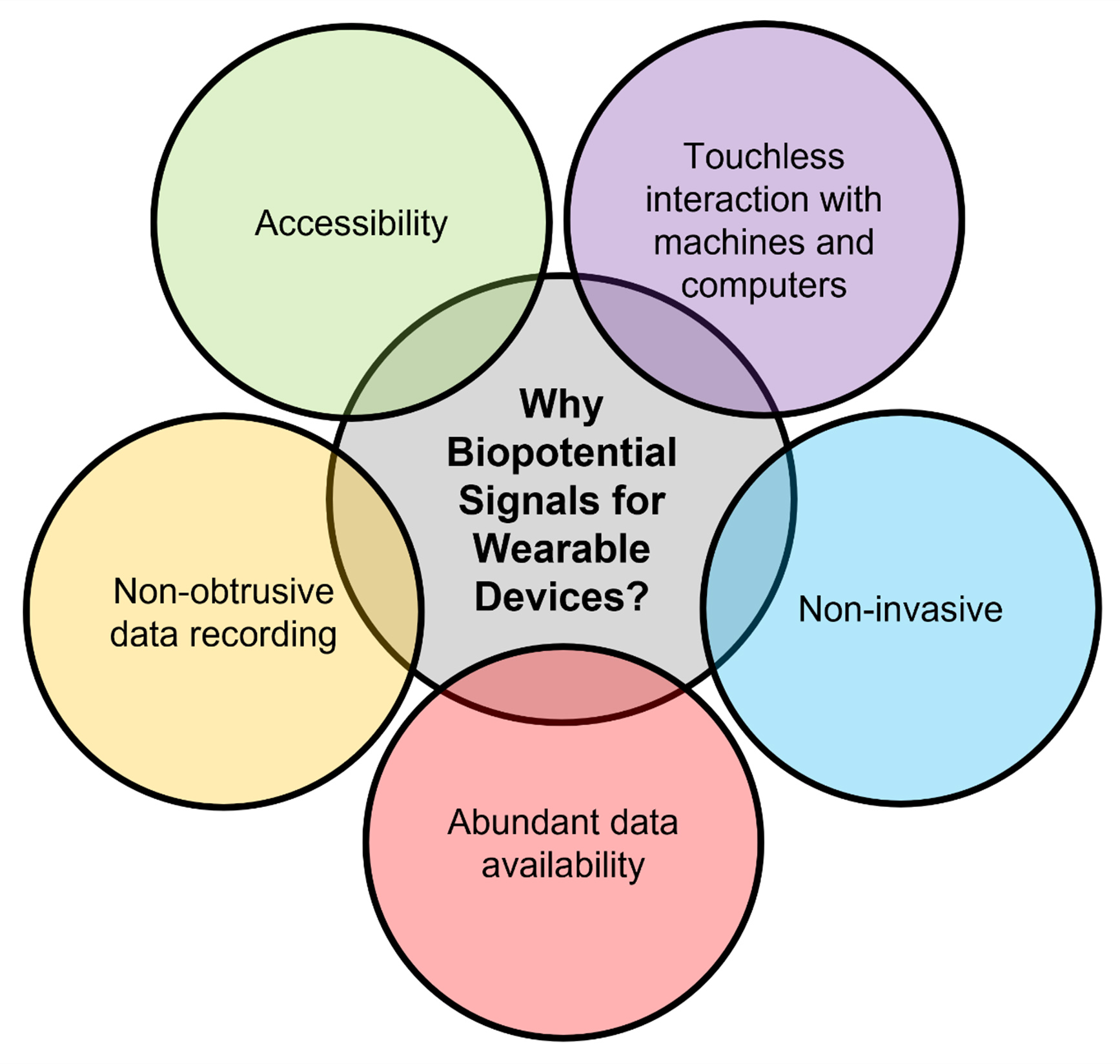
Wearable devices can utilize biopotential signals, such as EMG (from muscular activity) and EOG (from eye movement), for delivering intention-based output signals to allow applications such as gesture control, end-effector manipulation, and prosthesis. Unintentional or autonomous biopotential signals, such as ECG (from heartbeats), can allow for health monitoring applications. In HCI, such information is valuable in various industries, including medical healthcare, assistive robotics, lifestyle, and more. Figure 1 illustrates the possible HCI applications that can be controlled via wearable soft biopotential interfaces. Figure 2 illustrates the advantages of using biopotential signals for wearable devices.
Figure 1. A graphical representation of the possible wearable HCI applications controlled via wearable soft biopotential interfaces. HCI: Human-computer interaction.
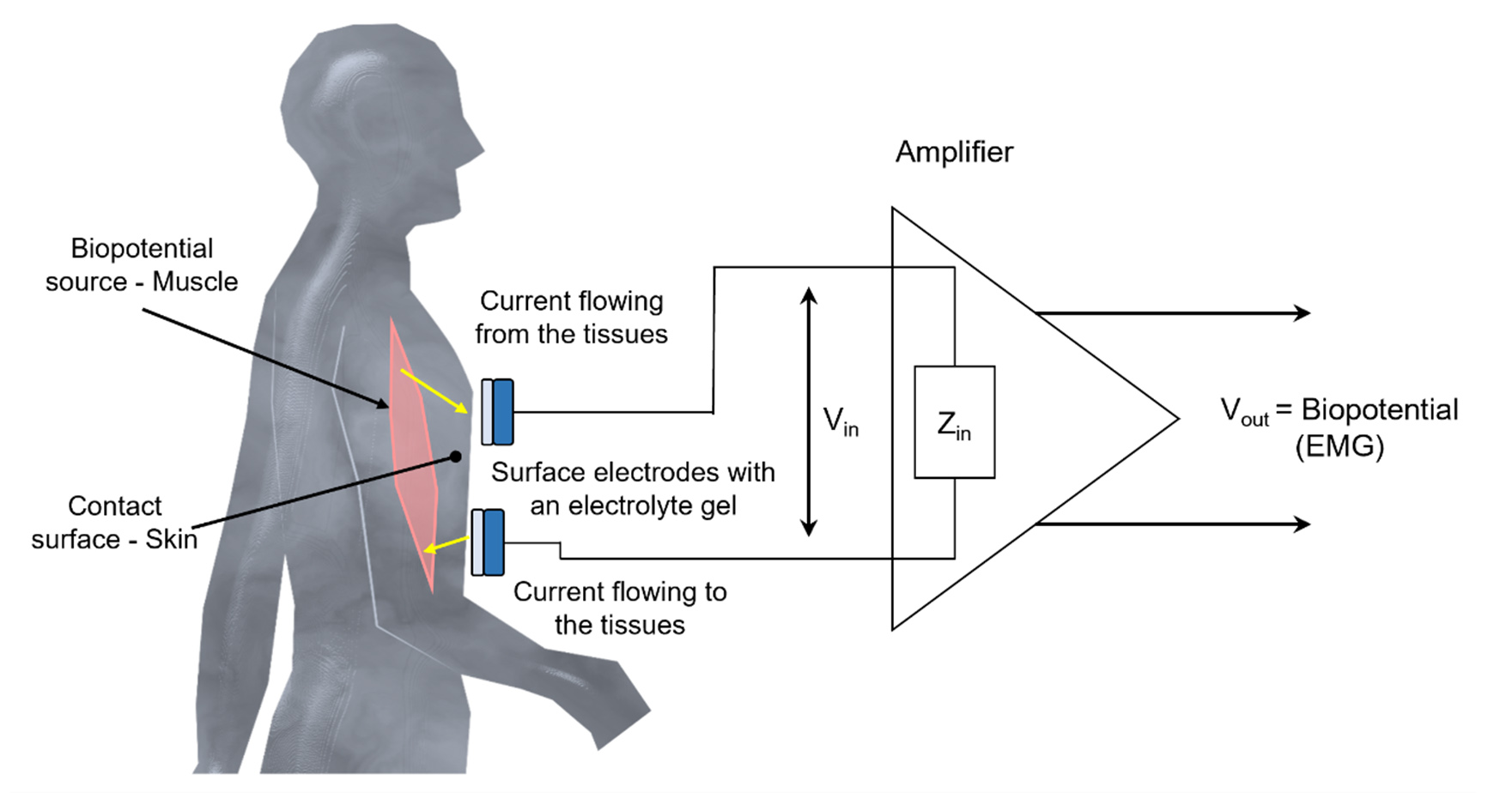
The most widely accepted and used interfaces for recording biopotentials are silver/silver-chloride (Ag/AgCl) electrodes[23]. These electrodes are the gold standard for conducting tests and research in the medical and bioinstrumentation industry[24]. Their performance has been constantly under study, and their parameters are well-defined, displaying remarkable consistency[25]. However, Ag/AgCl electrodes, due to their use of electrolyte gel, cannot be used for the long term, which is an important consideration for producing convenient wearable devices. Moreover, the materials used have a short life cycle. Other issues, such as inelasticity, obtrusiveness, and non-reusability, make them unfit for integration with wearable HCI devices[26-28]. Figure 3 shows the basic Ag/AgCl biopotential electrode and its electrical circuit model while interfacing with the skin. A usable biopotential signal is generally acquired by amplifying the surface recording by an electrode interface. Figure 4 shows a schematic for surface EMG (sEMG) biopotential signal acquisition.
Figure 3. Conventional disposable Ag/AgCl biopotential electrode interface and its circuit equivalent model. Ehc: Half-cell potential of electrode. Cd: capacitance of double layer in the electrode/electrolyte interface. Rd: resistance of double layer in the electrode/electrolyte interface. Rg: resistance in the electrolyte. Ese: potential drop from the ionic concentration difference between stratum corneum and electrolyte. Ce and Re: capacitance and resistance of the epidermis impedance, respectively. Ru: resistance representing dermis layers.
Figure 4. Biopotential signal measurement schematic representation. Zin is the input impedance of an amplifier. Vin is the voltage difference.
Integrating interfaces and sensors into wearable HCI devices requires embedding soft and advanced materials. Soft materials can improve the physical attributes of these electrode interfaces, such as conformability, safety, size, attachment, adaptability, efficiency, accuracy, and ergonomics, among others, while advanced engineering materials can improve conductivity, reliability, stability, and more. These attributes address some of the limitations of current technology[29-31]. Recent progress in areas such as soft material designs[32], nanomaterials[33], stretchable electronics[34-37], energy harvesting[38,39], and wireless communication[40,41] has shown promising results in improving the wearability and portability of wearable HCI devices.
Kwon et al. developed a fully equipped soft biopotential EMG wearable device that consists of a stretchable serpentine-designed interface and embedded bioelectronics[42]. Their work displayed excellent EMG recording capabilities by using machine learning algorithms and also demonstrated real-time wireless control of other devices. 3D printing technologies have opened up a new realm of possibilities by allowing rapid prototyping of soft interface structures and enabling supplication-specific designing[43-45]. Zhu et al. have successfully demonstrated the direct printing of biomedical devices on live human organs by using an adaptive 3D printing approach[46]. If such innovative methodologies are applied to the development of soft biopotential interfaces, the HCI capabilities of wearable devices can reach possibilities that are currently beyond our comprehension.
The paper reviews some recent works in soft biopotential electrode interfaces and discussion of their abilities with regard to wearability and possible HCI applications. For this study, we separate the four primary biopotential signal interfaces (EMG, EEG, ECG, and EOG) into their main sections and survey different types of soft electrode/interface technologies developed in the sub-sections. Each biopotential interface technique will be briefly explained in terms of its working methodology, its use, and the limitations of current technology. Finally, each sub-section will discuss recent works and their possible applications.
