Abstract
To compare the efficacy and safety of the proposed aflibercept biosimilar SCD411 and reference aflibercept in patients with neovascular age-related macular degeneration, this randomized, double-masked, parallel-group, multicenter study was conducted in 14 countries from 13 August 2020 to 8 September 2022. Patients with neovascular age-related macular degeneration. With subfoveal, juxtafoveal, or extrafoveal choroidal neovascularization were aged 50 years or older. Intravitreal injection of SCD411 or aflibercept (2.0 mg) were administered every 4 weeks for the first three injections and every 8 weeks until week 48. The primary efficacy endpoint was the change in best-corrected visual acuity from baseline to week 8 with an adjusted equivalence margin ofâ±â3.0 letters. Patients were randomly assigned to receive either SCD411 (nâ=â288) or reference aflibercept (nâ=â288). A total of 566 participants (98.3%) completed week 8 of the study. The least-squares mean difference of change in best-corrected visual acuity from baseline to week 8 (SCD411âaflibercept) wasâââ0.4 letters (90% confidence intervalâ=ââââ1.6 to 0.9). The incidence of ocular (69 of 287 [24.0%] vs. 71 of 286 [24.8%]) and serious ocular (5 of 287 [1.7%] vs. 3 of 286 [1.0%]) treatment-emergent adverse effects were similar between the SCD411 and aflibercept groups. Immunogenicity analysis revealed a low incidence of neutralizing antibody formation in both groups. In conclusion, SCD411 has equivalent efficacy compared with reference aflibercept in patients with neovascular age-related macular degeneration and has a comparable safety profile. The results support the potential use of SCD411 for the treatment of neovascular age-related macular degeneration.
Similar content being viewed by others
Introduction
Vascular endothelial growth factor (VEGF) is the main target for treatment of neovascular age-related macular degeneration (nAMD); thus, intravitreal injection of agent inhibiting the activity of VEGF has become the standard treatment for nAMD1,2,3,4. Aflibercept, first introduced as âVEGF-trapâ, is a soluble decoy receptor fusion protein approved by the US Food and Drug Administration in 20115 and the European Medicines Agency in 20126. Aflibercept has a biological advantage over ranibizumab because of its higher binding affinity to VEFG and longer duration of action7,8. However, the high cost of the original aflibercept is considered as a barrier for some patients and for some developing countries to access the treatment9. The original aflibercept comes off patent in 2023 in the USA and 2025 in Europe10.
Biosimilars are biological products that are highly similar to a reference product. They do not have clinically meaningful differences from the reference product in terms of safety, purity, and potency11,12. Biosimilars are relatively large and complex proteins that differ from generic drugs, which are small, simple, and well-defined structures that are easily characterized11.
SCD411 is a proposed aflibercept biosimilar that binds to VEGF-A and is produced in Chinese hamster ovary cells by using recombinant DNA technology. It has demonstrated similar structural characteristics, physicochemical properties, and biological activities similar to those of reference aflibercept in a series of in vitro and in vivo nonclinical studies. This phase 3 randomized clinical compared the efficacy, safety, pharmacokinetics, and immunogenicity of SCD411 with those of reference aflibercept.
Methods
Study design
This was a randomized, double-masked, parallel-group, multicenter phase 3 study conducted at 132 sites in 14 countries (e-Appendix 1 in Supplemental 1) from August 13, 2020 to September 8, 2022, and an interim analysis performed in August 2022. The study adhered to the International Council for Harmonization and Good Clinical Practice guidelines and the Declaration of Helsinki. The study protocol and protocol amendment were reviewed and approved by an independent ethics committee or institutional review board of each site (e-Appendix 2 in Supplemental 1) and registered at clinicaltrials.gov (NCT04480463, registered at 21/07/2020). A written informed consent was obtained from each participant prior to study participation. The study protocol, including treatment and statistical analysis methods and protocol amendment, is available in Supplemental 2. All imaging devices and the quality of the images obtained at each site were certified by the central reading center (Duke Reading Center, Duke University, NC, USA) prior to the initiation of the study.
