Preprint
Review
Utilization of Jackfruit (Artocarpus heterophyllus L.) Waste towards Sustainable Energy and Biochemicals: Attainment of Zero Waste Technologies
Altmetrics
Downloads
493
Views
107
Comments
0
A peer-reviewed article of this preprint also exists.
# Equal Contribution
This version is not peer-reviewed
Abstract
Valorization of food and fruit wastes has the potential for the production of sustainable energy and biochemicals. Approximately 70% of the weight of the original jackfruit (Artocarpus heterophyllus L.) fruit is lost during processing as waste in the form of peeled skin and core, both of which have not been utilized and, thus contribute to disposal as well as pollution issues. The major components, cellulose, and hemicellulose, can be biologically transformed easily into bioenergy sources like ethanol, methanol, and butanol; valuable phenolics and biotechnological products like pectin, citric acid, bromelain, ferulic acid, and vanillin; and many other products. These residues can also be utilized as essential sources for the biological transformation process leading to the production of numerous products with added value, such as phenolic antioxidants, phenolic flavor compounds, and organic acids. Thus, the value addition of jackfruit waste can support the sustainable solution towards food and nutritional security. In this way, zero waste can be achieved through novel biorefineries which are critically highlighted in this paper. Furthermore, novel technologies for the conversion of jackfruit wastes are summarized with recent findings.

Keywords:
Subject: Chemistry and Materials Science - Food Chemistry
Highlights
- Reports on diversity of jackfruits cultivation and uses in food/ diet nutrients.
- Discussed jackfruit wastes generation and also valorization methods for utility.
- Valorization techniques reported for value‐added products with zero‐wastes generation.
- Bioactive compounds and bioenergy generation from jackfruit wastes was targeted.
- Sustainable way of bioproducts generation, promotes biorefinery/ clean environment.
1. Introduction
Jackfruit (Artocarpus heterophyllus L.) tree is known to produce the huge fruits from its stems and it is unique in term of food utilization as vegetables and fruits. It is native plant and can be found in rain-forests especially in Western Ghats. People are well known about jackfruits and it was derived from Malayalam as chakka name. In India, it is grown/ cultivated at low elevation regions through all the states. Several wastes are generated from ripe fruits and this fruit waste is more palatable than wastes from raw fruit [1]. Jackfruit has shown the various nutrients like crude protein (CP~7.9%); crude fibre (CF~14.1%), calcium (0.8%) and phosphorus (0.1%). Except these nutrients, wastes from ripe and raw jackfruit is also utilized as potential substrates for energy production and nitrogen free radical (NFE~ 65%). The rind of the ripe fruit is good sources for cattle foods and this waste of jackfruit is non-edible portion (~59.2%) like perianth meal, rind and core meal. This waste matter is utilized for total dry meal recovery (11.6%) [1,2]. Some analysis was done jackfruits waste compositions (for perianth meal, rind and core meal) that contained ash (6 to 7.5%), carbohydrates (20-29%), crude protein (8- 10.6%), crude fate (1.7 to 7.3%) and crude fibres (12- 17.3%). Some studies were done utilization of jack fruits wastes and these are used for food, feed and other industries application. Some non-edible portion of jackfruit (as peels and axis) is reported with edible products (seed) and still these waste matters are underutilized at worldwide like Bangladesh, India or others countries [3]. Different waste portions of jackfruits are generated during juicy edible bulbs. Thick peels of jackfruits can be utilized for different valuable products generation such as biofuel, non-porous adsorbent and nutrient enriched cattle feeds. Normally non-porous adsorbent is used in removal of dye. Peel and central axis of the jackfruit is also utilized for pectin extraction [4]. And its seed power is reported to be used in various bakery products. From this fruit seed power, starch and protein fraction was isolated and then it was utilized in its purified form for food formulations at industries level [1,3,4].
Jackfruits have been analysed during different seasons by various workers [4,5,6,7] for their nutritional and antioxidant properties. The findings of these studies indicate that jackfruits serve as a valuable source in the development of nutraceuticals, which are currently in high demand worldwide. [5]. Jackfruits in different seasons are reported to differ in phytochemicals like phenolics, terpenoids, steroid, glycosides, saponins, alkaloids and tannins and these compounds are known to possess antioxidant properties. Diversity in secondary metabolites in jackfruits is reported and it is due to variation in functionally, nutritionally and medically important jackfruits wastes [4,5]. From jackfruit wastes, antibacterial and antioxidant activity agents/ compounds can be evaluated from the extracts that were obtained from methanol extract jackfruit leaves and stem barks and then it is applied as a peel-off mask. From extraction of those properties from jackfruit wastes, first raw material macerated using the methanol agent and then filtrates were evaporated for some time to get concentrated crude extract [6]. Then this extract was evaluated by different tests such as phytochemistry screening and also antibacterial tests on propionibakerium acnes and Staphylococcus aureus at different concentration of extracts using the DPPH (a,a-diphenyl-β-picrylhydrazyl) method [7].
For best evaluation of these phytochemicals, some additional test was done that can prove the characteristics of peel-off mask such as homogeneity, pH, organoleptic and irritation test. Some phytochemical screenings have proved for domination of tannin and saponin in their extracts [6,7]. In context to jackfruits wastes, these are rich sources of carbohydrates, protein, fats and phytochemicals. For these organics extraction or utilizations as a promising feedstock for valuable bioproducts including fuels/ chemicals synthesis. And several natures of pretreatments (like biological, physical,and chemicals including green solvents) were applied as effective valorization strategies for jackfruits waste matters [8]. The implementation of these strategies has facilitated the transformation of waste into products that possess added value, including but not limited to bioethanol, biogas, bioplastic, feeds, and functional compounds/food additives. The utilization of jackfruit waste for bioenergy production and recovery represents a promising avenue for sustainable and eco-friendly food waste-based renewable resources. This approach offers an economically feasible alternative to non-renewable fossil fuels [9].
Further efforts were done on efficient bioconversion tasks/ techniques, applied for jackfruits that can generate/ produce the valuable biomaterials/ chemicals and it is only to be achieved via microbial fermentation process. This conversion can help to get sustainable products with mitigation of jackfruits generation/ accumulation with support to a green environment. Some reports claimed the utility of jackfruit peels for the remediation of dye color from contaminated aquatic environments [8,9]. The implementation of said technology has the potential to facilitate the creation of an environmentally sustainable economic framework centered around the repurposing of waste materials. Many studies have carried out utilization of jackfruit waste for the production of value-added products, with the ultimate goal of mitigating waste generation and promoting environmental sustainability. In an attempt to utilize jackfruit waste, the production of plastic from jackfruit seed starch has also been carried out. The resulting plastic was strengthened through the incorporation of microcrystalline cellulose (MCC) derived from cocoa pod husks, with glycerol serving as the plasticizer [10]. This study aimed to identify the optimal mass and volume of microcrystalline cellulose (MCC) and glycerol concentration for the production of bioplastics in high yield. Before the bioplastic production, MCC, bio-production was done with cocoa pod husks, and this husk was subjected to a pretreatment task with the help of alkali agents, bleaching and HCl acid solution to get the effective hydrolysis [11].
During this effort, degree of crystallinity of MCC determination was done with help of analytical technique like XRD (X-ray diffraction), with functional groups determination by FTIR (Fourier-transform infrared) and also morphological properties analysis by scanning electron microscopy (SEM). Some researchers have gone on results for isolated MCC from pod husks and they discussed it as rod-like form with respective length (5-10µm) and diameter (11.63nm) with high crystallinity [10,12]. From isolated MCC utility in bioplastic synthesis, tensile property of bioplastic was determined at starch to MCC mass ratio (8:2). Further tasks were done in addition to 20% glycerol with measured tensile strength (0.637) and good elongation (at break of 7.04%). From analytical measurement by FTIR spectroscopy for bioplastic functional groups studies, it was found for more numbers of -OH groups at bioplastics and it was reinforced with filler MCC, with representation of hydrogen bond [10,13].
A thorough screening of literature reveals that there is a very limited understanding to how jackfruit waste can contribute to sustainable energy, and help attain zero waste. To ensure sustainable future for all by 2030, United Nations has developed blue prints through its various sustainable development goals (SDGs), and SDG 7 encourages sustainable utilization of bioresources in increasing the share of renewable energy in the global energy mix, and to provide sustainable energy services to all countries. During the past decades, efforts are continuously being made to use cleaner and greener technologies to utilize various bioresources, and thus novel technologies are being continuously implemented. In this review we aimed to (a) report the approaches/methods used in conversion of jackfruit waste to sustainable energy, and biochemical, (b) analyse the findings of various studies, and advances made in the field, (c) compare the advantages, limitations, and drawbacks of various technologies, and (d) the new trend of jackfruit waste as bio-absorbent for the benefit of pollution control.
2. Database Formation and Analysis
For developing this review we searched published articles dealing with jackfruit cultivation, nutrient values of its seeds, composition of jackfruit wastes, types of wastes, valorization techniques/strategies (physical, biological and chemical including green solvent), novel techniques/green extraction techniques for bioactive recovery, microbial fermentation for jackfruit waste conversion, value added products from jackfruit wastes (bioethanol, biogas, bioplastic, bioactive compound like phenolics, carotenoids, flavonoids, tannins, antioxidant etc.) from journals on ISI web of science, google scholar to create database. All the downloaded papers were thoroughly reviewed, and filtered to extract the pre-treatment methods employed and diversity of value added products derived from jackfruit peels, rags and other wastes. Besides, we examined the conversion technologies of fruit wastes, and their practical implementation, cost effective measures and limitations in the global context. Further, the potential of jackfruit waste conversion in the context of increasing renewable energy share to global energy mix, environmental safeguards are discussed in the paper.
3. Results and Discussion
3.1. Cultivation of Jackfruits with Impact to its Nutrients
Due to more demand for waste mitigation and also extraction of valuable products with bioactive properties, there is more jackfruit cultivation reported all over India with some other countries such as Burma and Malaysia with some locations in Brazil. It is now one of the remunerative and also more valuable fruits in India. This jackfruit tree belongs to the Moraceae family and is also native tree of India [14]. This plant is now cultivated throughout the tropical low land at both hemispheres. This jackfruit plant is widely grown in Western Ghats of India but its plantation is found in Bihar, West Bengal, Uttar Pradesh, Kerala, Tamil Nadu, Assam and Orissa. Some reports claimed its regular plantation in the U.P., especially marginal orchards. But in other parts this plant cultivation is reported as rare in plantations but jackfruit cultivation is found throughout South India up to an elevation of 2400 meters [15]. Some reports have discussed the jackfruit plantation in systematic and proper ways and this plant growth needs rich and well drained sandy loam soils. For this plant plantation, soil drainage showed more importance with proper evidence. Sudden decline of numerous jackfruit plants in areas that are suffering a sudden rise of water level [14,15]. Reports on jackfruit tolerance capacity especially for moisture stress was shown and it can be shown to some extent of tolerance due to presence of lime and chlorine Areas near the river beds can be found to be ideally suitable for jackfruit plants cultivation. Further information on jackfruit plantation is a warm humid plain and it is able to flourish in humid hill slopes up to an elevation of 1500 meters. Jackfruit can deteriorate at higher altitudes with satisfactory plantations in arid and warm plains of South India [16].
This jackfruit can provide unlimited scope for clone selection of promising strain/ species for multiple applications. Many types of these plants are available under various local names and it can be originated via clone selection. Gulabi, Champa, Hazarix are some examples of jackfruits. It needs storage conditions for jackfruit plantation. The impact to thick peels is found with good storage quality [17]. Jackfruits can be easily gone for cross pollination and then its seeds can be found to easily propagate. To date, the prevailing population of jackfruits is distributed across numerous trees, exhibiting variations in their morphological characteristics such as shape, size, and density of tubercles, as well as differences in rind color, bulb size, fiber content, and fruit quality and maturity [15,17]. The storage longevity of jackfruit has been determined to be approximately 6 weeks when exposed to temperatures ranging from 0.1 to 12.70 C, provided that the humidity level is maintained at eighty to ninety per cent. The initial quality and maturity stage at the time of harvest are crucial factors that can significantly impact the storage life of a product. The jackfruit's storage life can be extended, allowing for transportation to remote locations for marketing purposes, through the utilization of appropriate packaging and wrapping techniques [16,17].
Reports for jackfruit application in India are found as culinary and also table fruits. And jackfruit used for culinary purposes is mainly found in more states in India. In market demand, tender fruits in spring and also summer are used as population vegetables. People have enjoyed jackfruits with high demand and premier cost/ price and sometimes this jackfruit cost/ price can be reached at a high rate in the year [18]. Most people in the world used jackfruits in ripe form as tasty fruits with high sweet and also high nutritive values. In ripe form, jackfruits are good sources of vitamins A and C with some people believe in aiding in digestion process as ailments on a regular basis [17,18].
3.2. Compositions of Jackfruits Wastes
The waste produced from mature jackfruit fruits has been found to possess greater palatability compared to the waste generated from raw fruits. This waste material is composed of crude fiber, crude protein, and various minerals. It is also a significant source of energy, with a substantial quantity of NFE. The generation of rind from ripe fruit can serve as a source of nourishment for cattle [19]. Additional applications of jackfruit-derived fruits have been documented for the production of pickles, dehydrated leather, and thin papad, as well as for canning purposes. These fruits have also been utilized in conjunction with soft drinks such as nectar and squash. In certain literature, rind has been identified as a viable source of protein. Additionally, it has been reported that extracts derived from rind waste can be utilized in the fabrication of jelly [20]. Discussion of skin parts of fruits of jackfruit is reported for excellent cattle feed. Jackfruit plant/timber can be used for making furniture that can exhibit less chance of white ants infection/ attacks. Some efforts were done for latex extraction that contains resin. Latex from jackfruit bark can be used for making the plug hole in earthen vats and baskets and it has shown multiple applications for humankind [19,20].
Further, multiple reports are discussed for jackfruits parts (in terms of edible and non-edible parts) and non-edible parts are 70-80%, and out of this, outer rind, perianth, and central core of jackfruits is 60% under waste matters. A number of researches were done on biochemical composition by suitable analysis in jackfruits-based wastes with promising sources of health benefits. These bioactive compounds (i.e., valuable compounds/bioproducts) can be recovered/ generated by several microbial bioprocessing/extraction techniques in an eco-friendly way [21]. The peel of jackfruit is a rich source of cellulose, protein, starch, and pectin. The chemical composition of dried jackfruit seeds includes carbohydrates (76%), protein (18%), and lipids (2.1%). Numerous phytochemicals, isoflavones, lignin, and saponins, along with several essential nutrients, have been documented in the seeds of jackfruit waste [22]. Subsequent discourse revealed noteworthy sources of vitamins such as thiamine and riboflavin. The processing of jackfruit generates solid waste, including jackfruit peels, seeds, as well as latex. These waste products have been identified as contributors to environmental damage [22,23]. Researchers have put some effort into jackfruit wastes and they used eco-friendly feedstock/ sources for bioproducts synthesis in sustainable ways and this waste matter has shown the best biochemical compositions, helping in renewable mode bio-based products development. Further research has been conducted on the utilization of jackfruit peels as a sustainable source for the recovery of commercial pectin, biofuels, and other valuable products [24,25]. And Figure 1 shows several form of jackfruits wastes with nutrients.
Various types of Jackfruits Wastes
Wastes generation from various types of vegetable and fruits/cultivation processes are discussed and a fruit can generate usually 3.5 to 10 kg of waste by weight and some fruits can generate higher quantities of waste (up to 25 kg). In the case of jackfruit plants, the outer parts of the jackfruit shell are found to contain a conical apex, covered via a thick robbery wall. And its fruits matured conditions, a non-edible core can be found on a longitudinal axis, fused with the rags [26]. This part is then fused to the fruit’s rind part. Next, other wastes of jackfruit are bulbs and this bulb is composed of pulp, surrounded by seeds and it is found between the rags. Seeds numbers in jackfruits are nearly 100 to 500 and it can be found in nearly 18 to 25% of the weight of jackfruits. The kernel of the seeds can constitute nearly 90 to 95% of their weight and then pulp can account for 30% and it can be found between 70 to 80% of jackfruits components as non-edible [27]. In non-edible parts of fruits, outer rinds, perianth, and central core can consist of 60% of the total wastes of jackfruits as discarded parts. Jackfruit peels and its conical carpel apices are also important components of jackfruit wastes. Apart from this waste, jackfruit leaves can be also used for some medical benefits such as relieving fever, boils, wounds, and skin conditions [26,27]. And then young fruits of jackfruit can exhibit acrid and astringent properties and characteristics and it can work on removing the flatulence in human health. The jackfruit seeds can be found as rich sources of protein concentrations (5 to 6%). In each tree of jackfruit, nearly 100 to 200 fruits can be produced as it is a large and evergreen tree every year. Still time, very limited research was done on unutilized wastes of jackfruits and then peels and fiber found nearly 60% of whole fruits as the largest known edible fruits from any plants. The jackfruit pulp and seeds are known to contain various bioactive compounds in addition to waste nutrients. The availability of these compounds in the fruit of this plant can vary, as reported in a previous study [28]. The cultivation of this plant species is feasible during the monsoon season in coastal areas. The fruit of this plant is considered a cost-effective source of sustenance and is widely accessible at a low market value. Subsequent investigations were conducted on underutilized segments of jackfruit, with a focus on mitigating the accumulation of biowaste to promote the eradication of pathogenic microorganisms in augmented agricultural yields [29]. The nutritional and functional characteristics of jackfruit peels warrant further investigation for the purpose of extracting bioactive compounds that may be utilized in the pharmaceutical industry [29,30]. Figure 2 shows the different jackfruit wastes with its valorized products.
The outer layer of jackfruit is comprised of peel which exhibits a spiky pattern on its surface. The peel of jackfruit is considered non-edible and is regarded as a potential waste material. Normally this part of fruit is discarded and then recently it is used for fertilizer sources. These parts of jackfruit can be used to feed the cattle in villages. It is a good source of carbohydrate (up to 24%), protein (up to 9%) and fibers (17.3%) [31]. Jackfruit peels are utilized as valuable raw materials for functional ingredients like steroid, triterpenoids, saponin and carbohydrates. In jackfruit peel, more quantities of polyphenols are reported and it is linked to peel proximity with extrinsic domains. Some abiotic stress parameters/ factors (such as daylight, ultraviolet radiation and climatic induction) can impact the synthesis of polyphenol on the complete part of jackfruit peel [32]. Other bioactive compounds in antioxidants can aid the chemical reactions in the human body and then it helps to offset the damages, mediated by oxidation reactions. Pectin content in jackfruits is reported to contain 9 to 15% of dry weight and it can make it a valuable source of the polysaccharides [31,32]. Extraction of various compounds is also used in the textile, paper and biofuel sectors with rich sources of celluloses. This can provide the alternative options to commercialized celluloses in pharmaceutical industries [33,34].
3.3. Valorization Techniques for Zero-waste Generation
In current scenarios, a number of researches are going on the zero-waste generation concept and many strategies (i.e., various valorization routes) are applied to achieve it. This was achieved on bread waste (BW) as the model development. But, there are a number of challenges (i.e., technical processing steps), occurring in various kinds of food wastes like jackfruits waste also. Normally, any type of food wastes hydrolysis including the jackfruits wastes can be done by enzymatic hydrolysis with assisting of physical and chemical agent processes. These approaches can generate monomeric compounds like glucose/ others sugar [35]. These sugars from jackfruit wastes can be utilized for cultivation of suitable microbial systems with capability to use as carbon substrates and also efficiency to metabolite into fuels sources like ethanol/ butanol/ other bioproducts. One example is shown for BW valorization strategy with uses of Euglena gracilis algae cultivation medium with systematic evaluation [36].
This algal cultivation was found to future perspectives and also economic viability for biodiesel sources production. In context to valuable products except biodiesel, other targeted compounds like paramylon (β-1, 3-glucan) were synthesized from E. gracilis with high productivity of 1.93 g.L-1d-1 and this productivity was 24% higher than control strains. In this product synthesis, people have applied the approaches of zero-waste disposal and then bread waste residues (BWR) was hydrolysed by enzymatic hydrolysis process and later it was valorized into syngas [35,36]. It was offered by a greener pyrolysis process for BWR and carbon dioxide was used as raw material for valuable products synthesis. CO2-assisted valorization is an attractive technique not only towards efficient waste disposal, but in attaining to climate neutral or zero carbon emission. In reports, several types of agricultural wastes (licluding jackfruit wastes) are found to utilize as potential raw materials for the generation of valuable products like biofuels, biochar or also biopesticides with briquettes or others. Then biochar can be mixed with the soils and it can produce the carbon rich soils via contribution in carbon dioxides sequestration and soil fertility. So, jackfruit wastes can be anaerobically utilized for production of biogas, briquettes and also biochars that can improve the crop production [37]. And other bioprocess, jackfruit waste can be gone for anaerobic decomposition into biogas and also for briquette production. But at high temperature, these waste (jackfruit waste) can decompose to produce biochar and then it can apply into the soil. This is good approach for carbon dioxide sink with helping in mitigation of effect of climatic change. It can find for anaerobic digestion process for jackfruit waste utilization for biogas production. This process has shown the economic viability for bioproducts generation [36,37].
Researchers have applied a mechanistic functionality process for carbon dioxide (CO2) with systematic description. In this process, CO2 was reacted with volatile matter (VMs) that evolved from BWR and it has helped to reduce the concentration of carbon dioxide. And it was done with proceeding to oxidation of VMs. Further process (like consecutive gas-phase-reaction ~GPR) was done with exhibiting a critical role in enhanced CO formation [38].
Research endeavors were undertaken to identify the induction of gas-phase reactions (GPR) through the utilization of carbon dioxide (CO2). A five per cent by weight (wt%) nickel/silicon dioxide (Ni/SiO2) catalyst was employed for the purpose of studying the reaction kinetics involved in the process of mitigating CO2. During the aforementioned procedure, the gaseous pyrolytic products, namely H2 and CO2, were acquired in terms of molar concentration [39]. The catalytic pyrolysis process (Ni/SiO2) in the CO2 environment has resulted in a greater quantity of syngas, which is 2 and 6 times higher, correspondingly, compared to pyrolysis without the catalyst in the N2 environment [35,40]. This study explores various valorization techniques that can be applied to the green extraction process. The aim is to develop innovative delivery systems, such as nano-emulsions, for bioactive compounds derived from fruit and vegetable waste, including jackfruit waste.. And Figure 3 shows the complete jackfruit plants with edible and non-edible parts that later utilized as wastes and then converted into value-added products with zero-waste generation.
3.4. Advanced/ Green Extraction Techniques for Bioactive Recovery
In the valorization processes, researchers have applied various types of novel extraction techniques such as enzyme-assisted, microwave-assisted, ultrasound-assisted, high-hydrostatic pressure-assisted, pulsed-electrical field, and super-critical fluid extraction methods to derive various bioactive compounds that are used by food, pharmaceutical, cosmetic, and health-care industries [32,34]. These non-conventional green extraction techniques have the potential to zero waste, and thereby helping in sustainable energy conservation. The recent researches have also shown that various biofuels can be produced through microbial fermentation. Some examples of biofuels (like bioethanol up to 11-13%; biogas including methane via A.D process) with help of Saccharomyces cerevisivae are produced through microbial fermentation [41,42] . Similarly, microbial fermentation has been effectively demonstrated to yield other valuable products like bioactive compounds and other products [43]. Researchers have done sincere efforts in developing novel and efficient techniques that can recover the bioactive active compounds without any solvent contamination. Enzyme-assisted and pressurized liquid-assisted extraction are explored for jackfruit wastes applicable to other wastes that can recover the valuable products [44]. Microwave-assisted and ultraviolet-assisted extraction techniques have been employed to derive the pectin polysaccharides and antioxidant phenolics respectively [45,46,47],. Pulsed electric field-assisted and supercritical fluid-assisted techniques are also exploited for various natures of wastes valorization tasks that have recovered the bioactive compounds at high yield/ concentration [48]. Among the various green modes of extraction techniques, their effectiveness can be varied with properties of the source matrix, its chemical structures and also process parameters/ factors (solvent, pressure, time or temperature [43,48].
Novel technologies can provide alternatives to conventional techniques of extraction of bioactive waste from various food wastes and these techniques are known to use water as solvent rather than organic chemicals. These techniques showed the positive impacts for phytochemicals, scattering inside the cytoplasm [49]. Normally, hydrogen or hydrophobic bonds presence in the polysaccharide-lignin network/ complex can be found to be big challenges due to exhibiting difficulty in extraction of bioactive compounds [50]. So these green techniques can be found to have a sustainable and eco-friendly nature with capability to achieve higher yield of the products compared to conventional ones. And these techniques have received more attention in the last few years due to more advantages [49,50]. Some techniques in context to green/ non-conventional mechanisms are discussed below.