Soft electromyography interfaces
Electromyography (EMG) signals are biological signals generated from the electrical activities in the muscles. EMG recording is used as a technique that provides indirect information on muscle activity and even muscle condition[47-49]. sEMG is one of the most utilized muscle interface methods since it is non-invasive. Due to the reliability of this method to acquire bio-signals while being completely non-invasive, it has been extensively studied for decades[50].
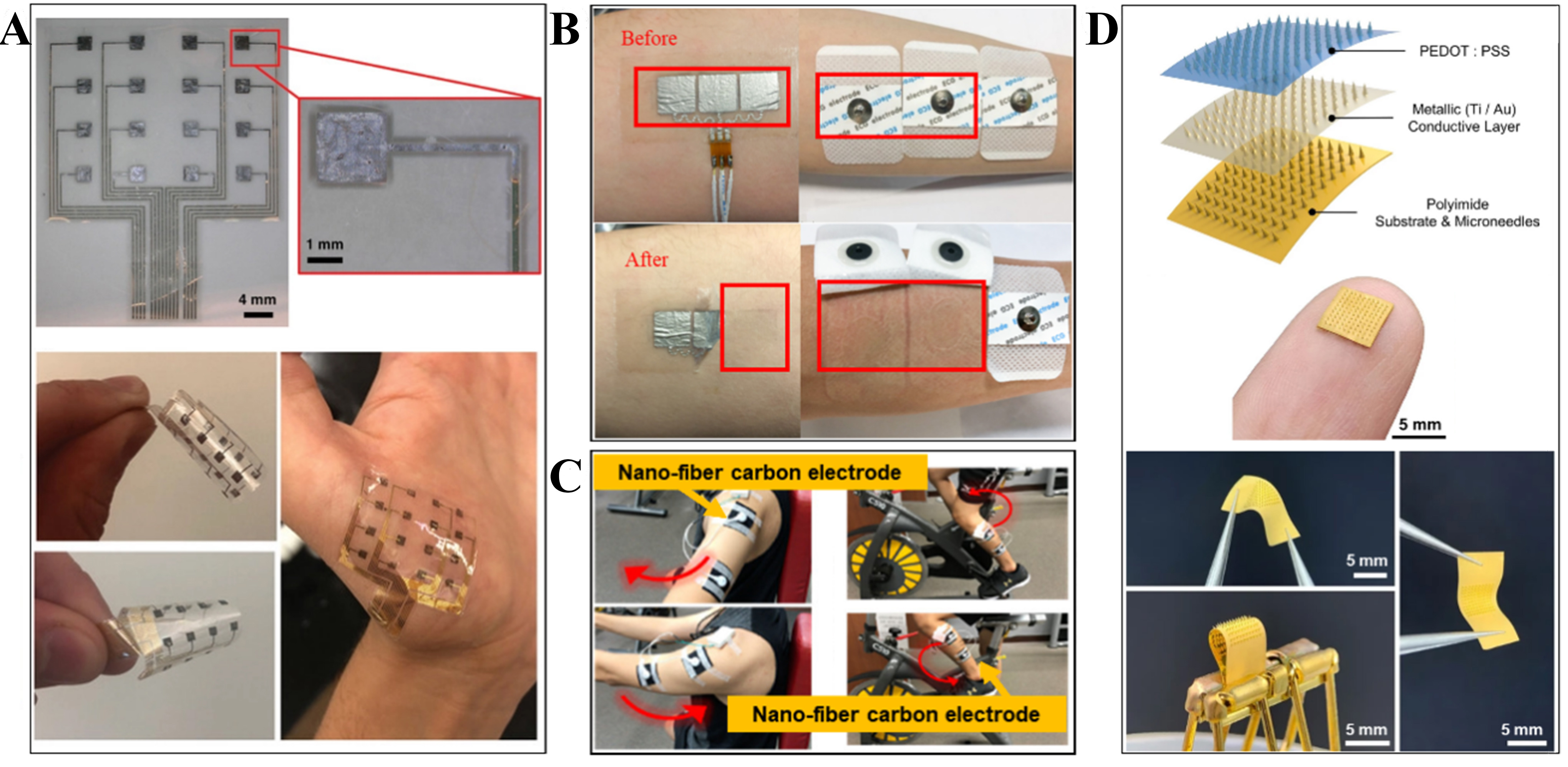
Ag/AgCl electrodes are the norm for analyzing biopotential signals non-invasively. However, while these Ag/AgCl electrodes have been used in several professional industries, such as medicine and bio-instrumentation, they hold an unimpressive share in the wearable device sector. To pass as a viable biopotential interface in wearable devices, using soft materials along with unique designs show great potential in shaping some of the newer sEMG interfaces. On the other hand, using soft materials can affect the conductivity of the sEMG interface, which inherently determines the quality of the output signal. Although recent advances in stretchable and flexible materials satisfy the mechanical requirements that are needed in wearable devices for HCI, it is equally important for these soft interfaces to exhibit competitive electrical performance. Figure 5 showcases different types of soft sEMG interfaces. Using soft materials along with unique designs has proved to be of great potential in shaping some of the newer sEMG interfaces to become more user-friendly and comply with the requirements needed in wearable devices for HMI.
Figure 5. EMG (A) Gel-free flexible HDsEMG electrode array. Reproduced with permission from Murphy et al.[52]. Copyright 2020 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim (B) Stretchable and flexible hydrographic-printing-based EMG electrode[57]. (C) Textile-based stretchable and flexible wearable EMG electrode interface. Reprinted (adapted) with permission from Huang et al.[59]. Copyright 2021 American Chemical Society. (D) Flexible Microneedle biopotential electrode array[60].
Flexible EMG interface
One of the critical features required for an sEMG interface to operate as a sensor on a wearable device is flexibility. Besides that, durability, biocompatibility, and size are some factors that are necessary as well.
The SLIP (Sub-Liner Interface for Prosthetics) electrode developed by Yeon et al. is a prime example of a flexible sEMG interface with HMI capabilities[51]. This sEMG was fabricated using polyimide film as the base substrate, with gold as the electrode material. The thickness of this electrode is around 80-100 μm. Since this electrode is developed using flexible PCB manufacturing techniques, it has good reproducibility. The group successfully demonstrated the human-machine interaction of the SLIP electrode by performing clinical trials on humans with lower-extremity amputation. While demonstrating walking using a prosthetic limb, it was found that the flexibility and sleek design of the electrode played a vital part in comfortable signal acquisition.
Another example of a flexible sEMG interface is the high-density sEMG (HDsEMG) electrode developed by Murphy et al.[52]. This sEMG interface is particularly interesting, showcasing a high-density and gel-free flexible electrode, a fitting candidate for wearable devices. It was fabricated using titanium carbide (Ti3c2Tx) Mxene encapsulated in Parylene-C. This electrode has demonstrated some desired characteristics such as decent skin conformability, hydrophilic surface terminations, excellent conductivity, low interfacial impedance, flexibility, and being around 8 μm thick with 16 recording channels. Since it is gel-free, unlike the Ag/AgCl electrodes, it has the potential to perform as a sensor in wearable devices. This array-based sEMG electrode can eventually support multiple wearable applications, as already demonstrated by the group. In Driscoll et al., with slight changes to the fabrication process, a similar MXene-based bioelectronic interface was developed[53]. Here, they not only performed high-fidelity biopotential signal recording but also demonstrated the stimulation capability through in-vivo experiments.
Soft-stretchable EMG interface
Human skin has a low modulus, making it stretchable and flexible[54]. Skin can also be dry and uneven, which adds some non-contact points between the electrode and the skin, resulting in insufficient coverage over the sensing area, lower resolution, and loss of reliable data[55]. Although flexible sEMG electrodes can bend, their inability to stretch and precisely conform to the surface of the skin can affect their performance. Therefore, it is important for the interfacing electrode to adapt to the constant stretching movement it is subjected to when attached to the surface of the skin.
Stretchable sEMG interfaces are sometimes called “e-skin” due to their ability to stretch akin to skin. Yu et al. developed a stretchable sEMG electrode using inkjet-printing technology using multiple custom-developed nanomaterial inks[56]. Although this sensor can sense multiple signals, we focus on its bio-signal acquisition and wearability performance. This stretchable interface uses PDMS as the base substrate and demonstrates high stretchability and good mechanical compliance. In addition, this inkjet-printed, four-channel, three-electrode, and serpentine-structured sEMG array electrode has the capability to recognize certain hand gestures after applying various machine learning algorithms. It has a wide range of wearable HMI applications, such as gesture-controlled IoT devices or robotic limbs.
Another type of stretchable and flexible sEMG electrode developed by Zeng et al. uses a hydrographic printing technique[57]. This technique allows the electrode to be directly transferred onto the skin, resembling an artificial tattoo. Since the electrode is essentially printed on the skin, the skin acts as an efficient stretchable natural substrate. In terms of recording sEMG signals, this electrode matched the performance of an Ag/AgCl electrode. When it comes to wearability, this electrode shows high conformability and long-term usage. The electrode is gel-free, which gives it an upper hand compared to the commercial electrodes, and upon detachment, it does not leave any distinctive marks, such as redness or swelling.
Textile-based EMG interface
Integrating sensor technology with existing clothing can enhance the user-friendly aspect of wearable devices. Apart from the physical aspect, such as wearability and comfortability, it improves the psychological aspect of wearable devices[58]. Since wearing clothes is a daily activity, built-in textile-based sEMG sensors can go unnoticed by the user, hence reducing the consciousness of wearing sensors.
A stretchable and flexible nanofiber carbon film-sensing electrode is developed by Huang and Chiu, which can perform EMG and ECG monitoring[59]. We focus on the EMG part. This textile-based EMG interface uses carbon as its conductive material, giving it an upper edge compared to metal-based conductive fabrics. This particular EMG interface has some excellent wearable parameters that can seamlessly blend into clothing, such as chemical resistance, washability, good skin contact, and wear resistance. Their experiments demonstrated various EMG applications, such as recording signals of upper and lower limb movement and finger movements. Such wearable EMG technology can provide multiple functions and applications ranging from healthcare monitoring to HMI applications such as gesture-controlled devices. This textile-based electrode could be used for long-term usage compared to Ag/AgCl.
Soft microneedle EMG interface
Microneedle EMG interfaces are not entirely non-invasive but can still be implanted on the skin without surgical techniques. In addition, microneedle EMGs have benefits over sEMG electrodes in acquiring more accurate biosignals. This is due to the micro-needle-like structures penetrating the skin and effectively reducing the impedance.
A polyimide-based microneedle array (MNA) developed by Li et al. is a soft EMG interface that can record high-quality biopotential signals[60]. Apart from excellent electrode-skin contact, using flexible material to fabricate this electrode allows it to have features such as long-term-wearability, bio-compatibility, and inexpensive manufacturing. The performance of this flexible MNA is noteworthy in terms of wearability aspects which were demonstrated through clinical studies. The electrode was used for long periods of time (8 hours/night for 44 nights) by multiple healthy subjects for the sleep-monitoring type of data accumulation. This successful study proved that these electrodes have the potential to substitute conventional clinical standard Ag/AgCl wet electrodes. Upon repeatedly penetrating the MNA electrode in the skin up to 100 times, no fractures were observed on the skin, and no inflammation or any other reactions were noticed. Some slight penetration marks are seen upon electrode detachment from the skin; however, they tend to fade within hours.
