Ch18 z7e nuclear
- 1. CHAPTER 18 Nuclear Chemistry 18.I Nuclear Stability & Radioactive Decay pp I
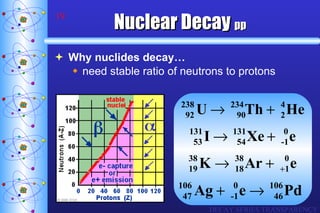
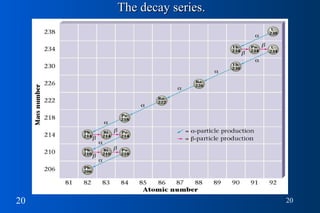
- 2. Black dots are stable nuclides. As A (atomic mass) increases, nº/p + ratio increases.
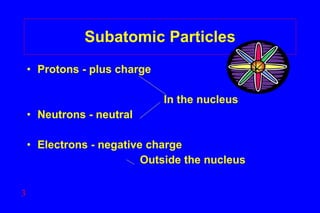
- 3. Subatomic Particles Protons - plus charge In the nucleus Neutrons - neutral Electrons - negative charge Outside the nucleus
- 4. Radiation Radiation comes from the nucleus of an atom. Unstable nucleus emits a particle or energy alpha beta gamma
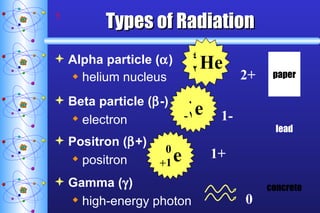
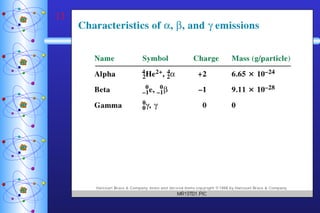
- 5. Types of Radiation Alpha particle ( ) helium nucleus paper 2+ Beta particle ( -) electron 1- lead Positron ( +) positron 1+ Gamma ( ) high-energy photon 0 concrete
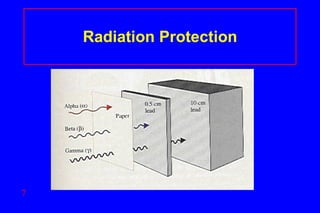
- 6. Radiation Protection Shielding alpha – paper, clothing beta – lab coat, gloves gamma- lead, thick concrete Limit time exposed Keep distance from source
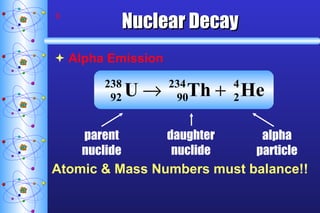
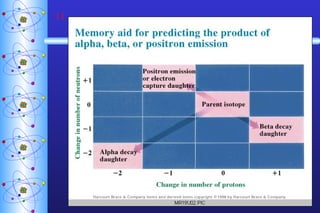
- 8. Nuclear Decay Alpha Emission Atomic & Mass Numbers must balance!! parent nuclide daughter nuclide alpha particle
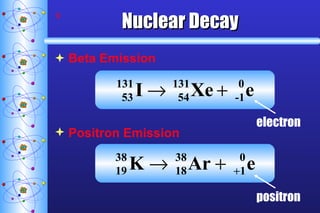
- 9. Nuclear Decay Beta Emission Positron Emission electron positron
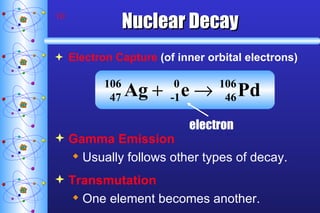
- 10. Nuclear Decay Electron Capture (of inner orbital electrons) Gamma Emission Usually follows other types of decay. Transmutation One element becomes another. electron
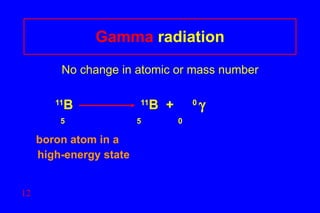
- 12. Gamma radiation No change in atomic or mass number 11 B 11 B + 0 5 5 0 boron atom in a high-energy state
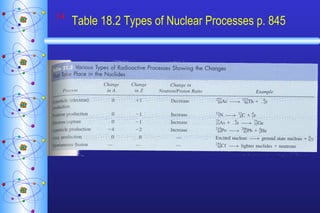
- 14. Table 18.2 Types of Nuclear Processes p. 845
- 15. Learning Check Write the nuclear equation for the beta emitter Cobalt-60. . . 60 Co 60 Ni + 0 e 27 28 -1
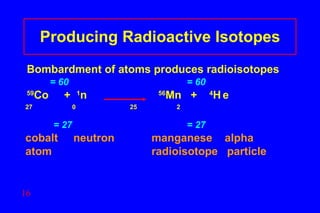
- 16. Producing Radioactive Isotopes Bombardment of atoms produces radioisotopes = 60 = 60 59 Co + 1 n 56 Mn + 4 H e 27 0 25 2 = 27 = 27 cobalt neutron manganese alpha atom radioisotope particle
