1. Introduction
Liquid-infused surfaces (LIS) are promising candidates for reducing drag, resisting biofouling and increasing heat transfer in liquid flows (Epstein et al. Reference Epstein, Wong, Belisle, Boggs and Aizenberg2012; Solomon, Khalil & Varanasi Reference Solomon, Khalil and Varanasi2014; Rosenberg et al. Reference Rosenberg, Van Buren, Fu and Smits2016; Sundin et al. Reference Sundin, Ciri, Leonardi, Hultmark and Bagheri2022). These surfaces consist of a solid surface texture with a lubricating liquid that is immiscible with the external fluid. The fluid–fluid interfaces and mobility of the lubricant give rise to a slipping effect of the external flow. LIS can self-repair and are not sensitive to hydrostatic pressure, thereby being more robust than superhydrophobic surfaces (SHS) if designed properly (Wong et al. Reference Wong, Kang, Tang, Smythe, Hatton, Grinthal and Aizenberg2011; Wexler, Jacobi & Stone Reference Wexler, Jacobi and Stone2015; Sundin, Zaleski & Bagheri Reference Sundin, Zaleski and Bagheri2021).
The functionality of LIS has been mostly characterised assuming perfectly clean external liquid flows. However, both in applications and in laboratory set-ups (Jacobi, Wexler & Stone Reference Jacobi, Wexler and Stone2015; Peaudecerf et al. Reference Peaudecerf, Landel, Goldstein and Luzzatto-Fegiz2017), LIS are exposed to trace amounts of surfactants. These are substances that can adsorb onto interfaces and alter the surface (or interfacial) tension. Surfactants influence phenomena such as foaming, wetting, dispersion and emulsification, appearing in a large variety of products, e.g. cleaning agents, paints, cosmetics, pharmaceuticals and motor oils (Rosen & Kunjappu Reference Rosen and Kunjappu2012). It has recently been acknowledged that traces of surfactants may induce Marangoni stresses that counteract the slip of SHS (Peaudecerf et al. Reference Peaudecerf, Landel, Goldstein and Luzzatto-Fegiz2017). As we demonstrate in this paper, the presence of surfactants in the system also modifies the slip of LIS. In applications where the mobility of the infusing liquid is crucial, it is necessary to understand the influence of surfactants on the performance, particularly when measured slip lengths deviate significantly from their expected values.
Surfactants adsorbed onto interfaces accumulate at stagnation points when subjected to flow, building up concentration gradients and corresponding Marangoni stresses. The Marangoni stress counteracts the shear stress from the overlying flow, reducing the slip length. Using numerical simulations, a significant slip length reduction of SHS has been observed at bulk concentrations ![]() $c_0 \approx 10^{-3}\,{\rm mol}\,{\rm m}^{-3}$ using properties of the surfactant sodium dodecyl sulphate (SDS) (Peaudecerf et al. Reference Peaudecerf, Landel, Goldstein and Luzzatto-Fegiz2017). Most recent experimental studies of SHS, which propose that surfactant gradients lead to slip degradation, have not added surfactants artificially (Kim & Hidrovo Reference Kim and Hidrovo2012; Bolognesi, Cottin-Bizonne & Pirat Reference Bolognesi, Cottin-Bizonne and Pirat2014; Peaudecerf et al. Reference Peaudecerf, Landel, Goldstein and Luzzatto-Fegiz2017; Song et al. Reference Song, Song, Hu, Du, Du, Choi and Rothstein2018; Temprano-Coleto et al. Reference Temprano-Coleto, Smith, Peaudecerf, Landel, Gibou and Luzzatto- Fegiz2021). Instead, the fluid systems have likely been contaminated by the surrounding environment. Indeed, it is generally accepted that surfactants appear as ‘hidden variables’ because their concentrations are unknown (Manikantan & Squires Reference Manikantan and Squires2020). Analytical models that relate the slip length to surfactant concentration are therefore crucial to interpreting experimental measurements.
$c_0 \approx 10^{-3}\,{\rm mol}\,{\rm m}^{-3}$ using properties of the surfactant sodium dodecyl sulphate (SDS) (Peaudecerf et al. Reference Peaudecerf, Landel, Goldstein and Luzzatto-Fegiz2017). Most recent experimental studies of SHS, which propose that surfactant gradients lead to slip degradation, have not added surfactants artificially (Kim & Hidrovo Reference Kim and Hidrovo2012; Bolognesi, Cottin-Bizonne & Pirat Reference Bolognesi, Cottin-Bizonne and Pirat2014; Peaudecerf et al. Reference Peaudecerf, Landel, Goldstein and Luzzatto-Fegiz2017; Song et al. Reference Song, Song, Hu, Du, Du, Choi and Rothstein2018; Temprano-Coleto et al. Reference Temprano-Coleto, Smith, Peaudecerf, Landel, Gibou and Luzzatto- Fegiz2021). Instead, the fluid systems have likely been contaminated by the surrounding environment. Indeed, it is generally accepted that surfactants appear as ‘hidden variables’ because their concentrations are unknown (Manikantan & Squires Reference Manikantan and Squires2020). Analytical models that relate the slip length to surfactant concentration are therefore crucial to interpreting experimental measurements.
Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020) developed an analytical theory to predict the effective slip length of SHS from relevant non-dimensional numbers in a two-dimensional channel flow with surfactants. The model assumed low concentrations and uniform interfacial concentration gradients. A high shear rate can result in the upstream part of an interface having almost no surfactant gradients, rendering the model inaccurate. Inspired by the analysis of buoyantly rising bubbles (Palaparthi, Papageorgiou & Maldarelli Reference Palaparthi, Papageorgiou and Maldarelli2006), Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020) found that the surface could enter this regime when interfacial surfactant advection overcomes interfacial diffusion and bulk exchange rates. However, they were unable to find a quantitative condition for the transition. The analytical model compared favourably with numerical simulations for low shear rates, containing four fitted parameters. Baier & Hardt (Reference Baier and Hardt2021) developed an analytical model for slip length degradation by insoluble surfactants. They considered a flow driven by an imposed shear stress and could therefore use the analytical flow solution of Philip (Reference Philip1972a).
The effects of surfactants on LIS have not been investigated thoroughly, although its importance has been acknowledged. Certain interfacial observations of LIS, that cannot be explained fully, have been attributed to the presence of surfactants. One example is the absence of interface deformations in the vicinity of stagnation points (Jacobi et al. Reference Jacobi, Wexler and Stone2015). The influence of surfactants on LIS drag reduction was also highlighted as a future challenge in a recent review (Hardt & McHale Reference Hardt and McHale2022).
There are indications that LIS are more sensitive to surfactants compared with SHS. Dodecane, hexane and other alkanes are promising infusing liquids for drag reduction applications (Van Buren & Smits Reference Van Buren and Smits2017). However, the interfaces of aqueous surfactant solutions and saturated hydrocarbons (e.g. alkanes) generally face a more significant decrease in surface tension than the interfaces of corresponding water–air systems (assuming only minor or no solubility of the surfactant in the hydrocarbon) (Rosen & Kunjappu Reference Rosen and Kunjappu2012). The relatively early publication by Gillap, Weiner & Gibaldi (Reference Gillap, Weiner and Gibaldi1968) reported this effect for sodium decyl sulphate and SDS, and various water–alkane interfaces (e.g. water–hexane with SDS).
Measurements have also shown that the surface tension of water–alkane interfaces can experience an initial decrease of several ![]() ${\rm mN}\,{\rm m}^{-1}$ at minuscule surfactant concentrations. Fainerman et al. (Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019) illustrated this effect for water–hexane interfaces with dodecyl and tridecyl dimethyl phosphine oxide (C
${\rm mN}\,{\rm m}^{-1}$ at minuscule surfactant concentrations. Fainerman et al. (Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019) illustrated this effect for water–hexane interfaces with dodecyl and tridecyl dimethyl phosphine oxide (C![]() $_{12}$DMPO and C
$_{12}$DMPO and C![]() $_{13}$DMPO, lowest concentration
$_{13}$DMPO, lowest concentration ![]() $c_0 = 10^{-6}\,{\rm mol}\,{\rm m}^{-3}$). This effect is also present for several other surfactants such as SDS and trimethyl ammonium bromides (C
$c_0 = 10^{-6}\,{\rm mol}\,{\rm m}^{-3}$). This effect is also present for several other surfactants such as SDS and trimethyl ammonium bromides (C![]() $_{n}$TAB). It is only recently that adsorption models for water–oil interfaces have been developed to account for such phenomena (Fainerman et al. Reference Fainerman2020).
$_{n}$TAB). It is only recently that adsorption models for water–oil interfaces have been developed to account for such phenomena (Fainerman et al. Reference Fainerman2020).
Based on the current knowledge about surfactants at water–oil interfaces, we have investigated the dependency of the slip length of LIS on surfactant concentration. The coupled system of equations for the flow and the surfactants has been solved numerically for transverse grooves in laminar shear flow. In contrast to SHS, it is necessary to resolve the flow both inside and outside the textures of LIS. We also developed an analytical model for the slip length in the presence of surfactants. The model can be used to predict the reduction of slip lengths if the surfactant type and concentration in bulk liquid are known. In settings where surfactants are hidden in the system, the model may be used to estimate the concentration of surfactants given measurements of the slip length.
The flow configuration, governing equations and numerical methods are described in the next section. Section 3 introduces the analytical model for an imposed Marangoni stress. Interfacial surfactant adsorption and desorption of surfactants using regular Frumkin kinetics are described and used for simulations in § 4, followed by the corresponding analytical model (§ 5). A more advanced model giving consistent surface tensions at minuscule concentrations is introduced in § 6. Higher applied shear stresses, resulting in highly skewed interfacial surfactant concentrations (the partial stagnant cap (SC) regime), are treated in § 7. Final remarks and conclusions are presented in §§ 8 and 9, respectively.
2. Configuration, governing equations and numerical method
We consider a LIS texture consisting of a periodic array of rectangular transverse grooves subjected to steady laminar flow. Due to the texture periodicity, it is sufficient to consider one interface unit cell with a single groove, sketched in figure 1(a). The groove depth was ![]() $k = 50\,\mathrm {\mu }{\rm m}$, the width
$k = 50\,\mathrm {\mu }{\rm m}$, the width ![]() $w = 2k$, and the pitch
$w = 2k$, and the pitch ![]() $p = 3k$ (groove centre-to-centre distance).
$p = 3k$ (groove centre-to-centre distance).
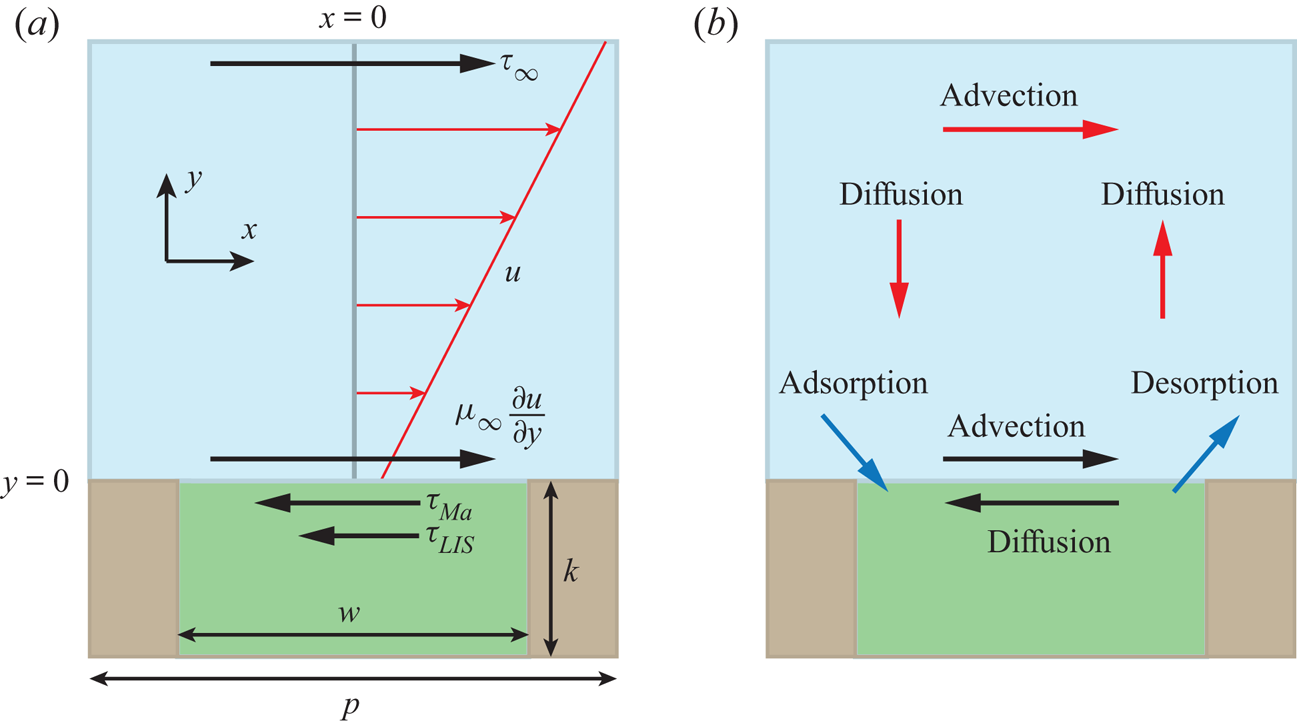
Figure 1. (a) Interface unit cell, illustrating the balance of stresses at the interface. The velocity profile of the external flow is also shown (red). (b) The physical processes transporting surfactants in the bulk and on the interface and controlling their exchange (red, black and blue arrows, respectively). The external liquid is blue, the infusing liquid green and the solid brown.
The external and infusing fluids have viscosities ![]() $\mu _\infty$ and
$\mu _\infty$ and ![]() $\mu _i$, respectively (
$\mu _i$, respectively (![]() $\mu _\infty = 1.0$ mPa s for water), forming interfaces aligned with the ridges between the grooves. Flat, non-deformable interfaces are assumed in this study so that the sole effect of the surfactants is the Marangoni force. An increased deformation due to a reduction in surface tension is expected to be of secondary importance (Landel et al. Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020).
$\mu _\infty = 1.0$ mPa s for water), forming interfaces aligned with the ridges between the grooves. Flat, non-deformable interfaces are assumed in this study so that the sole effect of the surfactants is the Marangoni force. An increased deformation due to a reduction in surface tension is expected to be of secondary importance (Landel et al. Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020).
The flow velocities and texture dimensions are small. Therefore, we use the Stokes equations for a steady incompressible flow,
where ![]() $P$ is the pressure,
$P$ is the pressure, ![]() $\mu$ is the fluid viscosity and
$\mu$ is the fluid viscosity and ![]() $\boldsymbol {u} = (u,v)$ is the fluid velocity with streamwise and wall-normal components
$\boldsymbol {u} = (u,v)$ is the fluid velocity with streamwise and wall-normal components ![]() $u$ and
$u$ and ![]() $v$, respectively. The streamwise coordinate is
$v$, respectively. The streamwise coordinate is ![]() $x$ with
$x$ with ![]() $x = 0$ in the centre of the considered fluid–fluid interface, and
$x = 0$ in the centre of the considered fluid–fluid interface, and ![]() $y$ is the wall-normal coordinate with
$y$ is the wall-normal coordinate with ![]() $y = 0$ at the interface. Equations (2.1a,b) are valid for both the external and infusing fluids. No-slip and impermeability conditions (
$y = 0$ at the interface. Equations (2.1a,b) are valid for both the external and infusing fluids. No-slip and impermeability conditions (![]() $\boldsymbol {u} = 0$) were used at solid boundaries. At the interface, the wall-normal velocity was
$\boldsymbol {u} = 0$) were used at solid boundaries. At the interface, the wall-normal velocity was ![]() $v = 0$ and the streamwise velocity
$v = 0$ and the streamwise velocity ![]() $u$ was assumed to be continuous. The balance of shear stress on the interface is (Leal Reference Leal2007)
$u$ was assumed to be continuous. The balance of shear stress on the interface is (Leal Reference Leal2007)
where ![]() $\gamma$ is the surface tension. The velocity gradients in (2.2) have been evaluated precisely above and below the interface (
$\gamma$ is the surface tension. The velocity gradients in (2.2) have been evaluated precisely above and below the interface (![]() $y = 0^+$ and
$y = 0^+$ and ![]() $0^-$, respectively). The imposed shear stress that drives the flow is assumed to be
$0^-$, respectively). The imposed shear stress that drives the flow is assumed to be ![]() $\tau _\infty$ at
$\tau _\infty$ at ![]() $y \to \infty$.
$y \to \infty$.
In order to simplify subsequent notation, we introduce
The balance of stresses is shown in figure 1(a). For no surfactants (![]() $\tau _{{ {Ma}}} = 0$), (2.2) relaxes to the classical interface condition, and for
$\tau _{{ {Ma}}} = 0$), (2.2) relaxes to the classical interface condition, and for ![]() $\tau _{LIS} = 0$, ideal (gas infused) SHS are regained.
$\tau _{LIS} = 0$, ideal (gas infused) SHS are regained.
The surfactants are assumed to be soluble in the water phase and can adsorb at the interfaces (see the sketch in figure 2). Surfactant transport mechanisms are illustrated in figure 1(b). An advection–diffusion equation governs the interfacial surfactant concentration ![]() $\varGamma$,
$\varGamma$,
where ![]() $u_s$ is the velocity of the interface plane (
$u_s$ is the velocity of the interface plane (![]() $y = 0$),
$y = 0$), ![]() $D_s$ is the interface diffusivity and
$D_s$ is the interface diffusivity and ![]() $S$ is a source term describing adsorption and desorption. The source term would be zero for insoluble surfactants. No-flux conditions apply at interface edges (
$S$ is a source term describing adsorption and desorption. The source term would be zero for insoluble surfactants. No-flux conditions apply at interface edges (![]() $\mathrm {d} \varGamma /\mathrm {d}\kern 0.06em x = 0$), and (2.4) is valid for
$\mathrm {d} \varGamma /\mathrm {d}\kern 0.06em x = 0$), and (2.4) is valid for ![]() $-w/2 < x < w/2$. The surface coverage of the interfacial surfactants is
$-w/2 < x < w/2$. The surface coverage of the interfacial surfactants is ![]() $\theta = \varGamma /\varGamma _m = \omega \varGamma$, where
$\theta = \varGamma /\varGamma _m = \omega \varGamma$, where ![]() $\varGamma _m$ is the maximum possible interface concentration and
$\varGamma _m$ is the maximum possible interface concentration and ![]() $\omega$ is the molar area. To increase brevity of the expressions throughout the paper, we use the non-dimensional surfactant concentration,
$\omega$ is the molar area. To increase brevity of the expressions throughout the paper, we use the non-dimensional surfactant concentration, ![]() $\theta$, but the theory is otherwise presented using dimensional quantities.
$\theta$, but the theory is otherwise presented using dimensional quantities.
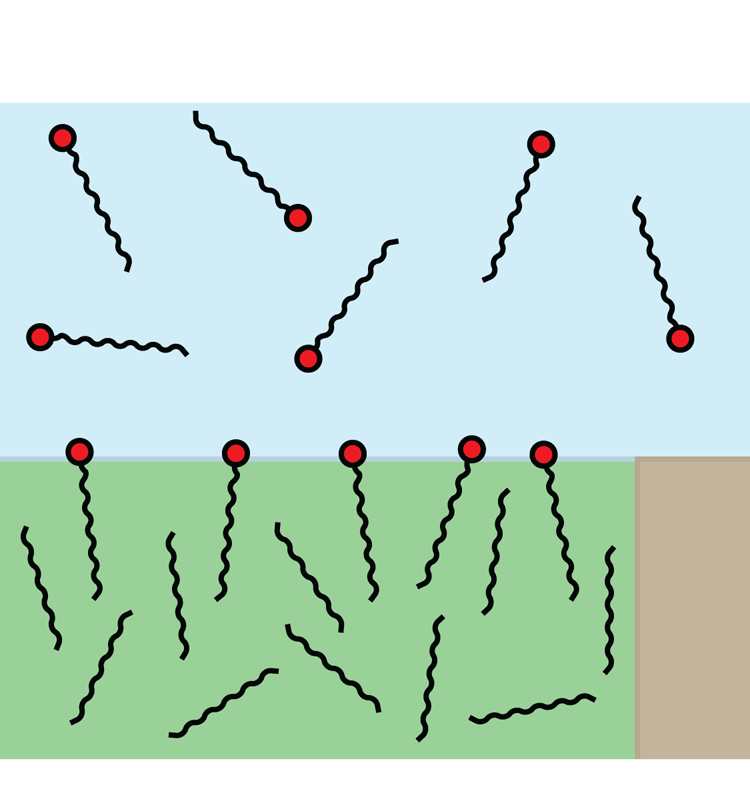
Figure 2. Schematic illustration of surfactants dissolved in water and adsorbed at a water–alkane interface. The interface is shown pinned to a corner of the solid texture. SDS and C![]() $_{12}$TAB molecules consist of hydrocarbon tails with 12 carbon atoms and hydrophilic head groups (red). Alkane molecules are also hydrocarbon chains. Dodecane and hexane molecules have
$_{12}$TAB molecules consist of hydrocarbon tails with 12 carbon atoms and hydrophilic head groups (red). Alkane molecules are also hydrocarbon chains. Dodecane and hexane molecules have ![]() $12$ and
$12$ and ![]() $6$ carbon atoms, respectively, here assumed to be arranged in straight chains. The different phases have the same colours as in figure 1.
$6$ carbon atoms, respectively, here assumed to be arranged in straight chains. The different phases have the same colours as in figure 1.
The equation governing bulk surfactant concentration ![]() $c$ is
$c$ is
where ![]() $D$ is the (bulk) diffusivity. The adsorption and desorption of surfactants at an interface balance the diffusive flux:
$D$ is the (bulk) diffusivity. The adsorption and desorption of surfactants at an interface balance the diffusive flux:
At solid boundaries, ![]() $\partial c/\partial y = 0$. Sufficiently far above the interface, we assume a constant surfactant concentration
$\partial c/\partial y = 0$. Sufficiently far above the interface, we assume a constant surfactant concentration ![]() $c_0$. The diffusivities were set to
$c_0$. The diffusivities were set to ![]() $D = D_s = 7.0 \times 10^{-10}\,{\rm m}^2\,{\rm s}^{-1}$, which also was used by Peaudecerf et al. (Reference Peaudecerf, Landel, Goldstein and Luzzatto-Fegiz2017).
$D = D_s = 7.0 \times 10^{-10}\,{\rm m}^2\,{\rm s}^{-1}$, which also was used by Peaudecerf et al. (Reference Peaudecerf, Landel, Goldstein and Luzzatto-Fegiz2017).
