Abstract
Organo- and hydrogels have been proposed in the restoration field to treat different types of surfaces. The possibility to retain solvents and to have a controlled and superficial action allowed to use these materials for the removal of very thin layers applied on ancient historical objects, when the under paint layers are particularly delicate and water sensitive. In the last years, an increased attention has been devoted to the proposal of more healthy products to guarantee the safeguard of the operators. Few attention has been devoted to the development of green methods which foresee the use of renewable and biodegradable materials. The aim of this paper is to test a green organo-gel for the cleaning of water sensitive surfaces like varnished egg tempera paintings. The gel has been tested experimented on mock ups varnished with natural and synthetic materials and has been validated on a small portion of a Cimabue painting for the removal of two varnishes applied on two different test areas of the painting.
Introduction
The application of sustainable approaches in the restoration of Cultural Heritage is a challenging task that should be addressed limiting the use of hazardous substances, both for human health and for the environment, without compromising the effectiveness of the procedure. Besides the possible damages to be fixed, the simple cleaning of a painting is generally devoted to the removal of aged protective varnishes, to re-establish the original appearance of the painted surfaces. The most common approach is based on the use of volatile organic solvents applied by cotton swabs onto the surface to be treated. However, this cleaning method has several drawbacks such as the fact that wet methods do not preserve the health of the restorers and generate wastes which would need to be disposed correctly to avoid environmental damages [1]. In addition, the solvent may diffuse into the porosity of the underneath layers causing phenomena like swelling and leaching that leave the paint surface brittle with loss of mechanical strength [2], [3], [4], [5]. The solubilised materials may migrate through the substrate and fill the porosities when the solvent is completely evaporated [1]. Moreover, the mechanical stresses applied on the surface when swabs are rolled may compromise the morphological and mechanical integrity of the painted surfaces [6].
At the beginning of XXI century, aqueous methods were introduced as an alternative to the traditional organic solvents to avoid the use of toxic organic substances. Surfactants, chelating agents and enzymes demonstrated a satisfactory cleaning action [7], [8], [9], [10]. Unfortunately aqueous systems can not being used indiscriminately over all kind of surfaces [11]. Indeed, water may act both as a reaction medium and as a high polarity solvent, with a high penetration ability into the microporosities of a paint layer. Water proved to be particularly aggressive when treating recent protein-based materials, such as the animal glue used in ground layers or the egg used as a medium in tempera technique [11], [12], [13]. Moreover, it has been demonstrated that aqueous cleaning may cause wrinkles in the hydrophilic layers and it may leach out small amount of proteinaceous components from not aged films [11], [13]. Reactions occur also within organic components such as water soluble inorganic pigments, leading to the dissolution and migration of metallic ions [4], [5].
In the last decades many efforts were focused in the development of “gelled-systems” capable to retain the liquid component and preventing its spreading and vertical diffusion. Cellulose ethers, carboxymethyl cellulose, and polyacrylic acid salified with strong bases were extensively used as gelling agents thanks to their capability to retain solvents [1], [10], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23].
The above-mentioned gels belong to the category of “physical gels” as weak interactions link them. However, the intrinsic limit of these gels is that they may leave gel residues on the surface after cleaning, requiring further washing with organic solvents in a free-form [19], [20]. In a proper cleaning procedure, the presence of gel residues should be avoided to prevent the painting by the risk of unknown alterations in the long-term. Physical gels produced with agar and gellum gum were proposed for the cleaning of water sensitive surfaces. Agar and gellum gum polysaccharides are capable to form a gel-like structure in the presence of water or water-based detergent solutions. The rigidity of the network allows the gels to be less prone to stick on the surface and thus to reduce the presence of residues after the cleaning. The above mentioned gels were proposed for the cleaning of superficial dirt and soiled materials from water-sensitive modern acrylic paintings [17], [21] and for the cleaning of paper and manuscripts [18], [22], [23]. The advantage in the use of rigid gel lies in the controlled releasing of water, compared to traditional swabbing, and in the avoiding of mechanical stress during cleaning. The intrinsic limit is the presence of water as cleaning agent, thus their use is limited to the removal of water-soluble matter.
Several “chemical gels” were proposed in recent years to avoid the presence of residues after the cleaning and to enhance the capability to retain the solvent. In chemical gels the polymeric chains form a strong 3D network cross linked by covalent bonds, that prevents the release of gel residues and is capable to retain the cleaning agent into the network more strongly compared to physical gels. Among this class of gels, acrylamide based hydrogels [24], hydroxyethyl methacrylate polymer semi-interpenetrated with poly-vinyl pyrrolidone chains [25], [26], poly vinyl alcohol based gels [27], [28] and polymethyl metacrylate organogels [29] were developed. Chemical gels were recently proposed for the cleaning of water-sensitive surfaces thanks to their high retentive capability. Cross-linked polyhydroxyethyl methacrylate and polyvinylpyrrolidone were employed for the removal of hydrophilic soiling material applied on acrylic/vinylic layers, showing an high retentive capability of the cleaning agent and a wide versatility as a fluid container [25], [26]. Polyvinyl alcohol based hydrogels were for instance employed for the removal of varnishes from tempera paint layer [27], [28]. Nevertheless, all above discussed systems still employ water as the main component of the gels, posing risks for the paintings connected to its use. Moreover, it should be noted that few attention has been devoted to the development of green methods, which foresee the use of renewable and biodegradable materials.