Participants
The inclusion criteria were patients aged 50Â years or older and had active subfoveal, juxtafoveal, or extrafoveal choroidal neovascularization (CNV) due to AMD in the study eye. The lesion activity was confirmed using fluorescein angiography (FA). Participants with best-corrected visual acuity (BCVA) letters between 73 and 35 (Snellen equivalent to 20/200â20/32) using the original series of the Early Treatment Diabetic Retinopathy Study (ETDRS) charts or 2702 series number charts in the study eye were included. Participants were excluded if they had prior ocular, systemic, or surgical treatment for nAMD, including anti-VEGF agents; had a total lesion sizeâ>â30.5 mm2 including blood, scars, atrophy, fibrosis, and neovascularization in the study eye; had subretinal hemorrhageâ>â50% of the total lesion area or presence of subfoveal blood 1 disc area or more; had scar or fibrosisâ>â50% of the total lesion or involving the foveal center; had other causes of CNV in the study eye; or had signs of nAMD in the fellow eye that required any treatment. The full list of inclusion and exclusion criteria is provided in e-Appendix 3 in Supplemental 1.
Intervention
An intravitreal injection, either 2.0Â mg (0.05Â mL) of SCD411 (SamChunDang Pharm. Co. Ltd., Seoul, Republic of Korea) or 2.0Â mg (0.05Â mL) of reference aflibercept (Eylea; Bayer HealthCare, Leverkusen, Germany), was administered every 4Â weeks for the first three injections and every 8Â weeks until week 48. Each study drug was provided as a single-use glass vial, containing 4Â mg of study drugs in 0.1Â mL solution. The other ingredients in the two study drugs were identical: polysorbate 20, sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate heptahydrate, sodium chloride, sucrose, and water for injection. The study drugs were stored in a refrigerator (2â8 â). The preparation and administration of the study drugs were conducted under controlled aseptic conditions. The study drugs were withdrawn into the syringe using a filter needle. Treatment of the fellow eye was allowed at any time during the study if the patient was newly diagnosed with neovascular AMD or had worsening neovascular AMD in the fellow eye. The fellow eye was treated with aflibercept according to the investigatorâs discretion. Fellow eye treatment injections were not permitted on the same day as the study eye treatment.
Randomization and masking
All participants were randomly assigned in a 1:1 ratio to receive SCD411 or Eylea intravitreal injections at baseline/randomization visit (day 1) using interactive response technology. Biostatistics was used to generate the randomization schedule using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA) for the interactive response technology, which was used to link the sequential subject randomization numbers to treatment codes. The participants, study site staff involved in subject management and study assessment, including visual acuity and image acquisition remained masked throughout the study, except for the intravitreal injection investigator and staff assigned to be unmasked for reporting of the interim analysis. The sponsor, the delegated contract research organization, and imaging teams were also masked to the study treatment.
Outcomes
Efficacy endpoint
The result of VIEW4 (VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet Age-related Macular Degeneration) studies indicated that the efficacy of treatment reaches a plateau at 12â16Â weeks. Consequently, The Committee for Medicinal Products for Human Use in European Medicines Agency recommended establishing the primary efficacy endpoint before reaching this plateau. As a result, the primary endpoint was changes in BCVA from baseline to week 8, as measured using the ETDRS letter score or 2702 charts.
The secondary efficacy endpoints included changes in BCVA from baseline to week 52, changes in central retinal thickness (CRT) from baseline to weeks 8 and 52, changes in the CNV area from baseline to weeks 8 and 52, percentage of participants who gained at least 15 letters in BCVA at weeks 8 and 52. The masked readers at the Duke Reading Center measured retinal thickness, defined as the distance between internal limiting membrane and Bruchâs membrane using their in-house software (DRCVisualizer).
Safety
The safety endpoints for the study included the incidence of treatment-emergent adverse effects (TEAEs), vital signs, and laboratory test results. Other safety parameters included electrocardiogram results, slit-lamp examination, dilated fundoscopy, intraocular pressure, and vision checks.
