Basic Science of Wound Healing
Basic Science of Wound Healing
Uploaded by
Marnia SulfianaCopyright:
Available Formats
Basic Science of Wound Healing
Basic Science of Wound Healing
Uploaded by
Marnia SulfianaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Copyright:
Available Formats
Basic Science of Wound Healing
Basic Science of Wound Healing
Uploaded by
Marnia SulfianaCopyright:
Available Formats
BASIC SCIENCE
Basic science of wound
healing
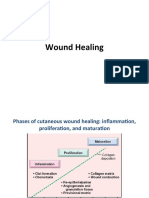
The wound healing process
Injury (including surgery) sets in motion a cascade of physiological responses which are coordinated and ordered, which if
uninterrupted result in wound repair. Such wounds are termed
acute wounds, whereas those wounds which fail to heal due to
interruption in the ordered process, due to infection or by an
underlying disease process are termed chronic or indolent
wounds.
Pauline Beldon
Abstract
Wound healing is a complicated process, dependent on the patients
underlying health and nutritional status and also upon the clinicians
ability to recognize stages of wound healing. For appropriate management, an understanding of the basic physiology of wound healing is
necessary.
Haemostasis
Within seconds of skin injury, vasoconstriction occurs to reduce
blood loss. As blood spills into the wound area, platelets come
into contact with and adhere to the wall of the injured blood
vessels and to the exposed collagen within the extracellular
matrix. This triggers platelets to release cytokines, growth factors
and numerous pro-inflammatory mediators resulting in platelet
aggregation and triggering the intrinsic and extrinsic coagulation
pathways, leading to fibrin clot formation. The clot seals the
disrupted blood vessels, preventing further blood loss.1 The clot
is sustained by the rapid intravascular accumulation of microvesicular tissue factor (TF) as microvesicles from white cells are
attracted to the platelet surface.
Growth factors produced by the platelets initiate the healing
cascade. Platelet-derived growth factor (PDGF) initiates the
chemotaxis of neutrophils and macrophages, smooth muscle
cells and fibroblasts, while also stimulating mitogenesis of
smooth muscle cells and fibroblasts.
Transforming growth factor-beta (TGF-b) attracts macrophages into the wound area and stimulates them to produce
additional cytokines, including fibroblast growth factor (FGF),
PDGF, tumour necrosis alpha (TNFa) and interleukin-1 (IL-1). In
addition TGF-b further enhances the fibroblast and smooth
muscle chemotaxis and modulates the expression of collagen and
collagenase. The purpose of PDGF and TGF-b is to stimulate
a response form the matrix to produce cells in preparation for the
rapid deposition of new connective tissue to rebuild the extracellular matrix. The clinical picture of haemostasis is clearly
demonstrated by wounds such as skin graft donor site wounds
which heal by regeneration (Figure 1).
Keywords Connective tissue; haemostasis; proliferation; regeneration;
wound healing
The process of wound healing is a survival mechanism which
commences immediately after the body sustains injury. It is the
process by which tissues damaged or destroyed by injury are
removed and the breach in tissue/skin integrity is restored.
Wound repair is performed either by spontaneous regeneration
of dermal tissue or by connective tissue repair. Both these occur
regardless of how the injury has been sustained, or whether the
injury is minor, major or confined to soft tissue trauma or is
a surgical wound. The mode of repair is primarily decided by the
depth of the tissue involved. In minor injuries involving the
epidermis, deep tissue regeneration is prompt, with re-epithelialization taking place via the adnexal processes within 7e10
days. However in wounds involving the loss of dermal tissue and
valuable adnexal processes such as hair follicles, sweat and
sebaceous glands, re-epithelialization and therefore regeneration
cannot occur, consequently the body relies upon the formation of
connective tissue to fill the wound deficit.
Wound healing by primary intention involves the close
approximation of the wound edges of a laceration or surgical
wound by sutures, clips or skin adhesive.