The source term and the Marangoni stresses are described in the following sections. We adopt surface tension and adsorption/desorption models for the two extensively studied surfactants SDS and C![]() $_{12}$TAB on water–air (Peaudecerf et al. Reference Peaudecerf, Landel, Goldstein and Luzzatto-Fegiz2017) and water–alkane interfaces (Fainerman et al. Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019). SDS can be found in personal care products, and C
$_{12}$TAB on water–air (Peaudecerf et al. Reference Peaudecerf, Landel, Goldstein and Luzzatto-Fegiz2017) and water–alkane interfaces (Fainerman et al. Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019). SDS can be found in personal care products, and C![]() $_{12}$TAB can, for example, be used to stabilise foam (Carey & Stubenrauch Reference Carey and Stubenrauch2009; Rosen & Kunjappu Reference Rosen and Kunjappu2012). These surfactants are illustrated schematically in figure 2. They have hydrophobic hydrocarbon tails with 12 carbon atoms but different hydrophilic (head) groups.
$_{12}$TAB can, for example, be used to stabilise foam (Carey & Stubenrauch Reference Carey and Stubenrauch2009; Rosen & Kunjappu Reference Rosen and Kunjappu2012). These surfactants are illustrated schematically in figure 2. They have hydrophobic hydrocarbon tails with 12 carbon atoms but different hydrophilic (head) groups.
2.1. Numerical method
The numerical simulations were performed using the finite-element solver FreeFem++ (Hecht Reference Hecht2012; Lācis et al. Reference Lcis, U., Sudhakar, Pasche and Bagheri2020). Velocities and surfactant concentrations were discretised using quadratic (![]() $P_2$) finite elements while linear (
$P_2$) finite elements while linear (![]() $P_1$) elements were used for the pressure. The system of (2.1a,b), (2.4) and (2.5) was solved iteratively. The domain consisted of one interface unit cell (figure 1). The external flow domain had a height of
$P_1$) elements were used for the pressure. The system of (2.1a,b), (2.4) and (2.5) was solved iteratively. The domain consisted of one interface unit cell (figure 1). The external flow domain had a height of ![]() $3k$. At the upper boundary, we imposed constant shear stress
$3k$. At the upper boundary, we imposed constant shear stress ![]() $\tau _\infty$, zero wall-normal stress and bulk surfactant concentration
$\tau _\infty$, zero wall-normal stress and bulk surfactant concentration ![]() $c_0$. Periodic boundary conditions were applied in the streamwise direction.
$c_0$. Periodic boundary conditions were applied in the streamwise direction.
The computational mesh used for the flow (bulk and cavity) and the bulk surfactants (![]() $y \ge 0$) was generated using a built-in tool. The cells were triangular, and the number of cells was prescribed at the different domain boundaries with a spacing of
$y \ge 0$) was generated using a built-in tool. The cells were triangular, and the number of cells was prescribed at the different domain boundaries with a spacing of ![]() $w/N$, where
$w/N$, where ![]() $N = 64$. The grid was refined close to the interface. For the simulations with a low flow speed (§§ 4 and 6), two refinements were made, reducing the sides of the cells by a factor of four in total, i.e.
$N = 64$. The grid was refined close to the interface. For the simulations with a low flow speed (§§ 4 and 6), two refinements were made, reducing the sides of the cells by a factor of four in total, i.e. ![]() $N = 256$. The simulations with artificially applied Marangoni stress (§ 3) also had
$N = 256$. The simulations with artificially applied Marangoni stress (§ 3) also had ![]() $N = 256$ at the interface. For simulations with high flow speed, we used four refinements (
$N = 256$ at the interface. For simulations with high flow speed, we used four refinements (![]() $N = 1024$, § 7). We have also performed grid refinement studies, see Appendix A.
$N = 1024$, § 7). We have also performed grid refinement studies, see Appendix A.
The interfacial surfactant transport equation (2.4) was solved on a one-cell-high mesh with equal cell spacing to the flow and bulk surfactant mesh in the streamwise direction. We imposed periodic boundary conditions in the ![]() $y$-direction. The interface velocity and the bulk surfactant concentration appearing in the source term (
$y$-direction. The interface velocity and the bulk surfactant concentration appearing in the source term (![]() $u_s$ and
$u_s$ and ![]() $c_s$, respectively) were taken at
$c_s$, respectively) were taken at ![]() $y = 0$. There were no variations in the
$y = 0$. There were no variations in the ![]() $y$-direction in the solution of the interfacial surfactant concentration; it exactly corresponds to the one-dimensional solution.
$y$-direction in the solution of the interfacial surfactant concentration; it exactly corresponds to the one-dimensional solution.
3. Analytical model of a viscous infusing liquid
To construct an analytical model of the flow over LIS with surfactants, we assume that the stresses ![]() $\tau _{LIS}$ and
$\tau _{LIS}$ and ![]() $\tau _{{ {Ma}}}$ are constant. Essentially, we combine the models presented by Schönecker, Baier & Hardt (Reference Schönecker, Baier and Hardt2014) and Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020). The former considered the flow over LIS without surfactants and assumed
$\tau _{{ {Ma}}}$ are constant. Essentially, we combine the models presented by Schönecker, Baier & Hardt (Reference Schönecker, Baier and Hardt2014) and Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020). The former considered the flow over LIS without surfactants and assumed ![]() $\tau _{LIS}$ was constant. The latter work considered flow over SHS and modelled the Marangoni stresses
$\tau _{LIS}$ was constant. The latter work considered flow over SHS and modelled the Marangoni stresses ![]() $\tau _{{ {Ma}}}$ as constant. In this section, we present the analytical model of our system and refer the reader to Appendix B for derivations.
$\tau _{{ {Ma}}}$ as constant. In this section, we present the analytical model of our system and refer the reader to Appendix B for derivations.
The (effective) slip length ![]() $b$ is defined by
$b$ is defined by
where ![]() $\left \langle \right \rangle$ gives the average in the streamwise direction (
$\left \langle \right \rangle$ gives the average in the streamwise direction (![]() $-p/2 < x \le p/2$), and
$-p/2 < x \le p/2$), and ![]() $U_s = \left \langle u \right \rangle$ at
$U_s = \left \langle u \right \rangle$ at ![]() $y = 0$ is the slip velocity. The resulting analytical expression of the effective slip length is (B24)
$y = 0$ is the slip velocity. The resulting analytical expression of the effective slip length is (B24)
where we have introduced the ideal SHS slip length (![]() $\mu _i/\mu _\infty = \tau _{{ {Ma}}}/\tau _\infty = 0$)
$\mu _i/\mu _\infty = \tau _{{ {Ma}}}/\tau _\infty = 0$)
with ![]() $\alpha = ({\rm \pi} /2)(w/p)$. The factor
$\alpha = ({\rm \pi} /2)(w/p)$. The factor ![]() $\beta _{LIS} = C_t/(1 + C_t)$ describes the effects due to the viscous infusing liquid, where
$\beta _{LIS} = C_t/(1 + C_t)$ describes the effects due to the viscous infusing liquid, where ![]() $C_t$ depends on the groove geometry and the viscosity ratio,
$C_t$ depends on the groove geometry and the viscosity ratio,
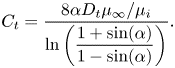 \begin{equation} C_t = \frac{8\alpha D_t\mu_\infty/\mu_i}{\ln\left(\dfrac{1 + \sin(\alpha)}{1 - \sin(\alpha)}\right)}. \end{equation}
\begin{equation} C_t = \frac{8\alpha D_t\mu_\infty/\mu_i}{\ln\left(\dfrac{1 + \sin(\alpha)}{1 - \sin(\alpha)}\right)}. \end{equation}
The parameter ![]() $D_t$ is a normalised maximum local slip length (B22). Equation (3.2) describes the slip length as a linear function of the Marangoni stress. Similarly, the velocity at the centre of the interface is (B25)
$D_t$ is a normalised maximum local slip length (B22). Equation (3.2) describes the slip length as a linear function of the Marangoni stress. Similarly, the velocity at the centre of the interface is (B25)
where
What remains is to find an explicit expression for ![]() $\tau _{{ {Ma}}}$. However, we can compare the expression for
$\tau _{{ {Ma}}}$. However, we can compare the expression for ![]() $b$ in (3.2) to simulations of (2.1a,b) and (2.2) with an artificially applied
$b$ in (3.2) to simulations of (2.1a,b) and (2.2) with an artificially applied ![]() $\tau _{{ {Ma}}}$. A comparison is shown in figure 3, with a convincing agreement. The two examined viscosity ratios were
$\tau _{{ {Ma}}}$. A comparison is shown in figure 3, with a convincing agreement. The two examined viscosity ratios were ![]() $\mu _i/\mu _\infty = 1.4$ and
$\mu _i/\mu _\infty = 1.4$ and ![]() $0.33$, corresponding to water–dodecane and water–hexane interfaces, respectively (table 1). For both viscosity ratios,
$0.33$, corresponding to water–dodecane and water–hexane interfaces, respectively (table 1). For both viscosity ratios, ![]() $b$ decreases from
$b$ decreases from ![]() $b_{SHS}\beta _{LIS}$ to
$b_{SHS}\beta _{LIS}$ to ![]() $0$ when
$0$ when ![]() $\tau _{{ {Ma}}}/\tau _\infty$ increases from
$\tau _{{ {Ma}}}/\tau _\infty$ increases from ![]() $0$ to
$0$ to ![]() $1$, as predicted by (3.2). The resulting slip length is higher for water–hexane interfaces (for the same
$1$, as predicted by (3.2). The resulting slip length is higher for water–hexane interfaces (for the same ![]() $\tau _{{ {Ma}}}/\tau _\infty$) because of the lower viscosity ratio.
$\tau _{{ {Ma}}}/\tau _\infty$) because of the lower viscosity ratio.

Figure 3. Comparison of (3.2) to simulation results where ![]() $\tau _{{ {Ma}}}$ was applied artificially. The viscosity ratio was set to
$\tau _{{ {Ma}}}$ was applied artificially. The viscosity ratio was set to ![]() $\mu _i/\mu _\infty = 1.4$ or
$\mu _i/\mu _\infty = 1.4$ or ![]() $0.33$ (corresponding to water–dodecane or water–hexane, respectively).
$0.33$ (corresponding to water–dodecane or water–hexane, respectively).
Table 1. Parameters used for SDS at water–air and water–dodecane interfaces and C![]() $_{12}$TAB at water–hexane interfaces. Reg. Frumkin refers to the model expressed by (4.1) and (4.2) and Adv. Frumkin to (6.1), (6.2) and (6.4). For all set-ups, we assume
$_{12}$TAB at water–hexane interfaces. Reg. Frumkin refers to the model expressed by (4.1) and (4.2) and Adv. Frumkin to (6.1), (6.2) and (6.4). For all set-ups, we assume ![]() $D = D_s = 7.0 \times 10^{-10}\,{\rm m}^2\,{\rm s}^{-1}$.
$D = D_s = 7.0 \times 10^{-10}\,{\rm m}^2\,{\rm s}^{-1}$.
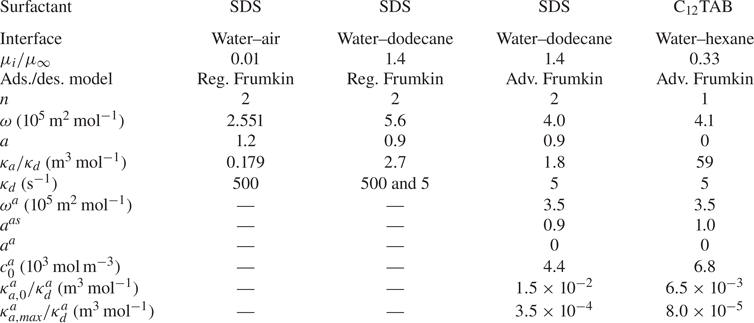
4. Adsorption and desorption with regular Frumkin kinetics
The interfacial adsorption and desorption rates determine the source term ![]() $S$. These rates have in recent studies of SHS been modelled by Frumkin kinetics (Peaudecerf et al. Reference Peaudecerf, Landel, Goldstein and Luzzatto-Fegiz2017; Landel et al. Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020), which are consistent with the Frumkin isotherm (Chang & Franses Reference Chang and Franses1995). Therefore, we also adopt Frumkin kinetics for this investigation. The source term becomes
$S$. These rates have in recent studies of SHS been modelled by Frumkin kinetics (Peaudecerf et al. Reference Peaudecerf, Landel, Goldstein and Luzzatto-Fegiz2017; Landel et al. Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020), which are consistent with the Frumkin isotherm (Chang & Franses Reference Chang and Franses1995). Therefore, we also adopt Frumkin kinetics for this investigation. The source term becomes
where ![]() $c_s$ is the value of
$c_s$ is the value of ![]() $c$ at the interfaces (dependent on
$c$ at the interfaces (dependent on ![]() $x$),
$x$), ![]() $a$ is a constant,
$a$ is a constant, ![]() $\kappa _{a}$ is the adsorption coefficient and
$\kappa _{a}$ is the adsorption coefficient and ![]() $\kappa _{d}$ is the desorption coefficient. If the surfactant molecules are mutually attractive,
$\kappa _{d}$ is the desorption coefficient. If the surfactant molecules are mutually attractive, ![]() $a$ is positive, and if they are repulsive,
$a$ is positive, and if they are repulsive, ![]() $a$ is negative (Manikantan & Squires Reference Manikantan and Squires2020). In equilibrium, adsorption and desorption fluxes balance (i.e.
$a$ is negative (Manikantan & Squires Reference Manikantan and Squires2020). In equilibrium, adsorption and desorption fluxes balance (i.e. ![]() $S = 0$), and it is sufficient to state
$S = 0$), and it is sufficient to state ![]() $\kappa _{a}/\kappa _{d}$ instead of the individual values of
$\kappa _{a}/\kappa _{d}$ instead of the individual values of ![]() $\kappa _{a}$ and
$\kappa _{a}$ and ![]() $\kappa _{d}$.
$\kappa _{d}$.
The expression for the surface tension (equation of state) corresponding to the Frumkin isotherm is
where ![]() $\gamma _c$ is the surface tension for
$\gamma _c$ is the surface tension for ![]() $\theta = 0$,
$\theta = 0$, ![]() $R = 8.314\,\mathrm {J}\,(\mathrm {K}\,\mathrm {mol})^{-1}$ is the universal gas constant,
$R = 8.314\,\mathrm {J}\,(\mathrm {K}\,\mathrm {mol})^{-1}$ is the universal gas constant, ![]() $T = 293\,\mathrm {K}$ (at
$T = 293\,\mathrm {K}$ (at ![]() $20\,^{\circ }{\rm C}$) is the absolute temperature and
$20\,^{\circ }{\rm C}$) is the absolute temperature and ![]() $n$ is a constant. The factor
$n$ is a constant. The factor ![]() $n$ can account for the adsorption of counter-ions (Chang & Franses Reference Chang and Franses1995; Fainerman et al. Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019). Without a supporting electrolyte, SDS has
$n$ can account for the adsorption of counter-ions (Chang & Franses Reference Chang and Franses1995; Fainerman et al. Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019). Without a supporting electrolyte, SDS has ![]() $n = 2$, which is the value used here. We assume that the data used for C
$n = 2$, which is the value used here. We assume that the data used for C![]() $_{12}$TAB were obtained using a solution with a sufficiently high concentration of supporting electrolyte so that
$_{12}$TAB were obtained using a solution with a sufficiently high concentration of supporting electrolyte so that ![]() $n = 1$. It is generally accepted that the equilibrium model (4.2) can be used in non-equilibrium conditions (Chang & Franses Reference Chang and Franses1995).
$n = 1$. It is generally accepted that the equilibrium model (4.2) can be used in non-equilibrium conditions (Chang & Franses Reference Chang and Franses1995).
We performed simulations of SDS on water–dodecane interfaces with regular Frumkin kinetics ((4.1) and (4.2)). We increased the concentration ![]() $c_0$ in steps starting from
$c_0$ in steps starting from ![]() $10^{-8}\,{\rm mol}\,{\rm m}^{-3}$. Equilibrium parameters were adapted from Fainerman et al. (Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019), compensated for not explicitly considering interactions of surfactant–alkane molecules (§ 6). These parameters are summarised in table 1 and result in an equilibrium-state surface tension shown in figure 4. The figure also contains experimental data from Fainerman et al. (Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019). Regular Frumkin kinetics can describe the measurements but not the correct asymptotic behaviour at minuscule concentrations.
$10^{-8}\,{\rm mol}\,{\rm m}^{-3}$. Equilibrium parameters were adapted from Fainerman et al. (Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019), compensated for not explicitly considering interactions of surfactant–alkane molecules (§ 6). These parameters are summarised in table 1 and result in an equilibrium-state surface tension shown in figure 4. The figure also contains experimental data from Fainerman et al. (Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019). Regular Frumkin kinetics can describe the measurements but not the correct asymptotic behaviour at minuscule concentrations.
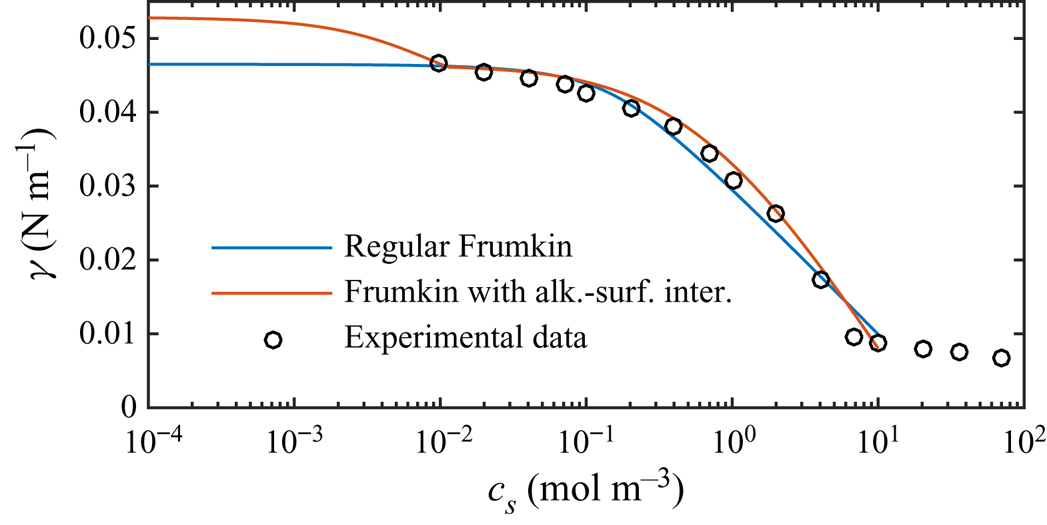
Figure 4. Equilibrium surface tension (![]() $S = 0$) of SDS on a water–dodecane interface, comparing experimental data of Fainerman et al. (Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019) with the regular Frumkin model and a Frumkin model taking into account alkane–surfactant interaction. The surface tension is
$S = 0$) of SDS on a water–dodecane interface, comparing experimental data of Fainerman et al. (Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019) with the regular Frumkin model and a Frumkin model taking into account alkane–surfactant interaction. The surface tension is ![]() $\gamma _c = 52.87\,{\rm mN}\,{\rm m}^{-1}$ for water–dodecane without surfactants at
$\gamma _c = 52.87\,{\rm mN}\,{\rm m}^{-1}$ for water–dodecane without surfactants at ![]() $20\,^{\circ }{\rm C}$ (Zeppieri, Rodríguez & López de Ramos Reference Zeppieri, Rodríguez and López de Ramos2001). In contrast to water–air interfaces, the regular Frumkin model does not exhibit the correct asymptotic behaviour when
$20\,^{\circ }{\rm C}$ (Zeppieri, Rodríguez & López de Ramos Reference Zeppieri, Rodríguez and López de Ramos2001). In contrast to water–air interfaces, the regular Frumkin model does not exhibit the correct asymptotic behaviour when ![]() $c_s \to 0$ at the water–oil interfaces.
$c_s \to 0$ at the water–oil interfaces.
Next, we consider the non-equilibrium system by imposing an external shear stress. As a reference to SDS on water–dodecane interfaces, we also simulated SDS on water–air interfaces, starting at ![]() $c_0 = 10^{-7}\,{\rm mol}\,{\rm m}^{-3}$. Air–water parameters for equilibrium were taken from Prosser & Franses (Reference Prosser and Franses2001). The relatively low imposed shear stress was
$c_0 = 10^{-7}\,{\rm mol}\,{\rm m}^{-3}$. Air–water parameters for equilibrium were taken from Prosser & Franses (Reference Prosser and Franses2001). The relatively low imposed shear stress was ![]() ${\tau _\infty = 0.33}$ mPa; the effects of increased shear stress are described in § 7. The non-equilibrium parameter
${\tau _\infty = 0.33}$ mPa; the effects of increased shear stress are described in § 7. The non-equilibrium parameter ![]() $\kappa _d = 500\,{\rm s}^{-1}$ of the air–water interfaces corresponds to the value reported by Chang & Franses (Reference Chang and Franses1995). This value was determined using empirically modified Langmuir–Hinshelwood kinetics. This model allows
$\kappa _d = 500\,{\rm s}^{-1}$ of the air–water interfaces corresponds to the value reported by Chang & Franses (Reference Chang and Franses1995). This value was determined using empirically modified Langmuir–Hinshelwood kinetics. This model allows ![]() $\kappa _d$ to vary with concentration and includes an exponential factor in the adsorption flux similar to the desorption flux (cf. (4.1)). The value used here corresponds to the lowest bulk concentration (
$\kappa _d$ to vary with concentration and includes an exponential factor in the adsorption flux similar to the desorption flux (cf. (4.1)). The value used here corresponds to the lowest bulk concentration (![]() $1.7\,{\rm mol}\,{\rm m}^{-3}$). For the water–dodecane system, we used both
$1.7\,{\rm mol}\,{\rm m}^{-3}$). For the water–dodecane system, we used both ![]() $\kappa _d = 500$ and
$\kappa _d = 500$ and ![]() $5\,{\rm s}^{-1}$, with minor differences. The latter implies a lower desorption rate. For all other simulations, we used
$5\,{\rm s}^{-1}$, with minor differences. The latter implies a lower desorption rate. For all other simulations, we used ![]() $\kappa _d = 5\,{\rm s}^{-1}$.
$\kappa _d = 5\,{\rm s}^{-1}$.
Figure 5(a) shows the resulting slip lengths as a function of ![]() $c_0$, and figure 5(b) provides an enlarged view. The water–air and water–dodecane results are shown in blue and red, respectively. Results for the advanced adsorption model are also included in the figure (yellow markers, see § 6). The slip length is approximately five times higher for the SHS without surfactants (
$c_0$, and figure 5(b) provides an enlarged view. The water–air and water–dodecane results are shown in blue and red, respectively. Results for the advanced adsorption model are also included in the figure (yellow markers, see § 6). The slip length is approximately five times higher for the SHS without surfactants (![]() $c_0 = 0\,{\rm mol}\,{\rm m}^{-3}$). As
$c_0 = 0\,{\rm mol}\,{\rm m}^{-3}$). As ![]() $c_0$ increases, the slip length of both systems decreases. The bulk concentration giving a significantly decreased slip length (
$c_0$ increases, the slip length of both systems decreases. The bulk concentration giving a significantly decreased slip length (![]() $b/(b_{SHS}\beta _{LIS}) \approx 0.5$) is around one order of magnitude smaller for the water–dodecane than the water–air system, marked by vertical dash-dotted lines in figure 5. For the water–air system and the water–dodecane systems (
$b/(b_{SHS}\beta _{LIS}) \approx 0.5$) is around one order of magnitude smaller for the water–dodecane than the water–air system, marked by vertical dash-dotted lines in figure 5. For the water–air system and the water–dodecane systems (![]() $\kappa _d = 500\,{\rm s}^{-1}$), these concentrations are
$\kappa _d = 500\,{\rm s}^{-1}$), these concentrations are ![]() $c_0 = 4\times 10^{-4}$ and
$c_0 = 4\times 10^{-4}$ and ![]() $5\times 10^{-5}\,{\rm mol}\,{\rm m}^{-3}$, respectively. This difference reflects the equilibrium surface tension behaviour. In order to explain the results in more detail, we continue to develop the analytical model of § 3 in § 5. This analytical model is also included in figure 5 (dashed lines).
