Am. J. Trop. Med. Hyg., 89(6), 2013, pp. 1203–1205
doi:10.4269/ajtmh.13-0436
Copyright © 2013 by The American Society of Tropical Medicine and Hygiene
Case Report: A Confirmed Case of Rickettsia parkeri Infection in a Traveler from Uruguay
Aránzazu Portillo, Concepción Garcı́a-Garcı́a, M. Mercedes Sanz, Sonia Santibáñez, José M. Venzal, and José A. Oteo*
Departamento de Enfermedades Infecciosas, Hospital San Pedro-Centro de Investigación Biomédica de La Rioja (CIBIR),
Logroño, La Rioja, Spain; Departamento de Parasitologı́a Veterinaria, Universidad de La República, Salto, Uruguay
Abstract. The first confirmed case of Rickettsia parkeri infection in Uruguay is reported. To date, in South America,
molecularly confirmed cases of human infection have been found in Argentina and probably, Brazil. Our patient
returned to Spain after a 7-day trip to Colonia Suiza (Southwestern Uruguay). He presented fever (39 °C), chills, and
two eschars (tache noire-like) surrounded by an indurated, erythematous halo on the inner side of the left ankle besides a
maculopapular rash on the legs. After treatment with doxycycline for 7 days, he fully recovered. R. parkeri infection was
diagnosed by molecular-based detection of the microorganism in a swab specimen of the eschar. Diagnosis was supported
by seroconversion between acute- and convalescent-phase sera specimens.
as antigens. Fragments of gltA and ompA rickettsial genes
were amplified from the swab sample. Partial gltA (285/285 bp)
and ompA (535/536 bp) sequences showed 100% and 99.8%
identity to the corresponding sequences of R. parkeri. Diagnostic antibodies against spotted fever group rickettsiae were
not detected in the acute serum specimen, but the convalescent specimen was positive for immunoglobulin G (IgG) at a
titer of 4,096 with both antigens. Doxycycline (100 mg/12 hours)
was administered for 7 days, and the patient fully recovered
(fever disappeared in the first 24 hours after initiation of
doxycycline therapy).
Previously considered non-pathogenic in humans, R. parkeri
was first described in Amblyomma maculatum ticks.12 In 2004,
Paddock and others1 described the first human cases associated
with this bacterium in the United States. At the same time,
this Rickettsia species was also suspected to be the responsible
agent for the tick-borne spotted fevers in Uruguay, because it
was amplified from one A. triste tick attached to a patient who
developed a rickettsial syndrome.13 Regarding clinical features,
it seems that R. parkeri causes a spotted fever syndrome that is
less severe than RMSF. Also, it can be differentiated from
RMSF by the presence of an eschar at the site of the tick
attachment.3 In South America, rickettsial illness caused by
R. parkeri has been described in Uruguay, Argentina, and
probably, Brazil.14 Cases from Argentina have been confirmed
Until recently, Rocky Mountain spotted fever (RMSF)
or Rickettsia rickettsii infection was the unique tick-borne
rickettsiosis known in the New World. However, during last
decades, new Rickettsia species have been identified as human
tick-borne pathogens, which is the case of R. parkeri. Human
cases caused by this microorganism and confirmed using molecular assays have been mainly described in North America,1–5
and retrospective analyses have shown that some cases
of RMSF could be now attributed to R. parkeri.6 In South
America, two molecularly confirmed cases of human infection
with R. parkeri have been reported in Argentina, and recent
molecular results strongly suggest that this infection is also
distributed in Brazil.7–9 Herein, we report a confirmed case
of R. parkeri human disease in a patient who returned to
Spain after acquiring the infection in Uruguay.