- 17. Learning Check NR2 What radioactive isotope is produced in the following bombardment of boron? 10 B + 4 He ? + 1 n 5 2 0
- 18. Solution NR2 What radioactive isotope is produced in the following bombardment of boron? 10 B + 4 He 13 N + 1 n 5 2 7 0 nitrogen radioisotope
- 19. Nuclear Decay pp Why nuclides decay… need stable ratio of neutrons to protons DECAY SERIES TRANSPARENCY
- 21. 18.2 Kinetics of Radioactive Decay Rate of decay is a 1st order process, which is . . . ln(N/N 0 ) = -kt (memorize -- not on AP sheet) N 0 = original number of nuclides at t = 0 N = nuclides remaining at time t Half-life (t 1/2 ) = time for nuclides to reach half their original value. t 1/2 = 0.693/k
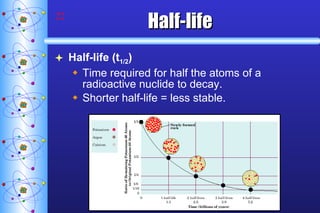
- 22. Half-life Half-life (t 1/2 ) Time required for half the atoms of a radioactive nuclide to decay. Shorter half-life = less stable.
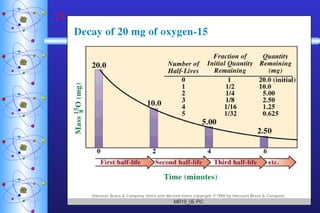
- 24. Examples of Half-Life Isotope Half life C-15 2.4 sec Ra-224 3.6 days Ra-223 12 days I-125 60 days C-14 5700 years U-235 710 000 000 years
- 25. Learning Check NR3 The half life of Iodine-123 is 13 hr. How much of a 64 mg sample of Iodine-123 is left after 26 hours?
- 26. Solution NR3 t 1/2 = 13 hrs 26 hours = 2 x t 1/2 Amount initial = 64mg Amount remaining = 64 mg x 1/2 x 1/2 = 16 mg
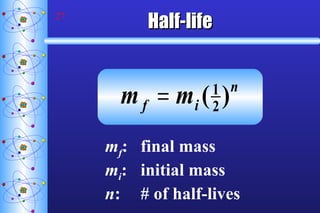
- 27. Half-life m f : final mass m i : initial mass n : # of half-lives
- 28. Half-life pp Fluorine-21 has a half-life of 5.0 seconds. If you start with 25 g of fluorine-21, how many grams would remain after 60.0 s? GIVEN: T 1/2 = 5.0 s m i = 25 g m f = ? total time = 60.0 s n = 60.0s ÷ 5.0s =12 WORK : m f = m i (1/2) n m f = (25 g)(0.5) 12 m f = 0.0061 g
- 29. Kinetics of Nuclear Decay Problems pp The rate constant for 99 43 Tc = 1.16 x 10 -1 /h What is its half life? . . . t 1/2 = 0.693/k = 0.693/(1.16 x 10 -1 /h) = 5.98 h It will take 5.98 hours for a given sample of technetium-99 to decrease to half the original number of nuclides.
