Abstract
The transduction of painful stimuli into the experience of pain involves several peripheral and central signaling pathways of the nervous system. The organization of these pathways parallels the main functions of pain: the assessment of noxious stimuli (where, what, how strong), and the negative emotion of unpleasantness. Multiple lines of evidence suggest that the thalamocortical (TC) system, which interprets ascending pain signals, has two main pathways which support these functions. We discuss the structural and functional findings that support the view that the lateral TC pathway is involved in discriminative assessment of pain, while the medial TC pathway gives rise to aversive emotions associated with pain. Our review focuses on acute pain, but we also discuss putative TC maladaptations in humans and animal models of pain that are thought to underlie pathological pain sensations.
Introduction
Pain is a vitally important sense for us, which protects us from damages to the body. However, pain can lose this function when it becomes chronic, and is signaled ceaselessly even in the absence of danger. When considering how pain signals become perceptions it is important to discriminate between acute and chronic pain because different neuronal mechanisms are involved in the genesis of the two types of pain, albeit with some overlap (Kuner R and H Flor 2016). While acute pain signals originate from the activation of peripheral receptors sensitive to noxious stimuli (nociceptors), the genesis of chronic pain is more complicated and is often uncoupled from the activation of peripheral nociceptors. Common to both acute and chronic pain is the critical role of the thalamus, which distributes pain signals within the brain, particularly between cortical areas. These thalamocortical (TC) interactions are thought to underlie the perception of pain as a sensory and unpleasant experience. We will begin with a description of the transmission of acute pain signals from the spinal cord to the TC system and then discuss the effects of deafferentation on the TC system, which potentially underlie pathological pain sensation.
Where are noxious stimuli encoded in the TC system?
As a first approximation, the neuronal pathways for pain sensation are similar to other major senses, particularly the tactile sensory pathways: receptors inside of, or on the surface of, the body transduce external stimuli into neuronal action potentials which ascend mainly through the spinal cord into the brain. While neuronal pain signals arise from a large variety of heterogeneous pain receptors (nociceptors) and ascend through several peripheral pain pathways, most nociceptive signals first arrive in the thalamus and are then distributed to cortical and subcortical structures. Thus, the thalamus is one of the brain areas most consistently activated by painful stimuli (Kobayashi K et al. 2009; Friebel U et al. 2011).
As summarized in the schematic in Figure 1A, the thalamus receives nociceptive signals from the body via two major input pathways in the spinal cord: the spinothalamic tract (STT) and the spinoreticulothalamic tract (SRT). The better-characterized STT conveys information about noxious and non-noxious stimuli directly to the lateral thalamus and to the medial thalamus. Nociceptive responses in the lateral thalamus have been most extensively studied in the ventroposterior lateral nucleus (VPL), which relays tactile, proprioceptive, and nociceptive signals to the somatosensory cortex. In contrast, the SRT is thought to relay nociceptive information specifically to the medial thalamus via an additional synaptic relay, the medullary reticular formation in the brainstem. Lateral and medial thalamus each innervate a specific set of cortical target areas: the lateral thalamus connects to the somatosensory cortex, while the medial thalamus innervates limbic cortices, such as the anterior cingulate cortex and insular cortex. These parallel spinothalamocortical pathways are referred to as lateral and medial respectively.
