Abstract
Multivesicular bodies (MVBs) are endocytic compartments that enclose intraluminal vesicles (ILVs) formed by inward budding from the limiting membrane of endosomes. In T lymphocytes, these ILV contain Fas ligand (FasL) and are secreted as 'lethal exosomes' following activation-induced fusion of the MVB with the plasma membrane. Diacylglycerol (DAG) and diacylglycerol kinase α (DGKα) regulate MVB maturation and polarized traffic, as well as subsequent secretion of pro-apoptotic exosomes, but the molecular basis underlying these phenomena remains unclear. Here we identify protein kinase D (PKD) family members as DAG effectors involved in MVB genesis and secretion. We show that the inducible secretion of exosomes is enhanced when a constitutively active PKD1 mutant is expressed in T lymphocytes, whereas exosome secretion is impaired in PKD2-deficient mouse T lymphoblasts and in PKD1/3-null B cells. Analysis of PKD2-deficient T lymphoblasts showed the presence of large, immature MVB-like vesicles and demonstrated defects in cytotoxic activity and in activation-induced cell death. Using pharmacological and genetic tools, we show that DGKα regulates PKD1/2 subcellular localization and activation. Our studies demonstrate that PKD1/2 is a key regulator of MVB maturation and exosome secretion, and constitutes a mediator of the DGKα effect on MVB secretory traffic.
Similar content being viewed by others
Main
Exosomes are nanovesicles that form as intraluminal vesicles (ILVs) inside multivesicular bodies (MVBs) and are then secreted by numerous cell types.1 ILVs are generated by inward budding of late endosome limiting membrane in a precisely regulated maturation process.2, 3 Two main pathways are involved in MVB maturation.4, 5 In addition to the ESCRT (endosomal complex required for traffic) proteins,6 there is increasing evidence that lipids such as lyso-bisphosphatidic acid (LBPA),7 ceramides8 and diacylglycerol (DAG)9 contribute to this membrane invagination process.
Exosomes participate in many biological processes related to T-cell receptor (TCR)-triggered immune responses, including T lymphocyte-mediated cytotoxicity and activation-induced cell death (AICD), antigen presentation and intercellular miRNA exchange.10, 11, 12, 13, 14, 15 The discovery of exosome involvement in these responses increased interest in the regulation of exosome biogenesis and secretory traffic, with special attention to the contribution of lipids such as ceramide and DAG, as well as DAG-binding proteins.14, 16, 17, 18, 19, 20, 21 These studies suggest that positive and negative DAG regulators may control secretory traffic. By transforming DAG into phosphatidic acid (PA), diacylglycerol kinase α (DGKα) is essential for the negative control of DAG function in T lymphocytes.22 DGKα translocates transiently to the T-cell membrane after human muscarinic type 1 receptor (HM1R) triggering or to the immune synapse (IS) after TCR stimulation; at these subcellular locations, DGKα acts as a negative modulator of phospholipase C (PLC)-generated DAG.23, 24
The secretory vesicle pathway involves several DAG-controlled checkpoints at which DGKα may act; these include vesicle formation and fission at the trans-Golgi network (TGN), MVB maturation, as well as their transport, docking and fusion to the plasma membrane.9, 16, 17, 18, 19, 20 The molecular components that regulate some of these trafficking processes include protein kinase D (PKD) family members.21 PKD1 activity, for instance, regulates fission of transport vesicles from TGN via direct interaction with the pre-existing DAG pool at this site.19 The cytosolic serine/threonine kinases PKD1, PKD2 and PKD3(ref. 21) are expressed in a wide range of cells, with PKD2 the most abundant isotype in T lymphocytes.25, 26 PKD have two DAG-binding domains (C1a and C1b) at the N terminus,21 which mediate PKD recruitment to cell membranes. Protein kinase C (PKC) phosphorylation at the PKD activation loop further promotes PKD autophosphorylation and activation.27
Based on our previous studies showing DGKα regulation of DAG in MVB formation and exosome secretion,9, 14, 28 and the identification of PKD1/2 association to MVB,14 we hypothesized that DGKα control of DAG mediates these events, at least in part, through PKD. Here we explored whether, in addition to its role in vesicle fission from TGN,19 PKD regulates other steps in the DAG-controlled secretory traffic pathway. Using PKD-deficient cell models, we analyzed the role of PKD1/2 in MVB formation and function, and demonstrate their implication in exosome secretory traffic.
Results
Pharmacological PKC inhibition limits exosome secretion in T lymphocytes
DGKα limits exosome secretion in T lymphocytes.9, 14, 28 This negative effect correlates with exosome secretion induced by addition of the cell-permeable DAG analog dioctanoyl glycerol.14
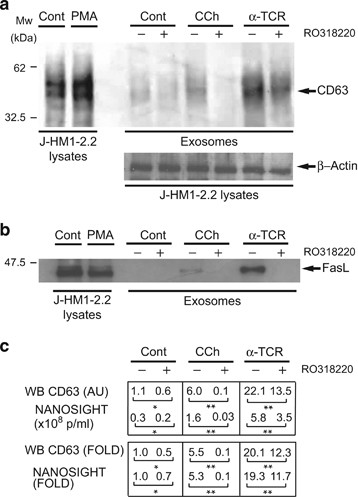
We first assessed the role of PKD in exosome secretion by inhibiting its upstream activator PKC. RO318220 is a broad range PKC inhibitor that prevents TCR-induced and phorbol myristate acetate (PMA)-induced PKD phosphorylation by PKC.29 RO318220 treatment inhibited PMA-induced, PKC-dependent phosphorylation of endogenous PKD1/2 and of PKD1 fused to GFP (GFP-PKD1) at the activation loop (pS744/S748)30 (Supplementary Figure S1A); the effect was similar for a PKD1 kinase-deficient mutant (D733A; GFP-PKD1KD).19, 31 Inhibitor treatment also impaired PKD autophosphorylation (pS916)27, 29 induced by carbachol (CCh) (Supplementary Figure S1B) or by anti-TCR (data not shown). We pretreated J-HM1-2.2 cells with RO318220, followed by anti-TCR or CCh stimulation to induce exosome secretion.14 Exosomes isolated from culture supernatants14, 32, 33, 34 were quantitated by WB using anti-CD63 or by NANOSIGHT, with similar results (Supplementary Figure S2). RO318220-pretreated J-HM1-2.2 cells showed a notable decrease in exosomal CD63 and Fas ligand (FasL; Figures 1a and b) after stimulation with anti-TCR or CCh. These results suggest that reducing PKC-dependent, PKD activation by RO318220 treatment results in less CD63 and FasL secretion into exosomes with a comparable decrease in the number of exosomes secreted (particles/ml culture supernatant; Figure 1c).
PKC regulates exosome secretion. (a) J-HM1-2.2 cells, alone or preincubated with RO318220, were stimulated with CCh (500âμM) or plate-bound anti-TCR mAb (UCHT1, 10âμg/ml) for 8âh to induce exosome secretion. Exosomes were isolated by sequential centrifugation. WB of exosome protein extracts were developed with anti-CD63, whereas WB of whole-cell lysates were developed with β-actin antibody to normalize to cell number. In the left panel, whole-cell lysates of PMA-treated J-HM1-2.2 cells are shown as a reference for CD63. (b) Whole J-HM1-2.2 and exosome lysates in a were analyzed with anti-FasL. In the left-side lanes, whole-cell lysates were run as a reference for FasL. (c) Top table: quantification of WB signal shown in a (mean arbitrary units; AUs) in parallel with NANOSIGHT data (mean particles/ml) for the same samples. Bottom, mean x-fold induction data are shown for CD63 in exosomes (WB) and exosome concentration (NANOSIGHT). S.D. was <5% of the mean in all cases; * not significant (NS); **Pâ¤0.05
PKD1/2 regulate exosome secretion in T lymphocytes
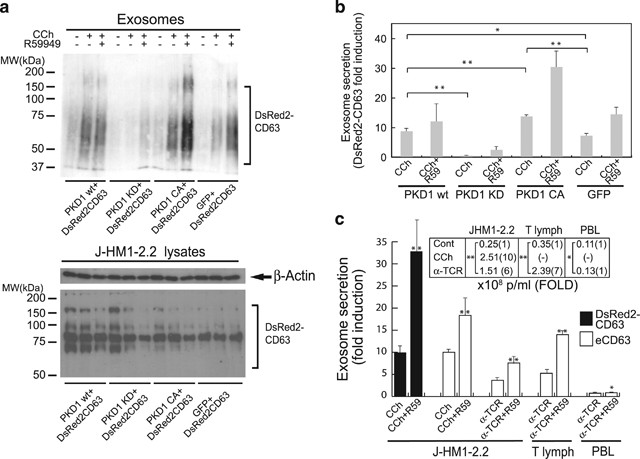
Although PKC inhibition can have many effects, our observations are compatible with PKD involvement in exosome biogenesis/secretion. To directly analyze PKD contribution, we used an exosome reporter secretion assay9, 14 based on transient expression of the exosome reporter DsRed2-CD63 and several GFP-PKD1 constructs, including GFP-PKD1, GFP-PKD1KD and a GFP-PKD1 version with phospho-mimetic glutamic residues that replace the PKC phosphorylation sites at S744/748 (GFP-PKD1CA). We analyzed exosome secretion by WB with anti-CD63 (Figure 2a, top). We normalized the results to the transfection efficiency of the exosome reporter and the number of viable exosome-secreting cells (Figure 2a, bottom).
