Silabenzene
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Siline[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| C5H6Si | |||
| Molar mass | 94.188 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||

A silabenzene is a heteroaromatic compound containing one or more silicon atoms instead of carbon atoms in benzene. A single substitution gives silabenzene proper; additional substitutions give a disilabenzene (3 theoretical isomers), trisilabenzene (3 isomers), etc.
Silabenzenes have been the targets of many theoretical and synthetic studies by organic chemists interested in the question of whether analogs of benzene with Group IV elements heavier than carbon, e.g., silabenzene, stannabenzene and germabenzene—so-called "heavy benzenes"—exhibit aromaticity.
Although several heteroaromatic compounds bearing nitrogen, oxygen, and sulfur atoms have been known since the early stages of organic chemistry, silabenzene had been considered to be a transient, un-isolable compound and was detected only in low-temperature matrices or as its Diels-Alder adduct for a long time. In recent years, however, a kinetically stabilized silabenzene and other heavy aromatic compounds with silicon or germanium atoms have been reported.
Synthesis
[edit]
Several attempts to synthesize stable silabenzenes have been reported from the late 1970s using well-known bulky substituents such as a tert-butyl (1,1-dimethylethyl) or a TMS (trimethylsilyl) group, but such silabenzenes readily react with themselves to give the corresponding dimer even at low temperature (below -100°C) due to the high reactivity of silicon-carbon π bonds. In 1978 Barton and Burns reported that flow pyrolysis of 1‑methyl-1‑allyl-1‑silacyclohexa-2,4‑diene through a quartz tube heated to 428 °C using either ethyne or perfluoro-2-butyne as both a reactant and a carrier gas afforded the methyl-1‑silylbenzene Diel-Alder adducts, 1‑methyl-1‑silabicyclo[2.2.2]octatriene or 1‑methyl-2,3‑bis(trifluoromethyl)-1‑silabicyclo[2.2.2]octatriene, respectively, by way of a retro-ene reaction.[2]
A computational investigation in 2013 points out a new route to stable silabenzenes at ambient conditions through Brook rearrangement.[3] The [1,3]-Si → O shift of TMS or triisopropylsilyl (TIPS) substituted precursors with tetrahedral silicon atoms to an adjacent carbonyl oxygen lead to aromatic Brook-type silabenzenes.
Following the synthesis of the naphthalene analog 2-silanaphthalene,[4][5] the first sila-aromatic compound, by Norihiro Tokitoh and Renji Okazaki in 1997, the same group reported thermally stable silabenzene in 2000 taking advantage of a new steric protective group.[6] A 9-silaanthracene derivative has been reported in 2002,[7] a 1-silanaphthalene also in 2002.[8]
A 1,4-disilabenzene was reported in 2002.[9] In 2007, 1,2-disilabenzene was synthesized via formal [2+2+2] cyclotrimerization of a disilyne (Si-Si triple bonded species) and phenylacetylene.[10]
Some theoretical studies suggest that the symmetric 1,3,5-trisilabenzene may be more stable than 1,2-disilabenzene.[11]
Properties and reactions
[edit]Isolated silabenzene reacts with various reagents at 1,2- or 1,4-positions to give diene-type products, so the aromaticity of the silabenzene is destroyed. It is different from benzene, which reacts with electrophiles to give not dienes but substituted benzenes, so benzene sustains its aromaticity. Silicon is a semi-metal element, so the Si-C π bond in the silabenzene is highly polarized and easily broken. The silabenzene is also light-sensitive; Ultraviolet irradiation gives the valence isomer, a silabenzvalene. The theoretical calculations and the NMR chemical shifts of silabenzenes, though, show that silabenzene is an aromatic compound in spite of the different reactivity from benzene and other classical aromatic compounds.
Hexasilabenzene
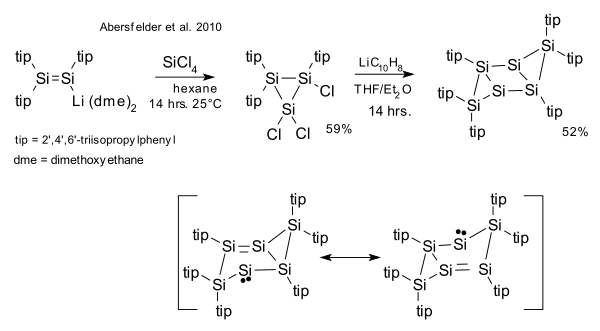
[edit]In calculations the all-silicon hexasilabenzene Si6H6 is predicted to have 6-fold symmetry [12] or a chair conformation.[13] It was shown that the deviation from planarity in hexasilabenzene is caused by the pseudo Jahn–Teller effect.[14] A stable hexasilaprismane has been known since 1993 [15] A compound isomeric with hexasilabenzene was first reported in 2010.[16] This compound is reported as stable and with according to X-ray crystallography a chairlike tricyclic silicon frame.