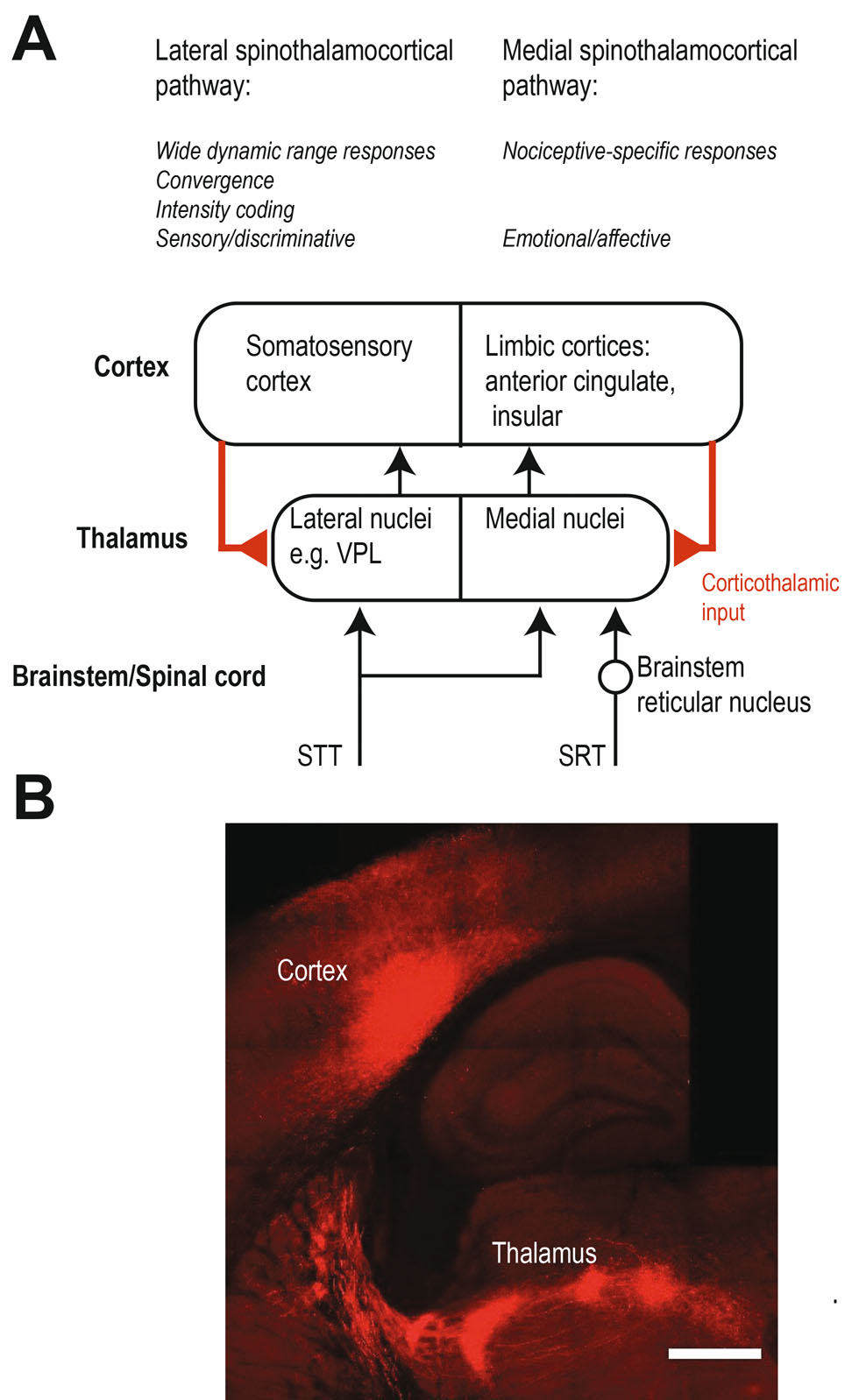
Pain associated spinothalamocortical pathways.ASchematic summarizing and contrasting the lateral and medial thalamic pain pathways. For simplicity, descending pathways are shown simplified and are confined to corticothalamic feedbackwithineach pathway. Other descending projections to the midbrain, brainstem and spinal cord as well as inhibitory connections are not shown.BCorticothalamic projections are modulating sensory signals in the thalamus including putative pain signals. Cortical projections from cortical layer 5 provide a strong excitatory “driver” input whereas projections from cortical layer 6 are believed to be weaker and rather have a “modulatory” function. The corticothalamic axonal projections can be visualized using viral technologies (adeno-associated virus encoding the fluorophore mCherry). The axons in this example innervate various nuclei in the lateral thalamus. Scale bar is 500 µm.
While it is widely accepted that nociceptive signals enter the TC system mainly via the STT and SRT (Figure 1A), it is not entirely clear which thalamic nuclei are functionally driven directly via spinal ascending pathways, due to the difficulty of measuring precise response latencies to nociceptive stimuli. In other sensory modalities, response latencies can be used to discriminate between mono- and polysynaptic activation after a stimulus, but it is difficult to apply pain stimuli with temporal precision sufficient to draw conclusions in this way. However, when latencies are reported, for example for the medial thalamus, the latencies are in fact rather long (Whitt JL et al. 2013). Hence, it is possible that some of the thalamic nuclei with nociceptive responses are activated polysynaptically by the cortex (Figure 1, corticothalamic input) or subcortical regions, rather than directly by the STT/SRT. In fact, parts of the thalamus – the higher-order thalamic nuclei – receive driving input from the cortex (Figure 1B). For example, the S1 cortex drives spiking in the posteriormedial nucleus (Groh A et al. 2014; Mease RA et al. 2016), and may be a source of nociceptive responses in this region (Masri R et al. 2009). The mediodorsal thalamus, part of the medial thalamus is in fact a higher-order nucleus which receives cortical driver input from layer 5 (Mitchell AS 2015). It is thus conceivable that the medial thalamus integrates nociceptive signals from ascending spinal and descending cortical pathways (Figure 1A).
