Answers
Answers
Uploaded by
Swastik DasCopyright:
Available Formats
Answers
Answers
Uploaded by
Swastik DasCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Copyright:
Available Formats
Answers
Answers
Uploaded by
Swastik DasCopyright:
Available Formats
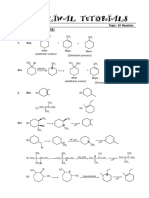
CHEMISTRY: ANSWER 1
1. CH3 CH3
Cl
+
Cl
(minor) (major)
CH3 CH3
Br
+
Br
(minor) (major)
CH 2Br
CH 2Cl
2. Br CH CH3
3. CMe 3 CMe 3
Br
(major) + (minor)
Br
CH3
H3C C CH 2Cl
No reaction
S. Bagchi Classes (School of Chemistry) | Salt Lake- CD-293 | Gariahat | 9830705367
CHEMISTRY: ANSWER 2
4. COOH
COOH
No reaction
5. A is i) CrO3/acetic anhydride/H2SO4
ii) H2O/H2SO4
or
i) CrO2Cl2
ii) H2O/H2SO4 (Eterd reaction)
or
i) CAN (Ceric Amnonium nitride)
ii) H+
6. CHO COOH
CHO CH 2OH
i) Concentration NaOH
+
ii) H / H2 O
A B
H 2 SO 4
O O
C
CH 2
O OH +
C OH
CH 2 OH C OH
+
H
CH 2 OH
OH
O
C OH C
O O
CH 2 CH 2
S. Bagchi Classes (School of Chemistry) | Salt Lake- CD-293 | Gariahat | 9830705367
CHEMISTRY: ANSWER 3
7. CHO
Only 1 CH 3 (any one) will react.
CH3
8. 5 > 2 > 3 > 1 > (free radical stability)
9. H3C CH CH CH2
OH
O O
H3C C C CH3
H3C CH CH CH2
OH
10. CH3
CH 3 CH 2 CH CH2 CH 3 CH CH2 H3C C CH CH2 (free radical stability)
CH3
11. O O
H3C C C CH3
12. i) alkaline KMnO4
ii) H+
B → E2 elimination though 30 alcohol.
13. O
H3C C OH + CH 3CH 2 COOH
O O
H3C C C CH 2CH 3
14. Propof’s rule: CH3CH2COOH + CH3CH2COOH
15. O
H3C CH CH CH2CH3
Same
16. O
H3C C CH CH CH CH3
CH3
More reactive (C = C) since free radical most stable at that position.
S. Bagchi Classes (School of Chemistry) | Salt Lake- CD-293 | Gariahat | 9830705367
CHEMISTRY: ANSWER 4
17. O O
CH 3 SH
H3C CH CH CH 2CH 3 H3C CH CHCH 2CH 3
+ SCH 3
H
+
H
H OH
+ H3C CH CH CH 2CH 3
O
SCH 3
CH 3 SH
H3C CH CH CH 2CH 3
(SN 1 like)
OH
H3C CH CH CH 2CH 3
SCH 3
18. CH 3 CH CH 2
OH OH
O
H3C C OH + CO 2 + H2O
19. (a) CH 3 COOH + CO 2 + H2O
(b) CH 3 COOH + CH 3CH 2COOH
O
(c) CH 3COOH + CH 3 C CH 2CH 3
(d) 2(CO 2 + H2O)
O O
(e) H3C C C CH3
O O
(f) CH 3 C C OH
O O
(g) HO C C OH
20. CH 3 CHO + CH 3CH 2CHO
CH 3CHO + CH 3CH 2CHO
CH 3COOH + CH 3CH 2COOH
CH 3COOH + CH 3CH 2COOH
CH 3COOH + CH 3CH 2COOH
S. Bagchi Classes (School of Chemistry) | Salt Lake- CD-293 | Gariahat | 9830705367
CHEMISTRY: ANSWER 5
21. HC CH CH CH CHO
HC CH CH CH COOH + HC CH CH CH CHO
(major)
HC CH CH CH CHO
HC CH CH CH CHO
22. 3 mole of HIO4 is required to break completely.
OH O O OH
OH
H3C CH + CH CH 2 C OH + HO CH CH 2 C CH 2 CH CH 2 CH + HO CH CH3
OH OH
OH OH OH OH
O O OH
O O O O O
H3C C H +H C CH 2 C OH + H C CH 2 C CH 2 CH CH 2 C H+H C CH 3
23. a X
b A, B, C
c A
d A, B, C
e A, B, C
H3C O OH H3C OH OH H3C O
OH HO H
H3C O
H3C CH CH 2 CH 2 COOH
HO H
OH
CH OH CH OH
H3C C O H3C C O CH OH
H3C C OH
COOH CH O
H3C CH OH H3C CH OH
(f) A, B, C
(g) X
(h) A, B, C
(i) X
S. Bagchi Classes (School of Chemistry) | Salt Lake- CD-293 | Gariahat | 9830705367
You might also like
- Sion CalculationDocument387 pagesSion CalculationHena AgrawalNo ratings yet
- 11acyl Halideisrael Jude P.Document16 pages11acyl Halideisrael Jude P.Angelo AstudilloNo ratings yet
- Oxidation QuestionsDocument4 pagesOxidation QuestionsSwastik DasNo ratings yet
- JEE Main Organic Compound Containing Halogens Important QuestionsDocument15 pagesJEE Main Organic Compound Containing Halogens Important QuestionsRuchitha VNo ratings yet
- Alkyl Halides AnsDocument2 pagesAlkyl Halides Anshattorininja1986No ratings yet
- Alkyl Halides QuesDocument5 pagesAlkyl Halides Queshattorininja1986No ratings yet
- Alkyl Halides QuesDocument5 pagesAlkyl Halides Quesvishesh17052007No ratings yet
- AlcoholPhenolandEthersDPPVI2024Solutions PDFDocument14 pagesAlcoholPhenolandEthersDPPVI2024Solutions PDFranaabhiruchiNo ratings yet
- C - Sol - Ch-26 - Aldehydes Ketones and Carboxylic AcidsDocument19 pagesC - Sol - Ch-26 - Aldehydes Ketones and Carboxylic AcidsHimanshi ChahalNo ratings yet
- Ex 2Document4 pagesEx 2Mahesh JagtapNo ratings yet
- C - Sol - Ch-26 - Aldehydes Ketones and Carboxylic AcidsDocument1 pageC - Sol - Ch-26 - Aldehydes Ketones and Carboxylic AcidsRishi KeshNo ratings yet
- Ape Assignment 3Document7 pagesApe Assignment 3Atharva KulkarniNo ratings yet
- +2 NEET IntelliQuest PCB-3 (28.01.2021) AnsKey - 10645292Document3 pages+2 NEET IntelliQuest PCB-3 (28.01.2021) AnsKey - 10645292swap2005sharmaNo ratings yet
- Chapter 5 HydrocarbonDocument25 pagesChapter 5 Hydrocarbonmeshal retteryNo ratings yet
- Peta Minda KimiaDocument36 pagesPeta Minda KimiaNATASHA 'ALIA BINTI ZULKIFLINo ratings yet
- Materi Reaksi KimiaDocument11 pagesMateri Reaksi KimiaRenn AgenaNo ratings yet
- Carbonyl Compounds and Carboxylic Acid - Med Easy - Yakeen 2.0 2024 (Legend)Document12 pagesCarbonyl Compounds and Carboxylic Acid - Med Easy - Yakeen 2.0 2024 (Legend)agrawaltwinkle2005No ratings yet
- CY2102Document3 pagesCY2102Prarabdha SharmaNo ratings yet
- 12TH PST-12 Eng-Guj Solu. (26-11-2024) - RamanDocument2 pages12TH PST-12 Eng-Guj Solu. (26-11-2024) - Ramanbathaniom04No ratings yet
- Hydrocarbon Njs2021Document25 pagesHydrocarbon Njs2021aarushagrawal0905No ratings yet
- Aldehydes Ketones: Subjective ProblemsDocument16 pagesAldehydes Ketones: Subjective ProblemsBhaskar AnandNo ratings yet
- Subject: Chemistry DATE: 17-04-2024: Carbonyl CompoundsDocument3 pagesSubject: Chemistry DATE: 17-04-2024: Carbonyl CompoundsPriyanshu BhagatNo ratings yet
- ReactionsDocument20 pagesReactionsknature0319100% (1)
- CLS ENG 22 23 XII Che Target 5 Level 1 Chapter 13Document62 pagesCLS ENG 22 23 XII Che Target 5 Level 1 Chapter 13Harsh JakharNo ratings yet
- Tutorial 12 SolutionDocument3 pagesTutorial 12 Solutionciraolwethu0No ratings yet
- Alkyl Halides & Aryl Halides-02 - Solved ProblemsDocument13 pagesAlkyl Halides & Aryl Halides-02 - Solved ProblemsRaju SinghNo ratings yet
- Ib Chem Answers 20Document4 pagesIb Chem Answers 20LE ZHAINo ratings yet
- Carbonyl CompoundDocument10 pagesCarbonyl CompoundParam shahNo ratings yet
- Problems 11-20-06 Chem231-SSCC: C H H C C C H CH C H CL C C H CH C H + CLDocument6 pagesProblems 11-20-06 Chem231-SSCC: C H H C C C H CH C H CL C C H CH C H + CLsarahNo ratings yet
- Carboxylic Acid & Derivatives-02 - Solved ProblemsDocument15 pagesCarboxylic Acid & Derivatives-02 - Solved ProblemsRaju SinghNo ratings yet
- Answers To AssignmentDocument1 pageAnswers To AssignmentIgbereyivwe TejiriNo ratings yet
- Chemistry Education Study Program: Ira Lestari, S.Si. M.Si Universit of TanjungpuraDocument23 pagesChemistry Education Study Program: Ira Lestari, S.Si. M.Si Universit of TanjungpuraTri indri yaniNo ratings yet
- Alcohol, Ether and PhenolDocument4 pagesAlcohol, Ether and PhenolKushagra SrivastavaNo ratings yet
- Carboxylic Acids and Its Derivatives DPP III 2022 SolutionsCopy_26586811Document9 pagesCarboxylic Acids and Its Derivatives DPP III 2022 SolutionsCopy_26586811boigaming328No ratings yet
- C - 8. (Alkyl & Aryl Hallide, Alcohol Ethers & Phenols) (Adv)Document20 pagesC - 8. (Alkyl & Aryl Hallide, Alcohol Ethers & Phenols) (Adv)revohe2640No ratings yet
- Alcohol Phenol EtherDocument3 pagesAlcohol Phenol EtherParam shahNo ratings yet
- CH NH CH CN O CH C O H CO: Circle and Name All 14 Functional Group From The Diagram BelowDocument9 pagesCH NH CH CN O CH C O H CO: Circle and Name All 14 Functional Group From The Diagram BelowFirdaus RamliNo ratings yet
- Hydrocarbons Pyqs SolnsDocument12 pagesHydrocarbons Pyqs Solnssaadvik1121No ratings yet
- Organic Review 4 MarkingDocument3 pagesOrganic Review 4 MarkingvickrantselvendranNo ratings yet
- C Sol Ch-21 HydrocarbonsDocument8 pagesC Sol Ch-21 Hydrocarbonsmysoftinfo.incNo ratings yet
- E1 Reactions - SolutionDocument3 pagesE1 Reactions - SolutionAshutosh KolseNo ratings yet
- 167245Document2 pages167245salman15assiriNo ratings yet
- 20 HaloalkanesDocument7 pages20 HaloalkanesizabelNo ratings yet
- Alcohols, Phenols & Ether - AnswersDocument6 pagesAlcohols, Phenols & Ether - AnswersK. RupaNo ratings yet
- 6d5245b7-95ec-4952-8c02-9dd44160cc05Document11 pages6d5245b7-95ec-4952-8c02-9dd44160cc05aryanlalpuraNo ratings yet
- NEET Alcohol Phenol and Ether Important Questions - Free PDF DownloadDocument25 pagesNEET Alcohol Phenol and Ether Important Questions - Free PDF DownloadPRANAV CHHAPARWALNo ratings yet
- 9.8.21 1655474586465Document6 pages9.8.21 1655474586465shintae2000No ratings yet
- Oxygen DPT 16 SolDocument2 pagesOxygen DPT 16 SolRamanaa SNo ratings yet
- Chapter 7 HaloalkanesDocument11 pagesChapter 7 HaloalkanesSandra JohnNo ratings yet
- 27 Alcohol Phenol Ether Formula Sheets Getmarks AppDocument15 pages27 Alcohol Phenol Ether Formula Sheets Getmarks AppFLASH FFNo ratings yet
- Photo ChemistryDocument26 pagesPhoto ChemistryAkowuah SamuelNo ratings yet
- Org Che Carboxylic AcidsDocument3 pagesOrg Che Carboxylic Acidskripalaalal4No ratings yet
- Short Notes by SK SirDocument8 pagesShort Notes by SK SirJay MeenaNo ratings yet
- Chemistry - 24 Jan - MorningDocument15 pagesChemistry - 24 Jan - MorningUju surtNo ratings yet
- Chemistry Test-Ii: Part-I Section-I Single Correct Choice Type 1. (D)Document19 pagesChemistry Test-Ii: Part-I Section-I Single Correct Choice Type 1. (D)aayushNo ratings yet
- Organic Chemistry: Preparations of Carbonyl Compounds Subjective QuestionsDocument6 pagesOrganic Chemistry: Preparations of Carbonyl Compounds Subjective QuestionsVandana ReddyNo ratings yet
- 9.8.2021 Test Paper - 2 Evolve Batch 1655474592306Document7 pages9.8.2021 Test Paper - 2 Evolve Batch 1655474592306shintae2000No ratings yet
- Sample MCQ Organic Chemistry Sem II PSCH203 BacklogDocument4 pagesSample MCQ Organic Chemistry Sem II PSCH203 BacklogganesanneelamuruganNo ratings yet
- How To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inDocument2 pagesHow To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inmyiitchemistry81% (16)
- A - 1 (Isomerism, Reaction Mechanism) - Question PaperDocument11 pagesA - 1 (Isomerism, Reaction Mechanism) - Question PaperSachin DedhiaNo ratings yet
- Balancing Chemical Equations: Things You Should Know (Questions and Answers)From EverandBalancing Chemical Equations: Things You Should Know (Questions and Answers)No ratings yet
- HSAB TheoryDocument11 pagesHSAB TheoryMahesh Babu SriNo ratings yet
- Chimistry MicroprojectDocument6 pagesChimistry MicroprojectAditya NikamNo ratings yet
- Crystal Field Theory, Spectrochemical Series, High Spin-Low Spin Complexes and Jahn-Teller Effect Crystal Field TheoryDocument2 pagesCrystal Field Theory, Spectrochemical Series, High Spin-Low Spin Complexes and Jahn-Teller Effect Crystal Field Theoryuvir iitmNo ratings yet
- Chapter 13 Chemical Change and Chemical BondDocument4 pagesChapter 13 Chemical Change and Chemical Bondsushila patel sushila patelNo ratings yet
- Tutorial QuestionDocument6 pagesTutorial QuestionopemipoalakindeNo ratings yet
- Sulphuric AcidDocument10 pagesSulphuric Acidgaming channel gaming channelNo ratings yet
- MERCAPTANDocument26 pagesMERCAPTANse1986No ratings yet
- Chapter 16 SmithDocument26 pagesChapter 16 SmithSandipan SahaNo ratings yet
- BS1001 LipidsDocument32 pagesBS1001 LipidsLiloyi LupiyaNo ratings yet
- Bonding Exam RevisionDocument19 pagesBonding Exam RevisionVaida MatulevičiūtėNo ratings yet
- Form 3 - Chemistry - Assignment - 237 - 1590689559732-CHEM-F3Document157 pagesForm 3 - Chemistry - Assignment - 237 - 1590689559732-CHEM-F3JosephNo ratings yet
- Classification of Org. CompdDocument7 pagesClassification of Org. CompdRaju SinghNo ratings yet
- MIXED BED Ion EXCHANGEDocument10 pagesMIXED BED Ion EXCHANGEMayette Rose SarrozaNo ratings yet
- Protecting Group HandoutDocument5 pagesProtecting Group HandoutRafaelle Sanvictores SilongNo ratings yet
- A+ Blog-Std-9-Chemistry-Chapter-3-Short Notes (Em)Document2 pagesA+ Blog-Std-9-Chemistry-Chapter-3-Short Notes (Em)mohammednisham5727No ratings yet
- Is 12308-5 - 1991 - 3Document1 pageIs 12308-5 - 1991 - 3Svapnesh ParikhNo ratings yet
- Organic ChemistryDocument32 pagesOrganic Chemistryj.obriain94No ratings yet
- Model 1 Exam Chapter 3 2024Document49 pagesModel 1 Exam Chapter 3 2024wg9xh2gw8dNo ratings yet
- CH 221 Tutorial Questions-1Document2 pagesCH 221 Tutorial Questions-1omary hassanNo ratings yet
- Chemistry 1 Zone A 2007Document20 pagesChemistry 1 Zone A 2007Lau Lee LingNo ratings yet
- Microsoft Word - Chem Model Question Papers - Master Trainers - 3Document15 pagesMicrosoft Word - Chem Model Question Papers - Master Trainers - 3HarishNo ratings yet
- General Chemistry The Essential Concepts 7th Edition Chang Solutions ManualDocument23 pagesGeneral Chemistry The Essential Concepts 7th Edition Chang Solutions Manualaffableamassor7h7100% (24)
- Octahedral vs. Tetrahedral GeometriesDocument3 pagesOctahedral vs. Tetrahedral GeometriesMa'arif A. SyafiiNo ratings yet
- Alcohols, Phenols, Ethers and ThiolsDocument22 pagesAlcohols, Phenols, Ethers and ThiolsJenan Usman JalilNo ratings yet
- Lewis Dot Structure PracticeDocument10 pagesLewis Dot Structure PracticeAngela MamonvilNo ratings yet
- Assignment 2 AnswerDocument5 pagesAssignment 2 AnswerVaibhav BacchavNo ratings yet
- Ib PPT 8 SL PDFDocument37 pagesIb PPT 8 SL PDFzarna nirmal rawalNo ratings yet
- IB-checklist-for-students-HL-Acids Ib Topic 8Document6 pagesIB-checklist-for-students-HL-Acids Ib Topic 8Hana BessalahNo ratings yet