Abstract
Hepatocellular carcinoma (HCC) has been associated with hepatitis C viral (HCV) infection as a potential risk factor. Nonetheless, the precise genetic regulatory mechanisms triggered by the virus, leading to virus-induced hepatocarcinogenesis, remain unclear. We hypothesized that HCV proteins might modulate the activity of aberrantly methylated HCC genes through regulatory pathways. Virus-host regulatory pathways, interactions between proteins, gene expression, transport, and stability regulation, were reconstructed using the ANDSystem. Gene expression regulation was statistically significant. Gene network analysis identified four out of 70 HCC marker genes whose expression regulation by viral proteins may be associated with HCC: DNA-binding protein inhibitor ID – 1 (ID1), flap endonuclease 1 (FEN1), cyclin-dependent kinase inhibitor 2A (CDKN2A), and telomerase reverse transcriptase (TERT). It suggested the following viral protein effects in HCV/human protein heterocomplexes: HCV NS3(p70) protein activates human STAT3 and NOTC1; NS2-3(p23), NS5B(p68), NS1(E2), and core(p21) activate SETD2; NS5A inhibits SMYD3; and NS3 inhibits CCN2. Interestingly, NS3 and E1(gp32) activate c-Jun when it positively regulates CDKN2A and inhibit it when it represses TERT. The discovered regulatory mechanisms might be key areas of focus for creating medications and preventative therapies to decrease the likelihood of HCC development during HCV infection.
1 Introduction
Malignant diseases annually claim many lives worldwide. For instance, initial findings for 2021 suggest that cancer had ascended to being the top 2 leading cause of mortality in the USA [1]. HCC stands as one of the most frequently diagnosed cancers [2]. The hepatitis B and C viruses represent significant risk contributors to the development of HCC. Longitudinal research has demonstrated an elevated likelihood of HCC development within the population infected by HCV [3]. The prevalence of HCV infection among HCC patients was 44%–66 % in Italy [4, 5], while in Japan, it was as high as 80 % [6]. A meta-analysis of case-control investigations revealed that patients positive for anti-HCV have a 17-fold greater probability of developing HCC compared to those who tested negative for anti-HCV [7]. HCV actively controls host biological processes in infected cells whose dysfunction can provoke HCC development [8].
The mechanisms underlying HCV effects on host gene methylation have been widely discussed [9, 10]. Of great interest are studies related to HCV’s effect on the transcriptional regulation of genes subject to aberrant methylation in HCC due to their extreme importance for HCC development. Such data can shed light on virus-induced carcinogenesis mechanisms after infection of normal cells in which gene methylation is unimpaired. It can also identify genes whose expression after hypermethylation or hypomethylation can potentially be modulated by viral proteins. Identifying host-virus regulatory pathways can identify the viral proteins underlying HCC risk factors. Such data could be of great importance in the search for new drugs that target these viral proteins.
Computational of protein-protein interaction (PPI) networks analyses have found wide applications for establishing HCC molecular mechanisms. For example, methylated differentially expressed genes (DEGs) in HCC have been examined using PPI networks [11–16]. However, analyses based on reconstructed gene networks describing gene expression regulation remain to be performed to identify the regulatory mechanisms of genes important for HCC with participating viral proteins.
Reconstructing regulatory gene networks is broadly used to study the molecular mechanisms underlying diseases and biological processes [17–19]. In prior research, we established the ANDSystem software and information system designed to facilitate the reconstruction and analysis of gene networks. This system leverages automated knowledge extraction methods applied to the content of scientific literature and factographic databases [20–22]. Specifically, the ANDSystem has been employed in various studies, such as reconstructing and analyzing the pre-eclampsia associome [23] and HCV interactome [24]. It also assisted identifying of novel susceptibility candidates for tuberculosis [25] and the discovery of potential candidate genes integral to the comorbidity of asthma and hypertension [26]. Furthermore, the software has been used in the analysis of programmed cell death modulation during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections [27], as well as in the identification of molecular mechanisms that involve nonstructural SARS-CoV-2 viral proteins in the metabolic dysregulation observed in SARSCoV-2-infected patients. This latter application was based on an analysis of plasma metabolomics and gene regulatory networks [28].
In this study, we employ the ANDSystem to reconstruct the regulatory pathways that may enable HCV proteins to modulate aberrantly methylated DEGs in HCC. The analysis examined 70 published HCC marker genes derived from genome-wide methylation analyses. Seven types of regulatory pathways that describe the influence of these genes by viral proteins were reconstructed. These pathways encompass PPIs, gene expression regulation, and the control of protein stability, activity, and transport. Among all the considered pathways, those involved in gene expression regulation were statistically significant. Out of 70 marker genes, 17 were differentially expressed in HCV-infected Huh 7.5 cells [29, 30]. Analysis of gene expression regulation pathways identified 7 out of 17 HCC marker genes potentially subject to regulation by HCV proteins, of which four had unidirectionally altered (upregulated) expression in HCC patients and HCV-infected Huh 7.5 cells: cyclin-dependent kinase inhibitor 2A (CDKN2A), flap endonuclease 1 (FEN1), DNA-binding protein inhibitor ID – 1 (ID1), and telomerase reverse transcriptase (TERT). Among those examined, we hypothesized that these genes might be responsible for the molecular mechanisms determining HCV as a risk factor for developing HCC.
Analyzing regulatory networks for these genes enabled us to make assumptions about viral protein effects on human protein functions in heterocomplexes formed due to PPIs between viral and human proteins in the considered networks. In particular, physical interactions between NS3 (p70) protein with human STAT3 cause its activation. NS3 can exert a similar effect on NOTC1 when heterocomplexed with it. Interactions between the protease NS5B (p68), envelope glycoprotein E2 (NS1), core viral (p21), and NS2-3 (p23) proteins with SETD2 are also expected to affect it positively. In contrast, interaction between viral proteins NS5A (p56) with human SMYD3 is expected to be inhibitory.
Of interest was the expected effect of interactions between viral protein NS3 (p70) and envelop glycoprotein E1 (gp32) with c-Jun. The network analysis indicated that NS3 and gp32 activate c-Jun when it positively regulates CDKN2A but inhibit it when it represses TERT.
Therefore, HCV protein modulation of host regulatory pathways may be important in HCC development during HCV infection. The findings of this study may provide valuable insights for the design of future experimental studies, such as the search for novel therapeutic targets or the development of pharmaceutical and prophylactic agents to mitigate the risk of HCC in the context of HCV infection.
2 Materials and methods
2.1 Analysis of genes exhibiting hypermethylation in HCC
Data on genes exhibiting aberrant methylation patterns in HCC were obtained from the scientific literature (Supplementary Table S1). Only those genes identified by the authors as the most significant were considered, creating a list of 70 genes. The largest gene groups were taken from a meta-analysis of differentially methylated genes [31] and an integrated analysis of microarray-based mRNA expression and methylation profiles [32]. Authors [31] used data on differential gene methylation in HCC tumor and adjacent tissue pairs, HCC tumor and normal tissue pairs, and HCC and normal serum pairs. They reported 22 marker genes, of which we excluded seven because their names did not allow us to accurately determine their identifiers in the Gene database: p16, p14, CDH1, p15, hMLH1, and p73. Ma et al. [32] identified novel aberrantly methylated DEGs using data from the GEO database: GSE19665 and GSE62232 for mRNA expression and GSE60753 for methylation. They identified 185 genes, including those downregulated and hypermethylated and those upregulated and hypomethylated. We took 35 genes for further analysis, which the authors listed among the central genes in their PPI network.