- 30. Kinetics of Nuclear Decay Problems pp How long for 87.5% of a sample of cobalt-60 to decay if t 1/2 = 5.26 years? Steps. . . What % is left? . . . 12.5% How many half-lives to get to this percent? 3. So, your answer to the problem is . . . 3 x 5.26 = 15.8 years.
- 31. Actual AP question: 1989 MC #68 pp If k = 0.023 min -1 how much of X was originally present if have 40. g after 60 min.? Your answer is . . . 160. g . Solution . . . t 1/2 = 0.693/k = 0.693/0.023 min -1 = 30 min. 60 minutes is 2 half-lives so going backwards 40. g to 80. g to 160. g.
- 32. 18.3 Nuclear Transformations Transmutation - change of one element into another. Particle and linear accelerators are used to synthesize new elements (currently up to element number 119). Difficult to characterize the chemical properties because with some only a few atoms are formed with very short half-lives.
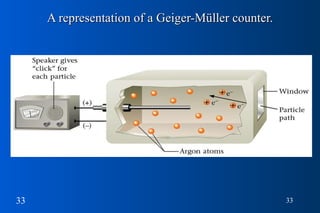
- 33. A representation of a Geiger-Müller counter.
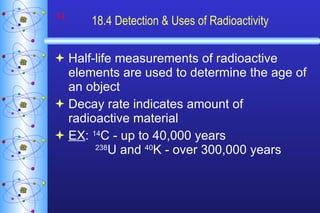
- 34. 18.4 Detection & Uses of Radioactivity Half-life measurements of radioactive elements are used to determine the age of an object Decay rate indicates amount of radioactive material EX : 14 C - up to 40,000 years 238 U and 40 K - over 300,000 years
- 35. Synthetic Elements Transuranium Elements elements with atomic #s above 92 synthetically produced in nuclear reactors and accelerators most decay very rapidly
- 36. Carbon-14 Dating You will have a test question like this! pp An ancient fire in an African cave has a 14 C decay rate of 3.1 cpm (cts per minute). If fresh wood has 13.6 cpm how old is the campfire if t 1/2 = 5730 years? Steps . . . Decay rates are directly proportional to nuclides so their ratio = N/N 0 What is the numerical ratio? Your answer . . . 3.1 cpm/13.6 cpm = 0.23 Use the two previous equations to solve (next slide).
- 37. Carbon-14 Dating You will have a test question like this! pp Ancient fire 14 C decay rate 3.1 cpm, fresh wood 13.6 cpm how old if t 1/2 = 5730 yrs ? 3.1 cpm/13.6 cpm = 0.23 = N/N 0 ln(N/N 0 ) = - k t and t 1/2 = 0.693/ k You want to solve for t (vs. t 1/2 ) so use t 1/2 to get k then plug into the 1st equation and solve for t. Your answer is . . . The campfire is 12 000 years old. ln( N/N 0 ) = ln( 0.23 ) = -(0.693/ 5730 ) t Se Ex 18.4A & 18.4B in Study Guide
- 38. Carbon-14 Dating You will have another test question like this ! pp A rock has ratio of Pb-206 to U-238 of 0.115. How old is it if t 1/2 of U-238 = 4.5 x 10 9 yrs ? Strategy: figure out N/N 0 of U-238, then use the 2 previous equations to get . . . 7.1 x 10 8 years . Calculations . . . Pb /U = 115/1000 so N 0 U238 = 1115, N = 1000 ln( 1000/1115 ) = -(0.693/ 4.5 x 10 9 ) t
- 39. Nuclear Medicine Radioisotope Tracers absorbed by specific organs and used to diagnose diseases Radiation Treatment larger doses are used to kill cancerous cells in targeted organs internal or external radiation source Radiation treatment using -rays from cobalt-60.