How are noxious stimuli encoded in the TC system: Specificity versus convergence?
The relatively high degree of selectivity of peripheral nociceptors for noxious stimuli suggests that nociceptive signals are conveyed and processed in pain-specific neuronal circuits, analogous to the modality-specific processing of visual or auditory information. This historical “specificity” hypothesis predicts that the brain has distinct areas which are specifically dedicated to pain processing by nociceptive specific (NS) neurons responsive only to only noxious stimuli. This hypothesis follows the “labeled lines” scheme in which somatosensory signals are conveyed via specific pathways which transmit unimodal information (touch, pain, proprioceptive, temperature, and itch) from the receptors to the unimodal brain centers. However, while appealing, this “Cartesian” model of pain is an oversimplification. The question of whether specific pain pathways exist proved to be more complicated and lies at the heart of a long-standing and now partly resolved debate of how the brain discriminates between nociceptive and non-nociceptive signals. In contrast to the “labeled lines” scheme, in the “convergence” view, unimodal signals converge along the pathways and the brain decodes the integrated neuronal activity patterns across groups of multimodal neurons (“patterned activity”, e.g. rate-encoded intensity). In line with this contemporary view, NS neurons are a minority in the spinal cord, and even more rare in thalamic nuclei. The majority of neurons in the lateral system are “wide dynamic range (WDR)” neurons which respond to both innocuous (non-noxious) and noxious stimuli.
It is now firmly established that both WDR and NS responses are found along the pain pathways in rodents, cats, and primates, showing that the convergence model and the specificity model of pain coexist. Given that nociceptive signals originate at primary nociceptors and later mix with innocuous signals, where along the pathway do these signals converge? The ratio between NS and WDR type responses gives a hint to the degree of this convergence: in primates, the ratio is approximately 1:2 in the dorsal horn neurons of the STT (Owens CM et al. 1992), and 1:4 in the lateral thalamus (Kenshalo DR, Jr. et al. 1980), while in the rat, the lateral thalamus may be devoid of NS neurons entirely (Patel R and AH Dickenson 2016). Hence, the convergence of noxious and innocuous signals appears to progressively increase as signals travel from the peripheral to central circuits and at the level of the lateral thalamus, neuronal responses are generally of the WDR type. The simultaneous transmission of noxious and innocuous signals by the same pathway may be the result of an evolutionary process in which an originally unimodal pathway gained additional functions. An advantage of multimodal transmission may be that in addition to the occurrence of a painful event, information about the quality of pain, such as the location and intensity are processed by the same circuits. In the following sections we take a more detailed look into the signaling of pain and pain-related signals.
What is encoded in TC pain pathways: sensory-discriminative versus emotional functions?
Pain as a useful sensation has two main functions: firstly, a sensory-discriminative function to assess the noxious stimulus (e.g. where, what, how strong), and secondly, an emotional function, to evoke aversive emotions, which are the basis for generating appropriate avoidance reactions and for forming memories to prevent future painful situations. It is now assumed that the segregation into lateral and medial pathways reflects these two functions, such that the lateral pathway carries out sensory-discriminative functions, while the medial pathway is dedicated to the emotional function. This functional segregation is already reflected by the input pathways to the thalamus. While upper layers in the spinal cord project to both lateral and medial thalamic nuclei, the deeper STT neurons (layers 7 and 8) project specifically to the medial thalamic nuclei, which are associated with emotional aspects of pain processing (Willis WD et al. 1979).