To the best of the Authors’ knowledge, a little attention has been devoted to the development of eco-compatible and sustainable systems able to simultaneously take care of the artworks, human and environmental safety. Within this scenario, we have recently prepared novel gel systems based on two or three bio-based components: polyhydroxybutyrate (PHB) a homopolymer belonging to the polyhydroxyalkanoates (PHA) family, γ-valerolactone (GVL) as organic solvent; in cases, triethyl citrate (TEC) was added as plasticizer [30]. These PHB-GVL or PHB-GVL-TEC gels have been proved to be thermoreversible and physically bonded by weak interactions. Moreover, since it was observed that the stiffness of these gels made them less prone to stick to the surface, they have been successfully applied for the removal of terpenic resins from oily paintings without leaving polymer residues after cleaning [30].
In this paper, the applicability of PHB-gel formulations in Cultural Heritage Conservation has been further deepened by testing the efficiency on water-sensitive works of art. Egg tempera was chosen as an example of water sensitive substrate thanks to the great diffusion of this artistic technique in Italy during the Renaissance. The exceptional case of study of the Cimabue altarpieces demonstrates the efficiency and potentialities of the cleaning system proposed.
Experimental
Gel preparation and characterisation
PHB GVL gels were produced according to a previously reported procedure [31].
Poly[(R)-3-hydroxybutyric acid] and γ-valerolactone were purchased from Sigma-Aldrich. PHB-GVL gel was prepared by solubilizing PHB (100 mg) in GVL (1 mL) at 130°C for 1 min. An opalescent and stiff gel was formed when cooling the solution at room temperature. As comparison, GVL was incorporated also in a traditional gelling system used in restoration composed by Carbopol® ULTREZ 21 and Ethomeen® C25, both purchased from CTSsrl (Italy). The GVL-Carbopol-Ethomeen gel was prepared by mixing Carbopol powder (100 mg) with Ethomeen (1 mL) at room temperature; once a homogenous solution was reached, GVL (5 mL) and deionized water (0.5 mL) were added. Gelification process happened immediately as water was added.
Tests for solvent evaporation were carried out in a TGA SDT Q600 TA Instruments apparatus, under nitrogen atmosphere, heating the sample at 50°C and keeping it isothermally for 90 min while recording the weight loss. Samples were prepared in order to contain comparable amount of solvent, besides all the other component of the mixture.
Cleaning methods
PHB-GVL, Carbopol-Ethomeen-GVL and Ethomeen-GVL were applied sandwiched in between two sheets of rice paper and left 5 min in contact with the surface. Then the gels were removed and the surface cleaned with a dry cotton swab.
Since benzyl alcohol is known to be highly retained by the paint layer, some tests with the neat solvent applied with cotton swabs were also performed for comparison.
Mock ups and real painting
The cleaning procedures were applied on mock ups composed by an egg tempera red burnt ochre paint layer applied onto a ground layer made of gypsum and animal glue and a closing layer composed by glue. A dammar or paraloid varnish was applied over the painting layer (Paraloid, dammar resin and red burnt ochre pigment were purchased from Kremer).
The cleaning approach was validated on a real tempera painting, “The Majesty of Santa Maria dei Servi” attributed to Cimabue (XIII) and located at the Chiesa Santa Maria dei Servi in Bologna (Italy).
Evaluation of the cleaning performances
The evaluation of the cleaning performances on the mock ups was performed using different micro destructive techniques.
Optical microscopy in visible and ultraviolet light (Olympus Optical Microscope BX51, Tokyo, Japan) was applied on cross sections before and after the cleaning procedure to establish the thickness of the varnish and assess the cleaning efficacy and effects. μ-FTIR spectroscopy (Thermo Scientific Nicolet iN10 Infrared Microscope) in Total Attenuated Reflection mode (ATR) analyses were performed on the treated surfaces to ascertain the varnish removal. Superficial analysis on mock ups were acquired in the range of 4000–675 cm−1 on untreated and treated areas with an ATR equipped with a germanium crystal, a spectral resolution of 4 cm−1 and an optical aperture of 200×200 μm2.
The solvent fraction retained by the paint layer after cleaning was determined by gas chromatography-mass spectrometry analysis using headspace solid phase micro extraction (HS-SPME) sampling method [30]. A Carboxen/Polydimethylsiloxane fibre was directly exposed in the headspace of sealed vials containing the sample fragment collected from treated areas at fixed times (1 h, 24 h, and 3 days after cleaning). The fibre is exposed at 150°C for 30 min to enhance the absorption of GVL or benzyl alcohol on the fibre coating. Then the SPME fibre is directly injected into the gas chromatograph connected to a quadrupole mass spectrometer (Agilent Technologies, Inc, Santa Clara, USA) and the analytes are thermally desorbed at 250°C for 10 min and separated by a HP-5 fused-silica capillary column (stationary phase poly[5% diphenyl/95% dimethyl]siloxane, 30 m, 0.25 mm i.d., 0.25 mm film thickness) using helium as carrier gas [31], [32].