PKD regulates exosome secretion. (a) J-HM1-2.2 cells were co-transfected with DsRed2-CD63 and the constructs GFP-PKD1WT, GFP-PKD1KD (kinase-dead) or GFP-PKD1CA (constitutively active). Cells were preincubated alone or with R59949 (R59, 10âμM) and stimulated with CCh (500âμM) to induce exosome secretion. Top, WB of exosome protein extracts developed with anti-CD63. For simplicity, R59949 alone was not included in these experiments; the inhibitor alone had no detectable effect on exosome secretion.9, 14 Bottom, lysates of exosome-secreting cells were developed with anti-β-actin and -CD63 for normalization. (b) Quantitation of results from three experiments similar to that in a. Exosome secretion by cells expressing the distinct constructs is expressed as x-fold induction (mean±S.D.) of three experiments, normalized to cell DsRed2-CD63 levels. (c) DsRed2-CD63-transfected J-HM1-2.2 cells, human T lymphoblasts or human PBL, alone or R59949-treated, were stimulated as indicated to induce exosome secretion. Normalized x-fold induction of exosome secretion was determined by WB analyses of DsRed2-CD63 and endogenous CD63 (eCD63). Inset, mean exosome concentration (particles/ml) secreted by the distinct cell types was determined by NANOSIGHT analyses; mean x-fold induction of exosome secretion is shown in brackets. S.D. was <5% of the mean in all cases; *not significant (NS); **Pâ¤0.05
CCh stimulation induced exosome secretion; this effect was increased by pretreatment with the DGKα inhibitor R59949.9, 14 GFP-PKD1WT expression did not markedly alter CCh-induced exosome secretion, whereas the GFP-PKD1KD mutant, which acts as a PKD1 dominant-inhibitory mutant,19 impaired exosome secretion even in the presence of the inhibitor (Figure 2b). These experiments support an endogenous PKD contribution to exosome secretion, although the lack of effect because of GFP-PDK1WT expression also suggests that DAG generation, directly or through PKC-dependent phosphorylation, is a limiting factor in PKD activation. To test this, we used the GFP-PKD1CA mutant that bypasses the PKC phosphorylation requirement, but not that for PLC-generated DAG.19, 31 GFP-PKD1CA-expressing cells showed enhanced exosome secretion in response to CCh stimulation compared with GFP-PKD1WT-expressing cells (Figure 2b), confirming the relevance of PKD phosphorylation by PKC for exosome secretion. Treatment with the DGKα inhibitor further increased exosome secretion by GFP-PKD1CA-expressing cells, which suggests that DGKα consumption of DAG controls PKD activation, not only through PKC-mediated phosphorylation, but also through direct DAG binding.
We compared exosome secretion by the J-HM1-2.2 cells with that of primary T lymphocytes, using various stimuli (CCh and anti-TCR for J-HM1-2.2 cells; anti-TCR for T lymphoblasts and peripheral blood lymphocytes, PBLs) (Figure 2c). T lymphoblasts and J-HM1-2.2 cells secreted comparable exosome numbers after TCR triggering, whereas exosome secretion by PBL was not enhanced following stimulation. DGKα inhibition enhanced exosome secretion in stimulated J-HM1-2.2 cells and T lymphoblasts (Figure 2c).
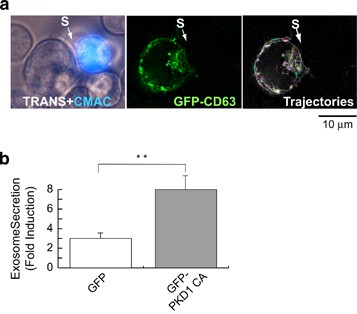
To evaluate the PKD1 effect on polarized exosome secretion in an IS model, we challenged GFP-CD63-expressing Jurkat cells with Staphylococcus enterotoxin E (SEE)-presenting Raji B cells,35 which allowed analysis of GFP-CD63+ MVB traffic and exosome secretion.9 Following conjugate formation, intracellular GFP-CD63+ MVB accumulated in Jurkat cells at the synapse contact area (Supplementary Video 1; interacting cells, top right), but not in unconjugated Jurkat cells (GFP-CD63-expressing cells; bottom left). MVB movement and concentration in the synapse area was recorded at 2âh post-conjugate formation (Supplementary Video 2) and vesicle trajectories plotted (Figure 3a). Although the vesicles showed random movement in conjugated cells, they tended to tether near the synapse area (Figure 3a). To examine exosome secretion, we challenged Jurkat cells co-expressing GFP-CD63 and GFP-PKD1CA with Raji cells, untreated or SEE pulsed (Supplementary Video 1). After synapse formation, we analyzed GFP-CD63+ exosomes, and expressed the results as normalized x-fold induction (Figure 3b). Expression of the GFP-PKD1CA mutant enhanced exosome secretion compared with an irrelevant control (GFP). PKD thus appears to be involved in activation-induced exosome secretion in T lymphocytes in polarized and non-polarized secretion models.
GFP-PKD1CA mutant expression enhances polarized exosome secretion. (a) GFP-CD63-expressing Jurkat cells were challenged (2âh) with SEE-pulsed (1âμg/ml), CMAC-labeled Raji cells (blue) to induce formation of synaptic conjugates (S, synapse) and MVB polarization toward the IS. Transmittance plus CMAC (blue; left panel), GFP-CD63 channel (center) and trajectories followed by GFP-CD63 vesicles (right) are shown (see also Supplementary Video 2). (b) Jurkat cells that coexpressed GFP-CD63 and GFP or GFP-PKD1CA were challenged with SEE-pulsed Raji cells; exosomes were isolated, quantitated by WB analysis of the GFP-CD63 reporter and normalized for cell number and cellular GFP-CD63 content. Exosome secretion is expressed as x-fold induction (mean±S.D.) of three independent experiments. **Pâ¤0.05
PKD1 regulates exosome secretion in a B-cell model
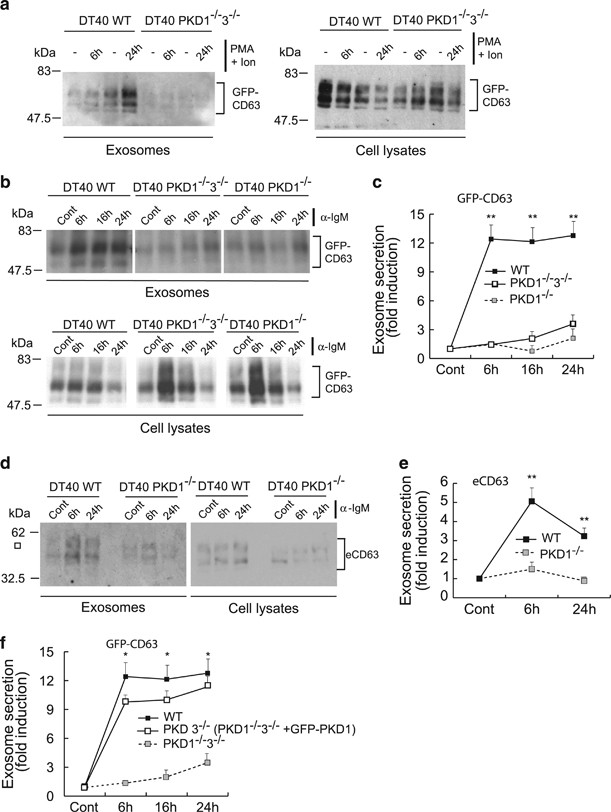
B-cell receptor stimulation with anti-IgM activates PKD1 and PKD3(ref. 36) and induces secretion of CD63+ exosomes, which participate in antigen presentation.37, 38, 39, 40 To extend results from T lymphocytes, we studied exosome secretion in DT40 chicken B lymphocytes that lacked their two PKD isotypes, PKD1 and PKD3.36 Wild-type and PKD1â/â3â/â DT40 cells were transfected with GFP-CD63 and exosome secretion was assessed after stimulation with PMA plus ionomycin or with anti-IgM. PMA plus ionomycin induced exosome secretion in WT, but not in PKD1â/â3â/â DT40 cells (Figure 4a). PKD1â/â3â/â cells bore a doxycycline-inducible Flag-tagged PKD3 transgene that, in the presence of tetracycline, induced Flag-PKD3 expression at levels comparable to those of endogenous PKD3 in WT DT40 cells36, 41 (Supplementary Figure S3). To determine the functional redundance of PKD1 and PKD3 in exosome secretion control, we induced Flag-PKD3 expression and stimulated WT, PKD1â/â3â/â and PKD1â/â DT40 cells with anti-IgM. In Flag-PKD3-expressing (PKD1â/â) DT40 cells, exosome secretion was not restored to the levels of WT DT40 controls (Figures 4b and c). Western blot results were comparable when we analyzed endogenous CD63 in exosomes secreted by WT and PKD1â/â DT40 cells (Figures 4d and e). When GFP-PKD1 was expressed in PKD1â/â3â/â cells to yield PKD3â/â DT40 cells, inducible exosome secretion was rescued to the values observed for WT DT40 cells (Figure 4f). PKD1 thus appears to be the essential isoform for inducible exosome secretion in chicken B lymphocytes.