The searching of a planar Si6 analogue to benzene has been extended to anionic cycles and structures bearing lithium atoms replacing hydrogens.[17] Through Density functional theory calculations, it has been shown that from a series of planar and tridimensional structures with molecular formula Si6Li2-8, the global minimum is a Si6Li6 planar ring. This particular ring has D2h symmetry with 4 lithium cations placed between two adjacent silicon atoms –forming three-center two-electron bonds –and two more Li cations located above and below the center of the ring’s plane. A highly symmetric D6h structure analogue to hexalithiumbenzene[18] was found to be higher in energy by 2.04 eV to respect to the minimum.[19]
Aromaticity was also tested using density functional calculations. DFT can be effectively used to calculate the aromaticity of various molecular systems[20] using the B3LYP hybrid density functional; this method has been proved to be the method of choice for computing delocalized systems.[21] The nucleus-independent chemical shifts (NICS)[22] was selected as the quantitative criterion to evaluate the aromatic character of the structures under study. The global minimum (D2h symmetry ring) and the D6h symmetry ring show values of −3.95 and −5.95, respectively. In NICS calculations, negative values indicate aromaticity.
More recently, using a novel genetic algorithm, a Si6Li6 three dimensional structure has been calculated to be more stable than planar isomers.[23]
See also
[edit]- 6-membered aromatic rings with one carbon replaced by another group: borabenzene, boratabenzene, silabenzene, germabenzene, stannabenzene, pyridine, phosphorine, arsabenzene, bismabenzene, pyrylium, thiopyrylium, selenopyrylium, telluropyrylium
References
[edit]- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 392. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Barton, T. J.; Burns, G. T. (1978). "Unambiguous generation and trapping of a silabenzene". Journal of the American Chemical Society. 100 (16): 5246. doi:10.1021/ja00484a075.
- ^ Rouf, Alvi Muhammad; Jahn, Burkhard O.; Ottosson, Henrik (14 January 2013). "Computational Investigation of Brook-Type Silabenzenes and Their Possible Formation through [1,3]-Si→O Silyl Shifts". Organometallics. 32 (1): 16–28. doi:10.1021/om300023s.
- ^ Tokitoh, N.; Wakita, K.; Okazaki, R.; Nagase, S.; von Ragué Schleyer, P.; Jiao, H. (1997). "A Stable Neutral Silaaromatic Compound, 2-{2,4,6-Tris[bis(trimethylsilyl)methyl]phenyl}- 2-Silanaphthalene". Journal of the American Chemical Society. 119 (29): 6951–6952. doi:10.1021/ja9710924.
- ^ Wakita, K.; Tokitoh, N.; Okazaki, R.; Nagase, S.; von Ragué Schleyer, P.; Jiao, H. (1999). "Synthesis of Stable 2-Silanaphthalenes and Their Aromaticity". Journal of the American Chemical Society. 121 (49): 11336–11344. doi:10.1021/ja992024f.
- ^ Wakita, K.; Tokitoh, N.; Okazaki, R.; Takagi, N.; Nagase, S. (2000). "Crystal Structure of a Stable Silabenzene and Its Photochemical Valence Isomerization into the Corresponding Silabenzvalene". Journal of the American Chemical Society. 122 (23): 5648–5649. doi:10.1021/ja000309i.
- ^ Takeda, N.; Shinohara, A.; Tokitoh, N. (2002). "The First Stable 9-Silaanthracene". Organometallics. 21 (2): 256–258. doi:10.1021/om0108301.
- ^ Takeda, N.; Shinohara, A.; Tokitoh, N. (2002). "Synthesis and Properties of the First 1-Silanaphthalene". Organometallics. 21 (20): 4024–4026. doi:10.1021/om0205041.
- ^ Kabe, Y.; Ohkubo, K.; Ishikawa, H.; Ando, W. (2000). "1,4-Disila(Dewar-benzene) and 1,4-Disilabenzene: Valence Isomerization of Bis(alkylsilacyclopropenyl)s". Journal of the American Chemical Society. 122 (15): 3775–3776. doi:10.1021/ja9930061.