$5\times 10^{-5}\,{\rm mol}\,{\rm m}^{-3}$, respectively. This difference reflects the equilibrium surface tension behaviour. In order to explain the results in more detail, we continue to develop the analytical model of § 3 in § 5. This analytical model is also included in figure 5 (dashed lines).
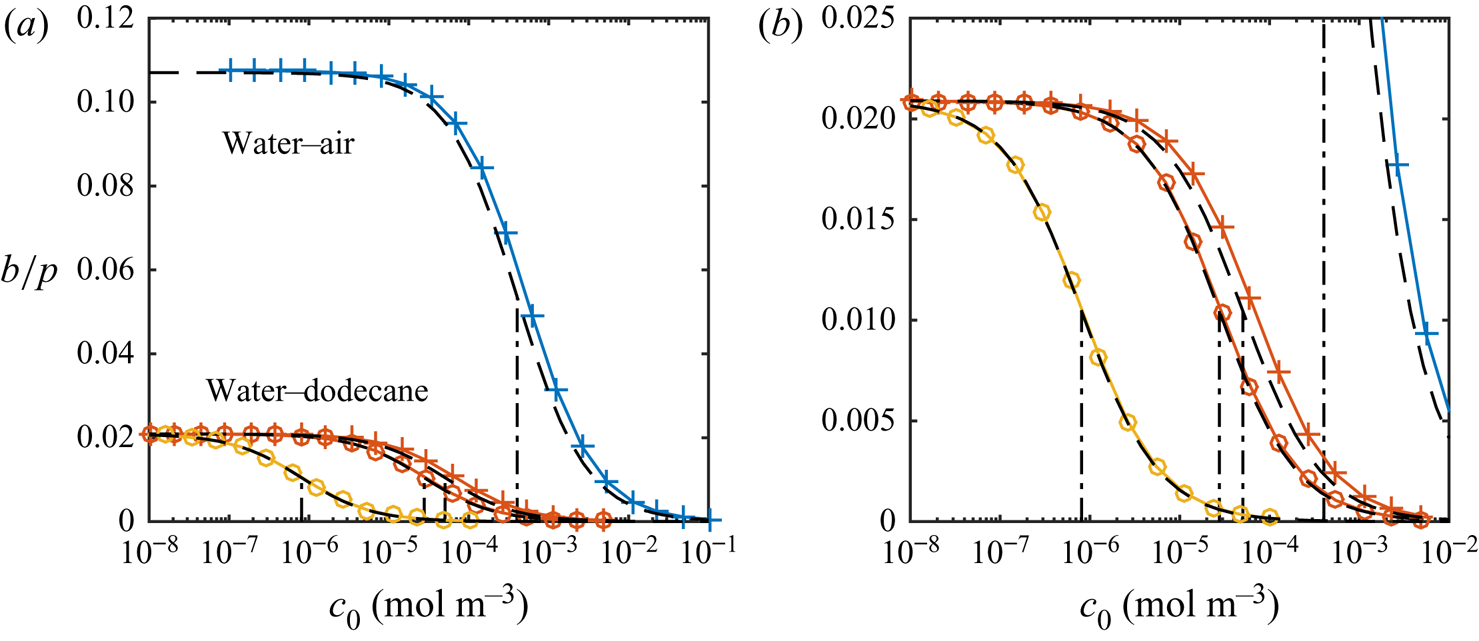
Figure 5. Parametric study of slip length at ![]() $\tau _\infty = 0.33$ mPa for different bulk concentrations of SDS with a water–air (blue) and a water–dodecane interface (red regular model, yellow advanced model), together with the analytical model (dashed lines). SHS and LIS results are shown in (a), and an enlarged view of the LIS results is shown in (b). Symbols refer to
$\tau _\infty = 0.33$ mPa for different bulk concentrations of SDS with a water–air (blue) and a water–dodecane interface (red regular model, yellow advanced model), together with the analytical model (dashed lines). SHS and LIS results are shown in (a), and an enlarged view of the LIS results is shown in (b). Symbols refer to ![]() $\kappa _d = 5$ (
$\kappa _d = 5$ (![]() $\circ$) and
$\circ$) and ![]() $500$ (
$500$ (![]() $+$) s
$+$) s![]() $^{-1}$. The vertical dash-dotted lines mark
$^{-1}$. The vertical dash-dotted lines mark ![]() $\alpha _{diff} + \alpha _S = 1$, corresponding to
$\alpha _{diff} + \alpha _S = 1$, corresponding to ![]() $b/(b_{SHS}\beta _{LIS}) \approx 1/2$ (5.16).
$b/(b_{SHS}\beta _{LIS}) \approx 1/2$ (5.16).
The analytical model predicts that the slip length becomes independent of ![]() $\kappa _d$ when this parameter is sufficiently large. At these desorption rates, diffusion of bulk surfactants becomes the limiting process (Damköhler number
$\kappa _d$ when this parameter is sufficiently large. At these desorption rates, diffusion of bulk surfactants becomes the limiting process (Damköhler number ![]() ${ {Da}}_\delta \gg 1$). Temprano-Coleto et al. (Reference Temprano-Coleto, Smith, Peaudecerf, Landel, Gibou and Luzzatto- Fegiz2021) recently discussed this independence, using
${ {Da}}_\delta \gg 1$). Temprano-Coleto et al. (Reference Temprano-Coleto, Smith, Peaudecerf, Landel, Gibou and Luzzatto- Fegiz2021) recently discussed this independence, using ![]() $\kappa _d = 0.75\,{\rm s}^{-1}$ with good agreement with the experimental results of SHS with surfactants naturally occurring in their experimental setting.
$\kappa _d = 0.75\,{\rm s}^{-1}$ with good agreement with the experimental results of SHS with surfactants naturally occurring in their experimental setting.
5. Analytical model of surfactant transport
The analytical model of surfactant transport discussed here uses similar core assumptions to previous works (Landel et al. Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020). It is assumed that interfacial concentrations are low (![]() $\theta \ll 1$) so that the governing equations (4.1) and (4.2) can be linearised. A second assumption is that bulk and interfacial surfactant concentrations vary linearly over the interfaces (figure 6). Such distributions imply approximately constant surface tension gradients, i.e. the uniformly retarded regime.
$\theta \ll 1$) so that the governing equations (4.1) and (4.2) can be linearised. A second assumption is that bulk and interfacial surfactant concentrations vary linearly over the interfaces (figure 6). Such distributions imply approximately constant surface tension gradients, i.e. the uniformly retarded regime.
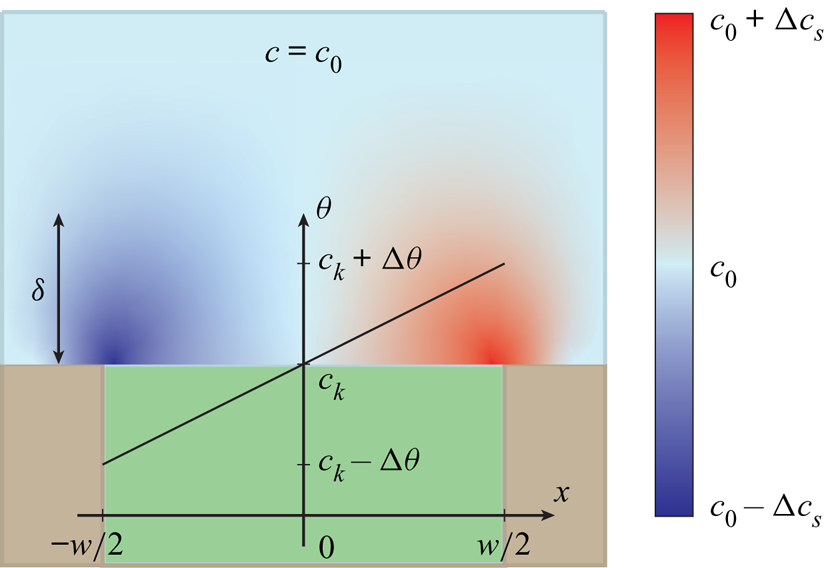
Figure 6. Illustration of the distributions of bulk and interfacial surfactant concentrations (![]() $c$ and
$c$ and ![]() $\theta$, respectively) of the analytical model. It is assumed that
$\theta$, respectively) of the analytical model. It is assumed that ![]() $c$ and
$c$ and ![]() $\theta$ vary linearly over the interfaces. The infusing liquid and the solid colours are the same as in figure 1.
$\theta$ vary linearly over the interfaces. The infusing liquid and the solid colours are the same as in figure 1.
5.1. Modelling bulk exchange
For a steady flow, there is a balance between adsorption and desorption processes. The integral of (2.6) over the interfaces must then be zero,
 \begin{equation} D\int_{{-}w/2}^{w/2}\left.\frac{\partial c}{\partial y}\right|_{y = 0}\, \mathrm{d}\kern0.06em x = \int_{{-}w/2}^{w/2}S \,\mathrm{d}\kern0.06em x = 0. \end{equation}
\begin{equation} D\int_{{-}w/2}^{w/2}\left.\frac{\partial c}{\partial y}\right|_{y = 0}\, \mathrm{d}\kern0.06em x = \int_{{-}w/2}^{w/2}S \,\mathrm{d}\kern0.06em x = 0. \end{equation}
This condition implies the existence of a point ![]() $x_0$ on an interface where
$x_0$ on an interface where ![]() $D\partial c/\partial y |_{y = 0} = S = 0$. As the wall-normal derivative of
$D\partial c/\partial y |_{y = 0} = S = 0$. As the wall-normal derivative of ![]() $c$ is zero at this location, we have
$c$ is zero at this location, we have ![]() $c_s \approx c_0$. The linearised source term (4.1) is
$c_s \approx c_0$. The linearised source term (4.1) is
where we have introduced the non-dimensional bulk surfactant concentration ![]() ${c_k = \kappa _ac_0/\kappa _d}$. From the linearised source term,
${c_k = \kappa _ac_0/\kappa _d}$. From the linearised source term,
Due to advection, ![]() $\theta$ decreases upstream and increases downstream of
$\theta$ decreases upstream and increases downstream of ![]() $x_0$. It is assumed that
$x_0$. It is assumed that ![]() $x_0 \approx 0$, that
$x_0 \approx 0$, that ![]() $\theta$ varies around
$\theta$ varies around ![]() $c_{k}$ by
$c_{k}$ by ![]() $\pm \Delta \theta$, and in the same way
$\pm \Delta \theta$, and in the same way ![]() $c_s$ around
$c_s$ around ![]() $c_0$ by
$c_0$ by ![]() $\pm \Delta c_s$. These assumed concentration distributions imply (using (2.6) and (5.2)),
$\pm \Delta c_s$. These assumed concentration distributions imply (using (2.6) and (5.2)),
where ![]() ${ {Da}} = \kappa _a w/(\omega D)$ is the Damköhler number (Temprano-Coleto et al. Reference Temprano-Coleto, Smith, Peaudecerf, Landel, Gibou and Luzzatto- Fegiz2021).
${ {Da}} = \kappa _a w/(\omega D)$ is the Damköhler number (Temprano-Coleto et al. Reference Temprano-Coleto, Smith, Peaudecerf, Landel, Gibou and Luzzatto- Fegiz2021).
From (5.2), a characteristic adsorption flux is given by
in ![]() ${\rm mol}\,({\rm s}\,{\rm m}^2)^{-1}$. Equation (5.3) entails that the characteristic desorption flux is the same:
${\rm mol}\,({\rm s}\,{\rm m}^2)^{-1}$. Equation (5.3) entails that the characteristic desorption flux is the same: ![]() $\kappa _d \theta /\omega \sim \kappa _d c_k/\omega = \kappa _a c_0/\omega$. A corresponding scale for the diffusive flux of bulk surfactants is
$\kappa _d \theta /\omega \sim \kappa _d c_k/\omega = \kappa _a c_0/\omega$. A corresponding scale for the diffusive flux of bulk surfactants is ![]() $D c_0/w$ (2.6). Hence,
$D c_0/w$ (2.6). Hence, ![]() ${ {Da}}$ expresses characteristic adsorption/desorption flux to diffusive flux of bulk surfactants. Corresponding rates are found by multiplication by
${ {Da}}$ expresses characteristic adsorption/desorption flux to diffusive flux of bulk surfactants. Corresponding rates are found by multiplication by ![]() $\omega$. The actual values of
$\omega$. The actual values of ![]() $\Delta \theta$ and
$\Delta \theta$ and ![]() $\Delta c_s$ are neglected, but we do include the characteristic sizes of
$\Delta c_s$ are neglected, but we do include the characteristic sizes of ![]() $\theta$ and
$\theta$ and ![]() $c_s$. This interpretation of
$c_s$. This interpretation of ![]() ${ {Da}}$ is also seen in (5.4); large diffusion flux and low adsorption result in smaller wall-normal derivative of
${ {Da}}$ is also seen in (5.4); large diffusion flux and low adsorption result in smaller wall-normal derivative of ![]() $c$.
$c$.
As pointed out by Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020), (5.4) can be used to estimate the boundary layer thickness ![]() $\delta$ of
$\delta$ of ![]() $c$. Approximating
$c$. Approximating ![]() $\delta$ by
$\delta$ by
 \begin{equation} \left.\frac{\partial c}{\partial y}\right|_{y = 0, x ={-}w/2} \approx \frac{\Delta c_s}{\delta} \implies \frac{\Delta c_s}{c_0} \approx \frac{\Delta \theta}{c_k}\frac{{{Da}} \dfrac{\delta}{w}}{1 + {{Da}} \dfrac{\delta}{w}}. \end{equation}
\begin{equation} \left.\frac{\partial c}{\partial y}\right|_{y = 0, x ={-}w/2} \approx \frac{\Delta c_s}{\delta} \implies \frac{\Delta c_s}{c_0} \approx \frac{\Delta \theta}{c_k}\frac{{{Da}} \dfrac{\delta}{w}}{1 + {{Da}} \dfrac{\delta}{w}}. \end{equation}
The modified Damköhler number ![]() ${ {Da}}_\delta = { {Da}} \delta /w$ considers the diffusion length scale of bulk surfactants to be
${ {Da}}_\delta = { {Da}} \delta /w$ considers the diffusion length scale of bulk surfactants to be ![]() $\delta$, which is more appropriate than
$\delta$, which is more appropriate than ![]() $w$ (Palaparthi et al. Reference Palaparthi, Papageorgiou and Maldarelli2006). We see that if the adsorption/desorption rate is much larger than the diffusion rate (
$w$ (Palaparthi et al. Reference Palaparthi, Papageorgiou and Maldarelli2006). We see that if the adsorption/desorption rate is much larger than the diffusion rate (![]() ${ {Da}}_\delta \gg 1$),
${ {Da}}_\delta \gg 1$), ![]() $\Delta c_s/c_0 \approx \Delta \theta /c_k$. Otherwise, if
$\Delta c_s/c_0 \approx \Delta \theta /c_k$. Otherwise, if ![]() ${ {Da}}_\delta \ll 1$,
${ {Da}}_\delta \ll 1$, ![]() $\Delta c_s/c_0 \approx { {Da}}_\delta \Delta \theta /c_k$.
$\Delta c_s/c_0 \approx { {Da}}_\delta \Delta \theta /c_k$.
The bulk surfactant transport equation (2.5) implies that ![]() $\delta$ depends on
$\delta$ depends on ![]() $w$ and Péclet number
$w$ and Péclet number ![]() ${ {Pe}} = Uw/D$, where
${ {Pe}} = Uw/D$, where ![]() $U = w\tau _\infty /\mu _\infty$ is the characteristic velocity at
$U = w\tau _\infty /\mu _\infty$ is the characteristic velocity at ![]() $y = w$. Diffusion between boundary layers of adjacent grooves also introduces a dependency on
$y = w$. Diffusion between boundary layers of adjacent grooves also introduces a dependency on ![]() $p$. An analytical estimate (Appendix C) resulted in
$p$. An analytical estimate (Appendix C) resulted in
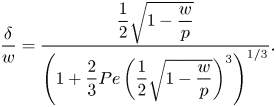 \begin{equation} \frac{\delta}{w} = \dfrac{\dfrac{1}{2}\sqrt{1 - \dfrac{w}{p}}}{\left(1 + \dfrac{2}{3}{{Pe}}\left(\dfrac{1}{2}\sqrt{1 - \dfrac{w}{p}}\right)^3\right)^{1/3}}. \end{equation}
\begin{equation} \frac{\delta}{w} = \dfrac{\dfrac{1}{2}\sqrt{1 - \dfrac{w}{p}}}{\left(1 + \dfrac{2}{3}{{Pe}}\left(\dfrac{1}{2}\sqrt{1 - \dfrac{w}{p}}\right)^3\right)^{1/3}}. \end{equation}
The left relation of (5.6) was also used to compute ![]() $\delta$ explicitly. It was found that
$\delta$ explicitly. It was found that ![]() $\delta /w = 0.3$ was a relatively good approximation for all tested configurations for the current geometry and
$\delta /w = 0.3$ was a relatively good approximation for all tested configurations for the current geometry and ![]() ${ {Pe}}$ (Appendix C and figure 12a,b), in agreement with (5.7). Deviations were around
${ {Pe}}$ (Appendix C and figure 12a,b), in agreement with (5.7). Deviations were around ![]() ${\pm }0.1$.
${\pm }0.1$.
5.2. Interfacial surfactant transport balance
The transport equation for the interfacial surfactant concentration (2.4) expresses a balance between advection, diffusion and adsorption/desorption (compare with figure 1b). We integrate this equation from ![]() $x = -w/2$ to
$x = -w/2$ to ![]() $x = x_0$ and use the boundary condition (2.6),
$x = x_0$ and use the boundary condition (2.6),
Using ![]() $x_0 \approx 0$ and (5.6) with the streamwise dependency (5.4), we have
$x_0 \approx 0$ and (5.6) with the streamwise dependency (5.4), we have
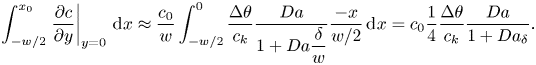 \begin{equation} \int_{{-}w/2}^{x_0} \left.\frac{\partial c}{\partial y}\right|_{y = 0} \,\mathrm{d}\kern0.06em x \approx \frac{c_0}{w}\int_{{-}w/2}^{0} \frac{\Delta \theta}{c_k}\frac{{{Da}}}{1 + {{Da}}\dfrac{\delta}{w}}\frac{-x}{w/2} \,\mathrm{d}\kern0.06em x = c_0\frac{1}{4}\frac{\Delta \theta}{c_k}\frac{{{Da}}}{1 + {{Da}}_\delta}. \end{equation}
\begin{equation} \int_{{-}w/2}^{x_0} \left.\frac{\partial c}{\partial y}\right|_{y = 0} \,\mathrm{d}\kern0.06em x \approx \frac{c_0}{w}\int_{{-}w/2}^{0} \frac{\Delta \theta}{c_k}\frac{{{Da}}}{1 + {{Da}}\dfrac{\delta}{w}}\frac{-x}{w/2} \,\mathrm{d}\kern0.06em x = c_0\frac{1}{4}\frac{\Delta \theta}{c_k}\frac{{{Da}}}{1 + {{Da}}_\delta}. \end{equation}
With (5.3) and ![]() $\mathrm {d} \theta /\mathrm {d}\kern 0.06em x|_{x=x_0} \approx 2\Delta \theta /w$, we obtain an expression for the interface centre velocity,
$\mathrm {d} \theta /\mathrm {d}\kern 0.06em x|_{x=x_0} \approx 2\Delta \theta /w$, we obtain an expression for the interface centre velocity,
with coefficients ![]() $c_1 \approx 2$ and
$c_1 \approx 2$ and ![]() $c_2 \approx 1/4$ resulting from the linear distributions. The interface Péclet number is defined by
$c_2 \approx 1/4$ resulting from the linear distributions. The interface Péclet number is defined by ![]() ${ {Pe}}_s = Uw/D_s$ and the Biot number is defined by
${ {Pe}}_s = Uw/D_s$ and the Biot number is defined by ![]() ${ {Bi}} = c_0D \omega { {Da}} /(U c_k) = \kappa _d w/U$.
${ {Bi}} = c_0D \omega { {Da}} /(U c_k) = \kappa _d w/U$.
The Péclet number ![]() ${ {Pe}}_s$ is a measure of characteristic advection to diffusion rates of the interfacial surfactants, which with (5.3) can be written as
${ {Pe}}_s$ is a measure of characteristic advection to diffusion rates of the interfacial surfactants, which with (5.3) can be written as
respectively. The characteristic adsorption/desorption fluxes (5.5) correspond to a rate ![]() $\kappa _d c_k$. Hence, the Biot number expresses the adsorption/desorption rate to the advection rate of interfacial surfactants.
$\kappa _d c_k$. Hence, the Biot number expresses the adsorption/desorption rate to the advection rate of interfacial surfactants.
For a specific value of ![]() $\Delta \theta /c_k$, (5.10) implies that for
$\Delta \theta /c_k$, (5.10) implies that for ![]() $u_s^0/U$ to be close to zero, the characteristic advection of interfacial surfactants must dominate (i) diffusion of interfacial surfactants (
$u_s^0/U$ to be close to zero, the characteristic advection of interfacial surfactants must dominate (i) diffusion of interfacial surfactants (![]() ${ {Pe}}_s \gg \Delta \theta /c_k$) and (ii) bulk exchange (cf. figure 1b). The bulk exchange may be limited by either adsorption/desorption rate (
${ {Pe}}_s \gg \Delta \theta /c_k$) and (ii) bulk exchange (cf. figure 1b). The bulk exchange may be limited by either adsorption/desorption rate (![]() ${ {Da}}_\delta \ll 1$) or diffusion of bulk surfactants (
${ {Da}}_\delta \ll 1$) or diffusion of bulk surfactants (![]() ${ {Da}}_\delta \gg 1$). If
${ {Da}}_\delta \gg 1$). If ![]() ${ {Da}}_\delta \ll 1$, we must have
${ {Da}}_\delta \ll 1$, we must have ![]() ${ {Bi}} \ll \Delta \theta /c_k$, i.e. advection of interfacial surfactants must dominate over the adsorption/desorption rate. If
${ {Bi}} \ll \Delta \theta /c_k$, i.e. advection of interfacial surfactants must dominate over the adsorption/desorption rate. If ![]() ${ {Da}}_\delta \gg 1$, then we must have
${ {Da}}_\delta \gg 1$, then we must have ![]() ${ {Bi}}/{ {Da}}_\delta \ll \Delta \theta /c_k$: advection of interfacial surfactants must dominate over the diffusion of bulk surfactants.