In the enzyme-assisted extraction (EAE) process, enzyme concentration, compositions, particle size, water to solid ratio and also hydrolysis time plays an important role and it can influence the yield / concentration of bioactive compounds. Some compounds like carotenoid extraction (from pumpkin waste), anthocyanin from (Crocus sativus/ grape fruit waste) were done via the EIE process [51,52]. Some bioactive compounds like phenolics (18-20 mg/g) were extracted from grape mare seed wastes with help of pectinase enzyme activity [53]. Other efforts were done for antioxidant phenol extraction from apple pomace by using the commercial enzyme Pectinex Ò® and then phenolics recovery (p to 87%) from grape residues using Celluclast Ò®. EAE based technique is very effective to enhance the recovery of enzymes like pectinase, cellulases and pectinases from jackfruit wastes [53,54].
The second extraction method is known as ultrasonic-assisted extraction (UAE), and it has a number of uses and benefits including a larger yield, desired quality, and a straightforward procedure with minimal impact on the environment. The UAE approach uses the ideal frequency range (20-2000 kHz) and is well renowned for being both straightforward and inexpensive. It can be effective at two different facts, such as diffusion over the cell wall and washing the contents following cell disintegration [55]. Due to wave creation of matrix expansion and compression, the UAE operating mechanism causes/generates cavitation phenomenon. The desired compounds are then extracted by causing the cell membrane to become permeable [56]. The researchers investigated the many processes of the UAE process, including the acceleration of mass transfer, the disintegration of the particles, and the improvement of solvent accessibility. For this technique, samples of liquid-liquid or liquid-solid processing are frequently employed. Pressure, temperature, frequency, and sonication duration are other parameters that might affect the UAE process [55,56]. Recent research on the UAE revealed the widespread use of this procedure and shown its effects on yield and compound characteristics. Some excellent instances of UAE frequency have been documented for energy at or above 20 kHz, which changes the physical-chemical characteristics of phytochemicals by causing the production of free radicals [57]. Figure 4 established the sophisticated processing methodologies employed for the extraction of bioactive compounds.
Some efforts on tannin extraction from Avaram shell is reported by application of UAE technique and it uses 100 W power. UAE based process has shown its impact in improved yield (160%) of tannins at 100W. This improvement in yield of tannin was explored and it was found due to improved mass transfer of cell components and a way of leaching of tannin by this power [58]. Others bioactive compounds like good yield (caffeic acid~64.3 µg/g, ferulic acid~ 1513 µg/g and p-coumaric acid~ 140 µg/g) of phenolic acids by UAE technique are reported with better improvement compared to conventional techniques (maceration extraction) for same bioactive from same wastes [59]. Temperature and prolonged time impact on UAE are found in the form of low yield of phenolic compounds from citrus peels. Some comparative studies were done on maceration and UAE technique performances in terms of consumed/ required time period for extraction of bioactive. And it was found to be a reduced time period (1h) for UAE technique compared to maceration assisted extraction (72h) for phenolic compounds from Punica granatum fruits. Extraction of polysaccharides by UAE technique is found with good yield with proving of efficient technique [57,58,59].
Another technique is pulse electric field-assisted extraction (PEF-AE) and its non-thermal process allowing direct current to produce. In this technique, application of high-voltage current/ pulse is passed through the materials that are kept/ placed between two electrodes for a short time period ( in range of microsecond to millisecond periods). These are based on the nature of products and also processing factors. During electric current passages through the suspension of cells, it can influence cell structures to be destroyed and then molecules can separate with respect to applied charge [60]. This technique can function in a batch and also in continuous modes. For this technique performance, various factors like field strength, energy, pulse number, temperature and material properties can affect/ influence the yield of extraction and it can be worked based on designing a process for better performance. Application of PEF assisted extraction can be found in phenolic compounds and also anthocyanins extraction process from different wastes [61]. PEF assisted extraction with maceration on grape skin can be applied for stability of compound during vinification and it can reduce the time of extraction. Some studies were done on untreated control samples along with PEF treated samples with maceration extraction process/ technique [60,61]. And it has shown improved color and also anthocyanin content/ yield with enhanced polyphenol contents in wastes like jackfruit. Further impact of PEF treatment can be found in the wind making process with reduced maceration time and also improved wine quality [63,64,65,66]. The cytomembrane in plant tissue's cell walls can influence the movement of intracellular material between cells. This approach for extracting bioactive substances is intriguing because it may trigger the cytomembrane in the tissue to disintegrate, which changes its permeable characteristics as well as increases mass transfer across the cells, leading to higher yields [64,65].
In context to advanced/ green extraction techniques, utilizes the electromagnetic radiation and it is transferred in the form of waves in the frequency range (300MHz- 300 GHz) with common uses of frequency of 2450MHz. This can be found to be equivalent to 600 to 700 W energy and this energy can be absorbed during the passage of microwave through suitable medium. This medium converts it into thermal energy via facilitating the processing [66,67]. Some bioactive compounds like flavonoids from Terminalia bellerica plant were reported by using this technique and microwave in MAE technique can result in maximum yield (83%) and it is higher yield while compared to conventional techniques (flavonoid yield~64%). MAE technique is an endothermic and spontaneous process and it is also influenced by operating conditions like temperature and feed ratio on flavonoid yield. Same extraction was applied for hesperidin compound extraction with better yield (48%) from skin of citrus unshiu fruits [68,69]. During this technique extraction temperature showed high impact on bioactive compound yield and at 140oC, it showed decreased hesperidin quantity/ content due to the interference of the other solubilized substance. This compound influence has been found in inhibition of hesperidin crystal [67,69]. Other conditions on waste matter maturity level in case of peels, matured peels have yielded less hesperidin contents compared to immature peels (more than 3 times). Some impacts of power in MAE technique is reported and it was found for phenolic compounds extraction from chokeberries. Better yield of phenolic compounds (420.1 equivalent mg gallic acid/100g. chokeberries) is reported at 300W for 5 min [70,71]. Extraction of silibinin from Silybum marianum waste with help of MAE technique is discussed with better yield (97.3%) and it is higher than conventional approaches. Similar study was done on this technique efficiency and it was reported for phenolic compounds from apple pomace wastes [72]. This compound extraction yield from this technique is influenced by several factors/ parameters such as solubility, dielectric constant, dissipation factor (d) and solvent nature. The higher recovery of flavonoid by MAE (up to 74%) is reported and it is better than traditional recovery / extraction process (up to 70.5%) with proof of efficient process [73,74].
During the supercritical fluid extraction process (SC-FE), desired compound extraction is carried out by using the solvent above the critical point (CP) and this CP can be found as a specific temperature (Tc) or pressure (Pc) point. But as above to CP, gas and liquid cannot exist as separate phases [75]. At CP, fluids/ solvents can exhibit the liquid (in terms of density) and salvation power/ gas (viscosity, diffusion and surface tension). These properties can facilitate the higher yield of bioactive compounds within a short time. In case of supercritical fluid extraction (SFE), there is a need for a good mobile tank (consisting of CO2 pump), solvent vessel, oven, controller and also a trapping vessel [75,76]. Now most of bioactive extraction is done by application of green technologies compared to conventional methods and in this context, supercritical CO2 extraction is discussed with better yield of naringin from citrus paradise. This approach uses the ethanol as a modifier (14% by wt.) at same process conditions like 58.6oC temperature and 9.5 MPa pressure [77].
This technique is applied for phenolic compound extraction from rice wine lees wastes with uses of Soxhlet extraction (SE) and SFE. Then it was compared for yield of bioactive compounds with reduced extraction (1 h compared to 6 h in case of traditional extraction technique) using the less ethanol needed with better yield of phenols (43%) [78]. In the process of SFE, carbon dioxide is a common solvent, used in food sector tasks and it is safe with easily attainable critical conditions (at 30.9 and 73.8 bar) for food processing. Some major limitations such as low polarity are disclosed but it can be improved by using polar solvents like methanol. Ethanol, dichloromethane and acetone) [77,78]. These can work as modifiers with capability to improve its solvating power and also enhance its extraction efficiency with minimum/ no interaction between analytes and matrices. Some parameters like low diffusibility of the solvent into matrix, extended extraction time, high-pressure requirement and expensive infrastructures can also be found some challenges for this technique [76,78]. Further, consistency and reproducibility during the continuous process can be found as some more limitation of this extraction technique and these can stop scalability of this technique [77,79].
3.5. Microbial Fermentation for Jackfruits Waste Conversion
For better utilization of jackfruits wastes, it needs several types of effective pretreatment process and it can be applied as physical, chemical and biological processes to hydrolyse its complex organic matter. These strategies help in valorization of jackfruits wastes into value-added products including fuels (bioethanol or biogas,)/ other bioproducts (bioplastic, feeds, or functional food additives) [80]. From jackfruit wastes, nowadays, bioenergy production and promotion is also carried out by several researchers groups and it can utilize the jackfruit waste as renewable resources and it is also an eco-friendly and cost-effective process to generate the alternative fuels options against fossil fuel [81]. In recent years, efficient bioconversion of jackfruit wastes into several types of fuel sources is done with the help of microbial fermentation process by using different microbes such as bacteria, yeast, fungi [74,75]. Yeast (like Saccharomyces cerevisivae for ethanol), bacteria (for methane, methanogen like Methanosarcina barkeri and Methanococcus maripaludis) and fungi (like Rhizopus oryzae MNT 006, Aspergillus oryzae MNT for ethanol) is reported for biofuel production [16,37,82]. And these efforts can help in reduction of environmental pollution and also help in bioremediation processes (i.e., several toxic dye removal) in water sources/ aquatic environments that were contaminated with dye color. Some review papers have addressed the color dye removal from water bodies with help of jackfruit waste uses with finding of utilization feasibility. This effort can solve the several serious ecological problems in jackfruit producing nations [81,83].
Several reports claim a waste generation quantity of jackfruit in the range of 5 to 7 kg wt. per fruit and this waste showed its potential for conversion into a wide range of bio-products such as biofuels, animal feeds or bioactive components and these are used in bakery and packaging material industries. In case of wastes (including jackfruit wastes also) materials hydrolysis, physical, chemical and biological pretreatments processes are applied and it helps in conversion into simple sugars and also in final production synthesis under effort of valorization [84]. Utilization of jackfruit wastes can result in several fuels products synthesis / production. These can be achieved by application of pretreatment and extraction steps via valorization of waste biomasses in an effective and successful manner. In recent years, numbers of vaporization technologies have developed for jackfruits waste hydrolysis and later these helped to conversion of desired products via utilization of several bioprocesses / green extraction steps and it can promote sustainable products utilization in the biorefinery and also the bioeconomic of the world [85]. Normally jackfruit is reported to contain 0- 80% non-edible parts and out of this quantity, 60% of jackfruit wastes is outer rind, perianth and central cores parts. Number of analyses for biochemical composition for jackfruit waste were done with utilization of these wastes with recovery of health benefits products [84,85]. The peels of jackfruits waste are good sources of protein, cellulose and pectin and then seed waste is good sources of carbohydrate (76%), protein (18%) and lipid content (2%). In context to bioenergy production from jackfruit wastes utilization, several types of pretreatment are applied as crucial steps with conversion capacity into complex forms of organic matter into simpler ones. Pretreatment step in the complex organic matter conversion process helps in enzymatic reactions of hydrolysis during the saccharification processes and this step can ensure the simple sugars form for fermentation via the help of different microbial agents [86]. Good example of jackfruit wastes conversion is found for ethanol production/ extraction process. This process utilizes the low-pressure and also high-intensity ultrasound processes that affect compositions and functionality of isolated proteins from jackfruit seeds [80]. Jackfruit waste is gone for several processing tasks by a variety of physical methods to develop the valuable products and some of these are irradiation, microwave processing, supercritical fluid and high pressure processing extraction as common and advanced processing methods [86,87].
In effort to hydrolysis of jackfruit wastes, several approaches/ methods of chemical treatment are applied in acidic or alkaline solution (low to high concentration) at temperature of 130oC and 210oC via mixing the waste matter. During the chemical agent assisted pretreatment process, waste matter is gone for a few minutes to few hours to get fermentable sugars and it can be dependent on pretreatment conditions [88,89]. Researchers have applied these processes for methane production, energy potential and also environmental benefits using jackfruit peels. The effect of methane generation via pretreatment of jackfruit wastes with 5% alkaline hydrogen peroxide (AHP) and it has shown its influence in production of enhanced methane yield and also biodegradability (up to 70%) while compared to untreated waste matter [90]. Some studies were done on analysis of potential of annual energy production from jackfruits, treated with 5% AHP solution. Alkaline extraction techniques were applied for isolation of starch from jackfruit seed wastes and then it has 18% starch from seeds [89,90].
During the conventional chemical and physical pretreatment process, high investment of reagents, machinery and energy is needed and in those treatment processes, it is needed before biological pretreatments. These can be applied for cellulosic and lignocellulosic matters for enzymatic saccharification processes [91,92]. During the biological treatment process, many living agents such as fungi/ bacteria are used and it utilizes less energy and is also an eco-friendly process. Many microbial agents are known to possess the cellulolytic and hemicellulolytics activities that can be utilized for jackfruits waste utilization task [93]. Among several microbial systems, Saccharomyces cerevisivae is applied for ethanol production from jackfruit wastes and ethanol contains the 35% oxygen contents and it can be utilized for burning process for production of lesser quantity of nitrogen and also particulate matters than the gasoline combustion process [90,93]. And Figure 5 discussed the pretreatment approaches for hydrolysis and also bioenergy development.
3.6. Value Added Products
In this context, huge quantity of various nature of fruits and vegetables wastes are generated due to different processing activities in food sector every year. And it can be excellent sources for different nature of valuable components like biofuels and polyphenols and these are utilized for different needs to human life [94]. Green extraction technology, often known as non-traditional approach, has been a significant subject of research in recent years [95]. These green technologies have replaced previous traditional methods due to their high yield, shortened process time, products of superior quality, and low waste creation [89,91]. During the pretreatment process, disruption of hydrogen bond is also found and it can occur easily after addition of chemical solvent and then it is heated at high temperature. This can cause dipole rotation of molecules and migration of ions. This changes in matter can occur with diffusion of the solvent and it can lead to the dissolution of the components [96]. Another mechanism during the pretreatment process is reported as evaporation of the moisture within cells and it can induce the high pressure on the cell wall and then change the physical properties of the materials [97]. This can lead the modification in porosities of biomaterials. This can occur with increased penetration of solvents with improved yield of biomaterials [98,99]. Various types of bioproducts from jackfruit wastes are discussed in below section.
3.7. Bioethanol
In context to value-added products recovery from jackfruit wastes, it has discussed bioethanol production from enzymatic hydrolysis and microbial fermentation of cellulosic biomass including jackfruit wastes and it is cost-effective and eco-friendly technique/ approach [100]. In this fuel synthesis process, jackfruit wastes like peel material are utilized as potential feedstock and this waste matter contains high quantities of carbohydrates contents/ %. Numbers of studies were done for saccharifying jackfruit rind matter via application of recombinant enzyme endoglucanase from Bacillus subtilis strain MUS1 microbes at temperature of 50oC and pH 5.0 with 15 mg/ ml substrates ratio/ quantity 101]. This recombinant enzyme from Bacillus subtilis can help in generation of more quantities of sugars during the saccharification process of jackfruits wastes [41,100]. From different types of wastes, bioethanol is produced as alternative energy sources. These wastes are good sources of natural products like carbohydrates and then it is utilized as potential feedstock for ethanol production with the hydrolysis and fermentation process. In this context, jackfruit seed is the best feedstock with rich sources of carbohydrates [101]. Number of research studies are done on determination of pH effect on carbohydrates hydrolysis that can be utilized for bioethanol production. For hydrolysis of carbohydrates from jackfruit seed, effective pretreatment with a pH dependent process was done by application of a separate fermentation hydrolysis (SHF) process [102].
This process has used the sulphuric acid solution as hydrolysing agents. Later, the fermentation process with help of Saccharomyces cerevisiae strain was used in a fermentor vessel at different pH values (such as 2, 3 and 5) for 70h period. From this experiment results, it was claimed for optimum glucose content (75%) and pH (3.0) that supported the high concentration of bioethanol (58%) in fermentation broth [101,102,[101,102,]. From this research work, it has discussed the fermentation stage role and it was found that high concentration of glucose can push the high concentration of bioethanol with linear relationship [103]. This published paper talked about the glucose concentration and high ethanol concentration from jackfruit seeds waste. This jackfruit seed showed the high potential for feedstock for bioethanol biosynthesis at cheap price [101,103].
Bioethanol production from fermenting raw matters like jackfruit waste is reported with the suitable microbial system. During the fermentation process, ethanol production jackfruit wastes is reported with application Saccharomyces cerevisiae strain. This yeast strain showed high capability for ethanol production and it is due to natural adaptation properties and also high tolerating sugar/ ethanol/ chemical inhibitor [104]. Ethanol can be synthesized by petroleum product/ by fermentation process. In the biological process, cellulose or hemicelluloses from jackfruit wastes is hydrolysed and then it is utilized for the ethanol production process. Most lignocellulosic biomass including jackfruit waste is rich sources of carbohydrates and then it needs the effective pretreatment process for fermentative sugars that can be used in ethanol production. Later, product separation and purification process is also needed to get the pure ethanol [101,104]. For this bioethanol production, jackfruit peels were selected as potential substrates and then enzyme/ microbial system (Saccharomyces cerevisiae) was used for getting the fermentative sugars and ethanol. Now, ethanol can be present in alcoholic drinks with more uses of this yeast in the bakery industry and fermented food and alcoholic drink preparation/ production [103,104]. Some efforts were done on jackfruit straw waste and then it was processed with fermentation as starter substrates. From this process, bioethanol was separated by a distillation process. Starter mass and fermentation time was checked to achieve the maximum ethanol yield with utilization of yeast like S. cerevisiae and urea as N- nutrients [105].
At optimum yeast mass (40g) of S. cerevisiae with distillate volume of 13.6 ml. It has been found after 96 h of fermentation rime, distillate / ethanol yield was 15.2 ml. But at optimal condition of fermentation, volume of bioethanol distillate was found to be 30 ml with the distillation temperature at 70-80oC. From this approach for ethanol production, its refractive index (1.354), density (0.367 g/ ml) and boiling points (71-72oC) was reported [106]. Another report, bioethanol production is discussed from utilization of Sri Lankan rotten fruits (without skin) including with jackfruits wastes. This ethanol was produced in a batch process with optimization of fermentation process parameters [106,106]. In optimization of fermentation process, some optimization techniques like Genetic algorithm (GA), Response Surface Methodology (RSM) and also Particle Swarm optimization (PSO) were discussed. During the bioethanol production, different overripe fruits were taken and these fruits were jackfruit, papaya and banana with uses of two microbes at three fermentation conditions. During these experiments, maximum ethanol yield with RSM (13.4 vol. %), GA (13.4 vol. %) and PSO (13. 36 vol. %) by the use of banana variety fruit fermentation and Pseudomonas mendocina microbial strain (ratio~ 1:1), pH (5.1) and temperature (35oC) [107,108].
3.8. Biogas
Biogas generation from different types of jackfruit wastes is a good effort for sustainable fuels sources. In this context, potential of biogas production is found from different fruit wastes like banana peels, jackfruit wastes, and pineapple wastes and these were gone for co-digestion process with cow drug and then this effort helped to provide alternative energy sources [109]. During these experiments, substrates from each fruit waste were sent to the co-digestion process with varying ratios (0%, 25%, and 50%) of cow dung. These were done in laboratory scale anaerobic digesters (up to capacity of 250 ml) and then it was run for 30 days to generate or produce the biogas from jackfruit (82.3 ml), banana fruit (189 ml) and also pineapple fruit waste/ peel (262 ml) is found [110]. In another experiment of this study, jackfruit waste, pineapple waste and banana peels were co-digested with 25% of cow dung and then biogas production from these fruits was increased by two to three times [110,111].
And 50% of cow dung with these fruits waste has improved the biogas yield by two folds. From these reported results, a mixture of jackfruit, banana peel and pineapple peel can be found much better in biogas production yield and it can help in the energy supply chain process for our daily needs [111]. During the biogas generation experiment, some efforts were done on experimental design, digester set-up, and volume and biogas composition determination that was produced from jackfruit waste, banana and pineapple peels with cow dung. And biochemical methane potential (BMP) assay protocol was applied for anaerobic digestion process [112]. Evaluation process for biogas quality attributes was done for process that used the from jackfruit waste, banana and pineapple peels with cow dung with batch digestion process. In this experiment, an anaerobic system was found to be 500 ml capacity and it was submerged in a 20 litre temperature regulating water bath and a 250 ml measuring cylinder for the generated biogas measurement task via displacement method. Temperature of the water bath was maintained at 36.5oC [109,112].
In context to value added products recovery, utilization of jackfruits was reported and it discussed the production of biogas, biochar and briquettes from jackfruit waste. In many developing countries, huge potential is found due to more quantity of organic waste accumulation and then it can be utilized for conversion into many types of fuel sources like biogas generation [37]. Waste organic matters in rural areas due to small holder farmers can be generated and then these can be utilized for different nature of biofuel sources. In context, biomass wastes like jackfruit waste can be managed to produce the bioenergy with mitigation in GHGs (greenhouse gasses) emission quantity via a well-managed way [113]. Now, decomposing of organic matter can be minimized via generation of biogas. Due to more quantities of agricultural waste like jackfruit waste can be utilized as cheap raw materials for production of bioproducts like biofuels, biochar and biopesticide with briquettes and others [114]. Biochar production from waste matter is good effort and then biochar can be mixed with the soils and it can help to produce soils rich in carbon with contribution in carbon dioxide sequestration and soil fertility [115]. Some papers have focussed on jackfruit waste utilization for biogas production from anaerobic digestion process with biochar and briquettes production. From the anaerobic process for jackfruit wastes utilization, biogas can be produced [110]. It needs a high temperature for jackfruit waste decomposition tasks to help in biochar production. This effort of research can help various products synthesize information with help in mitigation of climatic changes and also carbon dioxide sink in soil [37,115]. And Figure 6 discusses the jackfruit wastes with different microbial / chemical transformation approaches for many products synthesis to support the zero-waste generation.
3.9. Bioplastic
Bioplastic production from Bacillus megaterium strain JHA is reported with high capacity of this microbial stain and this microbe was isolated from oil contaminated soils and then it was tested in laboratory on glucose substrates consumption that can result in high quantity of polyhydroxyalkanoates (PHA) accumulation in this microbes [116]. This plastic was biosynthesized by Bacillus megaterium with utilization of jackfruit waste like seeds with some inorganic or organic matter/ compounds. This waste matter was found suitable for high accumulation of PHA inside microbial cells. Some additional efforts were done on characterization of the bioplastic layer that was formed inside the cell due to PHA [117]. Several viable applications of PHA were found in medical science, cosmetic and pharmaceutical products synthesis. For best synthesis of PHA, advanced research is needed with optimization of process parameters and it can promote huge quantities of PHA from cheaper feedstock like jackfruit wastes. This waste matter is good sources of carbon and nitrogen with finding an economically viable process for commercial scale [116,117]. Further PHA product is biodegradable plastic and showed it is an effective and durable process. In effort to jackfruit waste hydrolysis, biological pretreatment is a promising and eco-friendly process for complete conversion into fermentable sugars [118]. Biological pretreatment needs optimal process parameters to achieve complete hydrolysis of waste biomass. The effect of pretreatment like physical and chemical processes can find the impact of enzymatic conversion of waste biomasses into simple ones with achieving bioproducts synthesis [117,118]. And Table 1 discusses the more example of bioenergy/ other value-added products
3.10. Bioactive Compounds
Pectin used in the food sector and pharmaceutical / cosmetics sectors is reported to serve as various agents like emulsifiers, binders and stabilizers) with performing various functions [33]. Polysaccharides sources in jackfruit peels can help in human diet and then it can maintain good immunity activity with some protective functions like cancer, blood sugar, ulcer, and bad cholesterol [34]. In pharmaceutical industries, pectin is utilized as bonding agents for various formulations of pills and also multi-purposes delivery. In food sectors, use of jackfruit peels is used in papers, paints, optics and also environmental remediation with biofuel sectors. In case of phenolic compound yield from orange peel, improvement in yield (15%) was achieved by PEF at power of 7KV/cm 62].
Reports on greater yield of phenolic compounds (102.9 mg GAE/100g food waste) and flavonoid compounds (37.6% QE/100g FW) from various wastes, such as onion waste, are described in this approach, with superior yield improvement (2.2 and 2.7 times, respectively) compared to control samples. The effects of electric field intensity and extraction duration on phenolic and flavonoids compound yields are demonstrated [63]. Nowadays, efforts are being made in the food processing sector to produce nanoemulsions, which can be useful in the delivery system for various bioactive substances capable of performing various functions. These actions boost bioavailability, regulate ingredient discharge, alter product texture, as well as preserve the substance from degradation [98]. In this technique, intensive research is going on to make it more effective in terms of a broad range of bioactive extraction from any type of wastes including jackfruit waste. For the nanoemulsion process, the delivery system can proceed for bioactive compounds with understanding of specific functions especially in food-based delivery systems and bioavailability in the human body. Some recent studies are done for understanding mechanisms on desirable bio-accessibility, metabolism and absorption of the encapsulated compounds and these can help in alteration in their properties in the gastro-intestinal tract [99].
