Preprint
Article
Using Waste Concentrated Sodium Hydroxide Solution From Aluminum Production and Fabric Softener As Phase Transfer Agent in the Environmentally Friendly Biodiesel Synthesis
Altmetrics
Downloads
154
Views
56
Comments
0
Abstract
Red mud (RM) is a residue obtained from the production of alumina. It contains a high concentration of metal oxides and waste concentrated sodium hydroxide solution (pH = 13). To increase the value of RM, an environmentally friendly process of transesterification using waste cooking oil (WCO), MeOH and concentrated sodium hydroxide solution (CSHS) from aluminum production was proposed. Triglycerides of WCO reacted with MeOH at 60 oC to yield mixtures of fatty acid methyl esters (FAMEs) in the presence of 2.03% (w/w) CSHS/WCO using the CSHS (0.204 mol L-1, predetermined by potentiometric titration) from aluminum production by the Bayer process or with the addition of 0.68% (w/w) fabric softener (3% w/w cetyltrimethylammonium chloride in solution) as a phase transfer (PTA) agent. The addition of PTA to the catalyst resulted in a better yield of the products (greater than 98% yield). A simplified mechanism is presented to account for the experimental results.

Keywords:
Subject: Chemistry and Materials Science - Applied Chemistry
1. Introduction
Concentrated sodium hydroxide solution (CSHS) and red mud (RM) (bauxite residue, bauxite tailings, red sludge, or alumina refinery residues) are an industrial waste generated during the processing of bauxite into alumina using the Bayer process (over 95% of the alumina is produced globally through the Bayer process). With every tonne of alumina produced, approximately 1 to 1.5 tonnes of RM are also produced. Annual production of alumina in 2020 was over 133 million tonnes, resulting in the generation of over 175 million tonnes of RM Because of this large production and the material's high alkalinity (pH of 10.5-12.5) and potential leaching if not stored properly, it can pose a significant environmental hazard. Its storage is a critical environmental problem [2,3,4,5,6,7,8,9]. This material is typically stored in dams, which demands prior preparation of the disposal area and includes monitoring and maintenance throughout its useful life
A small residual amount of the sodium hydroxide (SH) used in the process remains with the residue. Various stages in the solid/liquid separation process have been introduced to recycle as much SH as possible from the residue back into the Bayer Process to make the process as efficient as possible and reduce production costs. These modifications also lower the final alkalinity of the residue, making it easier and safer to handle and store
The present study discusses the technical viability of RM valorization. The authors proposed the utilization of residual CSHS from the aluminum industry for the first time as a catalyst in the transesterification reaction in aqueous middle for the production of Fatty acid methyl esters (FAME).
FAME or biodiesel, according to the American Society for Testing and Materials (ASTM) and European standards (EN), is a fuel consisting of “long chain fatty acids of mono-alkyl esters derived from renewable fatty raw material such as animal fats or vegetable oils” FAME content needs to be higher than 96.5 wt.%. The total glycerol, including bound glycerol (in glycerides such as monoglycerides (MGs), diglycerides (DGs) and triglycerides (TGs) and unbound glycerol (free glycerol), needs to be limited to 0.24 and 0.25 wt.% by ASTM and EN standards, respectively.
In the search for an environmentally friendly method for biodiesel synthesis by transesterification of TGs, several alternatives for catalysis have been explored. In general, catalysts that can be used for producing biodiesel are divided into three categories: acidic, alkaline and biocatalysts. Acidic and alkaline catalysts are classified into two groups: homogeneous and heterogeneous catalysts.
Catalysts play a vital role in the transesterification process. Both the amount and type of catalyst affect the rate of reaction and conversion efficiency. Homogeneous catalysts function in the same phase as the reactants and can be categorized into homogeneous base catalysts and homogeneous acid catalysts. Currently, most FAME are produced by the base-catalyzed transesterification reaction because of its high conversion rate, negligible side reactions, and short reaction time. It is a low-pressure and low-temperature process, which occurs without the formation of intermediate substances. Despite these advantages, homogeneous base catalysts have some weaknesses. The production of biodiesel from feedstocks with a high free fatty acid (FFA) content is limited. It was reported by some researchers that homogeneous base catalysts are only effective for the FAME production via the transesterification process using the feedstocks with a FFA content of less than 2 wt % When FFA content is >2%, the catalyst reacts with FFA to produce soap and water. The soap inhibits the separation of FAME and glycerin, and the water can hydrolyze the esters in a reaction that competes with the transesterification.
In transesterification reactions of TGs catalyzed by homogeneous bases, FAME and glycerol are produced. Zhang, Stanciulescu, and Ikura (2009) demonstrated that the use of phase transfer agents (PTA) greatly increased the rate of the base-catalyzed transesterification reaction A product containing 96.5 wt% was obtained after only 15 min of rapid reaction at 60 oC in the presence of tetrabutylammonium hydroxide or acetate. The reaction was performed in the presence of MeOH, glycerol, refined and bleached soybean oil and the basic catalyst, without the presence of water.
Huang (2013) synthesized biodiesel from soybean oil and MeOH using K2CO3 and phase-transfer catalysis (TBAB). The yield of biodiesel could be changed by reaction factors such as the kind and amount of phase-transfer catalysis, the amount of K2CO3, reaction time and temperature, and the molar ratio of MeOH to soybean oil. The results show that the reaction conditions are as follows: 0.6% mass ratio of TBAB to soybean oil, 1.5% mass ratio of K2CO3 to soybean oil, 6:1 molar ratio of MeOH to soybean oil, 20 min reaction time, reaction temperature, 40 ˚C. The yield of biodiesel reached 95% under the optimum reaction conditions
Recently, we also studied the acid-catalyzed transesterification reaction using WCO and a solid acidic catalyst (SiO2-SO3H) in the presence of quaternary ammonium salts as co-catalysts in toluene and DMSO We, therefore, decided to study the transesterification reaction in a medium containing CSHS, utilizing the quaternary ammonium salts contained in fabric softener as the PTA. This study sought to utilize the industrial residues (RM; CSHS) to improve their value and decrease the environmental problems resulting from the storage of these residues. A commercial fabric softener (cetyl trimethyl ammonium chloride) was also used in the process because of its low cost. The glycerol residue was pretreated with sulfuric acid to produce Na2SO4, which can be used as a drying agent. The process is environmentally correct.
2. Experimental
2.1. Raw materials and chemicals
RM containing CSHS was collected from ALCOA (Juruti, PA, Brazil). WCO (soybean) was donated by the university restaurant, and it was filtered through silica gel, which removed any fatty acids (FA) and polar and polymeric substances prior to use. The physical parameters determined for the yellow oil were the viscosity (41.2 mPa) and the density (0.883 g.mL-1). MeOH (analytical grade) was supplied by Vetec, São Paulo, Brazil, and fabric softener (cetyltrimethylammonium chloride) was obtained from the local market.
2.2. Typical procedures
2.2.1. Standardization the waste CSHS generated during the processing of bauxite into alumina using the Bayer process.
CSHS (1.60 mL) was diluted to 100 mL. Subsequently, an aliquot of 1.00 mL was cremoved and diluted again to 100 mL. A 25 mL aliquot was collected from this solution and titrated with a 0.0017 M HCl solution using 0.48 mL of HCl solution. Titration was accomplished using a SI Analytics Titrator TitroLine® 7000 potentiometric titrator. The concentration of CSHS determined was 0.204 mol L-1.