${ {Bi}}/{ {Da}}_\delta \ll \Delta \theta /c_k$: advection of interfacial surfactants must dominate over the diffusion of bulk surfactants.
The Marangoni stress also dictates the velocity on the interface (3.5). This relationship and (5.10) give a condition for ![]() $\Delta \theta$, derived in the next section.
$\Delta \theta$, derived in the next section.
5.3. Corresponding slip length
Linearisation of the Marangoni stresses with surface tension (4.2) implies
where we have introduced the Marangoni number ![]() ${ {Ma}} = nRT/(\omega \mu _\infty U)$. We have chosen to neglect thermal effects (
${ {Ma}} = nRT/(\omega \mu _\infty U)$. We have chosen to neglect thermal effects (![]() $T$ is a constant). The gradient of the surface coverage
$T$ is a constant). The gradient of the surface coverage ![]() $\mathrm {d} \theta /\mathrm {d}\kern 0.06em x$ is generally positive (figure 6), meaning that
$\mathrm {d} \theta /\mathrm {d}\kern 0.06em x$ is generally positive (figure 6), meaning that ![]() $\mathrm {d} \gamma /\mathrm {d}\kern 0.06em x$ is negative, and the Marangoni stresses act in the negative streamwise direction, as shown by figure 1(a). By assuming linear interfacial concentrations,
$\mathrm {d} \gamma /\mathrm {d}\kern 0.06em x$ is negative, and the Marangoni stresses act in the negative streamwise direction, as shown by figure 1(a). By assuming linear interfacial concentrations, ![]() $\mathrm {d} \theta /\mathrm {d}\kern 0.06em x$ can be estimated as
$\mathrm {d} \theta /\mathrm {d}\kern 0.06em x$ can be estimated as ![]() $2\Delta \theta /w$, resulting in
$2\Delta \theta /w$, resulting in
It follows from (3.5) that
Equation (5.13) implies a scaling ![]() $nRT/(\omega w)$ of the Marangoni stress, neglecting
$nRT/(\omega w)$ of the Marangoni stress, neglecting ![]() $\Delta \theta$. As
$\Delta \theta$. As ![]() $\tau _\infty = \mu _\infty U/w$, the Marangoni number expresses the ratio of characteristic Marangoni to imposed shear stresses. If the characteristic size of
$\tau _\infty = \mu _\infty U/w$, the Marangoni number expresses the ratio of characteristic Marangoni to imposed shear stresses. If the characteristic size of ![]() $\theta$ is considered, the Marangoni number transforms to
$\theta$ is considered, the Marangoni number transforms to ![]() ${ {Ma}} c_k$.
${ {Ma}} c_k$.
The velocities expressed by (5.10) and (5.14) must be the same. This equality results in the expression
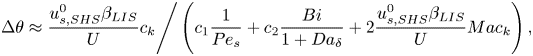 \begin{equation} \left.\Delta \theta \approx \frac{ u_{s,{SHS}}^0\beta_{LIS}}{U}c_k\right/\left(c_1 \frac{1}{{{Pe}}_s} + c_2\frac{{{Bi}}}{1 + {{Da}}_\delta} + 2\frac{u_{s,{SHS}}^0\beta_{LIS}}{U}{Ma} c_k\right), \end{equation}
\begin{equation} \left.\Delta \theta \approx \frac{ u_{s,{SHS}}^0\beta_{LIS}}{U}c_k\right/\left(c_1 \frac{1}{{{Pe}}_s} + c_2\frac{{{Bi}}}{1 + {{Da}}_\delta} + 2\frac{u_{s,{SHS}}^0\beta_{LIS}}{U}{Ma} c_k\right), \end{equation}
which by (5.13) gives ![]() $\tau _{{ {Ma}}}$. Equation (3.2) then gives the slip length
$\tau _{{ {Ma}}}$. Equation (3.2) then gives the slip length
We have introduced
where ![]() ${ {Pe}}_s' = wu_{s,{SHS}}^0\beta _{LIS}/D_s$ and
${ {Pe}}_s' = wu_{s,{SHS}}^0\beta _{LIS}/D_s$ and ![]() ${ {Bi}}' = \kappa _d w /(u_{s,{SHS}}^0\beta _{LIS}$) are the Péclet and Biot numbers, respectively, with more appropriate velocity scales. The two expressions of (5.17) represent the effects of interfacial surfactant diffusion and bulk exchange on the slip length, respectively. In order to reduce
${ {Bi}}' = \kappa _d w /(u_{s,{SHS}}^0\beta _{LIS}$) are the Péclet and Biot numbers, respectively, with more appropriate velocity scales. The two expressions of (5.17) represent the effects of interfacial surfactant diffusion and bulk exchange on the slip length, respectively. In order to reduce ![]() $b$, they must both be small,
$b$, they must both be small, ![]() $\alpha _{diff} \lesssim 1$ and
$\alpha _{diff} \lesssim 1$ and ![]() $\alpha _S \lesssim 1$. Based on the interpretations of the non-dimensional numbers,
$\alpha _S \lesssim 1$. Based on the interpretations of the non-dimensional numbers,
 $$\begin{gather}\alpha_{S}
\sim \dfrac{\dfrac{\text{adsorption/desorption
rate}}{\text{advection rate of interfacial surfactant}}}{1
+ \dfrac{\text{adsorption/desorption rate}}{\text{diffusion
rate of bulk surfactant}}}\dfrac{\text{imposed shear
stress}}{\text{Marangoni stress scale}}.
\end{gather}$$
$$\begin{gather}\alpha_{S}
\sim \dfrac{\dfrac{\text{adsorption/desorption
rate}}{\text{advection rate of interfacial surfactant}}}{1
+ \dfrac{\text{adsorption/desorption rate}}{\text{diffusion
rate of bulk surfactant}}}\dfrac{\text{imposed shear
stress}}{\text{Marangoni stress scale}}.
\end{gather}$$Returning to figure 5(a), we can plot the results also from the analytical model, showing a satisfactory agreement with the simulation results. Some central non-dimensional numbers are summarised in table 2.
If ![]() $\alpha _S \gg \alpha _{diff}$, the diffusion of interfacial surfactants is insignificant compared with the bulk exchange. In the opposite situation,
$\alpha _S \gg \alpha _{diff}$, the diffusion of interfacial surfactants is insignificant compared with the bulk exchange. In the opposite situation, ![]() $\alpha _S \ll \alpha _{diff}$, the interfacial diffusion dominates (insoluble limit). For the
$\alpha _S \ll \alpha _{diff}$, the interfacial diffusion dominates (insoluble limit). For the ![]() $\kappa _d = 500\,{\rm s}^{-1}$ water–dodecane and water–air systems, the bulk exchange is much faster than interfacial diffusion (
$\kappa _d = 500\,{\rm s}^{-1}$ water–dodecane and water–air systems, the bulk exchange is much faster than interfacial diffusion (![]() $\alpha _S \gg \alpha _{diff}$). The parameter
$\alpha _S \gg \alpha _{diff}$). The parameter ![]() $\alpha _S$ thereby governs the decrease of slip length for these systems. The values of
$\alpha _S$ thereby governs the decrease of slip length for these systems. The values of ![]() ${ {Da}}_\delta$ are
${ {Da}}_\delta$ are ![]() $97$ and
$97$ and ![]() $14$, respectively. As
$14$, respectively. As ![]() ${ {Da}}_\delta \gg 1$, the bulk exchange rate is limited by the diffusion of bulk surfactants, implying that
${ {Da}}_\delta \gg 1$, the bulk exchange rate is limited by the diffusion of bulk surfactants, implying that ![]() $\alpha _S$ depends on
$\alpha _S$ depends on ![]() $\kappa _d/\kappa _a$ (
$\kappa _d/\kappa _a$ (![]() ${ {Bi}}'/{ {Da}}_\delta$) instead of
${ {Bi}}'/{ {Da}}_\delta$) instead of ![]() $\kappa _d$ (
$\kappa _d$ (![]() ${ {Bi}}'$). For the water–dodecane system,
${ {Bi}}'$). For the water–dodecane system, ![]() $\kappa _d = 5\,{\rm s}^{-1}$ corresponds to
$\kappa _d = 5\,{\rm s}^{-1}$ corresponds to ![]() ${ {Da}}_\delta = 0.97$, below which the condition (5.16) becomes more strict as then
${ {Da}}_\delta = 0.97$, below which the condition (5.16) becomes more strict as then ![]() $\alpha _S$ becomes approximately proportional to
$\alpha _S$ becomes approximately proportional to ![]() $\kappa _d$.
$\kappa _d$.
Figure 7 illustrates how ![]() $\alpha _{diff}$ and
$\alpha _{diff}$ and ![]() $\alpha _S$ can be used to predict whether there will be a considerable decrease in slip length. These two parameters span a two-dimensional space. If
$\alpha _S$ can be used to predict whether there will be a considerable decrease in slip length. These two parameters span a two-dimensional space. If
the slip length has halved compared with surfactant-free interfaces (![]() $b/(b_{SHS}\beta _{LIS}) \approx 0.5$). In the region bounded by (5.20), the slip length decrease is larger and outside it is smaller. We use this limit to denote a significant slip length reduction, but other threshold values could also be used. As we only varied
$b/(b_{SHS}\beta _{LIS}) \approx 0.5$). In the region bounded by (5.20), the slip length decrease is larger and outside it is smaller. We use this limit to denote a significant slip length reduction, but other threshold values could also be used. As we only varied ![]() $c_0$,
$c_0$, ![]() $\alpha _S/\alpha _{diff}$ is constant, describing a straight line in the
$\alpha _S/\alpha _{diff}$ is constant, describing a straight line in the ![]() $(\alpha _{diff},\alpha _S)$ space, shown in figure 7 for the water–air and the
$(\alpha _{diff},\alpha _S)$ space, shown in figure 7 for the water–air and the ![]() $\kappa _d = 5\,{\rm s}^{-1}$ water–dodecane systems (cf. figure 5). The water–dodecane system has
$\kappa _d = 5\,{\rm s}^{-1}$ water–dodecane systems (cf. figure 5). The water–dodecane system has ![]() $\alpha _S$ and
$\alpha _S$ and ![]() $\alpha _{diff}$ of similar magnitude (
$\alpha _{diff}$ of similar magnitude (![]() $\alpha _S/\alpha _{diff} = 4.5$). Bulk exchange is more prominent than interfacial diffusion, but both are considerable. For the air–water system, bulk exchange dominates and the points are shifted towards the upper left corner of the figure.
$\alpha _S/\alpha _{diff} = 4.5$). Bulk exchange is more prominent than interfacial diffusion, but both are considerable. For the air–water system, bulk exchange dominates and the points are shifted towards the upper left corner of the figure.
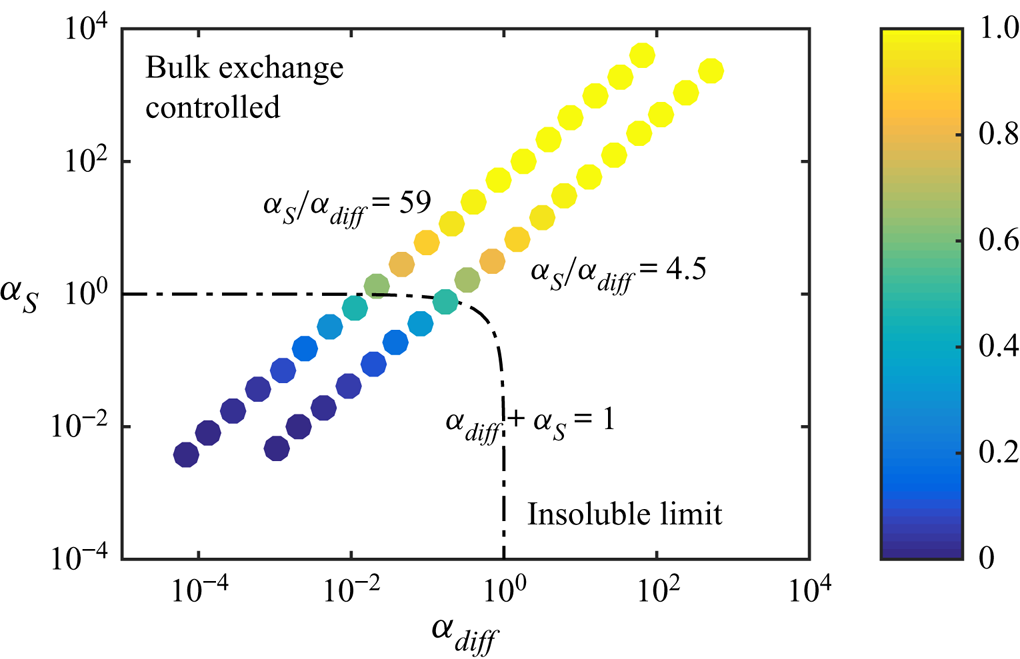
Figure 7. Normalised slip length ![]() $b/(b_{SHS}\beta _{LIS})$ of the SDS water–air and the SDS
$b/(b_{SHS}\beta _{LIS})$ of the SDS water–air and the SDS ![]() $\kappa _d = 5\,{\rm s}^{-1}$ water–dodecane systems (
$\kappa _d = 5\,{\rm s}^{-1}$ water–dodecane systems (![]() $\alpha _S/\alpha _{diff} = 59$ and
$\alpha _S/\alpha _{diff} = 59$ and ![]() $4.5$, respectively), plotted in the space spanned by
$4.5$, respectively), plotted in the space spanned by ![]() $\alpha _{diff}$ and
$\alpha _{diff}$ and ![]() $\alpha _S$. The dash-dotted line shows
$\alpha _S$. The dash-dotted line shows ![]() $\alpha _{diff} + \alpha _S = 1$.
$\alpha _{diff} + \alpha _S = 1$.
6. Adsorption and desorption taking into account alkane–surfactant interaction
The bulk exchange model presented in § 4 resulted in a more considerable surface tension decrease for the LIS than the SHS for the same SDS concentration. However, it cannot capture the initial decrease in surface tension at low concentrations appearing in water–alkane systems. To be able to describe this phenomenon, more advanced models are needed.
The water–alkane interface abnormalities must be caused by interactions between adsorbed surfactants and alkane molecules adjacent to the interface. The level of interaction between adsorbed surfactant and alkane molecules at the interface can be modelled by an effective alkane interface concentration ![]() $\varGamma ^a$ (Fainerman et al. Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019). It is associated with a molar area
$\varGamma ^a$ (Fainerman et al. Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019). It is associated with a molar area ![]() $\omega ^a \sim \omega$ and surface coverage
$\omega ^a \sim \omega$ and surface coverage ![]() $\theta ^a = \omega ^a\varGamma ^a$. This interaction has recently been investigated on molecular levels (Kartashynska et al. Reference Kartashynska, Lylyk, Aksenenko, Makievski, Vysotsky, Fainerman and Miller2020; Müller et al. Reference Müller, Bonthuis, Miller and Schneck2021). In this paper, we adopt a model consistent with the equilibrium model of Fainerman et al. (Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019). They assumed that the molar area
$\theta ^a = \omega ^a\varGamma ^a$. This interaction has recently been investigated on molecular levels (Kartashynska et al. Reference Kartashynska, Lylyk, Aksenenko, Makievski, Vysotsky, Fainerman and Miller2020; Müller et al. Reference Müller, Bonthuis, Miller and Schneck2021). In this paper, we adopt a model consistent with the equilibrium model of Fainerman et al. (Reference Fainerman, Aksenenko, Makievski, Nikolenko, Javadi, Schneck and Miller2019). They assumed that the molar area ![]() $\omega$ decreased with surface coverage. However, this decrease is only notable for higher concentrations (
$\omega$ decreased with surface coverage. However, this decrease is only notable for higher concentrations (![]() $\theta \sim 1$), and, therefore, we neglect this correction.
$\theta \sim 1$), and, therefore, we neglect this correction.
The interfacial and bulk surfactant concentrations are assumed to follow the same transport equations as the previous model ((2.4) and (2.5), respectively). However, the source term is (cf. (4.1))
where we have introduced the additional interaction constant ![]() $a^{as}$ and the total surface coverage
$a^{as}$ and the total surface coverage ![]() $\theta ^t = \theta + \theta ^a$. The adsorption term contains the total surface coverage, but the desorption term maintains its
$\theta ^t = \theta + \theta ^a$. The adsorption term contains the total surface coverage, but the desorption term maintains its ![]() $\theta$-dependency (together with an additional exponential factor).
$\theta$-dependency (together with an additional exponential factor).
We assume that the alkane molecules are in local equilibrium with the adsorbed surfactants, resulting in the corresponding expression
where ![]() $\kappa _a^a$ and
$\kappa _a^a$ and ![]() $\kappa _d^a$ are the adsorption and desorption coefficients of the alkane phase, respectively,
$\kappa _d^a$ are the adsorption and desorption coefficients of the alkane phase, respectively, ![]() $a^a$ is a constant and
$a^a$ is a constant and ![]() $c_0^a$ is the (constant) bulk alkane concentration. The current model assumes that the alkane adsorption coefficient depends on
$c_0^a$ is the (constant) bulk alkane concentration. The current model assumes that the alkane adsorption coefficient depends on ![]() $\theta$ by
$\theta$ by
where ![]() $\kappa _{a,0}^{a}$ and
$\kappa _{a,0}^{a}$ and ![]() $\kappa _{a,{max}}^a$ are constants. This expression implies that the alkane adsorption rate increases proportionally to
$\kappa _{a,{max}}^a$ are constants. This expression implies that the alkane adsorption rate increases proportionally to ![]() $\theta$ for minuscule concentrations. Without surfactants,
$\theta$ for minuscule concentrations. Without surfactants, ![]() $\theta ^a = 0$.
$\theta ^a = 0$.
The surface tension has been modelled by (cf. (4.2))
where
is the effective average molar area.
The results plotted in figure 5 show that this improved modelling lowers the critical concentration by an additional order of magnitude. Corresponding results for C![]() $_{12}$TAB and water–hexane LIS are shown in figure 8. We only used the more advanced adsorption/desorption model for C
$_{12}$TAB and water–hexane LIS are shown in figure 8. We only used the more advanced adsorption/desorption model for C![]() $_{12}$TAB (parameters given in table 1). This system manifests a stronger sensitivity to the surfactants, as
$_{12}$TAB (parameters given in table 1). This system manifests a stronger sensitivity to the surfactants, as ![]() $b/p$ decreases significantly at even lower concentrations. The simulations are illustrated in the
$b/p$ decreases significantly at even lower concentrations. The simulations are illustrated in the ![]() $(\alpha _{diff},\alpha _S)$ space in figure 8(c). In contrast to SDS (figure 7), C
$(\alpha _{diff},\alpha _S)$ space in figure 8(c). In contrast to SDS (figure 7), C![]() $_{12}$TAB have more prominent interfacial diffusion than bulk exchange (
$_{12}$TAB have more prominent interfacial diffusion than bulk exchange (![]() $\alpha _S/\alpha _{diff} = 0.30$). Therefore, the simulations are closer to the bottom right corner of the figure.
$\alpha _S/\alpha _{diff} = 0.30$). Therefore, the simulations are closer to the bottom right corner of the figure.
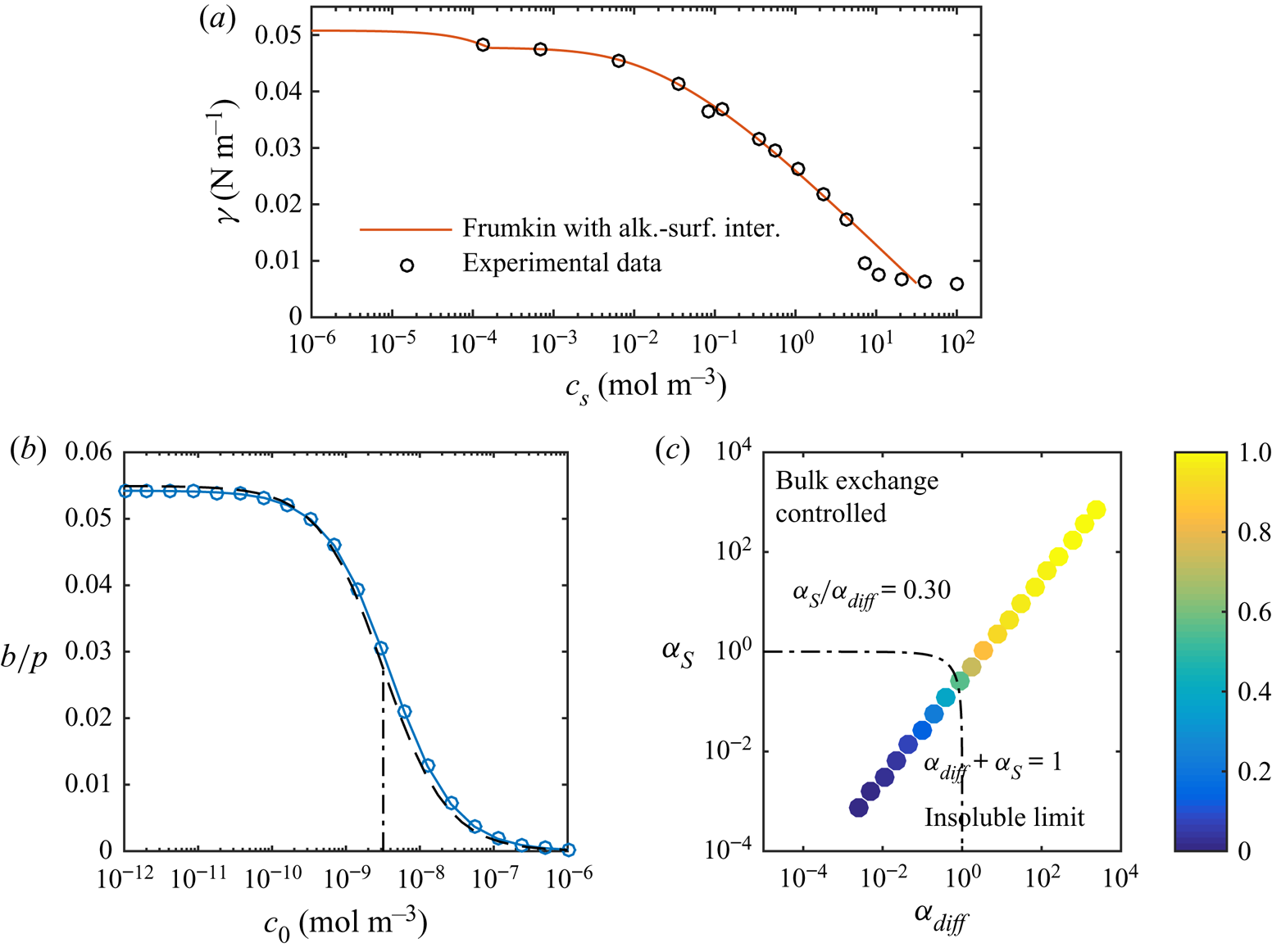
Figure 8. (a) Equilibrium surface tension of C![]() $_{12}$TAB at a water–hexane interface with experimental data from Pradines et al. (Reference Pradines, Fainerman, Aksenenko, Krägel, Mucic and Miller2010). Without surfactants, the surface tension is
$_{12}$TAB at a water–hexane interface with experimental data from Pradines et al. (Reference Pradines, Fainerman, Aksenenko, Krägel, Mucic and Miller2010). Without surfactants, the surface tension is ![]() $\gamma _c = 50.8\,{\rm mN}\,{\rm m}^{-1}$ for water–hexane (
$\gamma _c = 50.8\,{\rm mN}\,{\rm m}^{-1}$ for water–hexane (![]() $20\,^{\circ }{\rm C}$, Zeppieri et al. Reference Zeppieri, Rodríguez and López de Ramos2001). (b) Slip lengths at
$20\,^{\circ }{\rm C}$, Zeppieri et al. Reference Zeppieri, Rodríguez and López de Ramos2001). (b) Slip lengths at ![]() $\tau _\infty = 0.33$ mPa for different bulk concentrations of C
$\tau _\infty = 0.33$ mPa for different bulk concentrations of C![]() $_{12}$TAB at a water–hexane interface, together with the analytical model (dashed lines). The correspondence for SDS is shown in figure 5(a). (c) Normalised slip length,
$_{12}$TAB at a water–hexane interface, together with the analytical model (dashed lines). The correspondence for SDS is shown in figure 5(a). (c) Normalised slip length, ![]() $b/(b_{SHS}\beta _{LIS})$, plotted in the parameter space of
$b/(b_{SHS}\beta _{LIS})$, plotted in the parameter space of ![]() $\alpha _{diff}$ and
$\alpha _{diff}$ and ![]() $\alpha _S$. In both (b,c), the dashed-dotted lines are
$\alpha _S$. In both (b,c), the dashed-dotted lines are ![]() $\alpha _{diff} + \alpha _S = 1$, corresponding to
$\alpha _{diff} + \alpha _S = 1$, corresponding to ![]() $b/(b_{SHS}\beta _{LIS}) \approx 1/2$ (5.16).
