Quantum dots and their applications
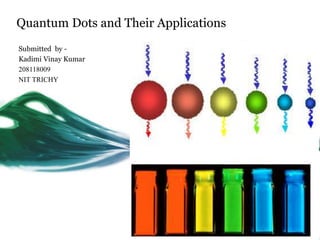
- 1. Quantum Dots and Their Applications Submitted by - Kadimi Vinay Kumar 208118009 NIT TRICHY
- 2. Presentation Content • What is a Quantum Dot ? • Why and how their optoeletronuc properies chages with size and shape ? • Overview of their fabrication techniques and applications.
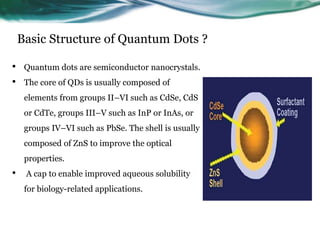
- 3. Basic Structure of Quantum Dots ? • Quantum dots are semiconductor nanocrystals. • The core of QDs is usually composed of elements from groups II–VI such as CdSe, CdS or CdTe, groups III–V such as InP or InAs, or groups IV–VI such as PbSe. The shell is usually composed of ZnS to improve the optical properties. • A cap to enable improved aqueous solubility for biology-related applications.
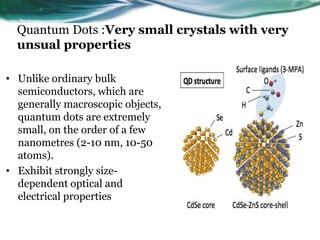
- 4. Quantum Dots :Very small crystals with very unsual properties • Unlike ordinary bulk semiconductors, which are generally macroscopic objects, quantum dots are extremely small, on the order of a few nanometres (2-10 nm, 10-50 atoms). • Exhibit strongly size- dependent optical and electrical properties
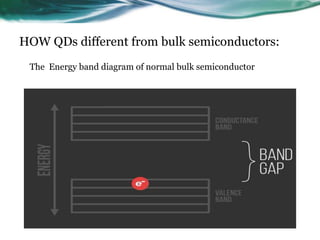
- 5. HOW QDs different from bulk semiconductors: The Energy band diagram of normal bulk semiconductor
- 6. The Band Gap variations with variation in QD size
- 8. WHY THESE VARIATIONS WITH SIZE ? • Band Theory: • Atomic orbital: a discrete set of energy levels. • If several atoms are brought together into a molecule, their atomic orbitals split, due to perturbation. • When a large number of atoms (of order 1020 or more) are brought together to form a solid, the number of orbitals becomes exceedingly large. • Difference in energy between them becomes very small so the levels may be considered to form continuous bands of energy rather than the discrete energy levels of the atoms in isolation. Band Theory
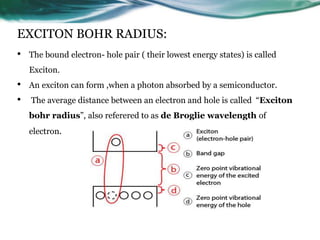
- 9. EXCITON BOHR RADIUS: • The bound electron- hole pair ( their lowest energy states) is called Exciton. • An exciton can form ,when a photon absorbed by a semiconductor. • The average distance between an electron and hole is called “Exciton bohr radius”, also referered to as de Broglie wavelength of electron.
- 10. What is happening when we are reducing the size ? • In a semiconductor crystallite whose size is smaller than twice the size of its exciton Bohr radius, the excitons are squeezed, leading to Quantum Confinement. • A particle behaves as if it were free when the confining dimension is large compared to the wavelength of the particle. During this state, the bandgap remains at its original energy due to a continuous energy state. • However, as the confining dimension decreases and reaches a certain limit, typically in nanoscale, the energy spectrum becomes discrete. As a result, the bandgap becomes size-dependent. This ultimately results in a blueshift in light emission as the size of the particles decreases.
- 12. Quantum Confinement: • The smaller you made the crystal the higher the energy of the electron will be • The kinetic energy of that electron will get increased. • The Uncertainty Principle states that the more precisely one knows the position of a particle, the more uncertainty in its momentum (and vice versa). • Therefore, the more spatially confined and localized a particle becomes, the broader the range of its momentum/energy. • This phenomena of increasing excitation energy with decreasing crystallite size is known as quantum confinement.
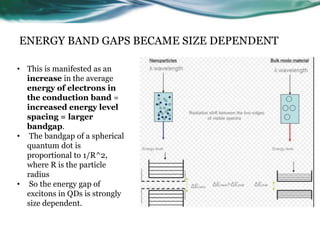
- 13. ENERGY BAND GAPS BECAME SIZE DEPENDENT • This is manifested as an increase in the average energy of electrons in the conduction band = increased energy level spacing = larger bandgap. • The bandgap of a spherical quantum dot is proportional to 1/R^2, where R is the particle radius • So the energy gap of excitons in QDs is strongly size dependent.
- 14. Quantum materials of other dimensions:
- 15. How QDs are getting fabricated: • There are three main ways to confine excitons in semiconductors: o Lithography o Colloidal synthesis o Epitaxy • Patterned Growth • Self-Organized Growth
- 16. Lithography • Quantum wells are covered with a polymer mask and exposed to an electron or ion beam. • The surface is covered with a thin layer of metal, then cleaned and only the exposed areas keep the metal layer. • Pillars are etched into the entire surface. • Multiple layers are applied this way to build up the properties and size wanted. • Disadvantages: slow, contamination, low density, defect formation.