Since microneedle EMGs can provide better quality signals compared to sEMG, their use can be extended to a wide range of wearable applications where near-accurate EMG signals can be beneficial.
Soft electroencephalogram interfaces
Numerous neurons in the brain are responsible for generating, transmitting, and processing mysterious electrophysiological signals[61]. Electroencephalogram (EEG) indicates recorded neural activities on the scalp surface. Although the resolution limitation of EEG, this non-invasive method of recording brain activities enables various applications, such as monitoring mental conditions and controlling machines. To effectively identify the recorded weak biopotentials, most of the EEG interfaces follow an international standard for electrode placement known as the 10-20 system[62]. By acquiring EEG signals, the brain can interact directly with the outside world without the intervention of the peripheral nervous system[63-65].
Generally, BCIs are implemented for various applications, including acquiring and amplifying signals, extracting and classifying features, controlling signals, and providing feedback[66-68]. These types of interfaces are categorized into invasive and non-invasive branches based on signal acquisition methods or contact between the electrode on the scalp of the patient[69]
Among the non-invasive techniques such as magnetoencephalography (MEG), near-infrared spectroscopy (NIRS), magnetic resonance imaging (MRI), functional MRI (fMRI), and EEG, EEG is considered one of the most non-invasive, realistic, and practical BCIs[70].
Due to the advantages of the EEG technique compared to other signals, such as direct measuring the cerebral activity, high-resolution mobility (1 ms) for use in clinical settings, ease and portability to clinical use, long-term stability, and adapting to multiple experimental paradigms, it is commonly used for measuring signals of different brain activities[71,72].
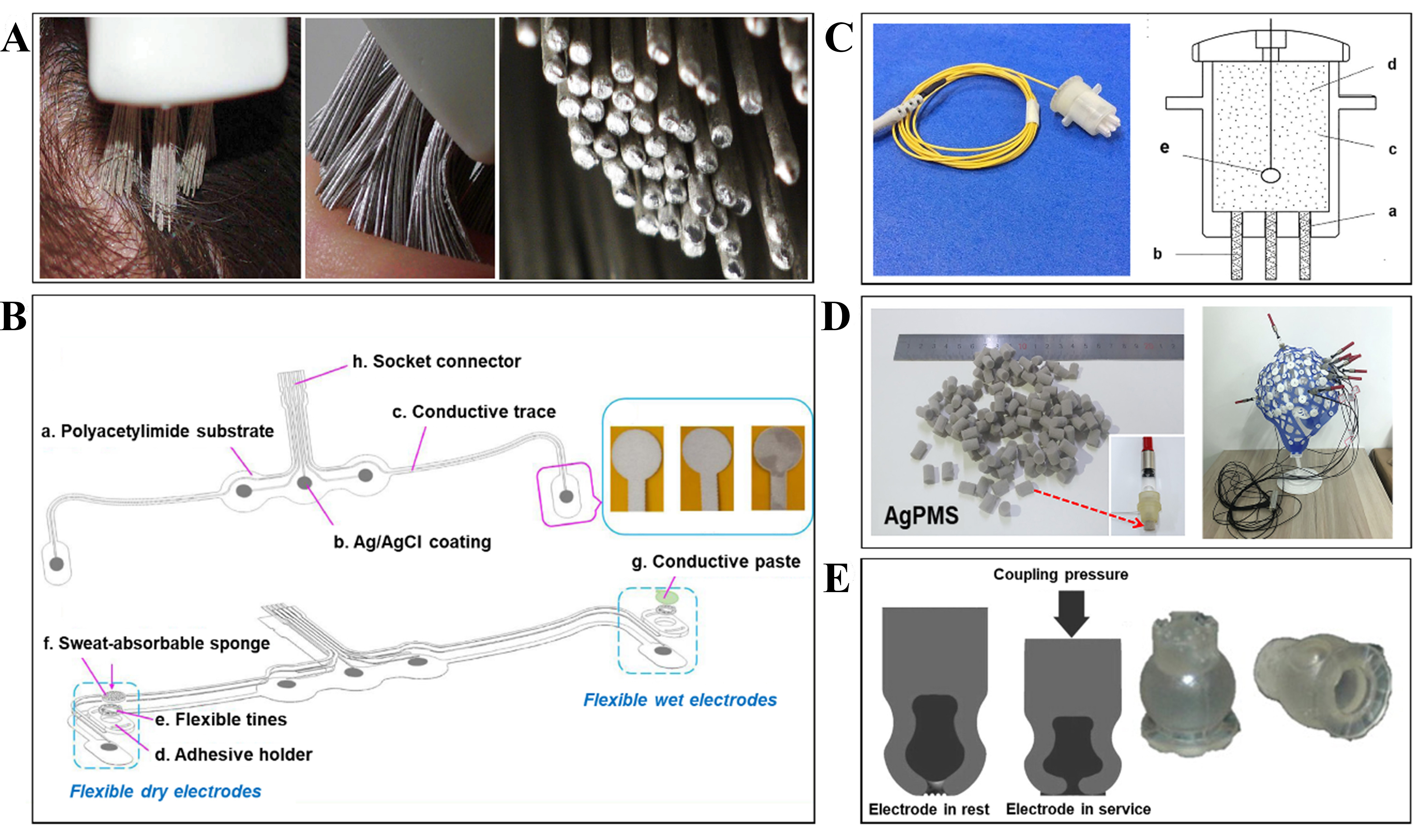
These types of non-invasive BCIs have been divided into wet, dry, and semi-dry electrodes according to the issue of whether the conductive gel is required for the electrode or not. However, it is important to note that most dry electrodes have unacceptably high contact impedances, while wet electrodes require lengthy setups and conductive pastes or gels to be used. Hence, semi-dry electrodes have been invented as a 3rd type of electrode to moderate the disadvantages of the two previous groups. Figure 6 shows a variety of flexible, semi-dry, and soft EEG interfaces. In the following, each type of electrode with its pros and cons will be addressed.
Figure 6. Flexible dry and semi-dry electrodes: (A) Silver-coated polymer conductive bristles dry electrode instead. Reproduced with permission[81]. Copyright 2011, the Authors. Published by IOP Publishing. (B) Schematic diagram of assembled screen-printed flexible Ag/AgCl electrode array. Reproduced with permission[83]. Copyright 2020, the Authors. Published by IOP Publishing. (C) Photo and schematic diagram of a single semi-dry electrode including porous ceramic pillars (a), a built-in reservoir (b), 3.5% saline solution (c), and sintered Ag/AgCl electrode (d). Reproduced with permission[86]. Copyright 2016, Elsevier. (D) Hollow cylinder electrode consisting of a PVC shell, infiltrated normal saline, and an AgPMS contact and assembled EEG electrode. Reproduced under the terms of an ACS AuthorChoice License[78]. Copyright 2019, Copyright American Chemical Society. (E) Diagram illustrating a semi-dry electrode that expels the hydrating agent. Manufactured polyurethane electrodes. Reproduced with permission[77]. Copyright 2013, Elsevier. EEG: electroencephalography.
Wet flexible EEG interface
In literature, this kind of electrode requires electrolytic substances to improve the conductivity of the scalp-electrode. In the commercial stage, these electrodes are composed of Ag/ AgCl disk and an infiltration substance, i.e., conductive gel, which contains electrochemical potential stability and infiltration ability. Due to the advantages mentioned above, wet electrodes are currently prevalently used.
However, a list of drawbacks exists that should be considered and solved. One of the common problems is the time-consuming procedure of cleaning up and controlling the amount of conductive gel for each electrode point. Another critical issue is reducing the moisturizing level of gel during the experiment, which results in poor EEG signal quality as an increasing impedance[73].
To mitigate these problems, dry and semi-dry electrodes were suggested.
Dry flexible EEG interface
Dry flexible electrodes are leveraged to overcome the inconvenience and unstable recording conditions of wet electrodes, as mentioned before. Indeed, dry electrodes make dry connections with skin that do not require conductive gel or any skin preparation and are desirable for portable and wearable electronic devices[74-76]. However, in addition to these benefits, dry electrodes have several drawbacks, including the fact that they are bulkier, more costly, and more prone to movement aberrations because there is no liquid contact[77].
Due to the lack of humid and electrolyte media in dry electrodes, an array of structural designs is implemented for improving the contact area, including the spongy[78], nanowire[79,80], microtip, and variable structural designs, or achieving highly flexible and stretchable structures and deformable conductors.
A wide range of brain-computer interactions can be performed using flexible dry EEG electrodes, as described by Grozea et al.[81], including recording alpha rhythms in the occipital region, testing event-related potentials using an oddball auditory paradigm, and implementing sensory-motor rhythms-based event-related desynchronization paradigms. The electrode was made by coating thin polymer bristles with silver-based conductive ink. Its bristles almost flex as easily as those in toothbrushes, and it is hard enough to pass through hair and reach the surface.
Compared to gel-based electrodes, the bristle sensors produced almost the same signal in the recorded frequency range between 7 to 44 Hz. Furthermore, it maintained mechanical and electrical contact, which is recommended for long-term usability. Compared to both gel-based electrodes and arrays of pin electrodes, this electrode demonstrated excellent comfortability.
To overcome the drawbacks of wet electrodes, a novel flexible dry electrode was proposed by Wang et al., which did not need any conductive gel and skin preparation[82]. This PDMS-based flexible electrode with pins structure indicated superior contact impedance compared to a standard wet electrode without skin preparation, while it was higher than that with a skin preparation. This flexible dry electrode is safer and more comfortable and can meet the requirement of high-quality EEG measurement.
Furthermore, Li et al. fabricated a unique flexible Ag/AgCl dry electrode array with a sweat-absorbable sponge for frontal EEG monitoring[83]. This coating exhibited exceptional non-polarizability and adhesion performance. Sweat absorption can be substantially aided by the sponge. Furthermore, it uses sweat as the electrolyte, effectively eliminating the risk of cross-interference or short circuits while lowering the contact impedance. All of the findings supported the viability of forehead EEG recording. Forehead EEG recording techniques make wearability more accessible. Golparvar et al. developed a graphene-based e-textile interface that can record brain waves[84]. This graphene-based textile EEG interface is made with a Dip-Dry-Reduce method. The main materials used are hydrophilic nylon textile and graphene oxide solution. The reliability of this wearable device was demonstrated by comparing it with commercial dry electrodes in EEG recording experiments. Another wearable e-textile EEG recording device developed by Carneiro et al. contains 11 electrodes for interfacing with the forehead site and an electronic circuit for signal processing[85]. Their design and fabrication process allows this soft latex-based interface to have two open sites, one to contact the epidermis and the other to allow connection with the electronics. The inclusion of the electronics system along with Lithium-Polymer batteries allows 24 hours of operation time, which is favorable for wearable device applications. For a variety of EEG recording and monitoring applications, flexible dry electrodes provide quick setup, user-friendliness, self-application, and wearer comfort. In general, the flexible dry electrodes presented rapid setup, user-friendliness, self-application, and wearer comfort for various applications of EEG recording and monitoring.
Semi-dry soft EEG interface
Several non-invasive electrode types, such as spring-like, porous, or sponge-like structures, and materials consisting of elastomers and hydrogels are known as semi-dry or quasi-dry electrodes[86-88].
The self-storage and gradual release of electrolytes make this type of electrode stand out from wet and dry electrodes[89]. Indeed, Semi-dry electrodes release electrolytes at a slower pace than wet electrode types, depending on the particular structural design or substance. Therefore, this value will have an impact on preserving low contact impedance and preventing short-circuit interference between electrodes. Despite these advantages, large-scale manufacture is limited due to specific structural designs or particular materials.