No formal statistical analysis of the safety data was performed. TEAEs were defined as adverse effects (AEs) that were reported or worsened on or after the first dose date of the study drug through 28Â days after the last dose date of the study drug. Adverse events were coded according to the Medical Dictionary for Regulatory Activities version 23.0 or above. Ocular TEAEs were separately summarized from non-ocular TEAEs. Ocular TEAEs were summarized for both the study and fellow eyes.
Immunogenicity
Immunogenicity analyses were performed at 0, 4, 8, 20, 36, and 52Â weeks. Blood samples were collected prior to intravitreal injection of the study drug and FA assessment when performed on the same date. The presence of neutralizing antibodies (Nab) was tested when anti-drug antibody (ADA) results were confirmed to be positive. ADA concentration with a signal above the cutoff point of 10.0Â ng/mL was defined as ADA positive.
Pharmacokinetics
Quantification of free and bound SCD411 and reference aflibercept was performed in patients who agreed to participate in the pharmacokinetic sub-study using blood samples before intravitreal injection and at 1, 3, 7, 14, and 28 days after the first (day 1) and third injections (week 8). Non-compartmental analysis on plasma concentrationâtime data were conducted using Phoenix WinNonlin® (version 8.3; Certara USA, Inc., Princeton, NJ, USA).
Statistical analysis
Sample size
A sample size of 266 participants per treatment arm was selected because it provided at least 80% power and a 5% significance level. Considering an approximately 5% loss from randomization through week 8, the total sample size required was 560.
Analysis sets
The full analysis set (FAS) included all randomized subjects who received at least one injection of the study drug. The per-protocol set (PPS) included all participants in the FAS, excluding those with significant protocol deviations. The safety set included all patients who received at least one injection of the study drug. This was the analysis set for safety and immunogenicity analyses. The pharmacokinetic analysis set (PK set) was used to estimate the pharmacokinetic endpoints and consisted of a subset of subjects in the FAS who had sufficient evaluable blood samples. Participants were analyzed according to the treatment they received.
Primary and secondary efficacy analyses
The equivalence margin for primary efficacy analysis was determined using data presented by the following studies: MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration)3, ANCHOR (Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration)13, and VIEW4 studies. For BCVA at week 8, a 90% confidence interval (CI) (equivalent to two one-sided test at an α of 0.05) with an equivalence margin of three letters was assessed. A mixed-effects model for repeated measures (MMRM) analysis was performed based on multiply imputed datasets. Rubinâs rules were used to combine the estimates from the MMRM analyses, and the estimated mean treatment differences at week 8, along with the corresponding 90% CIs, are presented. Equivalence was determined if the upper and lower CI limits for the difference between the treatments were within the equivalence margins. The analysis was based on both FAS and PPS.
The estimated mean treatment difference in the change in BCVA from baseline to week 52 and corresponding 90% CI from the same MMRM model used in the week 8 were determined. FAS and PPS were used for the analysis. The analysis of change in CRT from baseline as assessed using optical coherence tomography (OCT) and change in CNV area from baseline was based on the MMRM model, similar to that used for the analysis of change in BCVA from baseline, with the corresponding 95% CIs for the estimates of treatment differences at weeks 8 and 52. The percentage of participants who gainedââ¥â15 letters in BCVA at post-baseline visits (week 8 and week 52) compared with baseline was summarized, and 95% CI for the proportions in each treatment arm and difference in proportions between treatment arms were presented. The 95% CI for the proportions in each treatment arm were calculated using the ClopperâPearson method. The 95% CI for the difference in proportions between the treatment arms was based on the exact unconditional CI. The FAS was used for these analyses. Analyses were performed using SAS software (version 9.4).
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by an independent ethics committee or institutional review board of each site (e-Appendix 1 in Supplemental 1) and registered at clinicaltrials.gov (NCT04480463).