Exosome secretion in DT40 B lymphocytes. WT, PKD1â/â3â/â and PKD1â/â DT40 cells (PKD1â/â3â/â DT40 cells cultured with tetracycline to induce Flag-PKD3 expression; see Supplementary Figure S3) were transfected with GFP-CD63 and stimulated for the indicated times with PMA (10âng/ml) plus ionomycin (0.5âμg/ml) (a) or anti-IgM (10âμg/ml) (b). We analyzed GFP-CD63 in cell lysates and exosomes using WB and quantified the x-fold induction (mean±S.D.) of exosome secretion of GFP-CD63, normalized to cell GFP-CD63 levels (c). Statistical significance corresponds to exosome secretion at the same time points of WT versus PKD1â/â3â/â or PKD1â/â DT40 cells. In d, WT and PKD1â/â DT40 cells were stimulated with anti-IgM, exosomes isolated and endogenous CD63 (eCD63) analyzed in cell lysates and exosomes using WB; quantification is shown in e, normalized to cellular eCD63. Statistical significance corresponds to exosome secretion at the same time points of WT versus PKD1â/â DT40 cells. (f) WT, PKD1â/â3â/â DT40 cells and GFP-PKD1-transfected PKD1â/â3â/â DT40 cells (PKD3â/â DT40 cells), all of which expressed GFP-CD63, were anti-IgM-stimulated and exosome secretion analyzed as above. Statistical significance corresponds to exosome secretion at the same time points of WT versus PKD3â/â DT40 cells. *not significant (NS); **Pâ¤0.05
PKD2 regulates exosome secretion in primary T lymphocytes
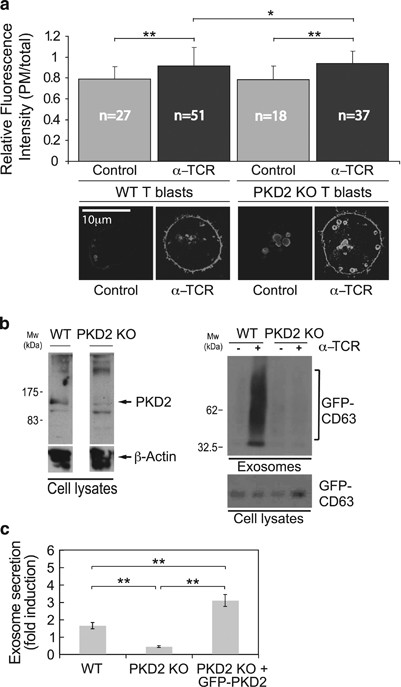
PKD2 is the main PKD isotype in human and mouse T lymphocytes.25, 26 We therefore analyzed T lymphoblasts from mice that lacked PKD2 (ref. 26) to determine its contribution to MVB traffic and exosome secretion. T lymphoblasts from WT and PKD2â/â mice were retrovirally transduced to express GFP-CD63, and then stimulated for 18âh with plate-bound anti-TCR to induce secretory traffic of MVB. Live-cell imaging of transduced WT T lymphoblasts showed numerous GFP-CD63+ vesicles with apparently random movement within the cell, as well as weak cell surface fluorescence that may reflect constitutive CD63 traffic42 (Supplementary Video 3). Following TCR stimulation, we consistently observed increased fluorescence at the plasma membrane, a finding compatible with GFP-CD63+ MVB degranulation43, 44 (Supplementary Video 4). After stimulation, some of these vesicles approached and apparently docked to the plasma membrane. Fluorescence at the docking point increased transiently and was later lost (Supplementary Video 5, trajectories 1 and 2; Supplementary Figure S4), suggesting TCR-induced MVB fusion to the plasma membrane.
The increase in cell surface GFP-CD63 after TCR stimulation was probably due to GFP-CD63 transfer from the MVB limiting membrane to the plasma membrane (Supplementary Video 5, Figure 5a). The ratio of plasma membrane to total GFP-CD63 fluorescence intensity after TCR stimulation increased similarly in PKD2â/â and WT T lymphoblasts (Figure 5a). After TCR stimulation, the mean velocity of CD63+ MVB was also similar in PKD2â/â and WT T lymphoblasts (data not shown). In contrast, WB analysis showed lack of inducible exosome secretion in PKD2â/â T lymphoblasts (Figure 5b). Lack of PKD2, therefore, did not alter TCR-induced MVB docking and fusion nor MVB velocity, but limited exosome secretion. PKD2 reconstitution of PKD2â/â T lymphoblasts enhanced TCR-triggered exosome secretion compared with PKD2â/â or WT T lymphoblasts (Figure 5c).
MVB secretory traffic and exosome secretion in PKD2â/â T lymphoblasts. GFP-CD63-transduced WT or PKD2â/â T lymphoblasts were stimulated with plate-bound anti-TCR (α-TCR, 2C11, 10âμg/ml; 6âh) to induce MVB degranulation and exosome secretion. (a) Top, quantitation of the GFP-CD63 fluorescence ratio (plasma membrane:total fluorescence) in WT and PKD2â/âT lymphoblasts. Bottom, representative epifluorescence images of unstimulated or stimulated cells. (b) WB analysis of cell PKD2 expression (left); WB analysis of exosome GFP-CD63 levels (right). (c) PKD2â/â and GFP-PKD2-transduced PKD2â/â T lymphoblasts, both transduced with GFP-CD63, were anti-TCR stimulated as in a and exosome secretion compared with that for WT T lymphoblasts. Results are quantified as x-fold induction (mean±S.D.) of exosome secretion of GFP-CD63, normalized to cell GFP-CD63 levels. *not significant (NS); **Pâ¤0.05
PKD involvement in MVB morphogenesis
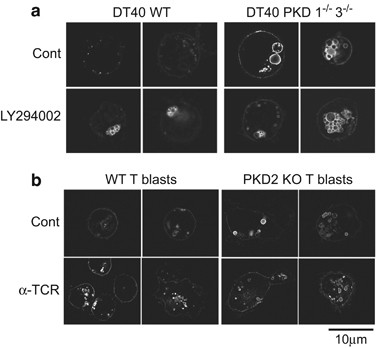
DGKα enzyme activity negatively regulates MVB maturation, as does expression of VPS4EQ, an ATPase-defective mutant of vacuolar protein sorting 4 that cooperates with the ESCRT complex.9 The molecular basis of the DGKα effect on MVB maturation is unclear, as DGKα is not known to regulate any ESCRT protein. Immature MVB consist of enlarged, ring-shaped CD63+ endocytic vesicles with no ILV.45 PKD2â/â T lymphoblasts showed enlarged CD63+ vesicles with an anomalous, ring-shaped structure (Figure 5a, bottom; Supplementary Video 6), which suggests that PKD2 participates in MVB maturation. Quantitative analysis showed clear differences in vesicle area in TCR-stimulated WT versus PKD2â/â T lymphoblasts (data not shown).
PI3K/Vps34 (phosphatidyl-inositol-3-phosphate kinase/vacuolar protein sorting 34) is a regulator of the ESCRT complex that participates in MVB maturation.45, 46 PI3K inhibition thus limits MVB maturation.46, 47, 48, 49 WT and PKD1â/â3â/â GFP-CD63+ DT40 cells were incubated alone or with the PI3K inhibitor LY294002. Inhibitor-treated WT DT40 cells formed a cluster of enlarged, ring-shaped CD63+ vesicles similar to those found constitutively in PKD1â/â3â/â DT40 cells (Figure 6a). CD63+ vesicles were comparable in size and shape in untreated and PI3K inhibitor-treated PKD1â/â3â/â DT40 cells (Figure 6a), and were similar to those in PKD2â/â T lymphoblasts (Figures 5a,6b,Supplementary Video 6). These data demonstrate that PKD regulates exosome secretion, at least in part through control of MVB morphogenesis.