Furthermore, regarding the usage of soft materials, the mechanical mismatch between electrodes and the human scalp is low[90]. As a result, semi-dry electrodes are advised for prolonged therapeutic usage, including sleep monitoring and rehabilitative treatment[91].
In 2019, Lin et al. reported a type of Gel-Free EEG Electrode with a high conductivity of 917 S/m due to the surface metallization by the silver nanowires (AgNWs). Compared to the conventional electrodes that contained conductive gel, the new electrode showed almost the same steady-state visual evoked potential (SSVEP) as that of the conventional one. In conclusion, the cost-effectiveness, simplicity of manufacture, flexibility, robustness, relatively low electrode-skin impedance, and mechanical stability of this electrode were its main strengths.
In addition, Li et al. proposed innovative passive semi-dry electrodes for recording EEG data from the hairy scalp. This ceramic-based electrode was able to attain a low and stable impedance and an SSVEP paradigm. Due to the capillary force through porous ceramic pillars, a regulated amount of saline solution could be released, which eliminates skin preparation and gel application. EEG signals were consistent, and electrode polarization voltage demonstrated a steady state that was similar to commercial gel-based Ag/AgCl electrodes. Semi-dry electrodes were applied to real-world practical EEG applications, such as brain-computer interfaces and wearable technology.
A quasi-dry electrode for EEG is fabricated by Mota et al.[77]. This polymer-based electrode was able to discharge 30 μl of a hydrating agent, which decreased the volume of gel needed to simply cover the contact sites of electrodes. The obtained legitimate EEG signals were comparable to those of commercial Ag/AgCl reference electrodes, demonstrating the suitability of electrodes for BCI applications.
Soft ECG interfaces
ECG is a non-invasive technique used for obtaining data on the electrical activity of a heart through small electrical changes caused by the continuous depolarization-repolarization cycle of the cardiac muscle[92]. In layman’s terms, an ECG signal is the recording of the electrical activity of the heart. This technique provides information on the autonomic nervous system responsible for the electrical activity of the heart[93,94]. An ECG signal has multiple waves that represent the specific state of the heart during a heartbeat cycle. This signal or wave is called the PQRST wave, where P indicates atrial contractions, QRS indicates ventricle contraction, and T indicates ventricle expansion[95]. Abnormalities in PQRST intervals can indicate heart-related problems. ECG recordings have been extensively used in the medical field to record or monitor the electrical activity of the heart in patients[96]. Abnormalities in ECG readings have proven to be essential for medical experts to determine the health condition of the patient[97].
Since ECG is mostly used for health monitoring, factors such as long-term usage, high stability, and stable attachment to the epidermal layer are of great importance and should be considered when developing wearable devices. Ag/AgCl electrodes are the industry standard for recording ECG signals, but they lack the wearability aspects mentioned in the previous section.
ECG monitoring can play a vital role as a wearable device. With the increased awareness of physical health and lifestyle maintenance, keeping track of the everyday performance of the body has become a necessity[98]. Many wearable technologies, such as smartwatches and fitness bands, already include heart-rate monitoring systems; however, most of these technologies have a different method for detecting these biopotential compared to the traditional ECG technique[99,100]. These devices generally use photoplethysmography (PPG) optical sensors that detect the change in blood volume during a cardiac cycle. These wearable devices have effectively shown impressive results as wearable heart-rate monitoring technology[101]. However, the PQRST wave cannot be precisely determined by this method, and a lot of vital data can go unmonitored. To record such delicate information, the ECG interfacing device should be placed on the skin. The inclusion of soft ECG interfaces can provide critical information in applications where detailed and accurate heart activity readings are needed to detect cardiac abnormalities in a subject under intensive care[102].
Flexible and Stretchable ECG interfaces
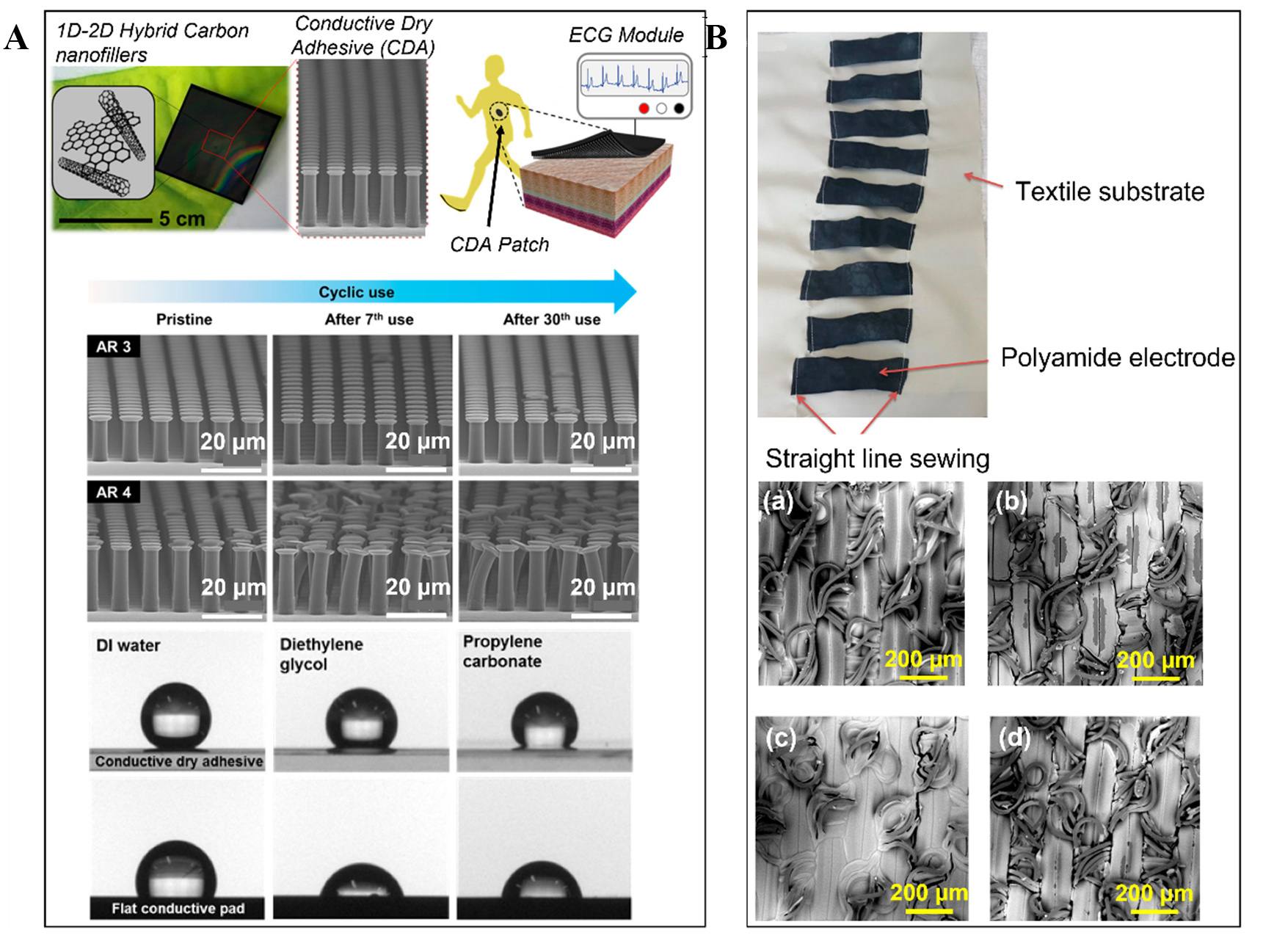
Inspired by the adhesion mechanism of the gecko’s feet and the advanced nanomaterials, these ECG electrodes developed by Kim et al. drastically improve the adhesion force on the skin while eliminating the need for an electrolyte gel[103]. The electrode is manufactured using materials such as PDMS and carbon-based nanofillers and is patterned in a carefully fabricated mold. This mushroom-shaped micropillar array electrode also exhibits flexibility and stretchability ( > 100%) while satisfying the electrical parameters. To add to its advantages, this bioinspired ECG electrode has a superhydrophobic surface that allows it to work when immersed underwater and also makes it prone to turning into a dusty surface. This property allows the electrode to be cleaned by simply washing it, allowing reusability up to a limit. Various experiments were carried out to demonstrate the unique properties of this electrode, such as adhesivity and superhydrophobicity.
Figure 7A shows the gecko-inspired ECG interface. Such bioinspired ECG electrode interfaces have benefits over traditional ECG electrodes. The ability to adequately perform while being immersed in water and not lose conformal contact while doing so is impressive. Moreover, when the user is in motion, the ECG readings can still facilitate meaningful data in real time, thus displaying great stability.
Figure 7. Soft ECG Electrode Interfaces: (A) Highly stretchable and conductive gecko-inspired dry ECG electrode. Reprinted (adapted) with permission from Taehoon Kim et al.[103]. Copyright 2016 American Chemical Society. (B) Washable textile-based ECG electrode interface (top) and SEM images of the washable PEDOT:PSS electrodes coated by S1 and S2 solutions (bottom) (a) S1 sample before washing; (b) S1 sample after 50 washes; (c) S2 sample before washing; (d) S2 sample after 50 washes[104].
Wearable textile-based ECG interfaces
Since health-monitoring wearable devices are meant to deal with extended periods of usage, apart from being imperceptive, it is also necessary to record and deliver data accurately in case of different motions it may be subjected to. Isolating motion artifacts can help provide reliable data, enhancing the reliability of wearable ECG devices. Wearable textile-based dry electrodes are favorable for such long-term applications. However, other factors, such as washability and retainment of signal quality, are necessary for such a type of soft electrode.
A poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) textile-based ECG electrode developed by Ankhili et al. is a wearable type of interface that can be washed and re-used[104]. The textile material used in this electrode is a polyamide textile fabric that absorbs a modified version of the PEDOT:PSS solution during its fabrication. This electrode was able to deliver ECG signals even after multiple washing cycles. However, it was found out in an experiment that after 50 washes, even though the ECG signals can be clearly monitored, a significant drop in can be seen in the signal-to-noise-ratio (SNR) values. The loss of SNR was inculpated by the fact that after washing the electrode, PEDOT:PSS was lost. Two samples of these interfaces were developed with two PEDOT:PSS solutions that had different viscosities (termed S1 and S2). Figure 7B shows the textile-based electrode interface and the effect on the textile-based ECG electrodes produced with solutions S1 and S2 after multiple washes.
Integrating soft sensors in clothing is a promising method for long-term monitoring applications. With significant efforts put into textile-based ECG interfaces, a stable and reliable wearable garment-type device can be developed to assist with athlete performance in real-time or patient health monitoring in real-time without discomfort.
Soft electrooculography interfaces
Electrooculography (EOG) is a technique used for recording the corneal-retinal potential difference[105-107]. EOG interfaces can allow effective communication for people with severe motor disabilities[108,109]. EOG has also seen its use in some of the newer HCI virtual reality systems wherein the user can navigate through a virtual environment using eye movements[110,111]. Using soft technology to develop EOG electrodes can improve their wearability. Since these types of electrodes are attached to the face of the user, being thin and transparent can enhance the user experience.