Consent
A written informed consent was obtained from each participant prior to study participation.
Results
Study populations
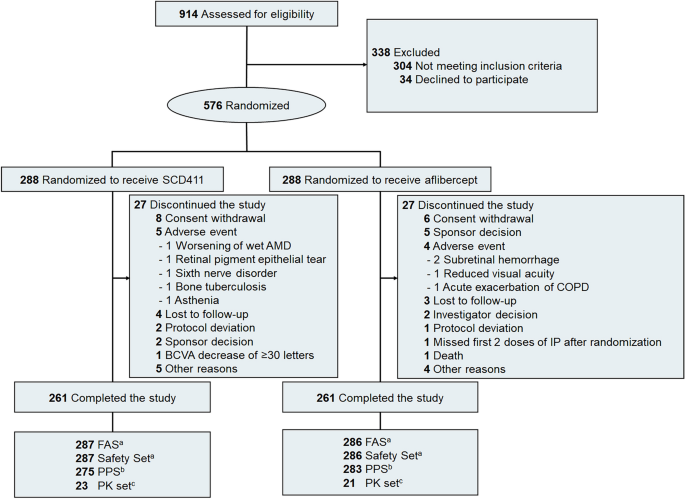
From August 13, 2020 to September 6, 2021, a total of 914 participants were screened, and 576 were randomly assigned to receive either SCD411 (nâ=â288) or reference aflibercept (nâ=â288). A total of 566 participants (98.3%) completed week 8 of the study (281 and 285 in the SCD411 and aflibercept groups, respectively). Five hundred and twenty-two participants (90.6%) completed until the end of the study (nâ=â261 per group). The most common reasons for discontinuing the study were withdrawal of consent from the participants (25.9%), AE (16.7%), and others (16.7%). The participantsâ disposition is shown in Fig. 1.
CONSORT diagram of participant disposition throughout the trial. AMD age-related macular degeneration, COPD chronic obstructive pulmonary disorder, IP investigational product; number, BCVA best-corrected visual acuity, FAS full analysis set, PPS per-protocol set, PK pharmacokinetics. aAll randomized participants who received at least 1 injection of the study drug. bAll participants in the FAS, excluding those with significant protocol deviations. CSubset of participants in the FAS who had sufficient evaluable blood samples.
Demographic and ocular baseline characteristics
The baseline demographic and ocular characteristics of the participants with FAS are summarized in Table 1. Overall, the demographic and baseline characteristics were comparable between the treatment groups. The mean (SD) age of the participants was 73.5 (8.3) years. This study included 277 (48.3%) men and 296 (51.7%) women. Majority of the participants were White (382 [66.7%]) or Asian (187 [32.6%]). The mean (SD) BCVA was 59.3 (10.7) letters. The mean CRT (SD) was 490.1 (172.7) μm, and the CNV area was 4.57 (4.23) mm2.
Efficacy endpoint results
As the primary efficacy endpoint, the least-square (LS) mean (SD) changes in BCVA score at week 8 from the baseline (FAS) were 5.5 (0.53) letters in the SCD411 group (nâ=â287) and 5.9 (0.52) letters in the aflibercept group (nâ=â286). The LS mean difference between the two groups (SCD411âaflibercept) for the estimated mean change in BCVA score from baseline was â 0.4 letters (90% CIâââ1.6 to 0.9). In the PPS, a comparable result for the LS mean changes in BCVA from baseline to week 8 was also confirmed (Fig. 2).
Secondary efficacy endpoints, including BCVA score change from baseline to week 52, changes in CRT and CNV area from baseline to weeks 8 and 52, and percentage of participants who gained at least 15 letters in BCVA at weeks 8 and 52, showed similar efficacy between the treatment groups (Tables 2, 3). In addition, the changes in the BCVA score and CRT from baseline at all visits to week 52 were comparable between the treatment groups (eFigs. 1, 2 in Supplemental 1).