Further studies were done loading capacity of bioactive compounds that are encapsulated form like nanoemulsion, exhibiting the better release properties of bioactive compounds [98,99]. Cultivation of jackfruit plants in the world is good and potential sources of valuable biomaterials and wastes from jackfruit plants is a good source of carbohydrates, fats, protein and also phytochemicals [97,99]. In the case of bioactive compounds from wastes of fruits/ vegetable sources, it showed a positive impact on human health via contributing to modulation of the metabolic processes and also cellular activities [94]. Some bioactive compounds showed their properties of antioxidants, anti-cancer, anti-inflammation and anti-allergenic. Some compounds contribute to anti-atherogenic activity and these properties of bioactive compounds can depend on the pathways and also their bioavailability in the human body. In categories of bioactive compounds, some are hydrophobic in nature and they showed less bioavailability in the human body [95]. In this context, some efforts have been made on technological advancement such as nano-emulsions application. And this effort helped in enhancing their stability and functional properties. Bioactive substances can be obtained via traditional and non-traditional methods, each with its own set of benefits and drawbacks [94,95]. And Table 2 shows the bioactive compounds with health benefits from jackfruit wastes.
4. Advantages, Limitations and Drawback of Various Valorization Techniques
The valorization techniques are basically two types (conventional, and novel-based). The technique preferred will depend on the parts of the fruit plants to be converted to useful bioactive compounds, mostly based on moisture content and composition of food waste to avoid energy-intensive processes. For an example, jackfruits leaves, barks, fruits can be easily valorized through various conventional methods such as maceration, percolation, decoction, reflux and through soxhlet extraction to develop various medicine for health care needs, while other bioactive compounds extraction need employment of advanced technologies [94,95,96,97]. The sustainability of valorization process is of paramount importance in efficient reduce, reuse and recycle, and in transitioning to circular bioeconomy. The end-use products derived from the fruit waste, and the scale of production also need to be looked into the environmental, ecological and social prospectives. The green technology/novel technologies employed are always more eco-friendly than the conventional approaches [86,89].
Application of efficient bioconversion of jackfruit wastes (like peels) can generate varieties of useful material by facilitation by microbial fermentation process and this material can be applied for reduction of water pollutant (like dye/ color removal under bioremediation) in contaminated aquatic system. This is good example of green economic model that is used for waste utilization [8]. Numbers of researches have claimed for utilization feasibility of jackfruit wastes and then these were converted into value-added products via reducing/ mitigating the waste quantity with protection of environment in a sustainable modes. Huge quantities of jackfruit wastes is generated due to modern practices and now interests have developed to convert it into varieties of applications and it is due jackfruit waste capabilities that keep diverse array of functions [119]. As discussed in earlier section about jackfruit waste nutrients amounts and varieties. This waste is good sources of carbohydrates, protein or mineral and these nutrients are utilized for cultivating the diverse groups of microbial system/ group that can be produced the various natures of metabolites like organic acids, polysaccharides, enzymes, or therapeutic compounds [8,119]. In conversion processes of jackfruit wastes, different type of pretreatments or bioprocesses like physic-chemical, biological and innovative green pretreatment method are discussed [88]. Further it requires/ needs to investigation of more genetically and biotechnologically advanced methods that can utilize the complete fractions of jackfruit wastes and then it converted to 100% into different products in direct ways with more functional natures. Further, it needs more researches on advances in technology, equipment, methodologies that can make necessary changes with promotion to biorefineries with generation of varieties of value-added products [8,88].
5. Prospect of Jackfruit Waste as a Bio-absorbant for Pollution Control
Recent studies have shown that the jackfruit peel is an effective low-cost bio-absorbant for removal of chromium and nickel from aqueous solution () . Besides, since jackfruit waste are rich source of pectin, they can be useful for economic removal of cadmium from water bodies/water subjected various chemical pollution [30]. Various reports have claimed for jackfruit leaves (JLP) utilization as important wastes and it is suitable agro-waste categories based materials and then it can utilize for efficient removal of metal (like lead~Pb –II form) from wastewater. Due to surface medication of jackfruit leaf power (JLP), it can able to remove high % of lead concentration [120]. Further the surface modification of jackfruit leave was achieved by application of chemical reagents like isopropyl alcohol (20%) and then followed by treatment with alkali sodium hydroxide (AIJLP) and tartaric acid (TIJLP). These modifications were confirmed by BET surface area (29 m2/g). And then chemical modification was resulted by 1.5 fold high/ increase and 2.5 fold increase in surface area due to AIJLP (50 m2/g) and TIJLP (72 m2/g) impacts [121]. After generating the low cost bioabsorbents from JLP, it can applied in batch experiment to confirm the lead adsorption and then it was put effort to find the optimal equilibrium conditions like pH, contact time and lead concentration. After that from developed bioabsorbsent, pore size and pore volume were also analysed by Barrett-Joyner-Halenda (BJH) model/ methods [120,121]. And this model has helped the adsorption mechanism. Recently by reusability studies it was confirmed that AIJLP can efficiently remove the lead contaminant/ pollutant up to 95% up to five cycles. And these results have recommendation of application of AIJLP for bioremediation task in wastewater [122]. Due to uses of low cost material like JLP from jackfruit leaves and later it was modified by chemical reagents to increase the surface area and it was checked for its cost (up to 11 USD/ kg) and it included the cost of collection of raw material, washing and then drying process and in last chemical reagents were used to get it final form. Some miscellaneous cost was also added. This is good examples of low cost bioadsorbesnt from jackfruit leaves [122,123].
6. Conclusions and Future Perspectives
This paper mainly focussed on jackfruit waste as a potential and sustainable source in augmenting energy requirement, and meeting the global challenges. There are plenty of this resource available in the world for sustainable conversion of jackfruit waste to a diverse bioactive compounds. Jackfruit trees can be found grown in wide ecological regions, and they can easily be raised as plantation with minimum agrocare. We found that huge quantities of jackfruit wastes are generated from many countries in the world and now it becomes a big challenge to utilize these wastes sources from the environment. Long-term accumulation and disposal to aquatic bodies can become a big threat to aquatic animals with the creation of water pollution. To avoid these issues, now researchers have applied the many approaches of valorization to recover biofuel/ other biochemicals in sustainable manners. These efforts can help in promotion of zero-waste generation from complete utilization of jackfruit wastes with production of bioactive compounds. These efforts in value-added products from jackfruit wastes can be found as cheap sources of feedstock with reduced price of bioproducts. In coming periods, how many green techniques like MAE, UAE and SCF-AE are applied with green extraction solvents to get more effective bioproducts with high yield compared to traditional technique. Different forms of jackfruit wastes can be mitigated in systematic manners via synthesis of a number of value-added products including cheap sources of adsorbent, nano-particles and also biogas and other biofuels. Energy sources from jackfruit wastes can be achieved by microbial fermentation with specific microbial cell systems and anaerobic digestion. Some application of biochar is discussed in the soil amendment process that increases the soil fertility and also carbon/ nitrogen content. This review can help to provide several ideas for sustainable fuel development with the maintenance of a clean environment. In this review, authors discuss the different natures of jackfruit wastes sources and then these were analysed/ studied for nutrients determination. Later different valorization techniques like green extraction, preparation of bioactive materials approaches, microbial fermentation were applied to generate/ recover the nutrients/ bioproducts. These conversion approaches are very effective to generate 100% products from these wastes without any further waste left in environment. This is good strategies to achieve the zero-wastes in different conversion process. Our objectives for this review was achieved due to mitigation of jackfruit wastes/ recovery of nutrients. These efforts are resulted into sustainable products development with zero-wastes in process.
Abbreviations
AgNO3: Silver nitrate; AgNPs: Silver nanoparticles; AHP: Alkaline hydrogen peroxide; ASPE: Artocarpus heterophyllus seed powder extract; BMP: Biochemical methane potential; BWR: Bread waste residues; CF: Crude fibre; CP: Critical point; CP: Crude protein; DPPH: a,a-Diphenyl-β-picrylhydrazyl; EAE: Enzyme assisted extraction; FTIR: Fourier-transform infrared; GA: Genetic algorithm; JSP: Jackfruit seed powder; MAE: Microwave-assisted extraction; MCC: Microcrystalline cellulose; NFE: Nitrogen free radical; Pc: pressure point; PEF-AE: Pulse electric field assisted extraction; PHA: Polyhydroxyalkanoates; POJ: Peel of jackfruit; PSO: Particle Swarm optimization; RSM: Response Surface Methodology; SC-FE: Supercritical fluid extraction process; SE: Soxhlet extraction; SEM: scanning electron microscopy; SHF: Separate fermentation hydrolysis; Tc: Specific temperature; UAE: Ultrasonic-assisted extraction; XRD: X-ray diffraction
References
- Thanh, L.P.; Truong, P.; Kha T.; Hang TT.T.; Jackfruit leaves can totally replace traditional grass in the diet of lactating dairy goats, J. Appl Animal Res. 50(1), (2022) ,97-102. [CrossRef]
- Ranasinghe, R.A.S.N.; Maduwanthi, S.D.T.; Marapana, R.A.U.J. Nutritional and Health Benefits of Jackfruit (Artocarpus heterophyllus Lam.): A Review. Int. J. Food Sci. 2019, 2019, 1–12. [CrossRef]
- Chavan, S.; Yadav, B.; Atmakuri, A.; Tyagi, R.; Wong, J.W.; Drogui, P. Bioconversion of organic wastes into value-added products: A review. Bioresour. Technol. 2021, 344, 126398. [CrossRef]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of Agro-Waste into Value-Added Bioproducts and Bioactive Compounds: Micro/Nano Formulations and Application in the Agri-Food-Pharma Sector. Bioengineering 2023, 10, 152. [CrossRef]
- Sreeja Devi, P.S.; Kumar, N.S.; Sabu, K.K. Phytochemical profiling and antioxidant activities of different parts of Artocarpus heterophyllus Lam. (Moraceae): A review on current status of knowledge. Futur J Pharm Sci. 7 (2021), 30. [CrossRef]
- Siregar, A.B.; Bulan, R.; Yusak, Y. Antibacterial & antioxidant properties of leave & stem bark extract of Artocarpus heterophyllus as the component of peel-off mask. Int J Sci Technol Eng. 5(4), (2018), 101–106.
- Brahma, R.; Ray, S. A Comprehensive Review on the Recent Advances in the Valorization of Jackfruit Waste for the Development of Value-Added Products. J. Food Technol. Res. 2022, 9, 120–134. [CrossRef]
- Sundarraj, A.A.; Ranganathan, T.V. Physicochemical characterization of Jackfruit Peel. Res J Pharm Biol Chem S. 8, (2017) 2285–95. [CrossRef]
- Begum, R.; Aziz, M.G.; Yusof, Y.A.; Saifullah; Uddin, M.B. Evaluation of gelation properties of jackfruit (Artocarpus heterophyllus) waste pectin. Carbohydr. Polym. Technol. Appl. 2021, 2, 100160. [CrossRef]
- Lubis, M.; Gana, A.; Maysarah, S.; Ginting, M.H.S.; Harahap, M.B. Production of bioplastic from jackfruit seed starch (Artocarpus heterophyllus) reinforced with microcrystalline cellulose from cocoa pod husk (Theobroma cacao L.) using glycerol as plasticizer. IOP Conf. Series: Mater. Sci. Eng. 2018, 309, 012100. [CrossRef]
- Cagasan, C.U.; Li̇ngatong, C.A.V.; Pore, K.M.T.; Ramada, R.V.; Restor, C.D.D.; Lauzon, R.D. Production and Quality Evaluation of Wine from Jackfruit Co-Products. Int. J. Life Sci. Biotechnol. 2021, 4, 340–352. [CrossRef]
- Widjaja, C.; Djojorahardjo, Y.; Kurniawan, A Irawaty, W.; Soetaredjo, F.E. Biorefinery concept on jackfruit peel waste: bio-oil upgrading. ARPN J Eng Appl Sci. 13 (2018) 2202–7.
- George, N.; Debroy, A.; Bhat, S.; Singh, S.; Bindal, S. Biowaste to Bioplastics: An Ecofriendly Approach for A Sustainable Future. J Appl Biotechnol Rep. 8(3) (2021) 221-233. [CrossRef]
- Meera, M.; Ruckmani, A.; Saravanan, R.; Prabhu, R.L. Anti-inflammatory effect of ethanolic extract of spine, skin and rind of Jack fruit peel – A comparative study. Nat. Prod. Res. 2017, 32, 2740–2744. [CrossRef]
- Ojwang, R.A.; Muge, E.K.; Mbatia, B.; Mwanza, B.; Ogoyi, D.O. Comparative Analysis of Phytochemical Composition and Antioxidant Activities of Methanolic Extracts of Leaves, Roots and Bark of Jackfruit (Artocapus heterophyllus) from Selected Regions in Kenya and Uganda. J. Adv. Biol. Biotechnol. 2017, 16, 1–13. [CrossRef]
- Soumya, B.; Gupta, P.; Vikas, R.; Pradeep, K. .Chemoenzymatic saccharification of Artocarpus heterophyllus Lam.(jackfruit) peel waste and its utilization for bioethanol production. Indian Forest, 145(12) (2019)1204-1209.
- Kahar, A.W.M.; Lingeswarran, M.; Hulwani, M.Z.A.; Ismail, H. Plasticized jackfruit seed starch: a viable alternative for the partial replacement of petroleum-based polymer blends. Polym. Bull. 2018, 76, 747–762. [CrossRef]
- Marichelvam, M.; Manimaran, P.; Sanjay, M.; Siengchin, S.; Geetha, M.; Kandakodeeswaran, K.; Boonyasopon, P.; Gorbatyuk, S. Extraction and development of starch-based bioplastics from Prosopis Juliflora Plant: Eco-friendly and sustainability aspects. Curr. Res. Green Sustain. Chem. 2022, 5, 100296. [CrossRef]
- Ahmed, M.; Nasar, A. Utilization of Jackfruit Peel as a Low-cost Adsorbent for the Removal of Methylene Blue Dye from Synthetically Polluted Water. Curr. Anal. Chem. 2021, 17, 1016–1026. [CrossRef]
- Butool, S.; Butool, M. Nutritional quality on value addition to jack fruit seed flour. Int J Sci Res. 4, (2015) 2406–11.
- Sundarraj, A.A.; Ranganathan, T.V. Phytochemical constituents and thin-layer chromatography evaluation of the ethanolic extract of jackfruit (Artocarpus integer) peel. J Pharm Res. 12(5), (2018) 717.
- Pathak, N.; Singh, P.; Singh, P.K.; Sharma, S.; Singh, R.P.; Gupta, A.; Mishra, R.; Mishra, V.K.; Tripathi, M. Biopolymeric nanoparticles based effective delivery of bioactive compounds toward the sustainable development of anticancerous therapeutics. Front. Nutr. 2022, 9, 963413. [CrossRef]
- Bhat, V.; Mutha, A.; Dsouza, M.R. Pharmacognostic and physiochemical studies of Artocarpus heterophyllus seeds. Int J ChemTech Res. 10(9), (2017) 525–536.
- Sundarraj, A.A.; Ranganathan, T.V. Phytochemical screening and spectroscopy analysis of jackfruit (Artocarpus integer Thumb. peel. Int Res J Pharm. 8(9) (2017) 151–159.
- Jagtap, U.B.; Bapat, V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crop. Prod. 2013, 46, 132–137. [CrossRef]
- Nur Hanani, Z.A.; Aelma Husna, A.B.; Nurul Syahida, S.; Nor Khaizura, M.A.B.; Jamilah, B. Effect of different fruit peels on the functional properties of gelatin/polyethylene bilayer films for active packaging. Food Packag. Shelf Life 2018, 18, 201–211. [CrossRef]
- Islam, M.R.; Haque, A.R.; Kabir, M.R.; Hasan, M.M.; Khushe, K.J.; Hasan, S.M.K. Fruit by-products: the potential natural sources of antioxidants and α-glucosidase inhibitors. J. Food Sci. Technol. 2021, 58, 1715–1726. [CrossRef]
- Jain, R.; Mendiratta, S.; Kumar, L.; Srivastava, A. Green synthesis of iron nanoparticles using Artocarpus heterophyllus peel extract and their application as a heterogeneous Fenton-like catalyst for the degradation of Fuchsin Basic dye. Curr. Res. Green Sustain. Chem. 2021, 4, 100086. [CrossRef]
- Jancy, S.; Shruthy, R.; Preetha, R. Fabrication of packaging film reinforced with cellulose nanoparticles synthesised from jack fruit non-edible part using response surface methodology. Int. J. Biol. Macromol. 2019, 142, 63–72. [CrossRef]
- Kalse, S.; Swami, S. Recent application of jackfruit waste in food and material engineering: A review. Food Biosci. 2022, 48. [CrossRef]
- Mahesh, R.; Surendrababu, K.; Kumar, S.S.; Anoop, C.T.; Nuhaiz, V.P. Emission characteristics of jackfruit peel oil with three hole nozzle. IOP Conf. Series: Mater. Sci. Eng. 2020, 993, 012010. [CrossRef]
- Mardarveran, P.; Mokhtar, N.M. Analysis of Artocarpus heterophyllus peel as a natural coagulant using response surface methodology (RSM). J. Technol. 82 (4) (2020). [CrossRef]
- Brahma, R.; Ray, S. In-depth analysis on potential applications of jackfruit peel waste: A systematic approach. Food Chem. Adv. 2022, 1. [CrossRef]
- Sundarraj, A.A.; Ranganathan, T.V.; Gobikrishnan, S. Optimized extraction and characterization of pectin from jackfruit (Artocarpus integer) wastes using response surface methodology. Internat. J. Biologic. Macromolec. 106, (2018) 698-703.
- Jung, J.-M.; Kim, J.Y.; Kim, J-H.; Kim, S.M.; Jung, S.; Song, H.; Kwon, EE. Choi, Y.-E. Zero-waste strategy by means of valorization of bread waste. J Cleaner Product. 365 (2022), 132795. [CrossRef]
- Zhang, C.; Kang, X.; Wang, F.; Tian, Y.; Liu, T.; Su, Y.; Qian, T.; Zhang, Y. Valorization of food waste for cost-effective reducing sugar recovery in a two-stage enzymatic hydrolysis platform. Energy 2020, 208. [CrossRef]
- Nsubuga, D.; Banadda, N.; Kabenge, I.; Wydra, K.D. Potential of Jackfruit Waste for Biogas, Briquettes and as a Carbondioxide Sink-A Review. J. Sustain. Dev. 2020, 13. [CrossRef]
- Zhang, C.; Ling, Z.; Yang, L.; Liu, Y.; Cao, T.; Sun, Y.; Liu, W.; Huo, S.; Zhang, Z.-H.; Su, H.; et al. Efficient caproate production from ethanol and acetate in open culture system through reinforcement of chain elongation process. J. Clean. Prod. 2023, 383. [CrossRef]
- Wu, Q.; Bao, X.; Guo, W.; Wang, B.; Li, Y.; Luo, H.; Wang, H.; Ren, N. Medium chain carboxylic acids production from waste biomass: Current advances and perspectives. Biotechnol Adv. 37(5), (2019), 599-615. [CrossRef]
- Daud, M.N.H.; Ahmad, R.; Abdullah, N.; Jabit, M.L.; Wibowo, A. Effect of storage on antioxidant activity and bioactive compound of Artocarpus heterophyllus J33 rind extract. Adv Appl Chem Biochem. 2019,1, 68–81.
- Nuriana, W.; Wuryantoro Ethanol Synthesis from Jackfruit (Artocarpus Heterophyllus Lam.) Stone Waste as Renewable Energy Source. Energy Procedia 2015, 65, 372–377. [CrossRef]
- Ginting, M.H.S.; Irvan; Misran, E.; Maulina, S. Potential of durian, avocado and jackfruit seed as raw material of bioethanol: a review. IOP Conf. Series: Mater. Sci. Eng. 2020, 801. [CrossRef]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioprocess. 2019, 6, 26. [CrossRef]
- Štambuk, P.; Tomašković, D.; Tomaz, I.; Maslov, L.; Stupić, D.; Kontić, J.K. Application of pectinases for recovery of grape seeds phenolics. 3 Biotech 2016, 6, 1–12. [CrossRef]
- Pathak, N.; Singh, S.; Singh, P.; Singh, P.K.; Singh, R.; Bala, S.; Thirumalesh, B.V.; Gaur, R.; Tripathi, M. Valorization of jackfruit waste into value added products and their potential applications. Front. Nutr. 2022, 9, 1061098. [CrossRef]
- Lal, A.N.; Prince, M.; Kothakota, A.; Pandiselvam, R.; Thirumdas, R.; Mahanti, N.K.; Sreeja, R. Pulsed electric field combined with microwave-assisted extraction of pectin polysaccharide from jackfruit waste. Innov. Food Sci. Emerg. Technol. 2021, 74. [CrossRef]
- Jiang, Z.; Shi, R.; Chen, H.; Wang, Y. Ultrasonic microwave-assisted extraction coupled with macroporous resin chromatography for the purification of antioxidant phenolics from waste jackfruit (Artocarpus heterophyllus Lam.) peels. J. Food Sci. Technol. 2019, 56, 3877–3886. [CrossRef]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, environment-friendly and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [CrossRef]
- Azooz, E.A.; Ridha, R.K.; Abdulridha, H.A. The Fundamentals and Recent Applications of Micellar System Extraction for Nanoparticles and Bioactive Molecules: A Review. Nano Biomed. Eng. 2021, 13. [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [CrossRef]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: a review. Int. J. Food Sci. Technol. 2018, 54, 1–13. [CrossRef]
- Nishad, S.; Saha, C. Kaur, Enzyme- and ultrasound-assisted extractions of polyphenols from Citrus sinensis (cv. Malta) peel: A comparative study. J Food Proc Preserv. 43 (8) (2019) 1-13. [CrossRef]
- Wan, N.; Kou, P.; Pang, H.-Y.; Chang, Y.-H.; Cao, L.; Liu, C.; Zhao, C.-J.; Gu, C.-B.; Fu, Y.-J. Enzyme pretreatment combined with ultrasonic-microwave-assisted surfactant for simultaneous extraction of essential oil and flavonoids from Baeckea frutescens. Ind. Crop. Prod. 2021, 174, 114173. [CrossRef]
- Rafińska, K.; Wrona, O.; Krakowska-Sieprawska, A.; Walczak-Skierska, J.; Kiełbasa, A.; Rafiński, Z.; Pomastowski, P.; Kolankowski, M.; Buszewski, B. Enzyme-assisted extraction of plant material – New functional aspects of the process on an example of Medicago sativa L.. Ind. Crop. Prod. 2022, 187. [CrossRef]
- Li, H.-Z.; Zhang, Z.-J.; Xue, J.; Cui, L.-X.; Hou, T.-Y.; Li, X.-J.; Chen, T. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants and rosmarinic acid from perilla leaves using response surface methodology. Food Sci. Technol. 2016, 36, 686–693. [CrossRef]
- Shen, X.; Shao, S.; Guo, M. Ultrasound-induced changes in physical and functional properties of whey proteins. Int. J. Food Sci. Technol. 2016, 52, 381–388. [CrossRef]
- ivković J, Šavikin K, Janković T, Ćujić N, Menković N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep Purif Technol 194 (2018) 40–47.
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT 2018, 101, 342–350. [CrossRef]
- Júnior, M.E.S.; Araújo, M.V.R.; Santana, A.A.; Silva, F.L.H.; Maciel, M.I.S. Ultrasound-assisted extraction of bioactive compounds from ciriguela (Spondias purpurea L.) peel: Optimization and comparison with conventional extraction and microwave. Arab. J. Chem. 2021, 14, 103260. [CrossRef]
- Carpentieri, S.; Jambrak, A.R.; Ferrari, G.; Pataro, G. Pulsed Electric Field-Assisted Extraction of Aroma and Bioactive Compounds From Aromatic Plants and Food By-Products. Front. Nutr. 2022, 8, 792203. [CrossRef]
- Pef-assisted Supercritical CO2 Extraction of Pigments from Microalgae Nannochloropsis Oceanica in a Continuous Flow System., 74, 97–102. [CrossRef]
- Pataro, G.; Carullo, D.; Siddique, A.B.; Falcone, M.; Donsì, F.; Ferrari, G. Improved extractability of carotenoids from tomato peels as side benefits of PEF treatment of tomato fruit for more energy-efficient steam-assisted peeling. J. Food Eng. 2018, 233, 65–73. [CrossRef]
- Pataro, G.; Bobinaitė, R.; Bobinas, Č..; Satkauskas, S.; Raudonis, R.; Visockis, M.; Ferrari, G.; Viskelis, P. Improving the Extraction of Juice and Anthocyanins from Blueberry Fruits and Their By-products by Application of Pulsed Electric Fields. Food Bioprocess Technol. 2017, 10, 1595–1605. [CrossRef]
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viskelis, P.; Ferrari, G. Application of pulsed electric field in the production of juice and extraction of bioactive compounds from blueberry fruits and their by-products. J. Food Sci. Technol. 2015, 52, 5898–5905. [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Obanijesu, E.O.; Alara, J.A.; Mudalip, S.K.A. Extract-rich in flavonoids from Hibiscus sabdariffa calyces: Optimizing microwave-assisted extraction method and characterization through LC-Q-TOF-MS analysis. J. Food Process. Eng. 2019, 43, e13339. [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2019, 51, 138–149. [CrossRef]
- Simić, V.M.; Rajković, K.M.; Stojičević, S.S.; Veličković, D.T.; Nikolić, N.Č.; Lazić, M.L.; Karabegović. I.T. Optimization of microwave assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Sep Purif Technol. 160 (2016) 89–97.
