Professional Documents
Culture Documents
Alpha Vs T: Temp 826°C 900°C 1020°C
Alpha Vs T: Temp 826°C 900°C 1020°C
Uploaded by
Purnima kumariOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Alpha Vs T: Temp 826°C 900°C 1020°C
Alpha Vs T: Temp 826°C 900°C 1020°C
Uploaded by
Purnima kumariCopyright:
Available Formats
PROBEM 2
a) α values
Temp alpha vs t
time 826°C 900°C 1020°C 0.300
1 0.035 0.05 0.1 0.250
2 0.076 0.15 0.18
0.200
3 0.106 0.14 0.22
4 0.13 0.17 0.24 0.150
5 0.148 0.192 0.255 0.100
6 0.16 0.21 0.262
0.050
7 0.178 0.23 0.27
0.000
0 1 2 3 4 5 6 7 8
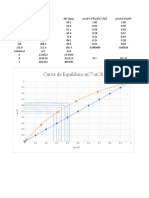
b) Plot g(α) v/s t
g(α) values =1-(2/3*α)-((1-α)^(2/3))
Temp
time 826°C 900°C 1020°C
0 0 0 0
1 0.000138273 0.000284 0.001164
2 0.000664468 0.002683 0.003922
3 0.001311175 0.002326 0.005983
4 0.001995286 0.003481 0.007197
5 0.002609215 0.00449 0.008191
6 0.003067874 0.005423 0.00868
7 0.003831811 0.006574 0.009259
c) Plot ln k v/s 1/T
From the plot of g(α) v/s t we get slope i.e. k rate constant as shown in table below. Then we plotted ln k v/s 1/T
which is known as Arrhenius Plot.
Slope of ln k v/s 1/T will give value of activation energy.
Temp
826°C 900°C 1020°C
k 0.0005 0.0009 0.0015
lnk -7.60090246 -7.01312 -6.50229
1/T 0.000909918 0.000853 0.000773
You might also like
- Alpha Vs T: Probem 2Document5 pagesAlpha Vs T: Probem 2Purnima kumariNo ratings yet
- FIZ2Document10 pagesFIZ2Andreea RaduNo ratings yet
- Batch Problema 1Document2 pagesBatch Problema 1Aylin Portillo OliveraNo ratings yet
- DIAGRAMA nC7-nC8Document2 pagesDIAGRAMA nC7-nC8Aylin Portillo OliveraNo ratings yet
- CH 15Document10 pagesCH 15denabanihani897No ratings yet
- Problem 3.42 PDFDocument2 pagesProblem 3.42 PDFKauê BrittoNo ratings yet
- 2) Comparison of Rate Law From LiteratureDocument4 pages2) Comparison of Rate Law From LiteratureEric WongNo ratings yet
- HW5 SolnsDocument11 pagesHW5 SolnsNaw AzNo ratings yet
- Medidas Experimento 01Document2 pagesMedidas Experimento 01Iago ManancezziNo ratings yet
- Experiment: X1 Vs Gamma 1,2 (Exp)Document5 pagesExperiment: X1 Vs Gamma 1,2 (Exp)Azizah Az ZahraNo ratings yet
- Calculadora Indice de LangelierDocument6 pagesCalculadora Indice de LangelierFábio SenaNo ratings yet
- HT Xlpe PDFDocument3 pagesHT Xlpe PDFrengaramanujanNo ratings yet
- Lab - 3a - Losses in Pipes and Pipe FittingsDocument13 pagesLab - 3a - Losses in Pipes and Pipe FittingsShehan FernandoNo ratings yet
- Calculate The 3 Control Limits For X-Bar and R Charts Based On The First 12 Samples ReflectingDocument6 pagesCalculate The 3 Control Limits For X-Bar and R Charts Based On The First 12 Samples ReflectingRamzi SaeedNo ratings yet
- Saturated Helium: Nama: Fanirazha Primesa C. NIM: 03031381621069 Kelas: ADocument10 pagesSaturated Helium: Nama: Fanirazha Primesa C. NIM: 03031381621069 Kelas: AFaniNo ratings yet
- Book 1Document3 pagesBook 1Arslan ArshadNo ratings yet
- MC 3Document2 pagesMC 3Aylin Portillo OliveraNo ratings yet
- Eco Semana 13Document11 pagesEco Semana 13Tu Mundo Mi MundoNo ratings yet
- Equation Solving ExamplesDocument10 pagesEquation Solving Examplesalif08042012No ratings yet
- Equation Solving ExamplesDocument10 pagesEquation Solving Examplesalif08042012No ratings yet
- Equation Solving ExamplesDocument10 pagesEquation Solving Examplessiswanto.micoNo ratings yet
- Pauta+solemne+2 V1Document22 pagesPauta+solemne+2 V1jean_pgallardoNo ratings yet
- Physics Report On ThermometersDocument3 pagesPhysics Report On ThermometersMiracle AndersonNo ratings yet
- Ohm's Law IDocument6 pagesOhm's Law IAndre JohnsonNo ratings yet
- Perhitungan Unit Hidrograf Metode Nakayasu Parameter HSS NakayasuDocument17 pagesPerhitungan Unit Hidrograf Metode Nakayasu Parameter HSS NakayasuAis(y)ahNo ratings yet
- UUT Lab ReportDocument10 pagesUUT Lab ReportAyong AnisNo ratings yet
- Diagrama Creciente) : Elavoracion de Hidrograma UnitarioDocument5 pagesDiagrama Creciente) : Elavoracion de Hidrograma Unitariodavid porrasNo ratings yet