Wound healing by secondary intention occurs when the
wound edges cannot be approximated, the wound is left open
and the defect will slowly fill with connective tissue. Such
wounds are slow to heal and prone to complications such as
infection. Commonly, this type of wound healing occurs in
patients with underlying co-morbidities (e.g. vascular or diabetic
ulcers, pressure ulcers) or in patients with post-surgical wound
dehiscence (itself often due to infection, haematoma or
mechanical tension).
Wound healing by tertiary intention, which is often called
delayed primary intention, involves the wound being left open
until any infection or contamination with non-viable tissue is
removed, the wound edges are then approximated and healing
continues as by primary intention.
Inflammation
Tissue injury and the activation of the coagulation cascade
stimulate the release of vascoactive cytokines such as prostaglandins, histamine and other amines from the granules released
by mast cells as a response to injury, which results in increased
local vasodilation and capillary permeability. The vessels
become more permeable, allowing the migration of monocytes
into the wound bed. Serous fluid also leaks into the wound bed
and the surrounding tissue, creating oedema. These are part of
the characteristic signs of inflammation. The patient is likely to
complain of increasing pain 24 hours after wounding or surgery
(hence the need for good analgesic provision).
During the period of inflammation neutrophils accumulate
within the wound area, their primary role being to act as
a wound cleanser. They are initially attracted to the wound area
by various chemotactic agents released from bacteria. Once in
the wound, they ingest the bacteria via phagocytosis. Neutrophils
Pauline Beldon RGN PGDipl is a Tissue Viability Nurse Consultant at
Epsom & St Helier University Hospitals NHS Trust, Surrey, UK. Conflicts
of interest: none declared.
SURGERY 28:9
409
2010 Elsevier Ltd. All rights reserved.
BASIC SCIENCE
environment. Macrophages also attract vascular endothelial cells
into the wound bed in preparation for angiogenisis.
The result of the inflammatory phase is control of bleeding
and the establishment of a clean wound bed. This phase should
last approximately 3 days in the surgical or acute wound closed
by primary intention. However should the wound be contaminated with non-viable or necrotic tissue or become infected, then
inflammation will be prolonged. This has consequences for the
patient as the process of inflammation is energetically costly.
Thus it is not unusual for patients with infected wounds or those
with non-viable tissue present to exhibit significant weight loss,
and this requires regular nutritional assessment and potentially
supplementary feeding. The (normal) inflammatory phase of
wound healing (Figure 3) should not be confused with the
(abnormal) inflammation secondary to clinical infection. A
raised temperature and white cell count indicate the latter and
helps avoid prescription of unnecessary antibiotic therapy.3
Figure 1 Clinical appearance of haemostasis. Donor site; superficial
wound which will heal via regeneration.
Proliferation of connective tissue
There is a wide overlap between the phase of inflammation and
the next phase of proliferation, when it is possible to see within
a wound bed both slough/necrotic tissue and burgeoning granulation tissue (Figure 4).
During the proliferative phase the wound defect is filled with
highly vascular connective tissue, commonly referred to as
granulation tissue. This process is instigated at the point of
wounding, when as a result of loss of vascularity in the wound
bed the wound environment has a low pH, reduced oxygen
tension and increased lactate, this initiates the release of vascular
endothelial growth factor (VEGF), basic fibroblast factor (bFF)
and TGF-b, all of which activate neovascularization or angiogenisis. The low oxygen tension caused by lack of blood supply
actually initiates the release of hypoxia-inducible factor (HIF),
which regulates the expression of VEGF. Endothelial cells in the
venules adjacent to the wound bed initiate capillary production
by projecting pseudopods into the wound bed. Simultaneously
the endothelial cells produce enzymes to breakdown the adjacent
extracellular matrix to create tissue defects into which the
capillary vessels infiltrate to form a network, from which will
arise the formation of arterioles and thus the vascularity is
filled with bacteria and debris produce the appearance of pus or
slough in a wound bed (Figure 2).