A 54-year-old man returned to Spain on December 16, 2012
after a 7-day trip to Uruguay. He did not notice any arthropod
bites. A risk factor for being bitten by ticks is walking in
grassy areas, and our patient had been walking barefoot along
a grassy area in Colonia Suiza (southwestern Uruguay). Two
days after arrival in Spain, he noticed two crusted lesions on
the inner side of the left ankle. The next day, he presented
with malaise, fever, and chills. He was treated with amoxicillinclavulanic acid and mupirocin cream for 4 days by a primary
care physician, but his symptoms persisted. On December 25,
he was admitted to the Hospital San Pedro in La Rioja (Spain)
with the presumptive diagnosis of cellulitis after probable
arthropod bite. Examination showed fever (39 °C) and two
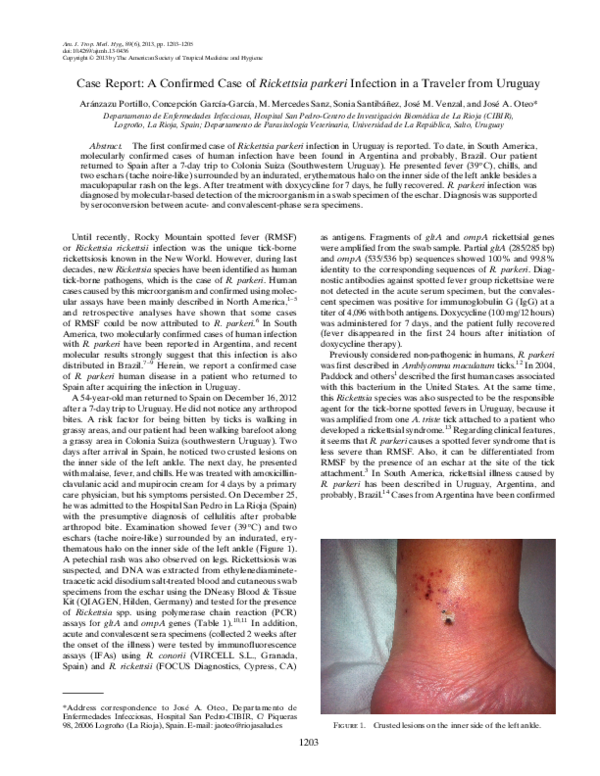
eschars (tache noire-like) surrounded by an indurated, erythematous halo on the inner side of the left ankle (Figure 1).
A petechial rash was also observed on legs. Rickettsiosis was
suspected, and DNA was extracted from ethylenediaminetetraacetic acid disodium salt-treated blood and cutaneous swab
specimens from the eschar using the DNeasy Blood & Tissue
Kit (QIAGEN, Hilden, Germany) and tested for the presence
of Rickettsia spp. using polymerase chain reaction (PCR)
assays for gltA and ompA genes (Table 1).10,11 In addition,
acute and convalescent sera specimens (collected 2 weeks after
the onset of the illness) were tested by immunofluorescence
assays (IFAs) using R. conorii (VIRCELL S.L., Granada,
Spain) and R. rickettsii (FOCUS Diagnostics, Cypress, CA)
*Address correspondence to José A. Oteo, Departamento de
Enfermedades Infecciosas, Hospital San Pedro-CIBIR, C/ Piqueras
98, 26006 Logroño (La Rioja), Spain. E-mail: jaoteo@riojasalud.es
Figure 1. Crusted lesions on the inner side of the left ankle.
1203
�1204
PORTILLO AND OTHERS
Table 1
Primers used for amplification of partial rickettsial genes
Primer sequence (5¢ ! 3¢)
Primer name
ompA
Rr190.70p
Rr190.701n
Rr190.70p
Rr190.602n
gltA
RpCS.877p
RpCS.1,258n
RpCS.896p
RpCS.1,233n
Amplified fragment (bp)
Annealing temperature ( °C)
Ref.