Studies of the responses of the lateral and medial thalamic neurons to noxious and innocuous stimuli led to the view that the lateral and medial pathway show some specificity for encoding the sensory and emotional aspects of nociception. For example, an expectation for the discriminative sensory function is that the neuronal responses encode properties of a noxious stimulus. Indeed, most neurons in the VPL respond to mechanical or heat stimulation in a graded manner, with low firing rates at innocuous intensities and highest firing rates at noxious intensities (Kenshalo DR, Jr. et al. 1980; Martin WJ et al. 1996). This suggests that the lateral thalamus encodes multimodal information (tactile, proprioceptive, and nociceptive) in the firing rates of WDR neurons. This scheme would allow the cortex to use a firing rate threshold to discriminate between spike trains corresponding to innocuous or noxious stimuli. In contrast, the medial pathway tends to be more specific for pain, reflected by mostly NS-type responses in medial thalamic nuclei (Whitt JL et al. 2013). While pain intensity coding is established for the lateral TC pathway, including human primary and secondary somatosensory cortices (Ploner M et al. 1999), the ability of the medial TC system to similarly discriminate nociceptive stimulus intensity is debated (Apkarian AV et al. 2011). In fact, intensity coding was recently compared between the rat medial and lateral TC areas, namely the VPL, mediodorsal thalamus, primary somatosensory and anterior cingulate cortex, finding intensity coding in all four areas, suggesting that pain intensity is encoded similarity in the lateral and medial system (Zhang Y et al. 2011).
In conclusion, the issues of specificity versus convergence discussed above is thought to be tightly linked to the segregation into discriminative and emotional pain circuits. In this view, the lateral pathway appears to follow the convergence scheme, while the medial pathway may be organized according to the specificity model. The lateral TC system simultaneously processes noxious and innocuous information in a multiplexed manner: the discrimination between innocuous and noxious stimuli and the intensity of noxious stimuli are encoded in the neuronal firing rates, while the location of pain is indicated in the somatotopic organization of the lateral pathway. The aversive (unpleasant) quality of pain on the other hand, is supplied possibly independent of discriminative parameters by the medial system (Rainville P et al. 1997), with the involvement of corticothalamic interactions. However, as noted above, there is increasing evidence that the medial pathway also contributes in discriminative aspects of nociception, which challenges the view that the lateral and medial pathways selectively mediate the sensory-discriminative and emotional/affective aspects of pain (Apkarian AV et al. 2011).
Maladaptation of TC pain pathways can lead to chronic pain
Rather counterintuitively, interruption or destruction of afferent sensory connections from the body often precipitates abnormal pain sensations, rather than a permanent loss of sensation. In fact, many chronic pain conditions are caused by deafferentations, in which the transmission of sensory signals is interrupted at some level along the pain pathways. For example, deafferentations can originate from injuries to the spinal cord, the loss of a limb, or stroke-associated lesions to the brainstem and thalamus and can lead to painful responses to innocuous stimuli (allodynia) and/or spontaneous severe pain sensations. The involvement of the TC pathway in this process has been investigated in humans and rodents, in which nerve injuries lead to characteristic changes in the TC activity.
Both animal and human studies suggest that pain related to deafferentation caused by peripheral nerve injury is accompanied by changes in spontaneous activity and increased sensitivity to somatosensory stimuli in the lateral thalamus. In humans, neuropathic pain is often associated with characteristic shifts of cortical EEG oscillations to lower frequencies and increased rhythmic high-frequency bursting activity in the thalamus (Jeanmonod D et al. 1996; Llinas RR et al. 1999; Sarnthein J and D Jeanmonod 2008; Walton KD and RR Llinas 2010). These two observations may be expressions of the same neuronal mechanism underlying this thalamocortical dysrhythmia. In fact, a series of experiments in animal models of limb and spinal cord injury clearly demonstrated a causal relationship between deafferentation and abnormal TC rhythmicity, although these studies did not focus on pain. Acute deafferentation caused an abnormal rhythmic synchronization of spontaneous neuronal spike patterns in the somatosensory cortex and thalamus and an associated shift of the EEG to lower frequencies (Aguilar J et al. 2010; Humanes-Valera D et al. 2014; Alonso-Calvino E et al. 2016). This reorganization of spiking activity resembles neuronal spike activity under deep anesthesia (which can be viewed as a form of deafferentation): periods of synchronized spike clusters alternate with periods of synchronized inactivity. Most remarkably, sensory responses to tactile stimulation in the thalamus and cortex were enhanced after the lesion, likely owing to the high level of synchronization caused by the deafferentation. Thus, the synchronization of TC networks may be the link between deafferentation and abnormal noxious responses observed in chronic pain patients.