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2017, 6, 189–196. [CrossRef]
- Vilariño, M.V.; Franco, C.; Quarrington, C. Food loss and Waste Reduction as an Integral Part of a Circular Economy. Front. Environ. Sci. 2017, 5. [CrossRef]
- Alara, O.R.; Abdurahman, N.H. Microwave-assisted extraction of phenolics from Hibiscus sabdariffa calyces: Kinetic modelling and process intensification. Ind. Crop. Prod. 2019, 137, 528–535. [CrossRef]
- Alara, O.R.; Abdurahman, N.H., Adbul Mudalip, S.K. Optimizing microwave-assisted extraction conditions to obtain Phenolic compounds-rich extract from Chromolaena odorata leaves. Chem. Eng. Technol. 42(9) (2019), 1733-1740. [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Azhari, N.H. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crop. Prod. 2018, 122, 533–544. [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Alara, J.A. Optimization of microwave-assisted extraction of phenolic compounds from Ocimum gratissimum leaves and its LC–ESI–MS/MS profiling, antioxidant and antimicrobial activities. J. Food Meas. Charact. 2020, 14, 3590–3604. [CrossRef]
- Espinosa-Pardo, F.A.; Nakajima, V.M.; Macedo, G.A.; Macedo, J.A.; Martínez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod. Process. 2016, 101, 1–10. [CrossRef]
- Argun, M. E.; Argun, M. Ş.; Arslan, F.N.; Nas, B.; Ates, H.; Tongur, S.; Cakmakcı, O. Recovery of valuable compounds from orange processing wastes using supercritical carbon dioxide extraction. J. Cleaner Prod. 375, (2022), 134169. [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2018, 11, 12–17. [CrossRef]
- Dey, T.K.; Banerjee, P.; Chatterjee, R.; Dhar, P. Designing of ω-3 PUFA enriched biocompatible nanoemulsion with sesame protein isolate as a natural surfactant: Focus on enhanced shelf-life stability and biocompatibility. Colloids Surfaces A: Physicochem. Eng. Asp. 2017, 538, 36–44. [CrossRef]
- de Andrade Lima, M.; Andreou, R.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Carbon Dioxide Extraction of Phenolic Compounds from Potato (Solanum tuberosum) Peels. Appl. Sci. 11, (2021), 3410. [CrossRef]
- Constance, E. C., Ifunanya, W. U., Amarachi, M. C. E., Idera, E. E. C., Ikechukwu, N., Ogechi, P. C., & Perpetua, O. C.. Evaluation of vinegar production properties of Garcina kola and Artocarpus heterophyllus. J. Appl. Chem. Sci. Internat. 12(2) (2021), 48-60.
- Nguyen, T.K.; That, N.T.T.; Nguyen, N.T.; Nguyen, H.T. Development of Starch-Based Bioplastic from Jackfruit Seed. Adv. Polym. Technol. 2022, 2022, 1–9. [CrossRef]
- Ochaikul, D.; Noiprasert, N.;Laoprasert, W.; Pookpun, S.Ethanol Production on Jackfruit Seeds by Selected Fungi and Yeast from Loog-pang. KMITL Sci. Tech. J. 2012, 12 (1). 1-5.
- Miah, R.A.; Alam, M.J.; Khatun, A.; Suhag, M.H.; Kayes, N. The Decolorization and Phytotoxic Efficiency of Jackfruit Seed on a Textile Dye Novacron Blue. J. Eng. Adv. 2022, 6–11. [CrossRef]
- Abid, M.K.; Bin Ibrahim, H.; Zulkifli, S.Z. Synthesis and Characterization of Biochar from Peel and Seed of Jackfruit plant waste for the adsorption of Copper Metal Ion from water. Res. J. Pharm. Technol. 2019, 12, 4182. [CrossRef]
- Widjaja, C.; Djojorahardjo, Y.; Kurniawan, A.; Irawaty, W.; Soetaredjo, F.E. Biorefinery concept on jackfruit peel waste: bio-oil upgrading. ARPN J Eng Appl Sci. 13 (2018) 2202–7.
- C.P., S.; Sebastian, D. Jackfruit outer rind: A sustainable feedstock for fermentable sugar production using recombinant endoglucanase from Bacillus subtilis MU S1. Environ. Technol. Innov. 2019, 16. [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass – An overview. Bioresour. Technol. 2016, 199, 76–82. [CrossRef]
- Wang, L.; Wei, B.; Cai, F.; Chen, C.; Liu, G. Recycling durian shell and jackfruit peel via anaerobic digestion. Bioresour. Technol. 2021, 343, 126032. [CrossRef]
- Nelluri, P.; Venkatesh, T.; Kothakota, A.; Pandiselvam, R.; Garg, R.; Eswaran, V.; Vaddevolu, U.B.P.; Venkatesh, R.; Khaneghah, A.M. Recent advances in non-thermal and thermal processing of jackfruit ( Artocarpus heterophyllus Lam ): An updated review. J. Food Process. Preserv. 2022, 46. [CrossRef]
- Rahmani, A.M.; Gahlot, P.; Moustakas, K.; Kazmi, A.; Ojha, C.S.P.; Tyagi, V.K. Pretreatment methods to enhance solubilization and anaerobic biodegradability of lignocellulosic biomass (wheat straw): Progress and challenges. Fuel 2022, 319, 123726. [CrossRef]
- Usmani, Z.; Sharma, M.; Diwan, D.; Tripathi, M.; Whale, E.; Jayakody, L.N.; Moreau, B.; Thakur, V.K.; Tuohy, M.; Gupta, V.K. Valorization of sugar beet pulp to value-added products: A review. Bioresour. Technol. 2021, 346, 126580. [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; Di Domenico, M.; Filho, V.F.d.S.; de Sena, R.F.; Machado, R.A.F.; Marangoni, C. Insights into the bioenergy potential of jackfruit wastes considering their physicochemical properties, bioenergy indicators, combustion behaviors, and emission characteristics. Renew. Energy 2020, 155, 1328–1338. [CrossRef]
- Ranasinghe, R.A.S.N.; Maduwanthi, S.D.T.; Marapana, R.A.U.J. Nutritional and Health Benefits of Jackfruit (Artocarpus heterophyllus Lam.): A Review. Int. J. Food Sci. 2019, 2019, 1–12. [CrossRef]
- Sreeletha, A.S.; Lini, J.J.; Dhanyalekshmi, C.S.; Sabu, K.R.; Pratap, C.R. Phytochemical, proximate, antimicrobial, antioxidant and FTIR analyses of seeds of Artocarpus heterophyllus Lam. Adv Biotechnol Microbiol. 2017, 5(1), 555–653.
- Burci, L.M.; da Silva, C.B.; de Oliveira, M.; Dalarmi, L.; Zanin, S.M.W.; Miguel, O.G.; Miguel, M.D. Determination of antioxidant, radical scavenging activity and total phenolic compounds of Artocarpus heterophyllus (Jackfruit) seeds extracts. J Med Plants Res. 2015, 9(40), 1013–1020.
- Jiang, T.-T.; Liang, Y.; Zhou, X.; Shi, Z.-W.; Xin, Z.-J. Optimization of a pretreatment and hydrolysis process for the efficient recovery of recycled sugars and unknown compounds from agricultural sweet sorghum bagasse stem pith solid waste. PeerJ 2019, 6, e6186. [CrossRef]
- Taheri, M.E.; Salimi, E.; Saragas, K.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Effect of pretreatment techniques on enzymatic hydrolysis of food waste. Biomass- Convers. Biorefinery 2020, 11, 219–226. [CrossRef]
- Adewuyi, Y.G.; Deshmane, V.G. Intensification of enzymatic hydrolysis of cellulose using high-frequency ultrasound: an investigation of the effects of process parameters on glucose yield. Energy Fuel. 29 (2015) 4998–5006. [CrossRef]
- Surh, J.; Decker, E.A.; McClements, D.J. Utilisation of spontaneous emulsification to fabricate lutein-loaded nanoemulsion-based delivery systems: factors influencing particle size and colour. Int. J. Food Sci. Technol. 2017, 52, 1408–1416. [CrossRef]
- ; Sundari, C.D.D.; Sunarya, R.R.; Suryaningsih, S. Optimization of bioethanol production from jackfruit straw waste through the addition of a starter and fermentation duration. 2023; 2572, 030014. [CrossRef]
- Arif, A.R.; Natsir, H.; Rohani, H.; Karim, A. Effect of pH fermentation on production bioethanol from jackfruit seeds (Artocarpus heterophyllus) through separate fermentation hydrolysis method. J. Physics: Conf. Ser. 2018, 979, 012015. [CrossRef]
- Nikolić, S.; Lazić, V.; Veljović, Đ.; Mojović, L. Production of bioethanol from pre-treated cotton fabrics and waste cotton materials. Carbohydr.polym. 164 (2017) 136-144.
- Brexó, R.P.; Sant’ana, A.S. Impact and significance of microbial contamination during fermentation for bioethanol production. Renew. Sustain. Energy Rev. 2017, 73, 423–434. [CrossRef]
- Yuvarani, M.; Immanuel, C.S. Dhas.Synthesis of Bioethanol from Artocarpus Heterophyllus Peel by Fermentation using Saccharomyces Cerevisiae at Low Cost. GRD J. Eng. 2 (12) (2017), 1-7.
- Zabed, H.; Faruq, G.; Sahu, J.N.; Azirun, M.S.; Hashim, R.; Boyce, A.N. Bioethanol Production from Fermentable Sugar Juice. Sci. World J. 2014, 2014, 1–11. [CrossRef]
- Kaewchana, A.; Techaparin, A.; Boonchot, N.; Thanonkeo, P.; Klanrit, P. Improved high-temperature ethanol production from sweet sorghum juice using Zymomonas mobilis overexpressing groESL genes. Appl. Microbiol. Biotechnol. 2021, 105, 9419–9431. [CrossRef]
- Rukshika, S.; Sisira, K.; Sanath, R.; Subramanium, S. Isolation and Characterization of an Ethanol Resistant Bacterium from Sap of Saccharum officinarum for Efficient Fermentation. Internat J Biotechnol Bioeng. 03(02) (2016) 15-20.
- Kularathne, I.W.; Gunathilaka, C.A.; Ratnaweera, A.C.; Kalpage, C.S.; Rajapakse, S.; Gamage, P. Optimization of Fermentation Process Parameters for Bioethanol Production from Sri Lankan Overripe Fruits. Eng. J. Inst. Eng. Sri Lanka 2021, 54, 77. [CrossRef]
- Mibulo, T.; Nsubuga, D.; Kabenge, I.; Kiggundu, N.; Wydra, K.D. Comparative Study of Biogas Production from Jackfruit Waste, Banana Peels, and Pineapple Peels Co-Digested with Cow Dung. J. Sustain. Bioenergy Syst. 2023, 13, 1–15. [CrossRef]
- Balamaze, J.; Muyonga, J.; Byaruhanga, Y. Production and utilization of jackfruit (Artocarpus heterophyllus) in Uganda. Afr. J. Food, Agric. Nutr. Dev. 2019, 19, 14289–14302. [CrossRef]
- Bijarchiyan, M.; Sahebi, H.; Mirzamohammadi, S. A sustainable biomass network design model for bioenergy production by anaerobic digestion technology: using agricultural residues and livestock manure. Energy, Sustain. Soc. 2020, 10, 1–17. [CrossRef]
- D’Adamo, I.; Falcone, P.M.; Huisingh, D.; Morone, P. A Circular Economy Model Based on Biomethane: What Are the Opportunities for the Municipality of Rome and Beyond? Rene Ener. 163, (2021) 1660-1672.
- Waluyo, J.; Pratiwi, Y. Analysis Proximate , Ultimate , and Thermal Gravimetric Based on Variations Dimensions of Briquettes from Waste Jackfruit Crust. Internat J Scient. Eng. Sci. 2(10), (2018) 36–39.
- Walsh, J.J.; Jones, D.L.; Chadwick, D.R.; Williams, A.P. Repeated application of anaerobic digestate, undigested cattle slurry and inorganic fertilizer N: Impacts on pasture yield and quality. Grass Forage Sci. 2018, 73, 758–763. [CrossRef]
- Westerholm, M.; Castillo, M.; Andersson, A.C.; Nilsen, P.J.; Schnürer, A. Effects of thermal hydrolytic pre-treatment on biogas process efficiency and microbial community structure in industrial- and laboratory-scale digesters. Waste Manag. 2019, 95, 150–160. [CrossRef]
- Zakaria, M.R.; Ariffin, H.; Abd-Aziz, S.; Hassan, M.A.; Shirai, Y. Improved Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Produced byComamonassp. EB172 Utilizing Volatile Fatty Acids by Regulating the Nitrogen Source. BioMed Res. Int. 2013, 2013, 1–7. [CrossRef]
- Gowda, V.; Shivakumar, S. Agrowaste-based Polyhydroxyalkanoate (PHA) production using hydrolytic potential of Bacillus thuringiensis IAM 12077. Braz. Arch. Biol. Technol. 2014, 57, 55–61. [CrossRef]
- Sehgal, R.; Gupta, R. Polyhydroxyalkanoate and its efficient production: an eco-friendly approach towards development. 3 Biotech 2020, 10, 1–14. [CrossRef]
- Nelluri, P.; Venkatesh, T.; Kothakota, A.; Pandiselvam, R.; Garg, R.; Eswaran, V.; Vaddevolu, U.B.P.; Venkatesh, R.; Khaneghah, A.M. Recent advances in non-thermal and thermal processing of jackfruit ( Artocarpus heterophyllus Lam ): An updated review. J. Food Process. Preserv. 2022, 46. [CrossRef]
- Gupta, R.; Gupta, S.K.; Gehlot, C.L.; Bahadur, I. Chemically modified jackfruit leaves as a low-cost agro-waste adsorbent for Pb(II) removal from synthetic wastewater. J. Hazard. Mater. Adv. 2023, 10. [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [CrossRef]
- Khan. A.U. A Review on Importance of Artocarpus heterophyllus L. (Jackfruit). J. Multidiscip. Appl. Nat. Sci., 2021. 1(2), 106-116.
- Zhang, L.; Lu, S.; Xiao, D.; Gu, M. Characterization of full pore size distribution and its significance to macroscopic physical parameters in tight glutenites. J. Nat. Gas Sci. Eng. 2017, 38, 434–449. [CrossRef]
Figure 1.
Production of jackfruits wastes.

Figure 2.
Jackfruit wastes valorization into different bioproducts.

Figure 3.
Schematic representation of jackfruit plants parts with its waste conversion into value-added products.
Figure 3.
Schematic representation of jackfruit plants parts with its waste conversion into value-added products.

Figure 4.
Green extraction for better yield of bioactive compounds from jackfruit wastes.

Figure 5.
Jackfruit wastes utilized for bioenergy task.
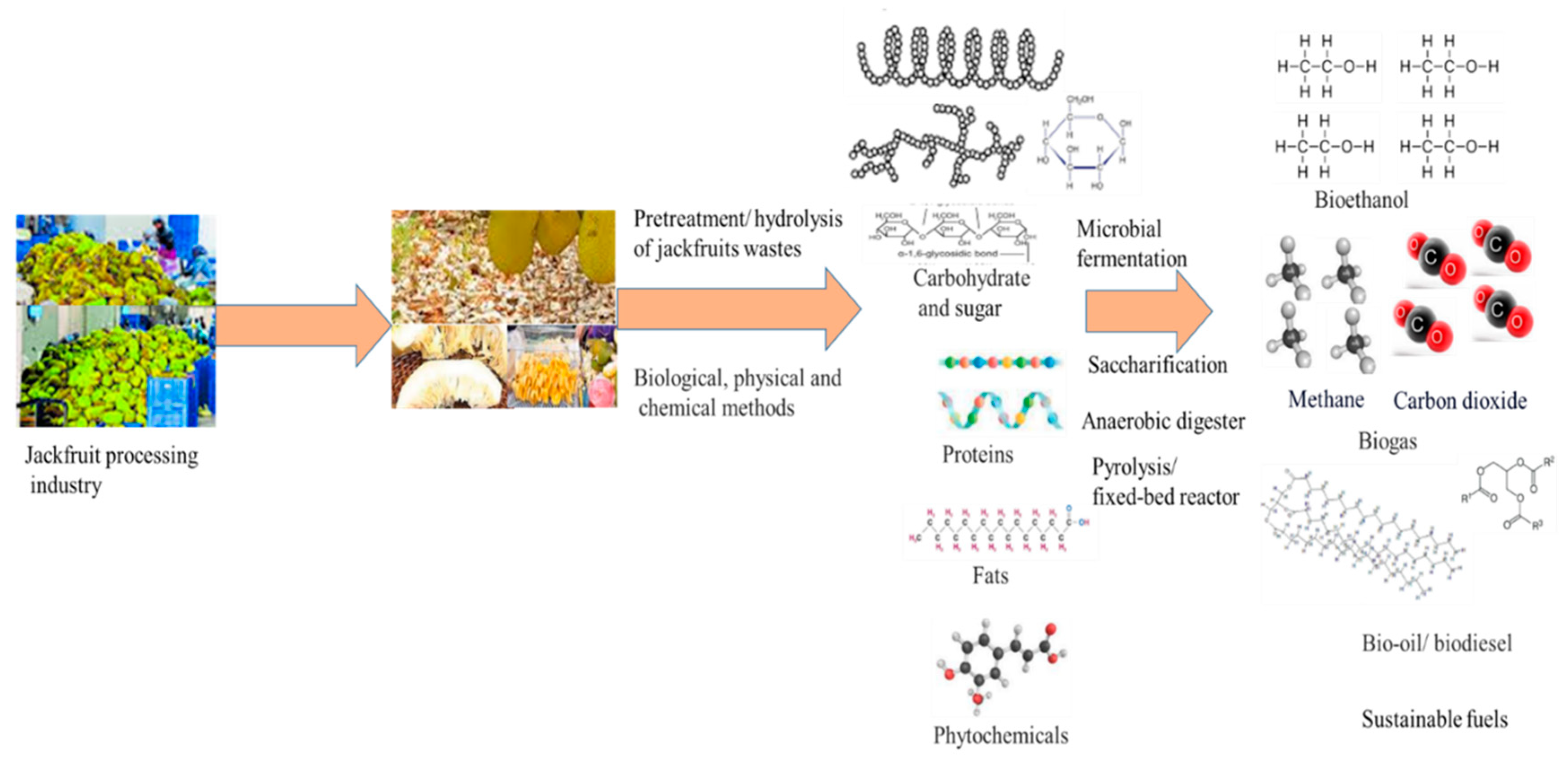
Figure 6.
Jackfruit wastes promoting the zero-waste generation and biorefinery promotion.

Table 1.
Different value-added products (i.e., biofuel, bioplastic, enzymes, pectin, nano-emulsion) synthesis from jackfruit waste conversion via valorization techniques.
Table 1.
Different value-added products (i.e., biofuel, bioplastic, enzymes, pectin, nano-emulsion) synthesis from jackfruit waste conversion via valorization techniques.
| Jackfruit wastes | Value-added products | Application | References |
|---|---|---|---|
| Jackfruit wastes with its different parts like peel/ skins | Bioethanol, biogas, bioplastics, are so on generated. | Biofuel is cheaper and greener than fossil fuels. Processed peel cleans dye-contaminated aquatic environments. | [7] |
| Durian shell and jackfruit peel | An increase of 103.8% in methane output and 69.8% in biodegradability. | Produces high levels of sustainable energy and fuel (2.0 109 MJ/year), while simultaneously decreasing coal usage (6.8 104 tons/year) and cutting emissions by 2.2 1010 particulate/year. | [9] |
| Jackfruit outer rind | Used as substrate for producing recombinant endoglucanase from Bacillus subtilis MU S1 | This enzyme helps in highest saccharification process (33.4 %) from jackfruit outer rind at 96 h of incubation | [11] |
| Jackfruit peel waste | Bio-oil upgrading is done by sub/super-critical fluids, solvent addition, and steam reforming | The application of bio=oil can substitute fuel for the commercial or industrial burner | [12] |
| Jackfruit seed starch was plasticized | Used to produce starch-based bioplastics | Four distinct kinds of bioplastics were manufactured in order to investigate the influence of the plasticizers and to characterise the features of the bioplastics that correspond to those plasticizers. | [18] |
| Utilization of jackfruit peel | There have been reports of the use of an adsorbent made from jackfruit peel. | Jackfruit peel adsorbent can biosorb maximally of232.55 mg/g of MB. | [19] |
| Artocarpus heterophyllus Lam. seed powder extract (ASPE) | Green production of silver nanoparticles from silver nitrate in water | ASPE may be utilized to synthesize AgNPs for nanomedicine in the future. It is eco-friendly and harmless. | [25] |
| Artocarpus heterophyllus peels | Green synthesis of iron nanoparticles is reported | Iron nanoparticles were highly catalytic, removing 87.5% in 20 minutes at 318 K. | [28] |
| Artocarpus heterophyllus (jackfruit) peel | Bio-based coagulating agent | The extract from the peel has the potential to serve as a bio-based coagulating agent alternative that is useful in the pre-treatment of wastewater. | [32] |
| Jackfruit seed powder (JSP) | The surface of JSP is used | Novacron blue textile dye can be decolorized using this substance. After a contact time of 60 minutes, the surface of JSP has been observed to adsorb 73% of Novacron blue. | [83] |
| Jackfruit waste feedstock | Biogas production was improved by chemical catalysts with maintaining the pH and C/N ratio. | Biogas is produced via decreasing the digestion time with improving the efficiency of digester unit by using jack fruit waste as raw material. This raw material contains significant quantity of fiber with small proportions of glucose. | [84] |
| Jackfruit waste together with peels (JP) as well as seeds (JS) | Bioenergy has optimal physicochemical, bioenergy indicators, combustion, and emission properties. | The bioenergy yields for JP and JS were 2.5 and 0.9 ha−1 yr−1 (dry basis), correspondingly. Low concentrations of CO, CO2, and SO2 may be released. | [92] |
| Jackfruit straw waste | Used as raw material for making bioethanol. | The amount of bioethanol distillate that could be produced under ideal circumstances was 30 ml. This research employed a distillation temperature range of 70 to 78 °C. | [100] |
| Jackfruit (Artocarpus heterophyllus) stone waste | Ethanol from these waste is found and it is used as renewable fuels | A quantity of Jackfruit flour was subjected to hydrolysis by the addition of 0.3 to 0.7 mL of alpha-amylase and 0.2 to 0.6 mL of glucoamylase. The resulting mixture was then subjected to a fermentation process lasting between 3 to 6 days. The yield of ethanol obtained from this process was found to be between 11 to 13%. | [41] |
| Renewable energy from jackfruit's seeds | Bioethanol (57.94%) is reported | The fermentation process for ethanol production is used with Saccharomyces cereviceae with a variation of pH values with 70 hours | [101] |
Table 2.
