Radial heat conduction
- 1. 1 Koya University Faculty of Engineering Chemical Engineering Department Laboratory of Heat transfer Experiment Number Two Radial heat conduction Instructor: Dr.Barham & Mrs.Anfal Name of Student: Aree Salah Tahir Experiment Contacted on: 12/ Nov/2014 Report Submitted on: 24/Nov/2014 Group:A
- 2. 2 List of content: Objectives……………............................................3 Introduction..………………………………… ….4 Background Theory ...……..…………...…..…….5 Procedure ………………..…………..……...……6 Equipment and components used………………...7 Table of Reading……………….…………......….8 Calculation…………...............................9-10-11-12 Discussion …..………………………….…...13-14 References ……………………...…………..…16
- 3. 3 Objectives: It allows us to obtain experimentally the coefficient of thermal conductivity of some unknown materials ,and in this way to understand the factors and parameters that affect the rate of heat transfer.
- 4. 4 Introduction: Heat transfer is a common phenomenon encountered in many areas in daily life. It is an important subject in natural sciences and even more so in engineering and the field of environmental physics. Knowledge about heat transfer is essential for the various possible ways to cut down on energy use. For example, to economize on home-heating one has to optimize insulation, i.e. minimize heat transfer. Improving cooling processes on the other hand implies maximizing heat transfer to the coolant. Engineering problems that involve heat transfer are generally concerned with optimization of heat transfer processes. For instance, in the development of novel materials that cannot sustain extreme temperatures heat transport towards the material is to be minimized, whereas the transport away from this material is to be maximized. Research on heat transfer focusses on electronic equipment, energy systems, fire and combustion systems, gas turbines, as well as manufacturing and materials processing. There is even a Journal of Heat Transfer whose emphasis is on energy transfer in applied thermodynamic processes in all fields of (mechanical) engineering and related industries. Therefore it is important to understand the basic concepts of heat transfer and the way it is mathematically described. You should be already familiar with the different mechanisms that result in heat transfer from the lecture. The most important heat transfer mechanisms are: • Conduction; on a microscopic or atomic scale kinetic energy is transferred through collisions between the atoms so that on a macroscopic scale thermal energy is transferred. • Convection; if the carrier of thermal energy is mobile, the thermal energy may be transferred through mass transfer. • Radiation; thermal energy may be transformed into electromagnetic energy, emitted, absorbed and with that it is transformed into thermal energy again {1}
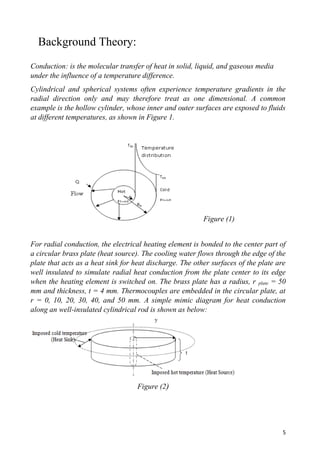
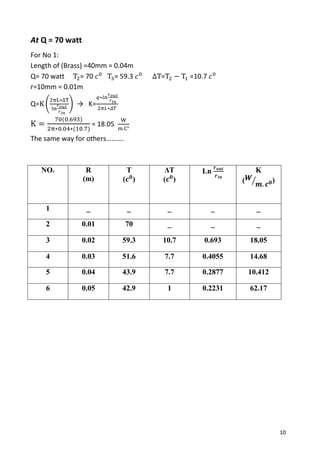
- 5. 5 Background Theory: Conduction: is the molecular transfer of heat in solid, liquid, and gaseous media under the influence of a temperature difference. Cylindrical and spherical systems often experience temperature gradients in the radial direction only and may therefore treat as one dimensional. A common example is the hollow cylinder, whose inner and outer surfaces are exposed to fluids at different temperatures, as shown in Figure 1. Figure (1) For radial conduction, the electrical heating element is bonded to the center part of a circular brass plate (heat source). The cooling water flows through the edge of the plate that acts as a heat sink for heat discharge. The other surfaces of the plate are well insulated to simulate radial heat conduction from the plate center to its edge when the heating element is switched on. The brass plate has a radius, r plate = 50 mm and thickness, t = 4 mm. Thermocouples are embedded in the circular plate, at r = 0, 10, 20, 30, 40, and 50 mm. A simple mimic diagram for heat conduction along an well-insulated cylindrical rod is shown as below: Figure (2)
- 6. 6 Procedure: 1. Set up the unit as per sect. 2.3 and adjust the cooling water flow rat (a cooling water flow rate of approx. 11th is required to dissipate a heater power of 90 watts at a temperature difference of 90 c) 2. Connect up the power and data cables appropriately. 3. Switch on the unit and adjust the desired temperature drop via the power setting on the control and display unit. 4. When the thermal conduction process has reached a steady state condition, I.e. the temperature at the individual measuring points are stable and no longer changing, note the measurement results at the individual measuring points and the electrical power supplied to the heater..