- ^ Kinjo, R.; Ichinohe, M.; Sekiguchi, A.; Takagi, N.; Sumimoto, M.; Nagase, S. (2007). "Reactivity of a Disilyne RSi≡SiR (R=SijPr(CH(SiMe3)2)2) Toward π-Bonds: Stereospecific Addition and a New Route to an Isolable 1,2-Disilabenzene". Journal of the American Chemical Society. 129 (25): 7766–7767. doi:10.1021/ja072759h. PMID 17542592.
- ^ Baldridge, K. K.; Uzan, O.; Martin, J. M. L. (2000). "The Silabenzenes: Structure, Properties, and Aromaticity". Organometallics. 19 (8): 1477–1487. doi:10.1021/om9903745.
- ^ Dewar, M. J. S.; Lo, D. H.; Ramsden, C. A. (1975). "Ground States of Molecules. XXIX. MINDO/3 Calculations of Compounds Containing Third Row Elements". Journal of the American Chemical Society. 97 (6): 1311–1318. doi:10.1021/ja00839a005.
- ^ Nagase, S.; Teramae, H.; Kudo, T. (1987). "Hexasilabenzene (Si6H6). Is the Benzene-Like D6h Structure Stable?". The Journal of Chemical Physics. 86 (8): 4513–4517. Bibcode:1987JChPh..86.4513N. doi:10.1063/1.452726.
- ^ Ivanov, A.; Boldyrev. A (2012). "Si6−nCnH6 (n = 0-6) Series: When Do Silabenzenes Become Planar and Global Minima?". J. Phys. Chem. A. 116 (38): 9591–9598. Bibcode:2012JPCA..116.9591I. doi:10.1021/jp307722q. PMID 22946702.
- ^ Sekiguchi, A.; Yatabe, T.; Kabuto, C.; Sakurai, H. (1993). "Chemistry of Organosilicon Compounds. 303. The "Missing" Hexasilaprismane: Synthesis, X-Ray Analysis and Photochemical Reactions". Journal of the American Chemical Society. 115 (13): 5853–5854. doi:10.1021/ja00066a075.
- ^ Abersfelder, K.; White, A.; Rzepa, H.; Scheschkewitz, D. (2010). "A Tricyclic Aromatic Isomer of Hexasilabenzene". Science. 327 (5965): 564–566. Bibcode:2010Sci...327..564A. doi:10.1126/science.1181771. PMID 20110501. S2CID 206523406.
- ^ Takahasi, M; Kawazoe, Y (2005). "Theoretical Study on Planar Anionic Polysilicon Chains and Cyclic Si6H Anions with D6h Symmetry". Organometallics. 24 (10): 2433–2440. doi:10.1021/om050025c.
- ^ Xie, Y; Schaefer, H (1991). "Hexalithiobenzene: a D6h Equilibrium Geometry with Six Lithium Atoms in Bridging Positions". Chemical Physics Letters. 179 (5, 6): 563–567. Bibcode:1991CPL...179..563X. doi:10.1016/0009-2614(91)87104-J.
- ^ Zdetsis, A (2007). "Stabilization of Flat Aromatic Si 6 Rings Analogous to Benzene: Ab initio Theoretical Prediction". The Journal of Chemical Physics. 127 (21): 214306. Bibcode:2007JChPh.127u4306Z. doi:10.1063/1.2805366. PMID 18067356.
- ^ De Proft, F; Geerlings, P (2001). "Conceptual and Computational DFT in the Study of Aromaticity". Chemical Reviews. 101 (5): 1451–1464. doi:10.1021/cr9903205. PMID 11710228.
- ^ Nedel, M; Houk, K; Tolbert, L; Vogel, E; Jiao, H; von Rague Schleyer, P (1998). "Bond Alternation and Aromatic Character in Cyclic Polyenes: Assessment of Theoretical Methods for Computing the Structures and Energies of Bismethano[14]annulenes". The Journal of Physical Chemistry A. 102 (36): 7191–7198. Bibcode:1998JPCA..102.7191N. doi:10.1021/jp9820976.
- ^ von Rague Schleyer, P; Maerker, C; Dransfeld, A; Jiao, H; van Eikema Hommes, N (1996). "Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe". Journal of the American Chemical Society. 118 (26): 6317–6318. doi:10.1021/ja960582d. PMID 28872872. S2CID 207152799.
- ^ Santos, J; Contreras, M; Merino, G (2010). "Structure and Stability of Si6Li6: Aromaticity vs Polarizability". Chemical Physics Letters. 496 (1–3): 172–174. Bibcode:2010CPL...496..172S. doi:10.1016/j.cplett.2010.07.026. hdl:10533/144740.