PKDs control MVB maturation. (a) WT and PKD1â/â3â/â DT40 cells were transfected with GFP-CD63 and treated with PI3K inhibitor (LY294002, 10âμM; 4âh). GFP-CD63 fluorescence was imaged by epifluorescence and definition improved by deconvolution. (b) GFP-CD63-transduced WT or PKD2â/â T lymphoblasts were stimulated with plate-bound anti-TCR (2C11, 10âμg/ml, 4âh) to induce MVB degranulation and GFP-CD63 relocalization to the plasma membrane and imaged by epifluorescence
DGKα regulates PKD1/2 activation
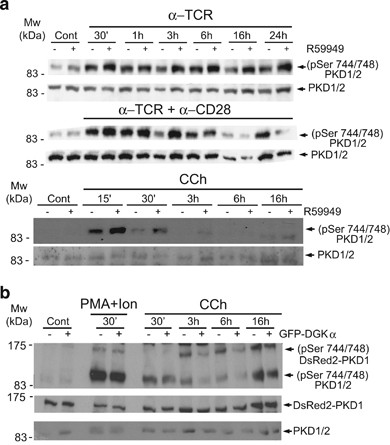
PKD positively controls MVB maturation and exosome secretion, whereas DGKα enzyme activity negatively regulates these processes,9 and the DGKα inhibitor did not rescue GFP-PKD1KD mutant inhibition of exosome secretion (Figure 2b). DGKα may thus control PKD1 activation in a pathway that governs MVB maturation and exosome secretion. We analyzed this using J-HM1-2.2 cells alone or treated with the DGKα inhibitor R59949, and activated with CCh, anti-TCR or anti-TCR plus anti-CD28. WB analysis of R59949-treated cells showed an increase in active PKD1/2, as measured by phosphorylation on S744/S748. This increase was most evident from 30âmin to 3-h post-stimulation (Figure 7a). R59949 treatment did not enhance PMA-induced PKD1/2 phosphorylation (data not shown), indicating that the effect was specific for the TCR and HM1R, which activate PKD1/2 via PLC-generated DAG.
DGKα regulates PKD1/2 phosphorylation. (a) J-HM1-2.2 cells, alone or pretreated with DGK inhibitor II (R59949; 10âμM), were stimulated for the indicated times with anti-TCR (10âμg/ml), anti-TCR plus anti-CD28 (10âμg/ml) or CCh (500âμM). WB of whole-cell lysates was developed with the indicated antibodies to measure phosphorylation of the PKD1/2 (pSer 744/748) activation loop. (b) J-HM1-2.2 cells were transfected with DsRed2-PKD1 alone (-) or co-transfected with GFP-DGKα (+), then stimulated with CCh (500âμM) or PMA (10âng/ml) plus ionomycin (0.5âμg/ml) for times indicated. Cell lysates were analyzed by WB as above to determine phosphorylation of endogenous PKD1/PKD2 and DsRed2-PKD1 activation loops
To test whether DGKα overexpression affects PKD1/2 activation, we transfected J-HM1-2.2 cells with DsRed2-PKD1 alone or co-transfected with GFP-DGKα, and stimulated them with CCh or PMA plus ionomycin (which induces DAG-independent PKD1/2 activation). WB analysis with anti-(pS744/748)PKD1/2 showed less DsRed2-PKD1 and endogenous PKD1/2 phosphorylation in CCh-treated, GFP-DGKα and DsRed2-PKD1 co-expressing cells than in cells that expressed DsRed2-PKD1 alone. This phosphorylation difference was not observed in PMA plus ionomycin-stimulated cells (Figure 7b).
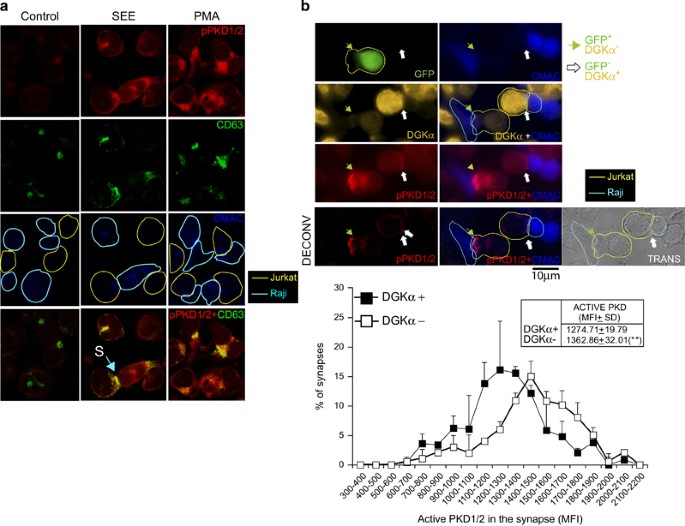
We tested the effect of attenuating DGKα expression on PKD activation in the IS model. Jurkat cells stimulated with PMA or SEE-pulsed Raji cells to induce synapse formation showed an increase in (pS916)PKD1/2 signal, which colocalized with CD63 (PMA) or synaptic membrane (SEE-pulsed Raji cells) (Figure 8a). We reduced DGKα expression by transfecting Jurkat cells with a bicistronic siRNA interference vector bearing GFP-coding sequence and DGKαâspecific siRNA.9 This approach allowed single-cell comparison of PKD1/2 activation in DGKα-silenced GFP+ cells with control GFP- cells during synapse formation (Figure 8b, top). Using image quantification of synapses, we compared the frequency distributions of PKD1/2 activation in synapses formed by DGKα-silenced cells and unsilenced Jurkat cells (Figure 8b, bottom). DGKα attenuation enhanced PKD activation, as indicated by an increase in mean fluorescence intensity of (pS916)PKD1/2 at the synaptic membrane. As PKD1 recruitment to the synapse depends on transient DAG production at this site, we measured DsRed2-PKD1 residence half-time at the synapse in DGKα-silenced cells by time-lapse video analysis (Supplementary Video 7). DGKα attenuation significantly enhanced mean DsRed2-PKD1 residence half-time at the synaptic membrane from 15â20 to 40âmin (Supplementary Figure S5). DAG consumption by DGKα thus limits PKD1/2 recruitment to and activation at the IS.
Interference with DGKα expression regulates PKD1/2 phosphorylation. (a) Co-cultured Jurkat and CMAC-labeled Raji cells were untreated (Control) or stimulated with SEE (1âμg/ml) or PMA (10âng/ml) (5âh). Confocal immunofluorescence images show phosphorylation of endogenous PKD1/2 (pSer916, red; top row), CD63 (green; second row), CMAC (Raji cells, blue; third row); bottom row shows merged images (yellow); S, synaptic contact. (b) Jurkat cells transfected with a GFP-containing bicistronic interference plasmid for human DGKα were stimulated with SEE-pulsed, CMAC (blue)-labeled Raji cells (5âh) to induce synapse formation. Top row, representative immunofluorescence analysis showing GFP+ Jurkat (green) and CMAC+ Raji (blue) cells. Interference with DGKα expression was assessed by intracellular staining of endogenous DGKα (orange; second row). Third row, pSer916 phosphorylation of endogenous PKD1/2 was assessed using anti-pPKD1 (pSer916, red) antibody in GFP+ (DGKαâ; green arrow) and GFPâ (DGKα+; white arrow) Jurkat cells forming synaptic conjugates. Bottom row, red fluorescence channel (pPKD1/2) signal-to-noise ratio was improved using deconvolution. The transmittance panel is also included to show two synaptic conjugates formed by Jurkat (yellow contour) and Raji (light blue contour) cells. The arrows indicate the synaptic contact areas. Bottom panel, analysis of mean fluorescence intensity distribution for pSer916 PKD1/2 in synapse-forming DGKα+ and DGKαâ Jurkat cells; results from four experiments (with at least 100 synapse-forming cells/condition) as in upper panels. Inset, MFI (mean±S.D.) for active PKD in both cell populations. **Pâ¤0.05
PKD2 controls CTL activity and AICD in T lymphoblasts
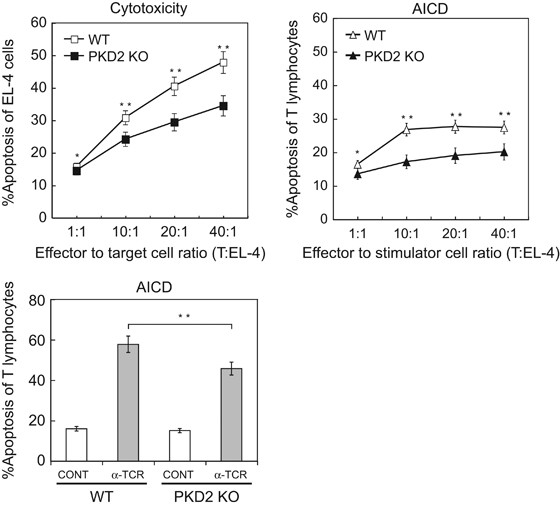
Cytotoxic T lymphocytes (CTLs) cytotoxic granules have a MVB structure and contain perforin, granzymes and FasL;44 ILVs from these MVBs are released to the synaptic cleft as exosomes.10 MVB degranulation and FasL secretion on exosomes mediates AICD of CD4+ T lymphocytes and Jurkat cells.14, 33 We next examined the effect of PKD2 deficiency on CTL activity and AICD. We used a murine IS model in which TCR-stimulated T lymphoblasts were challenged with Staphylococcus enterotoxin B (SEB)-pulsed, CMAC-labeled EL-4 cells. SEB binds to Vβ3,7,8,17-TCR-bearing T cells, which in mice make up a large proportion (~30%) of T lymphocytes,50 and induces T-cell activation and AICD.51 EL-4 syngeneic cells are used as target cells in murine CTL assays,52, 53 but also induce reactivation and AICD of effector T lymphocytes. After several transient synaptic contacts, CMAC+ EL-4 cells and T lymphoblasts both underwent apoptosis (plasma membrane blebbing; Supplementary Video 8). In mixed cultures at various effector:target/stimulator ratios, we used flow cytometry to quantitate the percentage of apoptosis (annexin V+ cells) in EL-4 cells (CTL activity marker) or T lymphoblasts (AICD marker) (Figure 9). CTL activity was lower in PKD2â/â than in WT T lymphoblasts, with a concomitant decrease in AICD (Figure 9, top). As only 30% of mouse T lymphoblasts express the SEB-responsive Vβ TCR, we restimulated T lymphoblasts with polyclonal anti-TCR with similar results (Figure 9, bottom). The lack of PKD2 thus reduced both AICD and CTL activity in primary T lymphoblasts.