The cleaning performance evaluation on the Cimabue painting was carried out both in situ and in laboratory on small fragments sampled before and after the application of the PHB-GVL gel. Laser scanning micro-profilometry was performed directly on the painting surface to figure out if any morphological modification occurred after the treatment. This non-invasive technique allows the acquisition of topographic maps of the surface with a depth resolution of 1 μm and a lateral resolution of 20 μm, suitable for morphological investigation of a 3D surface with micrometric resolution [33]. Further investigations of the cleaning performance were achieved by analyses of fragments sampled before and after the cleaning procedures with Optical Microscope in UV-Vis light and with FTIR microscopy in ATR mode.
Results and discussion
PHB-GVL characterisation
A full analytical characterization of gels similar to the one tested in this research and the biodegradability test of their components were reported by Samorì and co-workers [30]. The results demonstred that a thermoreversible physical gel was formed, exploiting the ability of the polymer to crystallize and swell in the presence of appropriate solvents such as GVL. All the gel formulations previously proposed showed the ability to swell at least 10 times their weight in solvent and slow down its evaporation, and such a feature, together with the stability of the products, accounted for their easy application as cleaning system for artworks. The use of plasticizers enhanced the handling properties. In the present investigation PHB, GVL gels were used without other additives to assess their cleaning performance through the application of an easier formulation: the obtained mixture lead to materials stiff enough to be handled by tweezers, as reported in Fig. 1. Such a stiffness allows an easy deployment and removal of the cleaning gel, without leaving any visible residue. The complex viscosity of the produced formulation steadily decreases with the frequency, as already reported for similar systems [33] while the storage and loss moduli (G′ and G″) behave as expected for gel-like systems, i.e. G′>G″ over the investigated frequencies. Preliminary TGA analyses of the volatilization of the GVL from PHB and Carbopol-Ethomeen-based gels was carried out and and results were compared to the behavior of the pure GVL (Fig. 2). Figure 2 displays that the PHB-based gel is the most effective system in terms of reduced volatilization of the solvent. Indeed, it is the most efficient in diminishing the initial solvent loss. Moreover, it slows down the solvent evaporation along the 90 min test, with a trend comparable to the Carbopol-Ethomeen gel. This overall behavior, thus, guarantees that the release of solvent is more controlled.

GVL-PHB gel.

TGA isothermal run at 50°C in nitrogen atmosphere comparing the weight loss of pure GVL (blue), PHB-based gel (red), and Carbopol-Ethomeen-based gel (green) formulations.
PHB-GVL cleaning performance
PHB-GVL gel was tested for the removal of resinous varnish (terpenic and acrylic resins) from egg-tempera paint reconstructions, prepared following ancient recipes [34].
Several tests were performed to evaluate: (a) the varnish removal ability of PHB-GVL gel and (b) the capability of PHB to retain the solvent in comparison with both the neat solvents (GVL and benzyl alcohol) applied by swabs, and a traditional gelled system (Carbopol-Ethomeen).
The cleaning performance of PHB-GVL gel, after 5 min contact with the surface, was compared to the performance of neat GVL and benzyl alcohol rolled on the surface for 30 s by cotton swabs. Benzyl alcohol is a powerful solvent for natural and synthetic varnish with high boiling point and polarity, but it produces high retention within the paint layer [5].