2.2 DEGs in HCV-infected cells
Two distinct sets of DEGs between mock-infected control and HCV-infected cells were created [1]: a compilation of 1844 significantly differentially expressed annotated genes identified in Huh 7.5 cells undergoing acute infection and proliferation at a 72-h mark [2, 30] a collection of 1886 genes derived from the analysis of the microarray-based dataset GSE66842 [29]. The GSE66842 dataset encompasses gene expression profiles of differentiated Huh7.5.1 cells subjected to infection with the HCV Jc1 clone. We used GEO2R [33] to screen for DEGs in 10-day post-infection samples in the dataset. We utilized an established threshold defined by a false discovery rate (FDR) below 0.05 to recognize differentially expressed genes.
2.3 Reconstructing regulatory pathways using the ANDSystem
Reconstruction of viral-protein-associated regulatory pathways containing HCC-associated genes (Table S1) was performed using the ANDSystem tool [21]. The ANDSystem knowledge base comprises a comprehensive gene network describing interactions between the objects of the ANDSystem’s ontology. This network was obtained via automated mining of scientific literature and factual databases. The ontology of the ANDSystem incorporates 13 distinct object types, including but not limited to proteins, genes, and metabolites, alongside 24 unique interaction types, such as expression, activity and stability regulation, physical interaction. Specific templates were used to search for pathways within the ANDSystem base of knowledge that dictate the structure of subgraphs embedded within the overarching gene network graph. Templates are defined as a sequential series of vertices that correspond to objects, where the edges that connect these vertices encapsulate the interactions between objects. Objects may be delineated either by a compilation of names or identifiers or, alternatively, by object type. In the first scenario, pathways containing objects from among those listed in the template will be searched. In the second case, any object of a given type can be included in the searched pathways.
We used seven different pathway templates (Table 1). The first object in all templates comprised HCV proteins, provided as a list of UniProt identifiers. The last object comprised a list of protein identifiers encoded by the genes in Table S1. The length of the templates varied from two to five objects. All intermediate objects were specified by their object type. Only human proteins and genes were considered intermediate objects. Interactions between objects in templates included PPIs, several regulatory interactions, and an expression-type relationship between a gene and its protein product. HCV proteins were only involved in PPIs with human proteins.
Templates for virus-host interaction pathways.
| N | Pathway templatea |
|---|---|
| 1. | Vp – PPI –> Tp |
| 2. | Vp – PPI –> Hp – PPI –> Tp |
| 3. | Vp – PPI –> Hp – Act/Stab/Pr/PPM/Tr –> Tp |
| 4. | Vp – PPI –> Hp – Exp reg –> Tg – Exp –> Tp |
| 5. | Vp – PPI –> Hp – Exp reg –> Hg – Exp –> Hp – Exp reg –> Tg – Exp –> Tp |
| 6. | Vp – PPI –> Hp – Exp reg –> Hg – Exp –> Hp – Act/Stab/Pr/PPM/Tr –> Tp |
| 7. | Vp – PPI –> Hp – Exp reg –> Hg – Exp –> Hp – PPI –> Tp |
-
aDesignations of objects and interactions: Vp, HCV proteins; Hp, any host proteins involved in the interactions; Hg, any host genes involved in the interactions; Tg, target gene (HCC marker gene); Tp, target protein (protein encoded by Tg); PPI, protein–protein interactions; Act/Stab/Pr/PPM/Tr, regulation of activity or stability or proteolysis or posttranslational modifications or transport (release); Exp reg, regulation of gene expression; Exp, gene expression (protein production).
3 Results
3.1 Identification of marker genes that can be regulated by viral proteins
We compiled a list of genes whose promoter hypermethylation or hypomethylation was accompanied by aberrant expression and associated with HCC in published data (Table S1). This list included 70 methylation-related DEGs. Most of these genes had been reported as HCC markers whose aberrant expression correlated with unfavorable disease prognoses. Regulatory pathways were reconstructed with the application of ANDSystem [21], intended to identify marker genes potentially regulated by viral proteins at the transcriptional regulation level, as illustrated in Table 1. We hypothesized that viral proteins could regulate such genes in normal and cancer cells, influencing the disease course and underlying risk factors. By deploying the ANDSystem software, the regulatory pathways that could potentially be modulated by HCV proteins influencing HCC marker genes were reassembled. This process was facilitated by templates that delineate various types of interactions between objects, as detailed in Table 2.
Significance of virus–host interaction pathway templates.
| Pathway template | Number of target genes | P-value | False discovery rate |
|---|---|---|---|
| 1. | 3 | 0.35 | 0.4 |
| 2. | 40 | 0.06 | 0.105 |
| 3. | 12 | 0.045 | 0.105 |
| 4. | 20 | 0.00017 | 0.0008 |
| 5. | 51 | 0.00023 | 0.0008 |
| 6. | 24 | 0.17 | 0.238 |
| 7. | 54 | 0.9 | 0.9 |
-
Values in bold were statistically significant (p < 0.0005).
In pathway templates, besides “expression regulation” interactions, we considered various interaction types, including PPIs; regulation of protein activity, stability, and transport (release); and post-translational modifications. Interestingly, among pathways involving all the regulation types mentioned above, only gene expression regulation represented by templates 4 and 5 was significant (Table 2). These pathways describe potential regulatory associations between HCV proteins and HCC marker genes with the participation of host mediator proteins and genes (Figures S1 and S2).
Nine HCV proteins, 19 host mediator proteins, and 20 HCC marker genes were involved in pathways associated with template 4 (Figures S1). Pathways reconstructed using template 5 contained 10 viral proteins, 51 HCC marker genes, 254 host mediator genes, and 344 host mediator proteins (Figures S2). According to the reconstructed regulatory pathways, HCV proteins possess the potential to modulate the expression of these hepatocellular carcinoma marker genes.
3.2 The employment of differential gene expression data derived from HCV-infected cell cultures
It should be noted that all reconstructed regulatory pathways are predictions and require further confirmation. In order to gain further insights, we analyzed previously published data on differential gene expression in HCV-infected cell cultures. Data concerning differential gene expression in Huh 7.5 cells acutely infected with HCV (72 h post-infection) were derived from the study by Papic et al. [30]. Additionally, another dataset, sourced from the work of Crouchet et al. [29] available in the GEO database (accession number GSE66842), included gene expression profiles of differentiated Huh 7.5.1 cells following infection with the HCV Jc1 clone. We used data representing 10 days post-infection in our analysis. In both studies, controls were mock-infected cells.
3.3 Reconstruction of regulatory pathways to differentially expressed genes in cell lines and patients
Of the 70 marker genes (Table S1), 17 were differentially expressed in HCV-infected cells (Table 3), of which seven were present in regulatory pathways (Figures S1 and S2). The data presented in Table 3 indicate a consistent pattern of alterations in gene expression among HCV-infected patients with HCC and corresponding cell lines. However, there are differences, which might reflect different aberrant methylation mechanisms in HCV presence and absence. The observed downregulation of tumor suppressor genes in patients, contrasted with their upregulation in HCV-infected cell lines, inversely mimicking the behavior of tumorigenic genes, suggests that not all regulatory pathways implicated in the transcriptional regulation of viral proteins necessarily contribute to the risk factors associated with HCC development. For further work, we considered only the gene group whose expression changed unidirectionally in patients and cell lines. Such genes may be of particular interest for studying the mechanisms through which HCV proteins influence HCC.
