Lab Manual GMB
Lab Manual GMB
Uploaded by
Deepika ChoudharyCopyright:
Available Formats
Lab Manual GMB
Lab Manual GMB
Uploaded by
Deepika ChoudharyCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Copyright:
Available Formats
Lab Manual GMB
Lab Manual GMB
Uploaded by
Deepika ChoudharyCopyright:
Available Formats
Experiment no.
1
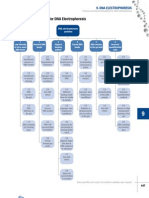
To perform Agarose gel electrophoresis Materials required:
An electrophoresis chamber and power supply Gel casting trays, which are available in a variety of sizes and composed of UV-transparent plastic. The open ends of the trays are closed with tape while the gel is being cast, then removed prior to electrophoresis.
Sample combs, around which molten agarose is poured to form sample wells in the gel. Electrophoresis buffer, usually Tris-acetate-EDTA (TAE) or Tris-borate-EDTA (TBE). Loading buffer, which contains something dense (e.g. glycerol) to allow the sample to "fall" into the sample wells, and one or two tracking dyes, which migrate in the gel and allow visual monitoring or how far the electrophoresis has proceeded.
Ethidium bromide, a fluorescent dye used for staining nucleic acids. NOTE: Ethidium bromide is a known mutagen and should be handled as a hazardous chemical - wear gloves while handling.
Transilluminator (an ultraviolet lightbox), which is used to visualize ethidium bromidestained DNA in gels. NOTE: always wear protective eyewear when observing DNA on a transilluminator to prevent damage to the eyes from UV light.
Procedure 1. For a 1% agarose gel, weigh out 1g of agarose into a flask and add 100ml of 1 x TBE or TAE. Heat solution in a microwave or on hot plate until agarose is completely dissolved and gives a clear solution. 2. Allow to cool until it has a temperature of 50 55 C; for 10 min (approx.). 3. Prepare gel casting tray by sealing ends of gel chamber with tape or appropriate casting system. Place appropriate number of combs in gel tray. 4. Add 5 l of ethidium bromide to cooled gel and pour into gel tray. Allow to cool for 15-30 min at room temperature. Gels can also be placed in a cold space and used the following day. 5. Remove comb(s), place in electrophoresis chamber and cover with buffer (TAE or TBE as used previously).
6. Add loading buffer to samples. As a guideline, add 1.5 l of 10x Loading Buffer to a 20-25 l PCR/DNA solution. For more concentrated DNA solutions (e.g. plasmids), prepare tubes with 8 l of 2x Loading Buffer and 2 l of plasmid. 7. Load DNA and standard (Ladder) onto gel. 8. Electrophorese at 100V for 1 h. 9. Visualize DNA bands using UV transilluminator or gel imaging system.
Reagents/Solutions
50 x TAE : 242g Tris base, 57.1 ml of glacial Acetic acid, 100ml of 0.5M EDTA (pH 8.0), Make up to 1 L with water. (set pH of EDTA with NaOH pellets; do not use HCl to alter the pH)
5 x TBE: 54g Tris base: 27.5g of boric acid: 20ml of 0.5M EDTA (pH 8.0), Make up to 1 L with water.
Trouble - shooting:
If you see faint or no bands on the gel:
There was insufficient quantity or concentration of DNA loaded on the gel. Increase the amount of DNA, but don't exceed 50 ng/ band.
The DNA was degraded. Avoid nuclease contamination. The DNA was electrophoresed off the gel. Electrophorese the gel for less time, use a lower voltage, or use a higher percent gel.
Improper W light source was used for visualization of ethidium bromide-stained DNA. Use a short wavelength (254 nm) W light for greater sensitivity. Note: For preparative gels, using a longer wavelength (312 nm) W light will minimize DNA degradation.
If you see smeared DNA bands:
The DNA was degraded. Avoid nuclease contamination. Too much DNA was loaded on the gel. Decrease the amount of DNA. Improper electrophoresis conditions were used. Do not allow voltage to exceed ~20 V/cm. Maintain a temperature <30 C during electrophoresis. Check that the electrophoresis buffer used had sufficient buffer capacity. This is done by checking the pH in the anode and cathode chambers.
There was too much salt in the DNA. Use ethanol precipitation to remove excess salts, prior to electrophoresis.