Safety
Exposure was similar between the SCD411 (nâ=â287) and aflibercept (nâ=â286) groups, including the mean (SD) number of injections (7.6 [1.32] vs. 7.6 [1.14]) and mean (SD) duration of drug exposure (317.1 [68.74] vs. 318.2 [61.36]). The number of participants treated with anti-VEGF in the fellow eye was not significantly different between the SCD411 (26 [9.1%]) and aflibercept (18 [6.3%]) groups. The incidence of ocular and non-ocular TEAEs was similar between the SCD411 and aflibercept groups (ocular TEAEs, 69 [24.0%] vs. 71 [24.8%]; serious ocular TEAEs, 5 [1.7%] vs. 3 [1.0%]; and non-ocular TEAEs, 128 [44.6%] vs. 130 [45.5%]). The number of TEAEs leading to study drug discontinuation was also similar between the SCD411 and aflibercept groups (ocular TEAEs, 2 [0.7%] in both groups; non-ocular TEAEs, 2 [0.7%] vs. 1 [0.3%]). No ocular or non-ocular TEAE that led to death were observed in either group. Ocular TEAE was not noted in the study eye inââ¥â5% of the patients, and the most common ocular TEAE was reduced visual acuity (13 [4.5%] in two groups), followed by conjunctival hemorrhage (SCD411, 8 [2.8%] vs. aflibercept, 6 [2.1%]). A retinal pigment epithelial tear was reported in three (1.0%) in the SCD 411 group and two (0.7%) in the aflibercept group (Table 4).
Pharmcokinetic results
PK analysis was performed for 23 participants in the SCD411 group and 21 participants in the aflibercept group, and the results are summarized in eFig. 3 in Supplemental 1. Following a single 2-mg intravitreal injection of SCD411 or Eylea on day 1 and at week 8, the mean free SCD411 and Eylea concentration versus time profiles showed similar PK properties.
Immunogenicity results
The cumulative incidence of ADA was similar between the two groups. ADA positivity was 7.1% in the SCD411 group and 6.9% in the aflibercept group at baseline and 20.2â40.0% in the SCD411 group and 18.8â52.3% in the aflibercept group during the study period. The incidence of Nab was low and similar between the two groups. Of the participants who provided blood samples for immunogenicity assessment at baseline, 1.1% of the participants in the SCD411 group and 2.2% of the participants in the aflibercept group were NAb-positive at baseline. The incidence of Nab-positive subjects remainedâ<â3% in the SCD411 group and mostly remainedâ<â4% in the aflibercept group during the study period. (eTable 1 in Supplemental 1).
Discussion
This phase 3, randomized, double-masked, parallel-group, multicenter study demonstrated that the proposed biosimilar SCD411 had equivalent efficacy, safety, immunogenicity, and pharmacokinetic profile for the treatment of nAMD to the reference aflibercept. The primary end-point of this study was achieved, because the difference in the change in BCVA from baseline to week 8 between the SCD411 and aflibercept groups was within a predefined equivalence margin. In addition, the results of the secondary end points supported the equivalent efficacy of SCD411 and reference aflibercept. Sensitivity analyses for the primary end point also showed robust equivalence of efficacy between the two groups.
Based on the safety data of the total population, SCD411 was well tolerated, with a favorable safety profile comparable with that of reference aflibercept. The incidences of ocular and non-ocular TEAEs were similar between the two treatment groups. The mean peak plasma concentrations of free SCD411 and reference aflibercept after week 8 were 50.7 and 43.0Â ng/mL, respectively and were more than 50-fold lower than the concentration of aflibercept required to inhibit the half of free VEGF activity14. This result was similar with those obtained by previous studies on nAMD, which ranged from 20.0 to 66.7Â ng/mL in patients with nAMD4,15. Considering the low systemic exposure of SCD411 along with reference aflibercept, the risk of systemic adverse effect would be low. Immunogenicity assessments showed that the observed immunogenicity leves were similar between the SCD411 and aflibercept groups with a low incidence of NAb positivity.