Health benefits from bioactive compounds that are recovered from jackfruit waste matters.
| Jackfruit wastes | Bioactive compounds | Health benefits | References |
|---|---|---|---|
| Jackfruit skins, leaves, and barks | Vitamins, minerals, and phytochemicals | The substance under consideration exhibits various properties such as anticarcinogenic, antimicrobial, antifungal, anti-inflammatory, wound healing, and hypoglycemic effects. | [2] |
| Agro-residues from jackfruit plants | Nanocapsules uses increase the bioactive efficacy | Bioactive in micro- and nanoencapsulation forms enhanced target site delivery in human body with more benefits | [4] |
| Jackfruits are known for their prickly outer bark and axis. | Flavonoids, stillbenoids, morin, artocarpin, dihydromorin and cynomacurin, | Because of their bioactivity, these chemicals have the potential to be developed into nutraceuticals with antioxidant characteristics. | [5] |
| Leave and stem bark extract of Artocarpus Heterophyllus | Extract is dominated with tannin and saponin | The methanol extract of Artocarpus stem and leaf bark has antibacterial and antioxidant properties, and the bark may be used topically as a peel-off mask. | [6] |
| Jackfruit peel | Functional food additives and pectin materials | These compounds are manipulated for food ingredient applications with providing of health benefit to gastro-intestinal tract. | [8] |
| Spine, skin and rind of jack fruit | Polyphenol as well as flavonoids | Crude ethanolic extracts undergo evaluation for their anti-inflammatory potential. | [14] |
| Fruit peel of jackfruit | Flavonoid, and also present some phenolic compounds is reported | Various phyto-constituents can use In different additives of human use with more health benefits |
[21] |
| Jackfruit seed extracts in three different solvents: methanol, hydroalcoholic, and aqueous. | Alkaloids, flavonoids, terpenoids, and so on | This extracts showed the antioxidant, anti-inflammatory and antibacterial activity | [23] |
| Artocarpus heterophyllus J33 rind parts | Proto-catechuic acid (PCA) antioxidant activity depends on its temperature: 25°C, 4°C, and -18°C. | The extract’s antioxidant activity was maintained because PCA is so stable. It has antioxidant properties and might be used in food and dietary supplements. | [40] |
| Seeds of jack fruit | The different solvent extracts showed the presence of fats, phenols and flavonoids. | Acetone extract demonstrated the greatest antibacterial activity towards Staphylococcus aureus and the greatest antifungal activity against Aspergillus flavus. | [94] |
| Jack fruit seeds | Total phenolic compounds in this seed extract confirmed by different screening tests | Total phenolic substances are identified as antioxidants with radical scavenging action. | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
05 July 2023
Posted:
05 July 2023
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
# Equal Contribution
This version is not peer-reviewed
Submitted:
05 July 2023
Posted:
05 July 2023
You are already at the latest version
Alerts
Abstract
Valorization of food and fruit wastes has the potential for the production of sustainable energy and biochemicals. Approximately 70% of the weight of the original jackfruit (Artocarpus heterophyllus L.) fruit is lost during processing as waste in the form of peeled skin and core, both of which have not been utilized and, thus contribute to disposal as well as pollution issues. The major components, cellulose, and hemicellulose, can be biologically transformed easily into bioenergy sources like ethanol, methanol, and butanol; valuable phenolics and biotechnological products like pectin, citric acid, bromelain, ferulic acid, and vanillin; and many other products. These residues can also be utilized as essential sources for the biological transformation process leading to the production of numerous products with added value, such as phenolic antioxidants, phenolic flavor compounds, and organic acids. Thus, the value addition of jackfruit waste can support the sustainable solution towards food and nutritional security. In this way, zero waste can be achieved through novel biorefineries which are critically highlighted in this paper. Furthermore, novel technologies for the conversion of jackfruit wastes are summarized with recent findings.

Keywords:
Subject: Chemistry and Materials Science - Food Chemistry
Highlights
- Reports on diversity of jackfruits cultivation and uses in food/ diet nutrients.
- Discussed jackfruit wastes generation and also valorization methods for utility.
- Valorization techniques reported for value‐added products with zero‐wastes generation.
- Bioactive compounds and bioenergy generation from jackfruit wastes was targeted.
- Sustainable way of bioproducts generation, promotes biorefinery/ clean environment.
1. Introduction
Jackfruit (Artocarpus heterophyllus L.) tree is known to produce the huge fruits from its stems and it is unique in term of food utilization as vegetables and fruits. It is native plant and can be found in rain-forests especially in Western Ghats. People are well known about jackfruits and it was derived from Malayalam as chakka name. In India, it is grown/ cultivated at low elevation regions through all the states. Several wastes are generated from ripe fruits and this fruit waste is more palatable than wastes from raw fruit [1]. Jackfruit has shown the various nutrients like crude protein (CP~7.9%); crude fibre (CF~14.1%), calcium (0.8%) and phosphorus (0.1%). Except these nutrients, wastes from ripe and raw jackfruit is also utilized as potential substrates for energy production and nitrogen free radical (NFE~ 65%). The rind of the ripe fruit is good sources for cattle foods and this waste of jackfruit is non-edible portion (~59.2%) like perianth meal, rind and core meal. This waste matter is utilized for total dry meal recovery (11.6%) [1,2]. Some analysis was done jackfruits waste compositions (for perianth meal, rind and core meal) that contained ash (6 to 7.5%), carbohydrates (20-29%), crude protein (8- 10.6%), crude fate (1.7 to 7.3%) and crude fibres (12- 17.3%). Some studies were done utilization of jack fruits wastes and these are used for food, feed and other industries application. Some non-edible portion of jackfruit (as peels and axis) is reported with edible products (seed) and still these waste matters are underutilized at worldwide like Bangladesh, India or others countries [3]. Different waste portions of jackfruits are generated during juicy edible bulbs. Thick peels of jackfruits can be utilized for different valuable products generation such as biofuel, non-porous adsorbent and nutrient enriched cattle feeds. Normally non-porous adsorbent is used in removal of dye. Peel and central axis of the jackfruit is also utilized for pectin extraction [4]. And its seed power is reported to be used in various bakery products. From this fruit seed power, starch and protein fraction was isolated and then it was utilized in its purified form for food formulations at industries level [1,3,4].
Jackfruits have been analysed during different seasons by various workers [4,5,6,7] for their nutritional and antioxidant properties. The findings of these studies indicate that jackfruits serve as a valuable source in the development of nutraceuticals, which are currently in high demand worldwide. [5]. Jackfruits in different seasons are reported to differ in phytochemicals like phenolics, terpenoids, steroid, glycosides, saponins, alkaloids and tannins and these compounds are known to possess antioxidant properties. Diversity in secondary metabolites in jackfruits is reported and it is due to variation in functionally, nutritionally and medically important jackfruits wastes [4,5]. From jackfruit wastes, antibacterial and antioxidant activity agents/ compounds can be evaluated from the extracts that were obtained from methanol extract jackfruit leaves and stem barks and then it is applied as a peel-off mask. From extraction of those properties from jackfruit wastes, first raw material macerated using the methanol agent and then filtrates were evaporated for some time to get concentrated crude extract [6]. Then this extract was evaluated by different tests such as phytochemistry screening and also antibacterial tests on propionibakerium acnes and Staphylococcus aureus at different concentration of extracts using the DPPH (a,a-diphenyl-β-picrylhydrazyl) method [7].
For best evaluation of these phytochemicals, some additional test was done that can prove the characteristics of peel-off mask such as homogeneity, pH, organoleptic and irritation test. Some phytochemical screenings have proved for domination of tannin and saponin in their extracts [6,7]. In context to jackfruits wastes, these are rich sources of carbohydrates, protein, fats and phytochemicals. For these organics extraction or utilizations as a promising feedstock for valuable bioproducts including fuels/ chemicals synthesis. And several natures of pretreatments (like biological, physical,and chemicals including green solvents) were applied as effective valorization strategies for jackfruits waste matters [8]. The implementation of these strategies has facilitated the transformation of waste into products that possess added value, including but not limited to bioethanol, biogas, bioplastic, feeds, and functional compounds/food additives. The utilization of jackfruit waste for bioenergy production and recovery represents a promising avenue for sustainable and eco-friendly food waste-based renewable resources. This approach offers an economically feasible alternative to non-renewable fossil fuels [9].
Further efforts were done on efficient bioconversion tasks/ techniques, applied for jackfruits that can generate/ produce the valuable biomaterials/ chemicals and it is only to be achieved via microbial fermentation process. This conversion can help to get sustainable products with mitigation of jackfruits generation/ accumulation with support to a green environment. Some reports claimed the utility of jackfruit peels for the remediation of dye color from contaminated aquatic environments [8,9]. The implementation of said technology has the potential to facilitate the creation of an environmentally sustainable economic framework centered around the repurposing of waste materials. Many studies have carried out utilization of jackfruit waste for the production of value-added products, with the ultimate goal of mitigating waste generation and promoting environmental sustainability. In an attempt to utilize jackfruit waste, the production of plastic from jackfruit seed starch has also been carried out. The resulting plastic was strengthened through the incorporation of microcrystalline cellulose (MCC) derived from cocoa pod husks, with glycerol serving as the plasticizer [10]. This study aimed to identify the optimal mass and volume of microcrystalline cellulose (MCC) and glycerol concentration for the production of bioplastics in high yield. Before the bioplastic production, MCC, bio-production was done with cocoa pod husks, and this husk was subjected to a pretreatment task with the help of alkali agents, bleaching and HCl acid solution to get the effective hydrolysis [11].
During this effort, degree of crystallinity of MCC determination was done with help of analytical technique like XRD (X-ray diffraction), with functional groups determination by FTIR (Fourier-transform infrared) and also morphological properties analysis by scanning electron microscopy (SEM). Some researchers have gone on results for isolated MCC from pod husks and they discussed it as rod-like form with respective length (5-10µm) and diameter (11.63nm) with high crystallinity [10,12]. From isolated MCC utility in bioplastic synthesis, tensile property of bioplastic was determined at starch to MCC mass ratio (8:2). Further tasks were done in addition to 20% glycerol with measured tensile strength (0.637) and good elongation (at break of 7.04%). From analytical measurement by FTIR spectroscopy for bioplastic functional groups studies, it was found for more numbers of -OH groups at bioplastics and it was reinforced with filler MCC, with representation of hydrogen bond [10,13].
A thorough screening of literature reveals that there is a very limited understanding to how jackfruit waste can contribute to sustainable energy, and help attain zero waste. To ensure sustainable future for all by 2030, United Nations has developed blue prints through its various sustainable development goals (SDGs), and SDG 7 encourages sustainable utilization of bioresources in increasing the share of renewable energy in the global energy mix, and to provide sustainable energy services to all countries. During the past decades, efforts are continuously being made to use cleaner and greener technologies to utilize various bioresources, and thus novel technologies are being continuously implemented. In this review we aimed to (a) report the approaches/methods used in conversion of jackfruit waste to sustainable energy, and biochemical, (b) analyse the findings of various studies, and advances made in the field, (c) compare the advantages, limitations, and drawbacks of various technologies, and (d) the new trend of jackfruit waste as bio-absorbent for the benefit of pollution control.
2. Database Formation and Analysis
For developing this review we searched published articles dealing with jackfruit cultivation, nutrient values of its seeds, composition of jackfruit wastes, types of wastes, valorization techniques/strategies (physical, biological and chemical including green solvent), novel techniques/green extraction techniques for bioactive recovery, microbial fermentation for jackfruit waste conversion, value added products from jackfruit wastes (bioethanol, biogas, bioplastic, bioactive compound like phenolics, carotenoids, flavonoids, tannins, antioxidant etc.) from journals on ISI web of science, google scholar to create database. All the downloaded papers were thoroughly reviewed, and filtered to extract the pre-treatment methods employed and diversity of value added products derived from jackfruit peels, rags and other wastes. Besides, we examined the conversion technologies of fruit wastes, and their practical implementation, cost effective measures and limitations in the global context. Further, the potential of jackfruit waste conversion in the context of increasing renewable energy share to global energy mix, environmental safeguards are discussed in the paper.
3. Results and Discussion
3.1. Cultivation of Jackfruits with Impact to its Nutrients
Due to more demand for waste mitigation and also extraction of valuable products with bioactive properties, there is more jackfruit cultivation reported all over India with some other countries such as Burma and Malaysia with some locations in Brazil. It is now one of the remunerative and also more valuable fruits in India. This jackfruit tree belongs to the Moraceae family and is also native tree of India [14]. This plant is now cultivated throughout the tropical low land at both hemispheres. This jackfruit plant is widely grown in Western Ghats of India but its plantation is found in Bihar, West Bengal, Uttar Pradesh, Kerala, Tamil Nadu, Assam and Orissa. Some reports claimed its regular plantation in the U.P., especially marginal orchards. But in other parts this plant cultivation is reported as rare in plantations but jackfruit cultivation is found throughout South India up to an elevation of 2400 meters [15]. Some reports have discussed the jackfruit plantation in systematic and proper ways and this plant growth needs rich and well drained sandy loam soils. For this plant plantation, soil drainage showed more importance with proper evidence. Sudden decline of numerous jackfruit plants in areas that are suffering a sudden rise of water level [14,15]. Reports on jackfruit tolerance capacity especially for moisture stress was shown and it can be shown to some extent of tolerance due to presence of lime and chlorine Areas near the river beds can be found to be ideally suitable for jackfruit plants cultivation. Further information on jackfruit plantation is a warm humid plain and it is able to flourish in humid hill slopes up to an elevation of 1500 meters. Jackfruit can deteriorate at higher altitudes with satisfactory plantations in arid and warm plains of South India [16].
This jackfruit can provide unlimited scope for clone selection of promising strain/ species for multiple applications. Many types of these plants are available under various local names and it can be originated via clone selection. Gulabi, Champa, Hazarix are some examples of jackfruits. It needs storage conditions for jackfruit plantation. The impact to thick peels is found with good storage quality [17]. Jackfruits can be easily gone for cross pollination and then its seeds can be found to easily propagate. To date, the prevailing population of jackfruits is distributed across numerous trees, exhibiting variations in their morphological characteristics such as shape, size, and density of tubercles, as well as differences in rind color, bulb size, fiber content, and fruit quality and maturity [15,17]. The storage longevity of jackfruit has been determined to be approximately 6 weeks when exposed to temperatures ranging from 0.1 to 12.70 C, provided that the humidity level is maintained at eighty to ninety per cent. The initial quality and maturity stage at the time of harvest are crucial factors that can significantly impact the storage life of a product. The jackfruit's storage life can be extended, allowing for transportation to remote locations for marketing purposes, through the utilization of appropriate packaging and wrapping techniques [16,17].
Reports for jackfruit application in India are found as culinary and also table fruits. And jackfruit used for culinary purposes is mainly found in more states in India. In market demand, tender fruits in spring and also summer are used as population vegetables. People have enjoyed jackfruits with high demand and premier cost/ price and sometimes this jackfruit cost/ price can be reached at a high rate in the year [18]. Most people in the world used jackfruits in ripe form as tasty fruits with high sweet and also high nutritive values. In ripe form, jackfruits are good sources of vitamins A and C with some people believe in aiding in digestion process as ailments on a regular basis [17,18].
3.2. Compositions of Jackfruits Wastes
The waste produced from mature jackfruit fruits has been found to possess greater palatability compared to the waste generated from raw fruits. This waste material is composed of crude fiber, crude protein, and various minerals. It is also a significant source of energy, with a substantial quantity of NFE. The generation of rind from ripe fruit can serve as a source of nourishment for cattle [19]. Additional applications of jackfruit-derived fruits have been documented for the production of pickles, dehydrated leather, and thin papad, as well as for canning purposes. These fruits have also been utilized in conjunction with soft drinks such as nectar and squash. In certain literature, rind has been identified as a viable source of protein. Additionally, it has been reported that extracts derived from rind waste can be utilized in the fabrication of jelly [20]. Discussion of skin parts of fruits of jackfruit is reported for excellent cattle feed. Jackfruit plant/timber can be used for making furniture that can exhibit less chance of white ants infection/ attacks. Some efforts were done for latex extraction that contains resin. Latex from jackfruit bark can be used for making the plug hole in earthen vats and baskets and it has shown multiple applications for humankind [19,20].
Further, multiple reports are discussed for jackfruits parts (in terms of edible and non-edible parts) and non-edible parts are 70-80%, and out of this, outer rind, perianth, and central core of jackfruits is 60% under waste matters. A number of researches were done on biochemical composition by suitable analysis in jackfruits-based wastes with promising sources of health benefits. These bioactive compounds (i.e., valuable compounds/bioproducts) can be recovered/ generated by several microbial bioprocessing/extraction techniques in an eco-friendly way [21]. The peel of jackfruit is a rich source of cellulose, protein, starch, and pectin. The chemical composition of dried jackfruit seeds includes carbohydrates (76%), protein (18%), and lipids (2.1%). Numerous phytochemicals, isoflavones, lignin, and saponins, along with several essential nutrients, have been documented in the seeds of jackfruit waste [22]. Subsequent discourse revealed noteworthy sources of vitamins such as thiamine and riboflavin. The processing of jackfruit generates solid waste, including jackfruit peels, seeds, as well as latex. These waste products have been identified as contributors to environmental damage [22,23]. Researchers have put some effort into jackfruit wastes and they used eco-friendly feedstock/ sources for bioproducts synthesis in sustainable ways and this waste matter has shown the best biochemical compositions, helping in renewable mode bio-based products development. Further research has been conducted on the utilization of jackfruit peels as a sustainable source for the recovery of commercial pectin, biofuels, and other valuable products [24,25]. And Figure 1 shows several form of jackfruits wastes with nutrients.
Various types of Jackfruits Wastes
Wastes generation from various types of vegetable and fruits/cultivation processes are discussed and a fruit can generate usually 3.5 to 10 kg of waste by weight and some fruits can generate higher quantities of waste (up to 25 kg). In the case of jackfruit plants, the outer parts of the jackfruit shell are found to contain a conical apex, covered via a thick robbery wall. And its fruits matured conditions, a non-edible core can be found on a longitudinal axis, fused with the rags [26]. This part is then fused to the fruit’s rind part. Next, other wastes of jackfruit are bulbs and this bulb is composed of pulp, surrounded by seeds and it is found between the rags. Seeds numbers in jackfruits are nearly 100 to 500 and it can be found in nearly 18 to 25% of the weight of jackfruits. The kernel of the seeds can constitute nearly 90 to 95% of their weight and then pulp can account for 30% and it can be found between 70 to 80% of jackfruits components as non-edible [27]. In non-edible parts of fruits, outer rinds, perianth, and central core can consist of 60% of the total wastes of jackfruits as discarded parts. Jackfruit peels and its conical carpel apices are also important components of jackfruit wastes. Apart from this waste, jackfruit leaves can be also used for some medical benefits such as relieving fever, boils, wounds, and skin conditions [26,27]. And then young fruits of jackfruit can exhibit acrid and astringent properties and characteristics and it can work on removing the flatulence in human health. The jackfruit seeds can be found as rich sources of protein concentrations (5 to 6%). In each tree of jackfruit, nearly 100 to 200 fruits can be produced as it is a large and evergreen tree every year. Still time, very limited research was done on unutilized wastes of jackfruits and then peels and fiber found nearly 60% of whole fruits as the largest known edible fruits from any plants. The jackfruit pulp and seeds are known to contain various bioactive compounds in addition to waste nutrients. The availability of these compounds in the fruit of this plant can vary, as reported in a previous study [28]. The cultivation of this plant species is feasible during the monsoon season in coastal areas. The fruit of this plant is considered a cost-effective source of sustenance and is widely accessible at a low market value. Subsequent investigations were conducted on underutilized segments of jackfruit, with a focus on mitigating the accumulation of biowaste to promote the eradication of pathogenic microorganisms in augmented agricultural yields [29]. The nutritional and functional characteristics of jackfruit peels warrant further investigation for the purpose of extracting bioactive compounds that may be utilized in the pharmaceutical industry [29,30]. Figure 2 shows the different jackfruit wastes with its valorized products.
The outer layer of jackfruit is comprised of peel which exhibits a spiky pattern on its surface. The peel of jackfruit is considered non-edible and is regarded as a potential waste material. Normally this part of fruit is discarded and then recently it is used for fertilizer sources. These parts of jackfruit can be used to feed the cattle in villages. It is a good source of carbohydrate (up to 24%), protein (up to 9%) and fibers (17.3%) [31]. Jackfruit peels are utilized as valuable raw materials for functional ingredients like steroid, triterpenoids, saponin and carbohydrates. In jackfruit peel, more quantities of polyphenols are reported and it is linked to peel proximity with extrinsic domains. Some abiotic stress parameters/ factors (such as daylight, ultraviolet radiation and climatic induction) can impact the synthesis of polyphenol on the complete part of jackfruit peel [32]. Other bioactive compounds in antioxidants can aid the chemical reactions in the human body and then it helps to offset the damages, mediated by oxidation reactions. Pectin content in jackfruits is reported to contain 9 to 15% of dry weight and it can make it a valuable source of the polysaccharides [31,32]. Extraction of various compounds is also used in the textile, paper and biofuel sectors with rich sources of celluloses. This can provide the alternative options to commercialized celluloses in pharmaceutical industries [33,34].
3.3. Valorization Techniques for Zero-waste Generation
In current scenarios, a number of researches are going on the zero-waste generation concept and many strategies (i.e., various valorization routes) are applied to achieve it. This was achieved on bread waste (BW) as the model development. But, there are a number of challenges (i.e., technical processing steps), occurring in various kinds of food wastes like jackfruits waste also. Normally, any type of food wastes hydrolysis including the jackfruits wastes can be done by enzymatic hydrolysis with assisting of physical and chemical agent processes. These approaches can generate monomeric compounds like glucose/ others sugar [35]. These sugars from jackfruit wastes can be utilized for cultivation of suitable microbial systems with capability to use as carbon substrates and also efficiency to metabolite into fuels sources like ethanol/ butanol/ other bioproducts. One example is shown for BW valorization strategy with uses of Euglena gracilis algae cultivation medium with systematic evaluation [36].
This algal cultivation was found to future perspectives and also economic viability for biodiesel sources production. In context to valuable products except biodiesel, other targeted compounds like paramylon (β-1, 3-glucan) were synthesized from E. gracilis with high productivity of 1.93 g.L-1d-1 and this productivity was 24% higher than control strains. In this product synthesis, people have applied the approaches of zero-waste disposal and then bread waste residues (BWR) was hydrolysed by enzymatic hydrolysis process and later it was valorized into syngas [35,36]. It was offered by a greener pyrolysis process for BWR and carbon dioxide was used as raw material for valuable products synthesis. CO2-assisted valorization is an attractive technique not only towards efficient waste disposal, but in attaining to climate neutral or zero carbon emission. In reports, several types of agricultural wastes (licluding jackfruit wastes) are found to utilize as potential raw materials for the generation of valuable products like biofuels, biochar or also biopesticides with briquettes or others. Then biochar can be mixed with the soils and it can produce the carbon rich soils via contribution in carbon dioxides sequestration and soil fertility. So, jackfruit wastes can be anaerobically utilized for production of biogas, briquettes and also biochars that can improve the crop production [37]. And other bioprocess, jackfruit waste can be gone for anaerobic decomposition into biogas and also for briquette production. But at high temperature, these waste (jackfruit waste) can decompose to produce biochar and then it can apply into the soil. This is good approach for carbon dioxide sink with helping in mitigation of effect of climatic change. It can find for anaerobic digestion process for jackfruit waste utilization for biogas production. This process has shown the economic viability for bioproducts generation [36,37].
Researchers have applied a mechanistic functionality process for carbon dioxide (CO2) with systematic description. In this process, CO2 was reacted with volatile matter (VMs) that evolved from BWR and it has helped to reduce the concentration of carbon dioxide. And it was done with proceeding to oxidation of VMs. Further process (like consecutive gas-phase-reaction ~GPR) was done with exhibiting a critical role in enhanced CO formation [38].
Research endeavors were undertaken to identify the induction of gas-phase reactions (GPR) through the utilization of carbon dioxide (CO2). A five per cent by weight (wt%) nickel/silicon dioxide (Ni/SiO2) catalyst was employed for the purpose of studying the reaction kinetics involved in the process of mitigating CO2. During the aforementioned procedure, the gaseous pyrolytic products, namely H2 and CO2, were acquired in terms of molar concentration [39]. The catalytic pyrolysis process (Ni/SiO2) in the CO2 environment has resulted in a greater quantity of syngas, which is 2 and 6 times higher, correspondingly, compared to pyrolysis without the catalyst in the N2 environment [35,40]. This study explores various valorization techniques that can be applied to the green extraction process. The aim is to develop innovative delivery systems, such as nano-emulsions, for bioactive compounds derived from fruit and vegetable waste, including jackfruit waste.. And Figure 3 shows the complete jackfruit plants with edible and non-edible parts that later utilized as wastes and then converted into value-added products with zero-waste generation.