2.2.2. Reacting the TGs from WCO with MeOH using CSHS as a catalyst without fabric softener.
The procedure utilized for the transesterification reaction was based on various trials to determine the optimum conditions for this reaction. A 150-mL round bottom flask containing CSHS (0.204 mol L-1; 0.569 mL, 0.116x10-3 mol NaOH, or 0.132% w/w of WCO) with MeOH (15 mL, 11.895 g, 371.27 mmol; or 1:73 molar ratio of WCO/MeOH) was stirred for 20 min at room temperature.
WCO (4.4170 g, 5.0 mL; 5.0583 mmol) and MeOH (15 mL, 11.895 g, 371.27 mmol) or 1:73 molar ratio of WCO/MeOH) were mixed in a second 150-mL round bottom flask equipped with a reflux condenser, and the mixture was refluxed for 20 min. at 60 oC. The addition of the contents of the first flask to the mixture containing WCO and MeOH was recorded as time zero. The mixture was heated under reflux at 60 oC for one hour. The mixture was cooled and transferred to a separatory funnel, where the biofuel-containing upper phase was separated from the lower phase containing glycerol by decantation. The MeOH was removed from the biodiesel phase on a rotary evaporator, purified by distillation and used in new reaction processes within this study. The recovered glycerol was stored for future treatments. The biofuel phase was dissolved in hexane (20 mL), extracted with 20 mL of a saturated solution of NaCl, dried over MgSO4 and concentrated.
2.2.3. Reacting the TGs from waste cooking oil with methanol, strong sodium hydroxide solution as catalyst and softener as PTA.
A 150-mL round bottom flask containing CSHS (0.204 mol L-1, 0.569 mL, 0.116x10-3 mols NaOH, 0.132% w/w of WCO) with MeOH (15 mL, 11.895 g, 371.27 mmol; or a molar ratio of WCO) was stirred for 20 min at room temperature.
WCO (4.4170 g, 5.0 mL, 5.0583 mmol, 873.22 g mol-1), MeOH (15 mL, 11.895 g, 371.27 mmol, 39.996 g mol-1 or 73:1 molar ratio to WCO) and fabric softener (0,68% w/w fabric softener, 3% w/w cetyltrimethylammonium chloride in solution/WCO, 0.5690 mL) were mixed in a second 150-mL round bottom flask equipped with a reflux condenser, and the mixture was refluxed for 20 min. at 60 oC. The addition of the contents of the first flask to the mixture containing WCO, MeOH and fabric softener mixture was recorded as time zero. The mixture was heated under reflux at 60 oC for 20.0 minutes. The mixture was cooled and transferred to a separatory funnel, where the biofuel-containing upper phase was separated from the lower phase containing glycerol by decantation. The MeOH was removed from the biodiesel phase on a rotary evaporator, purified by distillation and used in new reaction processes within this study. The recovered glycerol was stored for future treatments. The biofuel phase was dissolved in hexane (50 mL), extracted with 20 mL of a saturated solution of NaCl, dried over MgSO4 and concentrated.
2.3. WCO and biodiesel analysis
The official methods proposed by ISO 12966 were used to determine the compositional profile by gas chromatography using a flame ionization detector (GC-FID) (Shimadzu GC-2010). The chromatographic system used to separate and identify FFAs (wt%) included a cross-bound polyethyleneglycol capillary column (Supelco SP 2560, 100 m x 0.25 mm x 20 µm). The initial temperature was 60 °C for 2 min; the temperature increased to 220 °C at 10 °C.min-1, and finally, to 240 °C at 5 °C.min-1, where it was held for 7 min. The injector and detector temperatures were 350 °C, and the sample (0.5 µL injected) was dissolved in 99% iso-octane.
The European Standards (EN 14103) and the Brazilian Technical Standards Association (ABNT NBR 15908) were used to quantify FAMEs and remaining mono-, di- and triglycerides (MG, DG, TG) in the biodiesel. For quantification of FAMEs, a Thermo Trace GC-Ultra chromatograph was used, equipped with a flame ionization detector and a Thermo Scientific TR-BD (FAME) Capillary GC Column (L × I.D. 30 m × 0.25 mm, df 0.25 μm) containing a polyethylene glycol stationary phase, according to the EN 14103 analytical procedure. Pure methyl nonadecanoate (C19:0, Sigma-Aldrich) was used as an internal standard to normalize the peak areas of the chromatograms. The integration was achieved from the methyl hexanoate (C6:0) peak to that of the methyl nervonate (C24:1), including all the peaks identified as FAMEs. To analyze the FAME samples, approximately 100 mg (accuracy ± 0.1 mg) of homogenized sample and approximately 100 mg (accuracy ± 0.1 mg) of nonadecanoic acid methyl ester were weighed in a 10 mL vial and diluted with 10 mL of toluene before injection into the equipment. All the samples were prepared in duplicate. Chromatographic conditions are described as follows: (a) column temperature: 60 °C held for 2 min, programmed at 10 °C.min-1 to 200 °C, then programmed at 5 °C.min-1 to 240 °C; the final temperature was held for 7 min; (b) injector and detector temperature: 250 °C; (c) helium carrier gas flow rate: 1-2 mL.min-1, a minimum flow rate of 1 mL.min-1 was warranted when operating at the maximum temperature; (d) injected volume: 1 μL and (e) split flow: 100 mL.min-1.
For the quantification of the glycerides (MG, DG, and TG), a Shimadzu GC2010 chromatograph equipped with a flame ionization detector was used according to the ASTM D6584 analytical procedure. The chromatographic system was configured to separate and identify MG, DG and TG with a CrossbondTM 5% Phenyl/95% dimethylpolysiloxane capillary column (Zebron ZB-5HT, 30 m x 0.32 mm x 0.1 mm - Phenomenex, Torrence, CA) with on-column injection. The initial temperature in the capillary column was 50 ºC (1 min); the temperature increased to 180 ºC at 15 ºC min-, to 230 ºC at 7 ºC.min-1, and finally to 380 ºC at 20 ºC.min-1, where it was held for 10 min. The injector and detector temperatures were 380 ºC, and the sample (0.5 mL injected) was prepared using heptane 99%. 1H- and 13C-NMR spectra were recorded on Bruker Avance 400 and Avance 500 spectrometers. These data are included in the supplementary material.
3. Results and Discussion
The compositional profile analysis of the WCO used in this work is described in Table 1. The main Fatty acids (FAs) in that oil were linolenic (C18:2) and oleic acids (C18:1); accordingly, the mean molecular weight (MW) of the FAs was determined to be 277.41 g.mol-1, and the mean molecular mass (MM) of the TGs was 873.22 g.mol-1. This profile was considered for calculating the molar ratio of WCO: MeOH for the transesterification reaction. The composition of the WCO was very similar to that of soybean oil (SO) described in the literature
Lit [17]: Palmitic acid, 11.6%; stearic acid, 3.22%; oleic acid, 25.09%; linoleic acid, 52.93%; linolenic acid, 5.95%; others, 1.08%.
Sodium methoxide (MeONa), is formed by the deprotonation of MeOH and frequently prepared by treating MeOH with metallic sodium [18]:
2 Na + 2 CH3OH → 2 CH3ONa + H2
The resulting solution, MeONa/MeOH, which is colorless, is often used as a source of MeONa. The MeONa absorbs CO2 from the air to form MeOH and Na2CO3, thereby diminishing the alkalinity of the base.
CH3ONa + CO2 + H2O → 2 CH3OH + Na2CO3
MeONa is highly caustic and reacts with water to give MeOH and NaOH. In this work, we used a solution of CSHS (0.204 mol/L, determined by potentiometric titration) for the formation of MeONa, in which an excess of MeOH was used so that the MeONa formed could immediately react as a nucleophilic reagent with the ester carbonyl groups such as TGs, DGs and MGs. The water contained in the CSHS was solvated by excess MeOH through strong secondary interactions of hydrogen bonds, thus preventing the formation of sodium hydroxide from the inverse reaction of MeONa with water.