PKD2 regulates CTL activity and AICD. Top, WT and PKD2â/â T lymphoblasts were challenged at the indicated cell:cell ratios with SEB-pulsed EL-4 cells (1âμg/ml; 6âh) Apoptosis was assessed in EL-4 cells (cytotoxicity) or T lymphoblasts (AICD) with annexin V-PE by flow cytometry. Results are expressed as the percentage of apoptotic cells. Bottom, AICD was analyzed as above for WT and PKD2â/â T lymphoblasts stimulated with plate-bound anti-TCR (2C11, 10âμg/ml). Statistical significance corresponds to percentage of apoptosis of EL-4 and T lymphocytes at the same effector to target/stimulator cell ratio of WT versus PKD2 KO T lymphoblasts. *not significant (NS), **Pâ¤0.05
Discussion
Exosomes are constitutively secreted by a variety of cell lineages and tumor cells, but T and B lymphocytes show inducible exosome secretion upon TCR and BCR triggering.54, 55 IS formation leads to polarization and membrane fusion of MVB, which release ILV as FasL-containing exosomes that trigger Fas-dependent AICD,13, 33 maintaining T lymphoid homeostasis.56, 57 CTL degranulation leads to specific, TCR-controlled target cell apoptosis.55 MVB biogenesis and secretion are thus important for immune effector responses and T-cell homeostasis.
DGKα acts as negative regulator of MVB maturation and exosome secretion by T lymphocytes.9, 14 Here we identify PKD as the major DAG effector that regulates MVB maturation and exosome secretion in T and B lymphocytes. Using pharmacological inhibition, DGKα overexpression and siRNA-mediated DGKα silencing, we show that DGKα controls MVB-mediated secretory traffic at least in part by limiting PKD activation. DGKα inhibition enhances exosome secretion in cells that express a PKD1 mutant that bypasses PKC activation requirements (GFP-PKD1CA); this suggests that in addition to limiting PKD phosphorylation by PKC, DGKα controls direct PKD activation by DAG.
This negative DGKα role contrasts with its positive function in MVB polarization to the IS.9 The molecular basis of this dual DGKα contribution to MVB formation and polarization is not fully understood, but may be linked to its ability to transform DAG into PA. Recent studies showed that DGKα controls late endosome polarized traffic in various cell lineages. DGKα-deficient CTL show defects in formation of the DAG gradient at the IS, as well as microtubule-organizing center (MTOC) reorientation.58 In breast carcinoma cells, DGKα-mediated PA generation mediates integrin recycling at the tips of invasive pseudopodial structures during invasive migration.59 Additional studies are needed to determine whether DGKα-generated PA and/or its DAG consumption are necessary for MVB polarization to the IS.
The PKD effectors that contribute to regulation of MVB maturation are not known. The aberrant CD63+ endosome phenotype in PKD-deficient B and T cells resembles that was observed after PI3K inhibition (Figure 6a). This suggests a role for PKD upstream of PI3K and correlates with studies that identified PKD phosphorylation and activation of class III PI3K Vps34.60 PI3P is a master organizer of endosomal sorting, and promotes recruitment of several proteins that regulate various steps in trafficking, including endosomal fusion and ESCRT-dependent intraluminal sorting of cargoes.61 J-HM1-2.2 cells that express an ATPase-deficient mutant VPS4(ref. 9) have aberrant endosomes similar to those seen in DT40 cells after PI3K inhibition. The comparable anomalous shape of CD63+ vesicles as a result of PKD2 deficiency provides a molecular mechanism for DGKα-negative contribution to MVB maturation and suggests that PKD2 acts as a DAG sensor that also modulates membrane PI3P levels.
PKD2 is the major PKD isotype expressed in human and mouse T lymphocytes.25, 26 In Jurkat T cells, GFP-tagged PKD1 and endogenous PKD2 have identical regulatory mechanisms, as well as similar activation kinetics and subcellular localization in response to TCR.62 The effects observed for exogenously expressed PKD1 constructs may thus be the result of inhibition of endogenous PKD2 and activation of PKD2 downstream effectors. The PKD2â/â mouse model confirms the contribution of this isoform to exosome secretion, and extends findings from DT40 B and Jurkat T cells to primary T lymphocytes. In response to TCR triggering, PKD2â/â T lymphoblasts show severe inhibition of IL-2 secretion,26 although TCR-induced IL-2 gene expression was unaffected.63 Defective IL-2 secretion by PKD2â/â T cells and the impaired exosome secretion we describe here could indicate a general secretion defect because of loss of PKD2. Indeed, a recent phosphoproteomic study of PKD2â/â CTL identified several traffic-related proteins as PKD2 substrates.64
Our data characterize a role for PKD2 in primary T-cell AICD and coincide with studies showing that expression of a constitutively active PKD2 mutant in Jurkat cells enhances AICD.25 Although loss of PKD2 enhances thymic output during T lymphocyte development, it does not affect T-cell selection or inhibit expression of Vβ8 TCR,63 the major murine TCR repertoire recognized by SEB.50 PKD2â/â T lymphoblasts show no changes in TCR-triggered, transcriptional induction of genes that encode proteins involved in CTL activity and AICD, such as FasL and perforin.55, 63 It remains to be determined whether the splenomegaly and lymphadenopathy in TCR transgenic PKD2â/â mice are due exclusively to enhanced thymic output63 or whether there is a contribution by an AICD defect. The decrease in CTL activity of PKD2â/âversus WT T lymphoblast-containing mixed cultures is underestimated, as PKD2â/â T lymphocytes undergo less AICD than WT T lymphoblasts (Figure 9), leading to an increase in the number of effector PKD2â/â T lymphoblasts. This reinforces the role of PKD2 as an important regulator of CTL activity.
Several studies have established an essential role for PKD and its activator DAG in Golgi-dependent secretory function. DAG-mediated PKD activation regulates vesicle budding and fission from the TGN, a process negatively regulated by DGK.16, 19, 65 Our results here identify an additional PKD1/2 function as a major downstream effector of DGKα in the control of MVB traffic and exosome secretion in T and B cells. DGKα-mediated DAG consumption limits PKD subcellular location and activation in T ymphocytes during IS formation. PKD1/2 is necessary for MVB maturation and exosome secretion in T and some B cells, and thus contributes to crucial lymphocyte immune functions such as cytotoxic activity and AICD.
Materials and Methods
Cells and mice
J-HM1-2.2 Jurkat cells expressing HM1R66 were stimulated with CCh (Sigma, St. Louis, MO, USA; final concentration 500âμM)67 or plate-bound anti-CD3 antibody (10âμg/ml, UCHT1; BD Biosciences, San Jose, CA, USA), or PMA (10âng/ml) plus ionomycin (0.5âμg/ml). We used the DGK inhibitor II R59949 (10âμM; Calbiochem, Merck KGaA, Darmstadt, Germany), the PKC inhibitor RO318220 (0.5âμM; Roche, F. Hoffmann-La Roche AG, Basel, Switzerland) and the PI3K inhibitor LY294002 (10âμM; Promega, Madison, WI, USA). The human Raji B-cell and the EL-4 mouse T-cell lines (ATCC, Manassas, VA, USA) were used for IS experiments. Generation, culture and activation of PKD1â/â3â/â and PKD1â/â DT40 B-cell lines were described.36 Cells were stimulated with PMA/ionomycin or with mouse anti-chicken IgM (10âμg/ml, M4; Southern Biotech, Birmingham, AL, USA). C57BL/6 PKD2â/â mice and their WT littermates were bred and maintained in the Wellcome Trust Biocentre, University of Dundee (Scotland) in compliance with UK Home Office Animals (Scientific Procedures) Act 1986 guidelines. For activation of primary T cells, spleens were removed, mashed in cell strainers (Becton Dickinson, Franklin Lakes, NJ, USA), disaggregated, erythrocytes lysed and the splenocytes suspended in RPMI 1640 medium containing L-glutamine (Invitrogen, Carlsbad, CA, USA) with 10% heat-inactivated FCS (Gibco, Carlsbad, CA, USA), penicillin/streptomycin (Gibco) and 50âμM β-mercaptoethanol (Sigma). Splenocytes were activated for 48âh with 1âμg/ml anti-CD3 antibody (2C11) to trigger the TCR. Following activation, cells were washed to remove 2C11 antibody and cultured (0.5 à 106 cells/ml) in medium supplemented with IL-2 (20âng/ml).
Antibodies and reagents
We used anti-DGKα antibody (Abnova, Taipei City, Taiwan), anti-human TCR (UCHT1; BD Biosciences) and anti-mouse TCR (2C11; Santa Cruz, Dallas, TX, USA). Polyclonal anti-PKD, anti-pPKD (S744/748) and anti-pPKD (S916) were from Cell Signaling (Danvers, MA, USA). Rabbit polyclonal anti-FasL (CD95L; Q-20) was from Santa Cruz, anti-CD63 (NKI-C-3) from Oncogene (Cambridge, MA, USA) and anti-LBPA (clone 6C4) from Echelon (San Jose, CA, USA). Horseradish peroxidase-coupled secondary antibodies were from Dako (Glostrup, Denmark). CCh was from Sigma, annexin V-PE from Immunostep (Salamanca, Spain) and AlexaFluor-coupled secondary antibodies from Invitrogen. Staphylococcal enterotoxin E (SEE) and B (SEB) were from Toxin Technology (Sarasota, FL, USA) and cell tracker blue (CMAC) from Invitrogen.
Expression vectors, transfection assays and retroviral transduction
pEFbos-GFP and pEFGFP-DGKα have been described;14, 23 pEFGFP-C1-CD63 and pECFP-C1-CD63 were a generous gift from Dr. G Griffiths;68 pEFGFP-PKD1wt, pEFGFP-PKD1-D733A and pEFGFP-PKD1-S744E/S748E have been described.27 pDsRed2-CD63 and pDsRed2-PKD1wt were prepared by subcloning cDNA for human CD63 from pEFGFP-C1-CD63, or PKD1wt from pEFGFP-C1-PKD1wt, into the pDsRed2-C1 vector (BD Biosciences). Constructs were verified by sequencing. Expression vectors for GFP-VPS4 wt and GFP-VPS4EQ mutant were a gift of Dr. P Whitley.69 For transfection experiments, J-HM1-2.2 or DT40 cells were transiently transfected with 20â30âμg of plasmids as described,70 DGKα was silenced using the pSUPER RNAi System (pSR-GFP bicistronic or pSuper plasmids; Oligoengine, Seattle, WA, USA) with the appropriate hairpin.9
The retroviral vector pBMN-Z (Addgene, Cambridge, MA, USA) was HindIII- and NotI digested to remove LacZ; a 0.7-âkb insert bearing EGFP (NCBI AAB02574.1) was excised from EGFP-N1 (Clontech, Mountain View, CA, USA) using HindIII and NotI and inserted into pBMN in place of LacZ (pBMN-GFP). PKD2wt cDNA was excised from pCR-Blunt II-TOPO-PKD2WT using HindIII and NotI and inserted into the digested pBMN vector (pBMN-GFP-PKD2). Human CD63 was excised from pCR-Blunt-EFGFP-CD63 using NotI and inserted into digested pBMN vector to yield pBMN-GFP-hCD63. Constructs were verified by sequencing. Phoenix ecotropic packaging cells (Stanford University) were transfected with 5-10âμg plasmid (pBMN-GFP, pBMN-GFP-CD63 and pBMN-GFP-PKD2wt) using a standard calcium-phosphate transfection protocol. At ~12â18âh post-transfection, medium was renewed. After 24âh (37ºC, 5% CO2), retroviral supernatants were centrifuged briefly (500xg, 5âmin) to remove packaging cells and viral particles concentrated by high-speed centrifugation (20â000xg, 4âh, 4ºC). Supernatants were discarded and concentrated viral particles resuspended in 1âml medium, snap-frozen and stored at â80ºC. Primary spleen T cells (106 cells per well) were mixed with freshly thawed retrovirus supernatant (1âml) and polybrene (Sigma; 10âμg/ml). Plates were centrifuged (650xg, 60âmin), 1âml per well IL-2-containing medium (20âng/ml) was added and cells were incubated (24âh), then centrifuged to remove polybrene, resuspended in fresh medium with IL-2 and incubated as before. Infection efficiency was assessed at 48âh using epifluorescence microscopy to detect GFP+ cells. We infected PKD2â/â T lymphoblasts with helper-free ecotropic and amphotropic retrovirus stock (with pBMN-EGFP-CD63) to obtain GFP-CD63-expressing cells; for GFP-PKD2 reconstitution experiments, we infected PKD2â/â T lymphoblasts with a mixed retrovirus stock (pBMN-EGFP-CD63 and pBMN-GFP-PKD2).
Isolation and quantitation of exosomes
Exosomes were isolated from culture supernatants as described.32, 33, 34 There were no differences in β-actin levels in cell lysates, which indicates that the exosomes were produced by equal numbers of viable cells. Using standard protocols, supernatants of 20 à 106 cells were centrifuged sequentially at low speed, clarified71 and exosomes recovered by ultracentrifugation (100â000xg, 12âh, 4ºC).33 In experiments to quantify exosomes and analyze their size distribution, the final culture supernatant collected before ultracentrifugation was diluted (1/5) in HBSS and analyzed using NANOSIGHT (calibrated with 100 and 400ânm fluorescent calibration beads; Malvern, Malvern, UK). We analyzed exosomes from cells expressing the exosome reporters GFP-CD63 and DsRed2-CD63 in a similar protocol, using 106 J-HM1-2.2 cells or 4 à 106 DT40 cells. To measure endogenous CD63 in exosomes, we normalized WB signals for exosome-producing cell number to those for β-actin or endogenous CD63. To analyze GFP-CD63 or DsRed2-CD63 reporters in exosomes, we normalized WB signals for exosome CD63 to those in cell lysates for each condition tested.
CD63 is found in MVB, ILV and hence in exosomes; this protein and its chimeras (GFP-CD63) are used as reporters for MVB/exosomes,2, 8, 14 and allow quantitation of exosome secretion.9, 71 Western blot analysis of endogenous or chimeric CD63 in isolated exosomes is used to measure exosomes,9 as the signal correlates with nanoparticle concentration data (nanoparticles/ml) as confirmed by Nanopaticle Tracking Analysis (NTA)72, 73 (Supplementary Figure S2). In preliminary experiments, we probed WB of purified exosomes with another exosome marker (Lamp-1) and plasma membrane markers (CD45 and CD28), to exclude the presence of cell debris, apoptotic bodies and/or shedding vesicles (Supplementary Figure S2). For murine T lymphoblasts, culture supernatants of 18 Ã 106 uninfected cells were concentrated (Amicon Ultra; Millipore, Merck KGaA, Darmstadt, Germany,) and centrifuged sequentially. Exosomes were obtained from 2 Ã 106 spleen T lymphoblasts expressing the exosome reporter GFP-CD63.
Preparation of whole-cell and exosome lysates and western blot analysis
Exosomes were resuspended in 60âμl RIPA lysis buffer and 20âμl of this lysate were analyzed by WB. We generally obtained 50â150âμg protein in the pellet from 20 à 106 stimulated cells. For WB, each lane contained the total exosomal protein recovered from medium for the same number of untreated or stimulated cells. Cells and exosomes were lysed (10âmin) in RIPA buffer with protease inhibitors, proteins separated in reducing SDS-PAGE and transferred to Hybond ECL membranes (GE Healthcare, Wauwatosa, WI, USA). For CD63 detection, proteins were separated in non-reducing conditions.74 Blots were incubated with rabbit anti-pPKD or mouse anti-CD63 and developed with appropriate HRP-conjugated secondary antibodies using ECL reagents following standard protocols. Data were quantitated using Quantity One 4.4.0 software (Bio-Rad, Hercules, CA, USA).
Immunofluorescence
Living cells expressing GFP-CD63 were attached to fibronectin-coated glass-bottom 35âmm culture dishes (Mat-Tek, Ashland, MA, USA) at 24â48âh post-transfection, and stimulated in medium without phenol red (37âºC).70 ISs between transfected Jurkat cells and SEE-pulsed (1âμg/ml), CMAC-labeled Raji cells were analyzed as described.9 In mouse cell IS experiments, CMAC-labeled EL-4 cells were SEB-pulsed (1âμg/ml) and challenged with T lymphoblasts from WT mice at a 1â:â1 ratio. For time-lapse video experiments, we using an OKO-lab stage incubator on a Nikon Eclipse TiE microscope with a DS-Qi1MC digital camera and a Plan Apo VC 60x NA 1.4 objective. For video acquisition and analysis, we used NIS-AR software (Nikon, Tokyo, Japan). Epifluorescence images were deconvoluted with Huygens Deconvolution Software (Scientific Volume Image, Hilversum, Holland). Digital images were quantified with NIS-AR or ImageJ software (Rasband, WS, ImageJ, US National Institutes of Health, http://rsb.info.nih.gov/ij/). Video images of GFP-CD63 vesicle trajectories were plotted and analyzed with NIS-AR and ImageJ MJTrack plugin. Significance of results for single cells was determined using ANOVA to analyze at least 20 cells from different fields for at least three experiments per condition.
CTL and AICD assays
For CTL and AICD assays, EL-4 cells were SEB-pulsed (1âμg/ml) and challenged with WT and PKD2â/â mouse T lymphoblasts at various effector/target ratios in U-bottom 96-well culture plates (5âh), then annexin V-PE stained to analyze the percentage of apoptotic cells by flow cytometry. Forward and side scatter detectors were calibrated to optimize discrimination of the cell populations, to gate EL-4 and T lymphoblasts by size and complexity, and simultaneously measure percentage of apoptosis of EL-4 (CTL activity) and T lymphoblasts (AICD).
Abbreviations
- AICD:
-
activation-induced cell death
- APC:
-
antigen-presenting cell
- CMAC:
-
cell tracker blue
- CTL:
-
cytotoxic T lymphocyte
- DAG:
-
diacylglycerol
- DGKα:
-
diacylglycerol kinase α
- ESCRT:
-
endosomal complex required for traffic
- FasL:
-
Fas ligand
- ILV:
-
intraluminal vesicles
- HM1R:
-
muscarinic receptor
- IS:
-
immune synapse
- MVB:
-
multivesicular bodies
- PA:
-
phosphatidic acid
- PBL:
-
peripheral blood lymphocytes
- PKC:
-
protein kinase C
- PKD:
-
protein kinase D
- PLC:
-
phospholipase C
- PMA:
-
phorbol myristate acetate
- SEE:
-
Staphylococcus enterotoxin E
- SEB:
-
Staphylococcus enterotoxin B
- TGN:
-
trans-Golgi network
References
Lakkaraju A, Rodriguez-Boulan E . Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol 2008; 18: 199â209.
Raiborg C, Rusten TE, Stenmark H . Protein sorting into multivesicular endosomes. Curr Opin Cell Biol 2003; 15: 446â455.
Thery C, Zitvogel L, Amigorena S . Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2: 569â579.
Marsh M, van Meer G . Cell biology. No ESCRTs for exosomes. Science 2008; 319: 1191â1192.
Babst M . MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr Opin Cell Biol 2011; 23: 452â457.
Williams RL, Urbe S . The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol 2007; 8: 355â368.
Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Faure J et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 2004; 303: 531â534.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008; 319: 1244â1247.
Alonso R, Mazzeo C, Rodriguez MC, Marsh M, Fraile-Ramos A, Calvo V et al. Diacylglycerol kinase alpha regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ 2011; 18: 1161â1173.
Peters PJ, Geuze HJ, Van der Donk HA, Slot JW, Griffith JM, Stam NJ et al. Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. Eur J Immunol 1989; 19: 1469â1475.
Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G . The biogenesis and functions of exosomes. Traffic 2002; 3: 321â330.
Zuccato E, Blott EJ, Holt O, Sigismund S, Shaw M, Bossi G et al. Sorting of Fas ligand to secretory lysosomes is regulated by mono-ubiquitylation and phosphorylation. J Cell Sci 2007; 120: 191â199.
Monleon I, Martinez-Lorenzo MJ, Monteagudo L, Lasierra P, Taules M, Iturralde M et al. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol 2001; 167: 6736â6744.
Alonso R, Rodriguez MC, Pindado J, Merino E, Merida I, Izquierdo M . Diacylglycerol kinase alpha regulates the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. J Biol Chem 2005; 280: 28439â28450.
Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2011; 2: 282.
Litvak V, Dahan N, Ramachandran S, Sabanay H, Lev S . Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat Cell Biol 2005; 7: 225â234.
Roth MG . Lipid regulators of membrane traffic through the Golgi complex. Trends Cell Biol 1999; 9: 174â179.
Sprong H, van der Sluijs P, van Meer G . How proteins move lipids and lipids move proteins. Nat Rev Mol Cell Biol 2001; 2: 504â513.
Baron CL, Malhotra V . Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 2002; 295: 325â328.
Quann EJ, Merino E, Furuta T, Huse M . Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol 2009; 10: 627â635.
Van Lint J, Rykx A, Maeda Y, Vantus T, Sturany S, Malhotra V et al. Protein kinase D: an intracellular traffic regulator on the move. Trends Cell Biol 2002; 12: 193â200.
Merida I, Avila-Flores A, Merino E . Diacylglycerol kinases: at the hub of cell signalling. Biochem J 2008; 409: 1â18.
Sanjuan MA, Jones DR, Izquierdo M, Merida I . Role of diacylglycerol kinase alpha in the attenuation of receptor signaling. J Cell Biol 2001; 153: 207â220.
Sanjuan MA, Pradet-Balade B, Jones DR, Martinez AC, Stone JC, Garcia-Sanz JA et al. T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: a novel mechanism for Ras attenuation. J Immunol 2003; 170: 2877â2883.
Irie A, Harada K, Tsukamoto H, Kim JR, Araki N, Nishimura Y . Protein kinase D2 contributes to either IL-2 promoter regulation or induction of cell death upon TCR stimulation depending on its activity in Jurkat cells. Int Immunol 2006; 18: 1737â1747.
Matthews SA, Navarro MN, Sinclair LV, Emslie E, Feijoo-Carnero C, Cantrell DA . Unique functions for protein kinase D1 and protein kinase D2 in mammalian cells. Biochem J 2010; 432: 153â163.
Matthews SA, Rozengurt E, Cantrell D . Characterization of serine 916 as an in vivo autophosphorylation site for protein kinase D/Protein kinase Cmu. J Biol Chem 1999; 274: 26543â26549.
Alonso R, Mazzeo C, Merida I, Izquierdo M . A new role of diacylglycerol kinase alpha on the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. Biochimie 2007; 89: 213â221.
Matthews SA, Rozengurt E, Cantrell D . Protein kinase D. A selective target for antigen receptors and a downstream target for protein kinase C in lymphocytes. J Exp Med 2000; 191: 2075â2082.
Waldron RT, Rey O, Iglesias T, Tugal T, Cantrell D, Rozengurt E . Activation loop Ser744 and Ser748 in protein kinase D are transphosphorylated in vivo. J Biol Chem 2001; 276: 32606â32615.
Iglesias T, Waldron RT, Rozengurt E . Identification of in vivo phosphorylation sites required for protein kinase D activation. J Biol Chem 1998; 273: 27662â27667.
Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol 2002; 168: 3235â3241.
Martinez-Lorenzo MJ, Anel A, Gamen S, Monle nI, Lasierra P, Larrad L et al. Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. J Immunol 1999; 163: 1274â1281.
Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med 2002; 195: 1303â1316.
Montoya MC, Sancho D, Bonello G, Collette Y, Langlet C, He HT et al. Role of ICAM-3 in the initial interaction of T lymphocytes and APCs. Nat Immunol 2002; 3: 159â168.