- 7. 7 Equipment and components used: 1-display and control unit, 2-measuring object, 3-experimental set-up for radial heat conduction, 4-experimental set-up for linear heat conduction {2}
- 8. 8 Table of Reading: Temperature of barrel is 21.3 c Q=40watt →Tout=31.8 c Q=70watt → Tout=38 c Q T1 T2 T3 T4 T5 T6 40 0 50 43.5 X 33.9 33.5 70 0 70 59.3 X 43.9 42.9 Q T1 T2 T3 T4 T5 T6 40 0 50 43.5 38.7 33.9 33.5 70 0 70 59.3 51.6 43.9 42.9
- 9. 9 Calculation: At Q = 40 watt For No 1: Length of (Brass) =40mm = 0.04m Q= 40 watt T2= 50 푐0 T3= 43.5 푐0 ΔT=T2−T1 =6.5 푐0 r=10mm = 0.01m Q=K( 2πL∗ΔTln 푟표푢푡 푟푖푛 ) → K= 푞∗푙푛 푟표푢푡 푟푖푛 2휋퐿∗훥푇 K= 40(0.693) 2π∗0.04∗(6.5) = 16.98 Wm.C° The same way for others………. NO. R (m) T (풄ퟎ) ΔT (풄ퟎ) Ln 풓풐풖풕 풓풊풏 K (푾 풎.풄ퟎ⁄) 1 _ _ _ _ _ 2 0.01 50 _ _ _ 3 0.02 43.5 6.5 0.693 16.98 4 0.03 38.7 4.8 0.4055 13.45 5 0.04 33.9 4.8 0.2877 9.54 6 0.05 33.5 0.4 0.2231 88.81
- 10. 10 At Q = 70 watt For No 1: Length of (Brass) =40mm = 0.04m Q= 70 watt T2= 70 푐0 T3= 59.3 푐0 ΔT=T2−T1 =10.7 푐0 r=10mm = 0.01m Q=K( 2πL∗ΔTln 푟표푢푡 푟푖푛 ) → K= 푞∗푙푛 푟표푢푡 푟푖푛 2휋퐿∗훥푇 K= 70(0.693) 2π∗0.04∗(10.7) = 18.05 Wm.C° The same way for others………. NO. R (m) T (풄ퟎ) ΔT (풄ퟎ) Ln 풓풐풖풕 풓풊풏 K (푾 풎.풄ퟎ⁄) 1 _ _ _ _ _ 2 0.01 70 _ _ _ 3 0.02 59.3 10.7 0.693 18.05 4 0.03 51.6 7.7 0.4055 14.68 5 0.04 43.9 7.7 0.2877 10.412 6 0.05 42.9 1 0.2231 62.17
- 11. 11 For Q=40watt Volume=200ml & time=267.48 s Volume flow rate= V/T → Volume flow rate=200/267.48=0.74 Volume flow rate=0.74 *10-6 m3 /s Mass flow rate=0.74 *10-3 kg /s Q=m*cp* ΔT→ m*cp*(Tout-Tin) Cp=4.182 Qlose=(0.74 *10-3)* 4.182*10.5 Qlose=32 J/s =32watt Qactual=Qsupply - Qlose Qactual=8 watt
- 12. 12 For Q=70watt Volume=200ml & time=267.48 s Volume flow rate= V/T → Volume flow rate=200/267.48=0.74 Volume flow rate=0.74 *10-6 m3 /s Mass flow rate=0.74 *10-3 kg /s Q=m*cp* ΔT→ m*cp*(Tout-Tin) Cp=4.182 Qlose=(0.74 *10-3)* 4.182*16.7 Qlose=51.6 J/s =51.6watt Qactual=Qsupply - Qlose Qactual=18.4 watt
- 13. 13 Discussion: In this experiment we see that the relation between ( ΔT) & (Distance) is linear with decrease ΔT decrease Distance 50 43.5 38.7 33.9 33.5 0 10 20 30 40 50 60 0 0.01 0.02 0.03 0.04 0.05 0.06 Temperature(c) Distance(m) Plot between Temperature and Distance At Q=40 70 59.3 51.6 43.9 42.9 0 10 20 30 40 50 60 70 80 0 0.01 0.02 0.03 0.04 0.05 0.06 Temperature(c) Distance(m) Plot between Temperature and Distance At Q=70
- 14. 14 Question: Why did we count Qlose ? Answer: we count Qlose because it is importance to determin Qactual and we can determin Qactual by this equation : Qactual=Qsupply - Qlose ………………………………………………………………………………… We find the cp and density in this tabe by temperature http://www.engineeringtoolbox.com/water-thermal-properties-d_162.html For Q=40 Taverag = (Tin+Tout)/2 (21.3+31.8)/2 = 26.55 at this temperature(26.55) cp=4.18 density=996.82 …………. For Q=70 cp=4.179 density=995.95 ………….
- 15. 15 1-Why we neglect the first rate of temp. ? Because of the distance is zero and read temp.as minus. 2-Here are the factors that affect the rate of conduction A) Temperature difference B) Cross-sectional area C) Length (distance heat must travel) D) Time 3-In contact point will make error because when we join the peace of material will make a space that cause to heat losses. 4- To measure the temperature distribution for steady-state conduction of energy through a composite plane wall and determine the Overall Heat Transfer Coefficient for the flow of heat through a combination of different materials in series 5- By increasing area and ΔT thermal conductivity decrease: But by increasing ΔX, thermal conductivity increase 6- Why we neglect the first rate of temp. ? Because of the distance is zero and read temp.as minus. 7- What is the advantage of cooling water? To make the difference between the temp.
- 16. 16 References: 1- www.laserspectroscopy.ucc.ie/PY3011/Manual_HeatCond.pdf 2- http://www.gunt.de/static/WL372 3- http://www.engineeringtoolbox.com/water-thermal- properties-d_162.html