CH3OH (excess) + NaOH + H2O → CH3ONa + H2O + CH3OH
In an unprecedented process, biodiesel was produced by catalysis using a CSHS obtained from the aluminum industry for the transesterification reaction of WCO. However, the CSHS-catalyzed transesterification is a slow reaction at both initial and final reaction stages because it is limited by mass transfer between the polar water/MeOH/glycerol phase and the non-polar MGs, DGs and TGs phase. In our study we used fabric softener as a PTA to facilitate transfer of methoxide anion (MeO:-) between the polar and the non-polar phases to accelerate the transesterification reaction.
The benefits of transesterification by PTAs include no need for expensive aprotic solvents, such as THF, potentially simpler scaleup and higher activity (shorter reaction time). Experimental results showed that the transesterification reaction catalyzed by CSHS from the aluminum industry was enhanced with an effective PTA, indicated by a high FAME yield within a relatively short time (15 minutes). Individual operating variables such as molar ratios of MeOH to WCO, total OH- to WCO, PTA to CSHS catalyst and stirring accompanied by heating were investigated for transesterification with PTA.
Product analyses showed that a FAME content greater than 98.5 wt.% was achieved after only 5 min. The conditions of the transesterification reaction included fthe abric softener as PTA, a 6:1 molar ratio of MeOH/WCO, total OH-/WCO molar ratio of 0.22, PTA/NaOH containing CSHS with a molar ratio of 1 and 60 oC. Free and total glycerol contents in the final product after 5 min of rapid transesterification with PTA were lower than the maximum allowable limits in the standard specification for biodiesel.
3.1. Transesterification of TGs from WCO with MeOH using CSHS as catalyst without fabric softener
In the first phase of this study, a large excess of MeOH was mixed with previously standardized CSHS, and this mixture was added to the mixture containing WCO and MeOH and refluxed at 60 oC. The progress of the reaction was accompanied by TLC. The total consumption of TGs occurred after 40 minutes. The FAMEs and glycerides (MG, DG, and TG) contained in the biodiesel phase were confirmed by GC-FID using the methods defined in EN 14103 and ASTM D6584. The composition of the products (FAMEs and glycerides) is presented in Table 2; they represent the average values of five different measurements.
3.2. Transesterification of TGs from WCO with MeOH using CSHS as a catalyst in the presence of fabric softener.
In the second phase of this study, a large excess of MeOH was mixed with previously standardized CSHS, and this mixture was added to the mixture containing WCO, MeOH and fabric softener and refluxed at 60 oC. The progress of the reaction was accompanied by TLC. The total consumption of TGs occurred after 15 minutes. The FAMEs and glycerides (MG, DG, and TG) contained in the biodiesel phase were confirmed by GC-FID using the methods defined in EN 14103 and ASTM D6584.
The best reaction conditions obtained from experiments are includec CSHS (0.204 mol L-1, 0.569 mL, 0.1161x10-3 mols NaOH, 0.132% w/w of WCO); the molar ratio of MeOH and WCO was 73:1 and the reaction temperature was 60 ℃. The composition of the products (FAMEs and glycerides) after 5.0 minutes of reaction is presented in Table 2; they represent the average values of five different measurements.
Table 2.
Composition of the WCO feedstock and the product mixture using refluxing MeOH.
| Products | WCO (wt%) | CSHS without fabric softener | CSHS with fabric softener |
|---|---|---|---|
| Triacylglycerides (%) | 96.2 | 20.0 | 5.9 |
| Diacylglycerides (%) | 2.80 | 4.5 | 11.0 |
| Monoacylglycerides (%) | 1 | 55.6 | 22.9 |
| FAME (%) | 0 | 44.9 | 60.2 |
Table 2. Numerical data for Figure 2.
The yields for completed transesterification reactions are presented in Table 3.
The yields of FAMEs obtained using fabric softener as a PTA were slightly higher than those obtained without the use of fabric softener. However, the rate of the reaction in the presence of fabric softener was significantly higher (15 min vs. 40 min for completion of the reaction). The first possible explanation for this result is that the fabric softener acted as a PTA to facilitate the contact of the catalyst with the substrate through mass transfer between the polar water/MeOH/glycerol phase and the non-polar MGs, DGs and TGs phase. Fabric softener was employed to facilitate anion transfer between the polar water/MeOH/glycerol phase and the non-polar MG, DG and TG phase to accelerate the transesterification reaction. The proposed mechanism is shown below (Scheme 1):
FAME (biodiesel) is synthesized using the combination of WCO and MeOH with CSHS catalyst and PTA. The process includes following steps: (1) The reaction between MeOH and CSHS catalyst creates anion MeO-; (2) When MeO- and PTA (Q+X-) encounter each other, anion exchange of MeO- with X- in Q+X- creates the MeO-Q+ ion pair, which enters the WCO phase; (3) Nucleophilic attack (Acyl substitution reaction) occurs in the WCO phase, which creates FAME. Glyceryl enters into water/MeOH phase carrying Q+, reacts with H+X-, and creates glycerol and PTA. The whole catalysis cycle is completed.
The choice of PTA (Figure 1) was based on the economy of the process and the solubility of PTA in water/MeOH and MG, DG and TG phases. CSHS catalyzes the synthesis of FAME from WCO in the presence of the fabric softener as PTA. The hydrophobic carbon chain of PTA possesses a high lipophilicity and facilitates the transfer of anions from the aqueous phase to the WCO phase. The basicity of the anion is high so the dissociation in the WCO phase is difficult; therefore, the concentration of the anion would be low, which results in a low catalytic activity. Thus, the effect of the fabric softener is very efficient.
4. Conclusions
The use of PTA was explored for CSHS-catalyzed transesterification of TGs from WCO. The CSHS and mixtures of CSHS with fabric softener (PTA) containing cetyltrimethylammonium chloride were used successfully for the transesterification of TGs from WCO with MeOH. Analysis of the quantities of TGs, DGs, MGs and FAMEs in the products indicated different behaviors of the CSHS and mixtures of CSHS with fabric softener. The CSHS-catalyzed transesterification rate was enhanced with PTA, indicated by high FAME content obtained after only 15 min of reaction. The observed rapid transesterification can be explained by the fact that PTA can facilitate anion transfer between the polar water/MeOH/glycerol phase and non-polar WCO phase, overcome mass transfer limitations and speed up reaction rates. Product analyses showed that a FAME content higher than 98.5 wt.% was achieved after only 15 min of rapid transesterification (PTA) and 40 min without the presence of the PTA. Free and total glycerol contents in the final products after 15 min of transesterification with PTA (or 40 min in the absence of the PTA) were lower than the maximum legal limits in standard specifications for FAME.
5. Acknowledgements
The authors acknowledge the support by the SENAI CIMATEC and PRPPG/UFVJM in response to Resolução 15/2019 and the Fundação de Apoio à Pesquisa do Estado de Minas Gerais - FAPEMIG (Chamada Universal), 0004022 code.
References
- Evans, K. , "The History, Challenges and new developments in the management and use of Bauxite Residue", J. Sustain Metall. May 2016. [CrossRef]
- A.W. Bray, D.I. Stewart, R. Courtney, S.P. Rout, P.N. Humphreys, W.M. Mayes, I.T. Burke, Sustained Bauxite Residue Rehabilitation with Gypsum and Organic Matter 16 years after Initial Treatment, Environmental Science & Technology 52 (2018) 152-161. [CrossRef]
- I.T. Burke, W.M. Mayes, C.L. Peacock, A.P. Brown, A.P. Jarvis, K. Gruiz, Speciation of Arsenic, Chromium, and Vanadium in Red Mud Samples from the Ajka Spill Site, Hungary, Environmental Science & Technology 46 (2012) 3085-3092. [CrossRef]
- I.T. Burke, C.L. Peacock, C.L. Lockwood, D.I. Stewart, R.J.G. Mortimer, M.B. Ward, P. Renforth, K.