Previous clinical trials on intravitreal anti-VEGF injections for nAMD showed that changes in BCVA and CRT rapidly coccurred within the initial 8â12Â weeks of treatment and then stabilized thereafter3,4,13,16. In addition, the primary outcome of the VIEW studies was BCVA at week 524. Therefore, assessing the clinical outcomes at weeks 8 and 52 is a reasonable approach to evaluate the efficacy of SCD411 for the treatment of nAMD.
The results of this study are consistent with those of previous aflibercept clinical trials in terms of external validity. The mean BCVA change at week 8 was 5.9 letters, and it was similar to the BCVA change in the VIEW studies, which was approximately 6 letters. At week 52, the mean change of BCVA was 7.7 letters in the aflibercept group, compared with 7.9 letters in VIEW 1, 8.9 letters in VIEW 2, and 10.2 letters in ARIES (Age-related Macular Degeneration Requiring Intensive Intravitreal Aflibercept Treatment) studies of same treatment protocol4,17. The mean CRT change from baseline to week 52 wasâââ189.9 μm, compared withâââ128.5 μm in VIEW 1 and â 149.2 μm in VIEW 2 studies of same treatment protocol4.
Several studies have demonstrated a discrepancy between the outcomes of clinical trials of anti-VEGF agents and real-world data18,19. Longer-acting agents and lower treatment costs are required to overcome these compliance issues. Aflibercept has been known to exert a relatively longer duration of action; therefore, fewer clinic visits are possible4,7,20. A biosimilar product of aflibercept can provide additional treatment options for patients with nAMD whose condition is suitable for aflibercept and is expected to alleviate financial burdens on patients in some countries.
This study had several limitations. The number of participants in this study was sufficient to compare the equivalence in efficacy between SCD411 and the reference aflibercept, but it may not have been sufficient to observe very rare complications associated with the biosimilar product SCD411. Therefore, further large-scale studies are warranted. This study followed the participants for 52Â weeks. Although there was no significant difference in the changes in the BCVA and CRT from the baseline at all study visits, the SCD411 group showed a trend towards better BCVA and CRT changes at the end of the follow-up. In this aspect, additional studies assessing long-term clinical outcomes are required.
In conclusion, the proposed aflibercept biosimilar product, SCD411, had efficacy, safety, and immunogenicity equivalent to the reference aflibercept in patients with nAMD. These results support the use of SCD411 as a promising aflibercept biosimilar.
Data availability
Subject to specific criteria, conditions, and exceptions, SamChunDang Pharm. Co. Ltd. is committed to granting researchers access to individual deidentified participant data, provided their proposals satisfy the research criteria and meet all other applicable conditions. Interested researchers are kindly requested to submit their proposals directly to the corresponding author. In order to obtain access, data requestors are required to enter into a data access agreement with SamChunDang Pharm. Co. Ltd.
References
Holz, F. G., Pauleikhoff, D., Klein, R. & Bird, A. C. Pathogenesis of lesions in late age-related macular disease. Am. J. Ophthalmol. 137, 504â510. https://doi.org/10.1016/j.ajo.2003.11.026 (2004).
Avery, R. L. et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 113, 363â372. https://doi.org/10.1016/j.ophtha.2005.11.019 (2006).
Rosenfeld, P. J. et al. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 355, 1419â1431. https://doi.org/10.1056/nejmoa054481 (2006).
Heier, J. S. et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119, 2537â2548. https://doi.org/10.1016/j.ophtha.2012.09.006 (2012).
Drugs@FDA: FDA-Approved Drugs: Eylea. US Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125387 (Accessed 9 April 2023).
Eylea. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/eylea#authorisation-details-section (Accessed 9 April 2023).
Papadopoulos, N. et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15, 171â185. https://doi.org/10.1007/s10456-011-9249-6 (2012).
Stewart, M. W. Pharmacokinetics, pharmacodynamics and pre-clinical characteristics of ophthalmic drugs that bind VEGF. Expert Rev. Clin. Pharmacol. 7, 167â180. https://doi.org/10.1586/17512433.2014.884458 (2014).