Over the following the 2e3 days the neutrophils begin to
disappear from the wound bed via the process of apoptosis. The
process of wound cleansing then continues via monocytes,
which have been gradually infiltrating the wound bed since the
commencement of inflammation and are now activated to
become wound macrophages, arguably the most important cells
within the process of wound healing.2 Any inhibition of macrophage function will delay wound healing. Once activated,
macrophages release PDGF and TGF-b to further the work of
attracting fibroblasts and smooth muscle cells into the wound
site. Macrophages are highly phagocytic and remove all nonviable cells, bacteria-filled neutrophils, damaged extracellular
matrix, debris and any bacteria from the wound site. An added
function is that of the production of nitric oxide which has an
antimicrobial effect, enhanced by the hypoxic wound
Figure 3 Sloughy wound bed due to the clearance of debris, bacteria and
non-viable connective tissue by neutrophils.
Figure 2 The inflammation peri ulcer is due to wound healing.
SURGERY 28:9
410
2010 Elsevier Ltd. All rights reserved.
BASIC SCIENCE
In wounds healing by secondary intention longer time is
needed to produce the volume of connective tissue required to fill
the cavity. In such wounds the granulation tissue which is
formed due to the combined process of neovascularization and
collagen production is visible (Figure 5). Once more the patients
nutritional status plays a direct role in their ability to heal.
Granulation tissue is slow to form or friable in the patient with
poor nutritional status, thus increasing the potential for wound
infection. Appropriate wound management is vital: topical
negative pressure (TNP) dressings, which are infrequently
changed, can provide an occlusive environment, while
increasing the rate of neovascularization.5 Absorbent dressings
such as hydrofibre, alginate or foams are used to absorb excess
exudate and prevent skin maceration.
Contraction
The phenomenon of contraction only occurs in open wounds. It
is desirable since if the wound has the ability to contract, then
less connective tissue is required to fill the wound deficit and the
wound will heal more quickly. How contraction occurs is still
debated, with three competing theories. Berry et al.6 argue that
modified fibroblasts (myofibroblasts) are responsible for generating contractile forces which pull wound edges together. A
second theory7 suggests that wound fibroblasts act collectively to
contract the connective tissue matrix. A third theory8 proposes
that the newly formed collagen fibres are compacted by cellular
forces producing a pulling force on surrounding tissues. The
degree of contraction within the wound bed is determined by the
mobility of the surrounding tissue: in areas such as the abdomen
where the overlying tissue is very mobile contracture is easily
achieved, in contrast to skin over bony prominences. Wound
contracture can be undesirable in some wounds when cosmetic
deformities may arise. In such cases, wound healing by
secondary intention is undesirable and the wound defect should
be filled by skin graft if superficial, and by myocutaneous flap if
deep.
Figure 4 Wound bed containing both slough and burgeoning granulation
tissue.
restored. As new blood vessels intrude into the wound environment the oxygen tension rises and oxygen binds onto the HIF,
blocking its activity which in turn leads to decreased production
of VEGF.
The process of angiogenesis is interdependent on the
production of a new extracellular matrix (ECM) which acts as
a scaffold to support the newly formed blood vessels.
The predominant cell responsible for the formation of the
ECM is the fibroblast, these are attracted into the wound bed by
cytokines produced by macrophages and then transformed into
wound fibroblasts which have decreased proliferative behaviour,
but increased collagen production behaviour.
Collagen synthesis to form the new ECM is a multi-layered
process.
During the initial phase of wound healing (haemostasis) the
fibrin clot supports the migration of cells into the wound. This
fibrin matrix is gradually replaced by material comprised mainly
of fibronectin and hyaluronic acid, both of which promote cell
migration and proliferation. Fibroblasts attach to the provisional
fibrin matrix and begin collagen production. The early, so-called
type 3 collagen comprises approximately 30% of granulation
tissue and does not contribute to the tensile strength of the
wound. Hence any wound healing by secondary intention
requires supportive dressings during this stage (e.g., an abdominal support belt for the patient with a dehisced abdominal
wound). As collagen matures, type 3 collagen is converted to
type 1 collagen, normally found in dermal tissue.