- Gruiz, W.M. Mayes, Behavior of Aluminum, Arsenic, and Vanadium during the Neutralization of Red Mud Leachate by HCl, Gypsum, or Seawater, Environmental Science & Technology 47 (2013) 6527-6535. [CrossRef]
- Gelencsér, N. Kováts, B. Turóczi, Á. Rostási, A. Hoffer, K. Imre, I. Nyirő-Kósa, D. Csákberényi-Malasics, Á. Tóth, A. Czitrovszky, A. Nagy, S. Nagy, A. Ács, A. Kovács, Á. Ferincz, Z. Hartyáni, M. Pósfai, The Red Mud Accident in Ajka (Hungary): Characterization and Potential Health Effects of Fugitive Dust, Environmental Science & Technology 45 (2011) 1608-1615. [CrossRef]
- Gupta, VK, Sharma, S. Removal of Cadmium and Zinc from Aqueous Solutions Using Red Mud, Environmental Science & Technology 36 (2002) 3612-3617. [CrossRef]
- S. Ruyters, J. Mertens, E. Vassilieva, B. Dehandschutter, A. Poffijn, E. Smolders, The Red Mud Accident in Ajka (Hungary): Plant Toxicity and Trace Metal Bioavailability in Red Mud Contaminated Soil, Environmental Science & Technology 45 (2011) 1616-1622. [CrossRef]
- T.C. Santini, M.V. Fey, Spontaneous Vegetation Encroachment upon Bauxite Residue (Red Mud) As an Indicator and Facilitator of In Situ Remediation Processes, Environmental Science & Technology 47 (2013) 12089-12096. [CrossRef]
- E.C.D. Resende, C. Gissane, R. Nicol, R.J. Heck, M.C. Guerreiro, J.V. Coelho, L.C.A.d. Oliveira, P. Palmisano, F. Berruti, C. Briens, M. Schlaf, Synergistic co-processing of Red Mud waste from the Bayer process and a crude untreated waste stream from bio-diesel production, Green Chemistry 15 (2013) 496-510. [CrossRef]
- Silveira, N.C.G.; Martins, M.L.F.; Bezerra, A.C.S.; Araújo, F.G.S. Red Mud from the Aluminium Industry: Production, Characteristics, and Alternative Applications in Construction Materials—A Review. Sustainability 2021, 13, 12741. [Google Scholar] [CrossRef]
- Chandra, S. (1996-12-31). "Red Mud Utilization". Waste materials used in concrete manufacturing. pp. 292–295. ISBN 978-0-8155-1393-3.
- ASTM D6751-20a; License Agreement, Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM: Philadelphia, PA, USA, 2020.
- Patel, R.L.; Sankhavara, C. Biodiesel production from Karanja oil and its use in diesel engine: A review. Renew. Sustain. Energy Rev. 2017, 71, 464–474. [Google Scholar] [CrossRef]
- Zhang, Y. Stanciulescu, M, Ikura M (2009) Rapid transesterification of soybean oil with phase transfer catalysts. Applied Catalysis A: General 366: 176–183. [CrossRef]
- Huang, Y. Synthesis of biodiesel by phase transfer catalysis. Applied Mechanics and Materials Vols 291-294 (2013) pp 355-358. [CrossRef]
- Barbosa, SL, Rocha, ACP., Nelson, DL, Freitas, MS, Mestre, AAPF., Klein, SI, Clososki, G.C., Caires, FJ, Flumignan, DL, Santos, LK, Wentz, AP, Pasa, V.MD. and Rios, RDF (2022) Catalytic Transformation of Triglycerides to Biodiesel with SiO2-SO3H and Quaternary Ammonium Salts in Toluene or DMSO. Molecules 27: 953. [CrossRef]
- Martínez, G.; Sánchez, N.; Encinar, J.M.; González, J.F. Fuel properties of biodiesel from vegetable oils and oil mixtures. Influence of methyl esters distribution. Biomass Bioenergy 2014, 63, 22–32. [Google Scholar] [CrossRef]
- Chandran, K.; Kamruddin, M.; Ajikumar, P.K.; et al. (2006). "Kinetics of thermal decomposition of sodium methoxide and ethoxide". Journal of Nuclear Materials. 358 (2–3): 111–128. Bibcode:2006JNuM. 358..111C. ISSN 0022-3115. [CrossRef]
Scheme 1.
Mechanism of PTA combining FAME.

Figure 1.
Cetyltrimethylammonium chloride structure.
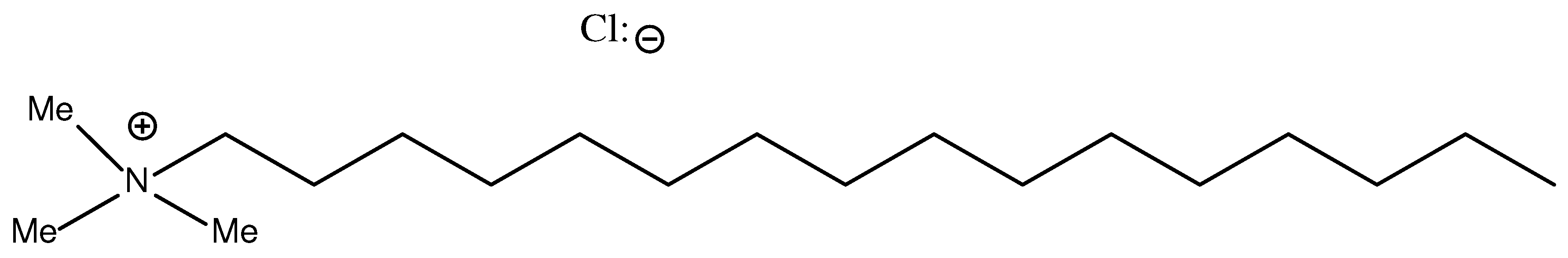
Table 1.
FAs composition of the WCO used in the present work.
| Fatty acid | Molecular Weight (g.mol-1) | wt% |
|---|---|---|
| Palmitic acid (C16:0) | 256.43 | 10.41 |
| Stearic acid (C18:0) | 284.48 | 3.91 |
| Oleic acid (C18:1) | 282.46 | 26.52 |
| Linoleic acid (C18:2) | 280.45 | 51.66 |
| Linolenic acid (C18:3) | 278.43 | 5.55 |
| Others | - | 1.95 |
| Average Molecular Weight of Fatty Acids (g.mol-1) | 277.41 | |
| Molar mass of TGs (g.mol-1) | 873.22 | |
Table 3.
Composition of the WCO feedstock and the product of transesterification of TGs from WCO with MeOH using CSHS as the catalyst in the presence of fabric softener and product of transesterification of TGs from WCO with MeOH using CSHS as catalyst without fabric softener.
Table 3.
