Assignment 1 and 2
Assignment 1 and 2
Uploaded by
Muhammad Idrees KhanCopyright:
Assignment 1 and 2
Assignment 1 and 2
Uploaded by
Muhammad Idrees KhanOriginal Title
Copyright
Share this document
Did you find this document useful?
Is this content inappropriate?
Copyright:
Assignment 1 and 2
Assignment 1 and 2
Uploaded by
Muhammad Idrees KhanCopyright:
Institute of Metallurgy and Materials Engineering
University of Punjab, Lahore
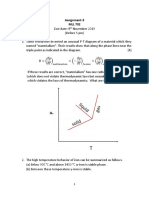
Assignment #1 [CLO 2]
Subject: Materials Thermodynamics & Kinetics (MME-223)
Total Marks: 10 (Equivalent to 4%)
Submission Deadline: 31st October 2022
Solve the following problems using Clapeyron or Clausius-Clapeyron
relations as appropriate.
Question 1: The vapor pressure p of Zn varies with temperature (T) in equilibrium with
−15780
Solid as ln p = − 0.755ln T + 19.25
T
−15250
Liquid as ln p = − 1.255ln T + 21.79
T
Find Tb, Ttp, Ptp, ∆Hsub, ∆Heva at Tb and Ttp, ∆Hmelting
Question 2: CaF2 has two solid allotropes, α (low temperature) and β (high temperature), β melts
to form liquid at the melting point. The vapor pressure of CaF2 vapors varies with temperature as
given below for different condensed phases.
−54350
CaF2 (α) : ln p = − 4.525ln T + 56.57
T
−53780
CaF2 (β) : ln p = − 4.525ln T + 56.08
T
−50200
CaF2 (L): ln p = − 4.525ln T + 53.96
T
Draw a schematic phase diagram for CaF2 showing all the transformations and label all the
characteristic points. And find Tb, Ttp, Ptp at all the triple points, ∆Hsub(α→v), ∆Hsub(β →v), ∆Heva,
∆Hmelting , ∆Hα→β
Question 3: The vapor pressure, P, (measured in mm Hg) of liquid arsenic, is given by
−2460 −6947
log P = + 6.69 and that of solid arsenic by log P = + 10.8
T T
Calculate the temperature at which the two forms of arsenic will have the same vapor pressure.
Also find out the pressure at that temperature.
Institute of Metallurgy and Materials Engineering
University of Punjab, Lahore
Assignment #2 [CLO 2]
Subject: Materials Thermodynamics & Kinetics (MME-223)
Total Marks: 10 (Equivalent to 4%)
Submission Deadline: 31st October 2022
Solve the following problems using Clapeyron or Clausius-Clapeyron
relations as appropriate.
Question 1: The saturated vapor pressure of liquid NdCl5 measured at 478 K and 520 K is 0.3045
atm and 0.8310 atm. Calculate the normal boiling point of NdCl5.
Question 2: The vapor pressures of liquid thorium tetraiodide at 1073 K and 1098 K are 62395 Pa
and 87993 Pa, respectively. Calculate its mean heat of vaporization.
Question 3: The heat of evaporation (∆Heva) of copper at its normal boiling point is 31172 kJ/mol.
The vapor pressure of copper at 1773 K is 34 Pa. Calculate the boiling point of copper.
Question 4: The densities of liquid and solid bismuth are 10.0 g/cm3 and 9.673 g/cm3,
respectively at the normal melting point 543 K. The heat of fusion (∆Hmelting) is 2.633 12.02
kJ/mol. Calculate the melting point of bismuth under a pressure of 100 atm, Atomic weight of
bismuth is 209.
Question 5: The melting point of cadmium at 1 atm is 594 K, and its heat of fusion is 57.15 kJ/kg
[you may like to convert into J/mol]. The volume change on the melting of cadmium is +0.0064
cm3/g [you may like to convert into cm3/mol]. Calculate the melting point of cadmium at 2 atm
pressure.
Question 6: Mercury melts at 234.13 K at 1 atm pressure. The densities of liquid and solid
mercury are 13.69 g/cm3 and 14.19 cm3 respectively at the normal melting point. The heat of
fusion of mercury is 9.75 kJ/kg). Calculate the melting point of mercury at 2 atm pressure.
Question 7: The densities of ice and liquid water at 1 atm and 273 K are 0.917 g/ cm3 and 0.9998
g/cm3 respectively. The heat of fusion of water is 334.72 kJ/kg. Calculate the melting point of ice
at 0.5 atm and 107 atm pressures.
You might also like
- OLGA - Modeling Buried Pipelines Without FEMTherm - 6620827 - 03100% (1)OLGA - Modeling Buried Pipelines Without FEMTherm - 6620827 - 034 pages
- IIIT RK Valley (Idupulapaya) Rajiv Gandhi University of Knowledge Technologies - Andhra PradeshNo ratings yetIIIT RK Valley (Idupulapaya) Rajiv Gandhi University of Knowledge Technologies - Andhra Pradesh2 pages
- To Identify Material Using Specific Heat As A Measurable Characteristic. Specific HeatNo ratings yetTo Identify Material Using Specific Heat As A Measurable Characteristic. Specific Heat5 pages
- Solution 10 (Brahmastra Test Series) @NeetoxphillicNo ratings yetSolution 10 (Brahmastra Test Series) @Neetoxphillic49 pages
- 5882347ac722925535ee4c0180cdfe4c7ab5075b43763534be1a54d23394374a4c8c25ca3eea4b912007d95beedf2d305961d593d4e95e49b5e0fcd6479beeaa24049_1608442713No ratings yet5882347ac722925535ee4c0180cdfe4c7ab5075b43763534be1a54d23394374a4c8c25ca3eea4b912007d95beedf2d305961d593d4e95e49b5e0fcd6479beeaa24049_16084427132 pages
- Lecture 8: Thermo Chemistry Applications in Metal ExtractionNo ratings yetLecture 8: Thermo Chemistry Applications in Metal Extraction6 pages
- Review Questions - Solutions: Multiple ChoiceNo ratings yetReview Questions - Solutions: Multiple Choice10 pages
- NCERT Solutions For Class 11 Chemistry 16may Chapter 6 ThermodynamicsNo ratings yetNCERT Solutions For Class 11 Chemistry 16may Chapter 6 Thermodynamics19 pages
- Chemistry Jee Main Shift-1 Total Questions Topic WiseNo ratings yetChemistry Jee Main Shift-1 Total Questions Topic Wise72 pages
- Numerical Simulation of Steel Ingot Solidification Process 2No ratings yetNumerical Simulation of Steel Ingot Solidification Process 24 pages
- CHEM 1000 Mid-Year Exam December 2002: Part A. 60 Marks. Answer Each Question (5 Marks Each)No ratings yetCHEM 1000 Mid-Year Exam December 2002: Part A. 60 Marks. Answer Each Question (5 Marks Each)7 pages
- Cbse Class Xi Physics Chap 11 - Thermal Properties of Matter - PracticeNo ratings yetCbse Class Xi Physics Chap 11 - Thermal Properties of Matter - Practice10 pages
- Day 74 - Daily MCQ Workout - 40 Revision MCQsNo ratings yetDay 74 - Daily MCQ Workout - 40 Revision MCQs5 pages
- Entropy, Gibbs Free Energy and Clausius-Clapeyron EquationNo ratings yetEntropy, Gibbs Free Energy and Clausius-Clapeyron Equation28 pages
- XI Chemistry Target Paper 2023 (Sir Nasim Zulfiqar)No ratings yetXI Chemistry Target Paper 2023 (Sir Nasim Zulfiqar)5 pages
- r05310803 Chemical Engineering Thermodynamics IINo ratings yetr05310803 Chemical Engineering Thermodynamics II8 pages
- Product Specifications Product Specifications: Ldf4Rn LDF4RN - 50A 50ANo ratings yetProduct Specifications Product Specifications: Ldf4Rn LDF4RN - 50A 50A3 pages
- Class 11 - Important Formulas Chapter 5 - Laws of MotionNo ratings yetClass 11 - Important Formulas Chapter 5 - Laws of Motion1 page
- Principle and Applications of The Background Oriented Schlieren (BOS) MethodNo ratings yetPrinciple and Applications of The Background Oriented Schlieren (BOS) Method10 pages
- GUIDE Group Exercise #17: This Study Resource WasNo ratings yetGUIDE Group Exercise #17: This Study Resource Was2 pages
- Laplace Transforms: The Laplace Transform of eNo ratings yetLaplace Transforms: The Laplace Transform of e2 pages
- Assessment: Grade 6 3rd Quarter - Module 4-5-60% (1)Assessment: Grade 6 3rd Quarter - Module 4-5-610 pages
- Rudy Rucker's Introduction To Speculations On The Fourth Dimension: Selected Writings Charles H. HintonNo ratings yetRudy Rucker's Introduction To Speculations On The Fourth Dimension: Selected Writings Charles H. Hinton15 pages
- Table of Content:: Mechanics of A Lamina Fibre Composites, FS20 Masoud Motavalli 1No ratings yetTable of Content:: Mechanics of A Lamina Fibre Composites, FS20 Masoud Motavalli 181 pages
- First Response Letter To Lexi Neal's "AQAL Cube Octo-Dynamics" by Giorgio Piacenza CabreraNo ratings yetFirst Response Letter To Lexi Neal's "AQAL Cube Octo-Dynamics" by Giorgio Piacenza Cabrera3 pages
- Maths Notes For Class 10 Chapter 13 Surface Areas and VolumesNo ratings yetMaths Notes For Class 10 Chapter 13 Surface Areas and Volumes5 pages