$b/(b_{SHS}\beta _{LIS}) \approx 1/2$ (5.16).
6.1. Analytical model with alkane–surfactant interaction
Even if the source term has been changed slightly, the analytical model developed in § 5 is essentially the same. The linear approximation of (6.1) is equal to (5.2). With the surfactant transport equations unchanged, the bulk exchange and the interfacial transport predictions do not need to be modified (§§ 5.1 and 5.2, respectively).
By linearising the alkane source term (6.2), an estimation of ![]() $\theta ^a$ can be found. We introduce the non-dimensional alkane concentrations
$\theta ^a$ can be found. We introduce the non-dimensional alkane concentrations ![]() $c_{k,0}^a = c_0^a\kappa _0^a/\kappa _{d}^a$ and
$c_{k,0}^a = c_0^a\kappa _0^a/\kappa _{d}^a$ and ![]() $c_{k,{max}}^a = c_0^a\kappa _{a,{max}}^a/\kappa _{d}^a$. For large concentrations,
$c_{k,{max}}^a = c_0^a\kappa _{a,{max}}^a/\kappa _{d}^a$. For large concentrations, ![]() $\theta ^a \approx c_{k,{max}}^a$, whereas for small concentrations,
$\theta ^a \approx c_{k,{max}}^a$, whereas for small concentrations, ![]() $\theta ^a \approx c_{k,0}^a\theta \approx c_{k,0}^a c_k$ (6.3). Capturing both cases,
$\theta ^a \approx c_{k,0}^a\theta \approx c_{k,0}^a c_k$ (6.3). Capturing both cases,
All simulations have resulted in values in the lower concentration interval (![]() $c_{k,0}^a \theta \le c_{k,{max}}^a$). It follows that
$c_{k,0}^a \theta \le c_{k,{max}}^a$). It follows that ![]() $\Delta \theta \approx \Delta \theta ^a/c_{k,0}^a$.
$\Delta \theta \approx \Delta \theta ^a/c_{k,0}^a$.
We use the linear approximation of the surface tension (6.4) to estimate the Marangoni stresses. With ![]() $c_{k,0}^a > 1$ (table 1),
$c_{k,0}^a > 1$ (table 1), ![]() $\theta ^a$ is assumed to be larger than
$\theta ^a$ is assumed to be larger than ![]() $\theta$ at low concentrations (6.6). With
$\theta$ at low concentrations (6.6). With ![]() $\omega _0 \approx \omega ^a$,
$\omega _0 \approx \omega ^a$,
where ![]() ${ {Ma}}^a = RT/(\omega ^a \mu _\infty U)$ (cf. (5.12)). Analogous to (5.13),
${ {Ma}}^a = RT/(\omega ^a \mu _\infty U)$ (cf. (5.12)). Analogous to (5.13), ![]() $\mathrm {d} \theta ^a/\mathrm {d}\kern 0.06em x \approx 2\Delta \theta ^a/w \approx 2c_{k,0}^a\Delta \theta /w$, giving
$\mathrm {d} \theta ^a/\mathrm {d}\kern 0.06em x \approx 2\Delta \theta ^a/w \approx 2c_{k,0}^a\Delta \theta /w$, giving
We now adapt the estimation of the slip length (5.16). Equations (3.5), (5.10) and (6.8) give an expression for ![]() $\Delta \theta$ corresponding to (5.15). The slip length is found from (3.2) and is identical to (5.16) if
$\Delta \theta$ corresponding to (5.15). The slip length is found from (3.2) and is identical to (5.16) if ![]() ${ {Ma}}$ is replaced by
${ {Ma}}$ is replaced by ![]() ${ {Ma}}^ac_{k,0}^a$, equivalent to redefining
${ {Ma}}^ac_{k,0}^a$, equivalent to redefining
The results from this analytical model are also shown in figures 5 and 8(b), in good agreement with the simulation results.
The sudden change in the gradient of ![]() $\gamma$ in figures 4 and 8(a) (
$\gamma$ in figures 4 and 8(a) (![]() $c_s = 1.1\times 10^{-2}$ and
$c_s = 1.1\times 10^{-2}$ and ![]() $1.6\times 10^{-4}\,{\rm mol}\,{\rm m}^{-3}$, respectively) coincide with switching the
$1.6\times 10^{-4}\,{\rm mol}\,{\rm m}^{-3}$, respectively) coincide with switching the ![]() $\theta$-dependency of
$\theta$-dependency of ![]() $\kappa _a^a$ in (6.3). However, the simulations are unaffected because they are performed at lower concentrations. In this interval,
$\kappa _a^a$ in (6.3). However, the simulations are unaffected because they are performed at lower concentrations. In this interval, ![]() $\partial \gamma /\partial \theta ^a \approx -RT/\omega ^a$ (6.7), which is independent of concentration. As
$\partial \gamma /\partial \theta ^a \approx -RT/\omega ^a$ (6.7), which is independent of concentration. As ![]() $\theta ^a$ depends linearly on
$\theta ^a$ depends linearly on ![]() $\theta$ (6.6), the total derivative with respect to
$\theta$ (6.6), the total derivative with respect to ![]() $\theta$ is constant, and with respect to
$\theta$ is constant, and with respect to ![]() $c_0$ in equilibrium because
$c_0$ in equilibrium because ![]() $\theta \approx c_k = \kappa _ac_0/\kappa _d$ (5.3). This concentration interval has no experimental data to validate the surface tension curve. A linear decrease in surface tension with concentration (i.e. constant derivative) is a reasonable first assumption.
$\theta \approx c_k = \kappa _ac_0/\kappa _d$ (5.3). This concentration interval has no experimental data to validate the surface tension curve. A linear decrease in surface tension with concentration (i.e. constant derivative) is a reasonable first assumption.
Another problem of the current adsorption/desorption model is that because ![]() $c_{k,0}^a$ usually is large, the ratio between
$c_{k,0}^a$ usually is large, the ratio between ![]() $\varGamma$ and
$\varGamma$ and ![]() $\varGamma ^a$ becomes unreasonably large at low concentrations, considering the number of alkane molecules that can interact with one surfactant molecule. A more realistic result is achieved by letting the surfactant molecules adsorb in two different states, described by the so-called reorientation model (Kartashynska et al. Reference Kartashynska, Lylyk, Aksenenko, Makievski, Vysotsky, Fainerman and Miller2020). We welcome future studies using more advanced adsorption/desorption models. However, for this investigation, we consider the current model sufficient.
$\varGamma ^a$ becomes unreasonably large at low concentrations, considering the number of alkane molecules that can interact with one surfactant molecule. A more realistic result is achieved by letting the surfactant molecules adsorb in two different states, described by the so-called reorientation model (Kartashynska et al. Reference Kartashynska, Lylyk, Aksenenko, Makievski, Vysotsky, Fainerman and Miller2020). We welcome future studies using more advanced adsorption/desorption models. However, for this investigation, we consider the current model sufficient.
7. SC regime
High shear stresses can result in a portion of the interface with almost no adsorbed surfactants. In such conditions, the interface is no longer in the uniformly retarded regime: an underlying assumption of the analytical model. The downstream part of the interface with surfactants becomes a SC in analogy to bubbles (Palaparthi et al. Reference Palaparthi, Papageorgiou and Maldarelli2006). The interface can be said to be in a partial SC regime. A slip length is partly regained because the flow is not decelerated outside the stagnant part. If the concentration of bulk surfactants cannot be reduced further, a partial SC regime can therefore be desirable to achieve. The partial SC regime was investigated by Baier & Hardt (Reference Baier and Hardt2021) for insoluble surfactants on SHS in the limit of large ![]() ${ {Pe}}_s'$. For LIS, the SC can improve the retention of the oil in the groove, but on the other hand, the stagnant part of the interface does not contribute to drag reduction (Fu et al. Reference Fu, Chen, Arnold and Hultmark2019).
${ {Pe}}_s'$. For LIS, the SC can improve the retention of the oil in the groove, but on the other hand, the stagnant part of the interface does not contribute to drag reduction (Fu et al. Reference Fu, Chen, Arnold and Hultmark2019).
This section shows that a SC eventually grows to cover the whole interface if the bulk concentration increases. The interface is then in the so-called full SC regime, again undesirable for drag reduction. There is no apparent difference in the distribution of ![]() $\theta$ between the full SC and the uniformly retarded regime, so distinguishing between them is a mere formality.
$\theta$ between the full SC and the uniformly retarded regime, so distinguishing between them is a mere formality.
To form a SC, we increased the imposed shear stress to ![]() $\tau _\infty = 0.33$ Pa (factor of 1000) by increasing the applied velocity gradient and thereby
$\tau _\infty = 0.33$ Pa (factor of 1000) by increasing the applied velocity gradient and thereby ![]() $U$. Relevant affected non-dimensional parameters are
$U$. Relevant affected non-dimensional parameters are ![]() ${ {Pe}}$,
${ {Pe}}$, ![]() ${ {Pe}}_s'$,
${ {Pe}}_s'$, ![]() ${ {Bi}}'$ and
${ {Bi}}'$ and ![]() ${ {Ma}}$, of which the first two increase linearly with
${ {Ma}}$, of which the first two increase linearly with ![]() $U$ and the latter decrease linearly (table 2). The increase in
$U$ and the latter decrease linearly (table 2). The increase in ![]() ${ {Pe}}$ makes the concentration boundary layer thinner (5.7). Interestingly,
${ {Pe}}$ makes the concentration boundary layer thinner (5.7). Interestingly, ![]() $\alpha _{diff}$ and
$\alpha _{diff}$ and ![]() $\alpha _S$ are independent of
$\alpha _S$ are independent of ![]() $U$ in the Stokes regime, except through
$U$ in the Stokes regime, except through ![]() $\delta$. The Reynolds number is
$\delta$. The Reynolds number is ![]() ${ {Re}} = \rho _\infty Uw/\mu _\infty = 3.3 \sim 1$ (with density
${ {Re}} = \rho _\infty Uw/\mu _\infty = 3.3 \sim 1$ (with density ![]() $\rho _\infty = 1000\,{\rm kg}\,{\rm m}^{-3}$ for water), so the Stokes equations are still valid to some extent, especially considering the lower velocity close to the interfaces.
$\rho _\infty = 1000\,{\rm kg}\,{\rm m}^{-3}$ for water), so the Stokes equations are still valid to some extent, especially considering the lower velocity close to the interfaces.
A partial SC requires that the advection rate of interfacial surfactants overcomes the interfacial diffusion and bulk exchange rates of the linear surfactant distribution. The non-dimensional numbers comparing these transport mechanisms are reiterated here for convenience, (cf. (5.18) and (5.19))
The quantitative limits of the SC formation can be understood from (5.15). This equation can be reformulated as
A linear interfacial surfactant distribution implies ![]() $\Delta \theta /c_k \le 1$ because we cannot have negative values of
$\Delta \theta /c_k \le 1$ because we cannot have negative values of ![]() $\theta$ (figure 6). Therefore, (7.4) cannot hold for large
$\theta$ (figure 6). Therefore, (7.4) cannot hold for large ![]() $\alpha _{diff}+ \alpha _S$ if
$\alpha _{diff}+ \alpha _S$ if
Instead, the surfactants are advected to the end stagnation point of the interface, where a larger gradient is created. The interface is then in the partial SC regime. The same limitation exists for ![]() $\Delta c_s/c_0$. However, from (5.6), we know that
$\Delta c_s/c_0$. However, from (5.6), we know that ![]() $\Delta c_s/c_0 \lesssim \Delta \theta /c_k$, so it does not provide any other peculiarities. If
$\Delta c_s/c_0 \lesssim \Delta \theta /c_k$, so it does not provide any other peculiarities. If ![]() $\alpha _{diff} + \alpha _S \ll 1$, (7.4) would again allow for a linear surfactant profile. Such interface is in the full SC regime. We suggest using (7.5) to distinguish between (i) the uniformly retarded regime (
$\alpha _{diff} + \alpha _S \ll 1$, (7.4) would again allow for a linear surfactant profile. Such interface is in the full SC regime. We suggest using (7.5) to distinguish between (i) the uniformly retarded regime (![]() $C_{SC} > 1$) and (ii) the partial and full SC regimes (
$C_{SC} > 1$) and (ii) the partial and full SC regimes (![]() $C_{SC} < 1$).
$C_{SC} < 1$).
Assuming that (7.5) is fulfilled, and considering the maximum value ![]() $\Delta \theta /c_k = 1$, we get the limit for
$\Delta \theta /c_k = 1$, we get the limit for ![]() $\alpha _{diff}+ \alpha _S$ above which no linear solution can exist as
$\alpha _{diff}+ \alpha _S$ above which no linear solution can exist as
Equation (7.6) is the lower limit for the partial SC regime, below which we enter the full SC regime.
We illustrate the limit (7.6) for SDS (advanced model) at ![]() $\tau _\infty = 0.33$ Pa in figure 9. These simulations were performed with a more refined grid (Appendix A). The region where linear solutions cannot exist is shown in grey. For the current parameters (table 2),
$\tau _\infty = 0.33$ Pa in figure 9. These simulations were performed with a more refined grid (Appendix A). The region where linear solutions cannot exist is shown in grey. For the current parameters (table 2),
In the grey region, the analytical model loses validity as expected. Equation (7.6) also holds for the simulation results of Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020), which are shown in Appendix D. Therefore, the transition between the partial and full SC regime appears consistent over a wide range of geometrical and surfactant parameters.
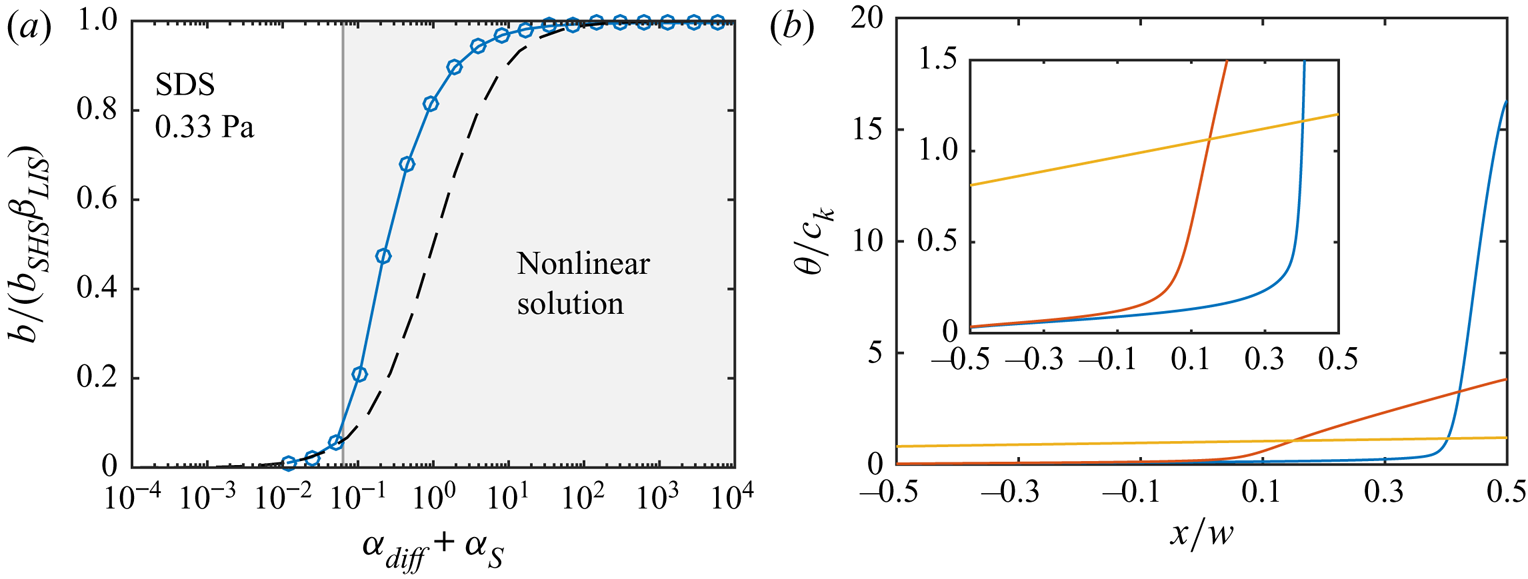
Figure 9. (a) The analytical model (dashed line) compared with simulations of SDS at ![]() $\tau _\infty = 0.33$ Pa. The limit (7.6) forms the boundary of the grey region, in which linear distributions of
$\tau _\infty = 0.33$ Pa. The limit (7.6) forms the boundary of the grey region, in which linear distributions of ![]() $\theta$ cannot exist. (b) Three distributions of
$\theta$ cannot exist. (b) Three distributions of ![]() $\theta /c_k$, going from a nonlinear (blue) to an intermediate (red) and a linear (yellow) for the smallest value of
$\theta /c_k$, going from a nonlinear (blue) to an intermediate (red) and a linear (yellow) for the smallest value of ![]() $\alpha _{diff} + \alpha _S$. The inset is identical, apart from a lower maximum vertical axis limit.
$\alpha _{diff} + \alpha _S$. The inset is identical, apart from a lower maximum vertical axis limit.
The limit for the bulk surfactant concentration corresponding to (7.6) is attained by using the definitions of ![]() $\alpha _{diff}$ and
$\alpha _{diff}$ and ![]() $\alpha _S$ ((5.17) or (6.9a,b)). The expression for the non-dimensional concentration is
$\alpha _S$ ((5.17) or (6.9a,b)). The expression for the non-dimensional concentration is
for the regular and the more advanced Frumkin model, respectively, where ![]() $c_k^a = c_{k,0}^a c_k$. No partial SC can exist above these concentrations. As
$c_k^a = c_{k,0}^a c_k$. No partial SC can exist above these concentrations. As ![]() $C_{SC}$ decreases,
$C_{SC}$ decreases, ![]() $c_k$ or
$c_k$ or ![]() $c_k^a$ increases towards a maximum value determined by the Marangoni number. The current parameters (7.7) correspond to
$c_k^a$ increases towards a maximum value determined by the Marangoni number. The current parameters (7.7) correspond to ![]() $94\,\%$ of the maximum concentration. The only flow-dependent quantity of the maximum concentration is
$94\,\%$ of the maximum concentration. The only flow-dependent quantity of the maximum concentration is ![]() $U$, which is determined by the imposed shear stress and the width of the grooves. Apart from
$U$, which is determined by the imposed shear stress and the width of the grooves. Apart from ![]() $T$, the other quantities depend only on the liquids and the surfactants.
$T$, the other quantities depend only on the liquids and the surfactants.
With the increase of slip length in the partial SC regime, a new condition for significant slip length reduction is needed in place of (5.20). Equation (7.6) can replace (5.20) if smaller than ![]() $1$ (this occurs if
$1$ (this occurs if ![]() $C_{SC} < 1/2$) because (5.20) remains valid in the full SC regime. However, it should be noted that (7.6) then gives
$C_{SC} < 1/2$) because (5.20) remains valid in the full SC regime. However, it should be noted that (7.6) then gives ![]() $b/b_{SHS}\beta _{LIS} < 0.5$ as predicted by the analytical model becoming valid at this
$b/b_{SHS}\beta _{LIS} < 0.5$ as predicted by the analytical model becoming valid at this ![]() $\alpha _{diff} + \alpha _S$. Still, we do think this is an appropriate condition. We summarise these limits by concluding that a significant slip length reduction occurs if
$\alpha _{diff} + \alpha _S$. Still, we do think this is an appropriate condition. We summarise these limits by concluding that a significant slip length reduction occurs if
 \begin{equation} \alpha_{diff} + \alpha_S < \begin{cases} 1, & \text{if } C_{SC} \ge 1/2, \\ \dfrac{1}{1 - C_{SC}} - 1, & \text{if } C_{SC} < 1/2. \end{cases} \end{equation}
\begin{equation} \alpha_{diff} + \alpha_S < \begin{cases} 1, & \text{if } C_{SC} \ge 1/2, \\ \dfrac{1}{1 - C_{SC}} - 1, & \text{if } C_{SC} < 1/2. \end{cases} \end{equation} The equations provided in this section are intended to serve as convenient tools to improve the understanding and classification of LIS with surfactants subjected to high shear stresses. We have not attempted to model the partial SC regime, which is a potential topic for future studies. However, it can be noted that, even in the partial SC regime, the analytical model can indicate the order of magnitude of the slip length. Uncertainties in parameters and adsorption models might be a larger concern; the difference between the analytical prediction and the simulation results of figure 9(a) can be compared with the deviations in slip length in figure 5. The error of the analytical model can, in particular, be expected to be minor if ![]() $C_{SC} \sim 1$, implying that the two conditions for significant slip length reduction ((5.20) and (7.9)) are similar.