The DNA was contaminated with protein. Use phenol extractions to remove protein prior to electrophoresis.
Small
DNA
bands
diffused
during
staining.
Add
the
ethidium bromide during electrophoresis. If you see anomalies DNA band migration:
Improper electrophoresis conditions were used. Do not allow voltage to exceed ~20 V/cm. Maintain a temperature <30 C during electrophoresis. Check that the electrophoresis buffer used had sufficient buffer capacity.
You might also like
- Agarose Gel ElectrophoresisDocument7 pagesAgarose Gel ElectrophoresisMahathir Mohmed79% (14)
- ChromatographyDocument31 pagesChromatographySelim Akhter86% (7)
- Agarose Gel ElectrophoresisDocument3 pagesAgarose Gel ElectrophoresisAbhijitBoruah100% (1)
- Agarose GelDocument8 pagesAgarose GelNazwa anhaNo ratings yet
- Lab5 (1)Document3 pagesLab5 (1)alooshmaroosh1011No ratings yet
- Campylobacter Jejuni and Campylobacter ColiDocument11 pagesCampylobacter Jejuni and Campylobacter ColinadbucukNo ratings yet
- Principles of Gel ElectrophoresisDocument6 pagesPrinciples of Gel ElectrophoresisCarmen Lopez100% (2)
- Electrophoresis 1Document8 pagesElectrophoresis 1Rishabh MasutaNo ratings yet
- Practical 06 MSC Botany MorningDocument4 pagesPractical 06 MSC Botany Morninghely shahNo ratings yet
- PMT 324 Molecular Biology of The GeneDocument6 pagesPMT 324 Molecular Biology of The GeneBlameless ArikoNo ratings yet
- Activity 2. Gel Extraction and PurificationDocument5 pagesActivity 2. Gel Extraction and Purificationgarcia.zaniabNo ratings yet
- Agarose Gel ElectrophoresisDocument5 pagesAgarose Gel ElectrophoresisSanmugam SubbaramaniamNo ratings yet
- SOPZoology pdfDocument11 pagesSOPZoology pdfaadithaysga018No ratings yet
- Agarose Gel ElectrophoresisDocument2 pagesAgarose Gel ElectrophoresisRian Lorenzo MascarinasNo ratings yet
- Agarose Gel Electrophoresis: ProcedureDocument6 pagesAgarose Gel Electrophoresis: ProcedureShaik BondNo ratings yet
- Experiment 9Document2 pagesExperiment 9pateldhairya82No ratings yet
- Lab 8 Gel Electrophoresis-1Document71 pagesLab 8 Gel Electrophoresis-1ramymilad538No ratings yet
- Agarose Gel Electrophoresis PDFDocument3 pagesAgarose Gel Electrophoresis PDF9001 Trisha BakshiBBT2No ratings yet
- Applied Zoology-II Practical ElectrophoresisDocument4 pagesApplied Zoology-II Practical ElectrophoresisAkshay BhiwalNo ratings yet
- Agarose Gel Electrophoresis (AGE)Document16 pagesAgarose Gel Electrophoresis (AGE)Nenita AlonzoNo ratings yet
- 9 DNA TroubleshootingDocument6 pages9 DNA TroubleshootingRicha AroraNo ratings yet
- Agarose Gel ElectrophoresisDocument10 pagesAgarose Gel Electrophoresisvenkat satyaNo ratings yet
- S.Y.Bsc Semester Iii Botany Paper IiDocument53 pagesS.Y.Bsc Semester Iii Botany Paper IiĐỗ Quang BìnhNo ratings yet
- Practical-BIO231-Agarose Gel Electrophoresis-FKDocument5 pagesPractical-BIO231-Agarose Gel Electrophoresis-FKmalik husnainNo ratings yet
- Agarose Gel ElectrophoresisDocument4 pagesAgarose Gel ElectrophoresisShailendra YadavNo ratings yet
- Western Blotting FINAL 2Document4 pagesWestern Blotting FINAL 2A NaNo ratings yet
- Protein Gel Electrophoresis Tips and Troubleshooting GuideDocument16 pagesProtein Gel Electrophoresis Tips and Troubleshooting Guidedrd@tiacnetNo ratings yet
- Protocolo Purificacion Desde GelDocument3 pagesProtocolo Purificacion Desde GelAriel ArayaNo ratings yet