3.4. Advanced/ Green Extraction Techniques for Bioactive Recovery
In the valorization processes, researchers have applied various types of novel extraction techniques such as enzyme-assisted, microwave-assisted, ultrasound-assisted, high-hydrostatic pressure-assisted, pulsed-electrical field, and super-critical fluid extraction methods to derive various bioactive compounds that are used by food, pharmaceutical, cosmetic, and health-care industries [32,34]. These non-conventional green extraction techniques have the potential to zero waste, and thereby helping in sustainable energy conservation. The recent researches have also shown that various biofuels can be produced through microbial fermentation. Some examples of biofuels (like bioethanol up to 11-13%; biogas including methane via A.D process) with help of Saccharomyces cerevisivae are produced through microbial fermentation [41,42] . Similarly, microbial fermentation has been effectively demonstrated to yield other valuable products like bioactive compounds and other products [43]. Researchers have done sincere efforts in developing novel and efficient techniques that can recover the bioactive active compounds without any solvent contamination. Enzyme-assisted and pressurized liquid-assisted extraction are explored for jackfruit wastes applicable to other wastes that can recover the valuable products [44]. Microwave-assisted and ultraviolet-assisted extraction techniques have been employed to derive the pectin polysaccharides and antioxidant phenolics respectively [45,46,47],. Pulsed electric field-assisted and supercritical fluid-assisted techniques are also exploited for various natures of wastes valorization tasks that have recovered the bioactive compounds at high yield/ concentration [48]. Among the various green modes of extraction techniques, their effectiveness can be varied with properties of the source matrix, its chemical structures and also process parameters/ factors (solvent, pressure, time or temperature [43,48].
Novel technologies can provide alternatives to conventional techniques of extraction of bioactive waste from various food wastes and these techniques are known to use water as solvent rather than organic chemicals. These techniques showed the positive impacts for phytochemicals, scattering inside the cytoplasm [49]. Normally, hydrogen or hydrophobic bonds presence in the polysaccharide-lignin network/ complex can be found to be big challenges due to exhibiting difficulty in extraction of bioactive compounds [50]. So these green techniques can be found to have a sustainable and eco-friendly nature with capability to achieve higher yield of the products compared to conventional ones. And these techniques have received more attention in the last few years due to more advantages [49,50]. Some techniques in context to green/ non-conventional mechanisms are discussed below.
In the enzyme-assisted extraction (EAE) process, enzyme concentration, compositions, particle size, water to solid ratio and also hydrolysis time plays an important role and it can influence the yield / concentration of bioactive compounds. Some compounds like carotenoid extraction (from pumpkin waste), anthocyanin from (Crocus sativus/ grape fruit waste) were done via the EIE process [51,52]. Some bioactive compounds like phenolics (18-20 mg/g) were extracted from grape mare seed wastes with help of pectinase enzyme activity [53]. Other efforts were done for antioxidant phenol extraction from apple pomace by using the commercial enzyme Pectinex Ò® and then phenolics recovery (p to 87%) from grape residues using Celluclast Ò®. EAE based technique is very effective to enhance the recovery of enzymes like pectinase, cellulases and pectinases from jackfruit wastes [53,54].
The second extraction method is known as ultrasonic-assisted extraction (UAE), and it has a number of uses and benefits including a larger yield, desired quality, and a straightforward procedure with minimal impact on the environment. The UAE approach uses the ideal frequency range (20-2000 kHz) and is well renowned for being both straightforward and inexpensive. It can be effective at two different facts, such as diffusion over the cell wall and washing the contents following cell disintegration [55]. Due to wave creation of matrix expansion and compression, the UAE operating mechanism causes/generates cavitation phenomenon. The desired compounds are then extracted by causing the cell membrane to become permeable [56]. The researchers investigated the many processes of the UAE process, including the acceleration of mass transfer, the disintegration of the particles, and the improvement of solvent accessibility. For this technique, samples of liquid-liquid or liquid-solid processing are frequently employed. Pressure, temperature, frequency, and sonication duration are other parameters that might affect the UAE process [55,56]. Recent research on the UAE revealed the widespread use of this procedure and shown its effects on yield and compound characteristics. Some excellent instances of UAE frequency have been documented for energy at or above 20 kHz, which changes the physical-chemical characteristics of phytochemicals by causing the production of free radicals [57]. Figure 4 established the sophisticated processing methodologies employed for the extraction of bioactive compounds.
Some efforts on tannin extraction from Avaram shell is reported by application of UAE technique and it uses 100 W power. UAE based process has shown its impact in improved yield (160%) of tannins at 100W. This improvement in yield of tannin was explored and it was found due to improved mass transfer of cell components and a way of leaching of tannin by this power [58]. Others bioactive compounds like good yield (caffeic acid~64.3 µg/g, ferulic acid~ 1513 µg/g and p-coumaric acid~ 140 µg/g) of phenolic acids by UAE technique are reported with better improvement compared to conventional techniques (maceration extraction) for same bioactive from same wastes [59]. Temperature and prolonged time impact on UAE are found in the form of low yield of phenolic compounds from citrus peels. Some comparative studies were done on maceration and UAE technique performances in terms of consumed/ required time period for extraction of bioactive. And it was found to be a reduced time period (1h) for UAE technique compared to maceration assisted extraction (72h) for phenolic compounds from Punica granatum fruits. Extraction of polysaccharides by UAE technique is found with good yield with proving of efficient technique [57,58,59].
Another technique is pulse electric field-assisted extraction (PEF-AE) and its non-thermal process allowing direct current to produce. In this technique, application of high-voltage current/ pulse is passed through the materials that are kept/ placed between two electrodes for a short time period ( in range of microsecond to millisecond periods). These are based on the nature of products and also processing factors. During electric current passages through the suspension of cells, it can influence cell structures to be destroyed and then molecules can separate with respect to applied charge [60]. This technique can function in a batch and also in continuous modes. For this technique performance, various factors like field strength, energy, pulse number, temperature and material properties can affect/ influence the yield of extraction and it can be worked based on designing a process for better performance. Application of PEF assisted extraction can be found in phenolic compounds and also anthocyanins extraction process from different wastes [61]. PEF assisted extraction with maceration on grape skin can be applied for stability of compound during vinification and it can reduce the time of extraction. Some studies were done on untreated control samples along with PEF treated samples with maceration extraction process/ technique [60,61]. And it has shown improved color and also anthocyanin content/ yield with enhanced polyphenol contents in wastes like jackfruit. Further impact of PEF treatment can be found in the wind making process with reduced maceration time and also improved wine quality [63,64,65,66]. The cytomembrane in plant tissue's cell walls can influence the movement of intracellular material between cells. This approach for extracting bioactive substances is intriguing because it may trigger the cytomembrane in the tissue to disintegrate, which changes its permeable characteristics as well as increases mass transfer across the cells, leading to higher yields [64,65].
In context to advanced/ green extraction techniques, utilizes the electromagnetic radiation and it is transferred in the form of waves in the frequency range (300MHz- 300 GHz) with common uses of frequency of 2450MHz. This can be found to be equivalent to 600 to 700 W energy and this energy can be absorbed during the passage of microwave through suitable medium. This medium converts it into thermal energy via facilitating the processing [66,67]. Some bioactive compounds like flavonoids from Terminalia bellerica plant were reported by using this technique and microwave in MAE technique can result in maximum yield (83%) and it is higher yield while compared to conventional techniques (flavonoid yield~64%). MAE technique is an endothermic and spontaneous process and it is also influenced by operating conditions like temperature and feed ratio on flavonoid yield. Same extraction was applied for hesperidin compound extraction with better yield (48%) from skin of citrus unshiu fruits [68,69]. During this technique extraction temperature showed high impact on bioactive compound yield and at 140oC, it showed decreased hesperidin quantity/ content due to the interference of the other solubilized substance. This compound influence has been found in inhibition of hesperidin crystal [67,69]. Other conditions on waste matter maturity level in case of peels, matured peels have yielded less hesperidin contents compared to immature peels (more than 3 times). Some impacts of power in MAE technique is reported and it was found for phenolic compounds extraction from chokeberries. Better yield of phenolic compounds (420.1 equivalent mg gallic acid/100g. chokeberries) is reported at 300W for 5 min [70,71]. Extraction of silibinin from Silybum marianum waste with help of MAE technique is discussed with better yield (97.3%) and it is higher than conventional approaches. Similar study was done on this technique efficiency and it was reported for phenolic compounds from apple pomace wastes [72]. This compound extraction yield from this technique is influenced by several factors/ parameters such as solubility, dielectric constant, dissipation factor (d) and solvent nature. The higher recovery of flavonoid by MAE (up to 74%) is reported and it is better than traditional recovery / extraction process (up to 70.5%) with proof of efficient process [73,74].
During the supercritical fluid extraction process (SC-FE), desired compound extraction is carried out by using the solvent above the critical point (CP) and this CP can be found as a specific temperature (Tc) or pressure (Pc) point. But as above to CP, gas and liquid cannot exist as separate phases [75]. At CP, fluids/ solvents can exhibit the liquid (in terms of density) and salvation power/ gas (viscosity, diffusion and surface tension). These properties can facilitate the higher yield of bioactive compounds within a short time. In case of supercritical fluid extraction (SFE), there is a need for a good mobile tank (consisting of CO2 pump), solvent vessel, oven, controller and also a trapping vessel [75,76]. Now most of bioactive extraction is done by application of green technologies compared to conventional methods and in this context, supercritical CO2 extraction is discussed with better yield of naringin from citrus paradise. This approach uses the ethanol as a modifier (14% by wt.) at same process conditions like 58.6oC temperature and 9.5 MPa pressure [77].
This technique is applied for phenolic compound extraction from rice wine lees wastes with uses of Soxhlet extraction (SE) and SFE. Then it was compared for yield of bioactive compounds with reduced extraction (1 h compared to 6 h in case of traditional extraction technique) using the less ethanol needed with better yield of phenols (43%) [78]. In the process of SFE, carbon dioxide is a common solvent, used in food sector tasks and it is safe with easily attainable critical conditions (at 30.9 and 73.8 bar) for food processing. Some major limitations such as low polarity are disclosed but it can be improved by using polar solvents like methanol. Ethanol, dichloromethane and acetone) [77,78]. These can work as modifiers with capability to improve its solvating power and also enhance its extraction efficiency with minimum/ no interaction between analytes and matrices. Some parameters like low diffusibility of the solvent into matrix, extended extraction time, high-pressure requirement and expensive infrastructures can also be found some challenges for this technique [76,78]. Further, consistency and reproducibility during the continuous process can be found as some more limitation of this extraction technique and these can stop scalability of this technique [77,79].
3.5. Microbial Fermentation for Jackfruits Waste Conversion
For better utilization of jackfruits wastes, it needs several types of effective pretreatment process and it can be applied as physical, chemical and biological processes to hydrolyse its complex organic matter. These strategies help in valorization of jackfruits wastes into value-added products including fuels (bioethanol or biogas,)/ other bioproducts (bioplastic, feeds, or functional food additives) [80]. From jackfruit wastes, nowadays, bioenergy production and promotion is also carried out by several researchers groups and it can utilize the jackfruit waste as renewable resources and it is also an eco-friendly and cost-effective process to generate the alternative fuels options against fossil fuel [81]. In recent years, efficient bioconversion of jackfruit wastes into several types of fuel sources is done with the help of microbial fermentation process by using different microbes such as bacteria, yeast, fungi [74,75]. Yeast (like Saccharomyces cerevisivae for ethanol), bacteria (for methane, methanogen like Methanosarcina barkeri and Methanococcus maripaludis) and fungi (like Rhizopus oryzae MNT 006, Aspergillus oryzae MNT for ethanol) is reported for biofuel production [16,37,82]. And these efforts can help in reduction of environmental pollution and also help in bioremediation processes (i.e., several toxic dye removal) in water sources/ aquatic environments that were contaminated with dye color. Some review papers have addressed the color dye removal from water bodies with help of jackfruit waste uses with finding of utilization feasibility. This effort can solve the several serious ecological problems in jackfruit producing nations [81,83].
Several reports claim a waste generation quantity of jackfruit in the range of 5 to 7 kg wt. per fruit and this waste showed its potential for conversion into a wide range of bio-products such as biofuels, animal feeds or bioactive components and these are used in bakery and packaging material industries. In case of wastes (including jackfruit wastes also) materials hydrolysis, physical, chemical and biological pretreatments processes are applied and it helps in conversion into simple sugars and also in final production synthesis under effort of valorization [84]. Utilization of jackfruit wastes can result in several fuels products synthesis / production. These can be achieved by application of pretreatment and extraction steps via valorization of waste biomasses in an effective and successful manner. In recent years, numbers of vaporization technologies have developed for jackfruits waste hydrolysis and later these helped to conversion of desired products via utilization of several bioprocesses / green extraction steps and it can promote sustainable products utilization in the biorefinery and also the bioeconomic of the world [85]. Normally jackfruit is reported to contain 0- 80% non-edible parts and out of this quantity, 60% of jackfruit wastes is outer rind, perianth and central cores parts. Number of analyses for biochemical composition for jackfruit waste were done with utilization of these wastes with recovery of health benefits products [84,85]. The peels of jackfruits waste are good sources of protein, cellulose and pectin and then seed waste is good sources of carbohydrate (76%), protein (18%) and lipid content (2%). In context to bioenergy production from jackfruit wastes utilization, several types of pretreatment are applied as crucial steps with conversion capacity into complex forms of organic matter into simpler ones. Pretreatment step in the complex organic matter conversion process helps in enzymatic reactions of hydrolysis during the saccharification processes and this step can ensure the simple sugars form for fermentation via the help of different microbial agents [86]. Good example of jackfruit wastes conversion is found for ethanol production/ extraction process. This process utilizes the low-pressure and also high-intensity ultrasound processes that affect compositions and functionality of isolated proteins from jackfruit seeds [80]. Jackfruit waste is gone for several processing tasks by a variety of physical methods to develop the valuable products and some of these are irradiation, microwave processing, supercritical fluid and high pressure processing extraction as common and advanced processing methods [86,87].
In effort to hydrolysis of jackfruit wastes, several approaches/ methods of chemical treatment are applied in acidic or alkaline solution (low to high concentration) at temperature of 130oC and 210oC via mixing the waste matter. During the chemical agent assisted pretreatment process, waste matter is gone for a few minutes to few hours to get fermentable sugars and it can be dependent on pretreatment conditions [88,89]. Researchers have applied these processes for methane production, energy potential and also environmental benefits using jackfruit peels. The effect of methane generation via pretreatment of jackfruit wastes with 5% alkaline hydrogen peroxide (AHP) and it has shown its influence in production of enhanced methane yield and also biodegradability (up to 70%) while compared to untreated waste matter [90]. Some studies were done on analysis of potential of annual energy production from jackfruits, treated with 5% AHP solution. Alkaline extraction techniques were applied for isolation of starch from jackfruit seed wastes and then it has 18% starch from seeds [89,90].
During the conventional chemical and physical pretreatment process, high investment of reagents, machinery and energy is needed and in those treatment processes, it is needed before biological pretreatments. These can be applied for cellulosic and lignocellulosic matters for enzymatic saccharification processes [91,92]. During the biological treatment process, many living agents such as fungi/ bacteria are used and it utilizes less energy and is also an eco-friendly process. Many microbial agents are known to possess the cellulolytic and hemicellulolytics activities that can be utilized for jackfruits waste utilization task [93]. Among several microbial systems, Saccharomyces cerevisivae is applied for ethanol production from jackfruit wastes and ethanol contains the 35% oxygen contents and it can be utilized for burning process for production of lesser quantity of nitrogen and also particulate matters than the gasoline combustion process [90,93]. And Figure 5 discussed the pretreatment approaches for hydrolysis and also bioenergy development.
3.6. Value Added Products
In this context, huge quantity of various nature of fruits and vegetables wastes are generated due to different processing activities in food sector every year. And it can be excellent sources for different nature of valuable components like biofuels and polyphenols and these are utilized for different needs to human life [94]. Green extraction technology, often known as non-traditional approach, has been a significant subject of research in recent years [95]. These green technologies have replaced previous traditional methods due to their high yield, shortened process time, products of superior quality, and low waste creation [89,91]. During the pretreatment process, disruption of hydrogen bond is also found and it can occur easily after addition of chemical solvent and then it is heated at high temperature. This can cause dipole rotation of molecules and migration of ions. This changes in matter can occur with diffusion of the solvent and it can lead to the dissolution of the components [96]. Another mechanism during the pretreatment process is reported as evaporation of the moisture within cells and it can induce the high pressure on the cell wall and then change the physical properties of the materials [97]. This can lead the modification in porosities of biomaterials. This can occur with increased penetration of solvents with improved yield of biomaterials [98,99]. Various types of bioproducts from jackfruit wastes are discussed in below section.
3.7. Bioethanol
In context to value-added products recovery from jackfruit wastes, it has discussed bioethanol production from enzymatic hydrolysis and microbial fermentation of cellulosic biomass including jackfruit wastes and it is cost-effective and eco-friendly technique/ approach [100]. In this fuel synthesis process, jackfruit wastes like peel material are utilized as potential feedstock and this waste matter contains high quantities of carbohydrates contents/ %. Numbers of studies were done for saccharifying jackfruit rind matter via application of recombinant enzyme endoglucanase from Bacillus subtilis strain MUS1 microbes at temperature of 50oC and pH 5.0 with 15 mg/ ml substrates ratio/ quantity 101]. This recombinant enzyme from Bacillus subtilis can help in generation of more quantities of sugars during the saccharification process of jackfruits wastes [41,100]. From different types of wastes, bioethanol is produced as alternative energy sources. These wastes are good sources of natural products like carbohydrates and then it is utilized as potential feedstock for ethanol production with the hydrolysis and fermentation process. In this context, jackfruit seed is the best feedstock with rich sources of carbohydrates [101]. Number of research studies are done on determination of pH effect on carbohydrates hydrolysis that can be utilized for bioethanol production. For hydrolysis of carbohydrates from jackfruit seed, effective pretreatment with a pH dependent process was done by application of a separate fermentation hydrolysis (SHF) process [102].
This process has used the sulphuric acid solution as hydrolysing agents. Later, the fermentation process with help of Saccharomyces cerevisiae strain was used in a fermentor vessel at different pH values (such as 2, 3 and 5) for 70h period. From this experiment results, it was claimed for optimum glucose content (75%) and pH (3.0) that supported the high concentration of bioethanol (58%) in fermentation broth [101,102,[101,102,]. From this research work, it has discussed the fermentation stage role and it was found that high concentration of glucose can push the high concentration of bioethanol with linear relationship [103]. This published paper talked about the glucose concentration and high ethanol concentration from jackfruit seeds waste. This jackfruit seed showed the high potential for feedstock for bioethanol biosynthesis at cheap price [101,103].
Bioethanol production from fermenting raw matters like jackfruit waste is reported with the suitable microbial system. During the fermentation process, ethanol production jackfruit wastes is reported with application Saccharomyces cerevisiae strain. This yeast strain showed high capability for ethanol production and it is due to natural adaptation properties and also high tolerating sugar/ ethanol/ chemical inhibitor [104]. Ethanol can be synthesized by petroleum product/ by fermentation process. In the biological process, cellulose or hemicelluloses from jackfruit wastes is hydrolysed and then it is utilized for the ethanol production process. Most lignocellulosic biomass including jackfruit waste is rich sources of carbohydrates and then it needs the effective pretreatment process for fermentative sugars that can be used in ethanol production. Later, product separation and purification process is also needed to get the pure ethanol [101,104]. For this bioethanol production, jackfruit peels were selected as potential substrates and then enzyme/ microbial system (Saccharomyces cerevisiae) was used for getting the fermentative sugars and ethanol. Now, ethanol can be present in alcoholic drinks with more uses of this yeast in the bakery industry and fermented food and alcoholic drink preparation/ production [103,104]. Some efforts were done on jackfruit straw waste and then it was processed with fermentation as starter substrates. From this process, bioethanol was separated by a distillation process. Starter mass and fermentation time was checked to achieve the maximum ethanol yield with utilization of yeast like S. cerevisiae and urea as N- nutrients [105].
At optimum yeast mass (40g) of S. cerevisiae with distillate volume of 13.6 ml. It has been found after 96 h of fermentation rime, distillate / ethanol yield was 15.2 ml. But at optimal condition of fermentation, volume of bioethanol distillate was found to be 30 ml with the distillation temperature at 70-80oC. From this approach for ethanol production, its refractive index (1.354), density (0.367 g/ ml) and boiling points (71-72oC) was reported [106]. Another report, bioethanol production is discussed from utilization of Sri Lankan rotten fruits (without skin) including with jackfruits wastes. This ethanol was produced in a batch process with optimization of fermentation process parameters [106,106]. In optimization of fermentation process, some optimization techniques like Genetic algorithm (GA), Response Surface Methodology (RSM) and also Particle Swarm optimization (PSO) were discussed. During the bioethanol production, different overripe fruits were taken and these fruits were jackfruit, papaya and banana with uses of two microbes at three fermentation conditions. During these experiments, maximum ethanol yield with RSM (13.4 vol. %), GA (13.4 vol. %) and PSO (13. 36 vol. %) by the use of banana variety fruit fermentation and Pseudomonas mendocina microbial strain (ratio~ 1:1), pH (5.1) and temperature (35oC) [107,108].
3.8. Biogas
Biogas generation from different types of jackfruit wastes is a good effort for sustainable fuels sources. In this context, potential of biogas production is found from different fruit wastes like banana peels, jackfruit wastes, and pineapple wastes and these were gone for co-digestion process with cow drug and then this effort helped to provide alternative energy sources [109]. During these experiments, substrates from each fruit waste were sent to the co-digestion process with varying ratios (0%, 25%, and 50%) of cow dung. These were done in laboratory scale anaerobic digesters (up to capacity of 250 ml) and then it was run for 30 days to generate or produce the biogas from jackfruit (82.3 ml), banana fruit (189 ml) and also pineapple fruit waste/ peel (262 ml) is found [110]. In another experiment of this study, jackfruit waste, pineapple waste and banana peels were co-digested with 25% of cow dung and then biogas production from these fruits was increased by two to three times [110,111].
And 50% of cow dung with these fruits waste has improved the biogas yield by two folds. From these reported results, a mixture of jackfruit, banana peel and pineapple peel can be found much better in biogas production yield and it can help in the energy supply chain process for our daily needs [111]. During the biogas generation experiment, some efforts were done on experimental design, digester set-up, and volume and biogas composition determination that was produced from jackfruit waste, banana and pineapple peels with cow dung. And biochemical methane potential (BMP) assay protocol was applied for anaerobic digestion process [112]. Evaluation process for biogas quality attributes was done for process that used the from jackfruit waste, banana and pineapple peels with cow dung with batch digestion process. In this experiment, an anaerobic system was found to be 500 ml capacity and it was submerged in a 20 litre temperature regulating water bath and a 250 ml measuring cylinder for the generated biogas measurement task via displacement method. Temperature of the water bath was maintained at 36.5oC [109,112].
In context to value added products recovery, utilization of jackfruits was reported and it discussed the production of biogas, biochar and briquettes from jackfruit waste. In many developing countries, huge potential is found due to more quantity of organic waste accumulation and then it can be utilized for conversion into many types of fuel sources like biogas generation [37]. Waste organic matters in rural areas due to small holder farmers can be generated and then these can be utilized for different nature of biofuel sources. In context, biomass wastes like jackfruit waste can be managed to produce the bioenergy with mitigation in GHGs (greenhouse gasses) emission quantity via a well-managed way [113]. Now, decomposing of organic matter can be minimized via generation of biogas. Due to more quantities of agricultural waste like jackfruit waste can be utilized as cheap raw materials for production of bioproducts like biofuels, biochar and biopesticide with briquettes and others [114]. Biochar production from waste matter is good effort and then biochar can be mixed with the soils and it can help to produce soils rich in carbon with contribution in carbon dioxide sequestration and soil fertility [115]. Some papers have focussed on jackfruit waste utilization for biogas production from anaerobic digestion process with biochar and briquettes production. From the anaerobic process for jackfruit wastes utilization, biogas can be produced [110]. It needs a high temperature for jackfruit waste decomposition tasks to help in biochar production. This effort of research can help various products synthesize information with help in mitigation of climatic changes and also carbon dioxide sink in soil [37,115]. And Figure 6 discusses the jackfruit wastes with different microbial / chemical transformation approaches for many products synthesis to support the zero-waste generation.
3.9. Bioplastic
Bioplastic production from Bacillus megaterium strain JHA is reported with high capacity of this microbial stain and this microbe was isolated from oil contaminated soils and then it was tested in laboratory on glucose substrates consumption that can result in high quantity of polyhydroxyalkanoates (PHA) accumulation in this microbes [116]. This plastic was biosynthesized by Bacillus megaterium with utilization of jackfruit waste like seeds with some inorganic or organic matter/ compounds. This waste matter was found suitable for high accumulation of PHA inside microbial cells. Some additional efforts were done on characterization of the bioplastic layer that was formed inside the cell due to PHA [117]. Several viable applications of PHA were found in medical science, cosmetic and pharmaceutical products synthesis. For best synthesis of PHA, advanced research is needed with optimization of process parameters and it can promote huge quantities of PHA from cheaper feedstock like jackfruit wastes. This waste matter is good sources of carbon and nitrogen with finding an economically viable process for commercial scale [116,117]. Further PHA product is biodegradable plastic and showed it is an effective and durable process. In effort to jackfruit waste hydrolysis, biological pretreatment is a promising and eco-friendly process for complete conversion into fermentable sugars [118]. Biological pretreatment needs optimal process parameters to achieve complete hydrolysis of waste biomass. The effect of pretreatment like physical and chemical processes can find the impact of enzymatic conversion of waste biomasses into simple ones with achieving bioproducts synthesis [117,118]. And Table 1 discusses the more example of bioenergy/ other value-added products
3.10. Bioactive Compounds
Pectin used in the food sector and pharmaceutical / cosmetics sectors is reported to serve as various agents like emulsifiers, binders and stabilizers) with performing various functions [33]. Polysaccharides sources in jackfruit peels can help in human diet and then it can maintain good immunity activity with some protective functions like cancer, blood sugar, ulcer, and bad cholesterol [34]. In pharmaceutical industries, pectin is utilized as bonding agents for various formulations of pills and also multi-purposes delivery. In food sectors, use of jackfruit peels is used in papers, paints, optics and also environmental remediation with biofuel sectors. In case of phenolic compound yield from orange peel, improvement in yield (15%) was achieved by PEF at power of 7KV/cm 62].