The role of thalamic bursts in pain is still puzzling (Saab CY and LF Barrett 2016): the occurrence of high-frequency bursts in thalamus associated with pain has been frequently reported in both humans, primates, and rodents (Lenz FA et al. 1989; Guilbaud G et al. 1990; Jeanmonod D et al. 1996; Weng HR et al. 2000; Hains BC et al. 2006), whereas the experimental enhancement of bursts or burst-like stimulation of the thalamus in mice has analgesic effects and suppression of bursts causes hyperalgesia (Huh Y and J Cho 2013). Furthermore, in anesthetized rats, the increase in bursting appears to be coupled to the spinal cord injury but not to allodynia (Gerke MB et al. 2003), and in the human thalamus bursting activity is prevalent in both pain and non-pain patients (Radhakrishnan V et al. 1999). Nevertheless, the anti-nociceptive function of thalamic bursts in animal models of pain on one hand and the abnormally high bursting activity and the associated shift in EEG in human chronic pain patients strongly argue for a role of bursting activity in pain. Since thalamic bursts are effectively regulated by corticothalamic feedback (Mease RA et al. 2014) (Figure 1) and corticothalamic feedback contributes to thalamic plasticity in response to changes in afferent activity, it is important to investigate corticothalamic feedback mechanisms as potential targets for pain intervention. For example, motor cortex stimulation is a classic pain treatment (Tsubokawa T et al. 1993; Pagano RL et al. 2012; Jiang L et al. 2014) which could in fact work via corticothalamic modulation of thalamic bursting activity. The difference in the results indicate the need for further investigation of the relationship between deafferentation and plasticity, and how this is linked to pain (Jutzeler CR et al. 2015).
Conclusion
Converging lines of evidence from human and animal studies point to a central role for the thalamus in pain disorders, but the network and single cell mechanisms by which thalamocortical interactions sculpt pain perceptions are still being unraveled. In this regard, mouse models are particularly promising, as the mouse offers an unprecedented access to genetic manipulations, including optogenetic and chemogenetic techniques. These approaches are still being refined, but offer novel opportunities to for cell-type specific functional investigations of the neuronal mechanisms of pain and pain relief.
About the authors

Alexander Groh studied biology and molecular- and cellular biology at the universities of Heidelberg, Amsterdam and Otago and received his Ph.D. from the University of Heidelberg after his thesis work with Bert Sakmann at the Max-Planck Institute for Medical Research Heidelberg. He was a postdoctoral fellow with Bert Sakmann at the Institute for Neuroscience at the Technical University Munich, followed by an independent group leader position at the same Institute. Alexander Groh is now leading a research group at the Klinikum rechts der Isar of the TU Munich and habilitated in 2017.

Rebecca A. Mease studied cognitive science at the Massachusetts Institute of Technology (1998–2002), and received her Ph.D. in 2010 from the University of Washington, in Neurobiology and Behavior with a concentration in computation neuroscience, advised by Adrienne Fairhall. She studied corticothalamic interactions as post-doctoral researcher with Bert Sakmann at the Technical University Munich and the MPI Martinsried (2010–2014).

Patrik Krieger studied chemistry at Stockholm University and received his Ph.D. in neuroscience in 2000 from Karolinska Institutet in Stockholm. As a Postdoc (2003–2007) with Bert Sakmann at the Max-Planck Institute for Medical Research, Heidelberg he investigated tactile perception in rodents. He then continued this research as a Group Leader at Karolinska Institutet and is presently Head of the Department of Systems Neuroscience at Ruhr-University Bochum.
Acknowledgements
Funding was provided by the DFG Collaborative Research Center SFB 1158 (AG, RAM) and SFB 874/A9 (PK).
References
Aguilar J, Humanes-Valera D, Alonso-Calvino E, Yague JG, Moxon KA, Oliviero A, Foffani G. (2010). Spinal cord injury immediately changes the state of the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience 30:7528-7537.10.1523/JNEUROSCI.0379-10.2010Search in Google Scholar
Alonso-Calvino E, Martinez-Camero I, Fernandez-Lopez E, Humanes-Valera D, Foffani G, Aguilar J. (2016). Increased responses in the somatosensory thalamus immediately after spinal cord injury. Neurobiol Dis 87:39-49.10.1016/j.nbd.2015.12.003Search in Google Scholar
Apkarian AV, Hashmi JA, Baliki MN. (2011). Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 152:S49-64.10.1016/j.pain.2010.11.010Search in Google Scholar
Friebel U, Eickhoff SB, Lotze M. (2011). Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain. NeuroImage 58:1070-1080.10.1016/j.neuroimage.2011.07.022Search in Google Scholar
Gerke MB, Duggan AW, Xu L, Siddall PJ. (2003). Thalamic neuronal activity in rats with mechanical allodynia following contusive spinal cord injury. Neuroscience 117:715-722.10.1016/S0306-4522(02)00961-2Search in Google Scholar
Groh A, Bokor H, Mease RA, Plattner VM, Hangya B, Stroh A, Deschenes M, Acsady L. (2014). Convergence of cortical and sensory driver inputs on single thalamocortical cells. Cereb Cortex 24:3167-3179.10.1093/cercor/bht173Search in Google Scholar PubMed PubMed Central
Guilbaud G, Benoist JM, Jazat F, Gautron M. (1990). Neuronal responsiveness in the ventrobasal thalamic complex of rats with an experimental peripheral mononeuropathy. Journal of neurophysiology 64:1537-1554.10.1152/jn.1990.64.5.1537Search in Google Scholar PubMed
Hains BC, Saab CY, Waxman SG. (2006). Alterations in burst firing of thalamic VPL neurons and reversal by Na(v)1.3 antisense after spinal cord injury. Journal of neurophysiology 95:3343-3352.10.1152/jn.01009.2005Search in Google Scholar PubMed
Huh Y, Cho J. (2013). Discrete pattern of burst stimulation in the ventrobasal thalamus for anti-nociception. PLoS One 8:e67655.10.1371/journal.pone.0067655Search in Google Scholar PubMed PubMed Central
Humanes-Valera D, Foffani G, Aguilar J. (2014). Increased cortical responses to forepaw stimuli immediately after peripheral deafferentation of hindpaw inputs. Sci Rep 4:7278.10.1038/srep07278Search in Google Scholar PubMed PubMed Central
Jeanmonod D, Magnin M, Morel A. (1996). Low-threshold calcium spike bursts in the human thalamus. Common physiopathology for sensory, motor and limbic positive symptoms. Brain 119 ( Pt 2):363-375.10.1093/brain/119.2.363Search in Google Scholar
Jiang L, Ji Y, Voulalas PJ, Keaser M, Xu S, Gullapalli RP, Greenspan J, Masri R. (2014). Motor cortex stimulation suppresses cortical responses to noxious hindpaw stimulation after spinal cord lesion in rats. Brain Stimul 7:182-189.10.1016/j.brs.2013.12.013Search in Google Scholar
Jutzeler CR, Curt A, Kramer JL. (2015). Relationship between chronic pain and brain reorganization after deafferentation: A systematic review of functional MRI findings. Neuroimage Clin 9:599-606.10.1016/j.nicl.2015.09.018Search in Google Scholar
Kenshalo DR, Jr., Giesler GJ, Jr., Leonard RB, Willis WD. (1980). Responses of neurons in primate ventral posterior lateral nucleus to noxious stimuli. Journal of neurophysiology 43:1594-1614.10.1152/jn.1980.43.6.1594Search in Google Scholar
Kobayashi K, Winberry J, Liu CC, Treede RD, Lenz FA. (2009). A painful cutaneous laser stimulus evokes responses from single neurons in the human thalamic principal somatic sensory nucleus ventral caudal (Vc). Journal of neurophysiology 101:2210-2217.10.1152/jn.91347.2008Search in Google Scholar
Kuner R, Flor H. (2016). Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci 18:20-30.10.1038/nrn.2016.162Search in Google Scholar
Lenz FA, Kwan HC, Dostrovsky JO, Tasker RR. (1989). Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain. Brain Res 496:357-360.10.1016/0006-8993(89)91088-3Search in Google Scholar
Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. (1999). Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proceedings of the National Academy of Sciences of the United States of America 96:15222-15227.10.1073/pnas.96.26.15222Search in Google Scholar PubMed PubMed Central