Composition of the WCO feedstock and the product of transesterification of TGs from WCO with MeOH using CSHS as the catalyst in the presence of fabric softener and product of transesterification of TGs from WCO with MeOH using CSHS as catalyst without fabric softener.
| CSHS without fabric softener | CSHS with fabric softener | |||
|---|---|---|---|---|
| Assay | Result (% w/w) |
Standard deviation | Result (% w/w) |
Standard deviation |
| FAME | 98.50 | 3.10 | 99.52 | 2.94 |
| Free glycerol | 0.01 | 0,01 | 0.01 | 0.01 |
| Total glycerol | 0.19 | 0.07 | 0.07 | 0.07 |
| Monoacylglycerol | 0.76 | 0.27 | 0.26 | 0.26 |
| Diacylglycerol | 0.23 | 0.12 | 0.06 | 0.12 |
| Triacylglycerol | 0.03 | 0.26 | 0.01 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
supplementary.docx (8.19MB )
Submitted:
12 April 2023
Posted:
13 April 2023
You are already at the latest version
Alerts
supplementary.docx (8.19MB )
Submitted:
12 April 2023
Posted:
13 April 2023
You are already at the latest version
Alerts
Abstract
Red mud (RM) is a residue obtained from the production of alumina. It contains a high concentration of metal oxides and waste concentrated sodium hydroxide solution (pH = 13). To increase the value of RM, an environmentally friendly process of transesterification using waste cooking oil (WCO), MeOH and concentrated sodium hydroxide solution (CSHS) from aluminum production was proposed. Triglycerides of WCO reacted with MeOH at 60 oC to yield mixtures of fatty acid methyl esters (FAMEs) in the presence of 2.03% (w/w) CSHS/WCO using the CSHS (0.204 mol L-1, predetermined by potentiometric titration) from aluminum production by the Bayer process or with the addition of 0.68% (w/w) fabric softener (3% w/w cetyltrimethylammonium chloride in solution) as a phase transfer (PTA) agent. The addition of PTA to the catalyst resulted in a better yield of the products (greater than 98% yield). A simplified mechanism is presented to account for the experimental results.

Keywords:
Subject: Chemistry and Materials Science - Applied Chemistry
1. Introduction
Concentrated sodium hydroxide solution (CSHS) and red mud (RM) (bauxite residue, bauxite tailings, red sludge, or alumina refinery residues) are an industrial waste generated during the processing of bauxite into alumina using the Bayer process (over 95% of the alumina is produced globally through the Bayer process). With every tonne of alumina produced, approximately 1 to 1.5 tonnes of RM are also produced. Annual production of alumina in 2020 was over 133 million tonnes, resulting in the generation of over 175 million tonnes of RM Because of this large production and the material's high alkalinity (pH of 10.5-12.5) and potential leaching if not stored properly, it can pose a significant environmental hazard. Its storage is a critical environmental problem [2,3,4,5,6,7,8,9]. This material is typically stored in dams, which demands prior preparation of the disposal area and includes monitoring and maintenance throughout its useful life
A small residual amount of the sodium hydroxide (SH) used in the process remains with the residue. Various stages in the solid/liquid separation process have been introduced to recycle as much SH as possible from the residue back into the Bayer Process to make the process as efficient as possible and reduce production costs. These modifications also lower the final alkalinity of the residue, making it easier and safer to handle and store
The present study discusses the technical viability of RM valorization. The authors proposed the utilization of residual CSHS from the aluminum industry for the first time as a catalyst in the transesterification reaction in aqueous middle for the production of Fatty acid methyl esters (FAME).
FAME or biodiesel, according to the American Society for Testing and Materials (ASTM) and European standards (EN), is a fuel consisting of “long chain fatty acids of mono-alkyl esters derived from renewable fatty raw material such as animal fats or vegetable oils” FAME content needs to be higher than 96.5 wt.%. The total glycerol, including bound glycerol (in glycerides such as monoglycerides (MGs), diglycerides (DGs) and triglycerides (TGs) and unbound glycerol (free glycerol), needs to be limited to 0.24 and 0.25 wt.% by ASTM and EN standards, respectively.
In the search for an environmentally friendly method for biodiesel synthesis by transesterification of TGs, several alternatives for catalysis have been explored. In general, catalysts that can be used for producing biodiesel are divided into three categories: acidic, alkaline and biocatalysts. Acidic and alkaline catalysts are classified into two groups: homogeneous and heterogeneous catalysts.
Catalysts play a vital role in the transesterification process. Both the amount and type of catalyst affect the rate of reaction and conversion efficiency. Homogeneous catalysts function in the same phase as the reactants and can be categorized into homogeneous base catalysts and homogeneous acid catalysts. Currently, most FAME are produced by the base-catalyzed transesterification reaction because of its high conversion rate, negligible side reactions, and short reaction time. It is a low-pressure and low-temperature process, which occurs without the formation of intermediate substances. Despite these advantages, homogeneous base catalysts have some weaknesses. The production of biodiesel from feedstocks with a high free fatty acid (FFA) content is limited. It was reported by some researchers that homogeneous base catalysts are only effective for the FAME production via the transesterification process using the feedstocks with a FFA content of less than 2 wt % When FFA content is >2%, the catalyst reacts with FFA to produce soap and water. The soap inhibits the separation of FAME and glycerin, and the water can hydrolyze the esters in a reaction that competes with the transesterification.
In transesterification reactions of TGs catalyzed by homogeneous bases, FAME and glycerol are produced. Zhang, Stanciulescu, and Ikura (2009) demonstrated that the use of phase transfer agents (PTA) greatly increased the rate of the base-catalyzed transesterification reaction A product containing 96.5 wt% was obtained after only 15 min of rapid reaction at 60 oC in the presence of tetrabutylammonium hydroxide or acetate. The reaction was performed in the presence of MeOH, glycerol, refined and bleached soybean oil and the basic catalyst, without the presence of water.
Huang (2013) synthesized biodiesel from soybean oil and MeOH using K2CO3 and phase-transfer catalysis (TBAB). The yield of biodiesel could be changed by reaction factors such as the kind and amount of phase-transfer catalysis, the amount of K2CO3, reaction time and temperature, and the molar ratio of MeOH to soybean oil. The results show that the reaction conditions are as follows: 0.6% mass ratio of TBAB to soybean oil, 1.5% mass ratio of K2CO3 to soybean oil, 6:1 molar ratio of MeOH to soybean oil, 20 min reaction time, reaction temperature, 40 ˚C. The yield of biodiesel reached 95% under the optimum reaction conditions
Recently, we also studied the acid-catalyzed transesterification reaction using WCO and a solid acidic catalyst (SiO2-SO3H) in the presence of quaternary ammonium salts as co-catalysts in toluene and DMSO We, therefore, decided to study the transesterification reaction in a medium containing CSHS, utilizing the quaternary ammonium salts contained in fabric softener as the PTA. This study sought to utilize the industrial residues (RM; CSHS) to improve their value and decrease the environmental problems resulting from the storage of these residues. A commercial fabric softener (cetyl trimethyl ammonium chloride) was also used in the process because of its low cost. The glycerol residue was pretreated with sulfuric acid to produce Na2SO4, which can be used as a drying agent. The process is environmentally correct.
2. Experimental
2.1. Raw materials and chemicals
RM containing CSHS was collected from ALCOA (Juruti, PA, Brazil). WCO (soybean) was donated by the university restaurant, and it was filtered through silica gel, which removed any fatty acids (FA) and polar and polymeric substances prior to use. The physical parameters determined for the yellow oil were the viscosity (41.2 mPa) and the density (0.883 g.mL-1). MeOH (analytical grade) was supplied by Vetec, São Paulo, Brazil, and fabric softener (cetyltrimethylammonium chloride) was obtained from the local market.
2.2. Typical procedures
2.2.1. Standardization the waste CSHS generated during the processing of bauxite into alumina using the Bayer process.
CSHS (1.60 mL) was diluted to 100 mL. Subsequently, an aliquot of 1.00 mL was cremoved and diluted again to 100 mL. A 25 mL aliquot was collected from this solution and titrated with a 0.0017 M HCl solution using 0.48 mL of HCl solution. Titration was accomplished using a SI Analytics Titrator TitroLine® 7000 potentiometric titrator. The concentration of CSHS determined was 0.204 mol L-1.