Differentially expressed marker genes in HCV-infected cells.
| Gene symbol | Crouchet et al. [29] | Papic et al. [30] | Expression in isolated | Expression in | Presence in regulatory | ||
|---|---|---|---|---|---|---|---|
| Fold change | FDR | Fold change | FDR | infected cellsb | HCC patients | pathwaysc | |
| CANX | – | – | 1,6 | 921,475 | Up | Up [34] | – |
| CDKN2A a | 2,74E−01 | 5 63E−02 | – | – | Up | Up [34, 35] | 4, 5 |
| CEP55 | 3,51E−01 | 3,11E−02 | – | – | Up | Up [36] | – |
| COL7A1 | 4,17E−01 | 1,69E−02 | – | – | Up | Up [37] | – |
| DSE | 4,66E−01 | 3,13E−03 | – | – | Up | Down [38] | – |
| FAT1 | 5,10E−01 | 4,59E−03 | – | – | Up | Up [39] | – |
| FEN1 | 5,81E−01 | 7,09E−03 | – | – | Up | Up [40] | 4, 5 |
| FNIP1 | – | – | 1,8 | 63,405 | Up | Down [34] | 5 |
| ID1 | 1,12 | 1,88E−04 | 2 | 445,256 | Up | Up [41] | 4, 5 |
| MCM6 | 6,06E−01 | 3,10E−03 | – | – | Up | Up [42] | – |
| NUSAP1 | 4,81E−01 | 1,30E−02 | – | – | Up | Up [43] | – |
| PROZ | −7,18E−01 | 5,85E−05 | – | – | Down | Up [44] | – |
| RARB | 2,23E−01 | 2,56E−02 | – | – | Up | Down [32] | 4, 5 |
| RASSF1 | 6,49E−01 | 1,60E−04 | 2,1 | 75,092 | Up | Down [34, 45] | 4, 5 |
| RFC4 | 5,05E−01 | 5,84E−03 | – | – | Up | Up [46] | – |
| RRAGD | 1,04 | 1,96E−05 | – | – | Up | Up [47] | – |
| TERT | – | – | 1,8 | 20,886 | Up | Up [48] | 4, 5 |
-
aThe signifcance value slightly exceeds 0.05. However, the gene is considered because of its importance. bHuh7.5.1 Huh7.5. cThe pathre specified by the number of pathway templates. Bold indicates genes whose expression changes coincide in patients and cell cultures.
3.4 Pathways with differentially expressed target marker and mediator genes
The next step in our analysis was reconstructing regulatory pathways in which both target marker and mediator genes are differentially expressed in HCV-infected cell lines (Figure 1). Our assumption was predicated on the notion that alterations in gene expression can correlate with the functional activities of viral proteins implicated in these regulatory pathways. The requirement for differential expression did not apply to proteins involved in PPIs with HCV proteins since their expression might not depend on viral proteins.
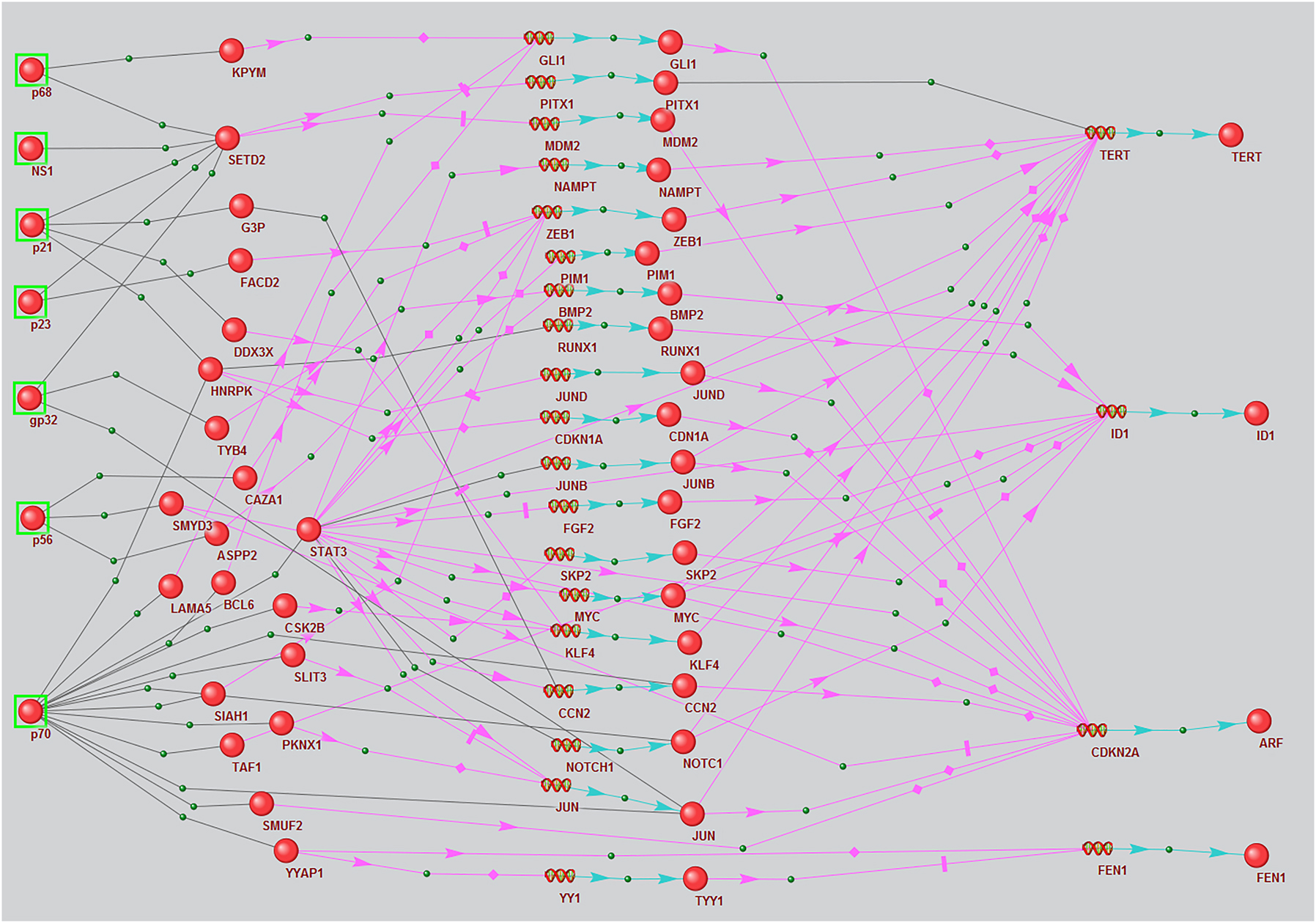
Regulatory pathways associated with viral protein modulation of the expression of HCC marker genes differentially expressed in infected cells, reconstructed using the ANDSystem according to combined templates 4 and 5. Balls with green frames denote viral proteins, other balls denote host proteins, spirals in the middle of the picture denote intermediate participant genes, and spirals on the right side denote marker genes. Black links between objects mean physical interaction, pink arrows – regulation of expression, blue arrows – production of a protein product from a gene.
3.5 ID1, FEN1, TERT, and CDKN2A regulatory pathways
Seven HCV proteins, 39 host mediator proteins, and four HCC marker genes are involved in pathways according to templates 4 and 5 (Figure 1). These marker genes were ID1, FEN1, TERT, and CDKN2A.
We reviewed individual regulatory pathways for each gene (Figure 2a–d). Three viral proteins, 10 human proteins, and six human genes were involved in regulating ID1 expression (Figure 2a). The viral proteins included gp32, core protein (p21), and NS3 (p70). NS3 had the most PPIs with human proteins.