- Molecular Biology Lab Manual FinalDocument19 pagesMolecular Biology Lab Manual FinalAnupriyaNo ratings yet
- Genomic Dna Isolation Protocol From BacteriaDocument4 pagesGenomic Dna Isolation Protocol From BacteriaMd Bulbul AhmedNo ratings yet
- Departments: SDMVM'S College of Agricultural BiotechnologyDocument45 pagesDepartments: SDMVM'S College of Agricultural BiotechnologyPAWANKUMAR S. K.No ratings yet
- ProtgelsDocument2 pagesProtgelsbettcorporationNo ratings yet
- Qualitative Alcohol PDFDocument9 pagesQualitative Alcohol PDFRoda Gayle RañadaNo ratings yet
- Separation of DNA by Agarose Gel ElectrophoresisDocument3 pagesSeparation of DNA by Agarose Gel ElectrophoresisSathish kumarNo ratings yet
- Plasmid Isolation From BacteriaDocument5 pagesPlasmid Isolation From BacteriaDeepak Ranjan SahooNo ratings yet
- BS5103 Genetics practical handout Nov 2022Document4 pagesBS5103 Genetics practical handout Nov 2022yfgm5ckgjzNo ratings yet
- Unit 2Document15 pagesUnit 2Anadi ChauhanNo ratings yet
- Dna07 2Document2 pagesDna07 2Sabesan TNo ratings yet
- POB Lab ManualDocument11 pagesPOB Lab ManualWEY LOON LIMNo ratings yet
- Gel ElectrophoresisDocument6 pagesGel ElectrophoresisEli NurlaeliNo ratings yet
- Agerose Gel ElectrophoresisDocument6 pagesAgerose Gel ElectrophoresisAnura BandaraNo ratings yet
- Single Cell Gel Electrophoresis (Comet) AssayDocument5 pagesSingle Cell Gel Electrophoresis (Comet) AssayCoțovanu IulianNo ratings yet
- Experiment:5: Agarose Gel ElectrophoresisDocument16 pagesExperiment:5: Agarose Gel ElectrophoresisANKIT KUMARNo ratings yet
- Molecular Biology - Amity University RajasthanDocument13 pagesMolecular Biology - Amity University Rajasthanabash_u1No ratings yet
- D1S80Document10 pagesD1S80mmarrinnaNo ratings yet
- DNA Gel ElectrophoresisDocument5 pagesDNA Gel ElectrophoresisTok WanNo ratings yet
- GE Lab 2Document14 pagesGE Lab 2goenkatarun33No ratings yet
- Agarose Gel Electrophoresis of DNA PDFDocument5 pagesAgarose Gel Electrophoresis of DNA PDFBiologistAhmedNo ratings yet
- BT0210 - Molecular Biology LaboratorymanualDocument29 pagesBT0210 - Molecular Biology LaboratorymanualsowjanyaNo ratings yet
- Lab 4 Agarose Gel ElectrophoresisDocument6 pagesLab 4 Agarose Gel Electrophoresisjimmy_ncop4702No ratings yet
- 5. Agarose gel مختبرDocument2 pages5. Agarose gel مختبرAbrar 111No ratings yet
- Agarose Gel Electrophoresis DNADocument5 pagesAgarose Gel Electrophoresis DNAAli Hasan100% (1)
- Gel ElphoDocument28 pagesGel ElphoEry NourikaNo ratings yet
- Mol Bio FinalDocument16 pagesMol Bio FinalBoka PolaNo ratings yet
- Restriction DigestionDocument3 pagesRestriction DigestionJesus PrattNo ratings yet
- Kraken KontilDocument4 pagesKraken KontilKraken KontilNo ratings yet
- AGAROSE GEL ELECTROPHORESIS - Assignment (AutoRecovered)Document19 pagesAGAROSE GEL ELECTROPHORESIS - Assignment (AutoRecovered)Sathvik BangiramaneNo ratings yet
- Telomerases: Chemistry, Biology, and Clinical ApplicationsFrom EverandTelomerases: Chemistry, Biology, and Clinical ApplicationsNeal F. LueNo ratings yet
- Section Cutting and Staining: A practical introduction to histological methods for students and practitionersFrom EverandSection Cutting and Staining: A practical introduction to histological methods for students and practitionersNo ratings yet