Matthews SA, Liu P, Spitaler M, Olson EN, McKinsey TA, Cantrell DA et al. Essential role for protein kinase D family kinases in the regulation of class II histone deacetylases in B lymphocytes. Mol Cell Biol 2006; 26: 1569â1577.
Muntasell A, Berger AC, Roche PA . T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J 2007; 26: 4263â4272.
Arita S, Baba E, Shibata Y, Niiro H, Shimoda S, Isobe T et al. B cell activation regulates exosomal HLA production. Eur J Immunol 2008; 38: 1423â1434.
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996; 183: 1161â1172.
Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem 2003; 278: 10963â10972.
Liu P, Scharenberg AM, Cantrell DA, Matthews SA . Protein kinase D enzymes are dispensable for proliferation, survival and antigen receptor-regulated NFkappaB activity in vertebrate B-cells. FEBS Lett 2007; 581: 1377â1382.
Rous BA, Reaves BJ, Ihrke G, Briggs JA, Gray SR, Stephens DJ et al. Role of adaptor complex AP-3 in targeting wild-type and mutated CD63 to lysosomes. Mol Biol Cell 2002; 13: 1071â1082.
Bossi G, Griffiths GM . Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med 1999; 5: 90â96.
Stinchcombe JC, Griffiths GM . Regulated secretion from hemopoietic cells. J Cell Biol 1999; 147: 1â6.
Granboulan M, Lankar D, Raposo G, Bonnerot C, Hivroz C . Phosphoinositide 3-kinase activation by Igbeta controls de novo formation of an antigen-processing compartment. J Biol Chem 2003; 278: 4331â4338.
Fernandez-Borja M, Wubbolts R, Calafat J, Janssen H, Divecha N, Dusseljee S et al. Multivesicular body morphogenesis requires phosphatidyl-inositol 3-kinase activity. Curr Biol 1999; 9: 55â58.
Futter CE, Collinson LM, Backer JM, Hopkins CR . Human VPS34 is required for internal vesicle formation within multivesicular endosomes. J Cell Biol 2001; 155: 1251â1264.
Reaves BJ, Bright NA, Mullock BM, Luzio JP . The effect of wortmannin on the localisation of lysosomal type I integral membrane glycoproteins suggests a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. J Cell Sci 1996; 109: 749â762.
Odorizzi G, Babst M, Emr SD . Phosphoinositide signaling and the regulation of membrane trafficking in yeast. Trends Biochem Sci 2000; 25: 229â235.
White J, Herman A, Pullen AM, Kubo R, Kappler JW, Marrack P . The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell 1989; 56: 27â35.
Izquierdo M, Grandien A, Criado ML, Robles S, Leonardo E, Albar JP et al. Blocked negative selection of developing T cells in mice expressing the baculovirus p35 caspase inhibitor. EMBO J 1999; 18: 101â111.
Morales-Kastresana A, Catalán E, Hervás-Stubbs S, Palazón A, Azpilikueta A, Bolaños E et al. Essential complicity of perforin-granzyme and FAS-L mechanisms to achieve tumor rejection following treatment with anti-CD137 mAb. J ImmunoTher Cancer 2013; 1: 3.
Pardo J, Bosque A, Brehm R, Wallich R, Naval J, Mullbacher A et al. Apoptotic pathways are selectively activated by granzyme A and/or granzyme B in CTL-mediated target cell lysis. J Cell Biol 2004; 167: 457â468.
Thery C, Ostrowski M, Segura E . Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; 9: 581â593.
de Saint Basile G, Menasche G, Fischer A . Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat Rev Immunol 2010; 10: 568â579.
Nagata S, Suda T . Fas and Fas ligand: lpr and gld mutations. Immunol Today 1995; 16: 39â43.
Krammer PH, Arnold R, Lavrik IN . Life and death in peripheral T cells. Nat Rev Immunol 2007; 7: 532â542.
Chauveau A, Le Floc'h A, Bantilan NS, Koretzky GA, Huse M . Diacylglycerol kinase alpha establishes T cell polarity by shaping diacylglycerol accumulation at the immunological synapse. Sci Signal 2014; 7: ra82.
Rainero E, Caswell PT, Muller PA, Grindlay J, McCaffrey MW, Zhang Q et al. Diacylglycerol kinase alpha controls RCP-dependent integrin trafficking to promote invasive migration. J Cell Biol 2012; 196: 277â295.
Eisenberg-Lerner A, Kimchi A . PKD is a kinase of Vps34 that mediates ROS-induced autophagy downstream of DAPk. Cell Death Differ 2012; 19: 788â797.
Morel E, Chamoun Z, Lasiecka ZM, Chan RB, Williamson RL, Vetanovetz C et al. Phosphatidylinositol-3-phosphate regulates sorting and processing of amyloid precursor protein through the endosomal system. Nat Commun 2013; 4: 2250.
Spitaler M, Emslie E, Wood CD, Cantrell D . Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity 2006; 24: 535â546.
Navarro MN, Sinclair LV, Feijoo-Carnero C, Clarke R, Matthews SA, Cantrell DA . Protein kinase D2 has a restricted but critical role in T-cell antigen receptor signalling in mature T-cells. Biochem J 2012; 442: 649â659.
Navarro MN, Goebel J, Hukelmann JL, Cantrell DA . Quantitative phosphoproteomics of cytotoxic T cells to reveal protein kinase d 2 regulated networks. Mol Cell Proteomics 2014; 13: 3544â3557.
Ghanekar Y, Lowe M . Signalling for secretion. Nat Cell Biol 2005; 7: 851â853.
Desai DM, Newton ME, Kadlecek T, Weiss A . Stimulation of the phosphatidylinositol pathway can induce T cell activation. Nature 1990; 348: 66â69.
Izquierdo M, Ruiz-Ruiz MC, López-Rivas A . Stimulation of the PtdIns turnover is a key event for Fas-dependent, activation-induced apoptosis in human T lymphocytes. J Immunol 1996; 157: 21â28.
Blott EJ, Bossi G, Clark R, Zvelebil M, Griffiths GM . Fas ligand is targeted to secretory lysosomes via a proline-rich domain in its cytoplasmic tail. J Cell Sci 2001; 114: 2405â2416.
Whitley P, Reaves BJ, Hashimoto M, Riley AM, Potter BV, Holman GD . Identification of mammalian Vps24p as an effector of phosphatidylinositol 3,5-bisphosphate-dependent endosome compartmentalization. J Biol Chem 2003; 278: 38786â38795.
Jambrina E, Alonso R, Alcalde M, RodrÃguez MCSerrano, A, MartÃnez-A C et al. Calcium influx through receptor-operated channel induces mitochondria-triggered paraptotic cell death. J BiolChem 2003; 278: 14134â14145.
Thery C, Amigorena S, Raposo G, Clayton A . Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006; Chapter 3: 22.
Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 2011; 7: 780â788.
Soo CY, Song Y, Zheng Y, Campbell EC, Riches AC, Gunn-Moore F et al. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology 2012; 136: 192â197.
Wang R, Dworak LJ, Lacy MJ . A panel immunoblot using co-incubated monoclonal antibodies for identification of melanoma cells. J Immunol Methods 2001; 249: 167â183.
Acknowledgements
We are indebted to Doreen Cantrell (University of Dundee, UK) for her generous and continuous support, reagents and superb advice. We acknowledge Elizabeth Emslie and Maria Navarro for their help and comments, Mark Ware (Nanosight Ltd, UK) for his support in NANOSIGHT studies, and Catherine Mark for excellent editorial assistance. RA received a doctoral fellowship from the Spanish Ministerio de Ciencia y TecnologÃa. This work was supported by grants from the Spanish Ministerio de EconomÃa y Competitividad (MINECO) Plan Nacional de Investigación CientÃfica (BFU2011-22849 to MI). IM is funded by MINECO grant BFU2013-47640-âP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by G Melino
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Mazzeo, C., Calvo, V., Alonso, R. et al. Protein kinase D1/2 is involved in the maturation of multivesicular bodies and secretion of exosomes in T and B lymphocytes. Cell Death Differ 23, 99â109 (2016). https://doi.org/10.1038/cdd.2015.72
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2015.72
This article is cited by
-
Addressing the role of PKD3 in the T cell compartment with knockout mice
Cell Communication and Signaling (2022)
-
Extracellular signals regulate the biogenesis of extracellular vesicles
Biological Research (2022)
-
Emerging role of exosomes in hematological malignancies
Clinical and Experimental Medicine (2022)
-
Comprehensive landscape of extracellular vesicle-derived RNAs in cancer initiation, progression, metastasis and cancer immunology
Molecular Cancer (2020)
-
Tumor-derived extracellular vesicles: insights into bystander effects of exosomes after irradiation
Lasers in Medical Science (2020)