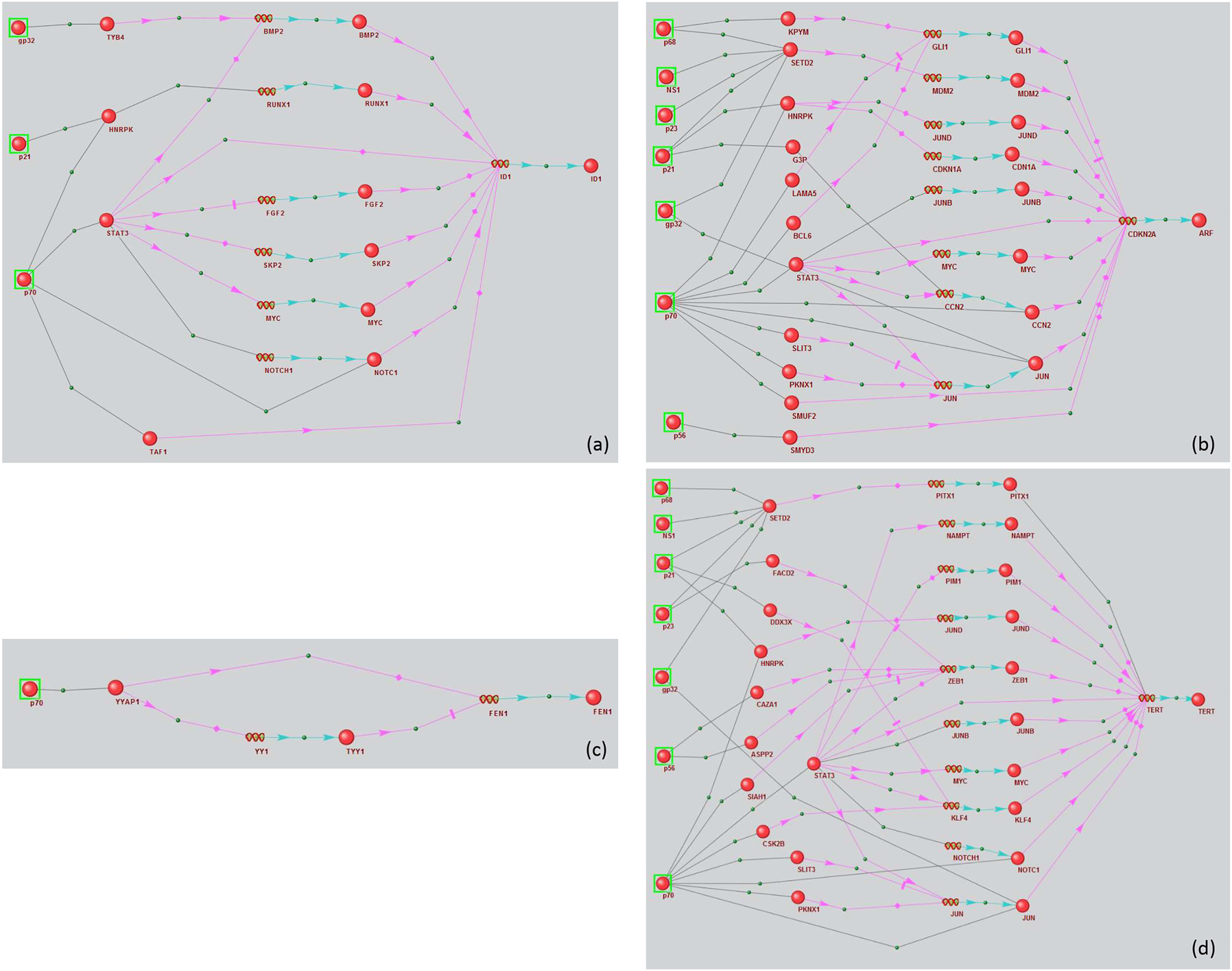
Regulatory networks of marker genes (a) ID1, (b) CDKN2A, (c) TERT, and (d) FEN1 modulated by HCV proteins. Black links between objects mean physical interaction, pink arrows – regulation of expression, blue arrows – production of a protein product from a gene.
Seven viral proteins, 19 human proteins, and eight human genes were involved in regulating CDKN2A (Figure 2b). The viral proteins included gp32, core protein (p21), NS5B (p68), protease NS2-3 (p23), NS5B (p56), NS1, and NS3 (p70).
The FEN1 regulatory network (Figure 2c) contained only one viral NS3 protein (p70) and two intermediate participants (the human YYAP1 protein and TYY1 gene and protein).
The TERT regulatory network involved seven viral proteins (gp32, p21, p23, p56, p68, p70, and NS1), 21 human proteins, and 10 human genes (Figure 2d). Among viral proteins, the NS3 protein had the most interactions with human proteins, consistent with the previous cases.
4 Discussion
The scientific literature has extensively discussed the regulation of host gene methylation by HCV proteins [9, 10]. Many genes subject to aberrant methylation contribute significantly to HCC pathophysiological mechanisms. Therefore, data on their transcriptional regulation, in which viral proteins are involved, may be of great interest. It is plausible to posit that viral protein regulatory interactions with these genes, potentially mediated through their transcription factors, could either repress or induce their expression. These effects could have similar consequences to hypermethylation or hypomethylation, provided HCV infection does not alter their methylation profile. In addition, viral regulation of the expression of already methylated genes can also change their expression profile. However, HCV’s effect on the transcriptional regulation of aberrantly methylated genes in HCC has not been systematically analyzed using reconstructed regulatory gene networks.
In this study, we employed the ANDSystem [21] to reconstruct and evaluate the statistical significance of regulatory pathways that potentially mediate the influence of hepatitis C virus proteins on host genes. We analyzed a set of previously reported genes aberrantly methylated in HCC patients in genome-wide methylation analyses (Table S1). This set included 70 genes previously characterized as epigenetic gatekeepers, HCC tumor suppressors, and protumorigenic factors.
Supplementing the data on anomalous methylation and gene expression sourced from patients, we incorporated data on differential gene expression in HCV-infected Huh 7.5 and 7.5.1 cells [29, 30]. Only 17 out of the 70 marker genes exhibited differential expression in HCV-infected cells.
It could be expected that viral proteins might regulate these genes through changes in their methylation status and effects on transcription factors. Indeed, 7 of the 17 DEGs were participants in regulatory pathways describing their expression regulation by viral proteins.
The virus-induced upregulation of tumorigenic gene expression and suppression of tumor suppressor gene expression in HCC patients may indicate the viral proteins’ contribution to HCC. Therefore, we considered the correspondence between the directions of marker gene expression changes in data for patients and cell cultures. A unidirectional change in gene expression in these datasets, activating tumorigenic genes or inhibiting tumor suppressor genes, suggests that they constitute one HCV pathological mechanism.
The next stage of the analysis was reconstructing regulatory networks with the requirement that all marker and mediator genes encoding transcription factors regulating marker genes show differential expression in HCV-infected cells (Figure 1). By incorporating this additional criterion, the count of marker genes within the regulatory network exhibiting unidirectional expression alterations in both HCC patients and cell lines was reduced to four: ID1, FEN1, CDKN2A, and TERT. We hypothesize that the influence of viral proteins on the expression of these genes might play a role in the mechanisms that render HCV a risk factor for HCC.