$C_{SC} \sim 1$, implying that the two conditions for significant slip length reduction ((5.20) and (7.9)) are similar.
8. Remarks
The analytical model has been developed for two-dimensional transverse grooves. However, similar surfactant transport processes are expected to be present for LIS with three-dimensional longitudinal grooves (Landel et al. Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020; Temprano-Coleto et al. Reference Temprano-Coleto, Smith, Peaudecerf, Landel, Gibou and Luzzatto- Fegiz2021). Therefore, the analytical model is expected to provide an indication of the effects of surfactants on the slip length also for such configurations, commonly used in experimental LIS studies (Jacobi et al. Reference Jacobi, Wexler and Stone2015; Wexler et al. Reference Wexler, Jacobi and Stone2015; Fu et al. Reference Fu, Chen, Arnold and Hultmark2019).
If the grooves are longer than those used here, the slip length tends to be larger. When disregarding changes in aspect ratio or solid fraction, ![]() $b_{SHS}\beta _{LIS}$ and
$b_{SHS}\beta _{LIS}$ and ![]() $u_{s,{SHS}}^0\beta _{LIS}$ increase proportional to
$u_{s,{SHS}}^0\beta _{LIS}$ increase proportional to ![]() $w$ (as given by (3.3) and (3.6)). The diffusion rate is suppressed by increasing the groove width. Thereby,
$w$ (as given by (3.3) and (3.6)). The diffusion rate is suppressed by increasing the groove width. Thereby, ![]() ${ {Pe}}_s'$ and
${ {Pe}}_s'$ and ![]() ${ {Da}}_\delta$ increase with
${ {Da}}_\delta$ increase with ![]() $w$ (even if the
$w$ (even if the ![]() $w$-dependency of
$w$-dependency of ![]() $\delta$ in (5.7) is non-trivial), whereas
$\delta$ in (5.7) is non-trivial), whereas ![]() ${ {Bi}}'$ is constant. Correspondingly, an increased groove width decreases
${ {Bi}}'$ is constant. Correspondingly, an increased groove width decreases ![]() $C_{SC}$ (7.5); increasing the groove width may make the interfaces enter the SC regime. As
$C_{SC}$ (7.5); increasing the groove width may make the interfaces enter the SC regime. As ![]() ${ {Ma}}$ and
${ {Ma}}$ and ![]() ${ {Ma}}^a$ are inversely proportional to
${ {Ma}}^a$ are inversely proportional to ![]() $w$,
$w$, ![]() $\alpha _{diff}$ decreases with
$\alpha _{diff}$ decreases with ![]() $w$, whereas
$w$, whereas ![]() $\alpha _S$ increases ((5.17) or (6.9a,b)). Therefore, with
$\alpha _S$ increases ((5.17) or (6.9a,b)). Therefore, with ![]() $C_{SC}$ decreasing and
$C_{SC}$ decreasing and ![]() $\alpha _S$ eventually dominating, the slip length becomes less sensitive to surfactants with increasing groove width, according to (7.9).
$\alpha _S$ eventually dominating, the slip length becomes less sensitive to surfactants with increasing groove width, according to (7.9).
For turbulent flows, the grooves would need to be larger or the imposed shear stress higher to generate a relevant drag reduction, even without surfactants. For ![]() $\tau _\infty = 0.33$ mPa and
$\tau _\infty = 0.33$ mPa and ![]() $0.33$ Pa, the viscous length scales correspond to
$0.33$ Pa, the viscous length scales correspond to ![]() $l_\nu = \mu _\infty /\sqrt {\rho _\infty \tau _\infty } = 1.7$ mm and
$l_\nu = \mu _\infty /\sqrt {\rho _\infty \tau _\infty } = 1.7$ mm and ![]() $55\,\mathrm {\mu }{\rm m}$, respectively. Assuming
$55\,\mathrm {\mu }{\rm m}$, respectively. Assuming ![]() $b/p \approx 0.02$ also for the turbulent flows (figure 5),
$b/p \approx 0.02$ also for the turbulent flows (figure 5), ![]() $b^+ = b/l_\nu \approx 1.7\times 10^{-3}$ and
$b^+ = b/l_\nu \approx 1.7\times 10^{-3}$ and ![]() $5.4\times 10^{-2}$, but these non-dimensional slip lengths would need to be
$5.4\times 10^{-2}$, but these non-dimensional slip lengths would need to be ![]() $b^+ \gtrsim 1$ to give meaningful drag reduction (Fu et al. Reference Fu, Arenas, Leonardi and Hultmark2017).
$b^+ \gtrsim 1$ to give meaningful drag reduction (Fu et al. Reference Fu, Arenas, Leonardi and Hultmark2017).
Some surfactants exhibit a critical micelle concentration (CMC), above which they can form micelles (clusters of surfactant molecules) instead of adsorbing at interfaces. Hence, the source term dependencies on ![]() $c_s$ ((4.1) and (6.1)) are invalid above the CMC. The CMC is
$c_s$ ((4.1) and (6.1)) are invalid above the CMC. The CMC is ![]() $8.2\,{\rm mol}\,{\rm m}^{-3}$ for SDS, above which the surface tension remains somewhat constant (Elworthy & Mysels Reference Elworthy and Mysels1966, cf. figure 4). C
$8.2\,{\rm mol}\,{\rm m}^{-3}$ for SDS, above which the surface tension remains somewhat constant (Elworthy & Mysels Reference Elworthy and Mysels1966, cf. figure 4). C![]() $_{12}$TAB holds a similar CMC (Klevens Reference Klevens1948, cf. figure 8a). The slip length reductions studied here occur at lower concentrations (figures 5a and 8b). Therefore, micelle formation does not affect our conclusions. Micelles can also act as monomer buffers (surfactants not part of micelles), dissociating to reduce monomer concentration gradients. They thereby give rise to a remobilisation effect where interface gradients are reduced (Manikantan & Squires Reference Manikantan and Squires2020).
$_{12}$TAB holds a similar CMC (Klevens Reference Klevens1948, cf. figure 8a). The slip length reductions studied here occur at lower concentrations (figures 5a and 8b). Therefore, micelle formation does not affect our conclusions. Micelles can also act as monomer buffers (surfactants not part of micelles), dissociating to reduce monomer concentration gradients. They thereby give rise to a remobilisation effect where interface gradients are reduced (Manikantan & Squires Reference Manikantan and Squires2020).
For ![]() $\theta \sim c_k \gg 1$, the analytical model could be inaccurate because nonlinearities in the Frumkin source term then become relevant. As discussed by Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020), such non-dimensional concentrations could appear below the CMC for ‘strong’ surfactants (
$\theta \sim c_k \gg 1$, the analytical model could be inaccurate because nonlinearities in the Frumkin source term then become relevant. As discussed by Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020), such non-dimensional concentrations could appear below the CMC for ‘strong’ surfactants (![]() $\kappa _d/\kappa _a$ much lower than the CMC, cf. table 1). However, they performed some simulations with
$\kappa _d/\kappa _a$ much lower than the CMC, cf. table 1). However, they performed some simulations with ![]() $c_k \gg 1$, which roughly agreed with the model (Appendix D). Peculiarities that might arise at high concentrations (e.g. for
$c_k \gg 1$, which roughly agreed with the model (Appendix D). Peculiarities that might arise at high concentrations (e.g. for ![]() $a \neq 0$) are out of the scope of the current investigation.
$a \neq 0$) are out of the scope of the current investigation.
We have isolated the effects of Marangoni stresses and viscous stresses imposed by the infusing liquids. However, surfactants might also cause additional rheological stresses at the interface, characterised by the (intrinsic) surface shear and dilatational viscosities (Manikantan & Squires Reference Manikantan and Squires2020). However, these surface viscosities are challenging to measure. Careful measurements have given a reliable upper limit of the surface shear viscosity for surfactants on water–air interfaces (Zell et al. Reference Zell, Nowbahar, Mansard, Leal, Deshmukh, Mecca, Tucker and Squires2014). If it is assumed that this upper limit holds for both surface viscosities, these extra stresses are irrelevant compared with the Marangoni stress at groove dimensions ![]() $w \gg 10\,\mathrm {\mu }{\rm m}$ (Landel et al. Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020). They can then be neglected for the geometries considered here.
$w \gg 10\,\mathrm {\mu }{\rm m}$ (Landel et al. Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020). They can then be neglected for the geometries considered here.
Finally, a possible extension of the current work would be to model surfactant solubility also in the oil phase. Such a model requires a second set of surfactant adsorption and desorption coefficients, describing surfactant exchange in the oil.
9. Conclusions
If surfactants are present in a flow over SHS or LIS, they might severely decrease the slip lengths of these surfaces. The surfactants adsorb at the fluid interfaces and are advected towards the downstream stagnation points, resulting in surface tension gradients that oppose the flow. Surface tension gradients are known as Marangoni stresses. Using numerical simulations of laminar flow over LIS with transverse grooves, we have explored how the effective slip length changes when the flow has a non-zero concentration of the commonly used surfactants SDS and C![]() $_{12}$TAB. The external fluid was water, and the infusing liquid an alkane. The surfactants have been assumed to be soluble in the water but insoluble in the alkane.
$_{12}$TAB. The external fluid was water, and the infusing liquid an alkane. The surfactants have been assumed to be soluble in the water but insoluble in the alkane.
For low applied shear stresses (![]() $\tau _\infty = 0.33$ mPa), the distribution of surfactants on an interface is approximately linear. It is then possible to construct an analytical theory for the LIS slip length, similar to what has been done for SHS (Landel et al. Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020). In the numerical simulations, we used classical Frumkin kinetics and a more advanced Frumkin model that considers interactions between the surfactants and the alkane molecules. Both models can be described by the (properly adjusted) analytical theory. The predicted slip length is given by (5.16), with parameters
$\tau _\infty = 0.33$ mPa), the distribution of surfactants on an interface is approximately linear. It is then possible to construct an analytical theory for the LIS slip length, similar to what has been done for SHS (Landel et al. Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020). In the numerical simulations, we used classical Frumkin kinetics and a more advanced Frumkin model that considers interactions between the surfactants and the alkane molecules. Both models can be described by the (properly adjusted) analytical theory. The predicted slip length is given by (5.16), with parameters ![]() $\alpha _{diff}$ and
$\alpha _{diff}$ and ![]() $\alpha _S$ specified by (5.17) or (6.9a,b), depending on the model.
$\alpha _S$ specified by (5.17) or (6.9a,b), depending on the model.
The interfacial surfactants might be swept towards the downstream stagnation point for large applied shear stresses (![]() $\tau _\infty = 0.33$ Pa). The interfaces are then in the partial SC regime. Some slip length is regained, as a large part of an interface does not have a significant surface tension gradient. However, this regime can only exist below a particular bulk concentration. Above this concentration, the surfactant distribution is linear, and the analytical model becomes accurate. The concentration is given by (7.8), with
$\tau _\infty = 0.33$ Pa). The interfaces are then in the partial SC regime. Some slip length is regained, as a large part of an interface does not have a significant surface tension gradient. However, this regime can only exist below a particular bulk concentration. Above this concentration, the surfactant distribution is linear, and the analytical model becomes accurate. The concentration is given by (7.8), with ![]() $1 - C_{SC}$ typically having a value close to unity.
$1 - C_{SC}$ typically having a value close to unity.
Surfactants in fluid systems are difficult to detect directly yet affect flows significantly; they act like hidden variables (Manikantan & Squires Reference Manikantan and Squires2020). The concentrations for which the slip is profoundly reduced for LIS and SHS can be assumed to correspond to those naturally occurring in experimental set-ups (Peaudecerf et al. Reference Peaudecerf, Landel, Goldstein and Luzzatto-Fegiz2017). Temprano-Coleto et al. (Reference Temprano-Coleto, Smith, Peaudecerf, Landel, Gibou and Luzzatto- Fegiz2021) estimated such naturally occurring surfactant concentrations to be ![]() $c_0 = 3\times 10^{-4}\,{\rm mol}\,{\rm m}^{-3}$ by comparing experimental results of SHS with polydimethylsiloxane (PDMS) textures to theoretical predictions (cf. figures 5 and 8b). It is the hope that the models presented here can be used to interpret experimental results of LIS both with and without artificially added surfactants. They can also aid the design of grooves to avoid severe performance degradation. For example, LIS can be designed to remain in the partial SC regime at similar concentrations. We also hope that by including infusing liquid viscosity in this model, we have taken a step towards three-dimensional models of LIS with textures more similar to those typically used in experimental set-ups.
$c_0 = 3\times 10^{-4}\,{\rm mol}\,{\rm m}^{-3}$ by comparing experimental results of SHS with polydimethylsiloxane (PDMS) textures to theoretical predictions (cf. figures 5 and 8b). It is the hope that the models presented here can be used to interpret experimental results of LIS both with and without artificially added surfactants. They can also aid the design of grooves to avoid severe performance degradation. For example, LIS can be designed to remain in the partial SC regime at similar concentrations. We also hope that by including infusing liquid viscosity in this model, we have taken a step towards three-dimensional models of LIS with textures more similar to those typically used in experimental set-ups.
Acknowledgements
We are thankful to Uǧis Lācis for his help in setting up the FreeFem++ framework.
Funding
This work was supported by SSF, the Swedish Foundation for Strategic Research (Future Leaders grant FFL15:0001).
Declaration of interests
The authors report no conflict of interest.
Appendix A. Grid convergence studies
The simulations of §§ 3, 4, and 6 were performed with a mesh spacing ![]() $w/N$ with
$w/N$ with ![]() ${N = 256}$ at the interface. The simulations of § 7 with the high shear stress
${N = 256}$ at the interface. The simulations of § 7 with the high shear stress ![]() $\tau _\infty = 0.33$ Pa were performed with
$\tau _\infty = 0.33$ Pa were performed with ![]() $N = 1024$. As
$N = 1024$. As ![]() $w = 100\,\mathrm {\mu }{\rm m}$,
$w = 100\,\mathrm {\mu }{\rm m}$, ![]() $N = 1024$ corresponds to a mesh spacing of
$N = 1024$ corresponds to a mesh spacing of ![]() $0.098\,\mathrm {\mu }{\rm m}$.
$0.098\,\mathrm {\mu }{\rm m}$.
In order to test the grid dependency of the simulations, three high shear stress simulations were repeated with ![]() $N = 256$. These cases included a highly skewed
$N = 256$. These cases included a highly skewed ![]() $\theta$ distribution (
$\theta$ distribution (![]() $c_0 = 10^{-10}\,{\rm mol}\,{\rm m}^{-3}$), a linear distribution (
$c_0 = 10^{-10}\,{\rm mol}\,{\rm m}^{-3}$), a linear distribution (![]() $c_0 = 10^{-4}\,{\rm mol}\,{\rm m}^{-3}$) and an intermediate case (
$c_0 = 10^{-4}\,{\rm mol}\,{\rm m}^{-3}$) and an intermediate case (![]() $c_0 = 5.5\times 10^{-6}\,{\rm mol}\,{\rm m}^{-3}$). Statistics are shown in figure 10. Overall, the results from the two grids agree well. The largest differences are found for the highly skewed distribution in
$c_0 = 5.5\times 10^{-6}\,{\rm mol}\,{\rm m}^{-3}$). Statistics are shown in figure 10. Overall, the results from the two grids agree well. The largest differences are found for the highly skewed distribution in ![]() $c_s$ (bulk surfactant concentration at the interface) and the peak of
$c_s$ (bulk surfactant concentration at the interface) and the peak of ![]() $\theta$, figures 10(e) and 10( f), respectively.
$\theta$, figures 10(e) and 10( f), respectively.

Figure 10. Statistics of flow with SDS for two grids and three values of ![]() $c_0$ (
$c_0$ (![]() $\tau _\infty = 0.33$ Pa). The parameter
$\tau _\infty = 0.33$ Pa). The parameter ![]() $N$ gives the cell spacing at the interface by
$N$ gives the cell spacing at the interface by ![]() $w/N$. The figures illustrate: (a) mean velocity profiles with (b) an enlarged view around the interface, (c) root-mean-squared velocity fluctuations (
$w/N$. The figures illustrate: (a) mean velocity profiles with (b) an enlarged view around the interface, (c) root-mean-squared velocity fluctuations (![]() $u_{rms} = \sqrt {\left \langle (u - \left \langle u \right \rangle )^2\right \rangle }$ and
$u_{rms} = \sqrt {\left \langle (u - \left \langle u \right \rangle )^2\right \rangle }$ and ![]() $v_{rms} = \sqrt {\left \langle v ^2\right \rangle }$ in the streamwise and wall-normal directions, respectively), (d) interfacial velocity, (e) bulk surfactant concentration at the interface and ( f) interfacial surfactant concentration. Velocities are normalised by
$v_{rms} = \sqrt {\left \langle v ^2\right \rangle }$ in the streamwise and wall-normal directions, respectively), (d) interfacial velocity, (e) bulk surfactant concentration at the interface and ( f) interfacial surfactant concentration. Velocities are normalised by ![]() $U = w\tau _\infty /\mu _\infty$ and decrease in magnitude with increasing
$U = w\tau _\infty /\mu _\infty$ and decrease in magnitude with increasing ![]() $c_0$. The maximum values of
$c_0$. The maximum values of ![]() $c_s/c_0$ and
$c_s/c_0$ and ![]() $\theta /c_k$ decrease with increasing
$\theta /c_k$ decrease with increasing ![]() $c_0$. Insets show enlarged views of (e)
$c_0$. Insets show enlarged views of (e) ![]() $c_s/c_0$ for the highest
$c_s/c_0$ for the highest ![]() $c_0$ and ( f)
$c_0$ and ( f) ![]() $\theta /c_k$ for all three
$\theta /c_k$ for all three ![]() $c_0$.
$c_0$.
Owing to the larger differences for the highly skewed distribution, we performed simulations with this ![]() $c_0$ using additional refinements giving
$c_0$ using additional refinements giving ![]() $N = 512$,
$N = 512$, ![]() $2048$ and
$2048$ and ![]() $4096$. The resulting distributions of
$4096$. The resulting distributions of ![]() $c_s$ are shown in figure 11(a), and the values of
$c_s$ are shown in figure 11(a), and the values of ![]() $\left \langle c _s\right \rangle$ in figure 11(b). The grid with
$\left \langle c _s\right \rangle$ in figure 11(b). The grid with ![]() $N = 1024$ had a relative difference in
$N = 1024$ had a relative difference in ![]() $\left \langle c _s\right \rangle$ of
$\left \langle c _s\right \rangle$ of ![]() $1.7\,\%$ to the finest grid (
$1.7\,\%$ to the finest grid (![]() $N = 4096$). The corresponding difference in the peak of
$N = 4096$). The corresponding difference in the peak of ![]() $\theta$ was
$\theta$ was ![]() $2.5\,\%$. We consider these differences acceptable.
$2.5\,\%$. We consider these differences acceptable.
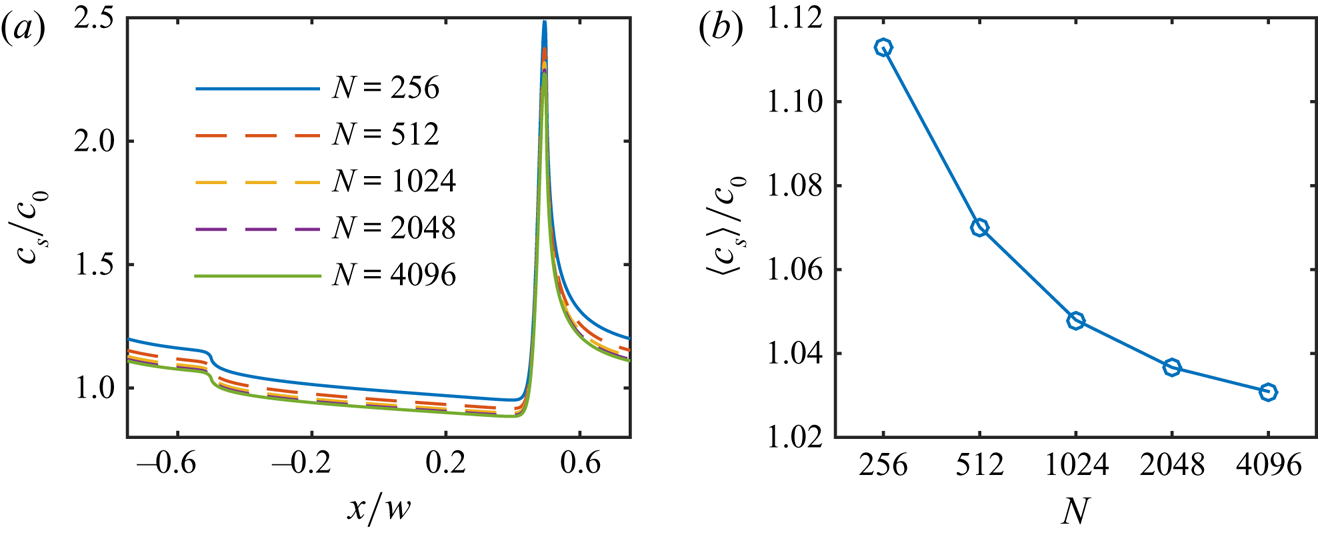
Figure 11. Grid convergence study for the lowest value of ![]() $c_0$, showing (a)
$c_0$, showing (a) ![]() $c_s/c_0$ and (b)
$c_s/c_0$ and (b) ![]() $\left \langle c _s\right \rangle /c_0$.
$\left \langle c _s\right \rangle /c_0$.
Appendix B. Analytical description of the flow
In the external flow (![]() $y > 0$), Stokes equations (2.1a,b) are equivalent to the biharmonic equation for the stream function
$y > 0$), Stokes equations (2.1a,b) are equivalent to the biharmonic equation for the stream function ![]() $\psi$,
$\psi$,
Owing to symmetry, we can limit the streamwise coordinate to ![]() $0 \le x \le p/2$. The streamwise velocity boundary condition is
$0 \le x \le p/2$. The streamwise velocity boundary condition is
and the shear-stress boundary conditions are
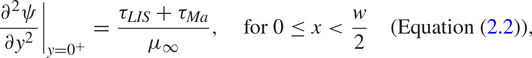 $$\begin{gather} \left.\frac{\partial^2 \psi}{\partial y^2}\right|_{y=0^+} = \frac{\tau_{LIS} + \tau_{{Ma}}}{\mu_\infty}, \quad \text{for} \ 0 \le x < \frac{w}{2} \quad \text{(Equation (2.2))}, \end{gather}$$
$$\begin{gather} \left.\frac{\partial^2 \psi}{\partial y^2}\right|_{y=0^+} = \frac{\tau_{LIS} + \tau_{{Ma}}}{\mu_\infty}, \quad \text{for} \ 0 \le x < \frac{w}{2} \quad \text{(Equation (2.2))}, \end{gather}$$
In the following, we use non-dimensional variables ![]() $X = 2x/w$,
$X = 2x/w$, ![]() $Y = 2y/w$,
$Y = 2y/w$, ![]() $\alpha = ({\rm \pi} /2)(w/p)$ and
$\alpha = ({\rm \pi} /2)(w/p)$ and ![]() $\tilde {\psi } = 8 \mu _\infty \psi /(\tau _\infty w^2)$.