- Physics Lab Report On Measuring Specific Heat Capacity Using Electrical MethodDocument3 pagesPhysics Lab Report On Measuring Specific Heat Capacity Using Electrical MethodMinh An Phan LeNo ratings yet
- Expt 6Document11 pagesExpt 6nooneNo ratings yet
- Laporan IqbalDocument5 pagesLaporan IqbalKekek LeliyanaNo ratings yet
- Tabla de Valores Capacitancia (PF) Distancia (MM) : Los Resultados Son: Parte 1. Capacitancia y ResistenciaDocument6 pagesTabla de Valores Capacitancia (PF) Distancia (MM) : Los Resultados Son: Parte 1. Capacitancia y Resistenciajonathan medianeroNo ratings yet
- DIAGRAMA nC6-nC7Document2 pagesDIAGRAMA nC6-nC7Aylin Portillo OliveraNo ratings yet
- LAB MEC424-Slider CrankDocument7 pagesLAB MEC424-Slider CrankAhmad Fadzlan93% (15)
- Contoh Hit Debit Dengan Current MetterDocument5 pagesContoh Hit Debit Dengan Current MetterAnna EmiliawatiNo ratings yet
- Jhardy MidtermDocument10 pagesJhardy MidtermJacob HardyNo ratings yet
- Contoh Hit Debit Dengan Current MetterDocument5 pagesContoh Hit Debit Dengan Current MetterAnna EmiliawatiNo ratings yet
- Resolución Práctica Calificada 3: Subcuenca 01 Subcuenca 02 TC TCDocument2 pagesResolución Práctica Calificada 3: Subcuenca 01 Subcuenca 02 TC TCNicole HuamaníNo ratings yet
- Practica Termo2Document5 pagesPractica Termo2Angel de JesusNo ratings yet
- Equilibrium DistillationDocument4 pagesEquilibrium DistillationEngr Anees AhmadNo ratings yet
- Vo Ve Co Ce Qe Lgce LG Qe 1/ce 1/qe Vo VeDocument6 pagesVo Ve Co Ce Qe Lgce LG Qe 1/ce 1/qe Vo VeoanaiulianaNo ratings yet
- PartyyyyDocument9 pagesPartyyyyMOH KHASAN AL FARUQ FARUQNo ratings yet
- Benceno-Etilbenceno RaoultDocument5 pagesBenceno-Etilbenceno RaoultGian GiancarlosNo ratings yet
- Tensione Di Vapore Formula Di AntoineDocument84 pagesTensione Di Vapore Formula Di AntoinealessandroNo ratings yet
- Software Exercise 1Document14 pagesSoftware Exercise 1MeraNo ratings yet
- Plantilla FormulasDocument10 pagesPlantilla FormulasjorgeluistreverasartilloNo ratings yet
- Curva de Calibracion: Pendiente (M) Intercepto (B) (Sy)Document2 pagesCurva de Calibracion: Pendiente (M) Intercepto (B) (Sy)Estefania GuzmanNo ratings yet
- Datos ExperimentalesDocument8 pagesDatos ExperimentalesKatty FloresNo ratings yet
- Swinburne University of Technology School of Engineering: Semester 1, 2019Document8 pagesSwinburne University of Technology School of Engineering: Semester 1, 2019Shehan FernandoNo ratings yet
- Swinburne University of Technology School of Engineering: Semester 1, 2019Document8 pagesSwinburne University of Technology School of Engineering: Semester 1, 2019Shehan FernandoNo ratings yet
- Temperatura: 25 °C (Ch3Cooc2H5) : (Naoh) 0,1M Tiempo (S) Conductividad (MS) 1/conductividad (Ms - 1)Document8 pagesTemperatura: 25 °C (Ch3Cooc2H5) : (Naoh) 0,1M Tiempo (S) Conductividad (MS) 1/conductividad (Ms - 1)Melissa QuinteroNo ratings yet
- Inflow Hydrograph: Time-Area CalculationDocument49 pagesInflow Hydrograph: Time-Area CalculationGan Chin PhangNo ratings yet
- Sup Med 0 Z Sup Z Med I (M) GZ Sup GZ Med Inf Z Inf GZ InfDocument2 pagesSup Med 0 Z Sup Z Med I (M) GZ Sup GZ Med Inf Z Inf GZ InfPop PollyNo ratings yet
- K Factor Quick Reference - Thermo Scientific Home PageDocument2 pagesK Factor Quick Reference - Thermo Scientific Home PageAbd AbuaishehNo ratings yet
- Lab Report E11Document9 pagesLab Report E11rbaldwin8No ratings yet
- Deck Slab DesignDocument18 pagesDeck Slab DesignNeelakandan PrakashNo ratings yet
- E 3.05E+01 3,532,921,654 1,221,044,674 fctm 2.60 37.87 Cracked β 1.00 17.40 L 8.00 X/l Moment ζ α I I M PDocument2 pagesE 3.05E+01 3,532,921,654 1,221,044,674 fctm 2.60 37.87 Cracked β 1.00 17.40 L 8.00 X/l Moment ζ α I I M PAbel BerhanmeskelNo ratings yet
- (Uas) - Rekayasa Hidrologi-Kevin Josua Ginting (212318045)Document5 pages(Uas) - Rekayasa Hidrologi-Kevin Josua Ginting (212318045)Kevin Josuaginting2No ratings yet
- Elastic Buckling of Column Under Varying Axial ForceDocument5 pagesElastic Buckling of Column Under Varying Axial ForcePatipol GunhomepooNo ratings yet
- Ejercicios en BiofarmaciaDocument8 pagesEjercicios en BiofarmaciaAlan Luis CVNo ratings yet