In wounds healing by primary intention collagen synthesis
peaks at day 5e6, although collagen synthesis and re-modulation
of collagen continue for several months. Clearly only a small
amount of collagen is required to heal the deficit in a sutured
wound and by day 5e6 it should be possible to palpate a healing
ridge under the intact suture line, formed by the deposition of
collagen.4 Wounds in which this healing ridge is absent may be
at risk of dehiscence. Commonly there are underlying complications (e.g. anaemia or past history of smoking, low oxygen
tension). Vitamin C deficiency can lead to reduced hydroxyproline which gives collagen molecules stabilization and aids the
tensile strength of the wound.
SURGERY 28:9
Epithelialization
In this final stage of visible wound healing, epithelial cells
migrate from the wound edges to resurface the wound defect.
Figure 5 Cavity wound containing granulation tissue.
411
2010 Elsevier Ltd. All rights reserved.
BASIC SCIENCE
resulting in abnormal scar formation, whether keloid or hypertrophic scarring. If the patient is severely malnourished then the
rate of collagen synthesis is delayed and can lead to late wound
breakdown.
Conclusion
Wound healing is a well-coordinated process, provided the
individual is nutritionally robust, haemodynamically well and
biochemically stable. However in those patients with wounds
healing by secondary intention it is not unusual to have at least
one complicating factor (commonly malnuturition) which needs
to be addressed. Clinicians need to recognize the processes and
factors involved in normal wound healing.
A
Figure 6 Partial dermal thickness wound, with islands of epithelium
visible.
REFERENCES
1 Verhamme P, Hoylaerts MF. Haemostasis and inflammation: two of
a kind? Thromb J 2009; 7: 1e3.
2 Diegleman RF, Evans MC. Wound healing: an overview of acute, fibrotic
and delayed healing. Front Biosci 2004; 9: 283e9.
3 Beldon P. Recognising wound infection. Nurs Times 2001; 97: 3e4.
4 Hunt TK, Van Winkle W. Normal repair. In: Hunt TK, Dunphy JE, eds.
Fundamentals of wound management. New York: Appleton-CenturyCrofts, 1997.
5 Hunter JE, Teot L, Horch R, Banwell PE. Evidence-based medicine:
vacuum-assisted closure in wound care management. Int Wound J
2007; 4: 256e69.
6 Berry DP, Harding KG, Stanton MR, Jasani B, Ehrlich HP. Human wound
contraction: collagen organization, fibroblasts, and myofibroblasts.
Plast Reconstr Surg 1998; 102: 124e34.
7 Rudolph R. Location of the force of wound contraction. Surg Gynaecol
Obstet 1979; 148: 547e51.
8 Kuhn MA, Smith PD, Hill DP, et al. In vitro fibroblast populated
collagen lattices are not good models on in vivo clinical wound
healing. Wound Repair Regen 2000; 8: 270e6.
9 Winter GD. Formation of the scab and the rate of re-epithelialisation in
the skin of the young domestic pig. Nature 1962; 193: 293e4.
In wounds healing by primary intention epithelialization occurs
concurrently with connective tissue deposition. In wounds
healing by secondary intention this process is delayed until the
wound defect is filled with granulation tissue. Epithelial cells can
only migrate over a moist, vascular wound surface, they are
inhibited by a dry or necrotic wound surface.9 Lateral migration
proceeds until the defect is covered, the cells then resume
upward migration and differentiation until the epidermis regains
its normal thickness and stratification (Figure 6).