$\tilde {\psi } = 8 \mu _\infty \psi /(\tau _\infty w^2)$.
B.1. An expression for the stream function
The stream function of Stokes flow over varying no-slip and no-shear stripes was found by Philip (Reference Philip1972a). However, it can be generalised to apply to varying no-slip and constant shear-stress stripes (Schönecker et al. Reference Schönecker, Baier and Hardt2014). The relevant form is
where ![]() $\tau _{LIS}$ and
$\tau _{LIS}$ and ![]() $\tau _{{ {Ma}}}$ are constants, and
$\tau _{{ {Ma}}}$ are constants, and ![]() $\mathrm {Im}\{\}$ designates the imaginary part. With complex coordinates
$\mathrm {Im}\{\}$ designates the imaginary part. With complex coordinates ![]() $\varTheta = X + {\rm i}Y$ and
$\varTheta = X + {\rm i}Y$ and ![]() $\bar {\varTheta } = X - {\rm i}Y$, the stream function can be re-written as
$\bar {\varTheta } = X - {\rm i}Y$, the stream function can be re-written as
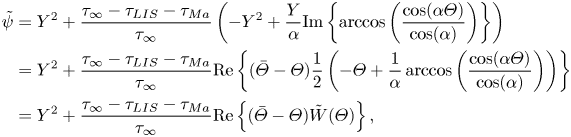 \begin{align} \tilde{\psi} &= Y^2 + \frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}\left({-}Y^2 + \frac{Y}{\alpha} \mathrm{Im}\left\{\arccos\left( \frac{\cos(\alpha\varTheta)}{\cos(\alpha)}\right)\right\}\right)\nonumber\\ &= Y^2 + \frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}\mathrm{Re}\left\{(\bar{\varTheta} - \varTheta)\frac{1}{2}\left(-\varTheta + \frac{1}{\alpha} \arccos\left( \frac{\cos(\alpha\varTheta)}{\cos(\alpha)}\right)\right)\right\} \nonumber\\ & = Y^2 + \frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}\mathrm{Re}\left\{(\bar{\varTheta} - \varTheta)\tilde{W}(\varTheta)\right\}, \end{align}
\begin{align} \tilde{\psi} &= Y^2 + \frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}\left({-}Y^2 + \frac{Y}{\alpha} \mathrm{Im}\left\{\arccos\left( \frac{\cos(\alpha\varTheta)}{\cos(\alpha)}\right)\right\}\right)\nonumber\\ &= Y^2 + \frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}\mathrm{Re}\left\{(\bar{\varTheta} - \varTheta)\frac{1}{2}\left(-\varTheta + \frac{1}{\alpha} \arccos\left( \frac{\cos(\alpha\varTheta)}{\cos(\alpha)}\right)\right)\right\} \nonumber\\ & = Y^2 + \frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}\mathrm{Re}\left\{(\bar{\varTheta} - \varTheta)\tilde{W}(\varTheta)\right\}, \end{align}where we have defined
and ![]() $\mathrm {Re}\{\}$ gives the real part.
$\mathrm {Re}\{\}$ gives the real part.
The wall-normal derivative of the stream function is
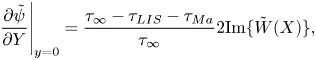 $$\begin{gather}\left.\frac{\partial \tilde{\psi}}{\partial Y}\right|_{y = 0} = \frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}2\mathrm{Im}\{\tilde{W}(X)\}, \end{gather}$$
$$\begin{gather}\left.\frac{\partial \tilde{\psi}}{\partial Y}\right|_{y = 0} = \frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}2\mathrm{Im}\{\tilde{W}(X)\}, \end{gather}$$
where ![]() $\tilde {W}'(\varTheta ) = \mathrm {d} \tilde {W}(\varTheta )/\mathrm {d} \varTheta$. On the interface (
$\tilde {W}'(\varTheta ) = \mathrm {d} \tilde {W}(\varTheta )/\mathrm {d} \varTheta$. On the interface (![]() $Y = 0$),
$Y = 0$), ![]() $0 \le X < 1$ and
$0 \le X < 1$ and ![]() $0 \le \alpha X < \alpha$. In addition,
$0 \le \alpha X < \alpha$. In addition, ![]() $0 < \alpha < {\rm \pi}/2$. In this interval,
$0 < \alpha < {\rm \pi}/2$. In this interval, ![]() $\tilde {W}(X)$ is imaginary, and outside (
$\tilde {W}(X)$ is imaginary, and outside (![]() $1 \le X \le {\rm \pi}/(2\alpha )$),
$1 \le X \le {\rm \pi}/(2\alpha )$), ![]() $\tilde {W}(X)$ is real. Hence, (B2) is satisfied. The velocity on the interface is
$\tilde {W}(X)$ is real. Hence, (B2) is satisfied. The velocity on the interface is
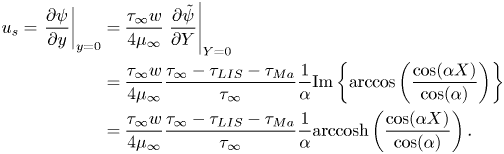 \begin{align} u_s = \left.\frac{\partial \psi}{\partial y}\right|_{y = 0} &= \frac{\tau_\infty w}{4\mu_\infty}\left.\frac{\partial \tilde{\psi}}{\partial Y}\right|_{Y = 0} \nonumber\\ &= \frac{\tau_\infty w}{4\mu_\infty}\frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}\frac{1}{\alpha}\mathrm{Im}\left\{\arccos\left( \frac{\cos(\alpha X)}{\cos(\alpha)}\right)\right\} \nonumber\\ &= \frac{\tau_\infty w}{4\mu_\infty}\frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}\frac{1}{\alpha} \mathrm{arccosh}\left( \frac{\cos(\alpha X)}{\cos(\alpha)}\right). \end{align}
\begin{align} u_s = \left.\frac{\partial \psi}{\partial y}\right|_{y = 0} &= \frac{\tau_\infty w}{4\mu_\infty}\left.\frac{\partial \tilde{\psi}}{\partial Y}\right|_{Y = 0} \nonumber\\ &= \frac{\tau_\infty w}{4\mu_\infty}\frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}\frac{1}{\alpha}\mathrm{Im}\left\{\arccos\left( \frac{\cos(\alpha X)}{\cos(\alpha)}\right)\right\} \nonumber\\ &= \frac{\tau_\infty w}{4\mu_\infty}\frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}\frac{1}{\alpha} \mathrm{arccosh}\left( \frac{\cos(\alpha X)}{\cos(\alpha)}\right). \end{align}The integral (Philip Reference Philip1972b)
which corresponds to the average of the integrand over the complete interface. The mean slip velocity is therefore
The velocity in the centre of the interface is
The second wall-normal derivative of the stream function is
 $$\begin{gather}\left.\frac{\partial^2 \tilde{\psi}}{\partial Y^2}\right|_{Y = 0} = 2 + \frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}4\mathrm{Re}\left\{\tilde{W}'(X)\right\}, \end{gather}$$
$$\begin{gather}\left.\frac{\partial^2 \tilde{\psi}}{\partial Y^2}\right|_{Y = 0} = 2 + \frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}4\mathrm{Re}\left\{\tilde{W}'(X)\right\}, \end{gather}$$
where ![]() $\tilde {W}''(\varTheta ) = \mathrm {d}^2 \tilde {W}(\varTheta )/\mathrm {d} \varTheta ^2$. We can compute
$\tilde {W}''(\varTheta ) = \mathrm {d}^2 \tilde {W}(\varTheta )/\mathrm {d} \varTheta ^2$. We can compute
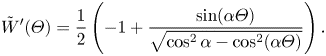 \begin{equation} \tilde{W}'(\varTheta) = \frac{1}{2}\left({-}1 + \frac{\sin (\alpha\varTheta)}{\sqrt{\cos^2 \alpha - \cos^2 (\alpha\varTheta)}} \right). \end{equation}
\begin{equation} \tilde{W}'(\varTheta) = \frac{1}{2}\left({-}1 + \frac{\sin (\alpha\varTheta)}{\sqrt{\cos^2 \alpha - \cos^2 (\alpha\varTheta)}} \right). \end{equation}
Hence, for ![]() $Y = 0$,
$Y = 0$,
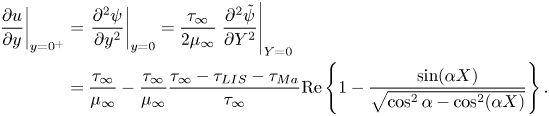 \begin{align} \left.\frac{\partial u}{\partial y}\right|_{y = 0^+} &= \left.\frac{\partial^2 \psi}{\partial y^2}\right|_{y = 0} = \frac{\tau_\infty}{2\mu_\infty}\left.\frac{\partial^2 \tilde{\psi}}{\partial Y^2}\right|_{Y = 0} \nonumber\\ &= \frac{\tau_\infty}{\mu_\infty} - \frac{\tau_\infty}{\mu_\infty}\frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}\mathrm{Re}\left\{1 - \frac{\sin (\alpha X)}{\sqrt{\cos^2 \alpha - \cos^2 (\alpha X)}} \right\}. \end{align}
\begin{align} \left.\frac{\partial u}{\partial y}\right|_{y = 0^+} &= \left.\frac{\partial^2 \psi}{\partial y^2}\right|_{y = 0} = \frac{\tau_\infty}{2\mu_\infty}\left.\frac{\partial^2 \tilde{\psi}}{\partial Y^2}\right|_{Y = 0} \nonumber\\ &= \frac{\tau_\infty}{\mu_\infty} - \frac{\tau_\infty}{\mu_\infty}\frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}\mathrm{Re}\left\{1 - \frac{\sin (\alpha X)}{\sqrt{\cos^2 \alpha - \cos^2 (\alpha X)}} \right\}. \end{align}
On the interface, ![]() $\sqrt {\cos ^2 \alpha - \cos ^2 (\alpha X)}$ is imaginary, and thereby the boundary condition (B3) is satisfied. However, the average shear stress at the surface (
$\sqrt {\cos ^2 \alpha - \cos ^2 (\alpha X)}$ is imaginary, and thereby the boundary condition (B3) is satisfied. However, the average shear stress at the surface (![]() $0 \le x \le p/2$) is
$0 \le x \le p/2$) is ![]() $\tau _\infty$, found by using
$\tau _\infty$, found by using ![]() $\tilde {W}(1) = -1/2$ and
$\tilde {W}(1) = -1/2$ and ![]() $\tilde {W}(p/w) = 0$.
$\tilde {W}(p/w) = 0$.
The first streamwise derivative of ![]() $\tilde {\psi }$ is
$\tilde {\psi }$ is
This expression is zero for ![]() $Y = 0$, showing that the wall-normal velocity is zero at the surface.
$Y = 0$, showing that the wall-normal velocity is zero at the surface.
B.2. Modelling the infusing-liquid shear stress
The interfacial shear stresses of the infusing liquid can be assumed to relate to the interface velocity by a local slip length ![]() $\zeta (x)$ (Schönecker et al. Reference Schönecker, Baier and Hardt2014),
$\zeta (x)$ (Schönecker et al. Reference Schönecker, Baier and Hardt2014),
where
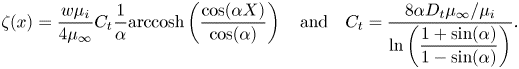 \begin{equation} \zeta(x) = \frac{w\mu_i}{4\mu_\infty}C_t\frac{1}{\alpha}\mathrm{arccosh}\left( \frac{\cos(\alpha X)}{\cos(\alpha)}\right) \quad \text{and} \quad C_t = \frac{8\alpha D_t\mu_\infty/\mu_i}{\ln\left(\dfrac{1 + \sin(\alpha)}{1 - \sin(\alpha)}\right)}. \end{equation}
\begin{equation} \zeta(x) = \frac{w\mu_i}{4\mu_\infty}C_t\frac{1}{\alpha}\mathrm{arccosh}\left( \frac{\cos(\alpha X)}{\cos(\alpha)}\right) \quad \text{and} \quad C_t = \frac{8\alpha D_t\mu_\infty/\mu_i}{\ln\left(\dfrac{1 + \sin(\alpha)}{1 - \sin(\alpha)}\right)}. \end{equation}
Here, ![]() $D_t = d_t/w$ is the maximum value of
$D_t = d_t/w$ is the maximum value of ![]() $\zeta (x)/w$. Together with (B10),
$\zeta (x)/w$. Together with (B10),
Hence, ![]() $\tau _\infty /(1 + C_t)$ is the interfacial shear stress of the infusing liquid in the absence of surfactants (
$\tau _\infty /(1 + C_t)$ is the interfacial shear stress of the infusing liquid in the absence of surfactants (![]() $\tau _{{ {Ma}}} = 0$).
$\tau _{{ {Ma}}} = 0$).
The non-dimensional slip length ![]() $D_t$ can be modelled by
$D_t$ can be modelled by
where
 \begin{equation} f(a) ={-}\frac{\ln\left(\dfrac{1 + \sin\left(\dfrac{{\rm \pi} a}{2}\right)}{1 - \sin\left(\dfrac{{\rm \pi} a}{2}\right)}\right)}{2a\ln 2 \left(1 + \dfrac{2\ln\left(\cos\left(\dfrac{{\rm \pi} a}{2}\right)\right)}{2a\, \mathrm{arctanh}(a) + \ln(1 - a^2)}\right)}, \quad g(a) = \frac{4}{\rm \pi} - \frac{4 - {\rm \pi}}{\rm \pi}a, \end{equation}
\begin{equation} f(a) ={-}\frac{\ln\left(\dfrac{1 + \sin\left(\dfrac{{\rm \pi} a}{2}\right)}{1 - \sin\left(\dfrac{{\rm \pi} a}{2}\right)}\right)}{2a\ln 2 \left(1 + \dfrac{2\ln\left(\cos\left(\dfrac{{\rm \pi} a}{2}\right)\right)}{2a\, \mathrm{arctanh}(a) + \ln(1 - a^2)}\right)}, \quad g(a) = \frac{4}{\rm \pi} - \frac{4 - {\rm \pi}}{\rm \pi}a, \end{equation}
![]() $\beta = 0.505/(2{\rm \pi} )$,
$\beta = 0.505/(2{\rm \pi} )$, ![]() $A = k/w$,
$A = k/w$, ![]() $a = w/p$ and
$a = w/p$ and ![]() $\mathrm {erf}(x)$ is the error function.
$\mathrm {erf}(x)$ is the error function.
B.3. Effective slip length
The effective slip length ![]() $b$ is (using (B12) and (B21))
$b$ is (using (B12) and (B21))
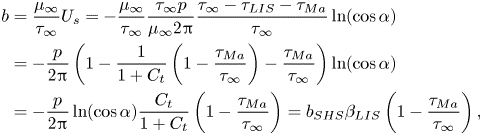 \begin{align} b &= \frac{\mu_\infty}{\tau_\infty}U_s ={-}\frac{\mu_\infty}{\tau_\infty}\frac{\tau_\infty p}{\mu_\infty 2{\rm \pi}}\frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}\ln( \cos \alpha) \nonumber\\ &={-}\frac{p}{2{\rm \pi}}\left(1 - \frac{1}{1 + C_t}\left(1 - \frac{\tau_{{Ma}}}{\tau_\infty}\right) - \frac{\tau_{{Ma}}}{\tau_\infty}\right)\ln( \cos \alpha) \nonumber\\ &={-}\frac{p}{2{\rm \pi}}\ln( \cos \alpha)\frac{C_t}{1 + C_t}\left(1 - \frac{\tau_{{Ma}}}{\tau_\infty}\right) = b_{SHS}\beta_{LIS} \left(1 - \frac{\tau_{{Ma}}}{\tau_\infty}\right), \end{align}
\begin{align} b &= \frac{\mu_\infty}{\tau_\infty}U_s ={-}\frac{\mu_\infty}{\tau_\infty}\frac{\tau_\infty p}{\mu_\infty 2{\rm \pi}}\frac{\tau_\infty - \tau_{LIS} - \tau_{{Ma}}}{\tau_\infty}\ln( \cos \alpha) \nonumber\\ &={-}\frac{p}{2{\rm \pi}}\left(1 - \frac{1}{1 + C_t}\left(1 - \frac{\tau_{{Ma}}}{\tau_\infty}\right) - \frac{\tau_{{Ma}}}{\tau_\infty}\right)\ln( \cos \alpha) \nonumber\\ &={-}\frac{p}{2{\rm \pi}}\ln( \cos \alpha)\frac{C_t}{1 + C_t}\left(1 - \frac{\tau_{{Ma}}}{\tau_\infty}\right) = b_{SHS}\beta_{LIS} \left(1 - \frac{\tau_{{Ma}}}{\tau_\infty}\right), \end{align}
where ![]() $b_{SHS} = -p\ln (\cos \alpha )/(2{\rm \pi} )$ is the slip length for
$b_{SHS} = -p\ln (\cos \alpha )/(2{\rm \pi} )$ is the slip length for ![]() $\mu _i/\mu _\infty \to 0$ and
$\mu _i/\mu _\infty \to 0$ and ![]() $\tau _{{ {Ma}}} = 0$, and
$\tau _{{ {Ma}}} = 0$, and ![]() $\beta _{LIS} = C_t/(1 + C_t)$ expresses the effects of the viscous infusing liquid. For SHS with
$\beta _{LIS} = C_t/(1 + C_t)$ expresses the effects of the viscous infusing liquid. For SHS with ![]() $\mu _i/\mu _\infty \to 0$,
$\mu _i/\mu _\infty \to 0$, ![]() $\beta _{LIS} \to 1$. The velocity at the centre of the interface (B13) becomes
$\beta _{LIS} \to 1$. The velocity at the centre of the interface (B13) becomes
Appendix C. Derivation of surfactant boundary layer thickness
In this section, we estimate ![]() $\delta /w$. The limits of low and high
$\delta /w$. The limits of low and high ![]() ${ {Pe}}$ are considered separately, and the final expression is formed as a combination of these two limits.
${ {Pe}}$ are considered separately, and the final expression is formed as a combination of these two limits.
C.1. Low Péclet numbers
For low ![]() ${ {Pe}}$, advection is assumed to be negligible, corresponding to (cf. (2.5))
${ {Pe}}$, advection is assumed to be negligible, corresponding to (cf. (2.5))
We integrate this equation one time in the streamwise and one time in the wall-normal direction.
For the wall-normal diffusion (second term) in the wall-normal direction,
where ![]() $f_y(x) = -2x/w$ for
$f_y(x) = -2x/w$ for ![]() $-w/2 < x < w/2$ and
$-w/2 < x < w/2$ and ![]() $0$ otherwise, using the assumed spatial dependency of (5.4) and (5.6) for the value at
$0$ otherwise, using the assumed spatial dependency of (5.4) and (5.6) for the value at ![]() $x = -w/2$. Integrating the result in the streamwise direction from
$x = -w/2$. Integrating the result in the streamwise direction from ![]() $-p/2$ to
$-p/2$ to ![]() $0$,
$0$,
 \begin{equation} \int_{{-}p/2}^0 - \frac{\Delta c_s}{\delta}f_y(x) \,\mathrm{d}\kern0.06em x = \int_{{-}w/2}^0 - \frac{\Delta c_s}{\delta}\frac{-2x}{w} \,\mathrm{d}\kern0.06em x ={-}\frac{\Delta c_s}{\delta}\frac{w}{4}. \end{equation}
\begin{equation} \int_{{-}p/2}^0 - \frac{\Delta c_s}{\delta}f_y(x) \,\mathrm{d}\kern0.06em x = \int_{{-}w/2}^0 - \frac{\Delta c_s}{\delta}\frac{-2x}{w} \,\mathrm{d}\kern0.06em x ={-}\frac{\Delta c_s}{\delta}\frac{w}{4}. \end{equation} For the streamwise diffusion, we need to assume a wall-normal dependency of ![]() $c$. We assume that
$c$. We assume that
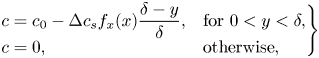 \begin{equation} \left. \begin{array}{ll@{}} c = c_0 - \Delta c_s f_x(x)\dfrac{\delta - y}{\delta}, & \text{for }0 < y < \delta, \\ c = 0, & \text{otherwise}, \end{array} \right\} \end{equation}
\begin{equation} \left. \begin{array}{ll@{}} c = c_0 - \Delta c_s f_x(x)\dfrac{\delta - y}{\delta}, & \text{for }0 < y < \delta, \\ c = 0, & \text{otherwise}, \end{array} \right\} \end{equation}
and ![]() $f_x(x)$ describes the streamwise dependency with
$f_x(x)$ describes the streamwise dependency with ![]() $f_x(-w/2) = 1$ and
$f_x(-w/2) = 1$ and ![]() $f_x(0) = f_x(-p/2) = 0$ (similarly to
$f_x(0) = f_x(-p/2) = 0$ (similarly to ![]() $f_y(x)$). The streamwise dependency is consistent with (5.4). The wall-normal dependency is consistent with (5.6) but not correct outside the interface (
$f_y(x)$). The streamwise dependency is consistent with (5.4). The wall-normal dependency is consistent with (5.6) but not correct outside the interface (![]() $x > w/2$ and
$x > w/2$ and ![]() $x < -w/2$) because there the wall-normal derivative at the wall needs to be zero. However, we think that it is a good enough approximation. Thus,
$x < -w/2$) because there the wall-normal derivative at the wall needs to be zero. However, we think that it is a good enough approximation. Thus,
where ![]() $f_x''(x) = \mathrm {d}^2 f_x(x)/\mathrm {d}\kern 0.06em x^2$. It can be noted that the alternative wall-normal distribution
$f_x''(x) = \mathrm {d}^2 f_x(x)/\mathrm {d}\kern 0.06em x^2$. It can be noted that the alternative wall-normal distribution ![]() $\exp (-y/\delta )$ would give a coefficient of
$\exp (-y/\delta )$ would give a coefficient of ![]() $1$ instead of
$1$ instead of ![]() $1/2$. Integrating the result in the streamwise direction,
$1/2$. Integrating the result in the streamwise direction,
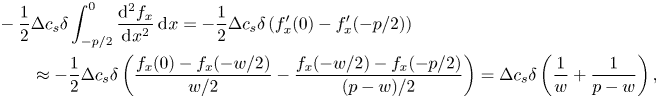 \begin{align} &- \frac{1}{2}\Delta c_s \delta \int_{{-}p/2}^0 \frac{\mathrm{d}^2 f_x}{\mathrm{d}\kern0.06em x^2} \,\mathrm{d}\kern0.06em x ={-} \frac{1}{2}\Delta c_s \delta\left(f_x'(0) - f_x'({-}p/2)\right) \nonumber\\ &\qquad \approx{-} \frac{1}{2}\Delta c_s \delta\left(\frac{f_x(0) - f_x({-}w/2)}{w/2} - \frac{f_x({-}w/2) - f_x({-}p/2)}{(p - w)/2}\right) = \Delta c_s \delta\left(\frac{1}{w} + \frac{1}{p - w}\right), \end{align}
\begin{align} &- \frac{1}{2}\Delta c_s \delta \int_{{-}p/2}^0 \frac{\mathrm{d}^2 f_x}{\mathrm{d}\kern0.06em x^2} \,\mathrm{d}\kern0.06em x ={-} \frac{1}{2}\Delta c_s \delta\left(f_x'(0) - f_x'({-}p/2)\right) \nonumber\\ &\qquad \approx{-} \frac{1}{2}\Delta c_s \delta\left(\frac{f_x(0) - f_x({-}w/2)}{w/2} - \frac{f_x({-}w/2) - f_x({-}p/2)}{(p - w)/2}\right) = \Delta c_s \delta\left(\frac{1}{w} + \frac{1}{p - w}\right), \end{align}
where ![]() $f_x'(x) = \mathrm {d} f_x(x)/\mathrm {d}\kern 0.06em x$.