2.2.2. Reacting the TGs from WCO with MeOH using CSHS as a catalyst without fabric softener.
The procedure utilized for the transesterification reaction was based on various trials to determine the optimum conditions for this reaction. A 150-mL round bottom flask containing CSHS (0.204 mol L-1; 0.569 mL, 0.116x10-3 mol NaOH, or 0.132% w/w of WCO) with MeOH (15 mL, 11.895 g, 371.27 mmol; or 1:73 molar ratio of WCO/MeOH) was stirred for 20 min at room temperature.
WCO (4.4170 g, 5.0 mL; 5.0583 mmol) and MeOH (15 mL, 11.895 g, 371.27 mmol) or 1:73 molar ratio of WCO/MeOH) were mixed in a second 150-mL round bottom flask equipped with a reflux condenser, and the mixture was refluxed for 20 min. at 60 oC. The addition of the contents of the first flask to the mixture containing WCO and MeOH was recorded as time zero. The mixture was heated under reflux at 60 oC for one hour. The mixture was cooled and transferred to a separatory funnel, where the biofuel-containing upper phase was separated from the lower phase containing glycerol by decantation. The MeOH was removed from the biodiesel phase on a rotary evaporator, purified by distillation and used in new reaction processes within this study. The recovered glycerol was stored for future treatments. The biofuel phase was dissolved in hexane (20 mL), extracted with 20 mL of a saturated solution of NaCl, dried over MgSO4 and concentrated.
2.2.3. Reacting the TGs from waste cooking oil with methanol, strong sodium hydroxide solution as catalyst and softener as PTA.
A 150-mL round bottom flask containing CSHS (0.204 mol L-1, 0.569 mL, 0.116x10-3 mols NaOH, 0.132% w/w of WCO) with MeOH (15 mL, 11.895 g, 371.27 mmol; or a molar ratio of WCO) was stirred for 20 min at room temperature.
WCO (4.4170 g, 5.0 mL, 5.0583 mmol, 873.22 g mol-1), MeOH (15 mL, 11.895 g, 371.27 mmol, 39.996 g mol-1 or 73:1 molar ratio to WCO) and fabric softener (0,68% w/w fabric softener, 3% w/w cetyltrimethylammonium chloride in solution/WCO, 0.5690 mL) were mixed in a second 150-mL round bottom flask equipped with a reflux condenser, and the mixture was refluxed for 20 min. at 60 oC. The addition of the contents of the first flask to the mixture containing WCO, MeOH and fabric softener mixture was recorded as time zero. The mixture was heated under reflux at 60 oC for 20.0 minutes. The mixture was cooled and transferred to a separatory funnel, where the biofuel-containing upper phase was separated from the lower phase containing glycerol by decantation. The MeOH was removed from the biodiesel phase on a rotary evaporator, purified by distillation and used in new reaction processes within this study. The recovered glycerol was stored for future treatments. The biofuel phase was dissolved in hexane (50 mL), extracted with 20 mL of a saturated solution of NaCl, dried over MgSO4 and concentrated.
2.3. WCO and biodiesel analysis
The official methods proposed by ISO 12966 were used to determine the compositional profile by gas chromatography using a flame ionization detector (GC-FID) (Shimadzu GC-2010). The chromatographic system used to separate and identify FFAs (wt%) included a cross-bound polyethyleneglycol capillary column (Supelco SP 2560, 100 m x 0.25 mm x 20 µm). The initial temperature was 60 °C for 2 min; the temperature increased to 220 °C at 10 °C.min-1, and finally, to 240 °C at 5 °C.min-1, where it was held for 7 min. The injector and detector temperatures were 350 °C, and the sample (0.5 µL injected) was dissolved in 99% iso-octane.
The European Standards (EN 14103) and the Brazilian Technical Standards Association (ABNT NBR 15908) were used to quantify FAMEs and remaining mono-, di- and triglycerides (MG, DG, TG) in the biodiesel. For quantification of FAMEs, a Thermo Trace GC-Ultra chromatograph was used, equipped with a flame ionization detector and a Thermo Scientific TR-BD (FAME) Capillary GC Column (L × I.D. 30 m × 0.25 mm, df 0.25 μm) containing a polyethylene glycol stationary phase, according to the EN 14103 analytical procedure. Pure methyl nonadecanoate (C19:0, Sigma-Aldrich) was used as an internal standard to normalize the peak areas of the chromatograms. The integration was achieved from the methyl hexanoate (C6:0) peak to that of the methyl nervonate (C24:1), including all the peaks identified as FAMEs. To analyze the FAME samples, approximately 100 mg (accuracy ± 0.1 mg) of homogenized sample and approximately 100 mg (accuracy ± 0.1 mg) of nonadecanoic acid methyl ester were weighed in a 10 mL vial and diluted with 10 mL of toluene before injection into the equipment. All the samples were prepared in duplicate. Chromatographic conditions are described as follows: (a) column temperature: 60 °C held for 2 min, programmed at 10 °C.min-1 to 200 °C, then programmed at 5 °C.min-1 to 240 °C; the final temperature was held for 7 min; (b) injector and detector temperature: 250 °C; (c) helium carrier gas flow rate: 1-2 mL.min-1, a minimum flow rate of 1 mL.min-1 was warranted when operating at the maximum temperature; (d) injected volume: 1 μL and (e) split flow: 100 mL.min-1.
For the quantification of the glycerides (MG, DG, and TG), a Shimadzu GC2010 chromatograph equipped with a flame ionization detector was used according to the ASTM D6584 analytical procedure. The chromatographic system was configured to separate and identify MG, DG and TG with a CrossbondTM 5% Phenyl/95% dimethylpolysiloxane capillary column (Zebron ZB-5HT, 30 m x 0.32 mm x 0.1 mm - Phenomenex, Torrence, CA) with on-column injection. The initial temperature in the capillary column was 50 ºC (1 min); the temperature increased to 180 ºC at 15 ºC min-, to 230 ºC at 7 ºC.min-1, and finally to 380 ºC at 20 ºC.min-1, where it was held for 10 min. The injector and detector temperatures were 380 ºC, and the sample (0.5 mL injected) was prepared using heptane 99%. 1H- and 13C-NMR spectra were recorded on Bruker Avance 400 and Avance 500 spectrometers. These data are included in the supplementary material.
3. Results and Discussion
The compositional profile analysis of the WCO used in this work is described in Table 1. The main Fatty acids (FAs) in that oil were linolenic (C18:2) and oleic acids (C18:1); accordingly, the mean molecular weight (MW) of the FAs was determined to be 277.41 g.mol-1, and the mean molecular mass (MM) of the TGs was 873.22 g.mol-1. This profile was considered for calculating the molar ratio of WCO: MeOH for the transesterification reaction. The composition of the WCO was very similar to that of soybean oil (SO) described in the literature
Lit [17]: Palmitic acid, 11.6%; stearic acid, 3.22%; oleic acid, 25.09%; linoleic acid, 52.93%; linolenic acid, 5.95%; others, 1.08%.
Sodium methoxide (MeONa), is formed by the deprotonation of MeOH and frequently prepared by treating MeOH with metallic sodium [18]:
2 Na + 2 CH3OH → 2 CH3ONa + H2
The resulting solution, MeONa/MeOH, which is colorless, is often used as a source of MeONa. The MeONa absorbs CO2 from the air to form MeOH and Na2CO3, thereby diminishing the alkalinity of the base.