Flexible and Stretchable EOG interfaces
This EOG electrode developed by Won et al. is fabricated using a plant-based bioplastic - polylactic acid (PLA) and PEDOT:PSS[112]. The conductive element, PEDOT:PSS is sandwiched between two substrate PLA layers. These materials make the interface soft, transparent, conductive, and biocompatible. Using fabrication techniques such as spin coating and laser cutting, thin Y-shaped kirigami patterned voids are created, which allow not only multidirectional stretchability and high areal coverage but also breathability. The fabrication process also allows rapid and scalable fabrication since it eliminates the use of time-consuming chemical-dependent patterning and etching processes generally used in microfabrication. Since there are no sacrificial layers, this soft device can be easily peeled off from the substrate. To accommodate external electrical contact, gold was deposited and used as a contact pad on PEDOT:PSS. The group also demonstrates the human-machine interaction capability of the interface by integrating it with a signal processing unit and switching various electric devices on and off by eye movements of the user wearing the soft electrode interface. Figure 8A shows images of the soft EOG interface showcasing the Kirigami structure and all its soft properties.
Figure 8. (A) Soft EOG interface with Y-shaped Kirigami motifs showcasing properties such as flexibility, stretchability, and transparency. Reprinted (adapted) with permission from Won et al.[112] Copyright 2021 American Chemical Society. (B) Imperceptible electrooculography graphene electronic tattoo. Reprinted (adapted) with permission from Ameri et al.[113]. Copyright 2018 Springer Nature. EOG: electrooculogram.
Since EOG interfacing is performed on the facial area of the body, transparency or imperceptibility becomes an important consideration when designing soft EOG interfaces. Ameri et al. developed a non-invasive graphene electronic tattoo (GET) type of EOG interface[113]. This imperceptible interface has highly desirable features such as breathability and transparency while being ultrasoft, as seen in Figure 8B. Additionally, the thickness of this GET EOG interface is 350 nanometers - making it ultrathin and allowing 85% optical transparency. This graphene-based interface is also capable of maintaining conformal contact with the skin without any adhesive. Lastly, the group demonstrates its functionality by controlling a quadcopter with eye movements.
There is evident scope for wearable applications using such soft EOG interface technology. Apart from controlling tangible devices in the real world, this type of interface has shown its practicality in controlling graphical user interfaces (GUI) in virtual reality environments[114]. Allowing the user to operate and navigate through a virtual environment using just eye movements can heighten the user experience. On the other hand, there might be certain limitations since eye movements can cause fatigue to the user.
Some soft EOG interfaces have also been used in sleep monitoring by integrating them into a sleeping mask[115,116]. However, users may feel discomfort wearing such masks during sleep and might prefer alternative methods of sleep tracking if needed. However, in some use cases, such technology can benefit patients who require sleep-tracking data that can provide useful insights to a medical expert to provide correct medical advice.
Innovative strategies for improved wearable soft interfaces
Soft biopotential interfaces in wearable devices can benefit from technologies such as soft electronics and optimized energy systems. Since wearable devices require a portable energy source to operate, most systems use battery systems. These battery systems face limitations when scaled down in size and can make the system bulky. Innovative strategies, such as energy harvesting systems and self-powered systems, have been integrated into these soft biopotential interface technologies to enhance wearability.
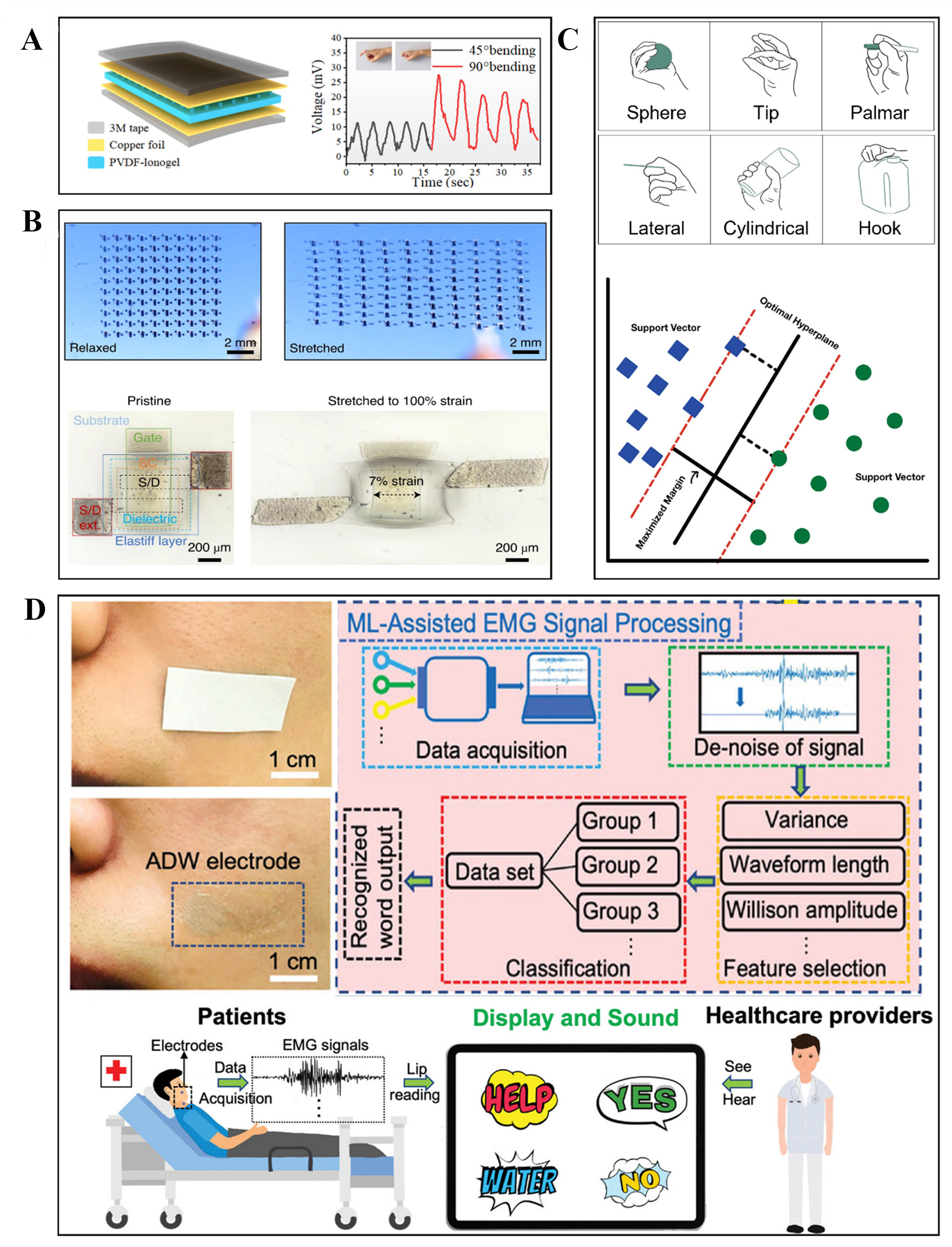
Li et al. developed a poly(vinylidene fluoride) (PVDF)-based self-powered wireless flexible wearable device that helps in overcoming the limitations of the battery system in wearable devices[117]. Apart from displaying high stretchability (1,500% of its original length), this PVDF-ionogel device has the ability to convert external pressure into electricity, which enables it to power itself. The electricity generated by this piezoelectric property is enough to charge a 4.7 μF 50 V capacitor to 0.7 V within 225 seconds. Although this device was used to measure different body movements, the inclusion of self-powered technology enhances its wearability aspect. The inclusion of such technology in soft biopotential interfaces can improve long-term usage in health monitoring.
Since biopotential interfaces are vastly used in healthcare and health monitoring areas, a slight imprecision in the electronic reading can result in an inaccurate diagnosis of the health of users. Stretchable devices containing electronics often have limited strain capabilities so as to not compromise the electronic performance. Wang et al., along with Bao. Z., developed a strain-insensitive intrinsically stretchable transistor array[118]. The stretchable device was fabricated using an all-elastomer process for applying local stiffness using styrene-ethylene-butylene-styrene (SEBS) as the main material. The device achieved stable electrical performance even under large strains. This property allows stretchable devices to have mechanical flexibility without compromising electrical stability. In soft biopotential interfaces, introducing such technology can make signal recording more precise and accurate. Figure 9A shows PVDF-ionogel self-powered soft and wearable device, and Figure 9B shows the strain-insensitive intrinsically stretchable transistor array under strain.
Figure 9. (A) Schematic of the PVDF-ionogel self-powered wearable device and the output voltages due to bending (above). Reprinted (adapted) with permission from Li et al.[117]. Copyright 2023 American Chemical Society. (B) Images of transistor array under the original and stretched states (above). Optical microscopic images of one transistor in the array under 0% and 100% global strain (below). Reprinted (adapted) with permission from Wang et al.[118]. Copyright 2021 Springer Nature. (C) The six hand gestures performed by the participants (above) and the support vector machine graph (below). Reprinted (adapted) with permission from Fatayerji et al.[119]. Copyright 2023 Springer Nature Switzerland AG. (D) AgNWs-D-sorbitol-WPU (ADW) sEMG enabled lip-reading system and the machine learning-assisted sEMG signal processing along with its application (below). Reprinted (adapted) with permission from Dong et al.[120]. Copyright 2023 Wiley-VCH GmbH. PVDF: poly(vinylidene fluoride).
Utilizing biopotential signals for an application sometimes requires massive computational post-processing and techniques such as machine learning. Fatayerji et al. investigate the performance of two supervised machine learning algorithms - the k-Nearest Neighbor (KNN) technique and the Support Vector Machine (SVM) technique, for hand gesture detection with sEMG signals[119]. Before these techniques are applied, the sEMG signal is first acquired and processed, followed by feature extraction. The required characteristics are highlighted by the feature extraction for recognizing the hand gesture from the obtained sEMG data. The experiments consisted of five participants performing six hand gestures, which were repeated 30 times for six seconds each. The six hand gestures and the SVM graph are shown in Figure 9C. These extracted features from 180 sEMG signals from every subject undergo KNN and SVM techniques. After applying these two techniques to the mined feature set, it was concluded that SVM showed a better overall accuracy score of 97.3%, while KNN was 91.6%. This suggests that applications requiring hand gesture recognition can utilize SVM classifiers as they have significant results in comparison to KNN.
Innovation is not only limited to the development of the interface but also its application. Dong et al. developed a self-adhesive, semi-transparent dry electrode that is specifically used for lip-reading by utilizing sEMG biopotential signals from the facial muscle movement[120]. Machine learning algorithms are applied in the processing, enabling the EMG signals to be converted into audible words, as shown in Figure 9D. Such applications can be extremely useful in the medical and healthcare industry as it enhances patient-medic communication. Moreover, the use of facial EMG can allow immersive interaction in a virtual world as well.
When direct comparisons are made to the standard Ag/AgCl electrode interface, the performance of these soft biopotential sensors has shown comparable or even better results. For instance, low skin-electrode contact impedance is an important factor for biopotential interfaces attached to the epidermal layer of the skin for recording signals. The flexible sEMG electrode by Zeng et al. was fabricated using hydrographic printing and showed an impedance value of around 10 KΩ at 500 Hz, whereas the traditional electrode interface had a value of around 14 KΩ at 500 Hz. Moreover, at lower frequencies, the flexible interface showed significantly lower impedance. Additionally, the SNR values Li et al. reported were comparable between their semi-dry EEG interface and conventional ‘wet’ EEG electrodes. Both devices showed SNR values of around 7 dB in the eyes open/close paradigm experiment. In other SSVEP paradigms, the semi-dry electrode interface performed slightly better. The Gecko-inspired dry ECG electrode displayed its adhesion capability by achieving over 30 cycles of adhesion to the skin. Moreover, the adhesion force was comparable to those of wet adhesive, -1.3 N/cm2. Qualitative analysis is made between traditional and soft biopotential interfaces concerning their wearability aspect, as shown in Table 1.