4.1 ID1’s regulatory network
ID1’s regulatory network is shown in Figure 2a. ID1 expression was upregulated in HCC patients and HCV-infected cells [41] (Table 3). There are three short pathways in the regulatory network that bind viral proteins to ID1; all involve NS3 (NS3/STAT3/ID1, NS3/NOTC1/ID1, and NS3/TAF1/ID1). While physical interaction between NS3 viral protein and STAT3 has been previously shown [49], their effects remain unclear. STAT3 is directly associated with ID1 upregulation [50]. In addition, STAT3 can regulate ID1 by regulating MYC and FGF2, whose expression is upregulated by STAT3 [51, 52]. It should be noted that MYC and FGF2 expression was elevated in HCV-infected Huh 7.5 cells [30]. Likewise, while NS3’s physical interaction with NOTC1 has been previously shown [49], its effects on NOTC1 function remain unclear. NS3 can also positively regulate NOTCH1 expression via STAT3. Data on physical interactions between STAT3 and NOTCH1 have been reported [53]. NOTCH1 then positively regulates ID1 expression [54].
Therefore, an analysis of the regulatory network indicates that PPIs between viral NS3 with human STAT3 accompany ID1 activation. In addition, NS3’s interaction with NOTC1 also positively affects its activity.
4.2 CDKN2A’s regulatory network
CDKN2A expression was upregulated in HCC patients and HCV-infected cells (Table 3). Similar to ID1, NS3 (p70) occupies a central place in the network with respect to interaction numbers with human proteins (Figure 2b).
SETD2 was the human protein targeted by the most viral proteins, interacting with five: gp32, core protein (p21), NS5B (p68), NS2-3 (p23), and NS1. The regulatory network contains five short pathways linking viral proteins to CDKN2A: NS3/CCN2/CDKN2A, NS3/JUN/CDKN2A, NS3/STAT3/CDKN2A, NS3/SMUF2/CDKN2A, and NS5A(p56)/SMYD3/CDKN2A. Four of these pathways involve the viral protein NS3.
Published data has documented PPIs between NS5A and SMYD3 [49, 55, 56]. These studies demonstrated that NS5A co-localizes with SMYD3 solely in the cytoplasm, inhibiting SMYD3’s nuclear localization [55]. The capacity of SMYD3 to suppress CDKN2A expression has also been explored in the literature [57]. The regulatory network analysis indicated that NS5A’s interaction with SMYD3 inhibits SMYD3, suppressing its ability to inhibit CDKN2A expression, consistent with increased CDKN2A expression with HCV infection. Another CDKN2A activation pathway involves viral protein-mediated suppression of its inhibitors and may involve NS3/CCN2/CDKN2A interactions. For example, data on CCN2’s ability to negatively affect CDKN2A expression has been reported [58]. NS3 can physically interact with CCN2 [49]. Therefore, it can be hypothesized that NS3’s interaction with CCN2 might prevent CCN2 from inhibiting CDKN2A expression.
The NS3/STAT3/MYC/CDKN2A pathway might be the CDKN2A activation pathway resulting from the positive effects of viral proteins on human proteins. The NS3 protein’s effects on human gene expression have been previously discussed [59]. It should be noted that positive CDKN2A regulators, such as MYC, JUN, and GLI1, are upregulated in HCV-infected cells [29, 30].
Therefore, this regulatory network analysis supports the following assumptions about viral protein effects on human protein functions due to their physical PPIs: NS5A inhibits SMYD3, NS3 inhibits CCN2, and NS3, gp32 activates c-Jun.
4.3 FEN1’s regulatory network
FEN1 is controlled by the following participants: viral NS3 (p70), human YYAP1 and YY1 (p70; Figure 2c). While a physical interaction between NS3 and YYAP1 has been previously reported [49], the effects of PPIs between NS3 and YYAP1 on YYAP1 function have not been previously described. YYAP1 can negatively regulate FEN1 expression by activating YY1 expression, a FEN1 transcriptional repressor [40, 60]. Interestingly, our reconstructed pathway indicates that increased FEN1 expression with HCV infection can be explained by NS3 interacting with YYAP1, suppressing its ability to activate YY1.
4.4 TERT’s regulatory network
Like ID1, FEN1, and CDKN2A, TERT expression was upregulated in HCC patients and HCV-infected cells (Table 3). Four short pathways from viral proteins to TERT can be seen in the regulatory net-work: NS3(p70)/STAT3/TERT, NS3(p70)/gp32/JUN/TERT, p23/p68/NS1/p21/SETD2/TERT, and NS3/NOTC1/TERT (Figure 2d). Three participants in these pathways (STAT3, SETD2, and NOTC1) are positively associated with TERT [61–65], while JUN inhibits TERT expression [66, 67]. Since TERT was activated with HCV infection, it can be hypothesized that the physical interactions of viral proteins with positive TERT regulators STAT3, SETD2, and NOTC1 may positively affect their activity.
Physical interactions between c-JUN and viral proteins NS3 and E1 appeared to inhibit c-JUN. Interestingly, c-JUN was involved in CDKN2A upregulation in the CDKN2A regulatory network, and it was assumed that viral proteins activated c-JUN. However, c-JUN is inhibited in the TERT network, and it can be hypothesized that these same viral proteins interfere with its activity.
STAT3 was a member of the NS3/STAT3/TERT pathway and was associated with nine other nodes in the network. Therefore, it can be called the hub of this regulatory network. STAT3 activation by NS3 was discussed above for the ID1 regulatory network. The effects of NOTCH signaling pathway activation by the NS3 protein have been previously discussed [68]. However, it should again be noted that our data did not support the effect of NS3’s physical interaction with NOTC1. There is also no published information on the functional effects of SETD2’s physical interactions with viral proteins. Based on our analysis, SET2D’s interactions with viral proteins NS2-3 (p23), NS5B (p68), NS1, and NS3 (p70) might be expected to positively affect its ability to activate TERT.
Therefore, this regulatory network analysis is consistent with our assumption that the NS3 protein activates STAT3, and also activates NOTC1. Interestingly, the expected effects of NS3 and gp32 interaction with c-Jun are likely opposing when c-Jun acts as a CDKN2A activator and a TERT repressor.
We discussed the expected effects of physical interactions between viral NS3 protein and STAT3 in the ID1, CDKN2A, and TERT regulatory networks. We also hypothesized that viral NS3 interaction with STAT3 positively affects STAT3 activity for all three networks. Consequently, the reconstituted regulatory networks serve as a foundation for generating hypotheses about the molecular mechanisms underpinning HCV proteins’ impact on pivotal genes associated with HCC. Our qualitative analyses of these networks allowed us to propose several hypotheses about the mechanisms of host-virus interactions and the role of specific viral proteins in them. However, it should be noted that a rigorous quantitative analysis of the reconstructed networks requires mathematical modeling of their dynamics. Many interactions in gene networks can contribute non-linearly to the regulation of gene expression, the signal interference from opposing pathways can also have non-linear effects.
Funding source: Ministry of Science and Higher Education of the Russian Federation within the ERA-NET Target identification and drug development in liver cancer (TAIGA)
Award Identifier / Grant number: No 075-15-2021-944
-
Research ethics: Not applicable.
-
Author contributions: The conceptual framework was provided by I.V.A., L.I.N., and K.N.A.; Methodological design was orchestrated by I.V.A., V.A.R., and A.E.A.; Software utilization was managed by D.P.S. and I.V.A.; Validation procedures were performed by A.E.A., K.T.M.; The investigation was conducted by V.A.S., V.A.R., G.A.D., A.A.V., R.P.M., I.N.V., I.T.V.; The initial draft of the manuscript was created by A.E.A. and I.V.A.; The manuscript underwent review and editing by K.T.M., I.V.A., A.E.A.; Data visualization was executed by D.P.S., A.E.A.; Supervision was provided by I.V.A.; Funding was acquired by I.V.A., L.I.N. All authors have agreed to be accountable for the entirety of this manuscript’s content and have approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Research funding: This research was funded by project No 075-15-2021-944 of the Ministry of Science and Higher Education of the Russian Federation within the ERA-NET “Target identification and drug development in liver cancer (TAIGA)”.