$f_x'(x) = \mathrm {d} f_x(x)/\mathrm {d}\kern 0.06em x$.
In contrast to the expression by Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020), (C7) accounts for diffusion between boundary layers of adjacent interfaces. This diffusion is relevant when the solid fraction is low (![]() $w \gtrsim p-w$).
$w \gtrsim p-w$).
C.2. High Péclet numbers
For high ![]() ${ {Pe}}$, we assume that streamwise advection balances wall-normal diffusion,
${ {Pe}}$, we assume that streamwise advection balances wall-normal diffusion,
We also integrate this equation in the wall-normal and streamwise directions. The integration of the wall-normal diffusion is the same as above. For the advection, we retain the wall-normal dependency of (C4) but replace ![]() $f_x(x)$ with another function,
$f_x(x)$ with another function, ![]() $g_x(x)$. As we neglect streamwise diffusion, we assume that the concentration at the stagnation point of the upstream interface is advected downstream,
$g_x(x)$. As we neglect streamwise diffusion, we assume that the concentration at the stagnation point of the upstream interface is advected downstream, ![]() $g_x(-p/2) = -1$. However, we consider
$g_x(-p/2) = -1$. However, we consider ![]() $g_x(0) = f_x(0) = 0$. Similar to Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020), we assume the Lévêque regime:
$g_x(0) = f_x(0) = 0$. Similar to Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020), we assume the Lévêque regime: ![]() $u = y\tau _\infty /\mu _\infty$. Thus,
$u = y\tau _\infty /\mu _\infty$. Thus,
Performing the streamwise integration,
 \begin{align}
& - \frac{1}{6}\frac{\tau_\infty}{\mu_\infty}\Delta c_s
\delta^2 \int_{{-}p/2}^0 g_x'(x) \,\mathrm{d}\kern0.06em x ={-}
\frac{1}{6}\frac{\tau_\infty}{\mu_\infty}\Delta c_s
\delta^2 (g_x(0) - g_x({-}p/2)) \nonumber\\
&\quad ={-}\frac{1}{6}\frac{\tau_\infty}{\mu_\infty}\Delta c_s
\delta^2 ={-}\frac{1}{6}{{Pe}}\Delta c_s
D\frac{\delta^2}{w^2}.
\end{align}
\begin{align}
& - \frac{1}{6}\frac{\tau_\infty}{\mu_\infty}\Delta c_s
\delta^2 \int_{{-}p/2}^0 g_x'(x) \,\mathrm{d}\kern0.06em x ={-}
\frac{1}{6}\frac{\tau_\infty}{\mu_\infty}\Delta c_s
\delta^2 (g_x(0) - g_x({-}p/2)) \nonumber\\
&\quad ={-}\frac{1}{6}\frac{\tau_\infty}{\mu_\infty}\Delta c_s
\delta^2 ={-}\frac{1}{6}{{Pe}}\Delta c_s
D\frac{\delta^2}{w^2}.
\end{align}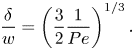 \begin{equation} \frac{\delta}{w} = \left(\frac{3}{2}\frac{1}{{{Pe}}} \right)^{1/3}. \end{equation}
\begin{equation} \frac{\delta}{w} = \left(\frac{3}{2}\frac{1}{{{Pe}}} \right)^{1/3}. \end{equation}C.3. Combined expression
The two expressions (C7) and (C11) can be combined into a single expression with the correct asymptotic behaviours,
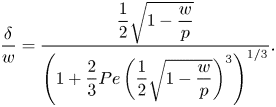 \begin{equation} \frac{\delta}{w} = \frac{\dfrac{1}{2}\sqrt{1 - \dfrac{w}{p}}}{\left(1 + \dfrac{2}{3}{{Pe}}\left(\dfrac{1}{2}\sqrt{1 - \dfrac{w}{p}}\right)^3\right)^{1/3}}. \end{equation}
\begin{equation} \frac{\delta}{w} = \frac{\dfrac{1}{2}\sqrt{1 - \dfrac{w}{p}}}{\left(1 + \dfrac{2}{3}{{Pe}}\left(\dfrac{1}{2}\sqrt{1 - \dfrac{w}{p}}\right)^3\right)^{1/3}}. \end{equation} In figure 12, (C12) is compared with values of ![]() $\delta /w$ from simulations of §§ 4 and 6, estimated using the left relation of (5.6). The value of
$\delta /w$ from simulations of §§ 4 and 6, estimated using the left relation of (5.6). The value of ![]() $\Delta c_s$ was computed using the minimum value of
$\Delta c_s$ was computed using the minimum value of ![]() $c_s$ (figure 12c). The concentration wall-normal derivative showed significant nonlinear behaviour close to the interface edges. Therefore, a reasonable value was determined by extrapolating a line to
$c_s$ (figure 12c). The concentration wall-normal derivative showed significant nonlinear behaviour close to the interface edges. Therefore, a reasonable value was determined by extrapolating a line to ![]() $x = -w/2$ with the same slope as around
$x = -w/2$ with the same slope as around ![]() $x = 0$ (figure 12d). There is a satisfactory agreement between the prediction and the simulation results.
$x = 0$ (figure 12d). There is a satisfactory agreement between the prediction and the simulation results.
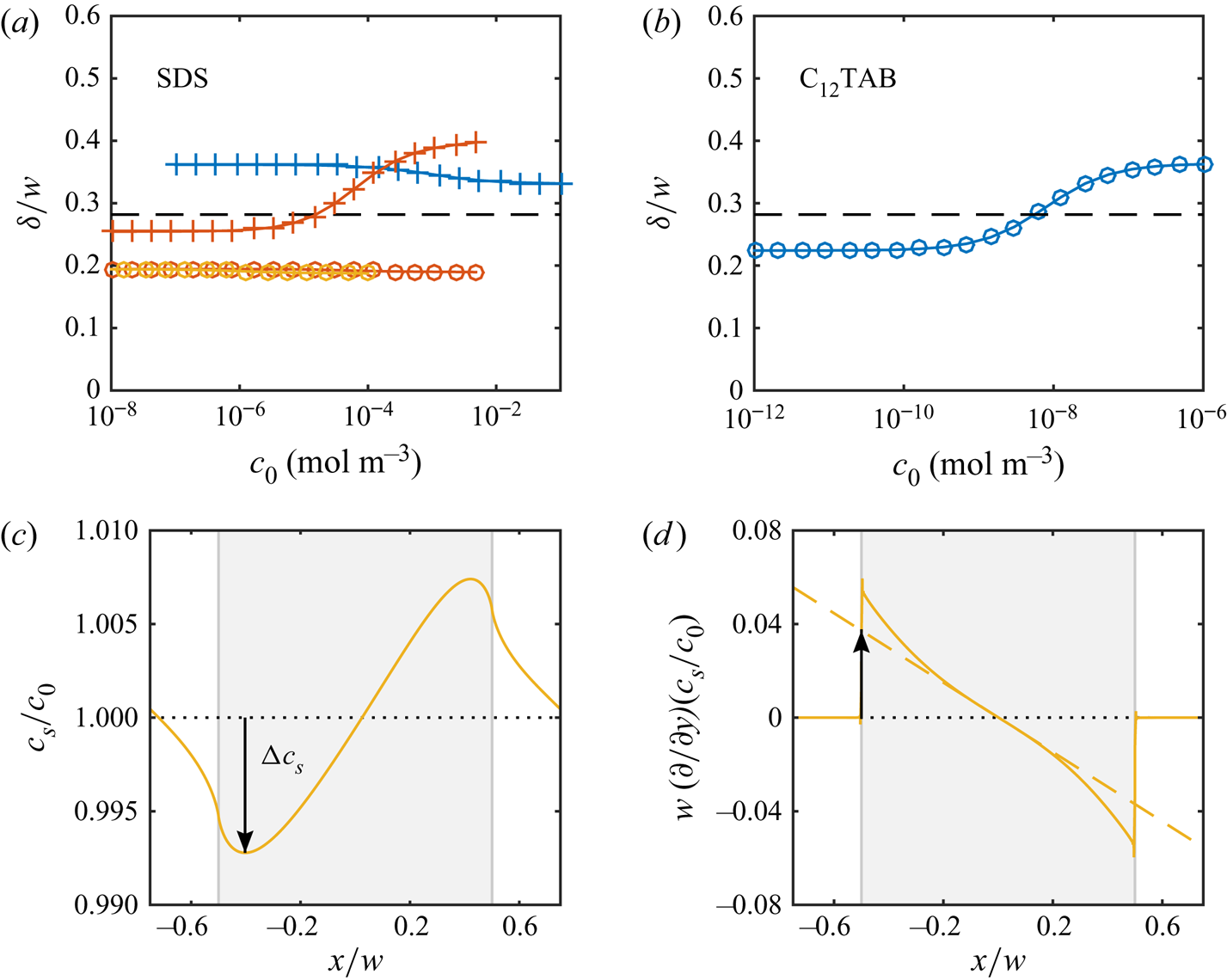
Figure 12. Comparison of ![]() $\delta /w$ computed from (C12) (dashed line) with estimations from simulations using (a) SDS (colours and symbols as in figure 5) C
$\delta /w$ computed from (C12) (dashed line) with estimations from simulations using (a) SDS (colours and symbols as in figure 5) C![]() $_{12}$TAB. The simulation estimates are based on the left relationship of (5.6), with
$_{12}$TAB. The simulation estimates are based on the left relationship of (5.6), with ![]() $\Delta c_s$ and
$\Delta c_s$ and ![]() $\partial c/\partial y|_{y = 0, x = -w/2}$ as the arrows in (c,d), respectively. The specific distributions in these two figures are from the lowest simulated SDS concentration using the Frumkin model with alkane–surfactant interaction,
$\partial c/\partial y|_{y = 0, x = -w/2}$ as the arrows in (c,d), respectively. The specific distributions in these two figures are from the lowest simulated SDS concentration using the Frumkin model with alkane–surfactant interaction, ![]() $c_0 = 10^{-10}\,\textrm {mol}\,\textrm {m}^{-3}$. The grey boxes in (c) and (d) indicate the interface, and the dotted lines correspond to
$c_0 = 10^{-10}\,\textrm {mol}\,\textrm {m}^{-3}$. The grey boxes in (c) and (d) indicate the interface, and the dotted lines correspond to ![]() $c_s/c_0 = 1$ and
$c_s/c_0 = 1$ and ![]() $\partial c_s/\partial y = 0$, respectively. The dashed line in (d) is a linear extrapolation from the centre of the interface.
$\partial c_s/\partial y = 0$, respectively. The dashed line in (d) is a linear extrapolation from the centre of the interface.
For the simulations with a higher ![]() ${ {Pe}}$ (§ 7), (C12) gives
${ {Pe}}$ (§ 7), (C12) gives ![]() $\delta /w = 0.068$. The assumption of linear interfacial surfactant distributions is valid for the highest simulated concentration, giving an estimate of
$\delta /w = 0.068$. The assumption of linear interfacial surfactant distributions is valid for the highest simulated concentration, giving an estimate of ![]() $\delta /w = 0.034$. Even if the relative difference between prediction and estimation is larger for the higher
$\delta /w = 0.034$. Even if the relative difference between prediction and estimation is larger for the higher ![]() ${ {Pe}}$, it does not influence the conclusions in any significant way.
${ {Pe}}$, it does not influence the conclusions in any significant way.
Appendix D. Reinterpretation of data from Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020)
Using the analytical model derived in this article, we reinterpreted the data from simulations of SHS (![]() $\beta _{LIS} = 1$) with surfactants in two-dimensional Poiseuille flow from Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020). These results are shown in figure 13, showing both
$\beta _{LIS} = 1$) with surfactants in two-dimensional Poiseuille flow from Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020). These results are shown in figure 13, showing both ![]() $b$ and
$b$ and ![]() $\gamma _{{ {Ma}}}$, where the latter is the averaged shear stress on the interface normalised by the average shear stress of the channel, corresponding to
$\gamma _{{ {Ma}}}$, where the latter is the averaged shear stress on the interface normalised by the average shear stress of the channel, corresponding to ![]() $\tau _{{ {Ma}}}/\tau _\infty$. Therefore,
$\tau _{{ {Ma}}}/\tau _\infty$. Therefore, ![]() $\gamma _{{ {Ma}}}$ can be compared with the analytical model of (5.16) (corresponding to equation (4.29) of Landel et al. Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020),
$\gamma _{{ {Ma}}}$ can be compared with the analytical model of (5.16) (corresponding to equation (4.29) of Landel et al. Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020),
with ![]() $\alpha _{diff}$ and
$\alpha _{diff}$ and ![]() $\alpha _S$ from (5.17). However, because of the different geometry,
$\alpha _S$ from (5.17). However, because of the different geometry, ![]() $b$ is not proportional to
$b$ is not proportional to ![]() $1 - \gamma _{{ {Ma}}}$, as (3.2) states for the geometry of the current work. Instead,
$1 - \gamma _{{ {Ma}}}$, as (3.2) states for the geometry of the current work. Instead,
for a channel half-height ![]() $h$ and the geometry-dependent constant
$h$ and the geometry-dependent constant ![]() $E_0$ (equation (4.34) of Landel et al. Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020). For
$E_0$ (equation (4.34) of Landel et al. Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020). For ![]() $b \ll 2h$, (3.2) and (D2) give
$b \ll 2h$, (3.2) and (D2) give ![]() $b_{SHS} \approx 2hE_0$. We plot the full expression (D2), marking the three points where nonlinear geometrical effects are present (
$b_{SHS} \approx 2hE_0$. We plot the full expression (D2), marking the three points where nonlinear geometrical effects are present (![]() $b/(2h) \ge 0.1$).
$b/(2h) \ge 0.1$).
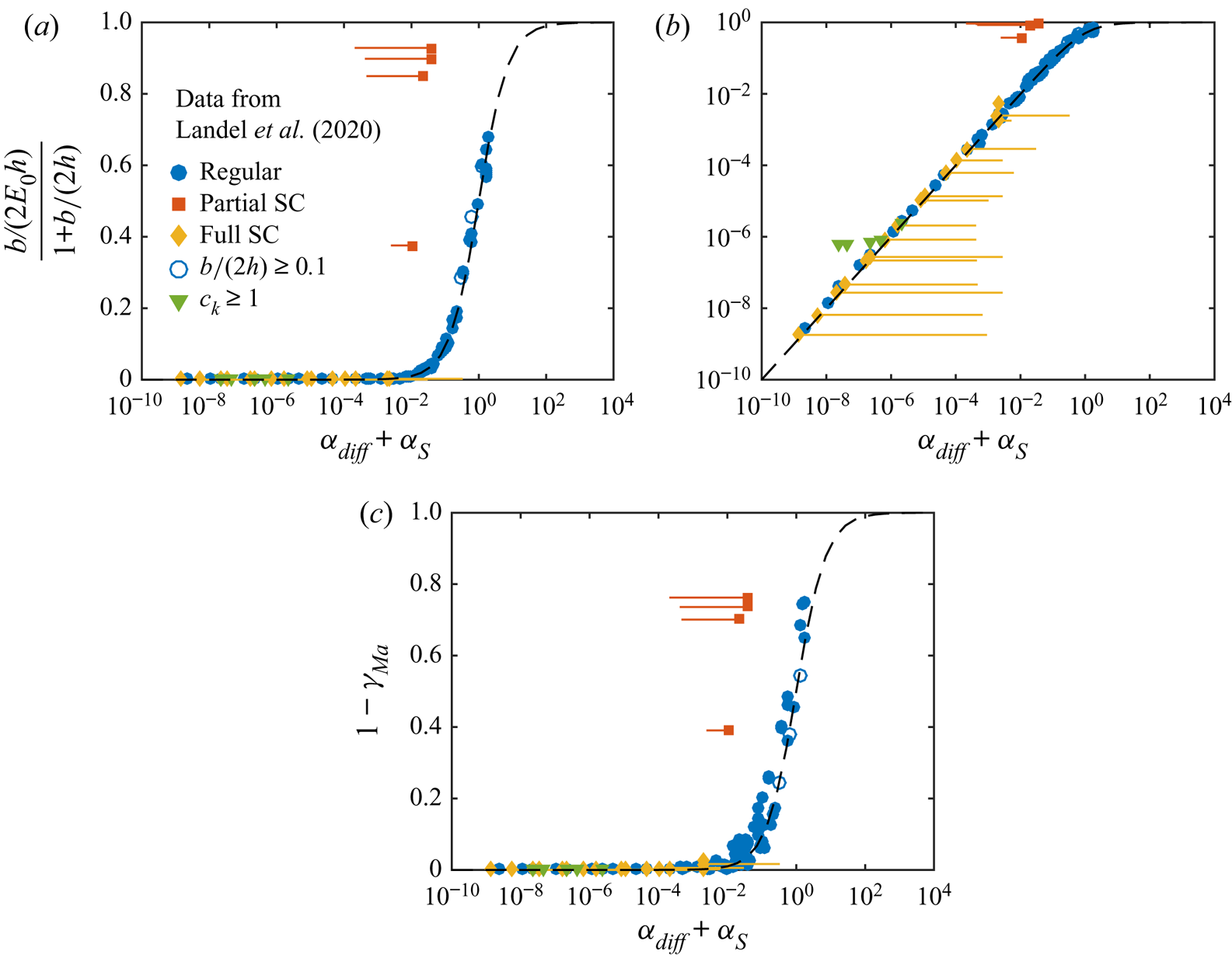
Figure 13. The analytical model (dashed line) compared with data from Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020), showing ![]() $b$ (by (D2)) with (a) linear and (b) logarithmic vertical axis, and (c)
$b$ (by (D2)) with (a) linear and (b) logarithmic vertical axis, and (c) ![]() $1 - \gamma _{{ {Ma}}}$. Points in the partial and full SC regimes and points with
$1 - \gamma _{{ {Ma}}}$. Points in the partial and full SC regimes and points with ![]() $b/(2h) \ge 0.1$ or
$b/(2h) \ge 0.1$ or ![]() $c_k \ge 1$ have been identified. Other points have been denoted as regular. For results in the partial or full SC regime, lines go from the points to the values of
$c_k \ge 1$ have been identified. Other points have been denoted as regular. For results in the partial or full SC regime, lines go from the points to the values of ![]() $\alpha _{diff} + \alpha _S$ given by (7.6).
$\alpha _{diff} + \alpha _S$ given by (7.6).
The total number of points is 137. In the figure, we have marked data points in the partial (4 points) and full SC (16 points) regimes as identified by Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020) and added a line going from the simulation results to ![]() $\alpha _{diff} + \alpha _S$ of (7.6). For interfaces in the partial SC regime, the simulation point is to the right of this value, whereas for interfaces in the full SC regime, the simulation points are to the left. This behaviour agrees with (7.6), as no linear distributions of
$\alpha _{diff} + \alpha _S$ of (7.6). For interfaces in the partial SC regime, the simulation point is to the right of this value, whereas for interfaces in the full SC regime, the simulation points are to the left. This behaviour agrees with (7.6), as no linear distributions of ![]() $\theta$ can exist above this
$\theta$ can exist above this ![]() $\alpha _{diff} + \alpha _S$, and the SC becomes partial. In addition to the SC points, we have identified five cases with
$\alpha _{diff} + \alpha _S$, and the SC becomes partial. In addition to the SC points, we have identified five cases with ![]() $c_k \ge 1$. None of these simulations resulted in
$c_k \ge 1$. None of these simulations resulted in ![]() $b/(2h) \ge 0.1$.
$b/(2h) \ge 0.1$.
Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020) did not use (7.5) to distinguish between uniform and SC regimes. All SC interfaces identified in figure 13 did fulfil (7.5). However, some points classified as uniformly retarded fulfilled (7.5) as well. Out of these, 6 points should be in the partial SC regime according to (7.6). The nonlinearity of their interfacial surfactant distributions should be visible upon inspection. However, with their ![]() $C_{SC}$ relatively close to
$C_{SC}$ relatively close to ![]() $1$ (minimum
$1$ (minimum ![]() $C_{SC} = 0.15$, cf.
$C_{SC} = 0.15$, cf. ![]() $0.059$ in 7.7), only a minor deviation from the predicted slip length is allowed; a smaller
$0.059$ in 7.7), only a minor deviation from the predicted slip length is allowed; a smaller ![]() $C_{SC}$ gives a lower
$C_{SC}$ gives a lower ![]() $\alpha _{diff} + \alpha _S$ limit where the analytically model becomes valid, allowing
$\alpha _{diff} + \alpha _S$ limit where the analytically model becomes valid, allowing ![]() $b$ to deviate further ((7.6) and figure 9a). Therefore, these SC presumably had a negligible influence on the results in figure 13.
$b$ to deviate further ((7.6) and figure 9a). Therefore, these SC presumably had a negligible influence on the results in figure 13.
The analytical model seems to fit ![]() $b$ better than
$b$ better than ![]() $\gamma _{{ {Ma}}}$. This observation appears to be consistent with what is reported by Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020);
$\gamma _{{ {Ma}}}$. This observation appears to be consistent with what is reported by Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020); ![]() $\gamma _{{ {Ma}}}$ is probably affected by numerical errors to a higher degree than
$\gamma _{{ {Ma}}}$ is probably affected by numerical errors to a higher degree than ![]() $b$. It can also be noted that
$b$. It can also be noted that ![]() $u_{s,{SHS}}^0$ (3.5) corresponds to
$u_{s,{SHS}}^0$ (3.5) corresponds to ![]() $2\mathcal {F}_0$ of Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020). It is exactly their leading-order expression for narrow grooves (equation (C24)).
$2\mathcal {F}_0$ of Landel et al. (Reference Landel, Peaudecerf, Temprano-Coleto, Gibou, Goldstein and Luzzatto-Fegiz2020). It is exactly their leading-order expression for narrow grooves (equation (C24)).































































