CH3ONa + CO2 + H2O → 2 CH3OH + Na2CO3
MeONa is highly caustic and reacts with water to give MeOH and NaOH. In this work, we used a solution of CSHS (0.204 mol/L, determined by potentiometric titration) for the formation of MeONa, in which an excess of MeOH was used so that the MeONa formed could immediately react as a nucleophilic reagent with the ester carbonyl groups such as TGs, DGs and MGs. The water contained in the CSHS was solvated by excess MeOH through strong secondary interactions of hydrogen bonds, thus preventing the formation of sodium hydroxide from the inverse reaction of MeONa with water.
CH3OH (excess) + NaOH + H2O → CH3ONa + H2O + CH3OH
In an unprecedented process, biodiesel was produced by catalysis using a CSHS obtained from the aluminum industry for the transesterification reaction of WCO. However, the CSHS-catalyzed transesterification is a slow reaction at both initial and final reaction stages because it is limited by mass transfer between the polar water/MeOH/glycerol phase and the non-polar MGs, DGs and TGs phase. In our study we used fabric softener as a PTA to facilitate transfer of methoxide anion (MeO:-) between the polar and the non-polar phases to accelerate the transesterification reaction.
The benefits of transesterification by PTAs include no need for expensive aprotic solvents, such as THF, potentially simpler scaleup and higher activity (shorter reaction time). Experimental results showed that the transesterification reaction catalyzed by CSHS from the aluminum industry was enhanced with an effective PTA, indicated by a high FAME yield within a relatively short time (15 minutes). Individual operating variables such as molar ratios of MeOH to WCO, total OH- to WCO, PTA to CSHS catalyst and stirring accompanied by heating were investigated for transesterification with PTA.
Product analyses showed that a FAME content greater than 98.5 wt.% was achieved after only 5 min. The conditions of the transesterification reaction included fthe abric softener as PTA, a 6:1 molar ratio of MeOH/WCO, total OH-/WCO molar ratio of 0.22, PTA/NaOH containing CSHS with a molar ratio of 1 and 60 oC. Free and total glycerol contents in the final product after 5 min of rapid transesterification with PTA were lower than the maximum allowable limits in the standard specification for biodiesel.
3.1. Transesterification of TGs from WCO with MeOH using CSHS as catalyst without fabric softener
In the first phase of this study, a large excess of MeOH was mixed with previously standardized CSHS, and this mixture was added to the mixture containing WCO and MeOH and refluxed at 60 oC. The progress of the reaction was accompanied by TLC. The total consumption of TGs occurred after 40 minutes. The FAMEs and glycerides (MG, DG, and TG) contained in the biodiesel phase were confirmed by GC-FID using the methods defined in EN 14103 and ASTM D6584. The composition of the products (FAMEs and glycerides) is presented in Table 2; they represent the average values of five different measurements.
3.2. Transesterification of TGs from WCO with MeOH using CSHS as a catalyst in the presence of fabric softener.
In the second phase of this study, a large excess of MeOH was mixed with previously standardized CSHS, and this mixture was added to the mixture containing WCO, MeOH and fabric softener and refluxed at 60 oC. The progress of the reaction was accompanied by TLC. The total consumption of TGs occurred after 15 minutes. The FAMEs and glycerides (MG, DG, and TG) contained in the biodiesel phase were confirmed by GC-FID using the methods defined in EN 14103 and ASTM D6584.
The best reaction conditions obtained from experiments are includec CSHS (0.204 mol L-1, 0.569 mL, 0.1161x10-3 mols NaOH, 0.132% w/w of WCO); the molar ratio of MeOH and WCO was 73:1 and the reaction temperature was 60 ℃. The composition of the products (FAMEs and glycerides) after 5.0 minutes of reaction is presented in Table 2; they represent the average values of five different measurements.
Table 2.
Composition of the WCO feedstock and the product mixture using refluxing MeOH.
| Products | WCO (wt%) | CSHS without fabric softener | CSHS with fabric softener |
|---|---|---|---|
| Triacylglycerides (%) | 96.2 | 20.0 | 5.9 |
| Diacylglycerides (%) | 2.80 | 4.5 | 11.0 |
| Monoacylglycerides (%) | 1 | 55.6 | 22.9 |
| FAME (%) | 0 | 44.9 | 60.2 |
Table 2. Numerical data for Figure 2.
The yields for completed transesterification reactions are presented in Table 3.
The yields of FAMEs obtained using fabric softener as a PTA were slightly higher than those obtained without the use of fabric softener. However, the rate of the reaction in the presence of fabric softener was significantly higher (15 min vs. 40 min for completion of the reaction). The first possible explanation for this result is that the fabric softener acted as a PTA to facilitate the contact of the catalyst with the substrate through mass transfer between the polar water/MeOH/glycerol phase and the non-polar MGs, DGs and TGs phase. Fabric softener was employed to facilitate anion transfer between the polar water/MeOH/glycerol phase and the non-polar MG, DG and TG phase to accelerate the transesterification reaction. The proposed mechanism is shown below (Scheme 1):
FAME (biodiesel) is synthesized using the combination of WCO and MeOH with CSHS catalyst and PTA. The process includes following steps: (1) The reaction between MeOH and CSHS catalyst creates anion MeO-; (2) When MeO- and PTA (Q+X-) encounter each other, anion exchange of MeO- with X- in Q+X- creates the MeO-Q+ ion pair, which enters the WCO phase; (3) Nucleophilic attack (Acyl substitution reaction) occurs in the WCO phase, which creates FAME. Glyceryl enters into water/MeOH phase carrying Q+, reacts with H+X-, and creates glycerol and PTA. The whole catalysis cycle is completed.
The choice of PTA (Figure 1) was based on the economy of the process and the solubility of PTA in water/MeOH and MG, DG and TG phases. CSHS catalyzes the synthesis of FAME from WCO in the presence of the fabric softener as PTA. The hydrophobic carbon chain of PTA possesses a high lipophilicity and facilitates the transfer of anions from the aqueous phase to the WCO phase. The basicity of the anion is high so the dissociation in the WCO phase is difficult; therefore, the concentration of the anion would be low, which results in a low catalytic activity. Thus, the effect of the fabric softener is very efficient.
4. Conclusions
The use of PTA was explored for CSHS-catalyzed transesterification of TGs from WCO. The CSHS and mixtures of CSHS with fabric softener (PTA) containing cetyltrimethylammonium chloride were used successfully for the transesterification of TGs from WCO with MeOH. Analysis of the quantities of TGs, DGs, MGs and FAMEs in the products indicated different behaviors of the CSHS and mixtures of CSHS with fabric softener. The CSHS-catalyzed transesterification rate was enhanced with PTA, indicated by high FAME content obtained after only 15 min of reaction. The observed rapid transesterification can be explained by the fact that PTA can facilitate anion transfer between the polar water/MeOH/glycerol phase and non-polar WCO phase, overcome mass transfer limitations and speed up reaction rates. Product analyses showed that a FAME content higher than 98.5 wt.% was achieved after only 15 min of rapid transesterification (PTA) and 40 min without the presence of the PTA. Free and total glycerol contents in the final products after 15 min of transesterification with PTA (or 40 min in the absence of the PTA) were lower than the maximum legal limits in standard specifications for FAME.
5. Acknowledgements
The authors acknowledge the support by the SENAI CIMATEC and PRPPG/UFVJM in response to Resolução 15/2019 and the Fundação de Apoio à Pesquisa do Estado de Minas Gerais - FAPEMIG (Chamada Universal), 0004022 code.