-
Data availability: Not applicable.
References
1. Ahmad, FB, Cisewski, JA, Anderson, RN. Provisional mortality data – United States, 2021. MMWR Morb Mortal Wkly Rep 2022;71:597–600. https://doi.org/10.15585/mmwr.mm7117e1.Search in Google Scholar PubMed PubMed Central
2. Yang, JD, Hainaut, P, Gores, GJ, Amadou, A, Plymoth, A, Roberts, LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589–604. https://doi.org/10.1038/s41575-019-0186-y.Search in Google Scholar PubMed PubMed Central
3. Goodgame, B, Shaheen, NJ, Galanko, J, El-Serag, HB. The risk of end stage liver disease and hepatocellular carcinoma among persons infected with hepatitis C virus: publication bias? Am J Gastroenterol 2003;98:2535–42. https://doi.org/10.1111/j.1572-0241.2003.07678.x.Search in Google Scholar PubMed
4. Fasani, P, Sangiovanni, A, De Fazio, C, Borzio, M, Bruno, S, Ronchi, G, et al.. High prevalence of multinodular hepatocellular carcinoma in patients with cirrhosis attributable to multiple risk factors. Hepatology 1999;29:1704–7. https://doi.org/10.1002/hep.510290604.Search in Google Scholar PubMed
5. Stroffolini, T, Andreone, P, Andriulli, A, Ascione, A, Craxì, A, Chiaramonte, M, et al.. Gross pathologic types of hepatocellular carcinoma in Italy. Oncology 1999;56:189–92. https://doi.org/10.1159/000011963.Search in Google Scholar PubMed
6. Yoshizawa, H. Hepatocellular carcinoma associated with hepatitis C virus infection in Japan: projection to other countries in the foreseeable future. Oncology 2002;62:8–17. https://doi.org/10.1159/000048270.Search in Google Scholar PubMed
7. Mittal, S, El-Serag, HB. Epidemiology of HCC: consider the population. J Clin Gastroenterol 2013;47:S2. https://doi.org/10.1097/mcg.0b013e3182872f29.Search in Google Scholar
8. D’souza, S, Lau, KC, Coffin, CS, Patel, TR. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroenterol 2020;26:5759–83. https://doi.org/10.3748/wjg.v26.i38.5759.Search in Google Scholar PubMed PubMed Central
9. Arora, P, Kim, EO, Jung, JK, Jang, KL. Hepatitis C virus core protein downregulates E-cadherin expression via activation of DNA methyltransferase 1 and 3b. Cancer Lett 2008;261:244–52. https://doi.org/10.1016/j.canlet.2007.11.033.Search in Google Scholar PubMed
10. Liu, M, Du, L, Cheng, X, Yuan, M, Shang, J, Shi, Y, et al.. CpG island methylation of suppressor of cytokine signaling-1 gene induced by HCV is associated with HCV-related hepatocellular carcinoma. Front Microbiol 2022;13:679593. https://doi.org/10.3389/fmicb.2022.679593.Search in Google Scholar PubMed PubMed Central
11. Sang, L, Wang, XM, Xu, DY, Zhao, WJ. Bioinformatics analysis of aberrantly methylated-differentially expressed genes and pathways in hepatocellular carcinoma. World J Gastroenterol 2018;24:2605–16. https://doi.org/10.3748/wjg.v24.i24.2605.Search in Google Scholar PubMed PubMed Central
12. Fan, G, Tu, Y, Chen, C, Sun, H, Wan, C, Cai, X. DNA methylation biomarkers for hepatocellular carcinoma. Cancer Cell Int 2018;18:140. https://doi.org/10.1186/s12935-018-0629-5.Search in Google Scholar PubMed PubMed Central
13. Cai, C, Xie, X, Zhou, J, Fang, X, Wang, F, Wang, M. Identification of TAF1, SAT1, and ARHGEF9 as DNA methylation biomarkers for hepatocellular carcinoma. J Cell Physiol 2020;235:611–18. https://doi.org/10.1002/jcp.28999.Search in Google Scholar PubMed
14. Zhen, L, Ning, G, Wu, L, Zheng, Y, Yang, F, Chen, T, et al.. Prognostic value of aberrantly expressed methylation genes in human hepatocellular carcinoma. Biosci Rep 2020;40:BSR20192593. https://doi.org/10.1042/bsr20192593.Search in Google Scholar PubMed PubMed Central
15. Cao, J, Zhang, R, Zhang, Y, Wang, Y. Combined screening analysis of aberrantly methylated-differentially expressed genes and pathways in hepatocellular carcinoma. J Gastrointest Oncol 2022;13:311–25. https://doi.org/10.21037/jgo-21-866.Search in Google Scholar PubMed PubMed Central
16. Kakar, MU, Mehboob, MZ, Akram, M, Shah, M, Shakir, Y, Ijaz, HW, et al.. Identification of differentially expressed genes associated with the prognosis and diagnosis of hepatocellular carcinoma by integrated bioinformatics analysis. BioMed Res Int 2022;2022:4237633. https://doi.org/10.1155/2022/4237633.Search in Google Scholar PubMed PubMed Central
17. Parikshak, NN, Gandal, MJ, Geschwind, DH. Systems biology and gene networks in neurodevelopmental and neurodegenerative disorders. Nat Rev Genet 2015;16:441–58. https://doi.org/10.1038/nrg3934.Search in Google Scholar PubMed PubMed Central
18. Hu, JX, Thomas, CE, Brunak, S. Network biology concepts in complex disease comorbidities. Nat Rev Genet 2016;17:615–29. https://doi.org/10.1038/nrg.2016.87.Search in Google Scholar PubMed
19. Morand, GB, Diaconescu, A, Ibrahim, I, Lamarche, G, Ruas, JS, Dalfen, J, et al.. Molecular prognostic indicators in HPV-positive oropharyngeal cancer: an updated review. Clin Exp Metastasis 2022;27:1–10. https://doi.org/10.1007/s10585-022-10148-9.Search in Google Scholar PubMed
20. Ivanisenko, VA, Saik, OV, Ivanisenko, NV, Tiys, ES, Ivanisenko, TV, Demenkov, PS, et al.. ANDSystem: an Associative Network Discovery System for automated literature mining in the field of biology. BMC Syst Biol 2015;9:S2. https://doi.org/10.1186/1752-0509-9-s2-s2.Search in Google Scholar PubMed PubMed Central
21. Ivanisenko, VA, Demenkov, PS, Ivanisenko, TV, Mishchenko, EL, Saik, OV. A new version of the ANDSystem tool for automatic extraction of knowledge from scientific publications with expanded functionality for reconstruction of associative gene networks by considering tissue-specific gene expression. BMC Bioinf 2019;20:34. https://doi.org/10.1186/s12859-018-2567-6.Search in Google Scholar PubMed PubMed Central
22. Ivanisenko, TV, Saik, OV, Demenkov, PS, Ivanisenko, NV, Savostianov, AN, Ivanisenko, VA. ANDDigest: a new web-based module of ANDSystem for the search of knowledge in the scientific literature. BMC Bioinf 2020;21:228. https://doi.org/10.1186/s12859-020-03557-8.Search in Google Scholar PubMed PubMed Central
23. Glotov, AS, Tiys, ES, Vashukova, ES, Pakin, VS, Demenkov, PS, Saik, OV, et al.. Molecular association of pathogenetic contributors to pre-eclampsia (pre-eclampsia associome). BMC Syst Biol 2015;9:S4. https://doi.org/10.1186/1752-0509-9-s2-s4.Search in Google Scholar
24. Saik, OV, Ivanisenko, TV, Demenkov, PS, Ivanisenko, VA. Interactome of the hepatitis C virus: literature mining with ANDSystem. Virus Res 2016;218:40–8. https://doi.org/10.1016/j.virusres.2015.12.003.Search in Google Scholar PubMed
25. Bragina, EY, Tiys, ES, Rudko, AA, Ivanisenko, VA, Freidin, MB. Novel tuberculosis susceptibility candidate genes revealed by the reconstruction and analysis of associative networks. Infect Genet Evol 2016;46:118–23. https://doi.org/10.1016/j.meegid.2016.10.030.Search in Google Scholar PubMed
26. Saik, OV, Demenkov, PS, Ivanisenko, TV, Bragina, EY, Freidin, MB, Goncharova, IA, et al.. Novel candidate genes important for asthma and hypertension comorbidity revealed from associative gene networks. BMC Med Genom 2018;11:15. https://doi.org/10.1186/s12920-018-0331-4.Search in Google Scholar PubMed PubMed Central
27. Ivanisenko, NV, Seyrek, K, Kolchanov, NA, Ivanisenko, VA, Lavrik, IN. The role of death domain proteins in host response upon SARS-CoV-2 infection: modulation of programmed cell death and translational applications. Cell Death Dis 2020;6:101. https://doi.org/10.1038/s41420-020-00331-w.Search in Google Scholar PubMed PubMed Central
28. Ivanisenko, VA, Gaisler, EV, Basov, NV, Rogachev, AD, Cheresiz, SV, Ivanisenko, TV, et al.. Plasma metabolomics and gene regulatory networks analysis reveal the role of nonstructural SARS-CoV-2 viral proteins in metabolic dysregulation in COVID-19 patients. Sci Rep 2022;12:19977. https://doi.org/10.1038/s41598-022-24170-0.Search in Google Scholar PubMed PubMed Central
29. Crouchet, E, Bandiera, S, Fujiwara, N, Li, S, El Saghire, H, Fernández-Vaquero, M, et al.. A human liver cell-based system modeling a clinical prognostic liver signature for therapeutic discovery. Nat Commun 2021;12:5525. https://doi.org/10.1038/s41467-021-25468-9.Search in Google Scholar PubMed PubMed Central
30. Papic, N, Maxwell, CI, Delker, DA, Liu, S, Heale, BS, Hagedorn, CH. RNA-sequencing analysis of 5’capped RNAs identifies many new differentially expressed genes in acute hepatitis C virus infection. Viruses 2012;4:581–612. https://doi.org/10.3390/v4040581.Search in Google Scholar PubMed PubMed Central
31. Zhang, C, Li, J, Huang, T, Duan, S, Dai, D, Jiang, D, et al.. Meta-analysis of DNA methylation biomarkers in hepatocellular carcinoma. Oncotarget 2016;7:81255–67. https://doi.org/10.18632/oncotarget.13221.Search in Google Scholar PubMed PubMed Central
32. Ma, Z, Liu, Y, Hao, Z, Hua, X, Li, W. DNA hypermethylation of aurora kinase A in hepatitis C virus-positive hepatocellular carcinoma. Mol Med Rep 2019;20:2519–32. https://doi.org/10.3892/mmr.2019.10487.Search in Google Scholar PubMed PubMed Central
33. Barrett, T, Wilhite, SE, Ledoux, P, Evangelista, C, Kim, IF, Tomashevsky, M, et al.. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res 2013;41:D991–5. https://doi.org/10.1093/nar/gks1193.Search in Google Scholar PubMed PubMed Central
34. Feng, Q, Stern, JE, Hawes, SE, Lu, H, Jiang, M, Kiviat, NB. DNA methylation changes in normal liver tissues and hepatocellular carcinoma with different viral infection. Exp Mol Pathol 2010;88:287–92. https://doi.org/10.1016/j.yexmp.2010.01.002.Search in Google Scholar PubMed PubMed Central
35. Luo, JP, Wang, J, Huang, JH. CDKN2A is a prognostic biomarker and correlated with immune infiltrates in hepatocellular carcinoma. Biosci Rep 2021;41:BSR20211103. https://doi.org/10.1042/bsr20211103.Search in Google Scholar
36. Li, M, Gao, J, Li, D, Yin, Y. CEP55 promotes cell motility via JAK2−STAT3−MMPs cascade in hepatocellular carcinoma. Cells 2018;7:99. https://doi.org/10.3390/cells7080099.Search in Google Scholar PubMed PubMed Central
37. Patil, MA, Chua, MS, Pan, KH, Lin, R, Lih, CJ, Cheung, ST, et al.. An integrated data analysis approach to characterize genes highly expressed in hepatocellular carcinoma. Oncogene 2005;24:3737–47. https://doi.org/10.1038/sj.onc.1208479.Search in Google Scholar PubMed
38. Liao, WC, Yen, HR, Liao, CK, Tseng, TJ, Lan, CT, Liu, CH. DSE regulates the malignant characters of hepatocellular carcinoma cells by modulating CCL5/CCR1 axis. Am J Cancer Res 2019;9:347–62.Search in Google Scholar
39. Zhu, HY, Cao, GY, Wang, SP, Chen, Y, Liu, GD, Gao, YJ, et al.. POU2F1 promotes growth and metastasis of hepatocellular carcinoma through the FAT1 signaling pathway. Am J Cancer Res 2017;7:1665–79.Search in Google Scholar
40. Zhang, Y, Liu, X, Liu, L, Chen, J, Hu, Q, Shen, S, et al.. Upregulation of FEN1 is associated with the tumor progression and prognosis of hepatocellular carcinoma. Dis Markers 2020;2020:2514090. https://doi.org/10.1155/2020/2514090.Search in Google Scholar PubMed PubMed Central
41. Wu, M, Zhou, Y, Fei, C, Chen, T, Yin, X, Zhang, L, et al.. ID1 overexpression promotes HCC progression by amplifying the AURKA/Myc signaling pathway. Int J Oncol 2020;57:845–57. https://doi.org/10.3892/ijo.2020.5092.Search in Google Scholar PubMed
42. Zheng, T, Chen, M, Han, S, Zhang, L, Bai, Y, Fang, X, et al.. Plasma minichromosome maintenance complex component 6 is a novel biomarker for hepatocellular carcinoma patients. Hepatol Res 2014;44:1347–56. https://doi.org/10.1111/hepr.12303.Search in Google Scholar PubMed
43. Satow, R, Shitashige, M, Kanai, Y, Takeshita, F, Ojima, H, Jigami, T, et al.. Combined functional genome survey of therapeutic targets for hepatocellular carcinoma. Clin Cancer Res 2010;16:2518–28. https://doi.org/10.1158/1078-0432.ccr-09-2214.Search in Google Scholar PubMed
44. Tsai, TH, Song, E, Zhu, R, Di Poto, C, Wang, M, Luo, Y, et al.. LC-MS/MS-based serum proteomics for identification of candidate biomarkers for hepatocellular carcinoma. Proteomics 2015;15:2369–81. https://doi.org/10.1002/pmic.201400364.Search in Google Scholar PubMed PubMed Central
45. Hu, L, Chen, G, Yu, H, Qiu, X. Clinicopathological significance of RASSF1A reduced expression and hypermethylation in hepatocellular carcinoma. Hepatol Int 2010;4:423–32. https://doi.org/10.1007/s12072-010-9164-8.Search in Google Scholar PubMed PubMed Central