Lancet, T. Age-related macular degeneration: Treatment at what cost? Lancet 392, 1090. https://doi.org/10.1016/s0140-6736(18)32291-8 (2018).
Kapur, M., Nirula, S. & Naik, M. P. Future of anti-VEGF: Biosimilars and biobetters. Int. J. Retina Vitreous 8, 3. https://doi.org/10.1186/s40942-021-00343-3 (2022).
Declerck, P., Danesi, R., Petersel, D. & Jacobs, I. The language of biosimilars: Clarification, definitions, and regulatory aspects. Drugs 77, 671â677. https://doi.org/10.1007/s40265-017-0717-1 (2017).
Lee, J. F., Litten, J. B. & Grampp, G. Comparability and biosimilarity: Considerations for the healthcare provider. Curr. Med. Res. Opin. 28, 1053â1058. https://doi.org/10.1185/03007995.2012.686902 (2012).
Brown, D. M. et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 355, 1432â1444. https://doi.org/10.1056/nejmoa062655 (2006).
Thai, H.-T. et al. A mechanism-based model for the population pharmacokinetics of free and bound aflibercept in healthy subjects. Br. J. Clin. Pharmacol. 72, 402â414. https://doi.org/10.1111/j.1365-2125.2011.04015.x (2011).
Avery, R. L. et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br. J. Ophthalmol. 98, 1636â1641. https://doi.org/10.1136/bjophthalmol-2014-305252 (2014).
Martin, D. F. et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 364, 1897â1908. https://doi.org/10.1056/NEJMoa1102673 (2011).
Mitchell, P. et al. Efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: The ARIES study: A randomized clinical trial. Retina 41, 1911â1920. https://doi.org/10.1097/iae.0000000000003128 (2021).
Rofagha, S., Bhisitkul, R. B., Boyer, D. S., Sadda, S. R. & Zhang, K. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 120, 2292â2299. https://doi.org/10.1016/j.ophtha.2013.03.046 (2013).
Holz, F. G. et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br. J. Ophthalmol. 99, 220â226. https://doi.org/10.1136/bjophthalmol-2014-305327 (2015).
Stewart, M. W. & Rosenfeld, P. J. Predicted biological activity of intravitreal VEGF Trap. Br. J. Ophthalmol. 92, 667â668. https://doi.org/10.1136/bjo.2007.134874 (2008).
Funding
Conduction of this study was funded by SamChunDang Pharm. Co. Ltd., Seoul, Republic of Korea.
Author information
Authors and Affiliations
Contributions
Concepts and design: Se Woong Kang, David M. Brown, Jaehwan Choi. Acquisition, analysis, or interpretation of the data: Se Woong Kang, Jaehwan Choi, Veeral S. Sheth, Agnieszka Nowosielska, Marta Misiuk-Hojlo, András Papp, David M. Brown, Yoreh Barak. Drafting of the manuscript: Se Woong Kang, Jaehwan Choi. Critical revision of the manuscript for important intellectual content: Veeral S. Sheth, Agnieszka Nowosielska, Marta Misiuk-Hojlo, András Papp, David M. Brown, Jae-Ho Lee, Yoreh Barak. Administration, technical, or material support: Se Woong Kang, Jae-Ho Lee. Supervision: Se Woong Kang.