Remodelling phase
This may extend to a year or more, during which fibroblasts
regulate the process of wound matrix breakdown by matrix
metalloproteinases (MMPs) and synthesis of new extracellular
matrix. This slow process increases the tensile strength of the
wound, but scar tissue is never more than 80% of the tensile
strength in unwounded tissue. Occasionally during the process of
remodelling an imbalance may occur, during which the process
of matrix degradation and that of synthesis are disrupted
SURGERY 28:9
412
2010 Elsevier Ltd. All rights reserved.
You might also like
- Part I - Antibiotics and The Human MicrobiomeDocument5 pagesPart I - Antibiotics and The Human MicrobiomeRejean Isip100% (3)
- The Art and Science of Thread Lifting: Based on Pinch AnatomyFrom EverandThe Art and Science of Thread Lifting: Based on Pinch AnatomyNo ratings yet
- White Label Training ManualDocument16 pagesWhite Label Training ManualAndreea Lorena Duroiu100% (6)
- Wound HealingDocument15 pagesWound HealingfgrehNo ratings yet
- Different Types of Meat and Its SourcesDocument6 pagesDifferent Types of Meat and Its SourcesJeric Enteria Cantillana67% (3)
- Traceability PPT Part 1Document33 pagesTraceability PPT Part 1Ozlem Mep100% (1)
- Biology of PeriodontalDocument78 pagesBiology of PeriodontalSudip Sen100% (1)
- Basic Principles of Wound Healing - UpToDateDocument8 pagesBasic Principles of Wound Healing - UpToDateNguyễn TrangtrangNo ratings yet
- Chronic Inflammation 1: DR Nahiid StephensDocument46 pagesChronic Inflammation 1: DR Nahiid Stephensmelinda0% (1)
- Antibiotic Prophylaxis: Francis Neil C. CaranayDocument11 pagesAntibiotic Prophylaxis: Francis Neil C. CaranayNdor BariboloNo ratings yet
- Invasion and Tumour MetastasisDocument33 pagesInvasion and Tumour MetastasisShimmering MoonNo ratings yet
- Evaluation of Wound Healing Potential of Cynodon DactylonDocument4 pagesEvaluation of Wound Healing Potential of Cynodon Dactylonchaitanya gNo ratings yet
- Cohort StudyDocument40 pagesCohort Studyபிரேம் குமார் ராஜாமணிNo ratings yet
- CH 30Document22 pagesCH 30Alejandra Oliveros VargasNo ratings yet
- SCMS V34i4 White Lesions in The Oral CavityDocument10 pagesSCMS V34i4 White Lesions in The Oral CavityTasneem Hussiny AbdallahNo ratings yet
- 01 - 007 Corticosteroids in DentistryDocument3 pages01 - 007 Corticosteroids in DentistryplsssssNo ratings yet
- Invoice HP CoverDocument1 pageInvoice HP CoverSurbhi JainNo ratings yet
- Asst - Prof. Meroj A. Jasem Ph.D. Student Molecular Immunology CourseDocument26 pagesAsst - Prof. Meroj A. Jasem Ph.D. Student Molecular Immunology CourseahmadNo ratings yet
- Evaluation of Wound Healing Activity of Leaves of Crinum AsiaticumDocument5 pagesEvaluation of Wound Healing Activity of Leaves of Crinum AsiaticumxiuhtlaltzinNo ratings yet
- Evaluation of Wound Healing Effect of Jasminum Grandiflorum in Albino Rats by Histopathological StudiesDocument4 pagesEvaluation of Wound Healing Effect of Jasminum Grandiflorum in Albino Rats by Histopathological StudiesAgung Tri LaksonoNo ratings yet
- Morphologic Patterns of Acute InflammationDocument51 pagesMorphologic Patterns of Acute Inflammationحفصه حسينNo ratings yet
- Oral BiopsiesDocument5 pagesOral BiopsiesDiego Morales100% (1)
- Electrotherapy in Wound Healing - HRDocument50 pagesElectrotherapy in Wound Healing - HRHitesh RohitNo ratings yet
- Extra-Oral Radiology DR Vineetha 2003 FormatDocument7 pagesExtra-Oral Radiology DR Vineetha 2003 FormatEshan VermaNo ratings yet
- The Wound Healing ProcessDocument15 pagesThe Wound Healing Processtabris76No ratings yet
- Oncogenic Viruses: Christopher B. Buck, Lee Ratner, and Giovanna TosatoDocument46 pagesOncogenic Viruses: Christopher B. Buck, Lee Ratner, and Giovanna TosatoJohan Rinto Even NapitupuluNo ratings yet
- Sepsis: Sepsis and Septic ShockDocument22 pagesSepsis: Sepsis and Septic ShockWialda Dwi rodyahNo ratings yet
- Inflammation SeminarDocument59 pagesInflammation SeminarVinod S Vinu100% (2)
- WOUND HEALING ACTIVITY OF METHONOLIC EXTRACT OF Martynia Annua L. (MARTYNIACEAE)Document5 pagesWOUND HEALING ACTIVITY OF METHONOLIC EXTRACT OF Martynia Annua L. (MARTYNIACEAE)xiuhtlaltzinNo ratings yet
- Mirobial DiagnosisDocument4 pagesMirobial Diagnosiswishvish_scribdNo ratings yet
- FicusDocument3 pagesFicusSugandha ShetyeNo ratings yet
- Studying Wound Healing Activities of Natural ProductsDocument47 pagesStudying Wound Healing Activities of Natural Productsmichael100% (1)
- Trigeminal Neuralgia: Trigeminal Neuralgia Is Sudden, Severe Facial Nerve PainDocument8 pagesTrigeminal Neuralgia: Trigeminal Neuralgia Is Sudden, Severe Facial Nerve Painrui.santiago582No ratings yet
- 2015 Antibiotic Prophylaxis DentalDocument5 pages2015 Antibiotic Prophylaxis DentalSyedMuhammadJunaid100% (2)
- Combined Dental Management of Patients With Medical ConditionsDocument65 pagesCombined Dental Management of Patients With Medical ConditionsJenny WangNo ratings yet
- Periodontal LigamentDocument11 pagesPeriodontal LigamentDANA ISABELLE PILAPILNo ratings yet
- Granulomatous Lesions of Oral CavityDocument120 pagesGranulomatous Lesions of Oral CavityMadhura ShekatkarNo ratings yet
- Unit 2 - Physiological Reaction To InjuryDocument90 pagesUnit 2 - Physiological Reaction To InjuryFabian Chapima100% (1)
- Saliva Composition and FunctionsDocument11 pagesSaliva Composition and FunctionsIngrid Johanna Alvarez ArangoNo ratings yet
- Viral Infections of The Oral CavityDocument33 pagesViral Infections of The Oral CavityChukwuenyem BlessingNo ratings yet
- Diseases of Nerves and MusclesDocument46 pagesDiseases of Nerves and MusclesAME DENTAL COLLEGE RAICHUR, KARNATAKANo ratings yet
- White Lesions - Part I (Lecture by DR - Eman Metwally @AmCoFam)Document11 pagesWhite Lesions - Part I (Lecture by DR - Eman Metwally @AmCoFam)AmericanCornerFamilyNo ratings yet
- Routes & Mechanisms of MetastasisDocument26 pagesRoutes & Mechanisms of MetastasisNeetu GuptaNo ratings yet
- Local Anaesthesia TechniquesDocument21 pagesLocal Anaesthesia TechniquesZaki Mubaraq100% (1)
- CMV & Ebv: A.ChancharoenDocument59 pagesCMV & Ebv: A.ChancharoenRapid MedicineNo ratings yet
- Introduction To MicrobiologyDocument6 pagesIntroduction To MicrobiologySeon u 'No ratings yet
- Role of Mast Cells in Periodontal DiseaseDocument26 pagesRole of Mast Cells in Periodontal DiseaseDpartment of PeriodontologyNo ratings yet
- Principles of Antiplatelet Therapy: DR Htet Htet Htethtet@Imu - Edu.MyDocument36 pagesPrinciples of Antiplatelet Therapy: DR Htet Htet Htethtet@Imu - Edu.MyAbby Liew100% (1)
- Frenectomy Z PlastyDocument4 pagesFrenectomy Z Plastyantonio dlNo ratings yet
- Hepatic Disorders 000Document58 pagesHepatic Disorders 000Ade Ratna RS100% (1)
- Review Article Cancrum OrisDocument3 pagesReview Article Cancrum OrisDesy AfrNo ratings yet
- Corticosteroids: Ghadi Mahmoud Elbarghathi Roll Number: 1950 5 Year 2021-2022Document26 pagesCorticosteroids: Ghadi Mahmoud Elbarghathi Roll Number: 1950 5 Year 2021-2022Ghadi ElbarghathiNo ratings yet
- Junctional EpitheliumDocument66 pagesJunctional EpitheliumSatya MeruguNo ratings yet
- 2 The Complement SystemDocument40 pages2 The Complement SystemJohn Louis RanetNo ratings yet
- Innate Immunity 11102018Document32 pagesInnate Immunity 11102018Thahir Anwar100% (1)
- Lec: TMJ DR - Nawres BahaaDocument13 pagesLec: TMJ DR - Nawres BahaaMarwa AlfuaadiNo ratings yet
- Review of Radiographic Techniques For The Paediatric PatientDocument50 pagesReview of Radiographic Techniques For The Paediatric Patientapi-3775747100% (3)
- Immunology Dental Caries PDFDocument29 pagesImmunology Dental Caries PDFTio AjhaNo ratings yet
- Acute and Chronic InflammationDocument52 pagesAcute and Chronic Inflammationjames20123100% (1)
- Maxillary Sinus Approaches-1Document23 pagesMaxillary Sinus Approaches-1Ahmed KhattabNo ratings yet
- ChemoMechanical Debridement - IrrigationDocument15 pagesChemoMechanical Debridement - IrrigationMiftah WiryaniNo ratings yet
- HandoutDocument2 pagesHandoutThe IntrovertNo ratings yet
- Diagnostics to Pathogenomics of Sexually Transmitted InfectionsFrom EverandDiagnostics to Pathogenomics of Sexually Transmitted InfectionsSunit Kumar SinghNo ratings yet
- Prontosan Askina Range Clinical and Scientific EvidenceDocument60 pagesProntosan Askina Range Clinical and Scientific EvidenceMarnia SulfianaNo ratings yet
- Archive of SID: The Effect of Ginger Biscuit On Nausea and Vomiting in Early PregnancyDocument6 pagesArchive of SID: The Effect of Ginger Biscuit On Nausea and Vomiting in Early PregnancyMarnia SulfianaNo ratings yet
- Best Practice & Research Clinical Endocrinology & MetabolismDocument12 pagesBest Practice & Research Clinical Endocrinology & MetabolismMarnia SulfianaNo ratings yet
- Accepted Manuscript: 10.1016/j.nut.2015.01.013Document20 pagesAccepted Manuscript: 10.1016/j.nut.2015.01.013Marnia SulfianaNo ratings yet
- Asian Nursing Research: Nejla Canbulat, PHD, Sevil - Inal, PHD, Hacer Sönmezer, MSCDocument6 pagesAsian Nursing Research: Nejla Canbulat, PHD, Sevil - Inal, PHD, Hacer Sönmezer, MSCMarnia SulfianaNo ratings yet
- Antimicrobial Agents: General ConsiderationDocument19 pagesAntimicrobial Agents: General ConsiderationMarnia SulfianaNo ratings yet
- Empirical Treatment of Sepsis in AdultsDocument11 pagesEmpirical Treatment of Sepsis in AdultsMarnia SulfianaNo ratings yet