46. Yang, WX, Pan, YY, You, CG. CDK1, CCNB1, CDC20, BUB1, MAD2L1, MCM3, BUB1B, MCM2, and RFC4 may be potential therapeutic targets for hepatocellular carcinoma using integrated bioinformatic analysis. BioMed Res Int 2019;2019:1245072. https://doi.org/10.1155/2019/1245072.Search in Google Scholar PubMed PubMed Central
47. Ding, L, Liang, X. Ras related GTP binding D promotes aerobic glycolysis of hepatocellular carcinoma. Ann Hepatol 2021;23:100307. https://doi.org/10.1016/j.aohep.2021.100307.Search in Google Scholar PubMed
48. Manganelli, M, Grossi, I, Corsi, J, D’Agostino, VG, Jurikova, K, Cusanelli, E, et al.. Expression of cellular and extracellular TERRA, TERC and TERT in hepatocellular carcinoma. Int J Mol Sci 2022;23:6183. https://doi.org/10.3390/ijms23116183.Search in Google Scholar PubMed PubMed Central
49. de Chassey, B, Navratil, V, Tafforeau, L, Hiet, MS, Aublin-Gex, A, Agaugué, S, et al.. Hepatitis C virus infection protein network. Mol Syst Biol 2008;4:230. https://doi.org/10.1038/msb.2008.66.Search in Google Scholar PubMed PubMed Central
50. Yu, H, Yue, X, Zhao, Y, Li, X, Wu, L, Zhang, C, et al.. LIF negatively regulates tumour-suppressor p53 through Stat3/ID1/MDM2 in colorectal cancers. Nat Commun 2014;5:5218. https://doi.org/10.1038/ncomms6218.Search in Google Scholar PubMed PubMed Central
51. Bromberg, J. Signal transducers and activators of transcription as regulators of growth, apoptosis and breast development. Breast Cancer Res 2000;2:86–90. https://doi.org/10.1186/bcr38.Search in Google Scholar PubMed PubMed Central
52. Bishop, JL, Thaper, D, Zoubeidi, A. The multifaceted roles of STAT3 signaling in the progression of prostate cancer. Cancers 2014;6:829–59. https://doi.org/10.3390/cancers6020829.Search in Google Scholar PubMed PubMed Central
53. Kalkan, R. Glioblastoma stem cells as a new therapeutic target for glioblastoma. Clin Med Insights Oncol 2015;9:95–103. https://doi.org/10.4137/cmo.s30271.Search in Google Scholar PubMed PubMed Central
54. Yoshida, Y, Hayashi, Y, Suda, M, Tateno, K, Okada, S, Moriya, J, et al.. Notch signaling regulates the lifespan of vascular endothelial cells via a p16-dependent pathway. PLoS One 2014;9:e100359. https://doi.org/10.1371/journal.pone.0100359.Search in Google Scholar PubMed PubMed Central
55. Chen, M, Gan, X, Yoshino, K, Kitakawa, M, Shoji, I, Deng, L, et al.. Hepatitis C virus NS5A protein interacts with lysine methyltransferase SET and MYND domain-containing 3 and induces activator protein 1 activation. Microbiol Immunol 2016;60:407–17. https://doi.org/10.1111/1348-0421.12383.Search in Google Scholar PubMed
56. Eberle, CA, Zayas, M, Stukalov, A, Pichlmair, A, Alvisi, G, Müller, AC, et al.. The lysine methyltransferase SMYD3 interacts with hepatitis C virus NS5A and is a negative regulator of viral particle production. Virology 2014;462–463:34–41. https://doi.org/10.1016/j.virol.2014.05.016.Search in Google Scholar PubMed PubMed Central
57. Jiang, LH, Hao, YL, Zhu, JW. Expression and prognostic value of HER-2/neu, STAT3 and SOCS3 in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 2019;43:282–91. https://doi.org/10.1016/j.clinre.2018.09.011.Search in Google Scholar PubMed
58. Rui, YF, Chen, MH, Li, YJ, Xiao, LF, Geng, P, Wang, P, et al.. CTGF attenuates tendon-derived stem/progenitor cell aging. Stem Cell Int 2019;2019:6257537. https://doi.org/10.1155/2019/6257537.Search in Google Scholar PubMed PubMed Central
59. Hassan, M, Ghozlan, H, Abdel-Kader, O. Activation of c-Jun NH2-terminal kinase (JNK) signaling pathway is essential for the stimulation of hepatitis C virus (HCV) non-structural protein 3 (NS3)-mediated cell growth. Virology 2005;333:324–36. https://doi.org/10.1016/j.virol.2005.01.008.Search in Google Scholar PubMed
60. Wang, J, Zhou, L, Li, Z, Zhang, T, Liu, W, Liu, Z, et al.. YY1 suppresses FEN1 overexpression and drug resistance in breast cancer. BMC Cancer 2015;15:50. https://doi.org/10.1186/s12885-015-1043-1.Search in Google Scholar PubMed PubMed Central
61. Bilsland, AE, Stevenson, K, Liu, Y, Hoare, S, Cairney, CJ, Roffey, J, et al.. Mathematical model of a telomerase transcriptional regulatory network developed by cell-based screening: analysis of inhibitor effects and telomerase expression mechanisms. PLoS Comput Biol 2014;10:e1003448. https://doi.org/10.1371/journal.pcbi.1003448.Search in Google Scholar PubMed PubMed Central
62. Deeb, D, Brigolin, C, Gao, X, Liu, Y, Pindolia, KR, Gautam, SC. Induction of apoptosis in pancreatic cancer cells by CDDO-me involves repression of telomerase through epige-netic pathways. J Carcinog Mutagen 2014;5:177. https://doi.org/10.4172/2157-2518.1000177.Search in Google Scholar PubMed PubMed Central
63. Chung, SS, Oliva, B, Dwabe, S, Vadgama, JV. Combination treatment with flavonoid morin and telomerase inhibitor MST-312 reduces cancer stem cell traits by targeting STAT3 and telomerase. Int J Oncol 2016;49:487–98. https://doi.org/10.3892/ijo.2016.3546.Search in Google Scholar PubMed PubMed Central
64. Sawangarun, W, Mandasari, M, Aida, J, Morita, KI, Kayamori, K, Ikeda, T, et al.. Loss of Notch1 predisposes oro-esophageal epithelium to tumorigenesis. Exp Cell Res 2018;372:129–40. https://doi.org/10.1016/j.yexcr.2018.09.019.Search in Google Scholar PubMed
65. Fragkiadaki, P, Tsoukalas, D, Fragkiadoulaki, I, Psycharakis, C, Nikitovic, D, Spandidos, DA, et al.. Telomerase activity in pregnancy complications (Review). Mol Med Rep 2016;14:16–21. https://doi.org/10.3892/mmr.2016.5231.Search in Google Scholar PubMed PubMed Central
66. Takakura, M, Kyo, S, Inoue, M, Wright, WE, Shay, JW. Function of AP-1 in transcription of the telomerase reverse transcriptase gene (TERT) in human and mouse cells. Mol Cell Biol 2005;25:8037–43. https://doi.org/10.1128/mcb.25.18.8037-8043.2005.Search in Google Scholar
67. Donati, B, Ciarrocchi, A. Telomerase and telomeres biology in thyroid cancer. Int J Mol Sci 2019;20:2887. https://doi.org/10.3390/ijms20122887.Search in Google Scholar PubMed PubMed Central
68. Iwai, A, Takegami, T, Shiozaki, T, Miyazaki, T. Hepatitis C virus NS3 protein can activate the Notch-signaling pathway through binding to a transcription factor. SRCAP. PloS one 2011;6:e20718. https://doi.org/10.1371/journal.pone.0020718.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jib-2023-0013).
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.