Reports on greater yield of phenolic compounds (102.9 mg GAE/100g food waste) and flavonoid compounds (37.6% QE/100g FW) from various wastes, such as onion waste, are described in this approach, with superior yield improvement (2.2 and 2.7 times, respectively) compared to control samples. The effects of electric field intensity and extraction duration on phenolic and flavonoids compound yields are demonstrated [63]. Nowadays, efforts are being made in the food processing sector to produce nanoemulsions, which can be useful in the delivery system for various bioactive substances capable of performing various functions. These actions boost bioavailability, regulate ingredient discharge, alter product texture, as well as preserve the substance from degradation [98]. In this technique, intensive research is going on to make it more effective in terms of a broad range of bioactive extraction from any type of wastes including jackfruit waste. For the nanoemulsion process, the delivery system can proceed for bioactive compounds with understanding of specific functions especially in food-based delivery systems and bioavailability in the human body. Some recent studies are done for understanding mechanisms on desirable bio-accessibility, metabolism and absorption of the encapsulated compounds and these can help in alteration in their properties in the gastro-intestinal tract [99].
Further studies were done loading capacity of bioactive compounds that are encapsulated form like nanoemulsion, exhibiting the better release properties of bioactive compounds [98,99]. Cultivation of jackfruit plants in the world is good and potential sources of valuable biomaterials and wastes from jackfruit plants is a good source of carbohydrates, fats, protein and also phytochemicals [97,99]. In the case of bioactive compounds from wastes of fruits/ vegetable sources, it showed a positive impact on human health via contributing to modulation of the metabolic processes and also cellular activities [94]. Some bioactive compounds showed their properties of antioxidants, anti-cancer, anti-inflammation and anti-allergenic. Some compounds contribute to anti-atherogenic activity and these properties of bioactive compounds can depend on the pathways and also their bioavailability in the human body. In categories of bioactive compounds, some are hydrophobic in nature and they showed less bioavailability in the human body [95]. In this context, some efforts have been made on technological advancement such as nano-emulsions application. And this effort helped in enhancing their stability and functional properties. Bioactive substances can be obtained via traditional and non-traditional methods, each with its own set of benefits and drawbacks [94,95]. And Table 2 shows the bioactive compounds with health benefits from jackfruit wastes.
4. Advantages, Limitations and Drawback of Various Valorization Techniques
The valorization techniques are basically two types (conventional, and novel-based). The technique preferred will depend on the parts of the fruit plants to be converted to useful bioactive compounds, mostly based on moisture content and composition of food waste to avoid energy-intensive processes. For an example, jackfruits leaves, barks, fruits can be easily valorized through various conventional methods such as maceration, percolation, decoction, reflux and through soxhlet extraction to develop various medicine for health care needs, while other bioactive compounds extraction need employment of advanced technologies [94,95,96,97]. The sustainability of valorization process is of paramount importance in efficient reduce, reuse and recycle, and in transitioning to circular bioeconomy. The end-use products derived from the fruit waste, and the scale of production also need to be looked into the environmental, ecological and social prospectives. The green technology/novel technologies employed are always more eco-friendly than the conventional approaches [86,89].
Application of efficient bioconversion of jackfruit wastes (like peels) can generate varieties of useful material by facilitation by microbial fermentation process and this material can be applied for reduction of water pollutant (like dye/ color removal under bioremediation) in contaminated aquatic system. This is good example of green economic model that is used for waste utilization [8]. Numbers of researches have claimed for utilization feasibility of jackfruit wastes and then these were converted into value-added products via reducing/ mitigating the waste quantity with protection of environment in a sustainable modes. Huge quantities of jackfruit wastes is generated due to modern practices and now interests have developed to convert it into varieties of applications and it is due jackfruit waste capabilities that keep diverse array of functions [119]. As discussed in earlier section about jackfruit waste nutrients amounts and varieties. This waste is good sources of carbohydrates, protein or mineral and these nutrients are utilized for cultivating the diverse groups of microbial system/ group that can be produced the various natures of metabolites like organic acids, polysaccharides, enzymes, or therapeutic compounds [8,119]. In conversion processes of jackfruit wastes, different type of pretreatments or bioprocesses like physic-chemical, biological and innovative green pretreatment method are discussed [88]. Further it requires/ needs to investigation of more genetically and biotechnologically advanced methods that can utilize the complete fractions of jackfruit wastes and then it converted to 100% into different products in direct ways with more functional natures. Further, it needs more researches on advances in technology, equipment, methodologies that can make necessary changes with promotion to biorefineries with generation of varieties of value-added products [8,88].
5. Prospect of Jackfruit Waste as a Bio-absorbant for Pollution Control
Recent studies have shown that the jackfruit peel is an effective low-cost bio-absorbant for removal of chromium and nickel from aqueous solution () . Besides, since jackfruit waste are rich source of pectin, they can be useful for economic removal of cadmium from water bodies/water subjected various chemical pollution [30]. Various reports have claimed for jackfruit leaves (JLP) utilization as important wastes and it is suitable agro-waste categories based materials and then it can utilize for efficient removal of metal (like lead~Pb –II form) from wastewater. Due to surface medication of jackfruit leaf power (JLP), it can able to remove high % of lead concentration [120]. Further the surface modification of jackfruit leave was achieved by application of chemical reagents like isopropyl alcohol (20%) and then followed by treatment with alkali sodium hydroxide (AIJLP) and tartaric acid (TIJLP). These modifications were confirmed by BET surface area (29 m2/g). And then chemical modification was resulted by 1.5 fold high/ increase and 2.5 fold increase in surface area due to AIJLP (50 m2/g) and TIJLP (72 m2/g) impacts [121]. After generating the low cost bioabsorbents from JLP, it can applied in batch experiment to confirm the lead adsorption and then it was put effort to find the optimal equilibrium conditions like pH, contact time and lead concentration. After that from developed bioabsorbsent, pore size and pore volume were also analysed by Barrett-Joyner-Halenda (BJH) model/ methods [120,121]. And this model has helped the adsorption mechanism. Recently by reusability studies it was confirmed that AIJLP can efficiently remove the lead contaminant/ pollutant up to 95% up to five cycles. And these results have recommendation of application of AIJLP for bioremediation task in wastewater [122]. Due to uses of low cost material like JLP from jackfruit leaves and later it was modified by chemical reagents to increase the surface area and it was checked for its cost (up to 11 USD/ kg) and it included the cost of collection of raw material, washing and then drying process and in last chemical reagents were used to get it final form. Some miscellaneous cost was also added. This is good examples of low cost bioadsorbesnt from jackfruit leaves [122,123].
6. Conclusions and Future Perspectives
This paper mainly focussed on jackfruit waste as a potential and sustainable source in augmenting energy requirement, and meeting the global challenges. There are plenty of this resource available in the world for sustainable conversion of jackfruit waste to a diverse bioactive compounds. Jackfruit trees can be found grown in wide ecological regions, and they can easily be raised as plantation with minimum agrocare. We found that huge quantities of jackfruit wastes are generated from many countries in the world and now it becomes a big challenge to utilize these wastes sources from the environment. Long-term accumulation and disposal to aquatic bodies can become a big threat to aquatic animals with the creation of water pollution. To avoid these issues, now researchers have applied the many approaches of valorization to recover biofuel/ other biochemicals in sustainable manners. These efforts can help in promotion of zero-waste generation from complete utilization of jackfruit wastes with production of bioactive compounds. These efforts in value-added products from jackfruit wastes can be found as cheap sources of feedstock with reduced price of bioproducts. In coming periods, how many green techniques like MAE, UAE and SCF-AE are applied with green extraction solvents to get more effective bioproducts with high yield compared to traditional technique. Different forms of jackfruit wastes can be mitigated in systematic manners via synthesis of a number of value-added products including cheap sources of adsorbent, nano-particles and also biogas and other biofuels. Energy sources from jackfruit wastes can be achieved by microbial fermentation with specific microbial cell systems and anaerobic digestion. Some application of biochar is discussed in the soil amendment process that increases the soil fertility and also carbon/ nitrogen content. This review can help to provide several ideas for sustainable fuel development with the maintenance of a clean environment. In this review, authors discuss the different natures of jackfruit wastes sources and then these were analysed/ studied for nutrients determination. Later different valorization techniques like green extraction, preparation of bioactive materials approaches, microbial fermentation were applied to generate/ recover the nutrients/ bioproducts. These conversion approaches are very effective to generate 100% products from these wastes without any further waste left in environment. This is good strategies to achieve the zero-wastes in different conversion process. Our objectives for this review was achieved due to mitigation of jackfruit wastes/ recovery of nutrients. These efforts are resulted into sustainable products development with zero-wastes in process.
Abbreviations
AgNO3: Silver nitrate; AgNPs: Silver nanoparticles; AHP: Alkaline hydrogen peroxide; ASPE: Artocarpus heterophyllus seed powder extract; BMP: Biochemical methane potential; BWR: Bread waste residues; CF: Crude fibre; CP: Critical point; CP: Crude protein; DPPH: a,a-Diphenyl-β-picrylhydrazyl; EAE: Enzyme assisted extraction; FTIR: Fourier-transform infrared; GA: Genetic algorithm; JSP: Jackfruit seed powder; MAE: Microwave-assisted extraction; MCC: Microcrystalline cellulose; NFE: Nitrogen free radical; Pc: pressure point; PEF-AE: Pulse electric field assisted extraction; PHA: Polyhydroxyalkanoates; POJ: Peel of jackfruit; PSO: Particle Swarm optimization; RSM: Response Surface Methodology; SC-FE: Supercritical fluid extraction process; SE: Soxhlet extraction; SEM: scanning electron microscopy; SHF: Separate fermentation hydrolysis; Tc: Specific temperature; UAE: Ultrasonic-assisted extraction; XRD: X-ray diffraction
References
- Thanh, L.P.; Truong, P.; Kha T.; Hang TT.T.; Jackfruit leaves can totally replace traditional grass in the diet of lactating dairy goats, J. Appl Animal Res. 50(1), (2022) ,97-102. [CrossRef]
- Ranasinghe, R.A.S.N.; Maduwanthi, S.D.T.; Marapana, R.A.U.J. Nutritional and Health Benefits of Jackfruit (Artocarpus heterophyllus Lam.): A Review. Int. J. Food Sci. 2019, 2019, 1–12. [CrossRef]
- Chavan, S.; Yadav, B.; Atmakuri, A.; Tyagi, R.; Wong, J.W.; Drogui, P. Bioconversion of organic wastes into value-added products: A review. Bioresour. Technol. 2021, 344, 126398. [CrossRef]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of Agro-Waste into Value-Added Bioproducts and Bioactive Compounds: Micro/Nano Formulations and Application in the Agri-Food-Pharma Sector. Bioengineering 2023, 10, 152. [CrossRef]
- Sreeja Devi, P.S.; Kumar, N.S.; Sabu, K.K. Phytochemical profiling and antioxidant activities of different parts of Artocarpus heterophyllus Lam. (Moraceae): A review on current status of knowledge. Futur J Pharm Sci. 7 (2021), 30. [CrossRef]
- Siregar, A.B.; Bulan, R.; Yusak, Y. Antibacterial & antioxidant properties of leave & stem bark extract of Artocarpus heterophyllus as the component of peel-off mask. Int J Sci Technol Eng. 5(4), (2018), 101–106.
- Brahma, R.; Ray, S. A Comprehensive Review on the Recent Advances in the Valorization of Jackfruit Waste for the Development of Value-Added Products. J. Food Technol. Res. 2022, 9, 120–134. [CrossRef]
- Sundarraj, A.A.; Ranganathan, T.V. Physicochemical characterization of Jackfruit Peel. Res J Pharm Biol Chem S. 8, (2017) 2285–95. [CrossRef]
- Begum, R.; Aziz, M.G.; Yusof, Y.A.; Saifullah; Uddin, M.B. Evaluation of gelation properties of jackfruit (Artocarpus heterophyllus) waste pectin. Carbohydr. Polym. Technol. Appl. 2021, 2, 100160. [CrossRef]
- Lubis, M.; Gana, A.; Maysarah, S.; Ginting, M.H.S.; Harahap, M.B. Production of bioplastic from jackfruit seed starch (Artocarpus heterophyllus) reinforced with microcrystalline cellulose from cocoa pod husk (Theobroma cacao L.) using glycerol as plasticizer. IOP Conf. Series: Mater. Sci. Eng. 2018, 309, 012100. [CrossRef]
- Cagasan, C.U.; Li̇ngatong, C.A.V.; Pore, K.M.T.; Ramada, R.V.; Restor, C.D.D.; Lauzon, R.D. Production and Quality Evaluation of Wine from Jackfruit Co-Products. Int. J. Life Sci. Biotechnol. 2021, 4, 340–352. [CrossRef]
- Widjaja, C.; Djojorahardjo, Y.; Kurniawan, A Irawaty, W.; Soetaredjo, F.E. Biorefinery concept on jackfruit peel waste: bio-oil upgrading. ARPN J Eng Appl Sci. 13 (2018) 2202–7.
- George, N.; Debroy, A.; Bhat, S.; Singh, S.; Bindal, S. Biowaste to Bioplastics: An Ecofriendly Approach for A Sustainable Future. J Appl Biotechnol Rep. 8(3) (2021) 221-233. [CrossRef]
- Meera, M.; Ruckmani, A.; Saravanan, R.; Prabhu, R.L. Anti-inflammatory effect of ethanolic extract of spine, skin and rind of Jack fruit peel – A comparative study. Nat. Prod. Res. 2017, 32, 2740–2744. [CrossRef]
- Ojwang, R.A.; Muge, E.K.; Mbatia, B.; Mwanza, B.; Ogoyi, D.O. Comparative Analysis of Phytochemical Composition and Antioxidant Activities of Methanolic Extracts of Leaves, Roots and Bark of Jackfruit (Artocapus heterophyllus) from Selected Regions in Kenya and Uganda. J. Adv. Biol. Biotechnol. 2017, 16, 1–13. [CrossRef]
- Soumya, B.; Gupta, P.; Vikas, R.; Pradeep, K. .Chemoenzymatic saccharification of Artocarpus heterophyllus Lam.(jackfruit) peel waste and its utilization for bioethanol production. Indian Forest, 145(12) (2019)1204-1209.
- Kahar, A.W.M.; Lingeswarran, M.; Hulwani, M.Z.A.; Ismail, H. Plasticized jackfruit seed starch: a viable alternative for the partial replacement of petroleum-based polymer blends. Polym. Bull. 2018, 76, 747–762. [CrossRef]
- Marichelvam, M.; Manimaran, P.; Sanjay, M.; Siengchin, S.; Geetha, M.; Kandakodeeswaran, K.; Boonyasopon, P.; Gorbatyuk, S. Extraction and development of starch-based bioplastics from Prosopis Juliflora Plant: Eco-friendly and sustainability aspects. Curr. Res. Green Sustain. Chem. 2022, 5, 100296. [CrossRef]
- Ahmed, M.; Nasar, A. Utilization of Jackfruit Peel as a Low-cost Adsorbent for the Removal of Methylene Blue Dye from Synthetically Polluted Water. Curr. Anal. Chem. 2021, 17, 1016–1026. [CrossRef]
- Butool, S.; Butool, M. Nutritional quality on value addition to jack fruit seed flour. Int J Sci Res. 4, (2015) 2406–11.
- Sundarraj, A.A.; Ranganathan, T.V. Phytochemical constituents and thin-layer chromatography evaluation of the ethanolic extract of jackfruit (Artocarpus integer) peel. J Pharm Res. 12(5), (2018) 717.
- Pathak, N.; Singh, P.; Singh, P.K.; Sharma, S.; Singh, R.P.; Gupta, A.; Mishra, R.; Mishra, V.K.; Tripathi, M. Biopolymeric nanoparticles based effective delivery of bioactive compounds toward the sustainable development of anticancerous therapeutics. Front. Nutr. 2022, 9, 963413. [CrossRef]
- Bhat, V.; Mutha, A.; Dsouza, M.R. Pharmacognostic and physiochemical studies of Artocarpus heterophyllus seeds. Int J ChemTech Res. 10(9), (2017) 525–536.
- Sundarraj, A.A.; Ranganathan, T.V. Phytochemical screening and spectroscopy analysis of jackfruit (Artocarpus integer Thumb. peel. Int Res J Pharm. 8(9) (2017) 151–159.
- Jagtap, U.B.; Bapat, V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crop. Prod. 2013, 46, 132–137. [CrossRef]
- Nur Hanani, Z.A.; Aelma Husna, A.B.; Nurul Syahida, S.; Nor Khaizura, M.A.B.; Jamilah, B. Effect of different fruit peels on the functional properties of gelatin/polyethylene bilayer films for active packaging. Food Packag. Shelf Life 2018, 18, 201–211. [CrossRef]
- Islam, M.R.; Haque, A.R.; Kabir, M.R.; Hasan, M.M.; Khushe, K.J.; Hasan, S.M.K. Fruit by-products: the potential natural sources of antioxidants and α-glucosidase inhibitors. J. Food Sci. Technol. 2021, 58, 1715–1726. [CrossRef]
- Jain, R.; Mendiratta, S.; Kumar, L.; Srivastava, A. Green synthesis of iron nanoparticles using Artocarpus heterophyllus peel extract and their application as a heterogeneous Fenton-like catalyst for the degradation of Fuchsin Basic dye. Curr. Res. Green Sustain. Chem. 2021, 4, 100086. [CrossRef]
- Jancy, S.; Shruthy, R.; Preetha, R. Fabrication of packaging film reinforced with cellulose nanoparticles synthesised from jack fruit non-edible part using response surface methodology. Int. J. Biol. Macromol. 2019, 142, 63–72. [CrossRef]
- Kalse, S.; Swami, S. Recent application of jackfruit waste in food and material engineering: A review. Food Biosci. 2022, 48. [CrossRef]
- Mahesh, R.; Surendrababu, K.; Kumar, S.S.; Anoop, C.T.; Nuhaiz, V.P. Emission characteristics of jackfruit peel oil with three hole nozzle. IOP Conf. Series: Mater. Sci. Eng. 2020, 993, 012010. [CrossRef]
- Mardarveran, P.; Mokhtar, N.M. Analysis of Artocarpus heterophyllus peel as a natural coagulant using response surface methodology (RSM). J. Technol. 82 (4) (2020). [CrossRef]
- Brahma, R.; Ray, S. In-depth analysis on potential applications of jackfruit peel waste: A systematic approach. Food Chem. Adv. 2022, 1. [CrossRef]
- Sundarraj, A.A.; Ranganathan, T.V.; Gobikrishnan, S. Optimized extraction and characterization of pectin from jackfruit (Artocarpus integer) wastes using response surface methodology. Internat. J. Biologic. Macromolec. 106, (2018) 698-703.
- Jung, J.-M.; Kim, J.Y.; Kim, J-H.; Kim, S.M.; Jung, S.; Song, H.; Kwon, EE. Choi, Y.-E. Zero-waste strategy by means of valorization of bread waste. J Cleaner Product. 365 (2022), 132795. [CrossRef]
- Zhang, C.; Kang, X.; Wang, F.; Tian, Y.; Liu, T.; Su, Y.; Qian, T.; Zhang, Y. Valorization of food waste for cost-effective reducing sugar recovery in a two-stage enzymatic hydrolysis platform. Energy 2020, 208. [CrossRef]
- Nsubuga, D.; Banadda, N.; Kabenge, I.; Wydra, K.D. Potential of Jackfruit Waste for Biogas, Briquettes and as a Carbondioxide Sink-A Review. J. Sustain. Dev. 2020, 13. [CrossRef]
- Zhang, C.; Ling, Z.; Yang, L.; Liu, Y.; Cao, T.; Sun, Y.; Liu, W.; Huo, S.; Zhang, Z.-H.; Su, H.; et al. Efficient caproate production from ethanol and acetate in open culture system through reinforcement of chain elongation process. J. Clean. Prod. 2023, 383. [CrossRef]
- Wu, Q.; Bao, X.; Guo, W.; Wang, B.; Li, Y.; Luo, H.; Wang, H.; Ren, N. Medium chain carboxylic acids production from waste biomass: Current advances and perspectives. Biotechnol Adv. 37(5), (2019), 599-615. [CrossRef]
- Daud, M.N.H.; Ahmad, R.; Abdullah, N.; Jabit, M.L.; Wibowo, A. Effect of storage on antioxidant activity and bioactive compound of Artocarpus heterophyllus J33 rind extract. Adv Appl Chem Biochem. 2019,1, 68–81.
- Nuriana, W.; Wuryantoro Ethanol Synthesis from Jackfruit (Artocarpus Heterophyllus Lam.) Stone Waste as Renewable Energy Source. Energy Procedia 2015, 65, 372–377. [CrossRef]
- Ginting, M.H.S.; Irvan; Misran, E.; Maulina, S. Potential of durian, avocado and jackfruit seed as raw material of bioethanol: a review. IOP Conf. Series: Mater. Sci. Eng. 2020, 801. [CrossRef]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioprocess. 2019, 6, 26. [CrossRef]
- Štambuk, P.; Tomašković, D.; Tomaz, I.; Maslov, L.; Stupić, D.; Kontić, J.K. Application of pectinases for recovery of grape seeds phenolics. 3 Biotech 2016, 6, 1–12. [CrossRef]
- Pathak, N.; Singh, S.; Singh, P.; Singh, P.K.; Singh, R.; Bala, S.; Thirumalesh, B.V.; Gaur, R.; Tripathi, M. Valorization of jackfruit waste into value added products and their potential applications. Front. Nutr. 2022, 9, 1061098. [CrossRef]
- Lal, A.N.; Prince, M.; Kothakota, A.; Pandiselvam, R.; Thirumdas, R.; Mahanti, N.K.; Sreeja, R. Pulsed electric field combined with microwave-assisted extraction of pectin polysaccharide from jackfruit waste. Innov. Food Sci. Emerg. Technol. 2021, 74. [CrossRef]
- Jiang, Z.; Shi, R.; Chen, H.; Wang, Y. Ultrasonic microwave-assisted extraction coupled with macroporous resin chromatography for the purification of antioxidant phenolics from waste jackfruit (Artocarpus heterophyllus Lam.) peels. J. Food Sci. Technol. 2019, 56, 3877–3886. [CrossRef]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, environment-friendly and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [CrossRef]
- Azooz, E.A.; Ridha, R.K.; Abdulridha, H.A. The Fundamentals and Recent Applications of Micellar System Extraction for Nanoparticles and Bioactive Molecules: A Review. Nano Biomed. Eng. 2021, 13. [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [CrossRef]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: a review. Int. J. Food Sci. Technol. 2018, 54, 1–13. [CrossRef]
- Nishad, S.; Saha, C. Kaur, Enzyme- and ultrasound-assisted extractions of polyphenols from Citrus sinensis (cv. Malta) peel: A comparative study. J Food Proc Preserv. 43 (8) (2019) 1-13. [CrossRef]
- Wan, N.; Kou, P.; Pang, H.-Y.; Chang, Y.-H.; Cao, L.; Liu, C.; Zhao, C.-J.; Gu, C.-B.; Fu, Y.-J. Enzyme pretreatment combined with ultrasonic-microwave-assisted surfactant for simultaneous extraction of essential oil and flavonoids from Baeckea frutescens. Ind. Crop. Prod. 2021, 174, 114173. [CrossRef]
- Rafińska, K.; Wrona, O.; Krakowska-Sieprawska, A.; Walczak-Skierska, J.; Kiełbasa, A.; Rafiński, Z.; Pomastowski, P.; Kolankowski, M.; Buszewski, B. Enzyme-assisted extraction of plant material – New functional aspects of the process on an example of Medicago sativa L.. Ind. Crop. Prod. 2022, 187. [CrossRef]
- Li, H.-Z.; Zhang, Z.-J.; Xue, J.; Cui, L.-X.; Hou, T.-Y.; Li, X.-J.; Chen, T. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants and rosmarinic acid from perilla leaves using response surface methodology. Food Sci. Technol. 2016, 36, 686–693. [CrossRef]
- Shen, X.; Shao, S.; Guo, M. Ultrasound-induced changes in physical and functional properties of whey proteins. Int. J. Food Sci. Technol. 2016, 52, 381–388. [CrossRef]
- ivković J, Šavikin K, Janković T, Ćujić N, Menković N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep Purif Technol 194 (2018) 40–47.