Corresponding author
Ethics declarations
Competing interests
S. W. K. received Grants from Novartis, Bayer, Samsung Bioepis, Roche, Altos Biologics, Opthea, Alexion Pharmaceuticals Inc., Hanlim Pharm. Co. Ltd., and SamChunDang Pharm. Co. Ltd. and serves as a board member and consultant for Bayer, Novartis, Roche, and SamChunDang Pharm. Co. Ltd. J. C. received non-financial supports from SamChunDang Pharm. Co. Ltd. V. S. S. received Grants from SamChunDang Pharm. Co. Ltd., Allergan, Opthea, Oxurion, Recens Medical, Roche, Regenxbio, Eyepoint, Genentech, Ionis, Novartis, Regeneron, Santen, Gyroscope, Chengdu Kanghong, SalutarisMD, NGM Biopharmaceuticals, Alimera Sciences, Outlook, 4D Molecular Therapeutics, Ashvattha Therapeutics, Olix pharmaceuticals, Janssen, and OcuTerra and received consulting fee from Genentech, Novartis, Alimera, EyePoint, Graybug, Apellis, Regeneron, and Vial and received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Genentech, Alimera, and Apellis. A. N. received travel grant from Bausch & Lomb and serves as a board member of Klinka Oczna and received equipment, materials, drugs, medical writing, gifts or other services from Kodiac, Ora, Altos, Xbrain, and Quilu. M. M. received grant from SamChunDang Pharm. Co. Ltd. A. P. received grants from Bayer, Novartis, Iveric Bio, Samsung Bioepis, Amgen, Oculis, Quilu, Roche, Xbrain, Formycon, Regeneron, Altos Biologics, Shanghai Henlius Biotech Inc., Kodiak Sciences Inc., Intas Pharmaceuticals Ltd., and SamChunDang Pharm. Co. Ltd. and received consulting fees from Bayer, Novartis, Roche, and Formycon and received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bayer, Novartis, and Roche and received travel grant from Novartis and serves as a board member of Bayer, Novartis, and Roche and a president of retina section of the Hungarian Ophthalmic Society. D. M. B. received grants from Adverum, Alderya, Alexion, Alkahest, Alimera, Amgen, Annexon, Apellis, Aura, Bayer, Boston Image Reading Center, Boehringer Ingelheim, Clearside Biomedical, Cleveland Clinic Foundation, Eyepoint, Gemini, Genentech/Roche, Graybug, Gyroscope, John Hopkins, Ionis, Irenix, Iveric, Kodiak, Lowery Medical Research Group, Lumithera, Nanoscope, NEI/NH, Neurotech, NGM, Novartis, Ocular Therapeutix, Ocuphire Pharma, Inc., OIRRC, ONS, Opthea, Opthotech, OPTOS, Oxurion, Oysterpoint, Regeneron, REGENXBIO, Roche, SamChunDang Pharm. Co. Ltd., Samsung, Sandoz, Santen, Senju, Stealth, Unity, Xbrane, and Zeiss and serves as a consultant for 4DMT, Adverum Biotechnologies Inc., AGTC, Alexion Pharmaceuticals Inc., Annexon Inc., Apellis Pharmaceuticals Inc., Bayer, Biogen MA Inc., Boehringer Ingelheim, Celltrion Inc., Chengdu Kanghong Pharmaceutical, Clearside Biomedical Inc., Coherus BioScience Inc., Gemini Therapeutics Inc., Glaukos, Heidelberg Engineering Inc., Iveric Bio Inc., Kodiak Sciences Inc., Lineage Cell Therapeutics, Medscape LLC, Molecular Partners AG, Novartis Pharma AG, Ocular Therapeutix, Ocuphire Pharma Inc., OPTOS PLC, Pontifax, PPD, Regeneron Pharmaceuticals Inc., RetinAI Medical AG, Ray Therapeutics Inc., SIMR Biotech, Samsung Bioepis Co. Ltd., Senju Pharmaceuticals Co.Ltd., SciNeuro Therapeutics Inc., Smilebiotek Zhuhai Limited, Stealth BioTherapeutics Inc., Verseon, and WebMD Global LLC and holds stock options in Adverum and Clearside Biomedical Inc. J. L. is an employee of SamChunDang Pharm. Co. Ltd. Y. B. received grant from SamChunDang Pharm. Co. Ltd.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, S.W., Choi, J., Sheth, V.S. et al. Comparison of the efficacy and safety of SCD411 and reference aflibercept in patients with neovascular age-related macular degeneration. Sci Rep 14, 14752 (2024). https://doi.org/10.1038/s41598-024-65815-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65815-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.