Martin WJ, Hohmann AG, Walker JM. (1996). Suppression of noxious stimulus-evoked activity in the ventral posterolateral nucleus of the thalamus by a cannabinoid agonist: correlation between electrophysiological and antinociceptive effects. The Journal of neuroscience : the official journal of the Society for Neuroscience 16:6601-6611.10.1523/JNEUROSCI.16-20-06601.1996Search in Google Scholar
Masri R, Quiton RL, Lucas JM, Murray PD, Thompson SM, Keller A. (2009). Zona incerta: a role in central pain. Journal of neurophysiology 102:181-191.10.1152/jn.00152.2009Search in Google Scholar PubMed PubMed Central
Mease RA, Krieger P, Groh A. (2014). Cortical control of adaptation and sensory relay mode in the thalamus. Proceedings of the National Academy of Sciences of the United States of America 111:6798-6803.10.1073/pnas.1318665111Search in Google Scholar
Mease RA, Sumser A, Sakmann B, Groh A. (2016). Corticothalamic Spike Transfer via the L5B-POm Pathway in vivo. Cereb Cortex 26:3461-3475.10.1093/cercor/bhw123Search in Google Scholar
Mitchell AS. (2015). The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neurosci Biobehav Rev 54:76-88.10.1016/j.neubiorev.2015.03.001Search in Google Scholar
Owens CM, Zhang D, Willis WD. (1992). Changes in the response states of primate spinothalamic tract cells caused by mechanical damage of the skin or activation of descending controls. Journal of neurophysiology 67:1509-1527.10.1152/jn.1992.67.6.1509Search in Google Scholar
Pagano RL, Fonoff ET, Dale CS, Ballester G, Teixeira MJ, Britto LR. (2012). Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: possible pathways for antinociception. Pain 153:2359-2369.10.1016/j.pain.2012.08.002Search in Google Scholar
Patel R, Dickenson AH. (2016). Neuronal hyperexcitability in the ventral posterior thalamus of neuropathic rats: modality selective effects of pregabalin. Journal of neurophysiology 116:159-170.10.1152/jn.00237.2016Search in Google Scholar
Ploner M, Freund HJ, Schnitzler A. (1999). Pain affect without pain sensation in a patient with a postcentral lesion. Pain 81:211-214.10.1016/S0304-3959(99)00012-3Search in Google Scholar
Radhakrishnan V, Tsoukatos J, Davis KD, Tasker RR, Lozano AM, Dostrovsky JO. (1999). A comparison of the burst activity of lateral thalamic neurons in chronic pain and non-pain patients. Pain 80:567-575.10.1016/S0304-3959(98)00248-6Search in Google Scholar
Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. (1997). Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277:968-971.10.1126/science.277.5328.968Search in Google Scholar PubMed
Saab CY, Barrett LF. (2016). Thalamic Bursts and the Epic Pain Model. Front Comput Neurosci 10:147.10.3389/fncom.2016.00147Search in Google Scholar PubMed PubMed Central
Sarnthein J, Jeanmonod D. (2008). High thalamocortical theta coherence in patients with neurogenic pain. NeuroImage 39:1910-1917.10.1016/j.neuroimage.2007.10.019Search in Google Scholar
Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. (1993). Chronic motor cortex stimulation in patients with thalamic pain. Journal of neurosurgery 78:393-401.10.3171/jns.1993.78.3.0393Search in Google Scholar
Walton KD, Llinas RR. (2010). Central Pain as a Thalamocortical Dysrhythmia: A Thalamic Efference Disconnection? In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man Boca Raton, FL.10.1201/9781439812105-c13Search in Google Scholar
Weng HR, Lee JI, Lenz FA, Schwartz A, Vierck C, Rowland L, Dougherty PM. (2000). Functional plasticity in primate somatosensory thalamus following chronic lesion of the ventral lateral spinal cord. Neuroscience 101:393-401.10.1016/S0306-4522(00)00368-7Search in Google Scholar
Whitt JL, Masri R, Pulimood NS, Keller A. (2013). Pathological activity in mediodorsal thalamus of rats with spinal cord injury pain. The Journal of neuroscience : the official journal of the Society for Neuroscience 33:3915-3926.10.1523/JNEUROSCI.2639-12.2013Search in Google Scholar PubMed PubMed Central
Willis WD, Kenshalo DR, Jr., Leonard RB. (1979). The cells of origin of the primate spinothalamic tract. J Comp Neurol 188:543-573.10.1002/cne.901880404Search in Google Scholar PubMed
Zhang Y, Wang N, Wang JY, Chang JY, Woodward DJ, Luo F. (2011). Ensemble encoding of nociceptive stimulus intensity in the rat medial and lateral pain systems. Molecular pain 7:64.10.1186/1744-8069-7-64Search in Google Scholar PubMed PubMed Central
Article note
German version available under https://doi.org/10.1515/nf-2017-0019
© 2017 by De Gruyter