Cleaning of egg-dammar painting layers
Figure 3a shows the cross section of the dammar varnished mock up before cleaning. After the application of PHB-GVL gel (Fig. 3b), a complete removal of the varnish layer, whose thickness ranges between 50 and 70 μm, was detected without affecting the paint layer underneath.
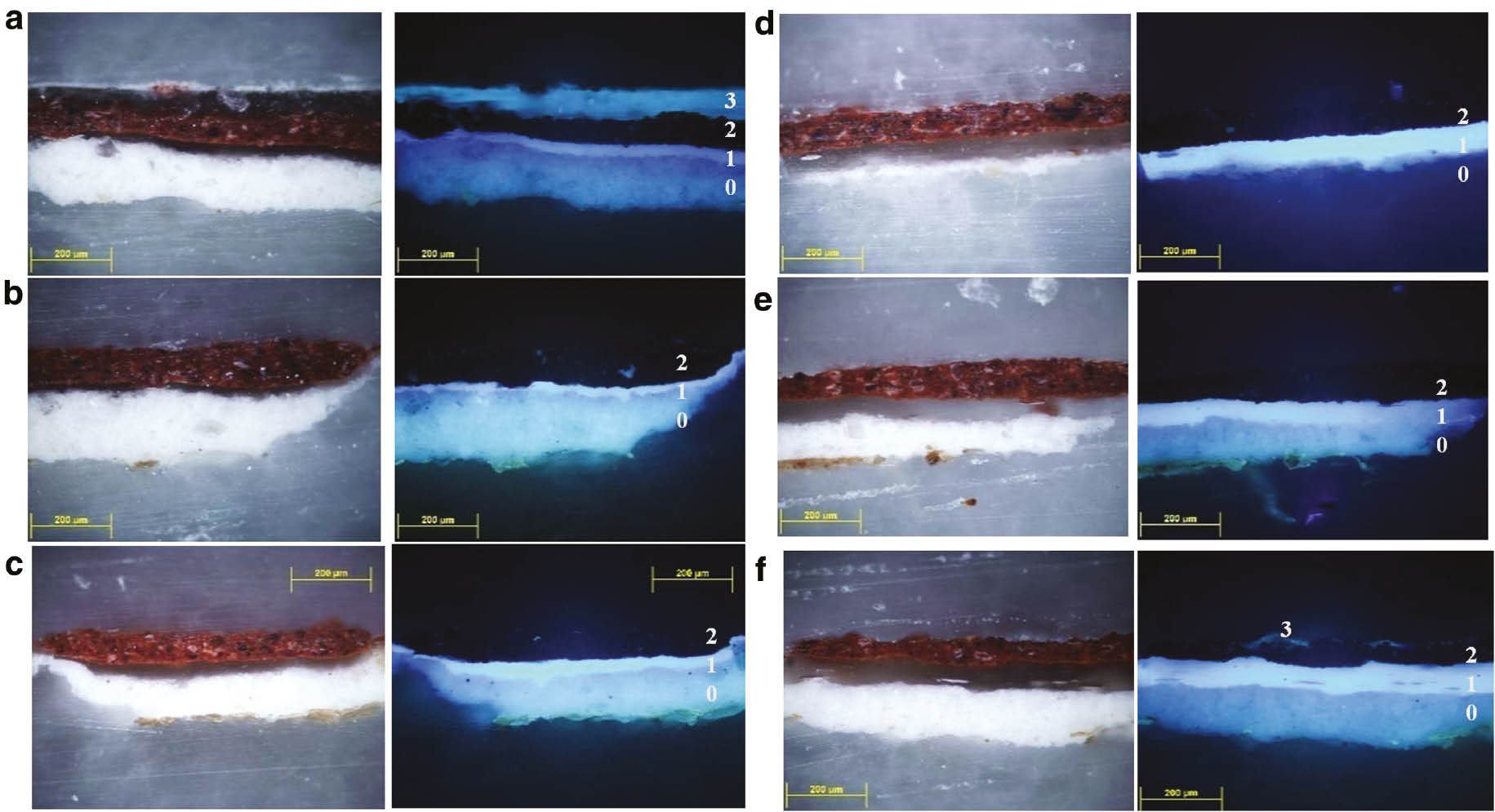
Cross-section microphotographs of egg-tempera paint reconstructions varnished with dammar before and after cleaning, image under visible light (left) and image under UV illumination (right). Numbers from 0 to 3 corresponds respectively to the ground layer, glue closing layer, paint layer and varnish layer: (a) Before cleaning, (b) after PHB-GVL, (c) after GVL swab cleaning, (d) after Benzyl alcohol swab cleaning (e) GVL-Carbopol-Ethomeen, (f) Benzyl alcohol Carbopol-Ethomeen.
The effective removal of the varnish accomplished by PHB-GVL gel was confirmed by ATR analyses performed on the surface of the mock up after the cleaning. Indeed, after the application of the gel the diagnostic peak of dammar resin at 1703 cm−1, ascribable to the C=O stretching vibrations, disappeared (Fig. 4b and e). Moreover, after the cleaning, the characteristic signals of the egg binder (mainly amide I and II at 1645, 1540 cm−1) and of the ochre pigment (calcium carbonate at 1384, 875 cm−1, sulfate at 1095 cm−1 and silicate at 1031 cm−1) were detected, as observed in unvarnished areas (Fig. 4a and e). PHB residues, recognizable by an intense peak at 1724 cm−1 (ester C=O bond vibration, Fig. 4c), are not apparently detected, but owing to the possible overlapping with the ester absorption from the egg medium, located at 1742 cm−1, their presence in low amount cannot be completely ruled out.

FTIR ATR spectra acquired on the surface of the dammar varnished mockup; (a) unvarnished area, (b) dammar reference, (c) PHB polymer reference, (d) GVL reference, (e) area treated with PHB-GVL gel, (f) area treated with GVL swab, (g) area treated with Benzyl alcohol swab, (h) area treated with Benzyl alcohol-Carbopol-Ethomeen gel, (i) area treated with GVL-Carbopol-Ethomeen gel. Bands of dammar are marked with asterisk (*), band at 1770 cm−1 of GVL with a triangle (▴).
FTIR analyses performed on cross sections after the cleaning were carried out to evaluate if varnish re-deposition occurred due to spreading of the solvent within the paint layer. No clear evidence of the presence of dammar was observed even if, due to band overlapping and the sensitivity of the technique, this phenomenon cannot be completely excluded.
Comparable results in terms of varnish removal capabilities were obtained with the application of neat GVL (Figs. 3c, 4f). On the other hand, some residues of the dammar were observed when using benzyl alcohol with swabs (Figs. 3d, 4g). This suggested that PHB-GVL gel ensures a satisfactory varnish removal, with the great advantage to avoid the mechanical stress caused by rolling the swabs over delicate surfaces. The performances of a more traditional gelling system were tested applying GVL or benzyl alcohol, dispersed in Carbopol-Ethomeen. The observation of the cross sections showed a good removal of the varnish after the application of GVL-Carbopol-Ethomeen gel (Fig. 3e), while spot residues of varnish remained after benzyl alcohol-Carbopol-Ethomeen (Fig. 3f). The results were confirmed by ATR investigation (Fig. 4h). Concerning the cleaning with GVL-Carbopol-Ethomeen, the spectra reveal that a residue of varnish is still present after cleaning and the presence of GVL is clearly detectable at 1770 cm−1 (Fig. 4i).