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT 2018, 101, 342–350. [CrossRef]
- Júnior, M.E.S.; Araújo, M.V.R.; Santana, A.A.; Silva, F.L.H.; Maciel, M.I.S. Ultrasound-assisted extraction of bioactive compounds from ciriguela (Spondias purpurea L.) peel: Optimization and comparison with conventional extraction and microwave. Arab. J. Chem. 2021, 14, 103260. [CrossRef]
- Carpentieri, S.; Jambrak, A.R.; Ferrari, G.; Pataro, G. Pulsed Electric Field-Assisted Extraction of Aroma and Bioactive Compounds From Aromatic Plants and Food By-Products. Front. Nutr. 2022, 8, 792203. [CrossRef]
- Pef-assisted Supercritical CO2 Extraction of Pigments from Microalgae Nannochloropsis Oceanica in a Continuous Flow System., 74, 97–102. [CrossRef]
- Pataro, G.; Carullo, D.; Siddique, A.B.; Falcone, M.; Donsì, F.; Ferrari, G. Improved extractability of carotenoids from tomato peels as side benefits of PEF treatment of tomato fruit for more energy-efficient steam-assisted peeling. J. Food Eng. 2018, 233, 65–73. [CrossRef]
- Pataro, G.; Bobinaitė, R.; Bobinas, Č..; Satkauskas, S.; Raudonis, R.; Visockis, M.; Ferrari, G.; Viskelis, P. Improving the Extraction of Juice and Anthocyanins from Blueberry Fruits and Their By-products by Application of Pulsed Electric Fields. Food Bioprocess Technol. 2017, 10, 1595–1605. [CrossRef]
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viskelis, P.; Ferrari, G. Application of pulsed electric field in the production of juice and extraction of bioactive compounds from blueberry fruits and their by-products. J. Food Sci. Technol. 2015, 52, 5898–5905. [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Obanijesu, E.O.; Alara, J.A.; Mudalip, S.K.A. Extract-rich in flavonoids from Hibiscus sabdariffa calyces: Optimizing microwave-assisted extraction method and characterization through LC-Q-TOF-MS analysis. J. Food Process. Eng. 2019, 43, e13339. [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2019, 51, 138–149. [CrossRef]
- Simić, V.M.; Rajković, K.M.; Stojičević, S.S.; Veličković, D.T.; Nikolić, N.Č.; Lazić, M.L.; Karabegović. I.T. Optimization of microwave assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Sep Purif Technol. 160 (2016) 89–97.
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2017, 6, 189–196. [CrossRef]
- Vilariño, M.V.; Franco, C.; Quarrington, C. Food loss and Waste Reduction as an Integral Part of a Circular Economy. Front. Environ. Sci. 2017, 5. [CrossRef]
- Alara, O.R.; Abdurahman, N.H. Microwave-assisted extraction of phenolics from Hibiscus sabdariffa calyces: Kinetic modelling and process intensification. Ind. Crop. Prod. 2019, 137, 528–535. [CrossRef]
- Alara, O.R.; Abdurahman, N.H., Adbul Mudalip, S.K. Optimizing microwave-assisted extraction conditions to obtain Phenolic compounds-rich extract from Chromolaena odorata leaves. Chem. Eng. Technol. 42(9) (2019), 1733-1740. [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Azhari, N.H. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crop. Prod. 2018, 122, 533–544. [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Alara, J.A. Optimization of microwave-assisted extraction of phenolic compounds from Ocimum gratissimum leaves and its LC–ESI–MS/MS profiling, antioxidant and antimicrobial activities. J. Food Meas. Charact. 2020, 14, 3590–3604. [CrossRef]
- Espinosa-Pardo, F.A.; Nakajima, V.M.; Macedo, G.A.; Macedo, J.A.; Martínez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod. Process. 2016, 101, 1–10. [CrossRef]
- Argun, M. E.; Argun, M. Ş.; Arslan, F.N.; Nas, B.; Ates, H.; Tongur, S.; Cakmakcı, O. Recovery of valuable compounds from orange processing wastes using supercritical carbon dioxide extraction. J. Cleaner Prod. 375, (2022), 134169. [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2018, 11, 12–17. [CrossRef]
- Dey, T.K.; Banerjee, P.; Chatterjee, R.; Dhar, P. Designing of ω-3 PUFA enriched biocompatible nanoemulsion with sesame protein isolate as a natural surfactant: Focus on enhanced shelf-life stability and biocompatibility. Colloids Surfaces A: Physicochem. Eng. Asp. 2017, 538, 36–44. [CrossRef]
- de Andrade Lima, M.; Andreou, R.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Carbon Dioxide Extraction of Phenolic Compounds from Potato (Solanum tuberosum) Peels. Appl. Sci. 11, (2021), 3410. [CrossRef]
- Constance, E. C., Ifunanya, W. U., Amarachi, M. C. E., Idera, E. E. C., Ikechukwu, N., Ogechi, P. C., & Perpetua, O. C.. Evaluation of vinegar production properties of Garcina kola and Artocarpus heterophyllus. J. Appl. Chem. Sci. Internat. 12(2) (2021), 48-60.
- Nguyen, T.K.; That, N.T.T.; Nguyen, N.T.; Nguyen, H.T. Development of Starch-Based Bioplastic from Jackfruit Seed. Adv. Polym. Technol. 2022, 2022, 1–9. [CrossRef]
- Ochaikul, D.; Noiprasert, N.;Laoprasert, W.; Pookpun, S.Ethanol Production on Jackfruit Seeds by Selected Fungi and Yeast from Loog-pang. KMITL Sci. Tech. J. 2012, 12 (1). 1-5.
- Miah, R.A.; Alam, M.J.; Khatun, A.; Suhag, M.H.; Kayes, N. The Decolorization and Phytotoxic Efficiency of Jackfruit Seed on a Textile Dye Novacron Blue. J. Eng. Adv. 2022, 6–11. [CrossRef]
- Abid, M.K.; Bin Ibrahim, H.; Zulkifli, S.Z. Synthesis and Characterization of Biochar from Peel and Seed of Jackfruit plant waste for the adsorption of Copper Metal Ion from water. Res. J. Pharm. Technol. 2019, 12, 4182. [CrossRef]
- Widjaja, C.; Djojorahardjo, Y.; Kurniawan, A.; Irawaty, W.; Soetaredjo, F.E. Biorefinery concept on jackfruit peel waste: bio-oil upgrading. ARPN J Eng Appl Sci. 13 (2018) 2202–7.
- C.P., S.; Sebastian, D. Jackfruit outer rind: A sustainable feedstock for fermentable sugar production using recombinant endoglucanase from Bacillus subtilis MU S1. Environ. Technol. Innov. 2019, 16. [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass – An overview. Bioresour. Technol. 2016, 199, 76–82. [CrossRef]
- Wang, L.; Wei, B.; Cai, F.; Chen, C.; Liu, G. Recycling durian shell and jackfruit peel via anaerobic digestion. Bioresour. Technol. 2021, 343, 126032. [CrossRef]
- Nelluri, P.; Venkatesh, T.; Kothakota, A.; Pandiselvam, R.; Garg, R.; Eswaran, V.; Vaddevolu, U.B.P.; Venkatesh, R.; Khaneghah, A.M. Recent advances in non-thermal and thermal processing of jackfruit ( Artocarpus heterophyllus Lam ): An updated review. J. Food Process. Preserv. 2022, 46. [CrossRef]
- Rahmani, A.M.; Gahlot, P.; Moustakas, K.; Kazmi, A.; Ojha, C.S.P.; Tyagi, V.K. Pretreatment methods to enhance solubilization and anaerobic biodegradability of lignocellulosic biomass (wheat straw): Progress and challenges. Fuel 2022, 319, 123726. [CrossRef]
- Usmani, Z.; Sharma, M.; Diwan, D.; Tripathi, M.; Whale, E.; Jayakody, L.N.; Moreau, B.; Thakur, V.K.; Tuohy, M.; Gupta, V.K. Valorization of sugar beet pulp to value-added products: A review. Bioresour. Technol. 2021, 346, 126580. [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; Di Domenico, M.; Filho, V.F.d.S.; de Sena, R.F.; Machado, R.A.F.; Marangoni, C. Insights into the bioenergy potential of jackfruit wastes considering their physicochemical properties, bioenergy indicators, combustion behaviors, and emission characteristics. Renew. Energy 2020, 155, 1328–1338. [CrossRef]
- Ranasinghe, R.A.S.N.; Maduwanthi, S.D.T.; Marapana, R.A.U.J. Nutritional and Health Benefits of Jackfruit (Artocarpus heterophyllus Lam.): A Review. Int. J. Food Sci. 2019, 2019, 1–12. [CrossRef]
- Sreeletha, A.S.; Lini, J.J.; Dhanyalekshmi, C.S.; Sabu, K.R.; Pratap, C.R. Phytochemical, proximate, antimicrobial, antioxidant and FTIR analyses of seeds of Artocarpus heterophyllus Lam. Adv Biotechnol Microbiol. 2017, 5(1), 555–653.
- Burci, L.M.; da Silva, C.B.; de Oliveira, M.; Dalarmi, L.; Zanin, S.M.W.; Miguel, O.G.; Miguel, M.D. Determination of antioxidant, radical scavenging activity and total phenolic compounds of Artocarpus heterophyllus (Jackfruit) seeds extracts. J Med Plants Res. 2015, 9(40), 1013–1020.
- Jiang, T.-T.; Liang, Y.; Zhou, X.; Shi, Z.-W.; Xin, Z.-J. Optimization of a pretreatment and hydrolysis process for the efficient recovery of recycled sugars and unknown compounds from agricultural sweet sorghum bagasse stem pith solid waste. PeerJ 2019, 6, e6186. [CrossRef]
- Taheri, M.E.; Salimi, E.; Saragas, K.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Effect of pretreatment techniques on enzymatic hydrolysis of food waste. Biomass- Convers. Biorefinery 2020, 11, 219–226. [CrossRef]
- Adewuyi, Y.G.; Deshmane, V.G. Intensification of enzymatic hydrolysis of cellulose using high-frequency ultrasound: an investigation of the effects of process parameters on glucose yield. Energy Fuel. 29 (2015) 4998–5006. [CrossRef]
- Surh, J.; Decker, E.A.; McClements, D.J. Utilisation of spontaneous emulsification to fabricate lutein-loaded nanoemulsion-based delivery systems: factors influencing particle size and colour. Int. J. Food Sci. Technol. 2017, 52, 1408–1416. [CrossRef]
- ; Sundari, C.D.D.; Sunarya, R.R.; Suryaningsih, S. Optimization of bioethanol production from jackfruit straw waste through the addition of a starter and fermentation duration. 2023; 2572, 030014. [CrossRef]
- Arif, A.R.; Natsir, H.; Rohani, H.; Karim, A. Effect of pH fermentation on production bioethanol from jackfruit seeds (Artocarpus heterophyllus) through separate fermentation hydrolysis method. J. Physics: Conf. Ser. 2018, 979, 012015. [CrossRef]
- Nikolić, S.; Lazić, V.; Veljović, Đ.; Mojović, L. Production of bioethanol from pre-treated cotton fabrics and waste cotton materials. Carbohydr.polym. 164 (2017) 136-144.
- Brexó, R.P.; Sant’ana, A.S. Impact and significance of microbial contamination during fermentation for bioethanol production. Renew. Sustain. Energy Rev. 2017, 73, 423–434. [CrossRef]
- Yuvarani, M.; Immanuel, C.S. Dhas.Synthesis of Bioethanol from Artocarpus Heterophyllus Peel by Fermentation using Saccharomyces Cerevisiae at Low Cost. GRD J. Eng. 2 (12) (2017), 1-7.
- Zabed, H.; Faruq, G.; Sahu, J.N.; Azirun, M.S.; Hashim, R.; Boyce, A.N. Bioethanol Production from Fermentable Sugar Juice. Sci. World J. 2014, 2014, 1–11. [CrossRef]
- Kaewchana, A.; Techaparin, A.; Boonchot, N.; Thanonkeo, P.; Klanrit, P. Improved high-temperature ethanol production from sweet sorghum juice using Zymomonas mobilis overexpressing groESL genes. Appl. Microbiol. Biotechnol. 2021, 105, 9419–9431. [CrossRef]
- Rukshika, S.; Sisira, K.; Sanath, R.; Subramanium, S. Isolation and Characterization of an Ethanol Resistant Bacterium from Sap of Saccharum officinarum for Efficient Fermentation. Internat J Biotechnol Bioeng. 03(02) (2016) 15-20.
- Kularathne, I.W.; Gunathilaka, C.A.; Ratnaweera, A.C.; Kalpage, C.S.; Rajapakse, S.; Gamage, P. Optimization of Fermentation Process Parameters for Bioethanol Production from Sri Lankan Overripe Fruits. Eng. J. Inst. Eng. Sri Lanka 2021, 54, 77. [CrossRef]
- Mibulo, T.; Nsubuga, D.; Kabenge, I.; Kiggundu, N.; Wydra, K.D. Comparative Study of Biogas Production from Jackfruit Waste, Banana Peels, and Pineapple Peels Co-Digested with Cow Dung. J. Sustain. Bioenergy Syst. 2023, 13, 1–15. [CrossRef]
- Balamaze, J.; Muyonga, J.; Byaruhanga, Y. Production and utilization of jackfruit (Artocarpus heterophyllus) in Uganda. Afr. J. Food, Agric. Nutr. Dev. 2019, 19, 14289–14302. [CrossRef]
- Bijarchiyan, M.; Sahebi, H.; Mirzamohammadi, S. A sustainable biomass network design model for bioenergy production by anaerobic digestion technology: using agricultural residues and livestock manure. Energy, Sustain. Soc. 2020, 10, 1–17. [CrossRef]
- D’Adamo, I.; Falcone, P.M.; Huisingh, D.; Morone, P. A Circular Economy Model Based on Biomethane: What Are the Opportunities for the Municipality of Rome and Beyond? Rene Ener. 163, (2021) 1660-1672.
- Waluyo, J.; Pratiwi, Y. Analysis Proximate , Ultimate , and Thermal Gravimetric Based on Variations Dimensions of Briquettes from Waste Jackfruit Crust. Internat J Scient. Eng. Sci. 2(10), (2018) 36–39.
- Walsh, J.J.; Jones, D.L.; Chadwick, D.R.; Williams, A.P. Repeated application of anaerobic digestate, undigested cattle slurry and inorganic fertilizer N: Impacts on pasture yield and quality. Grass Forage Sci. 2018, 73, 758–763. [CrossRef]
- Westerholm, M.; Castillo, M.; Andersson, A.C.; Nilsen, P.J.; Schnürer, A. Effects of thermal hydrolytic pre-treatment on biogas process efficiency and microbial community structure in industrial- and laboratory-scale digesters. Waste Manag. 2019, 95, 150–160. [CrossRef]
- Zakaria, M.R.; Ariffin, H.; Abd-Aziz, S.; Hassan, M.A.; Shirai, Y. Improved Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Produced byComamonassp. EB172 Utilizing Volatile Fatty Acids by Regulating the Nitrogen Source. BioMed Res. Int. 2013, 2013, 1–7. [CrossRef]
- Gowda, V.; Shivakumar, S. Agrowaste-based Polyhydroxyalkanoate (PHA) production using hydrolytic potential of Bacillus thuringiensis IAM 12077. Braz. Arch. Biol. Technol. 2014, 57, 55–61. [CrossRef]
- Sehgal, R.; Gupta, R. Polyhydroxyalkanoate and its efficient production: an eco-friendly approach towards development. 3 Biotech 2020, 10, 1–14. [CrossRef]
- Nelluri, P.; Venkatesh, T.; Kothakota, A.; Pandiselvam, R.; Garg, R.; Eswaran, V.; Vaddevolu, U.B.P.; Venkatesh, R.; Khaneghah, A.M. Recent advances in non-thermal and thermal processing of jackfruit ( Artocarpus heterophyllus Lam ): An updated review. J. Food Process. Preserv. 2022, 46. [CrossRef]
- Gupta, R.; Gupta, S.K.; Gehlot, C.L.; Bahadur, I. Chemically modified jackfruit leaves as a low-cost agro-waste adsorbent for Pb(II) removal from synthetic wastewater. J. Hazard. Mater. Adv. 2023, 10. [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [CrossRef]
- Khan. A.U. A Review on Importance of Artocarpus heterophyllus L. (Jackfruit). J. Multidiscip. Appl. Nat. Sci., 2021. 1(2), 106-116.
- Zhang, L.; Lu, S.; Xiao, D.; Gu, M. Characterization of full pore size distribution and its significance to macroscopic physical parameters in tight glutenites. J. Nat. Gas Sci. Eng. 2017, 38, 434–449. [CrossRef]
Figure 1.
Production of jackfruits wastes.

Figure 2.
Jackfruit wastes valorization into different bioproducts.

Figure 3.
Schematic representation of jackfruit plants parts with its waste conversion into value-added products.
Figure 3.
Schematic representation of jackfruit plants parts with its waste conversion into value-added products.

Figure 4.
Green extraction for better yield of bioactive compounds from jackfruit wastes.

Figure 5.
Jackfruit wastes utilized for bioenergy task.

Figure 6.
Jackfruit wastes promoting the zero-waste generation and biorefinery promotion.

Table 1.
Different value-added products (i.e., biofuel, bioplastic, enzymes, pectin, nano-emulsion) synthesis from jackfruit waste conversion via valorization techniques.
Table 1.
Different value-added products (i.e., biofuel, bioplastic, enzymes, pectin, nano-emulsion) synthesis from jackfruit waste conversion via valorization techniques.
| Jackfruit wastes | Value-added products | Application | References |
|---|---|---|---|
| Jackfruit wastes with its different parts like peel/ skins | Bioethanol, biogas, bioplastics, are so on generated. | Biofuel is cheaper and greener than fossil fuels. Processed peel cleans dye-contaminated aquatic environments. | [7] |
| Durian shell and jackfruit peel | An increase of 103.8% in methane output and 69.8% in biodegradability. | Produces high levels of sustainable energy and fuel (2.0 109 MJ/year), while simultaneously decreasing coal usage (6.8 104 tons/year) and cutting emissions by 2.2 1010 particulate/year. | [9] |
| Jackfruit outer rind | Used as substrate for producing recombinant endoglucanase from Bacillus subtilis MU S1 | This enzyme helps in highest saccharification process (33.4 %) from jackfruit outer rind at 96 h of incubation | [11] |
| Jackfruit peel waste | Bio-oil upgrading is done by sub/super-critical fluids, solvent addition, and steam reforming | The application of bio=oil can substitute fuel for the commercial or industrial burner | [12] |
| Jackfruit seed starch was plasticized | Used to produce starch-based bioplastics | Four distinct kinds of bioplastics were manufactured in order to investigate the influence of the plasticizers and to characterise the features of the bioplastics that correspond to those plasticizers. | [18] |
| Utilization of jackfruit peel | There have been reports of the use of an adsorbent made from jackfruit peel. | Jackfruit peel adsorbent can biosorb maximally of232.55 mg/g of MB. | [19] |
| Artocarpus heterophyllus Lam. seed powder extract (ASPE) | Green production of silver nanoparticles from silver nitrate in water | ASPE may be utilized to synthesize AgNPs for nanomedicine in the future. It is eco-friendly and harmless. | [25] |
| Artocarpus heterophyllus peels | Green synthesis of iron nanoparticles is reported | Iron nanoparticles were highly catalytic, removing 87.5% in 20 minutes at 318 K. | [28] |
| Artocarpus heterophyllus (jackfruit) peel | Bio-based coagulating agent | The extract from the peel has the potential to serve as a bio-based coagulating agent alternative that is useful in the pre-treatment of wastewater. | [32] |
| Jackfruit seed powder (JSP) | The surface of JSP is used | Novacron blue textile dye can be decolorized using this substance. After a contact time of 60 minutes, the surface of JSP has been observed to adsorb 73% of Novacron blue. | [83] |
| Jackfruit waste feedstock | Biogas production was improved by chemical catalysts with maintaining the pH and C/N ratio. | Biogas is produced via decreasing the digestion time with improving the efficiency of digester unit by using jack fruit waste as raw material. This raw material contains significant quantity of fiber with small proportions of glucose. | [84] |
| Jackfruit waste together with peels (JP) as well as seeds (JS) | Bioenergy has optimal physicochemical, bioenergy indicators, combustion, and emission properties. | The bioenergy yields for JP and JS were 2.5 and 0.9 ha−1 yr−1 (dry basis), correspondingly. Low concentrations of CO, CO2, and SO2 may be released. | [92] |
| Jackfruit straw waste | Used as raw material for making bioethanol. | The amount of bioethanol distillate that could be produced under ideal circumstances was 30 ml. This research employed a distillation temperature range of 70 to 78 °C. | [100] |
| Jackfruit (Artocarpus heterophyllus) stone waste | Ethanol from these waste is found and it is used as renewable fuels | A quantity of Jackfruit flour was subjected to hydrolysis by the addition of 0.3 to 0.7 mL of alpha-amylase and 0.2 to 0.6 mL of glucoamylase. The resulting mixture was then subjected to a fermentation process lasting between 3 to 6 days. The yield of ethanol obtained from this process was found to be between 11 to 13%. | [41] |
| Renewable energy from jackfruit's seeds | Bioethanol (57.94%) is reported | The fermentation process for ethanol production is used with Saccharomyces cereviceae with a variation of pH values with 70 hours | [101] |
Table 2.
Health benefits from bioactive compounds that are recovered from jackfruit waste matters.
| Jackfruit wastes | Bioactive compounds | Health benefits | References |
|---|---|---|---|
| Jackfruit skins, leaves, and barks | Vitamins, minerals, and phytochemicals | The substance under consideration exhibits various properties such as anticarcinogenic, antimicrobial, antifungal, anti-inflammatory, wound healing, and hypoglycemic effects. | [2] |
| Agro-residues from jackfruit plants | Nanocapsules uses increase the bioactive efficacy | Bioactive in micro- and nanoencapsulation forms enhanced target site delivery in human body with more benefits | [4] |
| Jackfruits are known for their prickly outer bark and axis. | Flavonoids, stillbenoids, morin, artocarpin, dihydromorin and cynomacurin, | Because of their bioactivity, these chemicals have the potential to be developed into nutraceuticals with antioxidant characteristics. | [5] |
| Leave and stem bark extract of Artocarpus Heterophyllus | Extract is dominated with tannin and saponin | The methanol extract of Artocarpus stem and leaf bark has antibacterial and antioxidant properties, and the bark may be used topically as a peel-off mask. | [6] |
| Jackfruit peel | Functional food additives and pectin materials | These compounds are manipulated for food ingredient applications with providing of health benefit to gastro-intestinal tract. | [8] |
| Spine, skin and rind of jack fruit | Polyphenol as well as flavonoids | Crude ethanolic extracts undergo evaluation for their anti-inflammatory potential. | [14] |
| Fruit peel of jackfruit | Flavonoid, and also present some phenolic compounds is reported | Various phyto-constituents can use In different additives of human use with more health benefits |
[21] |
| Jackfruit seed extracts in three different solvents: methanol, hydroalcoholic, and aqueous. | Alkaloids, flavonoids, terpenoids, and so on | This extracts showed the antioxidant, anti-inflammatory and antibacterial activity | [23] |
| Artocarpus heterophyllus J33 rind parts | Proto-catechuic acid (PCA) antioxidant activity depends on its temperature: 25°C, 4°C, and -18°C. | The extract’s antioxidant activity was maintained because PCA is so stable. It has antioxidant properties and might be used in food and dietary supplements. | [40] |
| Seeds of jack fruit | The different solvent extracts showed the presence of fats, phenols and flavonoids. | Acetone extract demonstrated the greatest antibacterial activity towards Staphylococcus aureus and the greatest antifungal activity against Aspergillus flavus. | [94] |
| Jack fruit seeds | Total phenolic compounds in this seed extract confirmed by different screening tests | Total phenolic substances are identified as antioxidants with radical scavenging action. | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Onion (Allium cepa L.) Skin Waste Valorization: Unveiling the Phenolic Profile and Biological Potential for the Creation of Bioactive Agents through Subcritical Water Extraction
Esther Trigueros
et al.
,
2024
Lignocellulosic Residues from Fruit Trees: Availability, Characterization, and Energetic Potential Valorization
Gianluca Cavalaglio
et al.
,
2024
Lignin Extraction from Waste Pine Sawdust Using a Biomass Derived Binary Solvent System
Solange Magalhães
et al.
,
2021
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated











