Naming Mixed Ionic and Covalent Compounds
Naming Mixed Ionic and Covalent Compounds
Uploaded by
api-325791445Copyright:
Available Formats
Naming Mixed Ionic and Covalent Compounds
Naming Mixed Ionic and Covalent Compounds
Uploaded by
api-325791445Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Copyright:
Available Formats
Naming Mixed Ionic and Covalent Compounds
Naming Mixed Ionic and Covalent Compounds
Uploaded by
api-325791445Copyright:
Available Formats
Naming Mixed Ionic and Covalent Compounds
Name the following compounds. Remember, they may be either ionic or covalent
compounds, so make sure you use the right naming method!
1) NaF __________________________________________
2) NF3 __________________________________________
3) Li2O __________________________________________
4) Al2S3 __________________________________________
5) MgSO4 __________________________________________
6) SiH4 __________________________________________
7) KNO3 __________________________________________
8) P2O5 __________________________________________
9) CH4 __________________________________________
10) Ca(OH)2 __________________________________________
Write the formulas for the following compounds. Remember, they may be either
ionic or covalent compounds, so make sure you use the right method!
11) lithium chloride __________________________________________
12) nitrogen trichloride __________________________________________
13) sodium oxide __________________________________________
14) dinitrogen trioxide __________________________________________
15) ammonia __________________________________________
16) diboron dihydride __________________________________________
17) potassium phosphide _________________________________________
18) oxygen difluoride __________________________________________
19) magnesium nitrate __________________________________________
20) aluminum carbonate __________________________________________
2004 Cavalcade Publishing, All Rights Reserved For chemistry help, visit www.chemfiesta.com
You might also like
- 5 Isotope Practice WorksheetDocument1 page5 Isotope Practice Worksheetapi-369690183No ratings yet
- Worksheet Grade 8Document5 pagesWorksheet Grade 8willadahNo ratings yet
- Atomic History Worksheet Answer KeyDocument1 pageAtomic History Worksheet Answer KeyRhuvy Ramos50% (2)
- CHEM 1211 Worksheet Covalent BondingDocument3 pagesCHEM 1211 Worksheet Covalent Bondingyash patel0% (1)
- Covalent Bonding WebquestDocument4 pagesCovalent Bonding Webquestapi-3031203990% (1)
- Acids and Bases - Worksheet Yr 9 ScienceDocument1 pageAcids and Bases - Worksheet Yr 9 ScienceCathy Billingham67% (3)
- Velocity Word Problems Assignment 0Document1 pageVelocity Word Problems Assignment 0api-3257914450% (4)
- Acceleration Practice ProblemsDocument2 pagesAcceleration Practice ProblemsDean Jezer0% (1)
- Ionic Covalent Bonds PractDocument2 pagesIonic Covalent Bonds PractRina NoviantiNo ratings yet
- Ib PPT 4 SL PDFDocument103 pagesIb PPT 4 SL PDFzarna nirmal rawalNo ratings yet
- Dot and Cross PracticeDocument4 pagesDot and Cross PracticeDeez NutsNo ratings yet
- Reaction of Metal Oxides With Acid WorksheetDocument1 pageReaction of Metal Oxides With Acid WorksheetRehan SadiqNo ratings yet
- Worksheet 10.6Document2 pagesWorksheet 10.6SavithaBroonan100% (1)
- Wave Properties WorksheetDocument4 pagesWave Properties WorksheetJohn Rudolf CatalanNo ratings yet
- Rates Practice Exam QuestionsDocument18 pagesRates Practice Exam QuestionsisheanesuNo ratings yet
- Reactions of Acids Homework Worksheet HADocument3 pagesReactions of Acids Homework Worksheet HASarah KKCNo ratings yet
- 2021 Grade 11 End of Term 1 Science Paper 2 TestDocument3 pages2021 Grade 11 End of Term 1 Science Paper 2 TestDavies Masumba100% (1)
- Physical and Chemical Properties-WorksheetDocument2 pagesPhysical and Chemical Properties-WorksheetMARGIE MEDINA100% (1)
- C5 Chemical Changes Exam QuestionsDocument10 pagesC5 Chemical Changes Exam QuestionsfrancescoNo ratings yet
- Lesson 2 WorksheetsDocument11 pagesLesson 2 WorksheetsyuiNo ratings yet
- Heating Curves Worksheet 2Document2 pagesHeating Curves Worksheet 2jules blancoNo ratings yet
- Redox Review With ANSWERS - 4Document13 pagesRedox Review With ANSWERS - 4AYESHA NAAZNo ratings yet
- Workbook - Oxidation and Reduction ReactionsDocument113 pagesWorkbook - Oxidation and Reduction ReactionsRudi Berlian100% (1)
- Ion Formation WorksheetDocument2 pagesIon Formation WorksheetKaren OrlanskiNo ratings yet
- Dot Structures Practice PacketDocument6 pagesDot Structures Practice Packetgoogley71No ratings yet
- Bonding and Lewis Structures WorksheetDocument2 pagesBonding and Lewis Structures WorksheetLarry MolinerosNo ratings yet
- Separation Techniques WorksheetDocument2 pagesSeparation Techniques WorksheetJESSAH MAE GAMUTANNo ratings yet
- What Is A Mole SummativeDocument8 pagesWhat Is A Mole Summativeapi-291560513No ratings yet
- Metals and Non Metals WorksheetDocument2 pagesMetals and Non Metals WorksheetKamaljeet Singh100% (1)
- Acid With Metals WorksheetDocument1 pageAcid With Metals WorksheetSumathi GanasenNo ratings yet
- Mole Ratio WorksheetDocument2 pagesMole Ratio WorksheetmillsaprunnerNo ratings yet
- Class: 7: Unit: 7 "Composition of Matter"Document20 pagesClass: 7: Unit: 7 "Composition of Matter"Imran khanNo ratings yet
- Resistance WorksheetDocument2 pagesResistance WorksheetArnulfo LavaresNo ratings yet
- Ionic Equation WorksheetDocument4 pagesIonic Equation WorksheetIrfana Begum Ikbal100% (1)
- Atoms, Molecules & IonsDocument5 pagesAtoms, Molecules & IonsSalman ZubaerNo ratings yet
- Worksheet On IGCSE Chemical EnergeticsDocument2 pagesWorksheet On IGCSE Chemical EnergeticsSamandarbek Numonov100% (1)
- Quiz Name OxidationDocument3 pagesQuiz Name OxidationAnony Mous100% (1)
- Neutralisation EquationsDocument1 pageNeutralisation EquationsYousha MalikNo ratings yet
- Trends in Group 2 Elements Alkaline Earth MetalsDocument52 pagesTrends in Group 2 Elements Alkaline Earth MetalsKemoy FrancisNo ratings yet
- Worksheet 6.1: Properties of MetalsDocument1 pageWorksheet 6.1: Properties of MetalsCally ChewNo ratings yet
- Specialized Cells - Science - Yr7Document7 pagesSpecialized Cells - Science - Yr7arslanplayz29No ratings yet
- Worksheet #1 Balancing Chemical EquationsDocument4 pagesWorksheet #1 Balancing Chemical EquationsMazanda YalinduaNo ratings yet
- Naming Ionic Compounds Worksheet I PDFDocument3 pagesNaming Ionic Compounds Worksheet I PDFgowrimanohar1975No ratings yet
- Solubility InvestigationDocument3 pagesSolubility InvestigationNoaNo ratings yet
- Elements, Compounds and Mixtures: Gen. ScienceDocument31 pagesElements, Compounds and Mixtures: Gen. ScienceMadhavi KapadiaNo ratings yet
- Reactions of Metals With Acids WorksheetDocument2 pagesReactions of Metals With Acids WorksheetRehan SadiqNo ratings yet
- Chemistry Solutes Solvents Solubility Solutions VCBCCTDocument17 pagesChemistry Solutes Solvents Solubility Solutions VCBCCTDIONYSUS100% (1)
- Giant Covalent Structures WorksheetDocument2 pagesGiant Covalent Structures WorksheetIsabel DurangoNo ratings yet
- WORKSHEET (Chemical Equations) PDFDocument4 pagesWORKSHEET (Chemical Equations) PDFnobodyNo ratings yet
- 6.4 Patterns in The Periodic Table WorksheetDocument3 pages6.4 Patterns in The Periodic Table WorksheetmrnejoeyguNo ratings yet
- Checkpoint Revision Worksheet # 1Document2 pagesCheckpoint Revision Worksheet # 1Sana Shakeel100% (1)
- Acids and Bases Unit Test 2011Document8 pagesAcids and Bases Unit Test 2011jkdfal1No ratings yet
- Worksheet On Atomic Structure, STD 8thDocument3 pagesWorksheet On Atomic Structure, STD 8thArshad KhanNo ratings yet
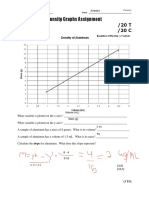
- Density Graphs Assignment: SNC1D1 Chemistry Date: NameDocument6 pagesDensity Graphs Assignment: SNC1D1 Chemistry Date: NameAmeera Vania100% (1)
- Six Types of Reaction Practice SheetDocument3 pagesSix Types of Reaction Practice SheetNikhil BhattNo ratings yet
- ICSE - Physical and Chemical Changes W1Document2 pagesICSE - Physical and Chemical Changes W1Hamisha KathuriaNo ratings yet
- Periodic Classification of ElementsDocument24 pagesPeriodic Classification of ElementsRanjit SinghNo ratings yet
- Algebraic Method To Balance Chemical EquationDocument3 pagesAlgebraic Method To Balance Chemical EquationBruce WalkerNo ratings yet
- O Level Biology Practice Questions And Answers: Coordination And ResponseFrom EverandO Level Biology Practice Questions And Answers: Coordination And ResponseNo ratings yet
- Energy Work PowerDocument17 pagesEnergy Work Powerapi-325791445No ratings yet
- Acceleration HelpDocument1 pageAcceleration Helpapi-325791445No ratings yet
- Speed Unitconversion-ExamplesDocument3 pagesSpeed Unitconversion-Examplesapi-325791445No ratings yet
- Speed Practice 2Document1 pageSpeed Practice 2api-325791445No ratings yet
- Speed PracticeDocument3 pagesSpeed Practiceapi-325791445No ratings yet
- Newton First LawDocument15 pagesNewton First Lawapi-325791445No ratings yet
- What Is Phys Sci IntroDocument10 pagesWhat Is Phys Sci Introapi-325791445No ratings yet
- ch12 Sec1 AsDocument15 pagesch12 Sec1 Asapi-325791445No ratings yet
- The Metric SystemDocument37 pagesThe Metric Systemapi-325791445No ratings yet
- Scientific NotationDocument14 pagesScientific Notationapi-325791445No ratings yet
- Experimental Design SheetDocument2 pagesExperimental Design Sheetapi-325791445No ratings yet
- Atoms CombiningDocument12 pagesAtoms Combiningshehryar khanNo ratings yet
- Classification of SolventsDocument4 pagesClassification of Solventsmohan raoNo ratings yet
- اهم 50 سؤال المتوقعة فى امتحان Chemistry للصف الثالث الثانوى اللغات-الامتحان التعليمىDocument14 pagesاهم 50 سؤال المتوقعة فى امتحان Chemistry للصف الثالث الثانوى اللغات-الامتحان التعليمىHajar HossamNo ratings yet
- Chapter 4. Aqueous Reactions and Solution Stoichiometry: Common Student MisconceptionsDocument7 pagesChapter 4. Aqueous Reactions and Solution Stoichiometry: Common Student MisconceptionsLeo NguyenNo ratings yet
- Board Assignment - AminesDocument5 pagesBoard Assignment - AminesHarsh GuptaNo ratings yet
- Lab 3 NewtonDocument9 pagesLab 3 NewtonKrishna KolluriNo ratings yet
- DNA StructureDocument39 pagesDNA StructureCitra Lusyana0% (1)
- Test 34 - Redox Reactions - Middle of PyramidDocument6 pagesTest 34 - Redox Reactions - Middle of PyramidJay PatelNo ratings yet
- Qoi0809t1 ConfDocument13 pagesQoi0809t1 ConfTahirat NasiruNo ratings yet
- Acid Base and Salt QuestionsDocument3 pagesAcid Base and Salt QuestionsBikash DasNo ratings yet
- Chemistry File Xii 2Document24 pagesChemistry File Xii 2AzkkNo ratings yet
- Handbook of Heterogeneous Catalytic Hydrogenation For Organic Synthesis 2001 2 PDFDocument747 pagesHandbook of Heterogeneous Catalytic Hydrogenation For Organic Synthesis 2001 2 PDFlidiane_nobre5659No ratings yet
- Compounds Containing The Carbonyl Group QuestionsDocument69 pagesCompounds Containing The Carbonyl Group QuestionsmarvellousadenugaNo ratings yet
- Proteins PDFDocument41 pagesProteins PDFSridhar BalaNo ratings yet
- Act 5 Lab Aromatic HC FinalDocument5 pagesAct 5 Lab Aromatic HC FinalJoses CalindasNo ratings yet
- Lecture Notes First Semester Yr 2 BPham BMLS BDSDocument57 pagesLecture Notes First Semester Yr 2 BPham BMLS BDSsriNo ratings yet
- Ujian 3 Form 4 KimiaDocument6 pagesUjian 3 Form 4 KimiaNazreen NashruddinNo ratings yet
- Co-Ordination Compounds PDFDocument1 pageCo-Ordination Compounds PDFYash PatelNo ratings yet
- Piccs 2017Document910 pagesPiccs 2017Reynee Shaira Lamprea MatulacNo ratings yet
- Aldehydes and Ketones - 1-MergedDocument94 pagesAldehydes and Ketones - 1-MergedseNo ratings yet
- Hs Code ChemicalDocument17 pagesHs Code ChemicaljazzloveyNo ratings yet
- Notes C16 121Document13 pagesNotes C16 121Amir HussainNo ratings yet
- PONTERAS (Alcohols, Phenols, Ethers)Document3 pagesPONTERAS (Alcohols, Phenols, Ethers)KARYLLE JUNE PONTERASNo ratings yet
- Mass Spectroscopy: - : Presented By:-Amruta S. Sambarekar 1 Year M.Pharm Dept. of Pharmaceutics M M C P, BelgaumDocument56 pagesMass Spectroscopy: - : Presented By:-Amruta S. Sambarekar 1 Year M.Pharm Dept. of Pharmaceutics M M C P, Belgaumsaurabh chaturvediNo ratings yet
- Synthesis of Coordination CompoundsDocument18 pagesSynthesis of Coordination CompoundsHaileeyssus TedlaNo ratings yet
- Carboxylic AcidDocument9 pagesCarboxylic AcidAbiot DibabaNo ratings yet
- New Microsoft Word DocumentDocument50 pagesNew Microsoft Word DocumentviraajNo ratings yet
- Chapter13 - Acids and BasesDocument44 pagesChapter13 - Acids and BasesXiaohan TangNo ratings yet
- Unit 09 Packet KeyDocument15 pagesUnit 09 Packet Keyjoeyt1026No ratings yet
- Macromolecules - Worksheet Answers - OdtDocument4 pagesMacromolecules - Worksheet Answers - OdtRachel JimenezNo ratings yet