Qualitative performance comparison of traditional vs. soft biopotential interfaces w.r.t. wearable devices requirement
| Interface type | Type | Conformability | Stretchability or flexibility | Period of usage | Electrolyte Gel | Bulkiness | Signal quality | ||
| Ag/AgCl Electrode Interface | Low | Absent | Short | Present | Medium | High | |||
| Soft Interface | EMG | High | Present | Long | Absent | Low | High | ||
| EEG | Average | Present | Long | Semi | Average | Average | |||
| ECG | High | Present | Long | Absent | Low | High | |||
| EOG | High | Present | Long | Absent | Low | High | |||
Neuromuscular stimulation with wearable non-invasive soft interfaces
Recording biopotential signals using skin-contact surface interfaces has a vast range of applications. Apart from recording biopotential signals, there are applications where the stimulation of nerves is required for rehabilitation purposes[121,122]. Non-invasive functional electrical stimulation (FES) can provide a pathway for non-surgical solutions for brain and nerve stimulation. Moreover, such wearable electroceuticals can allow for therapeutic electrostimulations without the limitations of invasive techniques.
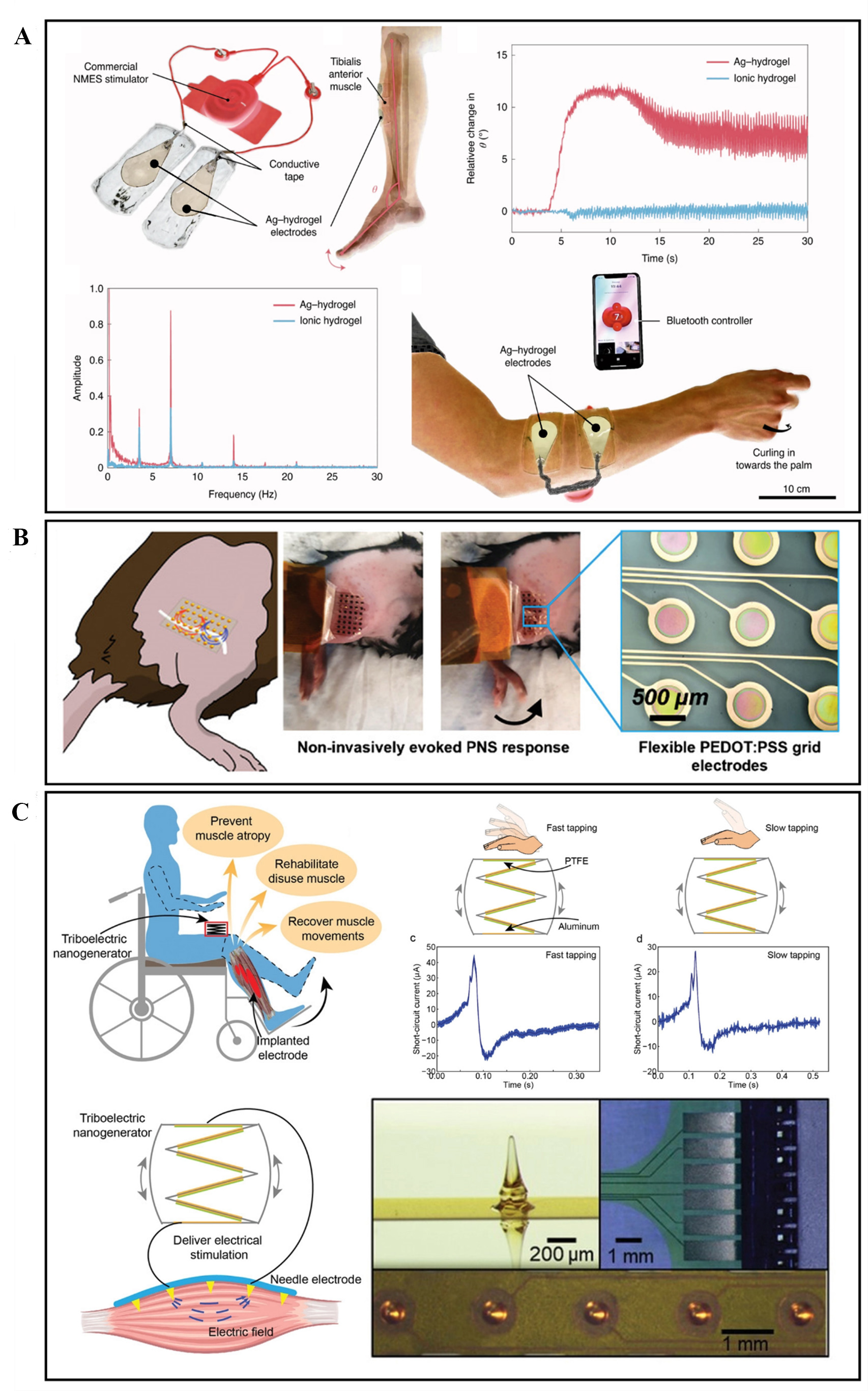
Ohm et al. developed a soft hydrogel-based interface and performed neuromuscular electrical stimulation[123]. The group stimulated the tibialis anterior muscle of the leg and observed angular movement at the ankle. The Ag-hydrogel interface performed better than the ionic hydrogel, as seen in Figure 10A. Additionally, the performance of the Ag-hydrogel electrode interface was comparable to the commercial electrodes. Similar experiments were repeated on the forearm muscles. With characteristics such as stretchability, biocompatibility, and high electrical conductivity, such a soft muscle-stimulating interface has the potential to replace conventional interfaces in the future.
Figure 10. (A) Electrically conductive Ag-polyacrylamide-alginate hydrogel composite used for neuromuscular stimulation of lower and upper limbs. Reprinted (adapted) with permission from Ohm et al..[123] Copyright 2021 Springer Nature Limited. (B) Non-invasive stimulation of peripheral nerves of a mouse using temporally interfering electrical fields from a flexible PEDOT:PSS electrode interface. Reprinted (adapted) with permission from Botzanowski et al.[124]. Copyright 2022 Wiley‐VCH GmbH. (C) Electrical muscle stimulation directly delivered by TENG for rehabilitation purposes. Reprinted (adapted) with permission from Wang et al.[125]. Copyright 2019 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. PEDOT:PSS: poly(3,4-ethylenedioxythiophene) polystyrene sulfonate; TENG: triboelectric nanogenerator.
Peripheral nerve stimulation (PNS) by using non-invasive methods has also been studied[124]. By using high-frequency sine-wave carriers, Botzanowski et al. showed that it is possible to temporally interfere at deep peripheral nerve targets. Temporal interference nerve stimulation (TINS) delivers effective outputs at a lower current than standard transcutaneous electrical stimulation. As seen in Figure 10B, this flexible PEDOT:PSS interface can non-invasively evoke a PNS response. Invasive methods are generally used to achieve better selectivity, and therefore, implantable electrodes are favored for PNS. However, this non-invasive interface uses high-frequency signals to perform efficient stimulation and is comparable to invasive methods.
Wang et al. developed a self-powered multichannel epimysial electrode interface to directly stimulate the muscle[125]. The self-powered system is based on triboelectric nanogenerator (TENG) technology, as seen in Figure 10C. With in-vivo experiments, the stimulation efficiency and stability are investigated for this TENG-based stimulator. This work shows the potential for wearable stimulators that eliminate the need for equipment such as waveform generators and power supplies.
CONCLUSION AND OUTLOOK
Non-invasive biopotential recording techniques, such as EMG, EEG, ECG, and EOG, play a crucial part in establishing valuable communication between users and machines. Since most of these biopotential signals are recorded non-invasively and require skin contact, several possibilities for wearable device applications emerge. Some of the aforementioned soft biopotential interface technologies have already successfully demonstrated human-machine interaction by controlling certain mechatronic devices. To achieve the full potential of a soft biopotential interface, extensive study is required in the sector of advanced materials, energy technology, and signal processing. Several limitations and challenges are still present in the current soft biopotential interface technologies that prohibit their immediate adoption in consumer devices. Even though soft interfaces allow miniaturization and improve conformability, their integration with conventional solid-structured electronics that are required for signal processing act as a barrier to achieving complete softness and flexibility of the device. With various soft materials and structural designs, another challenge that soft interfaces face is standardizing to the industry-ready parameters often leads to ineffective large-scale production. Another major issue to be considered is that human skin is constantly subjected to tiny wear and tear, leaving almost scars and cuts on the surface of the skin that might be invisible to the naked eye. This phenomenon can cause several issues, such as irritation, infection due to material type, loss of adhesion, and inefficiency in healing.
Further development of soft biopotential interfaces to accommodate practical wearable HMI applications is required. Along with soft interfaces, efforts should be made towards minimizing the footprint of signal processing units and energy storage units. Renewable energy and energy harvesting technologies can be involved in powering these untethered bio-interfaces. Wireless technology can help enable communication between the interface and its processing system and, in some cases, even provide power for operation. Besides health monitoring, signal recording, and data accumulation, more research should be carried out with soft non-invasive interfaces for FES, which can, in a way, provide bidirectional (recording and stimulation) applications in cases of physical rehabilitation.
Soft biopotential or neural implants have also been researched for many years. For such invasive technology to penetrate the wearable device market will require extraordinary endeavors towards the biocompatibility, biodegradability, and user-acceptance aspects. Discovering methods to implant soft electrodes and interfaces directly inside a user using minimum-to-no surgical techniques can lead to much more intricate, precise, and useful HCI applications.
Lastly, it is definite that the inclusion of soft biopotential electrode interfaces while developing HCI technologies can provide more options for wearable device applications that are sustainable, credible, and effective. To eliminate the need for Ag/AgCl electrodes completely, more efforts will focus on standardizing the characteristic parameters of soft biopotential interfaces and scaling their manufacturability to the industry level. The fast-paced and promising growth towards these soft biopotential interfaces does assure one thing - “getting used to” the rigid and bulky wearable devices is a phenomenon that our future generations will never have to experience.
DECLARATIONS
Authors’ contributionsInitiated the idea: Nagwade P, Parandeh S, Lee S
Did the literature review: Nagwade P, Parandeh S
Outlined the manuscript structure: Nagwade P, Parandeh S, Lee S
Wrote the manuscript draft: Nagwade P, Parandeh S
Designed and formatted the figures: Nagwade P
Reviewed and revised the manuscript: Nagwade P, Lee S
Supervision: Lee S
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis research was supported by a Korea Medical Device Development Fund grant funded by the Korean government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: 1711135031, KMDF_PR_20200901_0158-05), and it was also supported by the DGIST R&D Program of the Ministry of Science and ICT (22-SENS2-2).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Gong C. Human-machine interface: design principles of visual information in human-machine interface design. In: 2009 International Conference on Intelligent Human-Machine Systems and Cybernetics; 2009 Aug 26-27; Hangzhou, China. IEEE; 2009. p. 262-5.
2. Hristov H, Stavrev S. Generations of human-computer interactions: evolution, tendencies and perspectives. J Phys Conf Ser 2022;2339:012009.
3. Francés-Morcillo L, Morer-Camo P, Rodríguez-Ferradas MI, Cazón-Martín A. Wearable design requirements identification and evaluation. Sensors 2020;20:2599.