ATGGCGAATATTTCTCCAAAA
GTTCCGTTAATGGCAGCATCT
ATGGCGAATATTTCTCCAAAA
AGTGCAGCATTCGCTCCCCCT
631
46
10
532
48
10
GGGGGCCTGCTCACGGCGG
ATTGCAAAAAGTACAGTGAACA
GGCTAATGAAGCAGTGATAA
GCGACGGTATACCCATAGC
381
48
10
337
56
11
with molecular tools,7 whereas rickettsial taxonomy related to
Brazilian cases remains unclear.8,9 All confirmed and probable
cases referred to tick bites. Most presented an eschar at the tick
bite site besides a maculopapular rash that was accompanied
by fever, myalgias, or headache. As we observed in our patient,
the clinical course was benign in all published cases, with clinical resolution after doxycycline prescription.7 Recently, two
cases of spotted fever group rickettsiosis caused by a noncultured Rickettsia closely related to R. parkeri as well as
R. africae and R. sibirica have been reported in Brazil.8,9 To
date, whether these taxonomic names may be considered a
single species is discussed.15
R. parkeri is a common microorganism found in ticks from
South American countries.13,16–19 In Uruguay, R. parkeri is
present in a relatively high percentage of A. triste ticks.20
A. triste is present in at least 12 other Latin American countries, and it is probable that this infection is widely distributed
in most of the continent.21,22 Higher R. parkeri infection rates
among tick populations, compared with R. rickettsii, suggest
that R. parkeri rickettsiosis is likely to be misdiagnosed.23 In
conclusion, we must consider the possibility of rickettsiosis in
people returning from South America.
4.
5.
6.
7.
8.
9.
10.
Received July 29, 2013. Accepted for publication September 17, 2013.
Published online October 28, 2013.
Acknowledgments: The American Committee on Clinical Tropical
Medicine and Travelers’ Health (ACCTMTH) assisted with publication expenses.
Authors’ addresses: Aránzazu Portillo, Concepción Garcı́a-Garcı́a, M.
Mercedes Sanz, Sonia Santibáñez, and José A. Oteo, Departamento de
Enfermedades Infecciosas, Hospital San Pedro-CIBIR, Logroño, La
Rioja, Spain, E-mails: aportillo@riojasalud.es, cgarciag@riojasalud.es,
mmsanz@riojasalud.es, ssantibanez@riojasalud.es, and jaoteo@
riojasalud.es. José M. Venzal, Departamento de Parasitologı́a
Veterinaria, Universidad de La República, Salto, Uruguay, E-mail:
dpvuru@hotmail.com.
11.
12.
13.
14.
15.
REFERENCES
1. Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS,
Goddard J, McLellan SL, Tamminga CL, Ohl CA, 2004.
Rickettsia parkeri: a newly recognized cause of spotted
fever rickettsiosis in the United States. Clin Infect Dis 38:
805 – 811.
2. Whitman TJ, Richards AL, Paddock CD, Tamminga CL, Sniezek
PJ, Jiang J, 2007. Rickettsia parkeri infection after tick bite,
Virginia. Emerg Infect Dis 13: 334–336.
3. Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ,
Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA,
McLellan SL, Goddard J, Holman RC, Openshaw JJ, Sumner
16.
17.
18.
19.
JW, Zaki SR, Eremeeva ME, 2008. Rickettsia parkeri
rickettsiosis and its clinical distinction from Rocky Mountain
spotted fever. Clin Infect Dis 47: 1188–1196.
Cragun WC, Bartlett BL, Ellis MW, Hoover AZ, Tyring SK,
Mendoza N, Vento TJ, Nicholson WL, Eremeeva ME, Olano
JP, Rapini RP, Paddock CD, 2010. The expanding spectrum
of eschar-associated rickettsioses in the United States. Arch
Dermatol 146: 641–648.
Myers T, Lalani T, Dent M, Jiang J, Daly PL, Maguire JD,
Richards AL, 2013. Detecting Rickettsia parkeri infection from
eschar swab specimens. Emerg Infect Dis 19: 778–780.
Raoult D, Paddock CD, 2005. Rickettsia parkeri infection
and other spotted fevers in the United States. N Engl J Med
353: 626–627.
Romer Y, Seijo AC, Crudo F, Nicholson WL, Varela-Stokes A,
Lash RR, Paddock CD, 2011. Rickettsia parkeri rickettsiosis,
Argentina. Emerg Infect Dis 17: 1169–1173.
Spolidorio MG, Labruna MB, Mantovani E, Brandao PE,
Richtzenhain LJ, Yoshinari NH, 2010. Novel spotted fever
group rickettsiosis, Brazil. Emerg Infect Dis 16: 521–523.
Silva N, Eremeeva ME, Rozental T, Ribeiro GS, Paddock
CD, Ramos EAG, Favacho ARM, Reis MG, Dasch GA,
de Lemos ERS, Ko AI, 2011. Eschar-associated spotted
fever rickettsiosis, Bahia, Brazil. Emerg Infect Dis 17:
275–278.
Regnery RL, Spruill CL, Plikaytis BD, 1991. Genotypic identification of rickettsiae and estimation of intraspecies sequence
divergence for portions of two rickettsial genes. J Bacteriol
173: 1576–1589.
Choi YJ, Jang WJ, Ryu JS, Lee SH, Park KH, Paik HS, Koh YS,
Choi MS, Kim IS, 2005. Spotted fever group and typhus
group rickettsioses in humans, South Korea. Emerg Infect Dis
11: 237–244.
Parker RR, Kohls GM, Cox GW, Davis GE, 1939. Observations
on an infectious agent from Amblyomma maculatum. Public
Health Rep 54: 1482–1484.
Venzal JM, Portillo A, Estrada-Peña A, Castro O, Cabrera PA,
Oteo JA, 2004. Rickettsia parkeri in Amblyomma triste from
Uruguay. Emerg Infect Dis 10: 1493–1495.
Labruna MB, Mattar S, Nava S, Bermudez S, Venzal JM, Dolz G,
Abarca K, Romero L, de Sousa R, Oteo J, Zavala-Castro J,
2011. Rickettsioses in Latin America, Caribbean, Spain and
Portugal. Rev MVZ Córdoba 16: 2435–2457.
Walker DH, Ismail N, 2008. Emerging and re-emerging
rickettsioses: endothelial cell infection and early disease events.
Nat Rev Microbiol 6: 375–386.
Silveira I, Pacheco RC, Szabó MP, Ramos HG, Labruna MB,
2007. Rickettsia parkeri in Brazil. Emerg Infect Dis 13: 1111–1113.
Nava S, Elshewany Y, Eremeeva ME, Sumner JW, Mastropaolo
M, Paddock CD, 2008. Rickettsia parkeri in Argentina.
Emerg Infect Dis 14: 1894–1897.
Tomassone L, Conte V, Parrilla G, De Meneghi D, 2010.
Rickettsia infection in dogs and Rickettsia parkeri in Amblyomma
tigrinum ticks, Cochabamba Department, Bolivia. Vector Borne
Zoonotic Dis 10: 953–958.
Flores-Mendoza C, Florin D, Felices V, Pozo EJ, Graf PC,
Burrus RG, Richards AL, 2013. Detection of Rickettsia parkeri
from within Piura, Peru, and the first reported presence of
�RICKETTSIA PARKERI HUMAN INFECTION FROM URUGUAY
Candidatus Rickettsia andeanae in the tick Rhipicephalus
sanguineus. Vector Borne Zoonotic Dis 13: 505–508.
20. Venzal JM, Estrada-Peña A, Portillo A, Mangold AJ, Castro O,
De Souza CG, Félix ML, Pérez-Martı́nez L, Santibánez S,
Oteo JA, 2012. Rickettsia parkeri: a rickettsial pathogen
transmitted by ticks in endemic areas for spotted fever
rickettsiosis in southern Uruguay. Rev Inst Med Trop Sao
Paulo 54: 131–134.
1205
21. Guglielmone AA, Estrada-Peña A, Keirans JE, Robbins RG,
2003. Ticks (Acari: Ixodida) of the neotropical zoogeographic
region. International Consortium on Ticks and Tick-Borne
Diseases, Atalanta, Houten, The Netherlands, 173 pp.
22. Pacheco RC, Venzal JM, Richtzenhain LJ, Labruna MB, 2006.
Rickettsia parkeri in Uruguay. Emerg Infect Dis 12: 1804–1805.
23. Labruna MB, 2009. Ecology of Rickettsia in South America. Ann
N Y Acad Sci 1166: 156–166.
�
 Aránzazu Portillo
Aránzazu Portillo