References
- Evans, K. , "The History, Challenges and new developments in the management and use of Bauxite Residue", J. Sustain Metall. May 2016. [CrossRef]
- A.W. Bray, D.I. Stewart, R. Courtney, S.P. Rout, P.N. Humphreys, W.M. Mayes, I.T. Burke, Sustained Bauxite Residue Rehabilitation with Gypsum and Organic Matter 16 years after Initial Treatment, Environmental Science & Technology 52 (2018) 152-161. [CrossRef]
- I.T. Burke, W.M. Mayes, C.L. Peacock, A.P. Brown, A.P. Jarvis, K. Gruiz, Speciation of Arsenic, Chromium, and Vanadium in Red Mud Samples from the Ajka Spill Site, Hungary, Environmental Science & Technology 46 (2012) 3085-3092. [CrossRef]
- I.T. Burke, C.L. Peacock, C.L. Lockwood, D.I. Stewart, R.J.G. Mortimer, M.B. Ward, P. Renforth, K.
- Gruiz, W.M. Mayes, Behavior of Aluminum, Arsenic, and Vanadium during the Neutralization of Red Mud Leachate by HCl, Gypsum, or Seawater, Environmental Science & Technology 47 (2013) 6527-6535. [CrossRef]
- Gelencsér, N. Kováts, B. Turóczi, Á. Rostási, A. Hoffer, K. Imre, I. Nyirő-Kósa, D. Csákberényi-Malasics, Á. Tóth, A. Czitrovszky, A. Nagy, S. Nagy, A. Ács, A. Kovács, Á. Ferincz, Z. Hartyáni, M. Pósfai, The Red Mud Accident in Ajka (Hungary): Characterization and Potential Health Effects of Fugitive Dust, Environmental Science & Technology 45 (2011) 1608-1615. [CrossRef]
- Gupta, VK, Sharma, S. Removal of Cadmium and Zinc from Aqueous Solutions Using Red Mud, Environmental Science & Technology 36 (2002) 3612-3617. [CrossRef]
- S. Ruyters, J. Mertens, E. Vassilieva, B. Dehandschutter, A. Poffijn, E. Smolders, The Red Mud Accident in Ajka (Hungary): Plant Toxicity and Trace Metal Bioavailability in Red Mud Contaminated Soil, Environmental Science & Technology 45 (2011) 1616-1622. [CrossRef]
- T.C. Santini, M.V. Fey, Spontaneous Vegetation Encroachment upon Bauxite Residue (Red Mud) As an Indicator and Facilitator of In Situ Remediation Processes, Environmental Science & Technology 47 (2013) 12089-12096. [CrossRef]
- E.C.D. Resende, C. Gissane, R. Nicol, R.J. Heck, M.C. Guerreiro, J.V. Coelho, L.C.A.d. Oliveira, P. Palmisano, F. Berruti, C. Briens, M. Schlaf, Synergistic co-processing of Red Mud waste from the Bayer process and a crude untreated waste stream from bio-diesel production, Green Chemistry 15 (2013) 496-510. [CrossRef]
- Silveira, N.C.G.; Martins, M.L.F.; Bezerra, A.C.S.; Araújo, F.G.S. Red Mud from the Aluminium Industry: Production, Characteristics, and Alternative Applications in Construction Materials—A Review. Sustainability 2021, 13, 12741. [Google Scholar] [CrossRef]
- Chandra, S. (1996-12-31). "Red Mud Utilization". Waste materials used in concrete manufacturing. pp. 292–295. ISBN 978-0-8155-1393-3.
- ASTM D6751-20a; License Agreement, Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM: Philadelphia, PA, USA, 2020.
- Patel, R.L.; Sankhavara, C. Biodiesel production from Karanja oil and its use in diesel engine: A review. Renew. Sustain. Energy Rev. 2017, 71, 464–474. [Google Scholar] [CrossRef]
- Zhang, Y. Stanciulescu, M, Ikura M (2009) Rapid transesterification of soybean oil with phase transfer catalysts. Applied Catalysis A: General 366: 176–183. [CrossRef]
- Huang, Y. Synthesis of biodiesel by phase transfer catalysis. Applied Mechanics and Materials Vols 291-294 (2013) pp 355-358. [CrossRef]
- Barbosa, SL, Rocha, ACP., Nelson, DL, Freitas, MS, Mestre, AAPF., Klein, SI, Clososki, G.C., Caires, FJ, Flumignan, DL, Santos, LK, Wentz, AP, Pasa, V.MD. and Rios, RDF (2022) Catalytic Transformation of Triglycerides to Biodiesel with SiO2-SO3H and Quaternary Ammonium Salts in Toluene or DMSO. Molecules 27: 953. [CrossRef]
- Martínez, G.; Sánchez, N.; Encinar, J.M.; González, J.F. Fuel properties of biodiesel from vegetable oils and oil mixtures. Influence of methyl esters distribution. Biomass Bioenergy 2014, 63, 22–32. [Google Scholar] [CrossRef]
- Chandran, K.; Kamruddin, M.; Ajikumar, P.K.; et al. (2006). "Kinetics of thermal decomposition of sodium methoxide and ethoxide". Journal of Nuclear Materials. 358 (2–3): 111–128. Bibcode:2006JNuM. 358..111C. ISSN 0022-3115. [CrossRef]
Scheme 1.
Mechanism of PTA combining FAME.

Figure 1.
Cetyltrimethylammonium chloride structure.

Table 1.
FAs composition of the WCO used in the present work.
| Fatty acid | Molecular Weight (g.mol-1) | wt% |
|---|---|---|
| Palmitic acid (C16:0) | 256.43 | 10.41 |
| Stearic acid (C18:0) | 284.48 | 3.91 |
| Oleic acid (C18:1) | 282.46 | 26.52 |
| Linoleic acid (C18:2) | 280.45 | 51.66 |
| Linolenic acid (C18:3) | 278.43 | 5.55 |
| Others | - | 1.95 |
| Average Molecular Weight of Fatty Acids (g.mol-1) | 277.41 | |
| Molar mass of TGs (g.mol-1) | 873.22 | |
Table 3.
Composition of the WCO feedstock and the product of transesterification of TGs from WCO with MeOH using CSHS as the catalyst in the presence of fabric softener and product of transesterification of TGs from WCO with MeOH using CSHS as catalyst without fabric softener.
Table 3.
Composition of the WCO feedstock and the product of transesterification of TGs from WCO with MeOH using CSHS as the catalyst in the presence of fabric softener and product of transesterification of TGs from WCO with MeOH using CSHS as catalyst without fabric softener.
| CSHS without fabric softener | CSHS with fabric softener | |||
|---|---|---|---|---|
| Assay | Result (% w/w) |
Standard deviation | Result (% w/w) |
Standard deviation |
| FAME | 98.50 | 3.10 | 99.52 | 2.94 |
| Free glycerol | 0.01 | 0,01 | 0.01 | 0.01 |
| Total glycerol | 0.19 | 0.07 | 0.07 | 0.07 |
| Monoacylglycerol | 0.76 | 0.27 | 0.26 | 0.26 |
| Diacylglycerol | 0.23 | 0.12 | 0.06 | 0.12 |
| Triacylglycerol | 0.03 | 0.26 | 0.01 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Using Waste Concentrated Sodium Hydroxide Solution From Aluminum Production and Fabric Softener As Phase Transfer Agent in the Environmentally Friendly Biodiesel Synthesis
SANDRO LUIZ BARBOSA DOS SANTOS
et al.
,
2023
Environmentally Friendly New Catalyst Using Waste Alkaline Solution from Aluminum Production for the Synthesis of Biodiesel in Aqueous Medium
Sandro Luiz Barbosa
et al.
,
2023
Upgrading/Deacidification of Organic Liquid Phase by Liquid-Liquid Extraction Using Methanol/Water as Solvent
Nélio Teixeira Machado
et al.
,
2024
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated