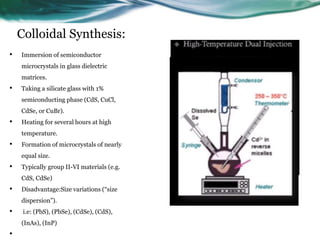
- 17. Colloidal Synthesis: • Immersion of semiconductor microcrystals in glass dielectric matrices. • Taking a silicate glass with 1% semiconducting phase (CdS, CuCl, CdSe, or CuBr). • Heating for several hours at high temperature. • Formation of microcrystals of nearly equal size. • Typically group II-VI materials (e.g. CdS, CdSe) • Disadvantage:Size variations (“size dispersion”). • i.e: (PbS), (PbSe), (CdSe), (CdS), (InAs), (InP) •
- 18. Cadmium-free quantum dots • In many regions of the world there is now, or soon to be, legislation to restrict and in some cases ban heavy metals in many household appliances such as IT & telecommunication equipment, Lighting equipment , Electrical & electronic tools, Toys, leisure & sports equipment. • For QDs to be commercially viable in many applications they MUST NOT CONTAIN cadmium or other restricted elements LIKE mercury, lead, chromium. • So research has been able to create non-toxic quantum dots using silicon and carbon.
- 19. APPLICATIONS • 1.QLED: • Quantum dots may some day light your homes, offices, streets, and entire cities. • Quantum dot LED’s can now produce any colour of light, including white. • Quantum dot LED’s are extremely energy efficient. They use only a few watts, while a regular incandescent lamp uses 30 or more watts for the same amount of light.
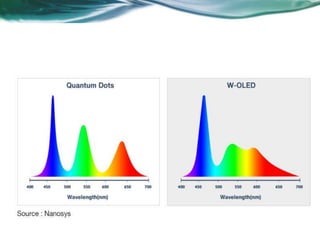
- 20. • The spectrum of photon emission is narrow, characterized by its fullwidth at half the maximum value-pure and saturated emission colors with narrow bandwidth- emission wavelength is easily tuned by changing the size of the quantum dots. • Advantages : Low power consumption – Ranger and accuracy color • Brightness: 50~100 times brighter than CRT and LCD displays ~40,000 cd/m2 • QLED screens are said to be twice as power efficient as OLED screens, and offer 30 to 40% improved brightness.
- 22. How QLEDs better than LEDS in Displays 1.Displays in any device generally uses 3 types of LEDs 1.RED 2.GREEN 3.BLUE 2.And rest of the colours will be generated using the combinations of thses 3 basic colours in various proportions.
- 23. Problem in LED display: 1.Since LEDS emits these 3 colours with broad colour profiles they will struggle to represent rest of the colours with high precision.
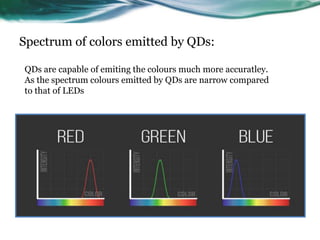
- 24. Spectrum of colors emitted by QDs: QDs are capable of emiting the colours much more accuratley. As the spectrum colours emitted by QDs are narrow compared to that of LEDs
- 25. QD solar cells: • Quantum dots may be able to increase the efficiency and reduce the cost of today's typical silicon photovoltaic cells. • Quantum dot photovoltaic would theoretically be cheaper to manufacture, as they can be made "using simple chemical reactions.
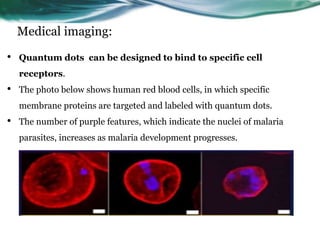
- 26. Medical imaging: • Quantum dots can be designed to bind to specific cell receptors. • The photo below shows human red blood cells, in which specific membrane proteins are targeted and labeled with quantum dots. • The number of purple features, which indicate the nuclei of malaria parasites, increases as malaria development progresses.
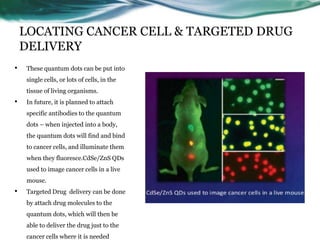
- 27. LOCATING CANCER CELL & TARGETED DRUG DELIVERY • These quantum dots can be put into single cells, or lots of cells, in the tissue of living organisms. • In future, it is planned to attach specific antibodies to the quantum dots – when injected into a body, the quantum dots will find and bind to cancer cells, and illuminate them when they fluoresce.CdSe/ZnS QDs used to image cancer cells in a live mouse. • Targeted Drug delivery can be done by attach drug molecules to the quantum dots, which will then be able to deliver the drug just to the cancer cells where it is needed
- 28. Programmable Matter • It is possible to build an ensemble of elements which can be programmed to change their physical properties in reality not just in simulation. • By the manipulation of quantum dots ,we can create materials that have some of the properties of real elements • Change the electron population in the quantum dots and you change the material . • EXAMPLE: A light weight breathable leather jacket that turns into high carbon steel when you fall off your motorcycle.
- 29. References • http://nextbigfuture.com/2006/05/quantum-dots-could-create-65- efficient.html • http://en.wikipedia.org/wiki/Quantum_dot • https://www.youtube.com/watch?v=7_B3tPkQ3XU&pbjreload=10 • https://www.youtube.com/watch?v=e-32eGL5bns • Google images
- 30. Thank you