Thus, PHB-GVL gel seems to have better performances both in terms of dammar varnish removal and solvent retention.
Cleaning of egg-paraloid painting layers
The effectiveness of the PHB-GVL gel was tested on water sensitive tempera layers varnished with an acrylic resin. Indeed, ancient varnishes were often removed and substituted, during maintenance operation. Thus, it is not unusual to find synthetic varnish applied on historical paintings. Figure 5 reports cross sections before and after cleaning with different procedures. After all the cleaning treatments, the acrylic varnish layer (of about 25−30 μm thick) are not detectable under UV fluorescence images.

Cross-section microphotographs of egg-tempera paint reconstructions varnished with paraloid before and after cleaning, image under visible light (left) and image under UV illumination (right). Numbers from 0 to 3 correspond respectively to the ground layer, glue closing layer, paint layer and varnish layer (a) before cleaning, (b) after PHB-GVL gel, (c) after GVL swab, (d) after GVL-Carbopol-Ethomeen gel and (e) after Benzyl alcohol-Carbopol-Ethomeen gel.
FTIR-ATR spectra reveal that the cleaning treatments allowed to achieve a consistent reduction of the varnish layer, as testified by the characteristic absorption bands related to amide I and II of egg binder (respectively at 1645 and 1540 cm−1) and to silicates in the range 1100–1030 cm−1 (Fig. 6). The presence of a very thin layer of varnish, not visible in optical microscopy, cannot be excluded by spectra because of the presence of the egg ester band at 1742 cm−1 that may overlapped with those of varnish at 1725 cm−1. Moreover, paraloid is a common synthetic product used as a protective coating and as a consolidating agent, thus its possible penetration into the paint layer cannot be excluded.

ATR spectra acquired on (a) an unvarnished area, (b) after PHB-GVL gel, (c) after GVL swab, (d) after GVL-Carbopol-Ethomeen, (e) after Benzyl alcohol swab, (f) after Benzyl alcohol Carbopol-Ethomeen, (g) Paraloid reference. The main peak of paraloid at 1725 cm−1 is labeled with an asterisk.
Also in this case, FTIR analyses performed on cross sections did not show the presence of paraloid within the paint layer even if due to band overlapping and the sensitivity of the technique low amount of varnish redopisited after the spreading of the solvent cannot be excluded.
In the spectra acquired after the application of GVL-Carbopol-Ethomeen and benzyl alcohol-Carbopol-Ethomeen gel (Fig. 6d and f) the carbonyl band is shifted and its relative intensity with respect to the proteinaceous bands is bigger than what observed in the unvarnished sample. This observation suggests a less effective cleaning efficacy compared to the other systems, as already observed in the case of dammar varnish.
Solvent retention study
Since the more the amount of the solvent released by the gel, the higher the risk of its interaction with the surface, attention was paid to the detection of the solvent retained within the underneath paint layer after the varnish removal. With this aim, both mock-ups treated with free solvents and with gelled systems were submitted to evaluation of treatment liquid remnants by using a HS-SPME approach, thus acquiring information about PHB capability to retain the solvent. Data related to the amount of residual solvent detected after 1 h, 24 h, and 3 days from the treatment are reported in Table 1.
Amount of solvent residues (wt%) after 1 h, 24 h and 3 days from the cleaning treatment.
| Cleaning system | Application time | wt% after 1 h | wt% after 24 h | wt% after 3 days |
|---|---|---|---|---|
| PHB-GVL | 5 min | 0.12±0.03 | 0.08±0.02 | 0.08±0.01 |
| CARBOP-GVL | 5 min | 0.41±0.03 | 0.14±0.02 | 0.06±0.01 |
| GVL swab | 30 s | 0.28±0.04 | 0.15±0.04 | 0.12±0.03 |
| CARBOP-BAL | 5 min | 2.74±1.31 | 0.69±0.48 | 0.47±0.17 |
| Benzyl alchohol swab | 30 s | 2.72±0.48 | 1.14±0.38 | 0.61±0.53 |
In particular, egg-dammar mock ups were used for the retention studies.
The amount of residual GVL in the samples is significantly lower than the residual benzyl alcohol even when the last is gelled with Carbopol-Ethomeen. When benzyl alcohol is gelled, the amount of solvent detected after 1 h is comparable to the amount detected after the cleaning with swabs.
The amount of GVL detected after 1 h from the application of PHB-GVL gel, instead, is lower than what observed using swabs. However, these differences tend to reduce after 24 h and, even more, after 3 days. This suggests that when swabs are used a more in depth absorption of the solvent occurs. This can cause leaching and swelling of the paint layer. Moreover, the application of gels has the advantage to avoid any mechanical stress on the painting surface. After 1 h from the treatment, the amount of GVL released from Carbopol-Ethomeen gel is much higher than PHB gel. This difference tends to reach a comparable value after 3 days, due to evaporation of the residual solvent, but it means that Carbopol-Ethomeen gel system entails a major risk to interact with the surface by time. Thus, PHB has not only the advantage to be a product derived by renewable sources, but also proved to guarantee a higher retention capability of GVL than Carbopol-Ethomeen systems.