- 40. Other Uses Food Irradiation radiation is used to kill bacteria Radioactive Tracers explore chemical pathways trace water flow study plant growth, photosynthesis Consumer Products ionizing smoke detectors - 241 Am
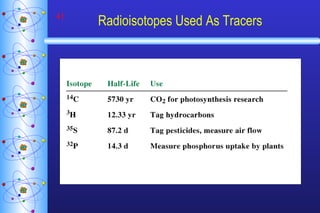
- 41. Radioisotopes Used As Tracers
- 42. 18.5 Thermodynamic Stability of the Nucleus Mass Defect - difference from mass of an atom & the mass of its individual particles. 4.00260 amu 4.03298 amu
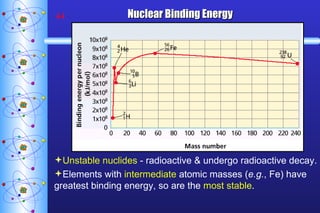
- 43. Nuclear Binding Energy Energy released when a nucleus is formed from nucleons. High binding energy = stable nucleus. E = mc 2 E: energy (J) m: mass defect ( kg ) c: speed of light (3.00 x 10 8 m/s)
- 44. Nuclear Binding Energy Unstable nuclides - radioactive & undergo radioactive decay. Elements with intermediate atomic masses ( e.g. , Fe) have greatest binding energy, so are the most stable .
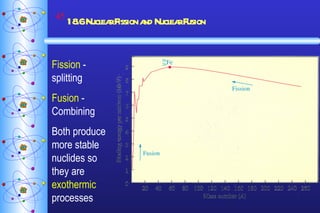
- 45. 18.6 Nuclear Fission and Nuclear Fusion Fission - splitting Fusion - Combining Both produce more stable nuclides so they are exothermic processes
- 46. A. Nuclear Fission Splitting a nucleus into two or more smaller nuclei 1 g of 235 U = 3 tons of coal
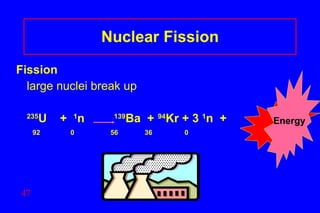
- 47. Nuclear Fission Fission large nuclei break up 235 U + 1 n 139 Ba + 94 Kr + 3 1 n + 92 0 56 36 0 Energy
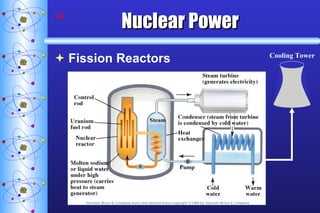
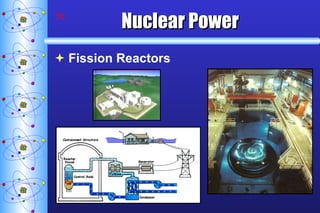
- 48. Nuclear Power Fission Reactors Cooling Tower
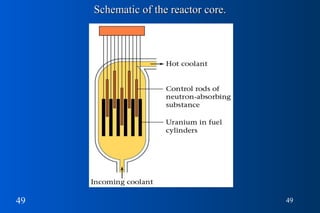
- 49. Schematic of the reactor core.
- 50. Nuclear Power Fission Reactors
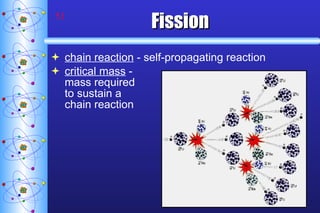
- 51. Fission chain reaction - self-propagating reaction critical mass - mass required to sustain a chain reaction
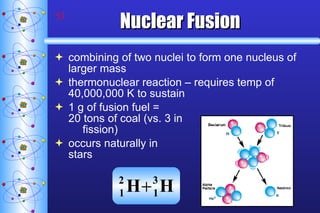
- 53. Nuclear Fusion combining of two nuclei to form one nucleus of larger mass thermonuclear reaction – requires temp of 40,000,000 K to sustain 1 g of fusion fuel = 20 tons of coal (vs. 3 in fission) occurs naturally in stars
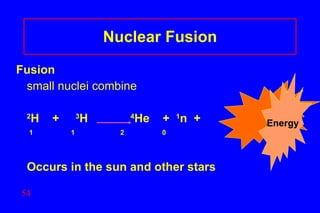
- 54. Nuclear Fusion Fusion small nuclei combine 2 H + 3 H 4 He + 1 n + 1 1 2 0 Occurs in the sun and other stars Energy
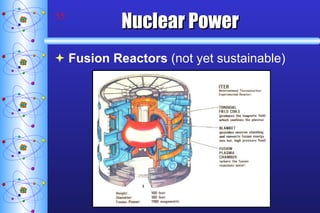
- 55. Nuclear Power Fusion Reactors (not yet sustainable)
- 56. Nuclear Power Fusion Reactors (not yet sustainable) Tokomak Fusion Test Reactor Princeton University National Spherical Torus Experiment
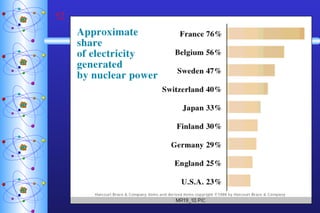
- 57. Fission vs. Fusion pp 235 U is limited danger of meltdown toxic waste thermal pollution fuel is abundant no danger of meltdown no toxic waste not yet sustainable FISSION FUSION
- 58. Learning Check NR4 Indicate if each of the following are Fission (2) fusion Nucleus splits Large amounts of energy released Small nuclei form larger nuclei Hydrogen nuclei react Energy
- 59. Solution NR4 Indicate if each of the following are Fission (2) fusion 1 Nucleus splits 1 + 2 Large amounts of energy released 2 Small nuclei form larger nuclei 2 Hydrogen nuclei react
- 60. E. Nuclear Weapons Atomic Bomb chemical explosion is used to form a critical mass of 235 U or 239 Pu fission develops into an uncontrolled chain reaction Hydrogen Bomb chemical explosion fission fusion fusion increases the fission rate more powerful than the atomic bomb
- 61. 18.7 Effects of Radiation pp Somatic - damage to the organism causing sickness or death. Genetic - damage to the genetic machinery causing birth defects.
- 62. Factors for Biological Effects of Radiation pp Energy - higher energy content (rads) causes more damage. Penetrating Ability - > - > Ionizing Ability - > - > (eating an -particle producer like Pu is very deadly) Chemical Properties Kr-85 is chemically inert, passes through quickly Sr-90 collects in bone and stays a long time in the body.
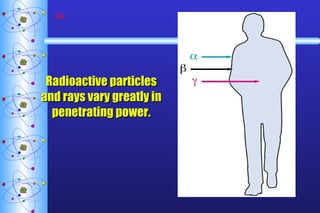
- 64. Radioactive particles and rays vary greatly in penetrating power.
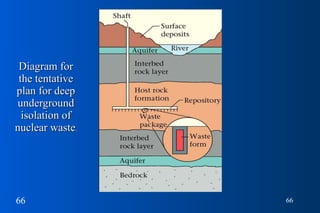
- 66. Diagram for the tentative plan for deep underground isolation of nuclear waste .
Editor's Notes
- Z5e 1022
- Hrw 705-707; z5e 1024-1025
- Hrw 706; z5e 1024
- Hrw 706; z5e 1024-1025
- Hrw 707; z5e 1024-1025
- Hrw 707; z5e 1027
- Hrw 710 z53 1027
- Z5e 1028
- Hrw 708; z5e 1028
- Hrw 708; z5e 1030
- Hrw 709; z5e 1028
- Hrw 709; z5e 1028
- Z5e text #21
- AP 1989 MC #68
- Z5e text #21
- Hrw 714; z53 1033-1044
- Hrw 715; z5e 1034
- Hrw none; z5e 1032
- Z5e 1035 SE 21.5
- Z5e 1035 SE 21.5
- Z5e 1035 SE 21.6
- Hrw 715; z5e 1037
- Hrw 715; z5e 1037
- Hrw 715; z5e 1038
- Hrw 701; Z5e 1038
- Hrw 701-702; Z5e 1038-40
- Hrw 702; z5e 1038-40
- Z5e 1042 Fig. 21.10
- Hrw 717; z5e 1042-1044
- Hrw 718; no 1044
- Hrw 718; z5e 1044
- Hrw 1044; no z5e
- Hrw 717; z5e 1042-1044
- Hrw 718
- Hrw 719; z5e 1047-1048
- None (z5e 1044-1047)
- Hrw none, z5e 1044-1047
- Hrw 717-719; z5e 1041-1042
- Hrw 716; z5e 1044
- Z5e 1048
- Hrw 713; z5e 1048
- Hrw 715; z5e 1038