- Management of Acute Gastroenteritis in Children: Pathophysiology in The UKDocument6 pagesManagement of Acute Gastroenteritis in Children: Pathophysiology in The UKMarnia SulfianaNo ratings yet
- Iso 18589-3 2023 (E)Document7 pagesIso 18589-3 2023 (E)Eduardo Gonzalo Villarreyes PeñaNo ratings yet
- Blink Device Company Launches TwitchView™ Neuromuscular TOF Monitor at American Society of Anesthesiologists Annual MeetingDocument2 pagesBlink Device Company Launches TwitchView™ Neuromuscular TOF Monitor at American Society of Anesthesiologists Annual MeetingPR.comNo ratings yet
- Motion To Appoint ReceiverDocument14 pagesMotion To Appoint ReceiverCBS 11 NewsNo ratings yet
- Counseling PrinciplesDocument52 pagesCounseling Principleslehsem20006985100% (2)
- Waste Management Design GuidelinesDocument40 pagesWaste Management Design GuidelinesPhilip PhamNo ratings yet
- INSTRUMENTS For UGDocument22 pagesINSTRUMENTS For UGPugazhenthi CNo ratings yet
- A Nurse-Driven Process For TimelyDocument7 pagesA Nurse-Driven Process For TimelyWardah Fauziah El SofwanNo ratings yet
- What's The Risk DoubletruckDocument1 pageWhat's The Risk DoubletruckHonolulu Star-AdvertiserNo ratings yet
- Larry Cook Natural Guide PDFDocument114 pagesLarry Cook Natural Guide PDFutpal_thakar100% (1)
- Conceptual LearningDocument9 pagesConceptual LearningFaris 2806100% (1)
- Cedar PointDocument3 pagesCedar PointChris HansonNo ratings yet
- Accidents-Emergencies ResultsDocument3 pagesAccidents-Emergencies ResultsRachel GonzálezNo ratings yet
- Individualized Orthodontic Treatment Plan1Document65 pagesIndividualized Orthodontic Treatment Plan1nagiNo ratings yet
- Future Trends in Nursing Research-PresentationDocument49 pagesFuture Trends in Nursing Research-PresentationSr theresejoseNo ratings yet
- Communication SkillsDocument3 pagesCommunication SkillsAruna PandyaNo ratings yet
- Sukshma and Sthula VyayamaDocument1 pageSukshma and Sthula VyayamaKomal MandeNo ratings yet
- Abstinence Report - June 2005Document29 pagesAbstinence Report - June 2005Ray StillNo ratings yet
- History of Science and Safety Movement: Chapter - 33Document8 pagesHistory of Science and Safety Movement: Chapter - 33surajNo ratings yet
- Drown Forest Fires in SoundDocument18 pagesDrown Forest Fires in SoundsssssssssNo ratings yet
- Facts About dsm-5-tr Psychiatric NewsDocument8 pagesFacts About dsm-5-tr Psychiatric NewslulumaryyyNo ratings yet
- Maxstar 140: With Auto-LinkDocument44 pagesMaxstar 140: With Auto-LinkABNo ratings yet
- Data Collection Methods and Research DesignDocument14 pagesData Collection Methods and Research DesignLakshmish Gopal100% (1)
- M.tech. Biotechnology (Effective From The Session - 2016-17)Document25 pagesM.tech. Biotechnology (Effective From The Session - 2016-17)Ravindra Mani TiwariNo ratings yet
- HOW Fit Are You? WorksheetDocument3 pagesHOW Fit Are You? WorksheetValeria Elizabeth Rojas RiveraNo ratings yet
- Philippine Nurses Licensure Examination!Document34 pagesPhilippine Nurses Licensure Examination!Mr Chan007No ratings yet
- Accessible, Affordable and Quality Healthcare For All: Sector ProfileDocument50 pagesAccessible, Affordable and Quality Healthcare For All: Sector ProfileswajitmishraNo ratings yet