Cleaning test on “the majesty” of Cimabue tests
After ascertaining the performance and safety of PHB-GVL gel on mock-ups, this formulation was finally tested as a cleaning agent on a real tempera painting, “The Majesty of Santa Maria dei Servi” attributed to Cimabue (XIII), currently exposed in the Church Santa Maria dei Servi in Bologna (Italy).
The GVL-PHB cleaning gel was applied for 5 min in two different areas (Fig. 7).

The GVL-PHB cleaning gel was applied for 5 min in two different areas. (a) The Majesty painted by Cimabue, test area 1 (b) during the GVL-PHB cleaning, (c) after the gel cleaning, (d) test area 2.
In the first area (Fig. 7b, c) the presence of an external varnish layer was clearly observable by naked eye so that it was possible to appreciate the effectiveness of the gel after the cleaning.
Two fragments were sampled from area 1 before and after the cleaning and observation of the cross sections with the optical microscope (Fig. 8) confirms that the thin varnish layer of about 5 μm was removed upon gel application.

Cross-section microphotographs of fragments sampled from area 1, image under visible light (left) and image under UV illumination (right) (a) before, (b) after PHB-GVL gel cleaning. Numbers correspond to the varnish, paint and ground layer, respectively from 2 to 0.
μATR-FTIR analyses on the cross sections (data not shown) allowed to identify the external varnish as a synthetic resin probably belonging to the acrylic family, as the main characteristic peak around 1724 cm−1 suggests. The paint layer is composed of an earth pigment, probably an ochre as suggested by the presence of carbonate, sulfate, and silicate (respectively at 1404, 1031, 976 cm−1). The distribution of the amide I and II bands at 1645 and 1550 cm−1 in the painting layer, characteristic for proteins, is consistent with the use of egg, the typical painting binder used at Cimabue times.
In the second area in which the treatment was applied (Fig. 7d) the presence of the varnish was not so easily identifiable at sight. Thus, some non-destructive investigations were preliminary performed using Optical Coherent Tomography (OCT) and Laser scanning 3D microprofilometry, directly in situ.
OTC was aimed at measuring the varnish thickness and to ascertain its removal in a non-destructive way; however, the varnish thickness was under the limit of detection of the method. Laser scanning 3D microprofilometry was performed by scanning an area of 3×4 cm which contemporary included both a cleaned and uncleaned area for the evaluation of morphological modifications after treatment. The topographic map of the area submitted to cleaning (traceable inside the square in Fig. 9), did not evidence any morphological differences with respect to the untreated area, suggesting that the cleaning procedure was extremely superficial and had not effect in modifying the roughness and morphology of the painting layer.
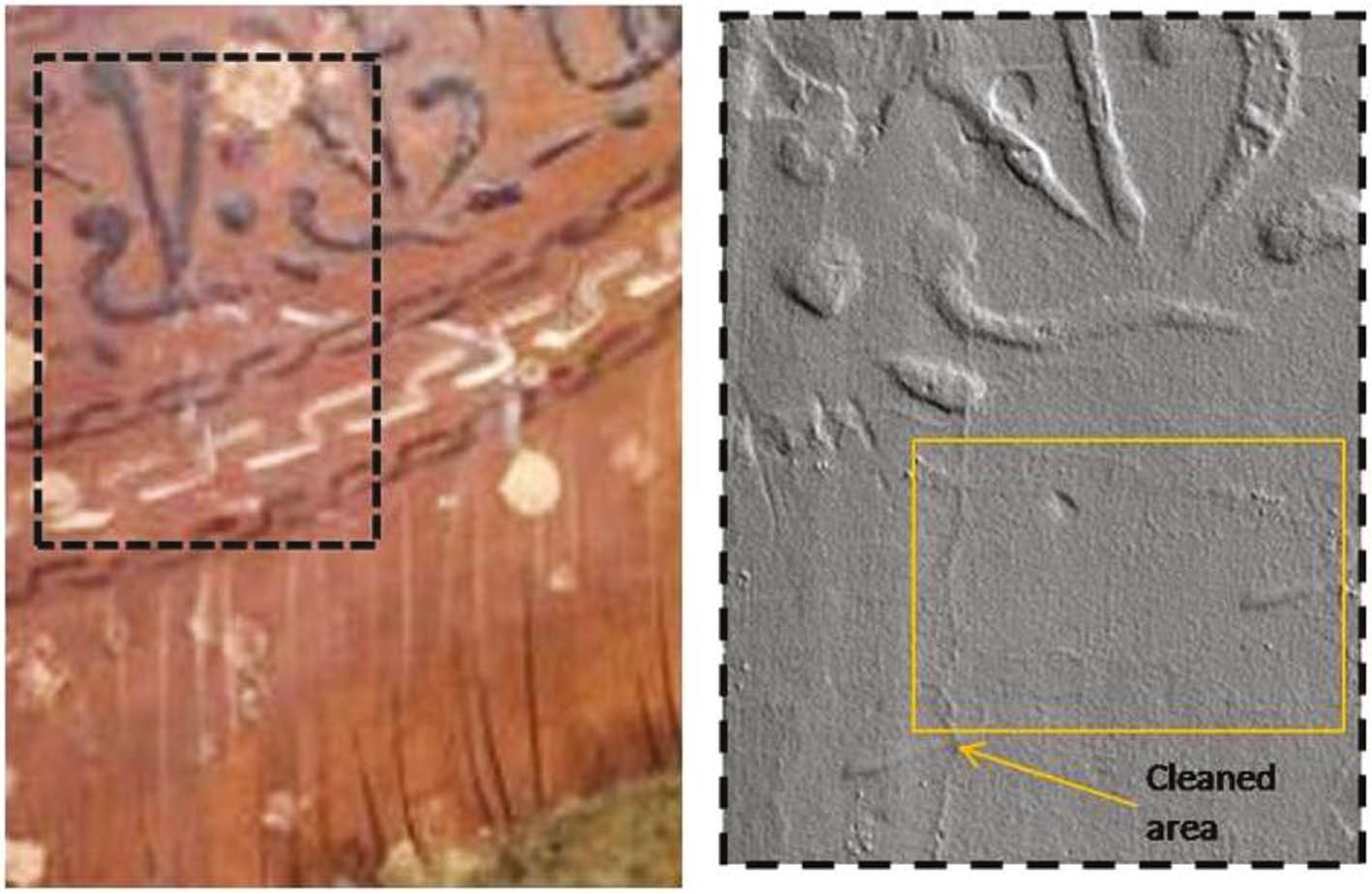
Image of area 2 analysed with micro-profilometry and partially treated with PHB-GVL gel (on the left); topographic map in grazing light (on the right) with in evidence the area cleaned with PHB-GVL gel (into the square).