4. Yin R, Wang D, Zhao S, Lou Z, Shen G. Wearable sensors-enabled human-machine interaction systems: from design to application. Adv Funct Mater 2021;31:2008936.
5. Shi Q, Dong B, He T, et al. Progress in wearable electronics/photonics-moving toward the era of artificial intelligence and internet of things. InfoMat 2020;2:1131-62.
6. Godfrey A, Hetherington V, Shum H, Bonato P, Lovell NH, Stuart S. From A to Z: wearable technology explained. Maturitas 2018;113:40-7.
7. Tang G, Shi Q, Zhang Z, He T, Sun Z, Lee C. Hybridized wearable patch as a multi-parameter and multi-functional human-machine interface. Nano Energy 2021;81:105582.
8. Park S, Jayaraman S. Enhancing the quality of life through wearable technology. IEEE Eng Med Biol Mag 2003;22:41-8.
9. Zhu M, Sun Z, Zhang Z, et al. Haptic-feedback smart glove as a creative human-machine interface (HMI) for virtual/augmented reality applications. Sci Adv 2020;6:eaaz8693.
10. Gao W, Emaminejad S, Nyein HYY, et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016;529:509-14.
11. Bifulco P, Cesarelli M, Fratini A, Ruffo M, Pasquariello G, Gargiulo G. A wearable device for recording of biopotentials and body movements. In: 2011 IEEE International Symposium on Medical Measurements and Applications; 2011 May 30-31; Bari, Italy. IEEE; 2011. p. 469-72.
12. Vehkaoja A, Lekkala J. Wearable wireless biopotential measurement device. Proceedings of 26th annual international conference of the IEEE engineering in medicine and biology society; 2004 Feb p. 2177-9; San Francisco, CA, USA. IEEE; 2004.
13. Cinar E, Sahin F. EOG controlled mobile robot using radial basis function networks. In: 2009 Fifth International Conference on Soft Computing, Computing with Words and Perceptions in System Analysis, Decision and Control; 2009 Feb 2-4; Famagusta . North Cyprus. IEEE; 2009. p. 1-4.
14. Sasaki M, Matsushita K, Rusyidi MI, et al. Robot control systems using bio-potential signals. AIP Conf Proc 2020;2217:020008.
15. Ding J, Tang Y, Zhang L, Yan F, Gu X, Wu R. A novel front-end design for bioelectrical signal wearable acquisition. IEEE Sensors J 2019;19:8009-18.
16. Magno M, Benini L, Spagnol C, Popovici E. Wearable low power dry surface wireless sensor node for healthcare monitoring application. In: 2013 IEEE 9th International Conference on Wireless and Mobile Computing, Networking and Communications (WiMob); 2013 Oct 7-9; Lyon, France.IEEE; 2013. p. 189-95.
18. Waldert S. Invasive vs. Non-invasive neuronal signals for brain-machine interfaces: will one prevail? Front Neuros 2016;10:295.
20. Pascual-Valdunciel A, Rajagopal A, Pons JL, Delp S. Non-invasive electrical stimulation of peripheral nerves for the management of tremor. J Neurol Sci 2022;435:120195.
21. Koutsou AD, Moreno JC, Del Ama AJ, Rocon E, Pons JL. Advances in selective activation of muscles for non-invasive motor neuroprostheses. J Neuroeng Rehabil 2016;13:56.
22. Caramenti M, Bartenbach V, Gasperotti L, Oliveira da Fonseca L, Berger TW, Pons JL. Challenges in neurorehabilitation and neural engineering. In: Pons JL, Raya R, González J, editors. Emerging Therapies in Neurorehabilitation II. Cham: Springer International Publishing; 2016. pp. 1-27.
23. Kruse J, Lee S. Biopotential electrode sensors in ECG/EEG/EMG systems. Analog Devices 2008;200:1 2. Available from: https://www.analog.com/en/technical-articles/biopotential-electrode-sensors-ecg-eeg-emg.html#/. [Last accessed on 21 Jun].
24. Picton TW, Bentin S, Berg P, et al. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiol 2000;37:127-52.
25. Albulbul A. Evaluating major electrode types for idle biological signal measurements for modern medical technology. Bioengineering 2016;3:20.
26. Bao S, Gia TN, Chen W, Westerlund T. Wearable health monitoring system using flexible materials electrodes. In: 2020 IEEE 6th World Forum on Internet of Things (WF-IoT); 2020 Jun 2-16; New Orleans, LA, USA. IEEE; 2020. p. 1-2.
27. Lopez-Gordo MA, Sanchez-Morillo D, Pelayo Valle F. Dry EEG electrodes. Sensors 2014;14:12847-70.
28. Fu Y, Zhao J, Dong Y, Wang X. Dry electrodes for human bioelectrical signal monitoring. Sensors 2020;20:3651.
29. Herbert R, Jeong JW, Yeo AW. Soft material-enabled electronics for medicine, healthcare, and human-machine interfaces. Materials 2020;13:517.
30. Herbert R, Kim JH, Kim YS, Lee HM, Yeo WH. Soft Material-enabled, flexible hybrid electronics for medicine, healthcare, and human-machine interfaces. Materials 2018;11:187.
31. Kim T, Cho M, Yu KJ. Flexible and stretchable bio-integrated electronics based on carbon nanotube and graphene. Materials 2018;11:1163.
32. Yu X, Xie Z, Yu Y, et al. Skin-integrated wireless haptic interfaces for virtual and augmented reality. Nature 2019;575:473-9.
33. Zhao Y, Hou N, Wang Y, et al. All-fiber structure covered with two-dimensional conductive MOF materials to construct a comfortable, breathable and high-quality self-powered wearable sensor system. J Mater Chem A 2022;10:1248-56.
34. Zheng Y, Yu Z, Zhang S, et al. A molecular design approach towards elastic and multifunctional polymer electronics. Nat Commun 2021;12:5701.
35. Matsuhisa N, Niu S, O'Neill SJK, et al. High-frequency and intrinsically stretchable polymer diodes. Nature 2021;600:246-52.
36. Jiang Y, Zhang Z, Wang YX, et al. Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics. Science 2022;375:1411-7.
37. Zheng YQ, Liu Y, Zhong D, et al. Monolithic optical microlithography of high-density elastic circuits. Science 2021;373:88-94.
38. Vallem V, Sargolzaeiaval Y, Ozturk M, Lai YC, Dickey MD. Energy harvesting and storage with soft and stretchable materials. Adv Mater 2021;33:e2004832.
39. Song Y, Wang N, Hu C, Wang ZL, Yang Y. Soft triboelectric nanogenerators for mechanical energy scavenging and self-powered sensors. Nano Energy 2021;84:105919.
40. Song Y, Min J, Yu Y, et al. Wireless battery-free wearable sweat sensor powered by human motion. Sci Adv 2020;6:eaay9842.
41. Park Y, Kwon K, Kwak SS, et al. Wireless, skin-interfaced sensors for compression therapy. Sci Adv 2020;6:eabe1655.
42. Kwon YT, Kim H, Mahmood M, Kim YS, Demolder C, Yeo WH. Printed, wireless, soft bioelectronics and deep learning algorithm for smart human-machine interfaces. ACS Appl Mater Interfaces 2020;12:49398-406.
43. Wei H, Li K, Liu WG, Meng H, Zhang PX, Yan CY. 3D printing of free-standing stretchable electrodes with tunable structure and stretchability. Adv Eng Mater 2017;19:1700341.
44. Zhou LY, Gao Q, Fu JZ, et al. Multimaterial 3D printing of highly stretchable silicone elastomers. ACS Appl Mater Interfaces 2019;11:23573-83.
45. Muth JT, Vogt DM, Truby RL, et al. Embedded 3D printing of strain sensors within highly stretchable elastomers. Adv Mater 2014;26:6307-12.
47. Farina D, Holobar A. Human?machine interfacing by decoding the surface electromyogram [Life Sciences]. IEEE Signal Process Mag 2015;32:115-20.
48. Farina D, Jiang N, Rehbaum H, et al. The extraction of neural information from the surface EMG for the control of upper-limb prostheses: emerging avenues and challenges. IEEE Trans Neural Syst Rehabil Eng 2014;22:797-809.
49. Holobar A, Farina D. Noninvasive neural interfacing with wearable muscle sensors: combining convolutive blind source separation methods and deep learning techniques for neural decoding. IEEE Signal Process Mag 2021;38:103-18.
50. Kollmitzer J, Ebenbichler GR, Kopf A. Reliability of surface electromyographic measurements. Clin Neurophysiol 1999;110:725-34.
51. Yeon SH, Shu T, Rogers EA, et al. Flexible dry electrodes for emg acquisition within lower extremity prosthetic sockets. In: 2020 8th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob). 2020. pp. 1088-95.
52. Murphy BB, Mulcahey PJ, Driscoll N, et al. A gel-free Ti3C2Tx-based electrode array for high-density, high-resolution surface electromyography. Adv Mater Technol 2020;5:2000325.
53. Driscoll N, Erickson B, Murphy BB, et al. MXene-infused bioelectronic interfaces for multiscale electrophysiology and stimulation. Sci Transl Med 2021;13:eabf8629.
54. Hammock ML, Chortos A, Tee BC, Tok JB, Bao Z. 25th anniversary article: the evolution of electronic skin (e-skin): a brief history, design considerations, and recent progress. Adv Mater 2013;25:5997-6038.
55. Goyal K, Borkholder DA, Day SW. Dependence of skin-electrode contact impedance on material and skin hydration. Sensors 2022;22:8510.
56. Yu Y, Li J, Solomon SA, et al. All-printed soft human-machine interface for robotic physicochemical sensing. Sci Robot 2022;7:eabn0495.
57. Zeng X, Dong Y, Wang X. Flexible electrode by hydrographic printing for surface electromyography monitoring. Materials 2020;13:2339.
58. Motti VG, Caine KE. Human factors considerations in the design of wearable devices. In: Proceedings of the Human Factors and Ergonomics Society Annual Meeting 2014;58:1820-4.
59. Huang C, Chiu C. Facile fabrication of a stretchable and flexible nanofiber carbon film-sensing electrode by electrospinning and its application in smart clothing for ECG and EMG monitoring. ACS Appl Electron Mater 2021;3:676-86.
60. Li J, Ma Y, Huang D, et al. High-performance flexible microneedle array as a low-impedance surface biopotential dry electrode for wearable electrophysiological recording and polysomnography. Nanomicro Lett 2022;14:132.
62. Tyagi A, Semwal S, Shah G. A review of EEG sensors used for data acquisition.In: National Conference on Future Aspects of Artificial intelligence in Industrial Automation (NCFAAIIA 2012), Proceedings published by International Journal of Computer Applications® (IJCA),2012:13-7. Acailable from: https://www.researchgate.net/publication/308259085_A_Review_of_Eeg_Sensors_used_for_Data_Acquisition.[Last accessed on 21 Jun]
63. Ganzer PD, Colachis SC 4th, Schwemmer MA, et al. Restoring the sense of touch using a sensorimotor demultiplexing neural interface. Cell 2020;181:763-773.e12.
64. Bouton CE, Shaikhouni A, Annetta NV, et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature 2016;533:247-50.
65. McFarland DJ, Sarnacki WA, Wolpaw JR. Electroencephalographic (EEG) control of three-dimensional movement. J Neural Eng 2010;7:036007.
66. McNaughton BL, O'Keefe J, Barnes CA. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J Neurosci Methods 1983;8:391-7.