- Microbial Enumeration: Marking BoxDocument10 pagesMicrobial Enumeration: Marking BoxNOR SYUHADA BINTI BAHARUDIN / UPMNo ratings yet
- Lab On A Chip: PaperDocument11 pagesLab On A Chip: Paperxianxian liuNo ratings yet
- Geneipure Bacterial Dna Purification Kit Manual: Genei GeneiDocument10 pagesGeneipure Bacterial Dna Purification Kit Manual: Genei GeneiHemant Kawalkar0% (1)
- Modern Analytical TechniquesDocument5 pagesModern Analytical TechniquesRia SinghNo ratings yet
- Atomic Emission Spectroscopy AsdaDocument18 pagesAtomic Emission Spectroscopy AsdaMark Cliffton BadlonNo ratings yet
- Using DMSO As Injection Solvent For HPLCDocument6 pagesUsing DMSO As Injection Solvent For HPLCemptybugNo ratings yet
- FluorescenceDocument40 pagesFluorescencefatemaNo ratings yet
- Quality Control in SARS COV-2 RNA TestingDocument63 pagesQuality Control in SARS COV-2 RNA TestingNanik AndianiNo ratings yet
- InFusion Cloning User ManualDocument15 pagesInFusion Cloning User ManualHannaNo ratings yet
- Separation Final Merged - RemovedDocument112 pagesSeparation Final Merged - RemovedBisma ShafiqNo ratings yet
- Activity 7 Isolation of DNA From OnionDocument4 pagesActivity 7 Isolation of DNA From OnionMikela DelatoreNo ratings yet
- CHAPTER 3 and 4-Final Final NewDocument43 pagesCHAPTER 3 and 4-Final Final Newkasahun AmareNo ratings yet
- Separation of Amino Acids by Paper ChromatographyDocument38 pagesSeparation of Amino Acids by Paper ChromatographyGenny Marantan83% (6)
- Lesson 1:: The Cell TheoryDocument24 pagesLesson 1:: The Cell Theorysyrine mendozaNo ratings yet
- Alan R. Hibbs (Auth.) - Confocal Microscopy For Biologists-Springer US (2004)Document460 pagesAlan R. Hibbs (Auth.) - Confocal Microscopy For Biologists-Springer US (2004)Viswanathan SundaramNo ratings yet
- Flash ChromatographyDocument24 pagesFlash Chromatographyyusuf230395bhimaniNo ratings yet
- Gel Electrophoresis ProjectDocument3 pagesGel Electrophoresis Projectvidhya sureshNo ratings yet
- MPAT SyllabusDocument2 pagesMPAT SyllabusCO71 Biresha GhodkeNo ratings yet
- Fundamental Chromatographic ParametersDocument12 pagesFundamental Chromatographic ParametersCindy ChandraNo ratings yet
- Prokaryotes VS EukaryotesDocument15 pagesProkaryotes VS EukaryotesGamu GamuNo ratings yet
- Jack Meyers - Microscope - Make - Up - Lab - Webuest - 1Document3 pagesJack Meyers - Microscope - Make - Up - Lab - Webuest - 1meyersj27No ratings yet
- Thin Layer Chromatography and Column Chromatography: Activity No. 11Document9 pagesThin Layer Chromatography and Column Chromatography: Activity No. 11Mary Jean SteffenNo ratings yet
- Microbiology Journal ListDocument4 pagesMicrobiology Journal ListKathirvel MaruthaiNo ratings yet
- Ncov 2019 Sequencing Protocol v3 Locost Bh42j8yeDocument18 pagesNcov 2019 Sequencing Protocol v3 Locost Bh42j8yeShavana RajkumarNo ratings yet
- 2210030313FC Apipah ApriantiDocument1 page2210030313FC Apipah Apriantitomo bukakaNo ratings yet
- How It Works?: Mueller Hinton Agar (MHA)Document9 pagesHow It Works?: Mueller Hinton Agar (MHA)dragusriyadi01No ratings yet
- 3.4 Structure and Function of Cells JONESDocument5 pages3.4 Structure and Function of Cells JONESJonathan GordonNo ratings yet
- Cell Parts and FunctionsDocument2 pagesCell Parts and FunctionsSheryl Lou AngelesNo ratings yet
- Microbial GeneticsDocument7 pagesMicrobial Geneticsshrey chaudharyNo ratings yet