Also in this case, two small fragments were sampled from area 2 before and after the application of the gel. As shown in Fig. 10, the the fragment sampled in the area before the cleaning presents three layers: (0) a ground layer of about 30 μm, (1) a paint layer of 15 μm, and (2) a varnish layer 5–8 μm. μATR-FTIR analyses show that the varnish was composed of a natural terpenic resin, as defined by the carbonyl diagnostic peak at 1706 cm−1. As previously observed the painting layer is composed of silicates and proteinaceous material. As expected, after PHB-GVL gel cleaning, the varnish layer appears consistentely reduced, ranging from 0 to less than 2 μm (see Fig. 10). Thus, the PHB-GVL gel showed a very good performance for the removal of both terpenic and of acrylic resin on a tempera painting, while preventing the damage of the surface induced by mechanical stresses.

Cross-section microphotographs of fragments sampled from area 2, image under visible light (left) and image under UV illumination (right), (a) before and (b) after PHB-GVL gel cleaning where a reduction of the varnish layer occurred.
Conclusion
In this work, a completely sustainable and bio-based cleaning method has been set up by using a PHB-GVL organogel for the removal of natural and synthetic varnishes from water-sensitive surfaces. The confinement of the solvent into a gelled-system in which the use of water is completely avoided in the ceiling process, allowed water sensitive painting surfaces to be treated.
FTIR, SPME and TGA analyses allowed to describe the efficacy of the gels in removing the varnishes and its capability to retain the solvent, thus a limited amount is detected after 24 h from the application. A better control of solvent release reduces its diffusion into the paint layer and then the risk of leaching and swelling of the binder. In particular, the experimental data proved the higher capability of PHB polymer to retain the solvent than traditional Carbopol-Ethomeen gel-systems.
Long-term analyses are actually in progress. However, first results obtained after few months from the application of the gels confirmed that no evident modifications occurred on the painting layers after the gel application.
The validation test of the proposed PHB-GVL system has been carried out on test areas in a Cimabue tempera painting (the “Majesty of Santa Maria dei Servi”, XIII century) proving its efficacy also in the removal of aged varnishes and its suitability for delicate surfaces, avoiding the morphological alteration of the surface.
In conclusion, PHB-GVL can be considered a novel cleaning method suitable for delicate water sensitive surfaces, when the mechanical action of swabbing must be avoided or when the use of hydrogels may have negative effects on the art work.
Article note
A collection of invited papers based on presentations at the 6th International IUPAC Conference on Green Chemistry (ICGC-6), Venice (Italy), 4–8 September 2016.
References
[1] P. Cremonesi. L’uso di solventi organici nella pulitura di opere policrome, Il Prato, Padova (2000).Search in Google Scholar
[2] A. Phenix. J. Am. Inst. Conserv.41, 43 (2002).Search in Google Scholar
[3] A. Phenix, K. Sutherland. Stud. Conserv.46, 47 (2001).Search in Google Scholar
[4] S. Michalski. Stud. Conserv.35, 85 (1990).Search in Google Scholar
[5] A. Phenix. “Effects of organic solvents on artists’ oil paint films: swelling,” New Insights into Clean. Paint., no. November 2010, p. 69, (2013).Search in Google Scholar
[6] G. Hedley, M. Odlyha, A. Burnstock, J. Tillinghast, C. Husband. Stud. Conserv.35, 98 (1990).Search in Google Scholar
[7] R. Wolbers. Cleaning Painted Surfaces: Acqueous Methods, Archetype, London (2000).Search in Google Scholar
[8] P. Cremonesi. L’uso di enzimi nella pulitura di opere policrome, Il Prato, Padova (2002).Search in Google Scholar
[9] J. Domingues, N. Bonelli, R. Giorgi, P. Baglioni. Appl. Physics. A. Mater. Sci. Process.114, 705 (2014).Search in Google Scholar
[10] P. Cremonesi. L’uso di tensioattivi e chelanti nella pulitura di opere policrome, 2nd ed., Il prato, Padova (2004).Search in Google Scholar
[11] A. Casoli, M. Berzioli, P. Cremonesi. “The chemistry of egg binding medium and its interactions with organic solvents and water,” in New Insights into the Cleaning of Paintings, pp. 39–44, (2010).Search in Google Scholar
[12] A. Karpowicz. Stud. Conserv.26, 153 (1981).Search in Google Scholar
[13] N. Khandekar, A. Phenix, J. Sharp. Conserv.18, 62 (1994).Search in Google Scholar
[14] P. Cremonesi. Materiali e metodi per la pulitura di opere policrome, Il prato, Padova (1997).Search in Google Scholar
[15] A. Casoli, P. Cremonesi, C. Isca, R. Groppetti, S. Pini, N. Senin. Cellulose20, 2027 (2013).Search in Google Scholar
[16] J. A. L. Domingues, N. Bonelli, R. Giorgi, E. Fratini, F. Gorel, P. Baglioni. Langmuir29, 2746 (2013).Search in Google Scholar
[17] P. Cremonesi. Stud. Conserv.61, 362 (2016).Search in Google Scholar
[18] C. Mazzuca, L. Micheli, M. Carbone, F. Basoli, E. Cervelli, S. Iannuccelli, S. Sotgiu, A. Palleschi. J. Colloid Interface Sci.416, 205 (2014).Search in Google Scholar
[19] D. Stulik, D. Miller, H. Khanjian, R. Wolbers, J. Carlson, W. C. Petersen, V. Dorge. J. Am. Inst. Conserv.48, 83 (2009).Search in Google Scholar
[20] A. Burnstock, R. White. “A preliminary assessment of the aging/degradation of Ethomeen C-12 residues from solvent gel formulations and their potential for inducing changes in resinous paint media,” Tradition and innovation: advances in conservation: contributions to the Melbourne Congress, 10–14 October 2000, vol. 1(March), pp. 34–38, 2000.Search in Google Scholar
[21] A. Volk, K. J. van der Berg. “Agar – A new tool for the surface cleaning of water sensitive oil paint?,” in Issues in Contemporary Oil Paint, K. J. et A. van der Berg (Ed.), pp. 389–406, Springer, Cham, Switzerland (2014).Search in Google Scholar
[22] C. Mazzuca, L. Micheli, E. Cervelli, F. Basoli, C. Cencetti, T. Coviello, S. Iannuccelli, S. Sotgiu, A. Palleschi. ACS Appl. Mater. Interfaces6, 16519 (2014).Search in Google Scholar
[23] S. Iannuccelli, S. Sotgiu. B. Pap. Gr. Annu.29, 25 (2010).Search in Google Scholar
[24] G. Pizzorusso, E. Fratini, J. Eiblmeier, R. Giorgi, D. Chelazzi, A. Chevalier, P. Baglioni. Langmuir28, 3952 (2012).Search in Google Scholar
[25] P. Baglioni, D. Chelazzi, R. Giorgi, G. Poggi. Langmuir29, 5110 (2013).Search in Google Scholar
[26] J. Domingues, N. Bonelli, R. Giorgi, E. Fratini, P. Baglioni. Int. J. Conserv. Sci.4, 715 (2013).Search in Google Scholar
[27] E. Carretti, C. Matarrese, E. Fratini, P. Baglioni, L. Dei. Soft Matter10, 4443 (2014).Search in Google Scholar
[28] E. Carretti, I. Natali, C. Matarrese, P. Bracco, R. Weiss, P. Baglioni, A. Salvini, L. Dei. J. Cult. Herit.11, 373 (2010).Search in Google Scholar
[29] P. Baglioni, N. Bonelli, D. Chelazzi, A. Chevalier, L. Dei, J. Domingues, E. Fratini, R. Giorgi, M. Martin. Appl. Phys. A Mater. Sci. Process.121, 857 (2015).Search in Google Scholar
[30] C. Samorì, P. Galletti, L. Giorgini, R. Mazzeo, L. Mazzocchetti, S. Prati, G. Sciutto, F. Volpi, E. Tagliavini. Chem. Select1, 4502 (2016).Search in Google Scholar
[31] A. G. Rombolà, G. Marisi, C. Torri, D. Fabbri, A. Buscaroli, M. Ghidotti, A. Hornung. J. Agric. Food Chem.63, 6660 (2015).Search in Google Scholar
[32] M. Ghidotti, D. Fabbri, A. Hornung. ACS Sustain. Chem. Eng.5, 510 (2016).Search in Google Scholar
[33] R. Fontana, A. Dal Fovo, J. Striova, L. Pezzati, E. Pampaloni, M. Raffaelli, M. Barucci. Appl. Phys. A Mater. Sci. Process.121, 957 (2015).Search in Google Scholar
[34] C. Cennini. Il libro dell’arte, Neri Pozza, Vicenza (1982).Search in Google Scholar
©2018 IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For more information, please visit: http://creativecommons.org/licenses/by-nc-nd/4.0/