68. Ponce CR, Lomber SG, Livingstone MS. Posterior inferotemporal cortex cells use multiple input pathways for shape encoding. J Neurosci 2017;37:5019-34.
69. Huang Z, Zhou Z, Zeng J, Lin S, Wu H. Flexible electrodes for non-invasive brain-computer interfaces: a perspective. APL Materials 2022;10:090901.
70. Abiri R, Borhani S, Sellers EW, Jiang Y, Zhao X. A comprehensive review of EEG-based brain-computer interface paradigms. J Neural Eng 2019;16:011001.
72. Orban M, Elsamanty M, Guo K, Zhang S, Yang H. A review of brain activity and EEG-Based brain-computer interfaces for rehabilitation application. Bioengineering 2022;9:768.
73. Li G, Wang S, Duan YY. Towards conductive-gel-free electrodes: understanding the wet electrode, semi-dry electrode and dry electrode-skin interface impedance using electrochemical impedance spectroscopy fitting. Sensor Actuat B-Chem 2018;277:250-60.
74. Pradhapan P, Velazquez ER, Witteveen JA, Tonoyan Y, Mihajlović V. The role of features types and personalized assessment in detecting affective state using dry electrode EEG. Sensors 2020;20:6810.
75. Marini F, Lee C, Wagner J, Makeig S, Gola M. A comparative evaluation of signal quality between a research-grade and a wireless dry-electrode mobile EEG system. J Neural Eng 2019;16:054001.
76. Baum U, Baum A, Deike R, et al. Eignung eines mobilen trockenelektroden-EEG-Gerätes im Rahmen der Epilepsiediagnostik. Klin Neurophysiol 2020;51:156-60.
77. Mota A, Duarte L, Rodrigues D, et al. Development of a quasi-dry electrode for EEG recording. Sensor Actuat A-Phys 2013;199:310-7.
78. Lin S, Liu J, Li W, et al. A flexible, robust, and gel-free electroencephalogram electrode for noninvasive brain-computer interfaces. Nano Lett 2019;19:6853-61.
79. Huang K, Liu J, Lin S, et al. Flexible silver nanowire dry electrodes for long-term electrocardiographic monitoring. Adv Compos Hybrid Mater 2022;5:220-8.
80. Lee JH, Hwang JY, Zhu J, et al. Flexible conductive composite integrated with personal earphone for wireless, real-time monitoring of electrophysiological signs. ACS Appl Mater Interfaces 2018;10:21184-90.
81. Grozea C, Voinescu CD, Fazli S. Bristle-sensors--low-cost flexible passive dry EEG electrodes for neurofeedback and BCI applications. J Neural Eng 2011;8:025008.
82. Wang L, Liu J, Yang B, Yang C. PDMS-based low cost flexible dry electrode for long-term EEG measurement. IEEE Sensors J 2012;12:2898-904.
83. Li G, Wu J, Xia Y, et al. Towards emerging EEG applications: a novel printable flexible Ag/AgCl dry electrode array for robust recording of EEG signals at forehead sites. J Neural Eng 2020;17:026001.
84. Golparvar A, Ozturk O, Yapici MK. Gel-free wearable electroencephalography (eeg) with soft graphene textiles. In: 2021 IEEE Sensors; 2021 31 Oct-3 Nov; Sydney, Australia. IEEE; 2021. p. 1-4.
85. Carneiro MR, de Almeida AT, Tavakoli M. Wearable and comfortable e-textile headband for long-term acquisition of forehead EEG signals. IEEE Sensors J 2020;20:15107-16.
86. Li G, Zhang D, Wang S, Duan YY. Novel passive ceramic based semi-dry electrodes for recording electroencephalography signals from the hairy scalp. Sensor Actuat B-Chem 2016;237:167-78.
87. Liu J, Lin S, Li W, et al. Ten-hour stable noninvasive brain-computer interface realized by semidry hydrogel-based electrodes. Research 2022;2022:9830457.
88. Krishnan A, Kumar R, Venkatesh P, Kelly S, Grover P. Low-cost carbon fiber-based conductive silicone sponge EEG electrodes. In: 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 2018 Jul 18-21; Honolulu, HI, USA. IEEE; 2018. p. 1287-90.
89. Tseghai GB, Malengier B, Fante KA, Langenhove L Van. The status of textile-based dry EEG electrodes. Autex Res J 2021;21:63-70.
90. He G, Dong X, Qi M. From the perspective of material science: a review of flexible electrodes for brain-computer interface. Mater Res Express 2020;7:102001.
91. Stevenson C, Chang Y, He C, et al. Emerging non-invasive brain-computer interface technologies and their clinical applications. In: Chaurasia, M.A., Juang, CF , editors. Emerging IT/ICT and AI technologies affecting society. Singaporer: Springer; 2023. p. 269-90.
92. Dupre A, Vincent S, Iaizzo PA. Basic ECG theory, recordings, and interpretation. In: Iaizzo PA, editor. Handbook of Cardiac Anatomy, Physiology, and Devices. Totowa, NJ: Humana Press; 2005. p. 191-201.
93. Frederiks J, Swenne CA, Kors JA, et al. Within-subject electrocardiographic differences at equal heart rates: role of the autonomic nervous system. Pflugers Arch 2001;441:717-24.
94. Park C, Youn I, Han S. Single-lead ECG based autonomic nervous system assessment for meditation monitoring. Sci Rep 2022;12:22513.
95. Madona P, Basti RI, Zain MM. PQRST wave detection on ECG signals. Gac Sanit 2021;35 Suppl 2:S364-9.
96. AlGhatrif M, Lindsay J. A brief review: history to understand fundamentals of electrocardiography. J Community Hosp Intern Med Perspect 2012;2:14383.
97. Martis RJ, Acharya UR, Adeli H. Current methods in electrocardiogram characterization. Comput Biol Med 2014;48:133-49.
98. Ramasamy S, Balan A. Wearable sensors for ECG measurement: a review. Sensor Review 2018;38:412-9.
99. Strik M, Ploux S, Weigel D, et al. The use of smartwatch electrocardiogram beyond arrhythmia detection. Trends Cardiovasc Med ;2023:S1050-1738(22)00153.
101. Abt G, Bray J, Benson AC. The validity and inter-device variability of the apple watch™ for measuring maximal heart rate. J Sports Sci 2018;36:1447-52.
102. Yu J, Park S, Kwon S, Cho K, Lee H. AI-based stroke disease prediction system using ECG and PPG bio-signals. IEEE Access 2022;10:43623-38.
103. Kim T, Park J, Sohn J, Cho D, Jeon S. Bioinspired, highly stretchable, and conductive dry adhesives based on 1D-2D hybrid carbon nanocomposites for all-in-one ECG electrodes. ACS Nano 2016;10:4770-8.
104. Ankhili A, Tao X, Cochrane C, Koncar V, Coulon D, Tarlet JM. Ambulatory evaluation of ECG signals obtained using washable textile-based electrodes made with chemically modified PEDOT:PSS. Sensors 2019;19:416.
105. Creel DJ. Chapter 33 - the electrooculogram. In: Levin KH, Chauvel P, editors. Handbook of Clinical Neurology. 2019. p. 495-9.
106. Soundariya RS, Renuga R. Eye movement based emotion recognition using electrooculography. In: 2017 Innovations in Power and Advanced Computing Technologies (i-PACT). 2017 Apr 21-22; Vellore, India.IEEE; 2017. p. 1-5.
107. Usakli AB, Gurkan S, Aloise F, Vecchiato G, Babiloni F. On the use of electrooculogram for efficient human computer interfaces. Comput Intell Neurosci 2010;2010:135629.
108. Choudhari AM, Porwal P, Jonnalagedda V, Mériaudeau F. An electrooculography based human machine interface for wheelchair control. Biocybern Biomed Eng 2019;39:673-85.
109. Ramkumar S, Emayavaramban G, Sathesh Kumar K, Macklin Abraham Navamani J, Maheswari K, Packia Amutha Priya P. Task identification system for elderly paralyzed patients using electrooculography and neural networks. In: Haldorai A, Ramu A, Mohanram S, Onn CC, editors. EAI International Conference on Big Data Innovation for Sustainable Cognitive Computing; 2019 Oct 19; Springer, Cham; 2020. p. 151-61.
110. Kumar D, Sharma A. Electrooculogram-based virtual reality game control using blink detection and gaze calibration. In: 2016 International Conference on Advances in Computing, Communications and Informatics (ICACCI); 2016 Sep 21-24; Jaipur, India. IEEE;2016. p. 2358-62.
112. Won Y, Lee JJ, Shin J, Lee M, Kim S, Gandla S. Biocompatible, transparent, and high-areal-coverage kirigami PEDOT:PSS electrodes for electrooculography-derived human-machine interactions. ACS Sens 2021;6:967-75.
113. Ameri SK, Kim M, Kuang IA, et al. Imperceptible electrooculography graphene sensor system for human-robot interface. npj 2D Mater Appl 2018:2.
114. Xiao J, Qu J, Li Y. An electrooculogram-based interaction method and its music-on-demand application in a virtual reality environment. IEEE Access 2019;7:22059-70.
115. Beach C, Karim N, Casson AJ. A graphene-based sleep mask for comfortable wearable eye tracking. In: 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 2019 Jul 23-27; Berlin, Germany. IEEE; 2019. p. 6693 6.
116. Liang S, Kuo C, Lee Y, et al. Development of an EOG-based automatic sleep-monitoring eye mask. IEEE Trans Instrum Meas 2015;64:2977-85.
117. Li W, Lin K, Chen L, Yang D, Ge Q, Wang Z. Self-powered wireless flexible ionogel wearable devices. ACS Appl Mater Interfaces 2023;15:14768-76.
118. Wang W, Wang S, Rastak R, et al. Strain-insensitive intrinsically stretchable transistors and circuits. Nat Electron 2021;4:143-50.
119. Fatayerji HR, Saeed M, Qaisar SM, Alqurashi A, Al Talib R. Application of wavelet decomposition and ma-chine learning for the semg signal based ges-ture recognition. In: Qaisar SM, Nisar H, Subasi A, editors. Advances in Non-Invasive Biomedical Signal Sensing and Processing with Machine Learning. Cham: Springer International Publishing; 2023. pp. 133-58.
120. Dong P, Song Y, Yu S, et al. Electromyogram-based lip-reading via unobtrusive dry electrodes and machine learning methods. Small 2023;19:e2205058.
121. Bi Z, Wang Y, Wang H, et al. Wearable EMG bridge-a multiple-gesture reconstruction system using electrical stimulation controlled by the volitional surface electromyogram of a healthy forearm. IEEE Access 2020;8:137330-41.
122. Wei Y, Yang K, Browne M, Bostan L, Worsley P. Wearable electrical stimulation to improve lymphatic function. IEEE Sens Lett 2019;3:1-4.
123. Ohm Y, Pan C, Ford MJ, Huang X, Liao J, Majidi C. An electrically conductive silver-polyacrylamide-alginate hydrogel composite for soft electronics. Nat Electron 2021;4:185-92.
124. Botzanowski B, Donahue MJ, Ejneby MS, et al. Noninvasive stimulation of peripheral nerves using temporally-interfering electrical fields. Adv Healthc Mater 2022;11:e2200075.
Cite This Article
How to Cite
Nagwade, P.; Parandeh S.; Lee S. Prospects of soft biopotential interfaces for wearable human-machine interactive devices and applications. Soft Sci. 2023, 3, 24. http://dx.doi.org/10.20517/ss.2023.12
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.






















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.